Inflammation and the pathophysiology of work-related musculoskeletal disorders (original) (raw)
. Author manuscript; available in PMC: 2006 Sep 1.
Published in final edited form as: Brain Behav Immun. 2006 May 2;20(5):423–429. doi: 10.1016/j.bbi.2006.03.001
Abstract
Work-related musculoskeletal disorders (MSDs) have accounted for a significant proportion of work injuries and workers' compensation claims in industrialized nations since the late 1980s. Despite epidemiological evidence for the role of repetition and force in the onset and progression of work-related MSDs, complete understanding of these important occupational health problems requires further elucidation of pathophysiological mechanisms of the tissue response, particularly in the early stage of these disorders. Results from several clinical and experimental studies indicate that tissue microtraumas occur as a consequence of performing repetitive and/or forceful tasks, and that this mechanical tissue injury leads to local and perhaps even systemic inflammation, followed by fibrotic and structural tissue changes. Here we review work linking inflammation and the development of work-related MSDs. We also propose a conceptual framework suggesting the potential roles that inflammation may play in these disorders, and how inflammation may contribute to pain, motor dysfunction, and to puzzling psychological symptoms that are often characteristic of patients with work-related MSDs.
Keywords: Work-related musculoskeletal disorder, Repetitive strain injury, Inflammation, Tissue injury
1. Introduction
Work-related musculoskeletal disorders (MSDs), also called overuse injuries, have accounted for a significant proportion of work injuries and workers' compensation claims in Western industrialized nations since the late 1980s. Recent epidemiological studies have greatly improved methods to distinguish the contribution of workplace and non-workplace risk factors to the development and severity of MSDs. It has become clear that both workplace and non-workplace factors, including psychosocial factors, may cause or exacerbate MSDs and that the key to controlling the impact of such disorders is prevention or early intervention. However, in order to plan effective treatments, the pathophysiological mechanisms underlying MSDs need to be further elucidated. Several recent clinical and experimental studies have been published indicating that inflammation plays a role in the development of tissue pathologies associated with these chronic disorders. We have undertaken experiments in a rat model of upper extremity work-related MSD in order to investigate early tissue and behavioral effects of performing highly repetitive and forceful forelimb intensive tasks. Here, we review our work and that of others examining both upper and lower extremity overuse MSDs showing the role of inflammation in the development of work-related MSDs.
2. Work-related musculoskeletal disorders—definitions and risk factors
The US Department of Labor defines work-related MSDs as injuries or disorders of the muscles, nerves, tendons, joints, cartilage, and spinal discs associated with exposure to risk factors in the workplace. MSDs include sprains, strains, tears, back pain, soreness, pain, carpal tunnel syndrome, musculoskeletal system, or connective tissue diseases and disorders, when the event or exposure leading to the injury or illness is bodily reaction/bending, climbing, crawling, reaching, twisting; overexertion; or repetition (Bureau of Labor Statistics, 2005). Several risk factors are associated with the development or exacerbation of MSDs in the workplace, including physical, biomechanical, individual predisposition, and psychosocial conditions.
Epidemiological research associates the onset and severity of hand and wrist overuse MSDs with the performance of repetitive and forceful hand-intensive tasks (see Barr et al., 2004 for review). MSDs are worsened by the performance of such tasks in the presence of awkward or fixed postures for long periods, vibration, and cold temperatures (Bernard, 1997; National Research Council, 2001). While exact dose-response relationships between work task demands and upper or lower extremity overuse MSDs are not clearly defined due to the vast number of work tasks that humans perform, several researchers have attempted to establish criteria for maximum acceptable forces and movements for tasks based on psychophysical outcomes (see Barr and Barbe, 2002 for a review). Silverstein et al. (1986) performed job analyses of industrial workers and defined high repetition rate as less than 30 s/cycle and low repetition rate as greater than 30 s/cycle. Despite the differences in methods of force estimation across a number of studies, there is a consensus that an exertion requiring less than 15% maximum grip force can be considered negligible to low and exertions requiring greater than 50% maximum grip force can be considered high (see Barr and Barbe, 2002 for a review).
