Inhibitory effects of JTV-519, a novel cardioprotective drug, on potassium currents and experimental atrial fibrillation in guinea-pig hearts (original) (raw)
Abstract
- We investigated the effects of JTV-519 (4-[3-(4-benzylpiperidin-1-yl)propionyl]-7-methoxy-2,3,4,5-tetrahydro-1,4-benzothiazepine monohydrochloride), a novel cardioprotective drug, on the repolarizing K+ currents in guinea-pig atrial cells by use of patch-clamp techniques. We also evaluated the effects of JTV-519 on experimental atrial fibrillation (AF) in isolated guinea-pig hearts.
- In atrial cells stimulated at 0.2 Hz, JTV-519 in concentrations of 0.3 and 1 μM slightly prolonged the action potential duration (APD). The drug also reversed the action potential shortening induced by the muscarinic agonist carbachol in a concentration-dependent manner.
- The muscarinic acetylcholine receptor-operated K+ current (_I_K.ACh) was activated by the extracellular application of carbachol (1 μM), adenosine (10 μM) or by the intracellular loading of GTPγS (100 μM). JTV-519 inhibited the carbachol-, adenosine- and GTPγS-induced _I_K.ACh with the IC50 values of 0.12, 2.29 and 2.42 μM, respectively, suggesting that the drug may inhibit _I_K.ACh mainly by blocking the muscarinic receptors.
- JTV-519 (1 μM) inhibited the delayed rectifier K+ current (_I_K). Electrophysiological analyses indicated that the drug preferentially inhibits _I_Kr (rapidly activating component) but not _I_Ks (slowly activating component).
- In isolated hearts, perfusion of carbachol (1 μM) shortened monophasic action potential (MAP) and effective refractory period (ERP), and lowered atrial fibrillation threshold (AFT). Addition of JTV-519 (1 μM) inhibited the induction of AF by prolonging MAP and ERP.
- We conclude that JTV-519 can exert antiarrhythmic effects against AF by inhibiting repolarizing K+ currents. The drug may be useful for the treatment of AF in patients with ischaemic heart disease.
Keywords: Antiarrhythmic effect, JTV-519, atrial fibrillation, delayed rectifier K+ current, muscarinic acetylcholine receptor-operated K+ current
Introduction
JTV-519 (4-[3-(4-benzylpiperidin-1-yl) propionyl]-7-methoxy-2,3,4,5-tetrahydro-1,4-benzothiazepine monohydrochloride) is a newly-synthesized cardioprotective drug. It has been reported that JTV-519 produced a protective effect against Ca2+ overload-induced myocardial injury and the effect was more potent than propranolol, verapamil and diltiazem (Kaneko, 1994). More recently it has been also demonstrated that this drug affords cardioprotection against ischaemia/reperfusion injury in isolated rat hearts through the activation of protein kinase C (Inagaki et al., 2000). In addition, JTV-519 has been shown to inhibit the Na+, Ca2+ and K+ currents in guinea-pig ventricular cells (Kimura et al., 1999). It is well-known that antiarrhythmic drugs possessing multichannel blocking action such as amiodarone and bepridil are effective against atrial fibrillation (AF) by inhibiting the several repolarizing K+ currents of atrial cells (Watanabe et al., 1996; Hara & Nakaya, 1995). Therefore, it is possible that JTV-519 possessing multichannel blocking actions may be useful for the treatment of AF, which is the primary target of pharmacotherapy with antiarrhythmic drugs. This study was undertaken to examine the effects of JTV-519 on the action potentials and the repolarizing K+ currents in isolated guinea-pig atrial cells using patch clamp techniques. Since JTV-519 potently inhibited the muscarinic acetylcholine receptor-operated K+ current (_I_K.ACh) and the rapid component of the delayed rectifier K+ current (_I_Kr) and prolonged the action potential in this study, we also evaluated the effects of JTV-519 on the experimental AF in Langendorff-perfused guinea-pig hearts.
Methods
Patch-clamp study
All experiments were performed under the regulations of the Animal Research Committee of the School of Medicine, Chiba University. Single atrial cells of the guinea-pig heart were isolated by an enzymatic dissociation method, as described previously (Ohmoto-Sekine et al., 1999). The heart was removed from open chest guinea-pigs (250–350 g) anaesthetized with pentobarbitone sodium and mounted on a modified Langendorff perfusion system for retrograde perfusion of the coronary circulation with a normal HEPES-Tyrode's solution. The perfused medium was changed to a nominally Ca2+-free Tyrode's solution and then to the solution containing 0.01–0.02% wt vol−1 collagenase (Wako, Osaka, Japan). After digestion, the heart was perfused with a high K+, low-Cl− solution, modified Kraftbrühe (KB) solution (Isenberg & Klockner, 1982; Nakaya et al., 1993). Atrial tissue was cut into small pieces in the modified KB solution and gently shaken to dissociate cells. The composition of the normal HEPES-Tyrode's solution was (in mM): NaCl 143, KCl 5.4, CaCl2 1.8, MgCl2 0.5, NaH2PO4 0.33, glucose 5.5 and HEPES-NaOH buffer (pH 7.4) 5.0. The composition of the modified KB solution was (in mM): KOH 70, 1-glutamic acid 50, KCl 40, taurine 20, KH2PO4 20, MgCl2 3, glucose 10, EGTA 1.0 and HEPES-KOH buffer (pH 7.4) 10.
Whole-cell membrane currents were recorded by the patch-clamp method (Hamill et al., 1981). Single atrial cells were placed in a recording chamber (1 ml volume) attached to an inverted microscope (model IMT-2, Olympus, Tokyo, Japan) and superfused with the HEPES-Tyrode's solution at a rate of 3 ml min−1. The temperature of the external solution was kept constant at 36.0±1.0°C. Patch pipettes were made from glass capillaries with a diameter of 1.5 mm using a vertical microelectrode puller (model PB-7, Narishige, Tokyo, Japan). They were filled with an internal solution, and their resistance was 2–4 M Ω. The composition of the pipette solution was (in mM): potassium aspartate 110, KCl 20, MgCl2 1.0, potassium ATP 5.0, potassium phosphocreatine 5.0, EGTA 10 and HEPES-KOH buffer (pH 7.4) 5.0. In some experiments GTP (100 μM) or GTPγS (100 μM) was also added to the pipette solution. The free Ca2+ concentration in the pipette solution was adjusted to pCa 8 according to the calculation by Fabiato & Fabiato (1979) with the correction of Tsien & Rink (1980). After the giga-ohm seal between the tip of the electrode and the cell membrane was established, the membrane patch was disrupted by more negative pressure to make the whole-cell voltage-clamp mode. The electrode was connected to a patch-clamp amplifier (model CEZ-2300, Nihon Kohden, Tokyo, Japan). Recording signals were filtered at 1 kHz bandwidth, and series resistance was compensated. Command pulse signals were generated by a 12-bit digital-to-analogue converter controlled by pCLAMP software (Axon Instruments Inc., Foster City, CA, U.S.A.). Current signals were digitized and stored on the hard disc of an IBM-compatible computer (Compaq Prolinea 4/50 with a 200 Mbyte hard disc, Houston, TX, U.S.A.). A liquid junction potential between the internal solution and the bath solution of −8 mV was corrected.
