Protein phosphatase 1 regulates the stability of the circadian protein PER2 (original) (raw)
Abstract
The circadian clock is regulated by a transcription/translation negative feedback loop. A key negative regulator of circadian rhythm in mammals is the PER2 (mammalian PERIOD 2) protein. Its daily degradation at the end of the night accompanies de-repression of transcription. CKIϵ (casein kinase I ϵ) has been identified as the kinase that phosphorylates PER2, targeting it for ubiquitin-mediated proteasomal degradation. We now report that PER2 degradation is also negatively regulated by PP1 (protein phosphatase 1)-mediated dephosphorylation. In Xenopus egg extract, PP1 inhibition by Inhibitor-2 accelerated mPER2 degradation. Co-immunoprecipitation experiments showed that PER2 bound to PP1c in transfected HEK-293 cells. PP1 immunoprecipitated from HEK-293 cells, mouse liver and mouse brain, dephosphorylated CKIϵ-phosphorylated PER2, showing that PER2 is a substrate for mammalian endogenous PP1. Moreover, over-expression of the dominant negative form of PP1c, the D95N mutant, accelerated ubiquitin and proteasome-mediated degradation of PER2, and shortened the PER2 half-life in HEK-293 cells. Over-expression of the PP1 inhibitors, protein phosphatase 1 holoenzyme inhibitor-1 and Inhibitor-2, confirmed these results. Thus PP1 regulates PER2 stability and is therefore a candidate to regulate mammalian circadian rhythms.
Keywords: casein kinase I ϵ, circadian rhythms, mammalian clock, phosphorylation, protein phosphatase 1, PER2
Abbreviations: β-TrCP, β-transducin repeat-containing protein; CIP, calf intestinal alkaline phosphatase; CKIϵ, casein kinase I ϵ; CLK, CLOCK; CRY, cryptochrome; DBT, DOUBLETIME; DTT, dithiothreitol; FRQ, FREQUENCY; FWD1, F-box and WD40 repeat-containing protein 1; HEK, human embryonic kidney; PER2, mammalian PERIOD 2; PHI-1, protein phosphatase 1 holoenzyme inhibitor 1; PP1, protein phosphatase 1; PP2A, protein phosphatase 2A; SCF, Skp/Cullin/F box complex; TWS, TWINS; WDB, WIDERBORST
INTRODUCTION
Circadian rhythms are an intrinsic 24 h cycle that co-ordinate many physiological processes. The mammalian circadian clock is composed of interacting feedback loops that generate rhythmical expression of the clock components. In the core loop two transcription factors, CLOCK and BMAL1, activate the transcription of the PER (mammalian PERIOD) and CRY (cryptochrome) genes through E-box elements. PER proteins associate with CRY and are phosphorylated by CKI (casein kinase I). The heterotrimer then translocates to the nucleus and represses its own transcription (reviewed in [1–3]).
To adjust the circadian cycle to approximately 24 h, transcriptional and post-translational modifications of the clock components are required. The proper function of the circadian clock relies on the regulated stability of the proteins, in addition to or instead of mRNA cycling. Several studies in Drosophila indicate that the cycling expression of the PER protein is required for circadian rhythms. In flies, the abundance of PER protein oscillates even when the PER gene is expressed from a constitutive promoter and its mRNA levels are constant [4–7]. Conversely, over-expression of either PER or TIM (TIMELESS) proteins eliminates behavioural rhythms [6]. In mammals, although CRY proteins are required to inhibit the transcription, the abundance of PER proteins determines the formation of the PER–CRY complexes as well as the translocation of CRY to the nucleus [8]. These findings emphasize the importance of the turnover of PER proteins.
Protein phosphorylation is an essential contributor to the delay between the signal and the negative feedback (reviewed in [9]). CKI phosphorylates PER, targeting it for degradation in both flies and mammals. In Drosophila, progressive phosphorylation of PER by DBT (DOUBLETIME) [10,11] leads to the recruitment of the ubiquitin ligase Slimb, which targets hyperphosphorylated PER for degradation in the 26 S proteasome [12,13]. Mutation of CKIϵ shortens rhythm in hamsters and mutation of CKIδ shortens rhythm in humans [14–16]. In mammalian cells, PER1 and PER2 degradation is regulated by CKIϵ/δ-dependent phosphorylation of specific sites, which leads to the recruitment of the ubiquitin ligase β-TrCP (β-transducin repeat-containing protein) and the further polyubiquitination and degradation of PER by the proteasome pathway [17,18]. These studies reveal that phosphorylation is absolutely required for targeting the degradation of PER proteins.
Rhythmic phosphorylation of PER proteins can be regulated by both changes in kinase activity, and changes in phosphatase activity. PP1 (protein phosphatase 1) and PP2A (protein phosphatase 2A) are the major serine/threonine phosphatases in the cell [19]. They regulate cellular processes ranging from metabolism to cell cycle progression and apoptosis. Despite sharing a structurally related core and a similar catalytic mechanism, a number of regulatory proteins provide high specificity for particular phosphorylated substrates as well as for specific sites within a substrate [19–23]. As phosphorylation is a reversible process, if CKI phosphorylates PER2 and promotes its degradation, a phosphatase must dephosphorylate it and therefore facilitate its stability. Although the role of several protein kinases in the circadian rhythm has been extensively studied, little is known about the circadian functions of protein phosphatases.
Inhibitors of PP1 and PP2A alter rhythms in dinoflagellates [24]. Both PP1 and PP2A are important for proper circadian timing in Neurospora, since mutations in both phosphatases independently alter the period [25,26]. The degradation rate of FRQ (FREQUENCY), the negative element of the feedback loop, is controlled by PP1. Strains with mutations in the PP1 catalytic subunit show a less stable FRQ, resulting in circadian phase advance and shorter periods. On the other hand, mutations in the PP2A regulatory subunit RGB-1 do not affect FRQ stability, but affect the normal rhythm leading to low amplitude and longer period [26]. RGB-1 is also required for dephosphorylation of the White Collar Complex and the subsequent activation of FRQ gene transcription [25].
In Drosophila, PP2A has been shown to regulate the circadian rhythms. Interference RNA against the PP2A regulatory subunits WDB (WIDERBORST) and TWS (TWINS) increased degradation of dPER and tws mutant flies displayed longer periods [27]. Drosophila CLK (CLOCK) is stabilized by PP2A-mediated dephosphorylation [28]. However, the phosphatases that regulate the mammalian clock have not been identified. In the present study, we identify PP1 as a regulator of mammalian PER2. PP1 interacts with and dephosphorylates mPER2. Over-expression of PP1 inhibitor, as well as a dominant negative PP1 mutant, accelerates the degradation of PER2 through the ubiquitin–proteasome pathway. PP1 may therefore be a significant regulator of circadian rhythm by altering the half-life of PER2.
