The subiculum: what it does, what it might do, and what neuroanatomy has yet to tell us (original) (raw)
Abstract
The subiculum is a pivotal but under-investigated structure positioned between the hippocampus proper and entorhinal and other cortices, as well as a range of subcortical structures. The subiculum has a range of electrophysiological and functional properties which are quite distinct from its input areas; given the widespread set of cortical and subcortical areas with which it interacts, it is able to influence activity in quite disparate brain regions. The rules governing plasticity of synaptic transmission in the hippocampal–subicular axis are poorly understood; this axis appears to share some properties in common with the hippocampus proper, but behaves quite differently in other respects. Equally, its functional properties are not well understood; it plays an important but ill-defined role in spatial navigation, mnemonic processing and control of the response to stress. Here, I review investigations of synaptic plasticity in the hippocampal–subicular pathway, recordings of subicular neurons in the freely moving behaving animal, the effects of behavioural and other stressors on subicular synaptic plasticity, and anatomical data on the dorso-ventral organization of the subiculum in relation to the hypothalamic–pituitary–adrenal (HPA) axis. I argue that there is a dorso-ventral segregation of function within the subiculum: the dorsal component appears principally concerned with the processing of information about space, movement and memory, whereas the ventral component appears to play a major regulatory role in the inhibition of the HPA axis.
Keywords: hippocampal formation, hypothalamus, prefrontal cortex, stress, subiculum, synaptic plasticity
Introduction
The hippocampal formation (HF) of the mammalian brain is conventionally defined as consisting of entorhinal cortex, dentate gyrus, Areas CA3 and CA1, and subiculum (O'Keefe & Nadel, 1978; Eichenbaum & Cohen, 2001) (Figs 1,2 and 7). The early components of the HF have been extensively investigated at anatomical, neurophysiological, biochemical and behavioural levels. By contrast, the subiculum is comparatively under-investigated; consensus on its anatomical description and definition, for example, has only recently emerged (Brodmann, 1909; Lorente de No, 1934; Witter & Groenewegen, 1990; Amaral & Witter, 1995; O'Mara et al. 2001). There is general agreement that the subiculum has three principal layers: a molecular layer, continuous with strata lacunosum-moleculare and radiatum of the adjacent hippocampal area CA1 field; an enlarged pyramidal cell layer containing the soma of principal neurons; and a polymorphic layer. The cell packing in the pyramidal layer of the subiculum is looser than that seen in hippocampal area CA1. The principal cell layer of the subiculum is populated with large pyramidal neurons: these are consistent in their shape and size and extend their apical dendrites into the molecular layer and their basal dendrites into deeper portions of the pyramidal cell layer. Among the pyramidal cells are many smaller neurons; these are considered the interneurons of the subiculum (Amaral & Witter, 1995).
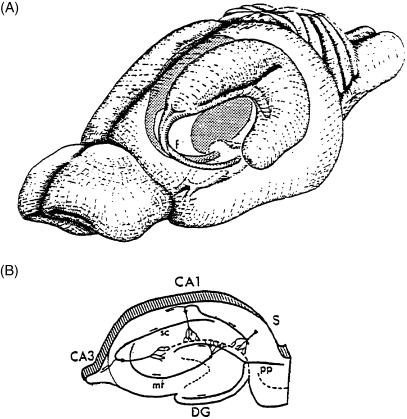
Fig. 1.
The hippocampal formation (A) and location of subiculum (B), indicated as ‘s’ in a section through the hippocampal formation. [From: Fuster, J.M.Memory in the Cerebral Cortex: An Empirical Approach to Neural Networks in the Human and Nonhuman Primate.Cambridge, MA:The MIT Press,1995,p. 26.Copyright MIT Press.]
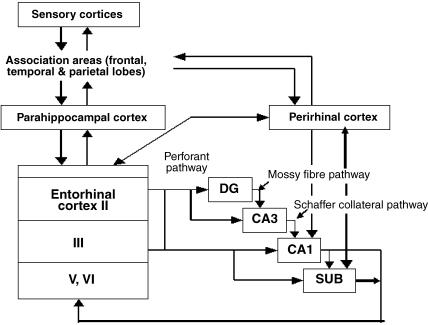
Fig. 2.
Intrinsic connections of the hippocampal formation, including the recently discovered projection from perirhinal cortex to CA1 and subiculum.
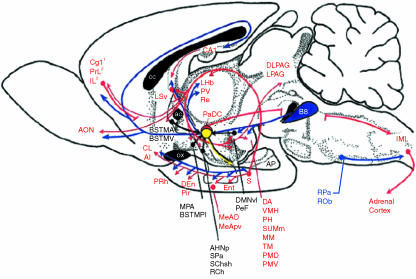
Fig. 7.
From C. A. Lowry (fig. 3). Functional subsets of serotonergic neurones: implications for control of the hypothalamic-pituitary-adrenal axis. Journal of Neuroendocrinology, 2002, 14, 911–923. Copyright Blackwell Publishing. 1Projections from the anterior cingulate to the dorsal hypothalamic area and lateral periaqueductal grey. 2Projections from the infralimbic and prelimbic cortices to the anterior hypothalamic nucleus, ventromedial hypothalamus and dorsolateral periaqueductal grey. ac, anterior commissure; AHNp, anterior hypothalamic nucleus, posterior part; AI, agranular insular cortex; AON, anterior olfactory nucleus; AP, anterior pituitary; B8, B8 serotonergic cell group, median raphe nucleus, interfascicular dorsal raphe nucleus; BSTMA, bed nucleus of the stria terminalis, medial division, anterior part; BSTMPI, bed nucleus of the stria terminalis, medial division, posterointermediate part; BSTMV, bed nucleus of the stria terminalis, medial division, ventral part; CA1, field CA1 of hippocampus; cc, corpus callosum; Cg1, cingulate cortex, area 1; CL, claustrum; DA, dorsal hypothalamic area; DEn, dorsal endopiriform nucleus; DLPAG, dorsolateral periaqueductal grey; DMNvl, dorsomedial hypothalamic nucleus, ventrolateral part; Ent, entorhinal cortex; IML, intermediolateral cell column; IL, infralimbic cortex; LHb, lateral habenular nucleus; LPAG, lateral periaqueductal grey; LSv, lateral septal nucleus, ventral part; MeAD, medial amygdaloid nucleus, anterodorsal part; MeApv, medial amygdaloid nucleus, posteroventral part; MM, medial mammillary nucleus, medial part; MPA, medial preoptic area; ox, optic chiasm; PaDC, paraventricular hypothalamic nucleus, dorsal cap; PeF, perifornical nucleus; PH, posterior hypothalamic area; Pir, piriform cortex; PMD, premammillary nucleus, dorsal part; PMV, premammillary nucleus, ventral part; PRh, perirhinal cortex; PrL, prelimbic cortex; PV, paraventricular thalamic nucleus; RCh, retrochiasmatic area; Re, reunions thalamic nucleus; ROb, raphe obscurus; RPa, raphe pallidus; S, subiculum; SChsh, suprachiasmatic nucleus, shell region; SPa, subparaventricular zone of the hypothalamus; SuMm, supramammillary nucleus, medial part; TM, tuberomammillary nucleus; VMH, ventromedial hypothalamic nucleus.
