Dual effect mediated by protease-activated receptors on the mechanical activity of rat colon (original) (raw)
Abstract
- The present study examined the mechanical effects of agonist enzymes and receptor-activating peptides for protease-activated receptor (PAR)-1 and PAR-2 on longitudinal and circular muscle of rat isolated colonic segments in the attempt to clarify the PAR functional role in intestinal motility.
- The responses to PAR-1 and PAR-2 activation were examined in vitro by recording simultaneously the changes of endoluminal pressure (index of circular muscle activity) and of isometric tension (index of longitudinal muscle activity).
- Both PAR-1 agonists, thrombin (0.1 nM – 3 μM) and SFLLRN-NH2 (1 nM – 3 μM), and PAR-2 agonists, trypsin (0.1 nM – 10 μM) and SLIGRL-NH2 (1 nM – 10 μM), induced different effects in the two muscular layers: a reduction of the spontaneous contractions in the circular muscle and a contractile effect or biphasic, relaxation followed by contraction, depending on the concentration, in the longitudinal muscle.
- The inhibitory effects were greatly reduced or abolished by apamin (0.1 μM) indicating that they mainly occur via activation of Ca2+-dependent small conductance, K+-channels.
- The responses to PAR-1 and PAR-2 were unaffected by tetrodotoxin (1 μM) or indomethacin (1 μM) suggesting that are independent by products of cyclooxygenase or by neural action potentials.
- These findings indicate that both PAR-1 and PAR-2 are functionally expressed in rat colon. PARs mediate changes of the mechanical activity of longitudinal and circular muscle which might explain the alterations of colonic motility observed during inflammatory conditions.
Keywords: Protease-activated receptor (PAR), intestinal motility, thrombin, trypsin, rat colon, potassium channel
Introduction
Certain serin proteases are known to regulate multiple cellular functions by activating specific receptors, called ‘protease-activated receptors' (PARs). PARs are members of a subfamily of G-protein-coupled receptors that play important roles in responses to injury, including inflammation and repair (Déry et al., 1998; Macfarlane et al., 2001). Four members of this family have so far been identified: PAR-1 and PAR-3, both preferentially activated by thrombin, PAR-2, selectively activated by trypsin or mast-cell tryptase and PAR-4, activated by thrombin or trypsin (Vu et al., 1991; Nystedt et al., 1994; Ishihara et al., 1997; Molino et al., 1997; Xu et al., 1998). The mechanism whereby proteases activate PARs involves the proteolytic cleavage and the unmasking of an N-terminal receptor sequence, that in turn acts as a tethered ligand, which activates the receptor itself (Déry et al., 1998). Short synthetic peptides (PAR-activating peptides), which have the same aminoacidic sequence of the tethered ligands of PAR-1, PAR-2 and PAR-4 are used to selectively activate the respective receptors, whereas PAR-3 does not respond to the presumed receptor-activating peptides (Ishihara et al., 1997). PARs are involved in different physiological or pathophysiological events including platelet aggregation, inflammation, vascular contraction/relaxation, neurodegeneration/neuroprotection (Déry et al., 1998; Macfarlane et al., 2001; Vergnolle et al., 2001).
The physiology and the pathophysiology of the gastrointestinal tract, including motility, have been suggested to be under PAR modulation since in the alimentary system PAR-1 and PAR-2 are highly expressed and, at least PAR-2, appear to be localized on the enterocytes, myocytes and on myenteric neurons (Hollenberg, 1999; Vergnolle, 2000). However, it remains to clarify the functional significance of the presence of different PARs in the same tissue (Hollenberg, 1999). A recent study in vivo has provided evidence for an increase of the mouse gastrointestinal transit in response to PAR-1 and PAR-2 activation (Kawabata et al., 2001). There is also in vitro evidence that PARs modulate smooth muscle mechanical activity throughout the gastrointestinal tract including the oesophagus (Al-Ani et al., 1995; Corvera et al., 1997; Hollenberg et al., 1997; 1999; Zheng et al., 1998; Cocks et al., 1999; Kawabata et al., 1999a 2000a, 2000b). However, so far, the investigations directed toward the evaluation of the intestinal motor effects induced by PAR activation have been mainly focused on the mechanical activity of longitudinal smooth muscle strips (Al-Ani et al., 1995; Saifeddine et al., 1996; Corvera et al., 1997; Zheng et al., 1998; Hollenberg et al., 1999; Kawabata et al., 1999b; Cocks et al., 1999; Tognetto et al., 2000) or of the muscularis mucosae (Kawabata et al., 2000a). Thrombin and trypsin, as well as PAR-1 and PAR-2 activating peptides, produce contraction of gastric longitudinal smooth muscle from rats or guinea pig (Hollenberg et al., 1993; 1997; 1999; Saifeddine et al., 1996; Zheng et al., 1998), an effect being blocked by the cyclo-oxygenase inhibitor, indomethacin, and by the tyrosin kinase inhibitor, genistein (Al-Ani et al., 1995; Saifeddine et al., 1996). However specie-specific variations have been documented. In fact, in longitudinal strips from mouse gastric fundus PAR-1 and PAR-2 mediate biphasic responses: relaxation followed by contraction. The relaxant component is eliminated by apamin or ryanodine, indicating that the effect is via ryanodine-sensitive and -insensitive activation of small-conductance, Ca2+-activated K+ channels (Cocks et al., 1999). Also in rat duodenal longitudinal smooth muscle the response to activation to PAR-1 is biphasic: an apamin-sensitive relaxation followed by contraction, which is partially dependent on intracellular and extracellular Ca2+ (Kawabata et al., 1999a; 2000b), while PAR-2 agonists elicit only contraction (Kawabata et al., 1999a). Further, PAR-2 activation inhibits spontaneous rhythmic contractions of the rat longitudinal colonic strips, but the mechanism of action is not yet known (Corvera et al., 1997).