EXPERIMENTAL
Plasmids
FLAG and myc-epitope tagged PER2 constructs, as well as β-TrCP(ΔFbox) where β-TrCP is β-transducin repeat-containing protein, were generated as described previously [17]. To generate GFP– and myc–PP1 expression vectors, PP1α cDNA was PCR amplified using primer pairs 5′-gggctcgaggccaccatgtccgacagcgagaagctc-3′ and 5′-gggaagctttttcttggctttggcagagtt-3′ and a rabbit PP1α cDNA as a template and subcloned into the XhoI and HindIII sites of pEGFP-N1-KS(−) and of pcDNA3 D95N. GFP- or myc-PP1 mutants were generated using the QuikChange Site-Directed mutagenesis kit (Stratagene) according to the manufacturer's protocol. The plasmids pCMV5-small t, pCMV1-Inhibitor-2 and pKVYFP-PHI-1 were generously provided by Dr Estelle Sontag (Department of Pathology, University of Texas Southwestern Medical Centre, Dallas, TX, U.S.A.), Dr Anna DePaoli-Roach (Department of Biochemistry and Molecular Biology, Indiana University School of Medicine, Indianapolis, IN, U.S.A) and Dr David Brautigan (Center for Cell Signaling and Department of Microbiology, University of Virginia School of Medicine, Charlottesville, VA, U.S.A.) respectively.
Cell culture and transfection
HEK- (human embryonic kidney)-293 cells were grown in Dulbecco's Modified Eagle's Medium (Gibco) supplemented with 10% foetal bovine serum and 100 units/μl penicillin and 100 μg/ml streptomycin (Invitrogen). Cells were maintained in a humidified incubator at 37 °C and 5% CO2. For transient transfections, cells were plated in individual wells of a six-well dish coated with poly-L-lysine (Sigma). Cells were transfected when 70% confluent, using Lipofectamine™ and Plus reagent (Invitrogen) according to the manufacturer's protocols. The total amount of transfected DNA was adjusted to 1 μg with empty vector. For immunoprecipitation experiments, 300 ng of PER2 and PP1 expression plasmids were used. For PER2 degradation experiments, 100 ng of PER2, 25–100 ng of PP1, 50 ng of Inhibitor-2, 25 ng of PHI-1 (protein phosphatase 1 holoenzyme inhibitor 1), 400 ng of βTrCP(ΔFbox) and 300 ng of small t expression constructs were transfected. Between 16 and 20 h after transfection, HEK-293 cells were treated with inhibitors as described in each Figure. After treatments, cells were washed three times with PBS and lysed in 200 μl of lysis buffer [0.1% Nonidet P40, 150 mM NaCl, 20 mM Hepes (pH 7.5), 1 mM EDTA, 2 mM DTT (dithiothreitol) and 1× Complete protease inhibitor mixture (Roche Applied Science)]. Cells were then mechanically sheared, and the lysates were centrifuged at 16000 g for 10 min at 4 °C. Before treatment with CIP (calf intestinal alkaline phosphatase; New England Biolabs), lysates containing 100 μg of total protein were diluted in 100 mM NaCl, 70 mM Tris/HCl (pH 7.5) and 10 mM MgCl2, in a reaction volume of 30 μl. Five units of CIP were added and the reaction was incubated for 1 h at 37 °C. The reactions were stopped by the addition of Laemmli sample buffer.
Circadian-timed tissue lysates
Two month old male Black 6 mice were entrained in 12 h light:12 h dark for 2 weeks. At the indicated times, animals were killed and livers were dissected and immediately frozen. Tissue samples were briefly homogenized in lysis buffer [0.1% Nonidet P40, 150 mM NaCl, 20 mM Hepes (pH 7.5), 1 mM EDTA, 2 mM DTT and 1× protease inhibitors cocktail]. After setting on ice for 30 min, homogenates were centrifuged at 16000 g and the soluble fraction was collected.
Degradation assay in Xenopus egg extracts
Xenopus egg extracts were prepared as described [29]. 35S-mPER2-(450–763) was synthesized using the TNT kit (Promega). Extracts (25 μl) were incubated with 1 μl 35S-mPER2-(450–763) at room temperature (24 °C) and 3 μl samples were removed at 0, 1.5 and 3 h. Samples were analysed as described previously [29].
Immunoprecipitation and Western blot
Myc epitope-tagged PER2 and PP1, FLAG–PER2 and endogenous PP1 were immunoprecipitated from pre-cleared cell lysates (300 μg) with 1 μg of anti-myc (9E10) or anti-FLAG (M5) antibodies and with 10 μg of anti-PP1α antibody (sc-6104) respectively. The cell lysates and the antibodies were incubated for 1 h on ice to allow formation of antigen–antibody complexes. Samples were then incubated with 10 μl of protein A agarose for 1 h at 4 °C with gentle agitation. Finally, the beads were collected and samples were resolved by SDS/PAGE and Western blotting was performed as described previously [17]. Immunoblotting with an anti-actin antibody (Sigma) was used as an internal control for the lysates (100 μg of total protein). Western blotting for PP1 (see Supplementary Figure 1 at http://www.BiochemJ.org/bj/399/bj3990169add.htm) was performed using an anti-pan PP1 antibody (E9; Santa Cruz, sc-7482).
The anti-PP2Ac affinity-purified rabbit polyclonal antibody 109-3AP has been described previously [30]. The rabbit polyclonal antibody against B55α was a gift from Dr Egon Ogris (Department of Medical Biochemistry, Max F. Perutz Laboratories, Vienna Biocentre, Medical University of Vienna, Austria).
mPER2 dephosphorylation assay
To generate the phosphatase substrate, 2 nmole MBP–mPER2-(450–763) and 1 nmole CKIϵ (Δ319) were incubated at 37 °C for 2 h in a 250 μl reaction mixture containing 30 mM Hepes (pH 7.5), 10 mM MgCl2, 100 μg/ml BSA, 250 μM ATP and 6.6 μM [γ-32P]ATP. Next, 600 μl of PP1 reaction buffer [50 mM Hepes (pH 7.5), 0.1 mM EDTA, 5 mM DTT, 0.025% Nonidet P40 and 1 mM MnCl2] was added and then unincorporated ATP was removed by sequential filtrations (Centricon MWCO=10 kDa) until no free 32PO4− was detected. For the in vitro phosphatase reaction, either mouse liver or mouse brain was lysed in lysis buffer containing 30 mM Hepes (pH 7.5), 150 mM NaCl, 0.1% Nonidet P40, 1 mM EDTA, 2 mM DTT and 1× protease inhibitor cocktail.
For immunoprecipitation-phosphatase assays, total lysates were first pre-cleared with Protein A agarose beads. Total protein (1.2 mg) was incubated with 10 μg anti-PP1α antibody and then 30 μl of Protein A was added as described above. Beads were washed three times and resuspended in 50 μl of PP1 reaction buffer. Then, 30 μl of CKIϵ-phosphorylated PER2 was added and the mixture was incubated at 37 °C for 1 h. The reaction was stopped by adding 130 μl of 100% TCA (trichloroacetic acid) and 60 μg of BSA in 100 μl of distilled water. Protein was precipitated on ice, centrifuged and the supernatant assessed by scintillation counting. The enzyme activity is expressed as percentage of released 32PO4−. For the calyculin A group, the beads containing immunoprecipitated PP1 were pre-incubated with 100 nM calyculin A for 15 min at room temperature (24 °C) before adding the substrate. Recombinant PP1 was from New England Biolabs.