Hippocampal area CA1 sends its primary projection to all regions of the subiculum, which in turn projects to many cortical and subcortical targets (Figs 2 and 7). The subiculum is therefore the major output structure of the hippocampus (Witter & Groenewegen, 1990; O'Mara et al. 2001). Amaral et al. (1991) suggest that the CA1 projection to the subiculum is organized in a simple pattern, with all portions of CA1 projecting to the subiculum, and all regions of subiculum receiving CA1 projections. Here, following Amaral et al. (1991), I use the term ‘proximal CA1’ to refer to the area bordering CA3 and ‘distal CA1’ for the area bordering the subiculum. The subiculum is similarly defined, with proximal subiculum bordering CA1 and distal subiculum bordering the presubiculum. To summarize these projections (Amaral et al. 1991; Fig. 3A): cells in proximal CA1 project to distal subiculum, cells in mid-CA1 project to mid-subiculum and cells in distal CA1 project across the CA1–subiculum border into proximal subiculum. Fibres arising in proximal CA1 travel to the subiculum mainly via the alveus and the deepest portion of the stratum oriens, whereas fibres originating in mid-CA1 do not enter the alveus but project to the subiculum through the deep parts of stratum oriens. The axons of distal CA1 cells travel directly to subiculum from all parts of stratum oriens (Amaral et al. 1991).
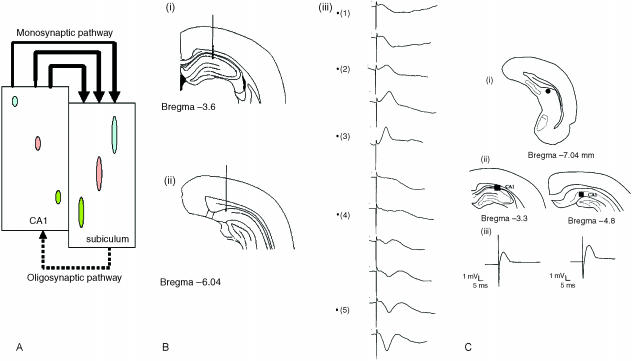
Fig. 3.
(A) Neurons in proximal CA1 project to distal subiculum, neurons in mid-CA1 project to mid-subiculum and neurons in distal CA1 project across the CA1–subiculum border into proximal subiculum. (B) Depth profile of subicular fEPSPs following stimulation in area CA1. (i) and (ii) indicate the positions of stimulating and recording electrodes located in area CA1 and subiculum, respectively; (iii) is a plot of fEPSPs following stimulation in successive locations as the stimulating electrode is moved towards area CA1 of the hippocampus. (C) Schematic drawings of the coronal sections indicating the positions of stimulating and recording electrodes located in dorsal subiculum and CA1, respectively (3.3 and 4.8 mm behind bregma; adapted from Paxinos & Watson, 1997). Also shown are the corresponding field potentials recorded after dorsal subiculum stimulation at the two sites in CA1.
Neurophysiological depth profiles of the CA1–subiculum projection, examining excitatory postsynaptic potentials evoked in the subiculum following stimulation of different sites by a bipolar stimulating electrode en route to hippocampal area CA1 of the rat in vivo, confirm this neuroanatomical analysis (O'Mara et al. 2001). Stimulating electrodes were aimed at area CA1 and the recording electrodes at the dorsal subiculum; after passing primary visual cortex and corpus callosum, the electrode was allowed to settle in dorsal subiculum (Fig. 3B). The stimulating electrode was then lowered slowly towards area CA1 of the hippocampus (Fig. 3B). Stimulation of the overlying cortex (either sensory or parietal cortex) did not produce a subicular response; the first subicular response was produced at the border of the cortex and cingulum. A large response was observed at the border of the cingulum and the alveus, characterized by a positive-going deflection in the subiculum (Fig. 3B). As the electrode was lowered further, it entered CA1 stratum oriens; the response at this point was characterized by a potential reversal. A large negative-going deflection was observed as the electrode lowered to the deeper parts of the oriens layer and the pyramidal layer of area CA1 of the hippocampus. The negative-going response observed in the subiculum after stimulation of the deeper layers of the stratum oriens and the pyramidal cell layer of the hippocampus confirms the anatomical connection between the two structures. Fibres arising in proximal CA1 travel to the subiculum mainly via the alveus and the deepest portion of the stratum oriens, whereas fibres originating in mid-CA1 do not enter the alveus but project through the deep parts of the stratum oriens. Axons of distal CA1 cells travel directly to the subiculum from all parts of the stratum oriens (Amaral et al. 1991). Combined single unit and morphological studies suggest that the CA1–subicular pathway is a monosynaptic projection (Gigg et al. 2000), and that it returns a minor oligosynaptic projection to CA1 (Commins et al. 2002). Finally, the subiculum receives cortical inputs from the entorhinal, perirhinal and prefrontal cortices, to which it returns important and prominent projections; it also receives inputs from and distributes to some other secondary and tertiary cortices. The particular pattern of convergence of these many cortical inputs onto subicular neurons will, in the model developed below, play a key role in determining the response properties of, in particular, dorsal subicular neurons.