Information is lacking concerning the responses of the intestinal circular smooth muscle to PAR-1 and PAR-2 activation. We have considered this aspect of study to be of interest because the evaluation of the influence of the PAR activation on the mechanical activity of the circular smooth muscle could contribute to define the physiological role of these receptors on the modulation of the intestinal motor activity. We have used an in vitro experimental approach which allows the simultaneous registration of the endoluminal pressure (index of the circular mechanical activity) and of the isometric tension (index of the longitudinal mechanical activity) from intestinal segments. Previously it has been reported that the changes in isometric tension and endoluminal pressure reflect the mechanical activity of longitudinal and circular muscle, respectively (Mulè et al., 1992; 1999a). Therefore, the aim of the present study was to examine in rat colon: (i) the differences in the mechanical responses induced by PAR-1 or PAR-2 activation; (ii) the differences in the PAR-evoked mechanical responses between longitudinal and circular muscle and (iii) the nature of the evoked responses evaluating, in particular, the possible involvement of an indirect action due to neural activation and/or release of prostanoids.
Methods
Experiments were authorized by the Ministero della Sanità (Rome, Italy). Adult male Wistar rats (250 – 400 g) were killed by cervical dislocation. The abdomen was immediately opened and the colon was removed distally to the caecum. The colonic lumen was cleaned with Krebs solution and segments of about 2 cm in length were cut. The preparation was then placed in a 5-ml horizontal organ bath continuously perfused with Krebs solution with the following composition (mM): NaCl 119, KCl 4.5, MgSO4 2.5, NaHCO3 25, KH2PO4 1.2, CaCl2 2.5 and glucose 11.1. The organ bath was bubbled with 95% O2 and 5% CO2 and maintained at a temperature of 37°C.
Recording of mechanical activity
As previously described (Mulè et al., 1995) the distal end of the intestinal segment was tied around the mouth of a J-tube, which was connected via a T-catheter to a standard pressure transducer (Ugo Basile, Biological Research Apparatus, Varese, Italy) and to a syringe for filling the preparation with Krebs solution. The ligated proximal end was secured with a silk thread to an isometric force transducer (DY2 Ugo Basile). Preparations, filled with 0.1 ml Krebs solution were subjected to an initial tension of 1 g and were allowed to equilibrate for at least 30 min before starting the experiment. Colonic contractions were monitored as changes in endoluminal pressure and isometric tension, which are mainly generated by circular or longitudinal muscle activity, respectively. Mechanical activities were recorded on an ink writer recorder (Gemini, Ugo Basile) (Figure 1).
Figure 1.
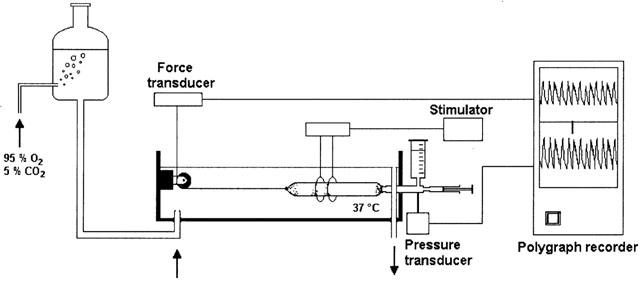
Experimental set-up used for the simultaneous recording of the intraluminal pressure and the isometric tension of the rat intestinal segments.
Design of study
At the beginning of each experiment, the preparation was challenged with carbachol until reproducible responses were obtained, to ensure that a stable and acceptable level of sensitivity had been reached before the experimental procedure was begun. Agonists were added into the bath in volumes of 50 μl after switching off the perfusion. Thrombin (0.1 nM – 3 μM) and trypsin (0.1 nM – 10 μM) were applied only once to each tissue preparation for at least 5 min until the response stabilized, because after a single application of each protease, the response did not completely recover. In another set of tissues, responses to cumulative concentrations of PAR-1 (SFLLRN-NH2) (0.1 nM – 10 μM) and PAR-2 (SLIGRL-NH2) (0.1 nM – 10 μM) activating peptides were obtained, applying each concentration to the tissue for 2 min. Desensitization to either SFLLRN-NH2 or SLIGRL-NH2 was performed repeatedly exposing the tissue to cumulative concentrations of PAR-1 or PAR-2 activating peptide over three consecutive periods (10 min interval between each curve). Responses to SFLLRN-NH2 were tested in SLIGRL-NH2 desensitized tissues and response to SLIGRL-NH2 were tested in SFLLRN-NH2 desensitized tissue. Moreover, we checked that the highly selective PAR-1 agonist TFLLR-NH2 mimicked the effects observed in response to SFLLRN-NH2. In some experiments, to confirm the specificity of the effect, the inactive control peptides, FSLLRN-NH2 and LSIGRL-NH2 for PAR-1 and PAR-2, respectively, were applied to the preparations. In experiments to examine the nature of the PAR-induced responses, we exclusively used the PAR-activating peptides, because their effects were reversible and reproducible. The responses to PAR-1 and PAR-2 activation were assessed in the absence and in the presence of the following drugs, at concentrations known to be effective in our and other systems (Serio et al., 1996; Kawabata et al., 1999a; Cocks et al., 1999; Corvera et al., 1997): apamin (0.1 μM), a small-conductance, Ca2+-activated K+ channel blocker; indomethacin (1 μM), a cyclo-oxygenase inhibitor; tetrodotoxin (TTX) (1 μM), a Na2+ channel blocker. These agents were added to the perfusing solution at least 20 min before testing the PAR-activating peptides. To prove that the TTX was effective the preparation was stimulated through a pair of platinum ring electrodes.