RESULTS
Phosphatase inhibitors increase PER2 degradation
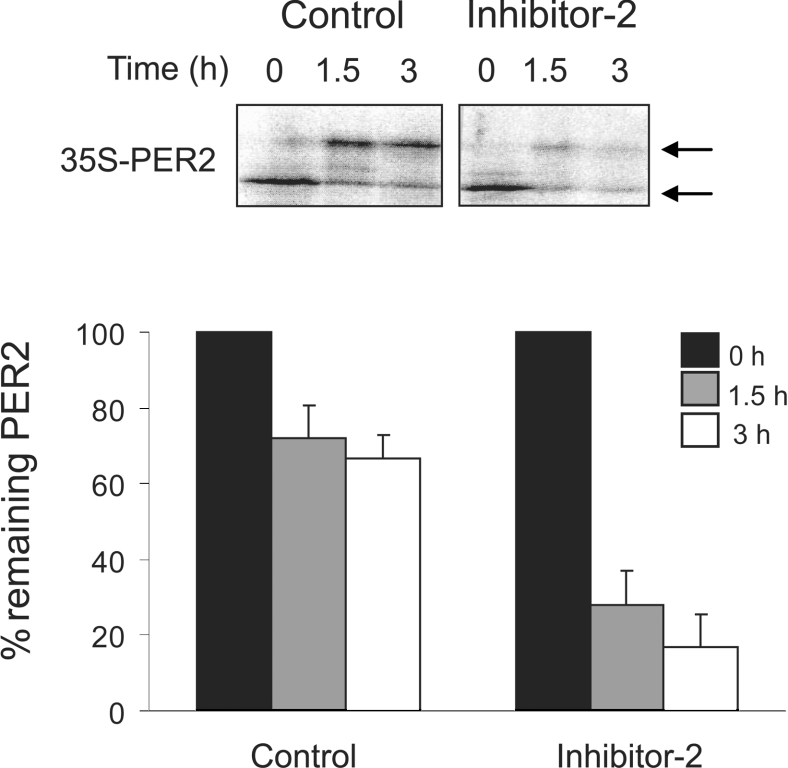
PER2 stability is regulated by the net phosphorylation of specific β-TrCP binding sites that, in turn, is determined by a balance between phosphorylation and dephosphorylation. We have demonstrated that inhibition of serine/threonine phosphatases with the cell-permeable phosphatase inhibitor calyculin A increased PER2 degradation (results not shown) [17]. However, calyculin A is a broad-spectrum inhibitor that inhibits PP1, PP2A, protein phosphatase 4 and protein phosphatase 5 [reviewed in 20]. In order to test PP1-specific (but not cell-permeable) inhibitors, we next examined the degradation of mPER2 in an in vitro system using Xenopus egg extracts. These extracts have been shown to recapitulate faithfully the phosphorylation-regulated, β-TrCP-dependent proteasomal degradation of β-catenin and allow the testing of non-cell permeable inhibitors [29,31]. In vitro translated 35S labelled mPER2-(450–763) was phosphorylated but stable when added to Xenopus egg extracts (Figure 1). However, in the presence of the PP1-specific inhibitor Inhibitor-2, PER2 was very efficiently degraded and 3 h after the addition of Inhibitor-2 less than 20% of PER2 remained. This suggests that in Xenopus egg extracts endogenous PP1 is a regulator of mPER2 stability.
Figure 1. Serine/threonine protein phosphatases regulate mPER2 stability.
Inhibition of PP1 stimulates PER2 degradation in Xenopus egg extract. Extracts supplemented with in vitro synthesized 35S-mPER2-(450–763) were incubated with the PP1-specific Inhibitor-2 (1 μM) or vehicle for 0, 1.5 and 3 h. 35S-mPER2-(450–763) abundance was analysed by SDS/PAGE and autoradiography (upper panel). Arrows indicate hypo- and hyper-phosphorylated forms of PER2. Three experiments were quantified using a Storm 860 gel imaging system and the averages (±S.E.) were plotted (lower panel). For each experimental group, the amount of 35S-mPER2-(450–763) present at time zero was set as 100%.
CKIϵ-phosphorylated PER2 is a substrate for mammalian PP1
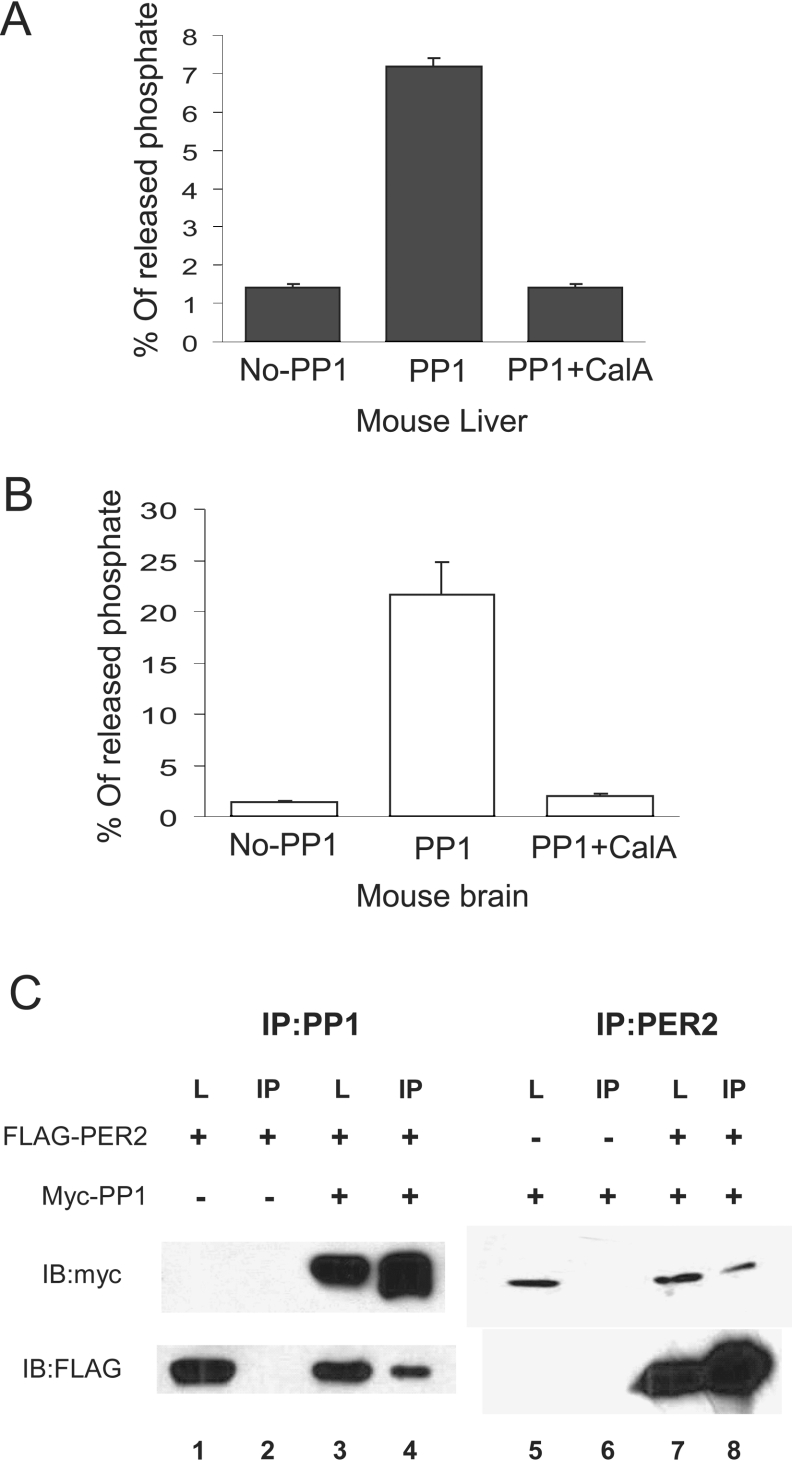
CKI phosphorylates PER2 on sites that promote its degradation. If PP1 is important for PER2 stability, then PP1 is expected to dephosphorylate CKIϵ-phosphorylated PER2. To test this, recombinant MBP–mPER2-(450–763) was phosphorylated in vitro by recombinant CKIϵ and then used as substrate in a dephosphorylation assay. PP1 immunoprecipitated from mouse tissues was used as a source of phosphatase. PP1 immunoprecipitated from mouse liver (Figure 2A), mouse brain (Figure 2B), and HEK-293 cells (results not shown) dephosphorylated CKIϵ-dependent sites on mPER2. The release of phosphate from 32P-MBP–mPER2 was due to PP1, since it was dependent on the presence of the specific antibody and inhibited by calyculin A. Recombinant PP1c also efficiently dephosphorylated CKIϵ-phosphorylated PER2-(450–763) in a concentration dependent manner, with 50 m-units approximately as active as PP1 immunoprecipitated from mouse liver under these conditions (results not shown). These studies indicate that CKIϵ-phosphorylated PER2 is a substrate for endogenous mammalian PP1.