Psychosocial risk factors in the workplace also contribute to MSDs. These factors are associated with levels of workplace stress, such as job content and demands, job control, and social support (National Research Council, 2001). Non-workplace factors may also contribute to the development and exacerbation of MSDs, such as similar physical or high stress levels in the home. Certain past or present medical conditions also represent comorbid risk factors for MSDs (National Research Council, 2001). Examples include past traumatic injury to the affected body part, systemic diseases that affect the musculoskeletal system, and diseases/disorders of the circulatory system. Women appear more susceptible than men to the development of MSDs, although this is highly industry-dependent. Advanced age or obesity may increase the impact of other risk factors on the severity of MSDs (National Research Council, 2001).
3. Musculotendinous injury and inflammation in the development of MSD
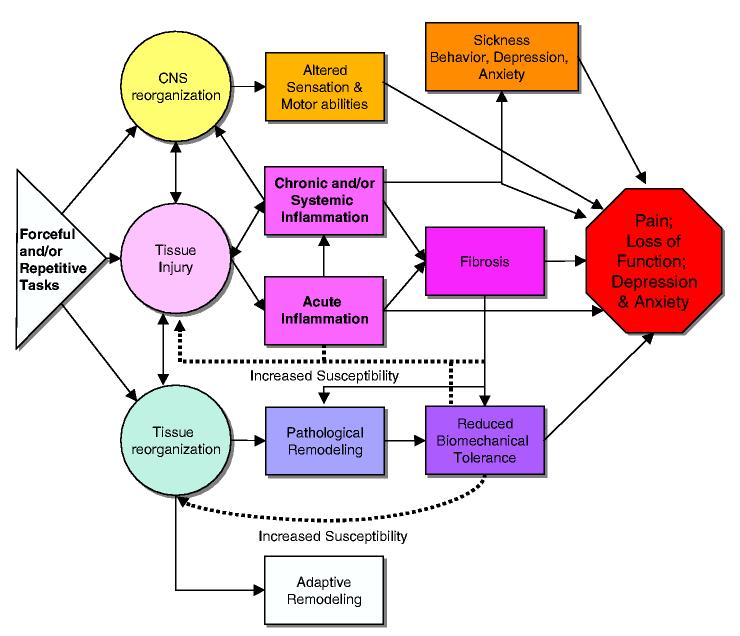
Musculotendinous injuries resulting from performing repetitive and/or forceful tasks are due to repeated overstretch, compression, friction, ischemia, and overexertion. We hypothesize that these injuries lead initially to an inflammatory response (Fig. 1). While the ultimate outcome of inflammation is to replace or repair injured tissues with healthy, regenerated tissue Copstead and Banadki, 2000, when continued task performance is superimposed upon injured and inflamed tissue a vicious cycle of injury, chronic or systemic inflammation, fibrosis, and perhaps even tissue breakdown may occur. The end result is often pain and loss of motor function.
Fig. 1.
Schematic diagram showing three primary pathways hypothesized to lead to work-related musculoskeletal disorders caused by repetitive and/or forceful hand-intensive tasks: CNS reorganization (reviewed in Barr et al., 2004), tissue injury, or tissue reorganization. With respect to the injury/inflammatory pathway, we hypothesize that repetitive tasks lead Wrst to tissue injury, then acute inflammation. Normally, this response would activate cellular mechanisms related to healing. However, with overuse MSDs, the continued cycle of tissue trauma induced by continued performance of a repetitive and/or forceful task hinders repair. Instead, a chronic inflammatory response (with associated immune cell activity causing secondary tissue injury) is stimulated along with a fibrogenic response. If task exposure is great enough to induce injury, inflammatory responses are induced with fibrosis often following. Continued performance of high demand tasks can also lead to structural disorganization (reduced biomechanical tolerance and pathological remodeling). However, if task exposure is low enough to avoid tissue injury, then inflammation is avoided and tissue reorganization moves into a beneficial adaptive remodeling. What is potentially intriguing about this graded response of musculoskeletal and peripheral nerve tissues to repetitive motion is the possibility that there is a threshold of activity below which the tissue response, whether accompanied by inflammation or not, leads to adaptive rather than degenerative long-term tissue changes. Interrelationships between components of these pathways are indicated, which illustrates the pathomechanical complexity that may contribute to an end point of pain, loss of function, and perhaps, sickness behaviors, depression, and/or anxiety.