There are extensive reciprocal connections between the subiculum and many subcortical structures (and particularly to various hypothalamic nuclei; see Fig. 7). Subcortical structures projecting to the subiculum include the ventral premammillary nucleus (to ventral subiculum), the medial septum/nucleus of the diagonal band, and all areas of the anteroventral (AV) and anteromedial (AM) nuclei of the thalamus (see Kohler, 1990; Canteras & Swanson, 1992; Risold et al. 1997). There is also some limited evidence of brainstem projections to the subiculum, possibly deriving from brainstem vestibular nuclei (M. P. Witter, pers. comm.). Ventral subiculum projects to the hypothalamus via the postcommissural fornix, the medial corticohypothalamic tract and the amygdala; these projections innervate the medial preoptic area, the ventromedial and dorsomedial nuclei, and ventral premammillary and medial mammillary nuclei. Lowry (2002) summarizes this extensive projection system as follows: ‘The ventral subiculum projection system projects to a distributed forebrain limbic system associated with inhibitory input to the hypothalamic–pituitary–adrenal (HPA) axis and the hypothalamic–spinal–adrenal (HSA). Inhibition of the HPA axis is thought to be mediated transynaptically via GABAergic neurones that project directly to the paraventricular nucleus or hypothalamic autonomic control systems. Neurones within the median raphe nucleus project extensively and selectively to the ventral subiculum projection system, including the medial hypothalamic defensive system associated with active emotional coping responses.’ Thus, the role of the subiculum is to act principally to inhibit the HPA axis, and thus it plays a key role in terminating or limiting the response of the HPA axis to stress.
Are there other non-HPA axis-related subcortical inputs to the subiculum? A particularly interesting candidate system that may provide endpoint input to the subiculum is the vestibular system. Some studies have examined functional activation of subcortical subicular inputs using metabolic markers (c-Fos; Vann et al. 2000a,b) or electrophysiological recordings (Wiener et al. 1995) and are suggestive of a strong, movement-related input, which is activated during exploratory locomotion (King et al. 1998). Additionally, several lesion studies have found deficits in spatial learning after thalamic lesions (Aggleton et al. 1996; Wiest et al. 1996; van Groen et al. 2002). The origin of these deficits is not clear, but anterior thalamic neurons reflect movement- and head-direction-related information, and the latter is lost after vestibular system lesion. Vestibular system activation influences hippocampal formation unit activity in the rodent and primate (O'Mara et al. 1994; Zugaro et al. 2001). Stimulation of vestibular regions induces field potentials in the hippocampal formation of anaesthetized guinea-pigs (Cuthbert et al. 2000), and vestibular influences have been implicated in the updating of hippocampal maps during self-motion and in path integration. Lesions of the subiculum do not lead to deficits in spatial learning in the watermaze in the same fashion as do lesions of the hippocampus proper; rather, the effects of ‘pure’ subicular lesions on spatial learning appear to be more readily interpretable as deficits in heading and bearing on a target, in addition to a deficit in precise localization of the position of the hidden platform (Morris et al. 1990).
Synaptic plasticity in the CA1–subiculum pathway
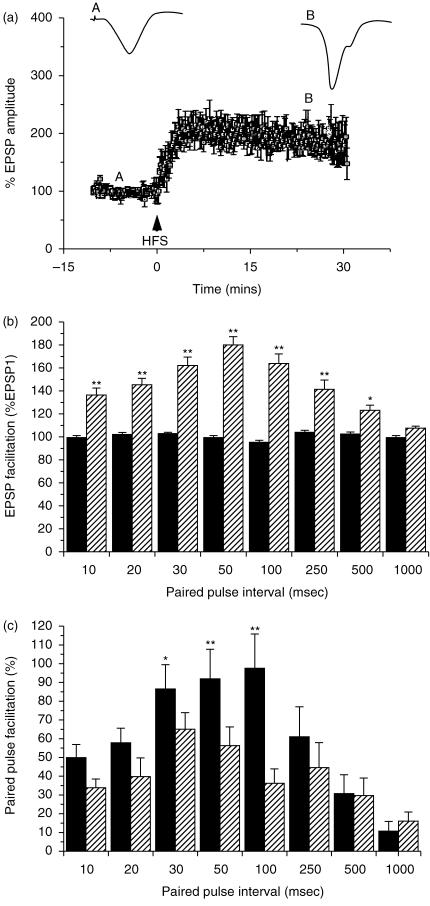
Long-term potentiation (LTP) is a popular model of the synaptic plasticity that may be engaged by the biological processes underlying learning and memory (Martin et al. 2000; Lynch, 2004). Most available studies of LTP have concentrated on the analysis of LTP occurring in ‘early’ components of the hippocampal circuit (for example, dentate gyrus and area CA1). Commins et al. (1998a; Fig. 4A) investigated if LTP could be induced in the CA1–subiculum projection and found that this projection does indeed sustain high-frequency stimulus (HFS)-induced LTP. In addition, input–output (I/O) curves relating stimulation voltages to excitatory postsynaptic potentials showed a leftward shift after HFS for all stimulation values, indicating that a given input elicits a greater response than prior to LTP induction. Studies of the interaction between LTP and paired-pulse facilitation (PPF – an elementary form of synaptic plasticity that is primarily presynaptic in origin) may throw light on the role of presynaptic factors in LTP. Commins et al. (1998b; Fig 4B) investigated PPF in the CA1–subiculum projection in vivo: PPF peaked at a 50 ms interstimulus interval (ISI) and was evident at ISIs from 10 to 500 ms; there was no PPF evident at 1000 ms ISI. After the induction of LTP, PPF decreases in magnitude across the middle range of ISI values tested (30, 50 and 100 ms); there was a positive correlation between initial PPF values and LTP, which increased as the ISI increases. Initial values and the change in PPF post-LTP were also negatively correlated.
Fig. 4.
(A) Effects of high-frequency stimulation (HFS) on the amplitude of fEPSPs; post-HFS fEPSP values are expressed as a percentage of the pre-HFS baseline. The insets are representative EPSPs pre- and post-LTP induction. The letters above the averaged data represent the time point from which the inset traces are taken. (B) Paired-pulse facilitation in the CA1–subiculum pathway for the intervals indicated. Bars represent mean peak amplitude for fEPSP1 (black) and fEPSP2 (hatched) (**P < 0.01, *P < 0.05). Data are normalized to fEPSP1 (100%). (C) Changes in PPF after LTP induction. Mean PPF before (black) and after (hatched) HFS that induced LTP (**P < 0.01, *P < 0.05).