Figure 2. Endogenous PP1 binds to and dephosphorylates CKIϵ-phosphorylated PER2.
(A) and (B) In vitro dephosphorylation of phospho-mPER2 by PP1. CKIϵ-phosphorylated PER2 was incubated with immunoprecipitated PP1 from mouse (A) liver and (B) brain pretreated with either calyculin A (100 nM) or DMSO, and 32PO4− release activity was assayed as described in the Experimental section. An immunoprecipitation performed without PP1 antibody was used as control for non-specific binding. (C) Over-expressed PP1 co-immunoprecipitates with mPER2. HEK-293 cells were transfected with myc–PP1 and FLAG–mPER2 as indicated. Cell lysates were immunoprecipitated separately with anti-myc (for PP1) or anti-FLAG (for mPER2) antibodies. The presence of mPER2 and PP1 was determined by Western blotting of the cell lysates and the pellets with anti-myc and anti-FLAG antibodies. The input samples are indicated by L (lysates), whereas IP indicates the pellet fractions. A representative of three experiments is shown.
PP1 interacts with PER2
As the in vitro phosphatase assay revealed that endogenous PP1 dephosphorylates PER2, we next asked whether these two proteins interact. To analyse PP1 and PER2 binding, FLAG–PER2 (full length) and myc–PP1α catalytic subunits were co-expressed in HEK-293 cells and their ability to bind each other was tested by co-immunoprecipitation assays. As shown in Figure 2(C), immunoprecipitation of PP1 with anti-Myc antibody pulled down PER2 (Figure 2C, lane 4, lower panel). Similarly, when PER2 was immunoprecipitated, PP1 co-precipitated (Figure 2C, lane 8, upper panel). In a parallel experiment we were unable to detect any interaction between PER2 and the PP2A catalytic subunit (results not shown), suggesting further that the interaction is specific to PP1.
Dominant negative PP1 accelerates PER2 degradation
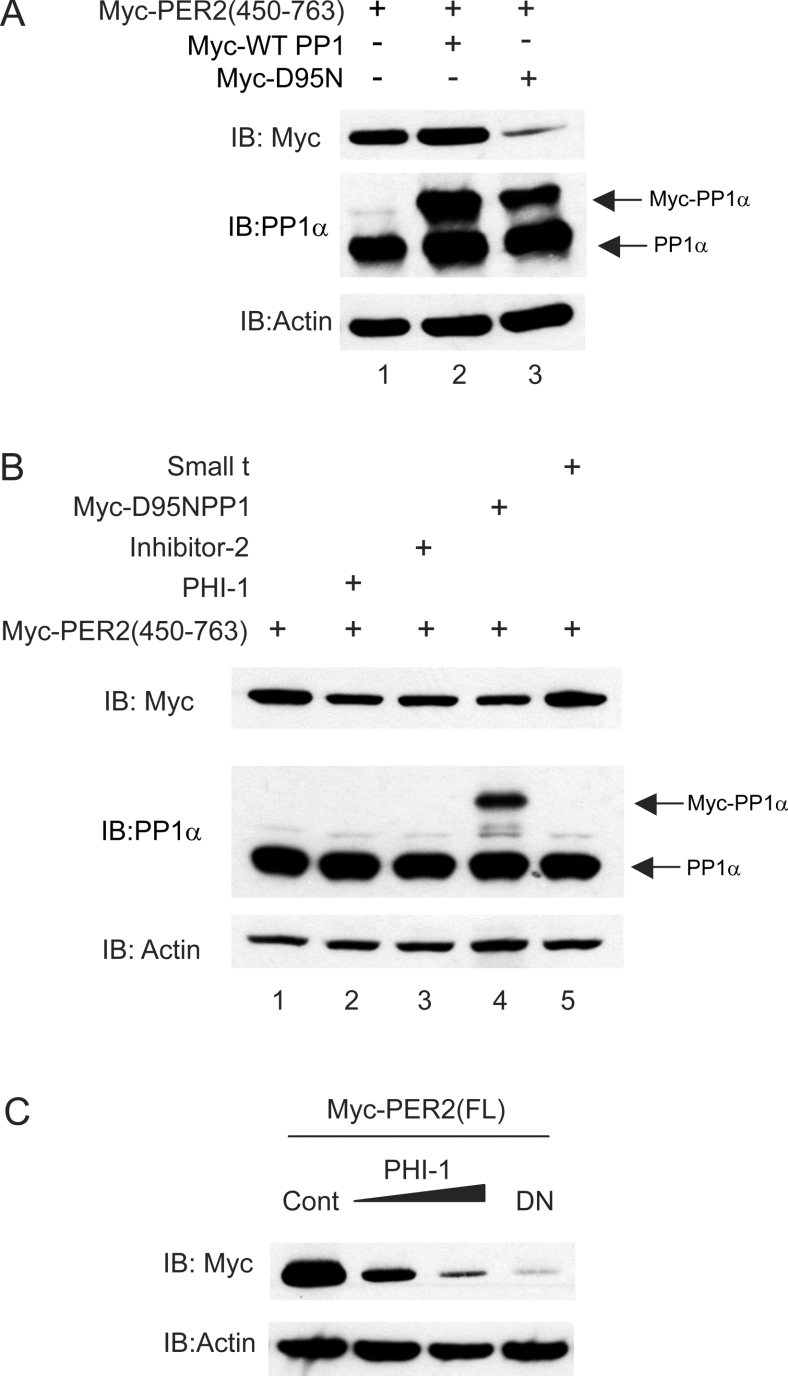
PP1 shares many invariant residues with PP2A, PP2B and other phosphatases, suggesting that these enzymes share a common catalytic mechanism. Sequence alignment shows a consensus sequence important for metal, phosphate binding and catalysis [32]. One of these invariant residues involved in the catalysis is an aspartic acid that in PP1 is residue 95. Mutation of this aspartic acid to asparagine has previously been demonstrated to impair markedly the catalytic activity of the enzyme [33]. Mutation of PP1 Asp95 to asparagine did not impair expression of the protein nor its ability to interact with PER2 in cells (results not shown). We therefore tested whether the PP1 mutant D95N acted as a dominant negative protein, by interacting with PER2 and preventing the dephosphorylation of the substrate by the endogenous enzyme.