Current-clamp experiments were also performed in the whole-cell recording mode at 36±1°C. External solution and pipette solution were the same as those used to record whole-cell membrane currents. The cells were stimulated by passing 2 ms currents through the pipette at a rate of 0.2 Hz. After stabilization of action potential configuration, effects of JTV-519 on the action potential in the presence or absence of carbachol (CCh 1 μM) were evaluated.
The _I_K.ACh was activated by the extracellular application of CCh (1 μM) or adenosine (10 μM) in the GTP-loaded atrial cells or by the intracellular loading of GTPγS, a nonhydrolyzable GTP analogue, in atrial cells held at −40 mV. Effects of various concentrations of JTV-519 on the _I_K.ACh activated in three different ways were examined. To calculate per cent inhibition of _I_K.ACh, the difference between the steady-state current in the solution containing either CCh (1 μM) or adenosine (10 μM) and the current level in the absence of any agonist was taken as 100% in the GTP-loaded cells. In the GTPγS-loaded cells, the difference between the persistent outward current in the absence of agonist and the initial current level just after the break of the patch membrane in the pipette was taken as 100%. In another series of experiments we examined the effects of JTV-519 on the current-voltage relationship of _I_K.ACh. The quasi-steady-state membrane current was recorded using a ramp pulse protocol, as previously described (Sakamoto et al., 1998). The membrane potential was held at −40 mV and depolarized first to +50 mV at a rate of 1.2 mV ms−1. It was then repolarized or hyperpolarized to −100 mV with a slope of −1.2 mV ms−1 during which time the change in the membrane current was automatically plotted against the membrane potential. The ramp voltage pulses were applied at appropriate timing while the membrane current at −40 mV was continuously monitored. Actually the ramp voltage pulses were delivered before, after the extracellular application of CCh, adenosine or the intracellular loading of GTPγS, and after the addition of JTV-519. When the GTPγS-induced _I_K.ACh was recorded, special care was employed in delivering the first ramp voltage pulse immediately after the rupture of the patch membrane.
The _I_K was elicited by delivering the depolarizing pulses from a holding potential of −40 mV after the inhibition of the L-type Ca2+ current (_I_Ca) by nisoldipine (1 μM), and effects of JTV-519 on _I_K were examined. The amplitude of the time-dependent current activated during depolarizing pulses (_I_K, depo), and the deactivating current (_I_K, tail) as the difference between the holding current and the peak current that was actually recorded upon the clamp back to the holding potential were measured, as described previously (Ohmoto-Sekine et al., 1999; Matsumoto et al., 1999). The _I_K of guinea-pig atrial myocyte reportedly consists of two components, rapid (_I_Kr) and slow (_I_Ks) (Sanguinetti & Jurkiewicz, 1991). In order to determine whether JTV-519 affects _I_Kr and/or _I_Ks, effects of JTV-519 on the _I_K were examined in the presence of E-4031 (5 μM), a selective blocker of _I_Kr (Sanguinetti & Jurkiewicz, 1990).
Isolated heart study
The heart was removed from the open-chest guinea-pigs (300–400 g) anaesthetized with pentobarbitone sodium. The aorta was cannulated and perfused at a constant pressure (800 mmH2O) of normal Tyrode's solution. The composition of the solution was (in mM): NaCl 125, KCl 4, CaCl2 1.8, MgCl2 0.5, NaH2PO4 1.8, glucose 5.5 and NaHCO3 25 (pH 7.4). The solution was aerated with a mixture of 95% O2 and 5% CO2 and maintained at 36.0±0.5°C.
The right atrium was stimulated with an external bipolar silver electrode. The stimuli were rectangular pulses of 2-ms duration at twice the diastolic threshold, delivered from an electronic stimulator (model SEC-2102, Nihon Kohden). The left atrial monophasic action potential (MAP) was recorded using an additional monopolar suction electrode with a diameter of 2.0 mm, attached to the walls of the left atria. The electrical signals were amplified by a bioelectric amplifier (model AB-620G, Nihon Kohden) at a time constant of 3 ms and recorded at a paper speed of 10–100 mm s−1 using a chart recorder (model 8K21, NEC San-ei Instruments, Tokyo, Japan).
Atrial effective refractory period (ERP) was determined using the standard extrastimulus technique. After every eighth basic right atrial stimulus (S1S1 200 ms), an extrastimulus (S2) was delivered with a shortening of the coupling interval (S1S2) in 5-ms steps until the S2 produced no atrial activity. ERP was defined as the longest S1S2 that failed to elicit atrial activity in response to S2. Conduction time (CT) from the right to the left atrium was measured as the time from the pacing spike to the first upstroke of left atrial monophasic action potential on the oscilloscope (model VC-11. Nihon Kohden).
Atrial fibrillation threshold (AFT) was measured by rapid atrial electrical stimulation as previously described (Watanabe et al., 1996). The fibrillating current that consisted of a train of 50 square wave pulse train was delivered to the right atrium after every eighth basic paced beat. The current was increased in increments of 0.1 mA from an intensity twice the diastolic threshold. The AFT was defined as the minimum amount of current required to induce AF which was sustained for at least 30 s. Sustained AF was terminated readily by perfusing normal Tyrode's solution. The stimulator used in this study was unable to deliver a current greater than 12 mA. If AF could not be induced by the current as high as 12 mA, the AFT was considered as more than 12 mA.
Initial measurements were made during perfusion with Tyrode's solution (control values). The same measurements were then repeated 10 min after changing to a Tyrode's solution containing 1 μM CCh and 10 min after the perfusion of the normal Tyrode's solution containing 1 μM CCh and 1 μM JTV-519.
Drugs
Drugs used in this study were as follows: JTV-519 (Japan Tobacco, Osaka, Japan), carbachol chloride (Tokyo Kasei, Tokyo, Japan), adenosine (Sigma, U.S.A.), nisoldipine (Bayer, Osaka, Japan), E-4031 (N-[4-[[1-[2-(6-methyl-2-pyridinyl) ethyl]-4-piperidinyl]carbonyl]phenyl]methanesulphonamide dihydrochloride dihydrate) (Eisai Co, Tokyo, Japan). JTV-519 was dissolved in dimethyl sulphoxide (DMSO) as a stock solution of 10 mM. The final concentration of DMSO was less than 0.3% and the same concentration of DMSO was applied during control period before the introduction of JTV-519. Nisoldipine was dissolved in ethanol as a stock solution of 10 mM. The final concentration of ethanol was less than 0.01%. It was confirmed that the concentrations of the solvents had no influence on the membrane currents. Other drugs were dissolved in distilled water.
Statistics
All data are presented as mean±s.e.mean. Student's _t_-test and analysis of variance (ANOVA) were used for the statistical analyses. _P_-values of <0.05 were considered significant. The concentration-effect data were fitted and the IC50 values were obtained using Delta Graph Professional (Delta Point, Polaroid Computing, Tokyo, Japan).