One of the challenges in studying workers is the diffculty in determining the causality between tissue and behavioral responses. A hypothesis of transient inflammation is supported by several clinical studies examining tenosynovial biopsies from patients with tendosynovial thickening as a result of carpal tunnel syndrome (CTS). Hirata et al. (2005) divided patients into symptom duration groups (<3, 4-7, 8-12, and >12 months). Edematous changes were found in these tissues in patients of <3 month duration. Prostaglandin E2 (PGE2) and vascular endothelial growth factor (VEGF) were increased in patients of 4-7 month symptom duration, while fibrotic changes were present in patients of longer symptom duration (>7 months). PGE2 is a factor believed to cause vasodilation, edema, and enhancement of cytokines that induce synoviocyte proliferation, while VEGF is associated with endothelial and vascular smooth muscle cell proliferation during chronic inflammation. In Hirata’s study, both molecules peak in the intermediate phase (4-7 months) of CTS-induced tendosynovial changes and appear to contribute to tissue remodeling. Hirata postulates that since PGE2 is thought to regulate the production of several molecules, that it may regulate VEGF production in tenosynovium. Freeland et al. (2002) also found increased levels of PGE2 and IL-6, but not IL-1, in tendosynovial tissues collected from 41 patients with idiopathic carpal tunnel syndrome at the time of carpal tunnel release. The increase in IL-6 is interesting. IL-6 has both inflammatory and anti-inflammatory properties, the latter primarily to suppress low-grade inflammation (Biffl et al., 1996). IL-6 is a tightly regulated cytokine normally not detectable in serum unless there is trauma, infection, or cellular stress, at which time IL-6 is an early cytokine responder. Pro-inflammatory effects of IL-6 include induction of cell growth and proliferation, and acute-phase responses, while its anti-inflammatory actions include inducing increases in serum IL-1 receptor antagonist and soluble TNF receptor (Biffl et al., 1996). These tenosynovial studies in CTS patients show an early inflammatory response, followed by an attempt at resolution that fails, and results in fibrosis.
Studies have also been performed on muscle tissue biopsied from patients with long-term chronic overuse syndromes. Trapezius muscle biopsies from male and female workers with either continuous or intermittent trapezius myalgia of at least 12 months duration show evidence of myopathic changes such as moth eaten and ragged, red type I muscle fibers, increased frequency of type II myofibers and atrophic myofibers consistent with muscle injury, and denervation/ischemic loss of muscle fibers, but no evidence of inflammation (Larsson et al., 2001). In contrast, Dennet and Fry (1988) examining the first dorsal interosseous muscle collected from 29 patients with painful chronic overuse syndrome found increased inflammatory cells as well as myopathic changes. While both studies found myopathic changes in muscles of these patients, they differ with respect to findings of inflammation. It is clear from human and animal studies that the interaction between exposure level, anatomical site, and the nature of the task produce different tissue responses with respect to magnitude and/or timing.
Only three published studies to date have looked for serum biomarkers indicative of underlying tissue changes in patients with overuse MSD. The first study, by Freeland et al. (2002) detected increased serum malondialdehyde, an indicator of cell stress, in patients with carpal tunnel syndrome, but no serum increases in PGE2, IL-1, or IL-6. A recent study by Kuiper et al. (2005), examined serum for biomarkers of collagen synthesis and degradation (but not for biomarkers of injury or inflammation) in construction workers involved in heavy manual materials handling. Both collagen synthesis and degradation products were increased in workers involved in heavy manual tasks, although the overall ratio of synthesis to degradation products remained the same as in sedentary workers. Kuiper’s results suggest that tissues undergo adaptive growth responses that protect them from unresolved degradation. In the third study, elevated plasma fibrinogen were present in subjects with low job control, linking perceived job stress with a biomarker of chronic inflammation (Clays et al., 2005). This was a large study of blue- and white-collar workers (n D 892), all without clinical heart disease. Although work-related MSD prevalence was not determined, and the authors suggest that these fibrinogen levels may be a link between job stress and future cardiovascular disease, a recently submitted study from our lab found increased pro-inflammatory cytokines in serum of patients with moderate and severe work-related MSD. Since inclusion in our study required duration of symptoms no longer than 12 weeks, these findings support the presence of an early inflammatory process in the development of work-related MSDs.