As Figure 3(A) demonstrates, co-expression of wild-type PP1 did not significantly increase the amount of PER2 present in the cells, suggesting that endogenous PP1 is not rate-limiting. In contrast, co-transfection of the D95N PP1 mutant led to a reduction in PER2 protein abundance compared with both the control and the wild-type PP1 over-expressing groups. This result is consistent with dominant negative PP1 causing decreased abundance of PER2 due to a decreased dephosphorylation and suggests that PP1 is required for the stability of cellular mammalian PER2.
Figure 3. PP1 inhibition increases PER2 degradation.
(A) and (B) Myc–mPER2-(450–763) was transiently expressed either alone, with PP1-WT or D95N in HEK-293 cells (A) or with polypeptide phosphatase inhibitors (B) as indicated. (C) Full length PER2 was co-expressed with either control empty plasmid, 100 or 200 ng of PHI-1 or D95N PP1 mutant. The amount of PER2 and PP1 in the cell lysates was analysed 18 h after transfection by Western blotting of lysates with anti-myc and anti-PP1α antibodies. Membranes were reprobed with anti-actin antibody as a loading control.
As a second approach to specifically inhibit intracellular PP1, two specific inhibitors, PHI-1 and Inhibitor-2, were used. The protein kinase C-activated and widely expressed PHI-1 was the first PP1 inhibitor described to inhibit the holoenzyme as well as the monomeric catalytic subunit of PP1 [34]. The heat-stable protein Inhibitor-2 is a well-characterized PP1 inhibitor [35] that can bind to PP1 already complexed to other targeting subunits [36,37]. Similar to that observed with the dominant negative PP1, cells over-expressing either PHI-1 or Inhibitor-2 showed a marked decrease in PER2 abundance the day after transfection (Figure 3B, compare lanes 2, 3 and 4 with lane 1). Full-length PER2 protein levels were also decreased when either dominant negative PP1 or increasing concentrations of PHI-1 were co-expressed (Figure 3C).
PP2A but not PP1 has been shown to alter PER stability in Drosophila melanogaster. We therefore tested whether expression of SV40 small t antigen, which inhibits a number of PP2A holoenzymes [38–40], had any effect on mammalian PER2 abundance. As Figure 3(B), lane 5 demonstrates, no effect of PP2A inhibition on mPER2 stability was observed. Additionally, in flies the mRNA levels of WDB and TWS, which encode the PP2A regulatory B subunits B55 and B56-2 respectively, were shown to cycle throughout the day. These oscillations in the PP2A regulatory subunits may cause circadian oscillation of PP2A activity that controls the stability of Drosophila PER [27]. In a similar manner to Drosophila, B subunits determine the activity and substrate specificity of the mammalian PP2A holoenzyme. We therefore tested whether mouse B55 or B56 subunits varied in abundance through the circadian cycle. No variation in abundance of PP2Ac, B55, B56β and B56δ was detected (see Supplementary Figure 1 at http://www.BiochemJ.org/bj/399/bj3990169add.htm and results not shown). Interestingly, no oscillations in PP1c subunit were found either (see Supplementary Figure 1 at http://www.BiochemJ.org/bj/399/bj3990169add.htm), suggesting that, as in flies, regulatory proteins rather than the catalytic subunit might cycle.
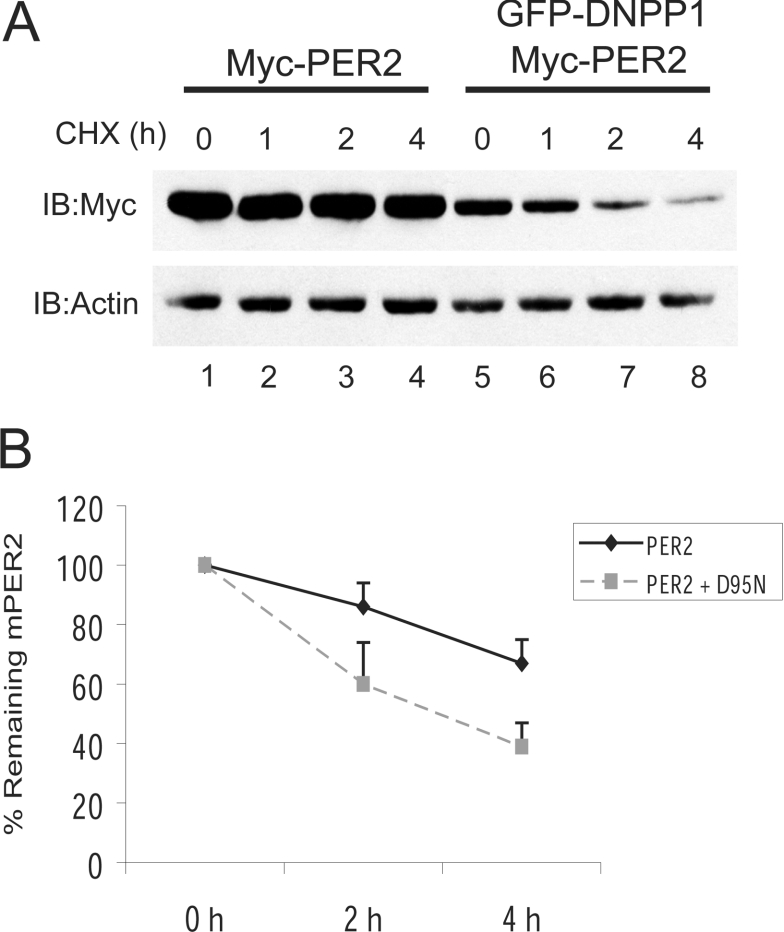
The PP1 dominant negative shortens PER2 half-life
Decreases in mPER2 abundance by PP1 inhibition could be due to increased degradation or decreased synthesis. We therefore investigated whether PP1 inhibition affects PER2 half-life. HEK-293 cells over-expressing myc-PER2-(450–763) were co-transfected with or without D95N PP1. To inhibit the de novo protein synthesis, 20 h later both experimental groups were treated with cycloheximide for up to 4 h. At the indicated time points, cells were lysed and the PER2 content in the cells was quantified. Since the protein synthesis is inhibited by the cycloheximide treatment, it is possible to survey the degradation, and therefore the half-life, of any protein of interest. In control conditions, PER2 was quite stable and had an estimated half-life close to 6 h. However, in cells co-expressing D95N PP1, the basal PER2 amount was reduced (Figure 4A, compare lane 1 with lane 5), which is consistent with an increased turnover of the protein, and it was more unstable than in the non-PP1 transfected cells. In fact, the dominant negative PP1 shortened PER2 half-life to about 3 h (Figure 4B). This result indicates that PER2 dephosphorylation by PP1 regulates protein stability.
Figure 4. Over expression of a dominant negative PP1 shortens PER2 half-life.
(A) HEK-293 transiently expressing myc–mPER2-(450–763) alone or with GFP–D95N PP1, were treated with cycloheximide (25 μg/ml) for up to 4 h. Samples were analysed at the indicated time points by SDS/PAGE and the amount of PER2 determined by Western blotting with anti-myc antibody. (B) The results shown in (A) and in three similar experiments were quantified using NIH (National Institutes of Health, Bethesda, MD, U.S.A.) Image Software and averaged. For each data point, the PER2 amount was normalized to actin. In each experimental group, the normalized amount of PER2 at time zero of the cycloheximide treatment was set to 100%.