Results
Effects of JTV-519 on the action potential
Effects of JTV-519 on the action potential of guinea-pig atrial cells in the absence and presence of muscarinic stimulation were examined in the current clamp mode. The baseline characteristics of action potentials recorded from single atrial myocytes stimulated at 0.2 Hz were as follows: resting membrane potential (RMP), −76.1±1.3 mV; action potential amplitude, 169.0±7.4 mV; action potential duration (APD) at 90% repolarization level (APD90), 102.7±6.7 ms (_n_=16). In the absence of any muscarinic agonist JTV-519 at concentrations of 0.3 and 1 μM insignificantly prolonged APD90 by 33.1±13.2% (_P_=0.16) and 41.0±16.6% (_P_=0.11) from the control, respectively (_n_=9) (Figure 1). The slight prolongation of APD90 was reverted toward the control after washout. CCh at a concentration of 1 μM markedly shortened APD90 from 97.8±7.2 to 18.4±2.9 ms (P<0.05) with a slight and insignificant increase in RMP (from −76.5±2.5 to −80.9±1.6 ms, _n_=7) in GTP (100 μM)-loaded single atrial cells. JTV-519 reversed the carbachol-induced action potential shortening in a concentration-dependent manner (Figure 1). The CCh-induced shortening of APD90 was reversed to 63.2±10.8% (P<0.05) and 118.2±20.8% (P<0.05) of the control after 0.3 and 1 μM JTV-519, respectively (_n_=7).
Figure 1.
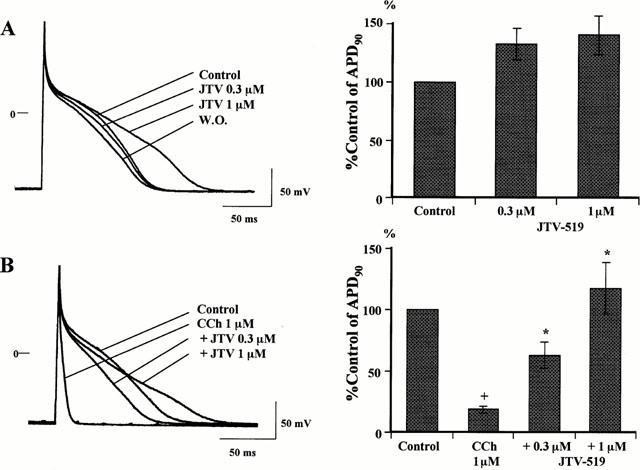
Effects of JTV-519 on the action potential in the absence (A) and presence of carbachol (1 μM) (B) in guinea-pig atrial cells. Superimposed records of action potentials recorded before (control), after exposure to JTV-519 (JTV) (0.3 and 1 μM) and washout (W.O.) of the drug are shown in A. Superimposed records of action potentials after exposure to carbachol (CCh) and carbachol plus JTV-519 are shown in B. Summarized data of changes of action potential duration at 90% repolarization level (APD90) after JTV-519 in the absence and presence of muscarinic stimulation are indicated on the right side. The values are expressed as mean±s.e.mean of 7–9 experiments. +P<0.05 vs control, *P<0.05 vs carbachol alone.
Effects of JTV-519 on the muscarinic acetylcholine receptor-operated K+ current
The _I_K.ACh is one of the important repolarizing currents in atrial cells and JTV-519 reversed the action potential shortening induced by muscarinic stimulation in this study. Therefore, we examined effects of JTV-519 on the _I_K.ACh induced by CCh, adenosine or intracellular loading of GTPγS in isolated atrial cells. Upon application of 1 μM CCh to the bath solution, an outward K+ current was rapidly activated at a holding potential of −40 mV. After the activation, the CCh-induced K+ current gradually declined despite the continuous presence of CCh, possibly because of a receptor desensitization (Carmeliet & Mubagwa, 1986; Kurachi et al., 1987). After the current had almost reached a steady level, JTV-519 was added to the bath solution. JTV-519 depressed the CCh-induced _I_K.ACh in a concentration-dependent manner (Figure 2). Recovery from the inhibition by JTV-519 was observed after washout. The IC50 value of JTV-519 for depressing the CCh-induced _I_K.ACh was 0.12 μM (Figure 2). Although CCh and adenosine act on different membrane receptors, i.e. M2 muscarinic-ACh receptor and A1-adenosine receptor, adenosine can also induce _I_K.ACh through the activation of pertussis toxin-sensitive GTP-binding protein in atrial cells (Kurachi et al., 1986). JTV-519 also inhibited the adenosine-induced current less effectively than the CCh-induced _I_K.ACh (Figure 2). The IC50 value of JTV-519 for depressing the adenosine-induced _I_K.ACh was 2.29 μM. We also evaluated the effects of JTV-519 on the _I_K.ACh induced by intracellular loading of GTPγS (100 μM), a nonhydrolyzable GTP analogue. In GTPγS-loaded cells, antagonist-resistant outward current was activated gradually and persisted even in the absence of any agonist. The GTPγS-induced K+ current was also inhibited by JTV-519 in a concentration-dependent manner (Figure 2). The IC50 value of JTV-519 for depressing the GTPγS-induced _I_K.ACh was 2.42 μM, which was very close to that of the adenosine-induced _I_K.ACh. These findings suggest that JTV-519 may interact with the M2 muscarinic-acetylcholine receptor in addition to its direct inhibition of K+ channel itself and/or G proteins.
Figure 2.
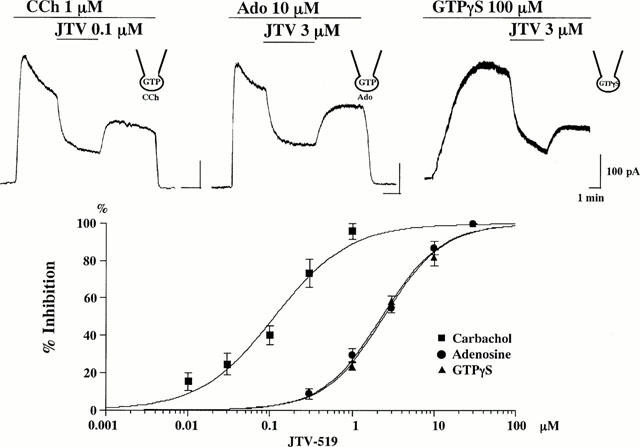
Effects of JTV-519 on the muscarinic acetylcholine receptor-operated K+ current (_I_K.ACh) in isolated guinea-pig atrial cells. _I_K.ACh was activated by the extracellular application of carbachol (CCh, 1 μM), adenosine (Ado, 10 μM) or intracellular loading of GTPγS (100 μM). Original current traces obtained from atrial cells held at −40 mV are shown in upper panels. Concentration-response curves for the inhibitory effects of JTV-519 on the _I_K.ACh activated by carbachol, adenosine and GTPγS are shown in lower panel. Per cent inhibition of the outward current is indicated on the ordinate and the concentrations of JTV-519 are on the abscissa. Values are expressed as mean±s.e.mean of 5–9 experiments.