A number of animal studies relate exercise loading of upper and lower extremity tendons to inflammatory changes. Backman et al. (1990) developed a controlled kicking rabbit model of Achilles tendinosis (150 repetition/min, 2 h/session, 3 days/week for 5-6 weeks). By week 5, inflammatory cells, matrix reorganization, and tissue necrosis were present in the tendon. Using this same model but at a slower repetition rate (75 repetitions/min), Archambault et al. (1997) observed hypercellularity, inflammatory cells, increased inflammatory cytokines, and increased mRNA of matrix molecules in the tendon by 6-8 weeks. When the kicking protocol was prolonged to 11 weeks, the inflammatory responses were apparently resolved. Instead, matrix reorganization processes, such as increased mRNA for collagen type III and matrix metalloproteinases, were observed (Archambault et al., 2001). Thus, in the higher demand kicking task, inflammation and tissue pathology were simultaneously present, while in the lower demand kicking task, inflammation preceeded matrix reorganization which may be a beneficial adaptive reorganization since no necrosis was observed. Soslowsky and colleagues developed a rat model of running-induced rotator cuff tendinopathy (Perry et al., 2005; see Barr et al., 2004 for a review). In a series of studies, they report evidence of inflammation and angiogenesis (hypercellularity; increased COX-2 and VEGF mRNA) after 4 weeks of running at a rate of 17 m/min on a decline, 1 h/day for 5 days/week. These changes persisted through 16 weeks. They also found tendon thickening and reduced biomechanical tissue tolerance, changes that increased with continued exposure. Thus, repetitive tendon overuse is associated with inflammation. The tendon tissue is unable to launch a successful healing response due to continued use, and becomes fibrotic and structurally damaged. Concerning differences in the varying length of the inflammatory response in these studies, as stated earlier, the interaction between exposure level, anatomical site, and the nature of the task produce different tissue responses with respect to magnitude and/or timing.
Chronic repetitive contraction of muscles also leads to inflammatory responses and fibrosis. Studies by Stauber and Willems (2002) (see Barr et al. (2004) for review) using a rat model of muscle forced-lengthening indicate that repeated muscle strains (3 days/week for 4-6 weeks) at fast velocities with short inter-stretch rests result in myopathic and inflammation, including muscle fibers that stain negative for desmin (a cellular protein, the loss of which indicates injury), increased complement C3 and macrophage infiltration. His studies also indicate that continued exposure to repeated muscle strains at fast velocities (25 mm/s) leads to muscle fibrosis, while repeated muscle strains at low velocities (10 mm/s) lead to compensatory, adaptive responses.
Our lab has developed a rat model of voluntary repetitive reaching and grasping with or without force in order to study the effects of such tasks on tissues. Our rats were trained to reach for a pellet of food or to pull on a bar for 2 h/day, 3 days/week, for 8-12 weeks. We found tissue injury and increased macrophage infiltration in upper extremity musculoskeletal tissues in as few as five weeks of performing a high repetition, low force (HRLF) task (4 reaches/min, <15% maximum grip strength; reviewed in Barr et al., 2004). Tissue macrophages were increased bilaterally in upper extremity tissues and in hind limb tissues, suggestive of widespread inflammation. The inflammatory response was transient in bone of HRLF rats, resolving in week 12 despite continued task performance. We examined musculotendinous tissues only to week 8, at which time there was no resolution of the macrophage response. There was no similar increase in macrophages in tissues of low repetition, low force (LRLF; 2 reaches/min) rats, indicating that the demands of the LRLF task were below a threshold capable of induced injury and/or inflammation. Serum analyses revealed increased levels of IL-1α in week 8 in the HRLF rats, but not in the LRLF rats (reviewed in Barr et al., 2002, 2004). We hypothesize that the net cytokine production in the lower demand task allows for the maintenance of homeostasis. The level of repeated incidents of mechanical tissue injury and local inflammation in the HRLF group, on the other hand, leads to a net production of serum IL-1α, a finding indicative of a systemic inflammatory response. These dose-dependent findings are similar to our recently submitted human study in which a systemic inflammatory mediator/marker response was greater in patients with moderate and severe MSD compared to mild.