Behavioural and endotoxic stressors and synaptic plasticity in the CA1–subicular pathway
A contemporary definition suggests stress involves heightened excitability or arousal, a perception of aversiveness and a lack of controllability over outcomes (Kim & Diamond, 2002). The stress response is controlled by the HPA axis, which is substantially regulated by the hippocampal formation. Behavioural stress (e.g. uncontrollable tailshock) and/or systemic stress (e.g. anoxia, infection) triggers the release of corticotropin-releasing hormone (CRH) from the hypothalamus into the portal circulation to the anterior pituitary, which releases adrenocorticotrophic-releasing hormone (ACTH) into the bloodstream, causing corticosterone (rat) or cortisol (human) release from the adrenal cortices. ACTH initiates ‘fight or flight’ responses, mobilizes energy stores, decreases reflex thresholds, and increases respiratory rate, muscle tension and gastric motility; these effects, if short-lived, are generally positive and behaviourally adaptive. Prolonged behavioural and/or systemic stress, however, among many diverse effects inhibits LTP, causes hippocampal atrophy, impairs hippocampal-dependent learning, contributes to brain ageing, causes many generalized behavioural changes, is implicated in many neuropsychiatric disorders and depresses the immune system (for reviews see Kim & Yoon, 1998; McEwen, 2000; Fuchs & Flugge, 2003; Sapolsky, 2003).
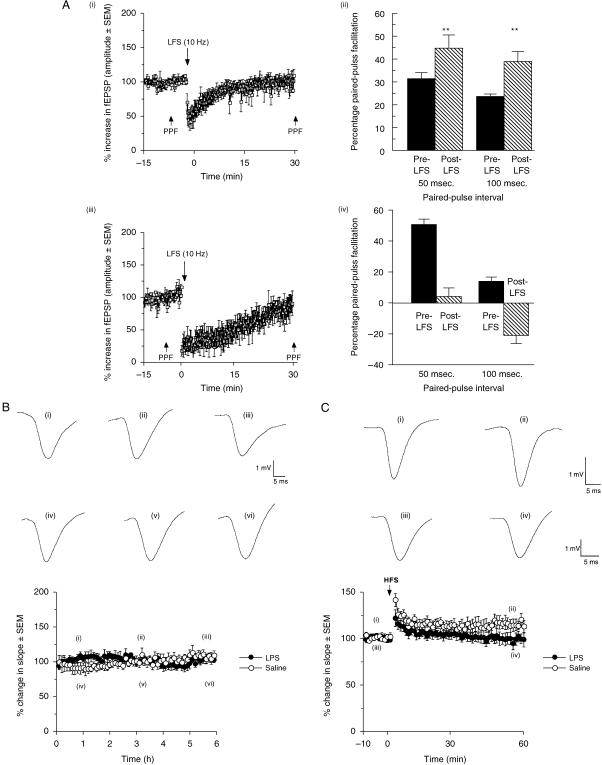
Although the subiculum substantially regulates the HPA axis stress response, the CA1–subicular axis is itself profoundly affected by behavioural and systemic stress. Standard long-term depression (LTD) induction protocols do not induce depression of synaptic transmission in the CA1–subiculum pathway (Anderson et al. 2000), but prior behavioural stress (inescapable photic stimulation) facilitates substantial and sustained LTD induction in this pathway (Commins & O'Mara, 2000; Fig. 5A). Behavioural stress also abolishes PPF by increasing the amplitude of the first excitatory postsynaptic potential (EPSP) of EPSP pairs at a short interval pair (50 ms), and causes paired-pulse depression with a long interval pair (100 ms). Thus, behavioural stress can regulate both basal and paired-pulse (presynaptic) synaptic transmission in this key hippocampal pathway. These data indicate that there is a dissociation between single-pulse stimulation and paired-pulse stimulation of the CA1–subiculum pathway and therefore suggest that there are previously undescribed mechanisms regulating transmission in this pathway. Commins et al. (2001) investigated the effects of systemic stress induced by lipopolysaccharide (LPS; a potent endotoxin which induces HPA axis changes similar to those induced by behavioural stress) on synaptic transmission/synaptic plasticity in the CA1–subicular pathway. Similar to behavioural stress, LPS blocked LTP induction and reduced PPF in the CA1–subicular pathway. Importantly, LPS did not affect baseline synaptic transmission in this pathway but did reduce the magnitude of PPF; thus, the effects of LPS on synaptic transmission in this pathway depend on the frequency and length of stimulation. LPS inhibits hippocampal-dependent spatial learning in the watermaze (Shaw et al. 2001). Thus, systemic stress induced by an LPS-induced primary immune response has similar consequences to behavioural stress on synaptic plasticity and learning in the CA1–subicular axis.
Fig. 5.
Effects of LFS (10 Hz) on the amplitude of fEPSPs. The post-LFS values are expressed as a percentage of the prestimulation baseline ( ± SEM). (ii) A bar chart showing percentage PPF both pre- and post-LFS for 50 and 100 ms ISIs. Note the increase in facilitation at both ISIs post-LFS. (iii) Effects of stress and LFS (10 Hz) on the amplitude of fEPSPs. The post-LFS values are expressed as a percentage of the prestimulation baseline ( ± SEM). (iv) A bar chart showing percentage PPF both pre- and post-LFS for the 50 and 100 ms ISIs. Note the decrease in facilitation at 50 ms ISI post-LFS and PPD at the 100 ms ISI. (B) Effects of LPS (closed circle) and saline (open circle) on synaptic transmission over a 6-h period. No significant differences were noted between the two groups. (C). LPS (closed circle) blocks LTP induction compared with saline-injected (open circle) animals.