The proteasome pathway mediates the dominant negative PP1-induced PER2 degradation
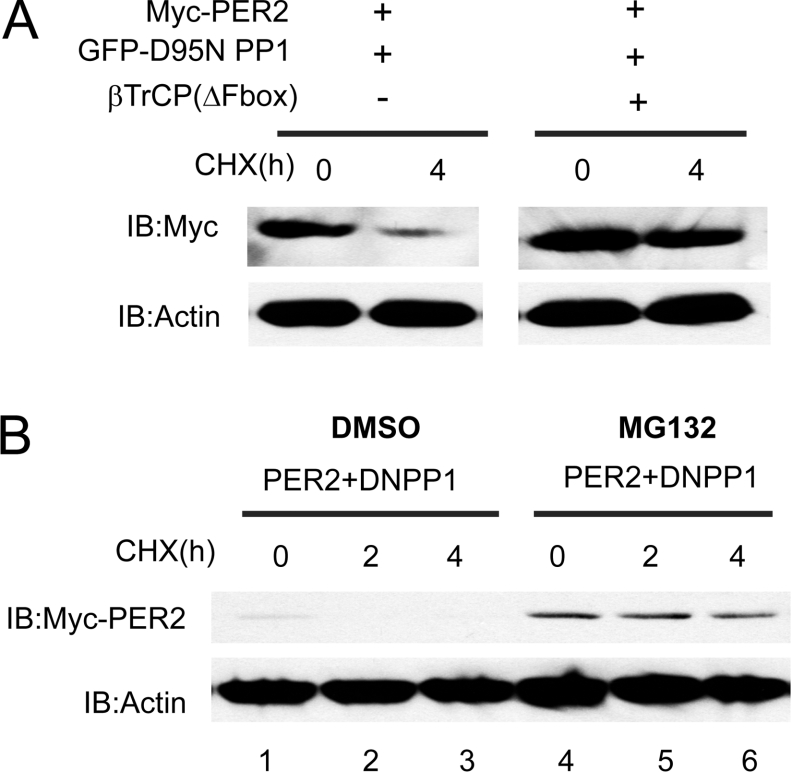
The SCF (Skp/Cullin/F box) ubiquitin–E3 ligase complexes target phosphorylated proteins to the ubiquitin–proteasome pathway. Drosophila Slimb, a member of the F-box/WD40 protein family of the ubiquitin ligase SCF complex targets PER to the proteasome [12,13]. Another F-box/WD-40 repeat-containing protein, FWD1 (F-box and WD40 repeat containing protein 1), mediates the degradation of FRQ, the protein that closes the negative feedback loop in the Neurospora clock [41]. In mammals, the Slimb homologue β-TrCP is involved in proteasomal degradation of hyperphosphorylated PER2 [17]. We therefore tested whether PP1 regulated the β-TrCP-mediated degradation of mPER2. As Figure 5(A) illustrates, mPER2 abundance (and hence its stability) 4 h after addition of cycloheximide was markedly decreased by co-expression of dominant negative PP1. Consistent with a role for β-TrCP in PP1-regulated PER2 stability, co-expression of dominant negative β-TrCP lead to an increase in the abundance of PER2 at time zero and increased PER2 stability after cycloheximide addition.
Figure 5. The ubiquitin and proteasome pathway mediates the dominant negative PP1-induced PER2 degradation.
(A) Dominant negative β-TrCP (ΔFbox) prevents the dominant negative PP1-induced PER2 degradation. HEK-293 cells were transfected with vectors expressing myc–mPER2-(450–763), dominant negative PP1 and dominant negative β-TrCP (ΔFbox) as indicated. Following transfection (after 18 h), cells were incubated with cycloheximide (25 μg/ml) for 0 and 4 h and then lysed. The amount of mPER2-(450–763) in the lysates was determined by immunoblotting with anti-myc antibody and Western blotting with anti-actin was used as a loading control. (B) Proteasome inhibition prevents the dominant negative PP1-induced PER2 degradation. HEK-293 cells transiently expressing myc–mPER2-(450–763) plus the dominant negative PP1 were incubated with the proteasome inhibitor MG132 (30 μM) or vehicle for 6 h. Cycloheximide (25 μg/ml) was added to the growth medium and cells were lysed at the indicated times. The Western blot shows the levels of PER2 and actin.
As a second test of the role of PP1 in the ubiquitin–proteasome mediated regulation of PER2, we examined whether the 26 S proteasome mediates the dominant negative PP1-induced PER2 degradation. We used MG132, which inhibits the 26 S proteasome and prevents PER2 degradation [17]. HEK-293 cells co-transfected with myc–mPER2-(450–763) plus GFP–D95N PP1 were pre-incubated with the proteasome inhibitor MG132 or vehicle for 6 h. Cells were then treated with cycloheximide and the stability of PER2 was determined by immunoblotting (Figure 5B). Treatment with MG132 led to an initial increase in PER2 protein abundance and to the stabilization of the protein throughout the experiment. These results indicate that in the presence of the proteasome inhibitor, dominant negative PP1 could no longer induce PER2 degradation. Dominant negative β-TrCP and MG132 blocked dominant negative PP1-induced degradation of PER2, indicating that PP1-regulated phosphorylation is required for the ubiquitin- and proteasome-mediated degradation.
DISCUSSION
The abundance of PER proteins is critical for initiating and resetting the negative feedback loop that drives circadian rhythms. PER degradation in the circadian cycle is required for establishing the 24 h long rhythm. Regulated phosphorylation and degradation of the PER proteins involve a dynamic balance between protein kinases and protein phosphatases. Previous studies demonstrate that CKI is the kinase that regulates PER2 stability. The data presented in the current paper indicate that PP1 is also an intracellular regulator of the phosphorylation state and the stability of mammalian PER2 protein. PP1 interacts with PER2 in vivo, dephosphorylates PER2 in vitro, and inhibition of PP1 caused hyperphosphorylation and proteasomal degradation of PER2 in cells. Taken together with data published previously, we propose that during the circadian cycle, the net phosphorylation and degradation of PER2 is a balance between CKI activity and PP1 activity. Since PP1 activity stabilizes PER2, this phosphatase (and consequently, its regulators) is a good candidate for regulating circadian rhythms in mammals.
The stability regulatory domain of the PER2 protein is formed in part by amino acids 450–763. This domain contains the CKI and β-TrCP binding sites [17] and responds to phosphorylation and dephosphorylation by CKI and PP1 respectively. In cells, PP1 catalytic subunits are targeted to substrates in part by binding to a large number of regulatory proteins. So far, around 65 of these PP1 binding proteins are currently identified, and many others may exist. These PP1 targeting and regulatory proteins determine the activity, localization and substrate specificity of the phosphatase [22]. We suspect that the PP1 catalytic subunit does not bind directly to PER2 but instead interacts with PER2 via a PP1 regulatory subunit. The best characterized binding site for PP1 is the RVXF motif [42,43], present in most PP1 regulators. This motif is not present in the PER2-(450–763) domain. The fact that the PER2-(450–763) lacks the RVXF motif but interacts with PP1 in immunoprecipitates from cell extracts is consistent with a model in which a specific PP1, regulator whose expression varies during the circadian cycle, is capable of targeting PP1 to PER2. If this regulatory protein displayed high expression it could lead to dephosphorylation and stabilization of PER2, whereas when its expression was low, phosphorylation of PER2 would be unopposed, leading to more rapid phosphorylation and degradation. Thus, oscillations in these PP1 regulators are a potential route to regulating PP1 activity and PER degradation. It is of note that mRNA expression of PP1 interactors PTG/PPP1R3C and Inhibitor-1/PPP1R1B have already been shown to oscillate during the circadian cycle (http://expression.gnf.org/cgi-bin/circadian/index.cgi and [44]).