Data analysis and statistics
The inhibitory response of the circular muscle to PAR activation was taken as the per cent change from the resting spontaneous activity (e.g., 100% corresponds to the abolition of spontaneous activity). In this view, the mean amplitude of the pressure waves was determined for 10 min before and after administration of agonists. The contractile response of the longitudinal muscle was defined as change in the resting tone (the bottom level of the tension oscillations) and was expressed as a percentage of the contraction caused by 10 μM carbachol. This concentration was demonstrated to induce a maximal contraction (about 2.5 g) in our preliminary experiments. All data obtained are expressed as means±s.e.mean. n indicates the number of animals from which intestinal segments were taken. The half-maximal contractile concentration (EC50) and the half-maximal inhibitory concentration (IC50) of the agonists were calculated by interpolation from the respective concentration-response curves. Statistical analysis was performed by means of Student's _t_-test or analysis of variance (ANOVA) followed by Bonferroni-test, when appropriate. P<0.05 was regarded as significant.
Drugs
The following drugs were used: carbamylcholine chloride (carbachol), apamin, tetrodotoxin (TTX), indomethacin, isoprenaline hydrochloride, trypsin (all purchased from Sigma, Chemical Corp. St Louis, MO, U.S.A.), thrombin (Calbiochem, Darmstadt, Germany), SFLLRN-NH2 (Bachem AG, Bubendorf, Switzerland). SLIGRL-NH2 and the inactive control peptides FSLLRN-NH2 and LSIGRL-NH2 were synthesized and supplied by Dr D. McMaster of the Peptide Synthesis Core Facility at the University of Calgary (Canada). TFLLR-NH2 was a kind gift by Dr N. Vergnolle (University of Calgary). The concentration, purity and composition of the peptides were determined by high-performance liquid chromatography, mass spectrometry and quantitative amino acid analysis. Thrombin was dissolved in 5% acetic acid; indomethacin in 2% Na2CO3. All other chemicals were dissolved in distilled water. Control tests showed that the solvent had no effect on the preparation.
Results
As previously described (Mulè et al., 1999a), isolated rat colon displayed spontaneous mechanical activity, consisting of rhythmic phasic changes in both intraluminal pressure (from 5 to 15 cm H2O) and isometric tension (from 0.5 to 1.5 g).
Thrombin (0.1 nM – 3 μM) produced different effects on the two muscular layers (Figure 2). In the circular muscle it induced an inhibitory response consisting in a concentration-dependent reduction in the amplitude of the spontaneous rhythmic contractions. In the longitudinal muscle it produced contractile effects, at least at the lowest concentrations tested. Both the effects occurred within 60 s after addition of the protease and were maintained until the washout. Thrombin, at concentrations over 1 μM, caused a biphasic response, relaxation followed by contraction, in the longitudinal muscle. The relaxation lasted about 2 min and ranged from 0.5 to 1.5 g depending on the concentration. The PAR-1 activating peptide, SFLLRN-NH2 (0.1 nM – 10 μM), which was added to the bath in a cumulative manner, mimicked the effects of the endogenous agonist, inducing inhibitory effects on circular muscle, longitudinal muscle contraction, which became a biphasic response at the highest concentrations (Figure 2). In order to confirm the specificity of the effect, we applied to the preparations the inactive control peptide FSLLRN-NH2 (0.01 – 10 μM) and this was without any effect (Figure 2).
Figure 2.
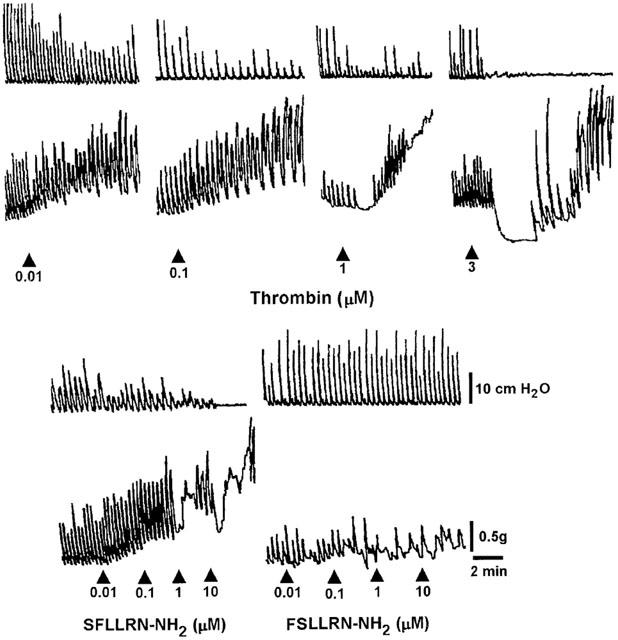
Representative recordings of the effects induced by thrombin and PAR-1 related peptides on the pressure waves (upper tracings) and on the tension oscillations (lower tracings) of an isolated segment of rat colon. The arrow indicates the application of the agonist. Only the more significant concentrations are shown. The PAR-1 agonist, SFLLRN-NH2, and the PAR-1 inactive control peptide, FSLLRN-NH2, were cumulatively added to the bath.