Recordings of subicular neurons in the freely moving, behaving animal
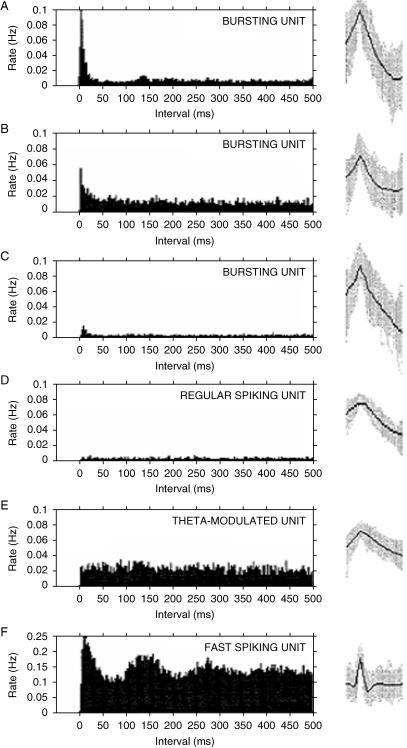
Understanding the neurocognitive functions of subiculum involves understanding the information represented by subicular neurons. Standardized methods have evolved for studying the discharge correlates of single neurons and neuronal ensembles (O'Keefe, 1979; O'Mara, 1995). Briefly, these require a freely moving rat to traverse mazes or open fields (often in search of food), and neuronal activity is recorded and correlated with the moment-to-moment position of the rat. These correlations are used to generate colour-coded contour maps representing the density of spike firing at all points occupied by the rat. Under these conditions, many hippocampal formation neurons (particular in area CA1) fire in a locally defined area of the maze (usually no more than a few per cent of the total maze area) and remain silent or fire at low rates (< 1 Hz) in other areas of the maze. The experimental apparatus may be shielded from the larger laboratory by means of curtains, to control the local cue set; this cue set may be manipulated by means of, for example, cue rotations or selective cue deletions. In a series of investigations of subicular neuron response properties under differing behavioural/task conditions, we have recorded subicular unit and EEG in rats and correlated neuronal activity with the animals' ongoing behaviour (Anderson & O'Mara, 2003, 2004). Units were classified into bursting and regular spiking units (similar to hippocampal CA1 ‘pyramidal’ units), fast-spiking units (putative inhibitory interneurons) and theta-modulated units (previously undescribed: similar to regular spiking units, but whose firing increases significantly during theta). We concluded that subicular units can be separated into at least four classes (bursting, regular spiking, theta-modulated and fast spiking) on the basis of the electrophysiological characteristics of their firing rate, spike duration, relationship with simultaneously recorded EEG and spike train time characteristics. We have also found that subicular bursting units show large variation in their propensity to burst (see also Staff et al. 2000). The analysis of unit firing against behavioural state revealed few significant differences between pre- and post-event flag firing rates, and these appeared to be related to arousal levels or movement. The ACHs for bursting, regular spiking, and the fast spiking unit classes are similar to those of Sharp & Green (1994); although the bursting units described here show more variation than Sharp (1997, 1999), it is possible that their ‘depolarized bursters’ are classified here as bursters. Sharp did not report theta-modulated units, but did not record EEG, so these units may have been assigned to the non-bursting class.
What are the discharge correlates of subicular neurons recorded while freely moving animals traverse mazes or open-field environments or engage in the exploration of objects in these environments? Our own recordings and those of others indicate that subicular units are not like hippocampal units during this sort of exploratory behaviour: subicular units tend to fire throughout the environment and show multiple peaks of activity; in general, subicular place fields appear to be of lower resolution and comprise much larger areas of comparable environments than those of area CA1 (O'Mara et al. 2000). What of subicular neuronal responses during object exploration in an open-field environment? The subiculum receives a direct projection from the perirhinal cortex, where neurons are responsive to the novelty or familiarity of objects encountered in the environment. Anderson & O'Mara (2004) made recordings of subicular neuronal activity during object exploration tasks that cause changes in the exploratory behaviour of rats and which are dependent upon the integrity of structures within the hippocampal formation. The exploratory behaviour of the rats was also modified in a manner consistent with them perceiving the novelty and familiarity of the objects used as part of the apparatus. Subicular cell firing, however, appeared to correlate best not with object novelty or familiarity, but with the concurrent location and speed of the rats within the task environment.
A neuroanatomically based model of subicular function
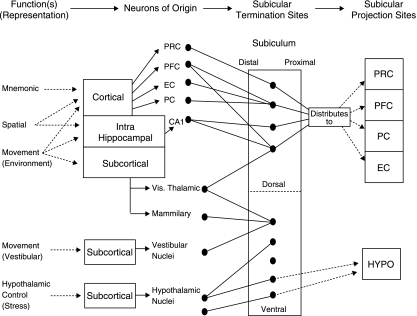
What are the functions of the subiculum, given its pivotal position as an interface between the hippocampus proper and key cortical and subcortical structures? We propose here that there is a fundamental dorso-ventral segregation of function within the subiculum: the dorsal component appears principally concerned with processing of information about space, movement and memory, whereas the ventral component is principally an interface between the hippocampal formation and the HPA axis, where it plays a major regulatory role in the inhibition of the HPA axis. I propose further that the subicular neurons have convergent inputs, both from within and from without the hippocampal formation, and that the particular pattern of convergence of neuronal inputs determines the response properties of subicular neurons in dorsal and ventral subiculum.
What are the implications of this model? As mentioned above, many hippocampal formation neurons, particularly in area CA1, fire strongly when an animal occupies a particular position in space – such cells have been named ‘place’ cells. Although the primary output of hippocampal area CA1 is the subiculum, subicular neurons do not show a clear place response when neuronal recordings are made under similar conditions to those of hippocampal neurons, despite the major input received from area CA1. By contrast, subicular neurons show multiple peaks of activity within an environment, and consistently are modulated by movement-related activity. Why is this? Thus, my simple working model suggests that multiple CA1 place cells converge on single subicular neurons (perhaps up to four or five) and that there is convergence of movement information onto single subicular neurons. These separate inputs generate a combined place and movement signal. The multiple peaks of place-related activity reflect separate place cell inputs, whereas the movement signal is assumed to derive primarily from tonic inputs from CA1 inputs, in addition to inputs from other cortical sources that converge on entorhinal cortex (particularly parietovestibular cortical inputs that are responsible for the movement signal apparent in subiculum). A consequent prediction is that microlesions of the inputs from entorhinal cortex to subiculum will substantially reduce the movement modulation of subicular neurons (as will carefully placed microlesions in CA1, although these latter lesions will also reduce the spatial selectivity of subicular neuronal response). Overall therefore I suggest that dorsal subiculum is a site of integration between hippocampal spatial information and whole-body movement-related information (primarily cortical in origin). Finally, I assume that cortical inputs from other areas (particularly prefrontal and perirhinal cortices) are important determinants of subicular neuronal response, giving rise to the possibility of subicular neurons that combine spatial and working memory information and neurons that combine spatial and object information. There is some evidence for the former possibility (Deadwyler & Hampson, 2004), but less for the latter (Anderson & O'Mara, 2003, 2004).