4. Peripheral nerve injury and inflammation in the development of MSD
In MSD, the primary causes of peripheral nerve trauma are over-stretch and compression of neuronal tissues during excursion (reviewed in Barr et al., 2004). Animal models of chronic nerve constriction injury using ligatures show that chronic compression leads to an upregulation of intraneural inflammatory cytokines, fibrosis, Schwann cell death, axonal demyelination, and declines in electrophysiological function. In our rat model, we found decreased nerve conduction velocity (NCV) in the median nerve at the wrist. By week 10 in HRLF rats, there was a small (9%) but significant decrease in NCV (Clark et al., 2003), demonstrating that nerve injury accumulates with continued task performance and leads to a clinically relevant loss of nerve function. The decrease in NCV was even greater (16%) in rats performing a high repetition, high force (HRHF; 4 reaches/min, 60% maximum grip strength) task for 12 weeks, indicating a positive dose-response relationship between task exposure level and loss of nerve function (Clark et al., 2004). Macrophages increased in nerves of both exposure groups as early as 3 weeks and persisted to 12 weeks. Neural fibrosis followed the peak inflammatory response temporally and was associated reduced conduction velocity. Transient but marked increases in several proinflammatory cytokines (IL-1α, IL-1β, and TNF-α) were also observed in median nerves of HRLF rats (Al-Shatti et al., 2005). The decline in inflammatory cytokines matched temporally with increases of an anti-inflammatory cytokine, IL-10, known to down-modulate production of pro-inflammatory cytokines. Last, we observed an increase in paw withdrawal threshold at 12 weeks in the HRHF rats, indicative of a loss in sensation most likely caused by fibrotic compression-induced axonal demyelination.
5. Behavioral changes that coincide with tissue inflammation in overuse MSDs
We have reported on motor behavioral indicators in our rat model that offer insight into the behavioral consequences of inflammation. Reach rate (RR; reaches/min) is an indicator of the animals' ability to maintain task pace, while reach movement pattern is an indicator of the quality of reaching. The HRLF group undergoes a significant decline in reach rate in week 6, which coincides with the peak inflammatory response, and a return toward baseline in week 8 (reviewed in Barr et al., 2004), which coincides with the resolution of tissue inflammation. The LRLF group, the group without injury or inflammatory changes, did not exhibit any change in reach rate (Barr et al., 2002). All HRLF animals developed progressively degraded reach movement patterns by 7 weeks of task performance, whereas only 60-70% of LRLF animals underwent reach movement pattern degradation. The HRHF group underwent a significant decline in RR as early as week 3, a persistent change, and significant decreases in sensation, grip strength and nerve conduction velocity by week 12 (Clark et al., 2004), matching the increased inflammatory and fibrotic tissue responses temporally. Thus, we observed dose-dependent motor behavioral decreases that match the timing and magnitude of tissue inflammatory responses.
Messner et al. (1999) also reported behavioral changes that develop in a rat model of eccentric loading of the Achilles tendon. With a repetitive task of 30 cycles/min, 1 h/day, 3 days/week for 7-11 weeks, they observed the development of a permanent limping gait that was associated with degenerative changes in the tendon outer sheath, angiogenesis and increased expression of neurochemicals often associated with pain. The association of motor behavioral changes with tissue changes in both our and Messner’s studies indicates that functional declines accompany tissue injury, inflammation and fibrosis/degeneration.
6. Role of pro-inflammatory cytokines in sickness behaviors, depression, and anxiety
The psychoneuroimmunological effects of pro-inflammatory cytokines, specifically IL-1β, TNF-α, and IL-6, have been extensively studied in humans and in animal models over the past decade for their contribution to a constellation of physiological and behavioral responses known collectively as the “sickness behaviors”. This response includes fever, weakness, listlessness, hyperalgesia, allodynia, decreased social interaction and exploration, somnolence, decreased sexual activity, and decreased food and water intake (amply reviewed by Capuron and Dantzer, 2003; Wieseler-Frank et al., 2005). Sickness behaviors can be induced by administration of exogenous cytokines to animals, whether the cytokines were injected peripherally or centrally. One mechanism of action, the immune-to-brain communication through activation of brain and spinal cord glial cells was reviewed by Wieseler-Frank et al. (2005). Activation of CNS glia and subsequent production of inflammatory cytokines can lead to hyperalgesia.