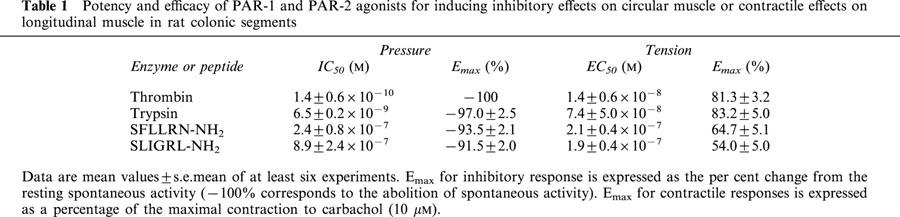
Also trypsin (0.1 nM – 10 μM) produced differential effects on the two muscular layers: a concentration-dependent reduction of the spontaneous phasic contraction in the circular muscle and contractile effects on the longitudinal muscle (Figure 3). The effects appeared within 1 min and persisted up to the washout. The PAR-2 selective agonist SLIGRL-NH2 (0.1 nM – 10 μM) caused similar effects, even if at the highest concentration (10 μM) it produced a biphasic response, relaxation followed by contraction (Figure 3). Once again, the inactive control peptide LSIGRL-NH2 (0.01 – 10 μM) failed to modify the mechanical activity of the preparation (Figure 3). Figure 4 shows the concentration-response curves for the suppression of the spontaneous contractions of the circular muscle and for the contractile effects on the longitudinal muscle induced by PAR-1 and PAR-2 agonists. Thrombin displayed more potency than other agonists. Concerning the inhibitory effects, thrombin and trypsin were more potent than the synthetic peptide, but they were as efficacious as the peptides, all drugs being able to abolish the spontaneous contractions. Concerning the contractile effects on the longitudinal muscle, thrombin and trypsin were more potent and more efficacious than the respective PAR-activating peptides (Table 1).
Figure 3.
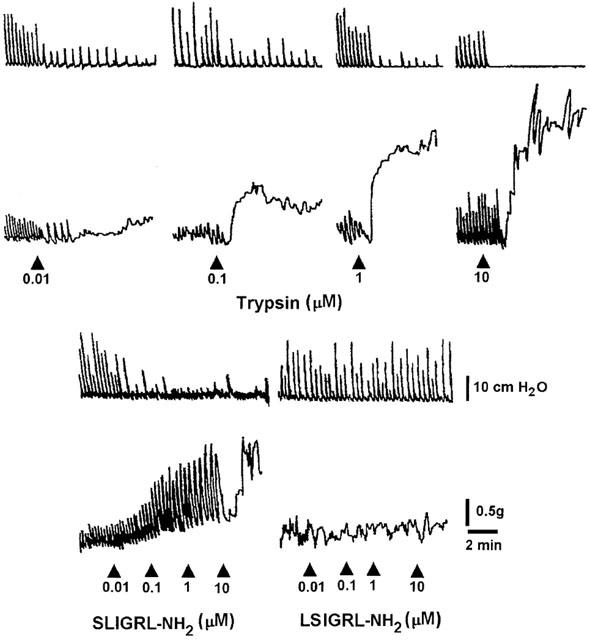
Representative recordings of the effects induced by trypsin and PAR-2 related peptides on the pressure waves (upper tracings) and on the tension oscillations (lower tracings) of an isolated segment of rat colon. The arrow indicates the application of the agonist. Only the more significant concentrations are shown. The PAR-2 agonist, SLIGRL-NH2, and the PAR-2 inactive control peptide, LSIGRL-NH2, were cumulatively added to the bath.
Figure 4.
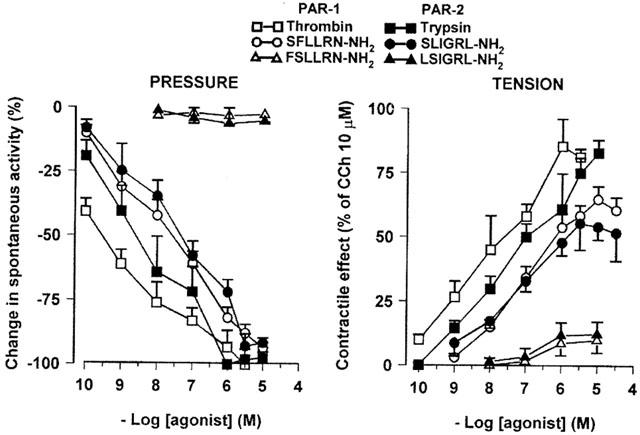
Concentration-response curves for effects evoked by PAR-1 and PAR-2 agonists on the pressure waves (left panel) and on the tension oscillations (right panel) of an isolated segment of rat colon. The inhibitory responses on circular muscle are expressed as the per cent change from the resting spontaneous activity (−100% corresponds to the abolition of spontaneous activity). The contractile effects on longitudinal muscle are expressed as a percentage of the maximal response to carbachol (10 μM). Each value is the mean±s.e.mean of 5 – 9 experiments.
Table 1.
Potency and efficacy of PAR-1 and PAR-2 agonists for inducing inhibitory effects on circular muscle or contractile effects on longitudinal muscle in rat colonic segments
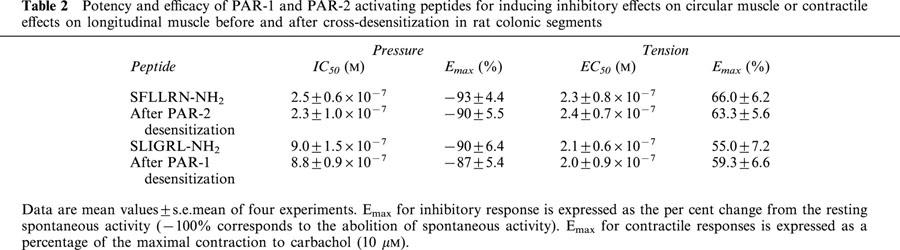
In order to rule out any crossover between the PAR-1 and PAR-2 activating peptides some experiments of desensitization were performed. The preparation desensitized to SLIGRL-NH2 remained fully responsive to SFLLRN-NH2 and vice versa. Data are shown in Table 2.