In order to examine the effects of JTV-519 on the current-voltage relationship of _I_K.ACh, another series of experiments was conducted. The quasi-steady-state current was recorded before, after the activation of _I_K.ACh and after the addition of JTV-519. As shown in Figure 3, the activation of _I_K.ACh by extracellular application of 1 μM CCh, 10 μM adenosine or intracellular loading of 100 μM GTPγS was more remarkable as the potential became more positive. The inhibitory effect of JTV-519 (0.1 μM) on the outward component of the CCh-induced current was voltage-independent. The fractional block of the CCh-induced _I_K.ACh by 0.1 μM JTV-519 was 0.51±0.10, 0.41±0.12 and 0.43±0.09 at −40, 0 and +40 mV, respectively, and there were no significant differences among the values of the block. Higher concentrations of JTV-519 were needed to inhibit the adenosine- or GTPγS-induced _I_K.ACh to a similar extent. The fractional block of the adenosine-induced _I_K.ACh by 3 μM JTV-519 was 0.71±0.07, 0.54±0.10 and 0.48±0.08 at −40, 0 and +40 mV, respectively. The fractional block of the GTPγS-induced _I_K.ACh by 3 μM JTV-519 was 0.82±0.04, 0.69±0.04, 0.64±0.07 at −40, 0 and +40 mV, respectively. The amount of block of the adenosine- or GTPγS-induced _I_K.ACh at −40 mV was significantly greater than that at 0 or +40 mV, possibly because of the additional inhibition of _I_K1 by 3 μM JTV-519.
Figure 3.
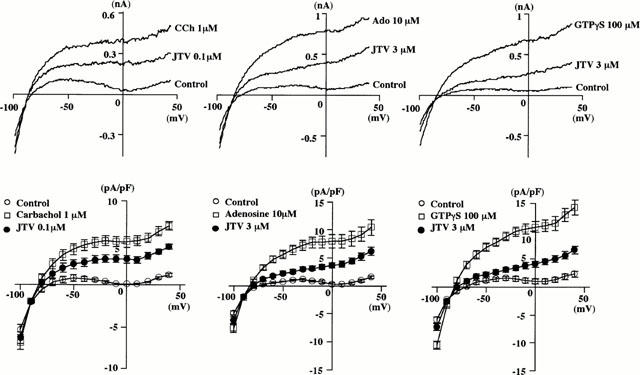
Effects of JTV-519 on the current-voltage relationships of the _I_K.ACh induced by carbachol (1 μM), adenosine (10 μM) and GTPγS (100 μM). The actual current traces of the quasi-steady-state current before, after the activation of _I_K.ACh and after the application of JTV-519 are superimposed in upper panels. The current density of the membrane current measured at each voltage is summarized in lower panels. Values are expressed as mean±s.e.mean of 4–5 experiments.
Effects of JTV-519 on the delayed rectifier K+ current
JTV-519 insignificantly prolonged atrial action potential in the absence of _I_K.ACh activation. Therefore, we examined effects of this drug on the _I_K which was important for the repolarization of the action potential in guinea-pig atrial cells. After the blockade of _I_Ca by 1 μM nisoldipine, membrane currents were elicited by 300-ms test pulses to various potentials from a holding potential of −40 mV at 0.1 Hz. Representative changes in the membrane currents and summarized data of current-voltage relations after 1 μM JTV-519 are shown in Figure 4. JTV-519 decreased the late outward current elicited by depolarizing test pulses (_I_K.depo), concomitantly with the decrease of the outward tail current after repolarization to the holding potential of −40 mV (_I_K.tail). The JTV-519-sensitive current was greatest at 0 mV of the test pulses. These findings suggest that JTV-519 inhibits _I_K in guinea-pig atrial cells. The IC50 value of JTV-519 for inhibiting the _I_K,depo at 0 mV was 0.41 μM (Figure 4).
Figure 4.
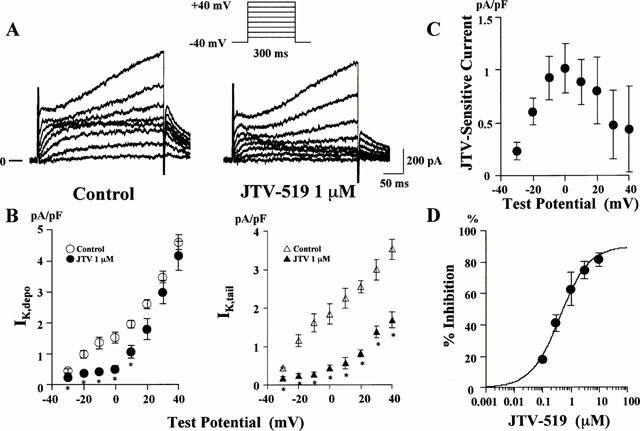
Effects of JTV-519 on the delayed rectifier K+ current in guinea-pig atrial cells. (A) Actual current traces elicited by 300 ms depolarizing pulses from a holding potential of −40 mV before (left) and after 1 μM JTV-519 (right). The external solution contained 1 μM nisoldipine. (B) Graphs showing _I_K measured at the end of 300 ms test pulse to the indicated test potential (_I_K,depo, left) and that measured after repolarization to −40 mV from the indicated test potential (_I_K,tail, right). (C) Current-voltage relation of JTV-519-sensitive _I_K during depolarization pulses to various potentials. (D) Concentration-response curve for the inhibitory effect of JTV-519 on _I_K,depo at 0 mV. Per cent inhibition of _I_K,depo is indicated on the ordinate and the concentrations of JTV-519 are on the abscissa. Values are expressed as mean±s.e.mean of 5–6 experiments. *P<0.05 vs control.
The _I_K of guinea-pig atrial cells has been reported to consist of two components, _I_Kr and _I_Ks (Sanguinetti & Jurkiewicz, 1991). _I_Kr is activated rapidly with mild depolarizations, whereas _I_Ks is activated slowly with a sigmoidal time course at more positive potentials. To test whether JTV-519 specifically blocks one or both components of _I_K, we examined the effect of JTV-519 on _I_K in the presence of the _I_Kr blocker E-4031. After the full inhibition of _I_Kr by 5 μM E-4031, JTV-519 at a concentration of 3 μM hardly affected _I_K,depo and _I_K,tail, as shown in Figure 5. These findings suggest that JTV-519 preferentially inhibits _I_Kr in guinea-pig atrial cells.
Figure 5.
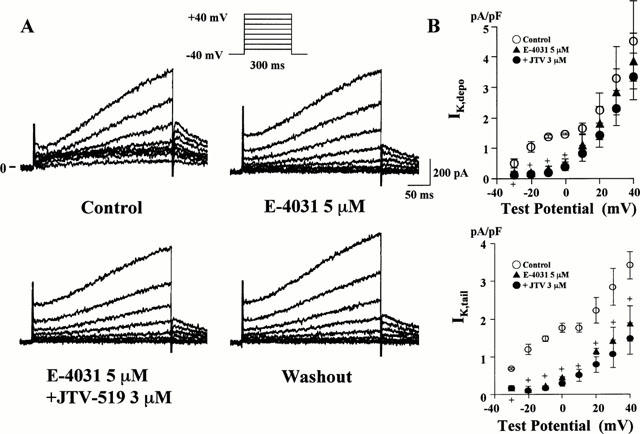
Effects of JTV-519 on the delayed rectifier K+ current (_I_K) in the presence of the _I_Kr blocker E-4031. (A) Current traces elicited by 300 ms depolarizing pulses from a holding potential of −40 mV in the control condition, in the presence of 5 μM E-4031, after the addition of 3 μM JTV-519 and after washout. (B) Graphs showing _I_K measured at the end of 300 ms test pulses to the indicated test potential (_I_K,depo, upper) and that measured after repolarization to −40 mV from the indicated potential (_I_K,tail, lower). Data represent mean±s.e.mean of five cells. +P<0.05 vs control.