PP2A has recently been implicated in core clock control in both Neurospora and in Drosophila. Of note, in Neurospora PP1 appeared to control the stability of FRQ, whereas in Drosophila circadian changes in the abundance of PP2A regulators controlled the stability of dPER. Using murine tissues, however, we were unable to identify circadian changes in the abundance of the cognate PP2A regulators. Our data therefore suggest that circadian regulation of PER2 may be controlled differently in flies and mammals. Alternatively, it is possible that the same phosphorylation sites on PER2 are regulated by both PP1 and PP2A in vivo. It will be important to test the effects of mutations in the appropriate phosphatase regulatory subunits on circadian rhythms. However, the number of circadian control proteins that are regulated by protein phosphorylation will complicate this genetic analysis. In vivo, mutation of circadian phosphatases may have diverse effects on substrate. It is to be hoped that by coupling direct biochemical analysis of substrates and enzymes, as performed in the present study, with genetic analysis of circadian rhythms a comprehensive picture of the clock mechanism will emerge.
Regulated phosphorylation and degradation of PER is important in controlling timing of the clock. Both kinases and phosphatases represent potential drug targets that could be used to manipulate circadian rhythms. In the present studies, specific inhibitors of PP1 were able to enhance the degradation of PER2. Since inhibition of CKI and the proteasome have already been shown to slow the clock, the current data suggests specific PP1 inhibitors could accelerate the clock. Such interventions, if they could be targeted specifically to circadian substrates, could provide therapeutic benefit in sleep disturbances, shift work, jet lag and potentially even seasonal affective disorder.
Online data
Supplemental Figure 1
Acknowledgments
We thank members of the laboratory, especially Erik Eide, Wojtek Swiatek and Xinghai Li for helpful discussions. This work was supported by NIH (National Institutes of Health, Bethesda, MD, U.S.A.) grant R01 GM060387 (to D. M. V.).
References
- 1.Reppert S. M., Weaver D. R. Co-ordination of circadian timing in mammals. Nature (London) 2002;418:935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- 2.Gachon F., Nagoshi E., Brown S. A., Ripperger J., Schibler U. The mammalian circadian timing system: from gene expression to physiology. Chromosoma. 2004;113:103–112. doi: 10.1007/s00412-004-0296-2. [DOI] [PubMed] [Google Scholar]
- 3.Emery P., Reppert S. M. A rhythmic Ror. Neuron. 2004;43:443–446. doi: 10.1016/j.neuron.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 4.Frisch B., Hardin P. E., Hamblen-Coyle M. J., Rosbash M., Hall J. C. A promoterless period gene mediates behavioral rhythmicity and cyclical per expression in a restricted subset of the Drosophila nervous system. Neuron. 1994;12:555–570. doi: 10.1016/0896-6273(94)90212-7. [DOI] [PubMed] [Google Scholar]
- 5.Vosshall L. B., Young M. W. Circadian rhythms in Drosophila can be driven by period expression in a restricted group of central brain cells. Neuron. 1995;15:345–360. doi: 10.1016/0896-6273(95)90039-x. [DOI] [PubMed] [Google Scholar]
- 6.Yang Z., Sehgal A. Role of molecular oscillations in generating behavioral rhythms in Drosophila. Neuron. 2001;29:453–467. doi: 10.1016/s0896-6273(01)00218-5. [DOI] [PubMed] [Google Scholar]
- 7.Cheng Y., Hardin P. E. Drosophila photoreceptors contain an autonomous circadian oscillator that can function without period mRNA cycling. J. Neurosci. 1998;18:741–750. doi: 10.1523/JNEUROSCI.18-02-00741.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee C., Etchegaray J. P., Cagampang F. R., Loudon A. S., Reppert S. M. Posttranslational mechanisms regulate the mammalian circadian clock. Cell. 2001;107:855–867. doi: 10.1016/s0092-8674(01)00610-9. [DOI] [PubMed] [Google Scholar]
- 9.Harms E., Kivimae S., Young M. W., Saez L. Posttranscriptional and posttranslational regulation of clock genes. J. Biol. Rhythms. 2004;19:361–373. doi: 10.1177/0748730404268111. [DOI] [PubMed] [Google Scholar]
- 10.Price J. L., Blau J., Rothenfluh A., Abodeely M., Kloss B., Young M. W. Double-time is a novel Drosophila clock gene that regulates PERIOD protein accumulation. Cell. 1998;94:83–95. doi: 10.1016/s0092-8674(00)81224-6. [DOI] [PubMed] [Google Scholar]
- 11.Kloss B., Rothenfluh A., Young M. W., Saez L. Phosphorylation of period is influenced by cycling physical associations of double-time, period, and timeless in the Drosophila clock. Neuron. 2001;30:699–706. doi: 10.1016/s0896-6273(01)00320-8. [DOI] [PubMed] [Google Scholar]
- 12.Ko H. W., Jiang J., Edery I. Role for Slimb in the degradation of Drosophila Period protein phosphorylated by Doubletime. Nature (London) 2002;420:673–678. doi: 10.1038/nature01272. [DOI] [PubMed] [Google Scholar]
- 13.Grima B., Lamouroux A., Chelot E., Papin C., Limbourg-Bouchon B., Rouyer F. The F-box protein slimb controls the levels of clock proteins period and timeless. Nature (London) 2002;420:178–182. doi: 10.1038/nature01122. [DOI] [PubMed] [Google Scholar]
- 14.Xu Y., Padiath Q. S., Shapiro R. E., Jones C. R., Wu S. C., Saigoh N., Saigoh K., Ptacek L. J., Fu Y. H. Functional consequences of a CKIδ mutation causing familial advanced sleep phase syndrome. Nature (London) 2005;434:640–644. doi: 10.1038/nature03453. [DOI] [PubMed] [Google Scholar]
- 15.Lowrey P. L., Shimomura K., Antoch M. P., Yamazaki S., Zemenides P. D., Ralph M. R., Menaker M., Takahashi J. S. Positional syntenic cloning and functional characterization of the mammalian circadian mutation tau. Science. 2000;288:483–492. doi: 10.1126/science.288.5465.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ralph M. R., Menaker M. A mutation of the circadian system in golden hamsters. Science. 1988;241:1225–1227. doi: 10.1126/science.3413487. [DOI] [PubMed] [Google Scholar]
- 17.Eide E. J., Woolf M. F., Kang H., Woolf P., Hurst W., Camacho F., Vielhaber E. L., Giovanni A., Virshup D. M. Control of mammalian circadian rhythm by CKIϵ-regulated proteasome-mediated PER2 degradation. Mol. Cell. Biol. 2005;25:2795–2807. doi: 10.1128/MCB.25.7.2795-2807.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shirogane T., Jin J., Ang X. L., Harper J. W. SCFβ-TRCP controls clock-dependent transcription via casein kinase 1-dependent degradation of the mammalian period-1 (Per1) protein. J. Biol. Chem. 2005;280:26863–26872. doi: 10.1074/jbc.M502862200. [DOI] [PubMed] [Google Scholar]