Table 2.
Potency and efficacy of PAR-1 and PAR-2 activating peptides for inducing inhibitory effects on circular muscle or contractile effects on longitudinal muscle before and after cross-desensitization in rat colonic segments
In order to examine the mechanism of action responsible for the PAR-induced responses, we exclusively used the PAR-activating peptides, since their effects were reversible and reproducible, testing them in the presence of different antagonists. Firstly, we examined the involvement of Ca2+-dependent K+ channels in the PAR-1- and PAR-2-mediated inhibitory effects. Apamin, an inhibitor of the small-conductance, Ca2+ activated K+, at a concentration of 0.1 μM, by itself, strongly augmented the spontaneous contractions of the circular muscle, as previously described (Mulè et al., 1999b). Apamin significantly reduced, but not abolished, the inhibitory responses on the circular muscle to PAR-1 and PAR-2 activating peptides. Apamin also abolished the relaxation in response to the highest concentrations of SFLLRN-NH2 (1 – 10 μM) and of SLIGRL-NH2 (10 μM). It significantly enhanced the contractile effects induced by PAR-1 or PAR-2 activating peptides (Figure 5). However, apamin failed to affect the inhibitory effects of isoproterenol (1 μM) or the contraction to carbachol (1 μM), concentrations that had been reported to be submaximal in our previous studies in the same preparation (Mulè & Serio, 1997; Mulè et al., 1999b).
Figure 5.
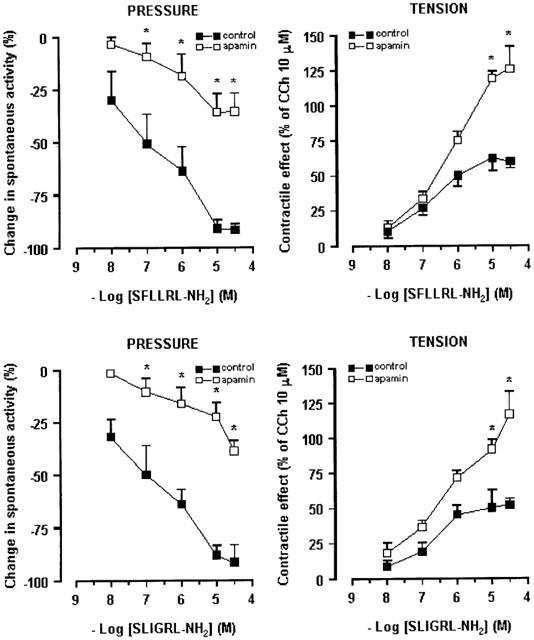
Concentration-response curves to PAR-1 and PAR-2 activating peptides, SFLLRN-NH2 and SLIGRL-NH2, respectively, in rat colonic segments in the absence or in the presence of apamin (0.1 μM). The suppression of spontaneous contractions of circular muscle (left panel) is expressed as the percent change from the resting spontaneous activity (−100% corresponds to the abolition of spontaneous activity). The contractile response on longitudinal muscle (right panel) is expressed as a percentage of the contraction to carbachol (10 μM). Each value is the mean±s.e.mean of six experiments. Apamin significantly antagonized the inhibitory responses and significantly enhanced the contractile effects induced by the highest concentration of PAR-1 agonist. *P<0.05, compared to the control value.
We treated also colonic segments with TTX to assess the role of enteric nerves in the responses evoked by PAR-1 or PAR-2 activation. As previously reported (Mulè et al., 1999a, 1999b) TTX (1 μM) resulted in an increase in the amplitude of spontaneous contractions of the circular muscle. However, TTX failed to affect both the inhibitory response of circular muscle and the contractile response of longitudinal muscle induced by PAR-1 or PAR-2 activating peptide (Figure 5). Lastly, to determine if the effects of PAR-1 and PAR-2 agonists were due to the release of prostanoids, we tested the response to SFLLRN-NH2 and SLIGRL-NH2 in the presence of indomethacin, an inhibitor of cyclooxygenase. Indomethacin (1 μM), which did not affect the spontaneous motility by itself, did not alter the inhibitory or the contractile responses to PAR-1 or PAR-2 activating peptide (Figure 6).
Figure 6.
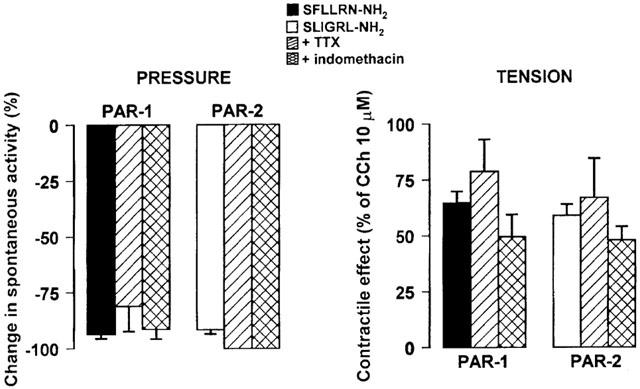
Histograms showing the suppression of spontaneous contractions of circular muscle (left panel) and the contractile effects on longitudinal muscle (right panel) exerted by the PAR-1 agonist, SFLLRN-NH2 (10 μM), or by the PAR-2 agonist, SLIGRL-NH2 (10 μM), in the absence or in the presence of TTX (1 μM) or indomethacin (1 μM). The inhibitory responses were expressed as the percent change from the resting spontaneous activity (−100% corresponds to the abolition of spontaneous activity). The contractile responses are expressed as a percentage of the maximal response to carbachol (10 μM). Each value is the mean±s.e.mean of five experiments. TTX and indomethacin failed to affect the inhibitory and the contractile responses to PAR-1 or PAR-2 activation at all concentrations tested (data not shown).