Effects of JTV-519 on experimental atrial fibrillation
In the control condition, AF could not be induced by a train of stimuli at an intensity up to 12 mA in Langendorff-perfused guinea-pig hearts. After the application of 1 μM CCh, MAP at 90% repolarization level (MAP90) was significantly decreased from 61.4±1.9 to 16.9±1.8 ms (_n_=11, P<0.05), as shown in Figure 6. Concomitantly, ERP was markedly decreased from 63.2±5.5 to 19.5±2.4 ms (P<0.05), and AFT was also decreased to 1.3±0.3 mA although CT was hardly changed. Addition of 1 μM JTV-519 significantly reversed the decreased MAP90 and ERP to 38.2±2.0 ms (P<0.05) and 38.3±5.3 ms (P<0.05), respectively (_n_=6), without significant change in CT (Figure 6). In the presence of 0.3 μM JTV-519, AF could be induced by a train of stimuli in two of five hearts. After the treatment with 1 μM JTV-519, AF could not be induced any longer even in the presence of CCh (_n_=6). Thus, JTV-519 concentration-dependently suppressed the CCh-induced AF in the isolated guinea-pig hearts.
Figure 6.
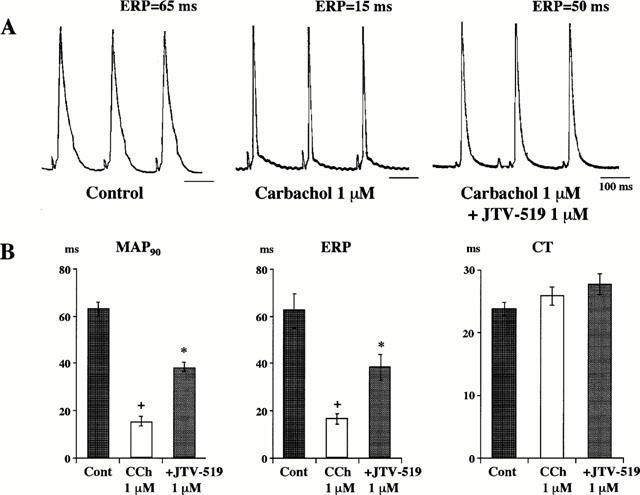
Effects of JTV-519 (1 μM) on the carbachol (CCh, 1 μM)-induced changes in monophasic action potential (MAP), effective refractory period (ERP) and conduction time (CT) in isolated guinea-pig hearts. Actual records of MAP during control period and after carbachol and carbachol plus JTV-519 are shown in A. Summarized data of changes in MAP at 90% repolarization level (MAP90), ERP and CT are shown in B. The values are expressed as mean±s.e.mean of six hearts. +P<0.05 vs control, *P<0.05 vs carbachol alone.
Discussion
JTV-519 is a benzothiazepine derivative possessing protective effects against myocardial injuries induced by Ca2+ overload and ischaemia/reperfusion (Kaneko, 1994; Inagaki et al., 2000). The underlying mechanism(s) of the cardioprotective effect have not been well established. The drug inhibited the myofibrillar overcontraction induced by high extracellular Ca2+ combined with epinephrine and/or caffeine (Kaneko, 1994). Kaneko et al. (1997) ascribed the cardioprotective effect to the inhibitory effect on annexin V. More recently Inagaki, et al. (2000) have demonstrated that JTV-519 activates specifically δ-isoform of protein kinese C through a receptor-independent mechanism and thereby induces pharmacological preconditioning. They concluded that the cardioprotective effect of JTV-519 cannot be attributed to the Ca2+ channel blocking action although the drug inhibited the L-type Ca2+ current (_I_Ca) in concentrations higher than 1 μM (Kimura et al., 1999).
In an electrophysiological study using patch clamp techniques JTV-519 was shown to inhibit the fast Na+ current (_I_Na), _I_Ca and the inward rectifier K+ current (_I_K1) in guinea-pig ventricular cells (Kimura et al., 1999). They reported that JTV-519 in concentrations higher than 1 μM inhibited _I_Na in a frequency- and voltage-dependent manner. The drug was shown to inhibit _I_Ca and _I_K1 in concentrations higher than 1 μM. The present study has demonstrated that JTV-519 potently inhibits the _I_K and the _I_K.ACh induced by 1 μM CCh in guinea-pig atrial cells with IC50 values of 0.41 and 0.12 μM, respectively. These IC50 values were a few to 10 times smaller than those to inhibit _I_Na, _I_Ca and _I_K1 in guinea-pig ventricular cells (Kimura et al., 1999). In addition, the concentrations to inhibit _I_K and _I_K.ACh were comparable to or smaller than those to elicit cardioprotective effects (Inagaki et al., 2000; Kaneko, 1994).
It has been reported that _I_K of guinea-pig atrial cells is composed of two components, _I_Kr and _I_Ks (Sanguinetti & Jurkiewicz, 1991). _I_Kr is activated rapidly with mild depolarizations, whereas _I_Ks is activated slowly with a sigmoidal time course at more positive potentials. In the present study the JTV-519-sensitive _I_K peaked around 0 mV and decreased during strong depolarizations. In addition, after full inhibition of _I_Kr by 5 μM E-4031 JTV-519 at a concentration of 3 μM hardly affected _I_K. These findings suggest that JTV-519 preferentially blocks _I_Kr. It is well-known that many antiarrhythmic drugs selectively or nonselectively inhibit _I_Kr. Flecainide and aprindine were shown to inhibit _I_Kr (Follmer & Colatsky, 1990; Ohmoto-Sekine et al., 1999) whereas quinidine, cibenzoline and bepridil were reported to block both _I_Kr and _I_Ks (Balser et al., 1991; Wang et al., 1996; 1999). In terms of class III antiarrhythmic drugs, sotalol, E-4031 and dofetilide selectively inhibited _I_Kr (Sanguinetti & Jurkiewicz, 1990; Jurkiewicz & Sanguinetti, 1993). Amiodarone was also shown to inhibit _I_Kr in rabbit ventricular cells (Varro et al., 1996) and in Xenopus oocytes expressing the human ether-a-go-go-related gene (HERG) (Kiehn et al., 1999).