I assume here, along with Lowry (2002), that ventral subiculum exerts a dynamic and inhibitory influence on the HPA axis, and therefore substantially orchestrates the stress response: Lowry suggests that ' [N]o neural system is so exquisitely poised to limit the activity of the HPA axis, as well as the autonomic and behavioural elements of the stress response to unconditioned stimuli' as is ventral subiculum (Fig. 7). The subiculum is therefore likely to have a pivotal role in the regulation of the response to stress: a straightforward prediction is that ventral subicular lesions should attenuate the HPA response to systemic and behavioural stressors, and this is what appears to occur (Mueller et al. 2004). There is, however, a differential effect of subicular lesions on behavioural and systemic stressors, and the differing roles of the multiple regulatory sites responsible for the response to differing stressors need further elaboration. I assume further here that the prefrontal cortical inputs to the hypothalamus are to the same neurons of the same hypothalamic nuclei as are those of subicular neurons, but that these prefrontal inputs are primarily to excitatory neurons (allowing for a rapid activation of the HPA axis in response to evaluations of extero- or interoceptive stimuli). A straightforward prediction is that the prefrontal–hypothalamic projection should show synaptic plasticity, and the strong possibility that there potentiation of this pathway should lead to a collateral heterosynaptic depression of the subicular input to the same hypothalamic nuclei (I am assuming here that the functional roles of prefrontal cortical and subicular projections to the hypothalamus are opposed to each other: that the prefrontal input is excitatory and the subicular input is inhibitory).
What neuroanatomy has yet to tell us
The model presented here revolves around two key hypotheses (Fig. 8): that there is a dorso-ventral segregation of function within subiculum, and that the particular pattern of convergence of inputs to subicular neurons determines the response properties of single subicular neurons. Are these hypotheses correct? A straightforward answer is not yet possible, because the neuroanatomy is as yet underdetermined, but the model clearly falls if the neuroanatomy turns out to be other than as predicted. The particular pattern of convergence of separate CA1 neurons (or neurons from other cortical areas) onto single subicular neurons has not yet been described; similarly, whether this projection is a straightforwardly feedforward monosynaptic excitatory projection (as assumed here) rather than a more complex polysynaptic or oligosynaptic projection involving complex feedforward and feedback elements is not yet known. Similarly, convergent projections from differing cortical areas leading to integrative and polymodal responses are assumed here to occur, but there are no data available yet to address this question in any meaningfully quantitative way. Another prediction here is that quantitative inputs to subiculum are segregated: the bulk of inputs to dorsal subiculum are either hippocampal or cortical in origin, whereas the bulk of inputs to ventral subiculum are subcortical in origin. The pattern of return projections is predicted to follow a similar fashion: the majority of dorsal subicular projections are returned to cortical sites, whereas the majority of ventral subicular projections are to subcortical sites. In respect of these latter projections, a further assumption is made: that projections from prefrontal cortex terminate directly in the same hypothalamic nuclei as those from ventral subiculum (allowing top-down cognitive control of the response to stress), whereas the projections from ventral subiculum to these neurons are mediated trans-synaptically via GABAergic neurons. Thus, the prefrontal cortex input is directly excitatory and the ventral subicular inputs are inhibitory. A straightforward neurophysiological investigation of the interaction between these projections can be made by placing stimulating electrodes in the subiculum and prefrontal cortex, respectively, and a recording electrode in the hypothalamus; the stimulating electrodes will be used to activate their respective projections to Hypothalamus. A similar anatomical experiment can be conducted by means of dual-labelling of these differing projection systems in order to determine particular sites of termination.
Fig. 8.
A model of subicular function(s) (see text for full details). Here, synaptic transmission and anatomical connectivity run from left to right (a deliberate simplification); information of differing types (mnemonic etc.) derives from various anteceding cortical and subcortical circuits, and is projected to the subiculum, converging in particular patterns, thereby giving rise to differing neuronal response types. EC, entorhinal cortex; Hypo, hypothalamus; PRC, perirhinal cortex; PFC, prefrontal cortex; PC, parietal cortex. For simplification no details of distal–proximal distribution of fibres is provided (but these do vary).
A further unknown is the role that intrasubicular associational fibres play: do these intrinsic projection fibres converge on neurons across whole dorsoventral subicular axis, and thus reduce segregation of function within the subiculum? Here it is assumed that such fibres have a role in maintaining neurophysiological tone and patency across the dorso-ventral subicular axis, but that they do not have any major functional role in terms of information representation by subicular neurons. Thus, this model predicts that there is anatomical ‘funnelling’ of information flow through the subiculum, and that the presence of associational fibres within subiculum does not prorogue this funnelling. A direct test of this hypothesis can be made by showing there is a double dissociation of function within the subiculum: dorsal subicular lesions should leave ventral subicular control of the HPA axis unaffected and ventral subicular lesions should leave the role of dorsal subiculum in spatial representation unaffected. Similarly, ventral lesions should leave synaptic transmission through the dorsal CA1–dorsal subiculum–entorhinal cortex axis unaffected, and dorsal lesions should leave synaptic transmission through the ventral CA1–ventral subiculum–entorhinal cortex axis unaffected. Morphological and neurophysiological diversity of differing neuronal types within the subiculum is a largely unexplored topic, as are the mechanisms of feedforward, feedback and lateral inhibition of intrinsic and extrinsic subicular projections. The model presented here is agnostic on these particular details.
Conclusions
Here I have reviewed some of the neurophysiological response properties and the neuroanatomy of the pivotal subicular component of the hippocampal formation. I have presented a model of the functions subserved by the subiculum, and have suggested a series of open anatomical questions that constrain this model. In particular, I suggest that there is an anatomically generated segregation of function within the subiculum, such that dorsal subiculum plays a particular role in the processing of spatial, mnemonic and movement information and ventral subiculum plays a particular role in the mediation of the hippocampal formation inhibitory control of the HPA axis.
Fig. 6.
Examples of normalized autocorrelation histograms (ACHs) for three bursting units (A–C), a regular spiking unit (D), a theta-modulated unit (E) and a fast-spiking unit (F). ACHs were normalized by dividing the number of intervals in each 1-ms bin by the total session time (s); this reveals the rate (Hz) of each interval. The corresponding overlaid spike waveforms (grey) and mean waveform (black) are shown to the right of each ACH.
Acknowledgments
My research has been supported by the Wellcome Trust, the Higher Education Authority Programme for Research in Third-Level Institutions and the Health Research Board. Thanks are due to Dr Richard Roche for assistance with manuscript preparation.