Discussion
The present study demonstrates that both PAR-1 and PAR-2 play a dual role in the control of the rat colonic motility, producing mainly contraction of the longitudinal muscle and suppression of contractility in the circular muscle.
So far, the studies concerning the intestinal mechanical effects induced by PAR-1 and PAR-2 activation have looked at the longitudinal muscle and the results have indicated that the responses can be relaxant, contractile or biphasic (relaxation followed by contraction) depending on the considered intestinal segment and on the animal species. In particular, PAR-1 and PAR-2 activating peptides induce contraction in rat gastric muscular strips (Al-Ani et al., 1995; Saifeddine et al., 1996), whereas they cause a biphasic response in mouse stomach (Cocks et al., 1999). PAR-1 activation evokes relaxation followed by contraction of rat duodenal longitudinal smooth muscle, while PAR-2 activation elicits muscular contraction (Kawabata et al., 1999a). This is the first study indicating that the mechanical effects due to PAR activation are different on the two muscular layers: suppression on the circular muscle and facilitation on the longitudinal one. The correspondence of the endoluminal pressure and tension recordings to the mechanical activity of circular and longitudinal muscle, respectively, was previously demonstrated (Mulè et al., 1992; 1999a). Because the two muscular layers show different behaviour during the propulsive activity, circular relaxation coupled with longitudinal contraction (Wood, 1998), our results might suggest a role of PAR-1 and PAR-2 in the facilitation of the intestinal transit. This hypothesis is consistent with the recent report of Kawabata et al. (2001), which demonstrated that activation of either PAR-1 or PAR-2 increases gastrointestinal transit in mice in vivo. It is likely that in physiological conditions the presence of the endogenous proteases is low, but during tissue trauma with plasma exudation or inflammatory events with mast-cell degranulation, the concentration of the endogenous ligands, thrombin and tryptase, enhances becoming sufficient to activate PAR-1 and PAR-2. The PAR-1 and PAR-2-mediated mechanical responses could have the purpose to expel the hypothetical novice agent from the intestinal lumen and might contribute to the alterations of colonic motility observed during inflammatory conditions. In fact, it is well known that the major changes in motility involved in the diarrhoea induced by inflammation are the suppression of spontaneous phasic contractions and increased ultrarapid propulsion (Jouet et al., 1995).
In addition, our study demonstrates for the first time that PAR-1, besides PAR-2, is functionally expressed in rat colon. Although SFLLRN-NH2 is reported to have a weak agonistic activity toward PAR-2 (Kawabata et al., 1999b), in our preparation an action of SFLLRN-NH2 on PAR-2 can be ruled out because the sensitivity and the maximum response to SFLLRN-NH2 were unaffected by prior desensitization of the tissue to SLIGRL-NH2. Moreover, the PAR-1 agonist analogue, TFLLR-NH2, known to be highly specific for PAR-1 with no PAR-2 activity (Hollenberg et al., 1997; Kawabata et al., 1999b), mimicked the SFLLRN-NH2-induced effects (data not shown). The specificity of SLISRL-NH2 as PAR-2, which is supported by previous evidence (Nystedt et al., 1994; Bohm et al., 1996), has been confirmed in our experiments. Taken together with the lack of activity of the control peptide analogues, FSLLRN-NH2 and LSIGRL-NH2, the response to SFLLRN-NH2 and SLIGRL-NH2 in the present study is considered to result from activation of PAR-1 and PAR-2, respectively.
Of interest is the observation that the PAR-1 and PAR-2 activating peptides induced also a relaxation of longitudinal muscle at the highest concentrations used. Several hypotheses could explain this observation. One possible explanation is that the relaxation is unmasked only when the tissue shows a significant tone, but this appears unlikely because isoproterenol was able to induce relaxation even in the resting conditions. Alternatively, contractile activity induced by PAR-1 and PAR-2 activating peptides at low concentrations could mask relaxant activity. This could be supported by a recent study in mouse in vivo indicating that the inhibitory effects due to PAR-1 and PAR-2 activation are overcome by excitatory effects (Kawabata et al., 2001). Moreover, a suggestive hypothesis is that another type of PAR might be present and mediate the relaxation of the longitudinal muscle. For example, it is known that PAR-4 responds to a higher concentration of thrombin. Kawabata et al. (2000a) reported that thrombin produces contraction via PAR-1 and relaxation at higher concentration via PAR-4 in the rat esophageal muscularis mucosae. On the other hand in human platelets PAR-4 is less sensitive to thrombin than PAR-1 (Kahn et al., 1998; 1999). PAR-4 can be activated also by trypsin (Kahn et al., 1998; Xu et al., 1998). Therefore future experiments using the PAR-4 activating peptide GYPGKF-NH2 may be useful to explain the present results.