In the present study JTV-519 prolonged APD in the absence and presence of muscarinic stimulation. Taking the potent inhibitory action of JTV-519 on the CCh-induced _I_K.ACh into consideration, it is not surprising that JTV-519 reversed the CCh-induced action potential shortening in a concentration-dependent manner. Slight prolongation of the action potential in the absence of CCh might be ascribed to the inhibition of _I_Kr by JTV-519. However, in guinea-pig ventricular cells the drug was reported to shorten APD (Kimura et al., 1999), which is apparently in conflict with the findings observed in atrial cells. It was reported that the current density of _I_Kr in atrial cells was 2.5 times higher than that measured in ventricular cells of guinea-pig (Sanguinetti & Jurkiewicz, 1991). Therefore, the _I_Kr blocking action of JTV-519 might be more important as a determinant of APD in atrial cells while the Na+ and Ca2+ channel blocking action might be more prominent in ventricular cells. Similar findings have been observed with the class Ib antiarrhythmic drug aprindine. Aprindine was a potent _I_Kr blocker and prolonged APD in atrial cells but not ventricular cells (Ohmoto-Sekine et al., 1999; Shirayama et al., 1991). Since JTV-519 might also inhibit _I_Ca weakly in atrial cells, the prolongation of APD resulting from _I_Kr inhibition might be partly offset and the APD prolongation by JTV-519 could not reach a statistical significance. Such a mild prolongation of APD after JTV-519 is different from consistent prolongation of atrial action potentials with pure class III antiarrhythmic drugs such as d,l-sotalol, E-4031 and MS-551 (Mori et al., 1995). These class III antiarrhythmic drugs are also potent _I_Kr blockers but possess little Ca2+ channel blocking action.
It is well-known that _I_K.ACh plays an important role in the repolarization of atrial action potential. Many antiarrhythmic drugs were reported to inhibit _I_K.ACh in isolated guinea-pig atrial cells (Nakajima et al., 1989; Inomata et al., 1993; Wu et al., 1994; Mori et al., 1995; Watanabe et al., 1996; Ohmoto-Sekine et al., 1999). Two mechanisms by which antiarrhythmic drugs inhibit _I_K.ACh have been proposed; some drugs block the muscarinic receptors and others inhibit the muscarinic K+ channel itself and/or GTP-binding proteins. Disopyramide, flecainide, aprindine and d,l-sotalol belong to the former group whereas quinidine, propafenone, cibenzoline and amiodarone belong to the latter group. In this study JTV-519 inhibited not only the current induced by CCh but also those induced by adenosine and GTPγS although higher concentrations of the drug were needed to inhibit the GTPγS- and adenosine-induced currents. These findings suggest that JTV-519 may inhibit the K+ current mainly by blocking the muscarinic receptors. However, this consideration is speculative and other explanations to such observations may be possible. If the G protein subtypes coupled to the M2 muscarinic and the A1 adenosine receptors are different, it is possible that JTV-519 might inhibit the CCh- and adenosine-induced _I_K.ACh with different potency. However, the concentration-response curve for the inhibitory effect of JTV-519 on the _I_K.ACh induced by GTPγS, which was expected to stimulate G proteins nonselectively, was superimposable with that for the inhibitory effect on the adenosine-induced _I_K.ACh. Previously we found that the class III antiarrhythmic drug MS-551 inhibited the _I_K.ACh activated in these three different ways in a similar fashion to JTV-519 (Mori et al., 1995), and that the class III antiarrhythmic drug interacted with muscarinic receptors of atrial membrane preparations in radioligand binding experiments (Uemura et al., 1995). In order to substantiate the interaction of JTV-519 with atrial muscarinic receptors directly, further studies using radioligand binding techniques may be needed.
Effects of JTV-519 on the current-voltage relationship of the _I_K.ACh activated by CCh, adenosine and GTPγS were evaluated using a ramp pulse protocol in this study. A low concentration (0.1 μM) of JTV-519 inhibited the CCh-induced _I_K.ACh voltage-independently. A higher concentration (3 μM) of JTV-519 was needed to inhibit the adenosine- or GTPγS-induced _I_K.ACh. Since JTV-519 at a concentration of 3 μM was reported to inhibit _I_K1 by about 50% in guinea-pig ventricular cells (Kimura et al., 1999), the drug at this concentration would be expected to decrease not only _I_K.ACh but also _I_K1 in atrial cells. Indeed the amount of block of the adenosine- or GTPγS-induced _I_K.ACh by 3 μM JTV-519 at −40 mV was greater than those at 0 or +40 mV in this study. Therefore, from the current-voltage relationships it would be difficult to evaluate accurately the electrophysiological mechanism of the inhibition of the adenosine- or GTPγS-induced _I_K.ACh by JTV-519. Further electrophysiological experiments including single _K_ACh channel recording may be needed to delineate the underlying mechanism.
As the mechanism involved in the establishment of AF, the wavelet hypothesis has been proposed from mapping studies in experimental AF (Allessie, 1995). It was established that several wavelets and the shortened wavelength were required for perpetuation of AF. The wavelength is designated as the product of refractory period and conduction velocity. Although AF could not be induced under a normal condition, it was easily induced by a high frequency atrial stimulation during CCh perfusion in isolated guinea-pig hearts, which is consistent with previous reports from our and other laboratories (Watanabe et al., 1996; Ohmoto-Sekine et al., 1999; Inoue et al., 1994). Since perfusion of CCh did not affect CT, the shortening of ERP resulting from the activation of _I_K.ACh seemed to underlie the shortening of the atrial wavelength and the induction of AF in this study. Addition of JTV-519 reverted the MAP duration and ERP toward the control, and the increase in ERP paralleled that of MAP. Since JTV-519 did not prolong CT significantly, the Na+ channel blocking action of JTV-519 might be trivial in this experimental model. Therefore, the inhibitory effect of JTV-519 on the experimental AF might be mainly due to the increase in ERP resulting from the blockade of _I_K.ACh.
Although initially AF terminated spontaneously within a few seconds, the repetitive induction of AF led to progressive prolongation of the duration of the induced paroxysms of AF in goats (Wijffels et al., 1995). The phenomenon is called as ‘AF begets AF'. Although the ionic mechanism(s) of the electrical remodelling are not well-defined, the shortenings of APD and ERP resulting from the decreased density of _I_Ca may play an important role (Yue et al., 1997). Since the L-type Ca2+ channel blocker verapamil has been found to prevent the electrical remodelling (Goette et al., 1996), JTV-519 with L-type Ca2+ channel blocking properties may also prevent AF-promoting electrophysiological remodelling. Further studies may be needed to test the hypothesis.
AF is a common complication of acute myocardial infarction with a reported incidence as high as 20% (Goldberg et al., 1990). Several studies have indicated that AF associated with acute myocardial infarction increases in-hospital and long-term mortality (Behar et al., 1992; Rathore et al., 2000). The present study has demonstrated that the cardioprotective drug JTV-519 shows antiarrhythmic efficacy against AF by inhibiting _I_K.ACh and _I_Kr. Therefore, JTV-519 may be useful for the prevention of AF in patients with ischaemic heart disease.
Acknowledgments
The authors thank Ms I. Sakashita for her secretarial work. This work was supported in part by a Grant-in-Aid from the Ministry of Education, Science, Sports and Culture of Japan and K. Watanabe Research Fund. We thank Japan Tobacco Inc. Central Pharmaceutical Research Institute (Osaka) for the generous supply of JTV-519.