References
- Aggleton JP, Hunt PR, Nagle S, Neave N. The effects of selective lesions within the anterior thalamic nuclei on spatial memory in the rat. Behav Brain Res. 1996;81:189–198. doi: 10.1016/s0166-4328(96)89080-2. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Dolorfo C, Alvarez-Royo P. Organization of CA1 projections to the subiculum: a PHA-L analysis in the rat. Hippocampus. 1991;1:415–435. doi: 10.1002/hipo.450010410. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Witter MP. Hippocampal formation. In: Paxinos G, editor. The Rat Nervous System. 2nd. New York: Academic Press; 1995. pp. 247–291. [Google Scholar]
- Anderson M, Commins S, O'Mara SM. The effects of low frequency and two-pulse stimulation protocols on synaptic transmission in the CA1-subiculum pathway in the anaesthetized rat. Neurosci Lett. 2000;279:181–184. doi: 10.1016/s0304-3940(99)00996-9. [DOI] [PubMed] [Google Scholar]
- Anderson MI, O'Mara SM. Analysis of recordings of single-unit firing and population activity in the dorsal subiculum of unrestrained, freely moving rats. J Neurophysiol. 2003;90:655–665. doi: 10.1152/jn.00723.2002. [DOI] [PubMed] [Google Scholar]
- Anderson M, O'Mara SM. Activity of subicular units on a spatial and non-spatial version of an open-field object exploration task. Exp Brain Res. 2004;159:519–529. doi: 10.1007/s00221-004-1977-z. [DOI] [PubMed] [Google Scholar]
- Brodmann K. Vergleichende Lokalisationslehre der Grosshirnrinde in ihren Prinzipien dargestellt auf Grund des Zellenbaues. Leipzig: Barth; 1909. [Google Scholar]
- Canteras NS, Swanson LW. Projections of the ventral subiculum to the amygdala, septum, and hypothalamus: a PHA-L anterograde tract-tracing study in the rat. J Comp Neurol. 1992;324:180–194. doi: 10.1002/cne.903240204. [DOI] [PubMed] [Google Scholar]
- Commins S, Gigg J, Anderson M, O'Mara SM. The projection from hippocampal area CA1 to the subiculum sustains long-term potentiation. Neuroreport. 1998a;9:847–850. doi: 10.1097/00001756-199803300-00015. [DOI] [PubMed] [Google Scholar]
- Commins S, Gigg J, Anderson M, O'Mara SM. Interaction between paired-pulse facilitation and long-term potentiation in the projection from hippocampal area CA1 to the subiculum. Neuroreport. 1998b;9:4109–4113. doi: 10.1097/00001756-199812210-00019. [DOI] [PubMed] [Google Scholar]
- Commins S, O'Mara SM. Interactions between paired-pulse facilitation, low-frequency stimulation, and behavioral stress in the pathway from hippocampal area CA1 to the subiculum. Dissociation of baseline synaptic transmission from paired-pulse facilitation and depression of the same pathway. Psychobiology. 2000;28:1–11. [Google Scholar]
- Commins S, O'Neill LA, O'Mara SM. The effects of the bacterial endotoxin lipopolysaccharide on synaptic transmission and plasticity in the CA1-subiculum pathway in vivo. Neuroscience. 2001;102:273–280. doi: 10.1016/s0306-4522(00)00498-x. [DOI] [PubMed] [Google Scholar]
- Commins S, Aggleton JP, O'Mara SM. Physiological evidence for a possible projection from dorsal subiculum to hippocampal area CA1. Exp Brain Res. 2002;146:155–160. doi: 10.1007/s00221-002-1158-x. [DOI] [PubMed] [Google Scholar]
- Cuthbert PC, Gilchrist DP, Hicks SL, MacDougall HG, Curthoys IS. Electrophysiological evidence for vestibular activation of the guinea pig hippocampus. Neuroreport. 2000;11:1443–1447. doi: 10.1097/00001756-200005150-00018. [DOI] [PubMed] [Google Scholar]
- Deadwyler SA, Hampson RE. Differential but complementary mnemonic functions of the hippocampus and subiculum. Neuron. 2004;42:465–476. doi: 10.1016/s0896-6273(04)00195-3. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Cohen NJ. From Conditioning to Conscious Recollection: Memory Systems of the Brain. Oxford: Oxford University Press; 2001. [Google Scholar]
- Fuchs E, Flugge G. Chronic social stress: effects on limbic brain structures. Physiol Behav. 2003;79:417–427. doi: 10.1016/s0031-9384(03)00161-6. [DOI] [PubMed] [Google Scholar]
- Gigg J, Finch DM, O'Mara SM. Responses of rat subicular neurons to convergent stimulation of lateral entorhinal cortex and CA1 in vivo. Brain Res. 2000;884:35–50. doi: 10.1016/s0006-8993(00)02878-x. [DOI] [PubMed] [Google Scholar]
- van Groen T, Kadish I, Wyss JM. Role of the anterodorsal and anteroventral nuclei of the thalamus in spatial memory in the rat. Behav Brain Res. 2002;132:19–28. doi: 10.1016/s0166-4328(01)00390-4. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Yoon KS. Stress: metaplastic effects in the hippocampus. Trends Neurosci. 1998;21:505–509. doi: 10.1016/s0166-2236(98)01322-8. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Diamond DM. The stressed hippocampus, synaptic plasticity and lost memories. Nat Rev Neurosci. 2002;3:453–462. doi: 10.1038/nrn849. [DOI] [PubMed] [Google Scholar]
- King C, Recce M, O'Keefe J. The rhythmicity of cells of the medial septum/diagonal band of Broca in the awake freely moving rat: relationships with behaviour and hippo-campal theta. Eur J Neurosci. 1998;10:464–477. doi: 10.1046/j.1460-9568.1998.00026.x. [DOI] [PubMed] [Google Scholar]
- Kohler C. Subicular projections to the hypothalamus and brainstem: some novel aspects revealed in the rat by the anterograde PHA-L tracing method. Prog Brain Res. 1990;83:59–69. doi: 10.1016/s0079-6123(08)61241-8. [DOI] [PubMed] [Google Scholar]
- Lorente de No F. Studies on the structure of the cerebral cortex. Continuation of the study on the ammonic system. J Psychol Neurol. 1934;46:113–117. [Google Scholar]
- Lowry CA. Functional subsets of serotonergic neurones: Implications for control of the hypothalamic-pituitary-adrenal axis. J Neuroendocrinol. 2002;14:911–923. doi: 10.1046/j.1365-2826.2002.00861.x. [DOI] [PubMed] [Google Scholar]