Why are there more types of PARs in the same tissue? In our preparations PAR-1 and PAR-2 mediate the same effects, therefore the possibility that they are arranged to cause a different response can be ruled out. It has been suggested that multiple PARs might be present in a single tissue to serve as a backup system that could respond effectively to different levels of protease (Kahn et al., 1998). Alternatively, the PARs might respond selectively to site-targeted proteases (Hollenberg, 1999). The observation that in rat colon the endogenous and exogenous agonists of PAR-1 were more potent than the PAR-2 agonists, but showed the same efficacy, could suggest that they, besides responding to protease of different origin, are set to signal a different degree of tissue injury, as proposed elsewhere (Cocks & Moffatt, 2000). Moreover, consistent with other reports (Saifeddine et al., 1996), in our preparation, proteases were more potent than the respective synthetic peptides. Differences in potency can be due to the inefficient presentation of the peptide to the binding domains of the receptor, compared with the tethered peptide (Déry et al., 1998) or to a more rapid inactivation by proteolysis (Godin et al., 1994).
Our results also suggest that PAR-1 and PAR-2 inhibitory effects on circular muscle and relaxation on longitudinal muscle involve the activation of the small-conductance Ca2+-activated, K+ channels, that are apamin-sensitive. Apamin also facilitated the contractile effects. The specificity of the effect of apamin on PAR-mediated responses is demonstrated by the observation that the toxin did not affect the contraction induced by carbachol and the relaxation induced by isoproterenol at submaximal concentrations. These findings are in agreement with other observations in vivo (Kawabata et al., 2001) and in vitro preparations (Kawabata et al., 1999a; 2000b; Cocks et al., 1999).
Although the mechanical effects induced by PAR-1 and PAR-2 activation are reported to be TTX-insensitive (Saifeddine et al., 1996; Corvera et al., 1997; Cocks et al., 1999; Kawabata et al., 1999a; Tognetto et al., 2000), recent studies indicate that PAR-1 and PAR-2 can be present in the enteric neurons in guinea-pig and porcine small intestine (Corvera et al., 1999; Green et al., 2000). Therefore, we treated the tissue with TTX to verify a neural involvement in the PAR-1 and PAR-2 responses. Our results concerning TTX indicate that both inhibitory and contractile responses to PAR-1 and PAR-2 are independent by propagation of neural action potential. Moreover, because activation of PAR-1 and PAR-2 can induce release of products of arachidonic acid in other gastrointestinal preparations (Saifeddine et al., 1996; Kong et al., 1997; Vergnolle et al., 1998; Tognetto et al., 2000) we tested the PAR-1 and PAR-2 response in the presence of indomethacin. The inhibitory or contractile responses to PAR-1 and PAR-2 were unaffected by the inhibitor of cyclooxygenase indicating that in our preparation they do not depend on products of cyclooxygenase.
In conclusion, both PAR-1 and PAR-2 are functionally expressed in rat colon. They play a dual role in the control of the rat colonic motility, producing mainly contraction of the longitudinal muscle and suppression of contractility in the circular muscle. This might contribute to the alterations of colonic motility observed during inflammatory conditions.
Acknowledgments
This work was supported by a grant from Ministero dell'Università e della Ricerca scientifica, Italy. We thank Dr N. Vergnolle (University of Calgary) for supplying TFLLR-NH2.
Abbreviations
PAR
protease-activated receptor
TTX
tetrodotoxin
References
- AL-ANI B., SAIFEDDINE M., HOLLENBERG M.D. Detection of functional receptors for the proteinase-activated-receptor-2-activating polypeptide, SLIGRL-NH2, in rat vascular and gastric smooth muscle. Can. J. Physiol. Pharmacol. 1995;73:1203–1207. doi: 10.1139/y95-172. [DOI] [PubMed] [Google Scholar]
- BOHM S.K., KONG W., BROMME D., SMEEKENS S.P., ANDERSON D.C., CONNOLLY A., KHAN M., NELKEN N.A., COUGHLIN S.R., PAYAN D.G., BUNNETT N.W. Molecular cloning, expression and potential functions of the human proteinase-activated receptor-2. Biochem. J. 1996;314:1009–1016. doi: 10.1042/bj3141009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COCKS T.M., MOFFATT J.D. Proteinase-activated receptors: sentries for inflammation. Trends Pharmacol. Sci. 2000;21:103–108. doi: 10.1016/s0165-6147(99)01440-6. [DOI] [PubMed] [Google Scholar]
- COCKS T.M., SOZZI V., MOFFATT J.D., SELEMIDIS S. Protease-activated receptors mediate apamin-sensitive relaxation of mouse and guinea pig gastrointestinal smooth muscle. Gastroenterology. 1999;116:586–592. doi: 10.1016/s0016-5085(99)70180-0. [DOI] [PubMed] [Google Scholar]