Abbreviations
AF
atrial fibrillation
AFT
atrial fibrillation threshold
ANOVA
analysis of variance
APD
action potential duration
APD90
APD at 90% repolarization level
CCh
carbachol
CT
conduction time
ERP
effective refractory period
HERG
human ether-a-go-go-related gene
_I_Ca
L-type Ca2+ current
_I_K
delayed rectifier K+ current
_I_K.ACh
muscarinic acetylcholine receptor-operated K+ current
_I_K1
inward rectifier K+ current
_I_K,depo
time-dependent current of _I_K during depolarizing pulses
_I_Kr
rapid component of _I_K
_I_Ks
slow component of _I_K
_I_K,tail
tail current of _I_K
_I_Na
fast Na+ current
MAP
monophasic action potentials
MAP90
MAP at 90% repolarization level
RMP
resting membrane potential
References
- ALLESSIE M.A.Reentrant mechanisms underlying atrial fibrillation Cardiac Electrophysiology: From Cell to Bedside 1995Philadelphia, PA: W.B. Saunders Company; 562–566.2nd edn. eds. Zipes, D.P. & Jalife, J., pp [Google Scholar]
- BALSER J.R., BENETT P.B., HONDEGHEM L.M., RODEN D.M. Suppression of time-dependent outward current in guinea pig ventricular myocytes: Action of quinidine and amiodarone. Circ. Res. 1991;69:519–529. doi: 10.1161/01.res.69.2.519. [DOI] [PubMed] [Google Scholar]
- BEHAR S., ZAHAVI Z., GOLDBOURT U., REICHER-REISS H., for the SPRINT Study Group Long-term prognosis of patients with paroxysmal atrial fibrillation complicating acute myocardial infarction. Eur. Heart J. 1992;13:45–50. doi: 10.1093/oxfordjournals.eurheartj.a060046. [DOI] [PubMed] [Google Scholar]
- CARMELIET E., MUBAGWA K. Desensitization of the acetylcholine-induced increase of potassium conductance in rabbit cardiac Purkinje fibres. J. Physiol. (Lond.) 1986;371:239–255. doi: 10.1113/jphysiol.1986.sp015971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FABIATO A., FABIATO F. Calculator programs for computing the composition of solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J. Physiol (Paris). 1979;75:463–505. [PubMed] [Google Scholar]
- FOLLMER C.H., COLATSKY T.J. Block of delayed rectifier potassium current, IK, by flecainide and E-4031 in cat ventricular myocytes. Circulation. 1990;82:289–293. doi: 10.1161/01.cir.82.1.289. [DOI] [PubMed] [Google Scholar]
- GOETTE A., HONEYCUTT C., LANGBERG J.J. Electrical remodeling in atrial fibrillation: Time course and mechanisms. Circulation. 1996;94:2968–2974. doi: 10.1161/01.cir.94.11.2968. [DOI] [PubMed] [Google Scholar]
- GOLDBERG R.J., SEELEY D., BECKER R.C. Impact of atrial fibrillation on the in-hospital and long-term survival of patients with acute myocardial infarction: a community wide perspective. Am. Heart J. 1990;119:996–1001. doi: 10.1016/s0002-8703(05)80227-3. [DOI] [PubMed] [Google Scholar]
- HAMILL O.P., MARTY A., NEHER E., SAKMANN B., SIGWORRTH F.J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- HARA Y., NAKAYA H. SD-3212, a new class I and IV antiarrhythmic drug: a potent inhibitor of the muscarinic acetylcholine-receptor-operated potassium current in guinea-pig atrial cells. Br. J. Pharmacol. 1995;116:2750–2756. doi: 10.1111/j.1476-5381.1995.tb17237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INAGAKI K., KIHARA Y., HAYASHIDA W., IZUMI T., IWANAGA Y., YONEDA T., TAKEUCHI Y., SUYAMA K., MUSO E., SASAYAMA S. Anti-ischemic effect of a novel cardioprotective agent, JTV-519, is mediated through specific activation of δ-isoform of protein kinase C in rat ventricular myocardium. Circulation. 2000;101:797–804. doi: 10.1161/01.cir.101.7.797. [DOI] [PubMed] [Google Scholar]
- INOMATA N., OHNO T., ISHIHARA T., AKAIKE N. Antiarrhythmic agents act differently on the activation phase of the ACh-response in guinea-pig atrial myocytes. Br. J. Pharmacol. 1993;108:111–115. doi: 10.1111/j.1476-5381.1993.tb13448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INOUE M., INOUE D., ISHIBASHI K., SAKAI R., SHIRAYAMA T., ASAYAMA J., NAKAGAWA M. Effect of E-4031 on the atrial fibrillation threshold in guinea pig atria: Comparative study with class I antiarrhythmic drugs. J. Cardiovasc. Pharmacol. 1994;24:534–541. doi: 10.1097/00005344-199410000-00003. [DOI] [PubMed] [Google Scholar]
- ISENBERG G., KLOECKNER U. Calcium tolerant ventricular myocytes prepared by preincubation in a “KB medium”. Pflügers Arch. 1982;395:6–18. doi: 10.1007/BF00584963. [DOI] [PubMed] [Google Scholar]
- JURKIEWICZ N.K., SANGUINETTI M.C. Rate-dependent prolongation of cardiac action potentials by a methanesulfonanilidine class III antiarrhythmic agent. Specific block of rapidly activating delayed rectifier K+ current by dofetilide. Circ. Res. 1993;72:75–83. doi: 10.1161/01.res.72.1.75. [DOI] [PubMed] [Google Scholar]
- KANEKO N. New 1,4-benzothiazepine derivative, K201, demonstrates cardioprotective effects against sudden cardiac cell death and intracellular calcium blocking action. Drug. Dev. Res. 1994;33:429–438. [Google Scholar]
- KANEKO N., MATSUDA R., TODA M., SHIMAMOTO K. Inhibition of annexin V-dependent Ca2+ movement in large unilamellar vesicles by K201, a new 1,4-benzothiazepine derivative. Biochim. Biophys. Acta. 1997;1330:1–7. doi: 10.1016/s0005-2736(97)00132-6. [DOI] [PubMed] [Google Scholar]
- KIEHN J., THOMAS D., KARLE C.A., SCHOLS W., KUBIER W. Inhibitory effects of the class III antiarrhythmic drug amiodarone on cloned HERG potassium channels. Naunyn-Schmiedeberg's Arch. Pharmacol. 1999;359:212–219. doi: 10.1007/pl00005344. [DOI] [PubMed] [Google Scholar]
- KIMURA J., KAWAHARA M., SAKAI E., YATABE J., NAKANISHI H. Effects of a novel cardioprotective drugs, JTV-519, on membrane currents of guinea pig ventricular myocytes. Jpn. J. Pharmacol. 1999;79:275–281. doi: 10.1254/jjp.79.275. [DOI] [PubMed] [Google Scholar]