- 19.Gallego M., Virshup D. M. Protein serine/threonine phosphatases: life, death, and sleeping. Curr. Opin. Cell Biol. 2005;17:197–202. doi: 10.1016/j.ceb.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 20.Honkanen R. E., Golden T. Regulators of serine/threonine protein phosphatases at the dawn of a clinical era? Curr. Med. Chem. 2002;9:2055–2075. doi: 10.2174/0929867023368836. [DOI] [PubMed] [Google Scholar]
- 21.Cegielska A., Moarefi I., Fanning E., Virshup D. M. T-antigen kinase inhibits simian virus 40 DNA replication by phosphorylation of intact T antigen on serines 120 and 123. J. Virol. 1994;68:269–275. doi: 10.1128/jvi.68.1.269-275.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ceulemans H., Bollen M. Functional diversity of protein phosphatase-1, a cellular economizer and reset button. Physiol. Rev. 2004;84:1–39. doi: 10.1152/physrev.00013.2003. [DOI] [PubMed] [Google Scholar]
- 23.Janssens V., Goris J. Protein phosphatase 2A: a highly regulated family of serine/threonine phosphatases implicated in cell growth and signalling. Biochem. J. 2001;353:417–439. doi: 10.1042/0264-6021:3530417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Comolli J., Taylor W., Rehman J., Hastings J. W. Inhibitors of serine/threonine phosphoprotein phosphatases alter circadian properties in Gonyaulax polyedra. Plant Physiol. 1996;111:285–291. doi: 10.1104/pp.111.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schafmeier T., Haase A., Kaldi K., Scholz J., Fuchs M., Brunner M. Transcriptional feedback of Neurospora circadian clock gene by phosphorylation-dependent inactivation of its transcription factor. Cell. 2005;122:235–246. doi: 10.1016/j.cell.2005.05.032. [DOI] [PubMed] [Google Scholar]
- 26.Yang Y., He Q., Cheng P., Wrage P., Yarden O., Liu Y. Distinct roles for PP1 and PP2A in the Neurospora circadian clock. Genes Dev. 2004;18:255–260. doi: 10.1101/gad.1152604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sathyanarayanan S., Zheng X., Xiao R., Sehgal A. Posttranslational regulation of Drosophila PERIOD protein by protein phosphatase 2A. Cell. 2004;116:603–615. doi: 10.1016/s0092-8674(04)00128-x. [DOI] [PubMed] [Google Scholar]
- 28.Kim E. Y., Edery I. Balance between DBT/CKIϵ kinase and protein phosphatase activities regulate phosphorylation and stability of Drosophila CLOCK protein. Proc. Natl. Acad. Sci. U.S.A. 2006;103:6178–6183. doi: 10.1073/pnas.0511215103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li X., Yost H. J., Virshup D. M., Seeling J. M. Protein phosphatase 2A and its B56 regulatory subunit inhibit Wnt signaling in Xenopus. EMBO J. 2001;20:4122–4131. doi: 10.1093/emboj/20.15.4122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCright B., Virshup D. M. Identification of a new family of protein phosphatase 2A regulatory subunits. J. Biol. Chem. 1995;270:26123–26128. doi: 10.1074/jbc.270.44.26123. [DOI] [PubMed] [Google Scholar]
- 31.Salic A., Lee E., Mayer L., Kirschner M. W. Control of β-catenin stability: reconstitution of the cytoplasmic steps of the wnt pathway in Xenopus egg extracts. Mol. Cell. 2000;5:523–532. doi: 10.1016/s1097-2765(00)80446-3. [DOI] [PubMed] [Google Scholar]
- 32.Myles T., Schmidt K., Evans D. R., Cron P., Hemmings B. A. Active-site mutations impairing the catalytic function of the catalytic subunit of human protein phosphatase 2A permit baculovirus-mediated overexpression in insect cells. Biochem. J. 2001;357:225–232. doi: 10.1042/0264-6021:3570225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang J., Zhang Z., Brew K., Lee E. Y. Mutational analysis of the catalytic subunit of muscle protein phosphatase-1. Biochemistry. 1996;35:6276–6282. doi: 10.1021/bi952954l. [DOI] [PubMed] [Google Scholar]
- 34.Eto M., Karginov A., Brautigan D. L. A novel phosphoprotein inhibitor of protein type-1 phosphatase holoenzymes. Biochemistry. 1999;38:16952–16957. doi: 10.1021/bi992030o. [DOI] [PubMed] [Google Scholar]
- 35.Huang F. L., Glinsmann W. H. Separation and characterization of two phosphorylase phosphatase inhibitors from rabbit skeletal muscle. Eur. J. Biochem. 1976;70:419–426. doi: 10.1111/j.1432-1033.1976.tb11032.x. [DOI] [PubMed] [Google Scholar]
- 36.Terry-Lorenzo R. T., Elliot E., Weiser D. C., Prickett T. D., Brautigan D. L., Shenolikar S. Neurabins recruit protein phosphatase-1 and inhibitor-2 to the actin cytoskeleton. J. Biol. Chem. 2002;277:46535–46543. doi: 10.1074/jbc.M206960200. [DOI] [PubMed] [Google Scholar]
- 37.Eto M., Elliott E., Prickett T. D., Brautigan D. L. Inhibitor-2 regulates protein phosphatase-1 complexed with NimA-related kinase to induce centrosome separation. J. Biol. Chem. 2002;277:44013–44020. doi: 10.1074/jbc.M208035200. [DOI] [PubMed] [Google Scholar]
- 38.Pallas D. C., Shahrik L. K., Martin B. L., Jaspers S., Miller T. B., Brautigan D. L., Roberts T. M. Polyoma small and middle T antigens and SV40 small t antigen form stable complexes with protein phosphatase 2A. Cell. 1990;60:167–176. doi: 10.1016/0092-8674(90)90726-u. [DOI] [PubMed] [Google Scholar]
- 39.Sontag E., Fedorov S., Kamibayashi C., Robbins D., Cobb M., Mumby M. The interaction of SV40 small tumor antigen with protein phosphatase 2A stimulates the map kinase pathway and induces cell proliferation. Cell. 1993;75:887–897. doi: 10.1016/0092-8674(93)90533-v. [DOI] [PubMed] [Google Scholar]
- 40.Chen W., Possemato R., Campbell K. T., Plattner C. A., Pallas D. C., Hahn W. C. Identification of specific PP2A complexes involved in human cell transformation. Cancer Cell. 2004;5:127–136. doi: 10.1016/s1535-6108(04)00026-1. [DOI] [PubMed] [Google Scholar]
- 41.He Q., Cheng P., Yang Y., He Q., Yu H., Liu Y. FWD1-mediated degradation of FREQUENCY in Neurospora establishes a conserved mechanism for circadian clock regulation. EMBO J. 2003;22:4421–4430. doi: 10.1093/emboj/cdg425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Egloff M. P., Johnson D. F., Moorhead G., Cohen P. T., Cohen P., Barford D. Structural basis for the recognition of regulatory subunits by the catalytic subunit of protein phosphatase 1. EMBO J. 1997;16:1876–1887. doi: 10.1093/emboj/16.8.1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wakula P., Beullens M., Ceulemans H., Stalmans W., Bollen M. Degeneracy and function of the ubiquitous RVXF motif that mediates binding to protein phosphatase-1. J. Biol. Chem. 2003;278:18817–18823. doi: 10.1074/jbc.M300175200. [DOI] [PubMed] [Google Scholar]
- 44.Ueda H. R., Chen W., Adachi A., Wakamatsu H., Hayashi S., Takasugi T., Nagano M., Nakahama K., Suzuki Y., Sugano S., et al. A transcription factor response element for gene expression during circadian night. Nature (London) 2002;418:534–539. doi: 10.1038/nature00906. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1