- Lynch MA. Long-term potentiation and memory. Physiol Rev. 2004;84:87–136. doi: 10.1152/physrev.00014.2003. [DOI] [PubMed] [Google Scholar]
- Martin SJ, Grimwood PD, Morris RG. Synaptic plasticity and memory: an evaluation of the hypothesis. Ann Rev Neurosci. 2000;23:649–711. doi: 10.1146/annurev.neuro.23.1.649. [DOI] [PubMed] [Google Scholar]
- McEwen BS. The neurobiology of stress: from serendipity to clinical relevance. Brain Res. 2000;886:172–189. doi: 10.1016/s0006-8993(00)02950-4. [DOI] [PubMed] [Google Scholar]
- Morris RG, Schenk F, Tweedie F, Jarrard LE. Ibotenate lesions of hippocampus and/or subiculum. Dissociating components allocentric spatial learning. Eur J Neurosci. 1990;2:1016–1028. doi: 10.1111/j.1460-9568.1990.tb00014.x. [DOI] [PubMed] [Google Scholar]
- Mueller NK, Dolgas CM, Herman JP. Stressor-selective role of the ventral subiculum in regulation of neuroendocrine stress responses. Endocrinology. 2005;146:1650–1673. doi: 10.1210/en.2004-0097. [DOI] [PubMed] [Google Scholar]
- O'Keefe J, Nadel L. The Hippocampus as a Cognitive Map. Oxford: Clarendon Press; 1978. [Google Scholar]
- O'Keefe J. A review of the hippocampal place cells. Prog Neurobiol. 1979;13:419–439. doi: 10.1016/0301-0082(79)90005-4. [DOI] [PubMed] [Google Scholar]
- O'Mara SM, Rolls ET, Berthoz A, Kesner RP. Neurons responding to whole-body motion in the primate hippocampus. J Neurosci. 1994;14:6511–6523. doi: 10.1523/JNEUROSCI.14-11-06511.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Mara SM. Spatially selective firing properties of hippocampal formation neurons in rodents and primates. Prog Neurobiol. 1995;45:253–274. doi: 10.1016/0301-0082(94)00050-r. [DOI] [PubMed] [Google Scholar]
- O'Mara SM, Commins S, Anderson M. Synaptic plasticity in the hippocampal area CA1-subiculum projection: implications for theories of memory. Hippocampus. 2000;10:447–456. doi: 10.1002/1098-1063(2000)10:4<447::AID-HIPO11>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- O'Mara SM, Commins S, Anderson M, Gigg J. The subiculum: a review of form, physiology and function. Prog Neurobiol. 2001;64:129–155. doi: 10.1016/s0301-0082(00)00054-x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego: London: Academic; 1997. [Google Scholar]
- Risold PY, Thompson RH, Swanson LW. The structural organization of connections between hypothalamus and cerebral cortex. Brain Res Rev. 1997;19:197–254. doi: 10.1016/s0165-0173(97)00007-6. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Stress and plasticity in the limbic system. Neurochem Res. 2003;28:1735–1742. doi: 10.1023/a:1026021307833. [DOI] [PubMed] [Google Scholar]
- Sharp PE. Subicular cells generate similar spatial firing patterns in two geometrically and visually distinctive environments: comparison with hippocampal place cells. Behav Brain Res. 1997;85:71–92. doi: 10.1016/s0166-4328(96)00165-9. [DOI] [PubMed] [Google Scholar]
- Sharp PE. Subicular place cells expand or contract their spatial firing pattern to fit the size of the environment in an open field but not in the presence of barriers: comparison with hippocampal place cells. Behav Neurosci. 1999;113:643–662. doi: 10.1037//0735-7044.113.4.643. [DOI] [PubMed] [Google Scholar]
- Sharp PE, Green C. Spatial correlates of firing patterns of single cells in the subiculum of the freely moving rat. J Neurosci. 1994;14:2339–2356. doi: 10.1523/JNEUROSCI.14-04-02339.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw KN, Commins S, O'Mara SM. Lipopolysaccharide causes deficits in spatial learning in the watermaze but not in BDNF expression in the rat dentate gyrus. Behav Brain Res. 2001;28:47–54. doi: 10.1016/s0166-4328(01)00232-7. [DOI] [PubMed] [Google Scholar]
- Staff NP, Jung H-Y, Thiagarajan T, Yao M, Spruston N. Resting and active properties of pyramidal neurons in subiculum and CA1 of rat hippocampus. J Neurophysiol. 2000;84:2398–2408. doi: 10.1152/jn.2000.84.5.2398. [DOI] [PubMed] [Google Scholar]
- Vann SD, Brown MW, Erichsen JT, Aggleton JP. Fos imaging reveals differential patterns of hippocampal and parahippocampal subfield activation in rats in response to different spatial memory tests. J Neurosci. 2000a;20:2711–2718. doi: 10.1523/JNEUROSCI.20-07-02711.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vann SD, Brown MW, Erichsen JT, Aggleton JP. Using fos imaging in the rat to reveal the anatomical extent of the disruptive effects of fornix lesions. J Neurosci. 2000b;20:8144–8152. doi: 10.1523/JNEUROSCI.20-21-08144.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiener SI, Korshunov VA, Garcia R, Berthoz A. Inertial, substratal and landmark cue control of hippocampal CA1 place cell activity. Eur J Neurosci. 1995;7:2206–2219. doi: 10.1111/j.1460-9568.1995.tb00642.x. [DOI] [PubMed] [Google Scholar]
- Wiest G, Baumgartner C, Deecke L, et al. Effects of hippocampal lesions on vestibular memory in whole-body rotations. J Vestibular Res. 1996;6:4S–S17. [Google Scholar]
- Witter MP, Groenewegen HJ. The subiculum: cytoarchitectonically a simple structure, but hodologically complex. In: Storm-Mathisen J, Zimmer J, Otterson OP, editors. Understanding the Brain Through the HippocampusProgress in Brain Research. Amsterdam: Elsevier; 1990. pp. 47–58. [DOI] [PubMed] [Google Scholar]
- Zugaro MB, Tabuchi E, Fouquier C, Berthoz A, Wiener SI. Active locomotion increases peak firing rates of anterodorsal thalamic head direction cells. J Neurophysiol. 2001;86:692–702. doi: 10.1152/jn.2001.86.2.692. [DOI] [PubMed] [Google Scholar]