In our model, animals exhibit dose-dependent task avoidance (decreased duration) over weeks of task performance (Clark et al., 2003, 2004). The HRLF group declined in duration in week 3, and then regained baseline duration by week 6. The task avoidance in week 3 matches the onset of inflammatory cytokine production, while the recovery to baseline duration matches temporally increased production of IL-10, an anti-inflammatory cytokine. This lack of participation often took the form of the animal curling up and falling asleep for some portion of the task session. Such behavior might indicate either malaise or decreased appetite.
Recent attention has also been given to the possible role of serum circulating cytokines in the etiology of depression and other mood disorders, particularly among cancer patients treated with cytokine therapy (Capuron and Dantzer, 2003). Cohen et al. (1997) have also speculated that the elevation of serum IL-6 produces fatigue, which then may be responsible for decreases in an individual’s ability to perform functionally. The possibility for patients with chronic inflammatory conditions to succumb to the depressive effects of local and systemic pro-inflammatory cytokines has implications in the management of overuse MSDs.
7. Psychoneuroimmunological changes in workers with MSDs?
Symptoms of depression, anxiety, heightened job stress, more anger with their employer, higher pain ratings, greater reactivity to pain, enhanced feelings of being overwhelmed by pain, and low confidence in problem solving abilities have been reported in numerous epidemiological and clinical studies of patients with MSDs (Clays et al., 2005; Gold et al., 2006; Shaw et al., 2002). One recent study reports significantly reduced pressure pain thresholds in automobile manufacturing workers (n 460) with physical examination findings of upper extremity MSDs (D Gold et al., 2006). The reduced pressure pain thresholds were associated with female gender, increasing age, and decreased grip strength.Shaw et al. (2002) examined factors correlating with functional limitations in 165 workers' compensation claimants. Multiple regression analyses showed that presence of hand symptoms, feeling overwhelmed by pain, low confidence in problem solving, and greater ergonomic risk factor exposures were significant predictors of functional motor limitations. Gold and Shaw did not include examination of serum for biomarkers of injury or inflammation, making it difficult to know if there was an underlying inflammatory process driving these symptoms (Clays' findings of increased serum fibrinogen were described earlier). Thus, although there is strengthening evidence of relationships between inflammation, cytokines, sickness behavior, depression, and anxiety, this relationship has yet to be studied in patients with MSDs. However, the possible role of serum cytokines in patients with MSDs in contributing to psychosocial problems is compelling and should be explored further.
8. How may inflammation be involved in the early development of work-related MSDs?
We have developed a conceptual model of tissue and behavioral changes associated with work-related MSDs (Fig. 1). We hypothesize that performance of repetitive and/or forceful tasks may induce MSDs through three primary pathways: (1) CNS reorganization, (2) tissue injury, and (3) tissue reorganization. These pathways have several points of interactions and connections, which eventually lead to pain or discomfort, loss of motor or sensory function, and perhaps, sickness behaviors, depression or anxiety. The CNS reorganization pathway was discussed previously in Barr et al., 2004. This review focused on clinical and experimental studies showing the induction of inflammation following injury occurring as a consequence of performing such tasks, subsequent tissue reorganization (pathological or adaptive), and on possible implications of systemic inflammation. Motor and sensory changes, including pain, develop as a consequence. The extent of these changes is dependent on task exposure (duration and level). A systemic response may be stimulated by cytokines released into the blood stream by injured tissues and immune cells. Circulating cytokines can stimulate global responses such as widespread increase in macrophages, local and distant tissue sensitization, and perhaps the induction of sickness behaviors, depression or anxiety, as may cytokine elevation in peripheral nerve tissues.
Acknowledgments
We thank our many colleagues who have contributed to the work and ideas discussed in this review. The experimental work in the authors' laboratories was supported by NIOSH OH03970 (to M.F.B.), NIAMS AR46426 (to A.E.B.), and the American Physical Therapy Foundation.