- KURACHI Y., NAKAJIMA T., SUGIMOTO T. On the mechanism of activation muscarinic K+ channels by adenosine in isolated atrial cells: Involvement of GTP-binding proteins. Pflügers Arch. 1986;407:264–274. doi: 10.1007/BF00585301. [DOI] [PubMed] [Google Scholar]
- KURACHI Y., NAKAJIMA T., SUGIMOTO T. Short-term desensitization of muscarinic K+ channel current in isolated atrial myocytes and possible role of GTP-binding proteins. Pflügers Arch. 1987;410:227–233. doi: 10.1007/BF00580270. [DOI] [PubMed] [Google Scholar]
- MATSUMOTO Y., OGURA T., UEMURA H., SAITO T., MASUDA Y., NAKAYA H. Histamine H1-receptor-mediated modulation of the delayed rectifier K+ current in guinea-pig atrial cells: opposite effects on IKs and IKr. Br. J. Pharmacol. 1999;128:1545–1553. doi: 10.1038/sj.bjp.0702918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORI K., HARA Y., SAITO T., MASUDA Y., NAKAYA H. Anticholinergic effects of class III antiarrhythmic drugs in guinea-pig atrial cells: different molecular mechanisms. Circulation. 1995;91:2834–2843. doi: 10.1161/01.cir.91.11.2834. [DOI] [PubMed] [Google Scholar]
- NAKAJIMA T., KURACHI Y., ITO H., TAKIKAWA R., SUGIMOTO T. Anti-cholinergic effects of quinidine, disopyramide and procainamide in isolated atrial myocytes: mediation by different molecular mechanisms. Circ. Res. 1989;64:297–303. doi: 10.1161/01.res.64.2.297. [DOI] [PubMed] [Google Scholar]
- NAKAYA H., TOHSE N., TAKEDA Y., KANNO M. Effects of MS-551, a new class antiarrhythmic drug, on action potential and membrane currents in rabbit ventricular myocytes. Br. J. Pharmacol. 1993;109:157–163. doi: 10.1111/j.1476-5381.1993.tb13546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OHMOTO-SEKINE Y., UEMURA H., TAMAGAWA M., NAKAYA H. Inhibitory effects of aprindine on the delayed rectifier K+ current and the muscarinic acetylcholine receptor-operated K+ current in guinea-pig atrial cells. Br. J. Pharmacol. 1999;126:751–761. doi: 10.1038/sj.bjp.0702334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RATHORE S.S., BERGER A.K., WEINFURT K.P., SCHULMAN K.A., OETGEN W.J., GERSH B.J., SOLOMON A.J. Acute myocardial infarction complicated by atrial fibrillation in the elderly: prevalence and outcomes. Circulation. 2000;101:969–974. doi: 10.1161/01.cir.101.9.969. [DOI] [PubMed] [Google Scholar]
- SAKAMOTO N., UEMURA H., HARA Y., SAITO T., MASUDA Y., NAKAYA H. Bradykinin B2-receptor-mediated modulation of membrane currents in guinea-pig cardiomyocytes. Br. J. Pharmacol. 1998;125:283–292. doi: 10.1038/sj.bjp.0702060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANGUINETTI M.C., JURKIEWICZ N.K. Two components of cardiac delayed rectifier K+ current: Differential sensitivity to block by class III antiarrhythmic agents. J. Gen. Physiol. 1990;96:194–214. doi: 10.1085/jgp.96.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANGUINETTI M.C., JURKIEWICZ N.K. Delayed rectifier outward K+ current is composed of two currents in guinea-pig atrial cells. Am. J. Physiol. 1991;260:H393–H399. doi: 10.1152/ajpheart.1991.260.2.H393. [DOI] [PubMed] [Google Scholar]
- SHIRAYAMA T., INOUE D., INOUE M., TATSUMI T., YAMAHARA Y., ASAYAMA J., NAKAGAWA M. Electrophysiological effects of sodium channel blockers on guinea pig left atrium. J. Pharmacol. Exp. Ther. 1991;259:650–659. [PubMed] [Google Scholar]
- TSIEN R.Y., RINK T.J. Neutral carrier ion-sensitive microelectrodes for measurement of intracellular free calcium. Biochim. Biophys. Acta. 1980;559:623–638. doi: 10.1016/0005-2736(80)90205-9. [DOI] [PubMed] [Google Scholar]
- UEMURA H., HARA Y., ENDOU M., MORI K., NAKAYA H. Interaction of class III antiarrhythmic drugs with muscarinic M2 and M3 receptors: radioligand binding and functional studies. Naunyn-Schmiedeberg's Arch. Pharmacol. 1995;353:73–79. doi: 10.1007/BF00168918. [DOI] [PubMed] [Google Scholar]
- VARRO A., VIRAG L., PAPP J.G. Comparison of the chronic and acute effects of amiodarone on the calcium and potassium currents in rabbit isolated cardiac myocytes. Br. J. Pharmacol. 1996;117:1181–1186. doi: 10.1111/j.1476-5381.1996.tb16713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG D.W., KIYOSUE T., SATO T., ARITA M. Comparison of the effects of class I anti-arrhythmic drugs, cibenzoline, mexiletine and flecainide, on the delayed rectifier K+ current of guinea-pig ventricular myocytes. J. Mol. Cell. Cardiol. 1996;28:893–903. doi: 10.1006/jmcc.1996.0084. [DOI] [PubMed] [Google Scholar]
- WANG J.C., KIYOSUE T., KIRIYAMA K., ARITA M. Bepridil differentially inhibits two delayed rectifier K+ currents, IKr and IKs, in guinea-pig ventricular myocytes. Br. J. Pharmacol. 1999;128:1733–1738. doi: 10.1038/sj.bjp.0702959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATANABE Y., HARA Y., TAMAGAWA M., NAKAYA H. Inhibitory effect of amiodarone on the muscarinic acetylcholine receptor-operated potassium current in guinea-pig atrial cells. J. Pharmacol. Exp. Ther. 1996;279:617–624. [PubMed] [Google Scholar]
- WIJFFELS M.C.E.F., KIRCHHOF C.J.H.J., DORLAND R., ALLESSIE M.A. Atrial fibrillation begets atrial fibrillation: a study in awake chronically instrumented conscious goats. Circulation. 1995;92:1954–1968. doi: 10.1161/01.cir.92.7.1954. [DOI] [PubMed] [Google Scholar]
- WU S.N., NAKAJIMA T., YAMASHITA T., HAMADA E., HAZAMA H., IWASAWA K., OMATA M., KURACHI Y. Molecular mechanism of cibenzoline-induced anticholinergic action in single atrial myocytes: Comparison with effects of disopyramide. J. Cardiovasc. Pharmacol. 1994;23:618–623. doi: 10.1097/00005344-199404000-00014. [DOI] [PubMed] [Google Scholar]
- YUE L., FENG J., GASPO R., LI G.R., WANG Z., NATTEL S. Ionic remodeling underlying action potential changes in a canine model of atrial fibrillation. Circ. Res. 1997;81:512–525. doi: 10.1161/01.res.81.4.512. [DOI] [PubMed] [Google Scholar]