References
- Al-Shatti T, Barr AE, Safadi F, Amin M, Barbe MF. Increase in pro- and anti-inflammatory cytokines in median nerves in a rat model of repetitive motion injury. J. Neuroimmunol. 2005;167(12):13–22. doi: 10.1016/j.jneuroim.2005.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archambault JM, Hart DA, Herzog W. Response of rabbit Achilles tendon to chronic repetitive loading. Connect. Tissue Res. 2001;42(1):13–23. doi: 10.3109/03008200109014245. [DOI] [PubMed] [Google Scholar]
- Archambault JM, Herzog W, Hart D. The effect of load history in an experimental model of tendon repetitive motion disorders; Proceedings of the Marconi Research Conference; Marshall, CA. 1997. [Google Scholar]
- Backman C, Boquist L, Friden J, Lorentzon R, Toolanen G. Chronic Achilles paratenonitis with tendinosis: an experimental model in the rabbit. J. Orthop. Res. 1990;8:541–547. doi: 10.1002/jor.1100080410. [DOI] [PubMed] [Google Scholar]
- Barr AE, Amin M, Barbe MF. Dose-response relationship between reach repetition and indicators of inflammation and movement dysfunction in a rat model of work-related musculoskeletal disorder; Proceedings of the HFES 46th Annual Meeting; 2002.pp. 1486–1490. [Google Scholar]
- Barr AE, Barbe MF. Pathological tissue changes associated with repetitive movement: a review of the evidence. Phys. Ther. 2002;82(2):173–187. doi: 10.1093/ptj/82.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr AE, Barbe MF, Clark BD. Work-related musculoskeletal disorders of the hand and wrist: epidemiology, pathophysiology, and sensorimotor changes. J. Orthop. Sports Phys. Ther. 2004;34(10):610–627. doi: 10.2519/jospt.2004.34.10.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard BP, editor. Department of Health and Human Services, National Institute of Occupational Safety and Health. Washington: 1997. Musculoskeletal Disorders (MSDs) and Workplace Factors: A Critical Review of Epidemiologic Evidence for Work-related Musculoskeletal Disorders of the Neck, Upper Extremity, and Low Back. DC. Publication No. 97-141. [Google Scholar]
- Biffl WL, Moore EE, Moore FA, Peterson VM. Interleukin-6 in the injured patient. Marker of injury or mediator of inflammation. Ann. Surg. 1996;224:647–664. doi: 10.1097/00000658-199611000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bureau of Labor Statistics . Lost-worktime injuries and illnesses: characteristics and resulting days away from work, 2003. United States Department of Labor News; 2005. USDL 05-521, March 30. [Google Scholar]
- Capuron L, Dantzer R. Cytokines and depression: the need for a new paradigm. Brain Behav. Immun. 2003;17:S119–S124. doi: 10.1016/s0889-1591(02)00078-8. [DOI] [PubMed] [Google Scholar]
- Clark BD, Al-Shatti TA, Barr AE, Amin M, Barbe MF. Performance of a high-repetition, high-force task induces carpal tunnel syndrome in rats. J. Orthop. Sports Phys. Ther. 2004;34:244–254. doi: 10.2519/jospt.2004.34.5.244. [DOI] [PubMed] [Google Scholar]
- Clark BD, Barr AE, Safadi FF, Beitman L, Al-Shatti T, Barbe MF. Median nerve trauma in a rat model of work-related musculoskeletal disorder. J. Neurotrauma. 2003;20:681–695. doi: 10.1089/089771503322144590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clays E, De Bacquer D, Delanghe J, Kittel F, Van Renterghem L, De Backer G. Associations between dimensions of job stress and biomarkers of inflammation and infection. J. Occup. Environ. Med. 2005;47(9):878–883. doi: 10.1097/01.jom.0000171056.22917.ad. [DOI] [PubMed] [Google Scholar]
- Cohen HJ, Pieper CF, Harris T, Rao KM, Currie MS. The association of plasma IL-6 levels with functional disability in community-dwelling elderly. J. Gerontol. A. Biol. Sci. Med. Sci. 1997;52:M201–M208. doi: 10.1093/gerona/52a.4.m201. [DOI] [PubMed] [Google Scholar]
- Copstead L-E, Banadki JL. Pathophysiology: Biological and Behavioral Perspectives. second ed W.B. Saunders; Philadelphia: 2000. pp. 198–201. [Google Scholar]
- Dennet X, Fry HJH. Overuse syndrome: a muscle biopsy study. Lancet. 1988;23:905–908. doi: 10.1016/s0140-6736(88)91714-x. [DOI] [PubMed] [Google Scholar]
- Freeland AE, Rucci MA, Barbieri RA, Angel MF, Nick TG. Biomechanical evaluation of serum and flexor tenosynovium in carpal tunnel syndrome. Microsurgery. 2002;22:378–385. doi: 10.1002/micr.10065. [DOI] [PubMed] [Google Scholar]
- Gold JE, Punnett L, Katz JN. Pressure pain thresholds and musculoskeletal morbidity in automobile manufacturing workers. Int. Arch. Occup. Environ. Health. 2006;79(2):128–134. doi: 10.1007/s00420-005-0005-3. [DOI] [PubMed] [Google Scholar]
- Hirata H, Tsujii M, Yoshida T, Yoshida KI, Morita A, Okuyama N, Nagakura T, Sugimoto T, Fujisawa K, Uchida A. MMP-2 expression is associated with rapid proliferative arteriosclerosis in the flexor tenosynovium and pain severity in carpal tunnel syndrome. J. Pathol. 2005;205:443–450. doi: 10.1002/path.1709. [DOI] [PubMed] [Google Scholar]
- Kuiper JI, Verbeek JH, Everts V, Straub JP, Frings-Dresen MH. Serum markers of collagen metabolism: construction workers compared to sedentary workers. Occup. Environ. Med. 2005;62(6):363–367. doi: 10.1136/oem.2004.016998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson B, Björk J, Elert J, Lindman R, Gerdle B. Fibre type proportion and fibre size in trapezius muscle biopsies from cleaners with and without myalgia and its correlation with ragged red fibres, cytochrome-c-oxidase-negative fibres, biomechanical output, perception of fatigue, and surface electromyography during repetitive forward flexions. Eur. J. Appl. Physiol. 2001;84:492–502. doi: 10.1007/s004210100409. [DOI] [PubMed] [Google Scholar]
- Messner K, Wei Y, Andersson B, Gillquist J, Räsänen T. Rat model of Achilles tendon disorder. Cells Tissues Organs. 1999;165:30–39. doi: 10.1159/000016671. [DOI] [PubMed] [Google Scholar]
- National Research Council and Institute of Medicine . Musculoskeletal Disorders and the Workplace. National Academy Press; Washington, DC: 2001. 2001. [Google Scholar]
- Perry SM, McIlhenny SE, Hoffman MC, Soslowsky LJ. Inflammatory and angiogenic mRNA levels are altered in a supraspinatus tendon overuse animal model. J. Shoulder Elbow Surg. 2005;14:79S–83S. doi: 10.1016/j.jse.2004.09.020. [DOI] [PubMed] [Google Scholar]
- Shaw WS, Feuerstein M, Lincoln AE, Miller VI, Wood PM. Ergonomic and psychosocial factors affect daily function in worker’s compensation claimants with persistent upper extremity disorders. J. Occup. Environ. Med. 2002;44:606–615. doi: 10.1097/00043764-200207000-00004. [DOI] [PubMed] [Google Scholar]
- Silverstein BA, Fine LJ, Armstrong TJ. Hand wrist cumulative trauma disorders in industry. Br. J. Ind. Med. 1986;42:779–784. doi: 10.1136/oem.43.11.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauber WT, Willems MET. Prevention of histopathic changes from 30 repeated stretches of active rat skeletal muscles by long interstretch rest times. Eur. J. Appl. Physiol. 2002;88:94–99. doi: 10.1007/s00421-002-0672-7. [DOI] [PubMed] [Google Scholar]
- Wieseler-Frank J, Maier SF, Watkins LR. Immune-to-brain communication dynamically modulates pain: physiological and pathological consequences. Brain Behav. Immun. 2005;19:104–111. doi: 10.1016/j.bbi.2004.08.004. [DOI] [PubMed] [Google Scholar]