- CORVERA C.U., DÉRY O., MCCONALOGUE K., BÖHM S.K., KHITIN L.M., CAUGHEY G.H., PAYAN D.G., BUNNETT N.W. Mast cell tryptase regulates rat colonic myocytes through proteinase-activated receptor 2. J. Clin. Invest. 1997;100:1383–1393. doi: 10.1172/JCI119658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CORVERA C.U., DÉRY O., MCCONALOGUE K., GAMP P., THOMA M., AL-ANI B., CAUGHEY G.H., HOLLENBERG M.D., BUNNETT N.W. Thrombin and mast cell tryptase regulate guinea-pig myenteric neurons through proteinase-activated receptors-1 and -2. J. Physiol. 1999;517:741–756. doi: 10.1111/j.1469-7793.1999.0741s.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DÉRY O., CORVERA C.U., STEINHOFF M., BUNNETT N.W. Proteinase-activated receptors: novel mechanisms of signaling by serine proteases. Am. J. Physiol. 1998;274:C1429–C1452. doi: 10.1152/ajpcell.1998.274.6.C1429. [DOI] [PubMed] [Google Scholar]
- GODIN D., MARCEAU F., BEAULE C., RIOUX F., DRAPEAU G. Aminopeptidase modulation of the pharmacological responses to synthetic thrombin receptor agonists. Eur. J. Pharmacol. 1994;253:225–230. doi: 10.1016/0014-2999(94)90195-3. [DOI] [PubMed] [Google Scholar]
- GREEN B.T., BUNNETT N.W., KULKARNI-NARLA A., STEINHOFF M., BROWN D.R. Intestinal type 2 proteinase-activated receptors: expression in opioid-sensitive secretomotor neural circuits that mediate epithelial ion transport. J. Pharmacol. Exp. Therap. 2000;295:410–416. [PubMed] [Google Scholar]
- HOLLENBERG M.D. Protease-activated receptors: PAR-4 and counting: how long is the course. Trends Pharmacol. Sci. 1999;20:271–273. doi: 10.1016/s0165-6147(99)01333-4. [DOI] [PubMed] [Google Scholar]
- HOLLENBERG M.D., LANIYONU A.A., SAIFEDDINE M., MOORE G.J. Role of the amino- and carboxyl-terminal domains of thrombin receptor-derived polypeptides in biological activity in vascular endothelium and gastric smooth muscle: evidence for receptor subtypes. Mol. Pharmacol. 1993;43:921–930. [PubMed] [Google Scholar]
- HOLLENBERG M.D., SAIFEDDINE M., AL-ANI B., GUI Y. Proteinase-activated receptor 4 (PAR4): action of PAR4-activating peptides in vascular and gastric tissue and lack of cross-reactivity with PAR1 and PAR2. Can. J. Physiol. Pharmacol. 1999;77:458–464. [PubMed] [Google Scholar]
- HOLLENBERG M.D., SAIFEDDINE M., AL-ANI B., KAWABATA A. Proteinase activated receptor: structural requirements for activity, receptor cross-reactivity, and receptor selectivity of receptor-activating peptides. Can. J. Physiol. Pharmacol. 1997;75:832–841. [PubMed] [Google Scholar]
- ISHIHARA H., CONNOLLY A.J., ZENG D., KAHN M.L., ZHENG Y.W., TIMMONS C., TRAM T., COUGHLIN S.R. Protease-activated receptor 3 is a second thrombin receptor in humans. Nature. 1997;386:502–506. doi: 10.1038/386502a0. [DOI] [PubMed] [Google Scholar]
- JOUET P., SARNA S.K., SINGARAM C., RYAN R.P., HILLARD C.J., TELFORD G.L., FINK J., HENDERSON J.D. Immunocytes and abnormal gastrointestinal motor activity during ileitis in dogs. Am. J. Physiol. 1995;269:G913–G924. doi: 10.1152/ajpgi.1995.269.6.G913. [DOI] [PubMed] [Google Scholar]
- KAHN M.L., NAKANISHI-MATSUI M., SHAPIRO M.J., ISHIHARA H., COUGHLIN S.R. Protease-activated receptors 1 and 4 mediate activation of human platelets by thrombin. J. Clin. Invest. 1999;103:879–887. doi: 10.1172/JCI6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAHN M.L., ZHENG Y.W., HUANG W., BIGORNIA V., ZENG D., MOFF S., FARESE R.V., JR, TAM C., COUGHLIN S.R. A dual thrombin receptor system for platelet activation. Nature. 1998;394:690–694. doi: 10.1038/29325. [DOI] [PubMed] [Google Scholar]
- KAWABATA A., KURODA R., KUROKI N., NISHIKAWA H., KAWAI K. Dual modulation by thrombin of the motility ofl rat esophageal muscularis mucosae via two distinct protease-activated receptors (PARs): a novel role for PAR-4 as opposed to PAR-1. Br. J. Pharmacol. 2000a;131:578–584. doi: 10.1038/sj.bjp.0703590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAWABATA A., KURODA R., KUROKI N., NISHIKAWA H., KAWAI K., ARAKI H. Characterization of protease-activated receptor-1-mediated contraction and relaxation in the rat duodenal smooth muscle. Life Sciences. 2000b;67:2521–2530. doi: 10.1016/s0024-3205(00)00835-3. [DOI] [PubMed] [Google Scholar]
- KAWABATA A., KURODA R., NAGATA N., KAWAO N., MASUKO T., NISHIKAWA H., KAWAI K. In vivo evidence that protease-activated receptors 1 and 2 modulate gastrointestinal transit in the mouse. Br. J. Pharmacol. 2001;133:1213–1218. doi: 10.1038/sj.bjp.0704211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAWABATA A., KURODA R., NISHIKAWA H., KAWAI K. Modulation by protease-activated receptors of the rat duodenal motility in vitro: possible mechanisms underlying the evoked contraction and relaxation. Br. J. Pharmacol. 1999a;128:865–872. doi: 10.1038/sj.bjp.0702755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAWABATA A., SAIFEDDINE M., AL-ANI B., LEBLOND L., HOLLENBERG M.D. Evaluation of proteinase-activated receptor-1 (PAR-1) agonists and antagonists using a cultured cell receptor desensitization assay: activation of PAR-2 by PAR-1 targeted ligands. J. Pharmacol. Exp. Therap. 1999b;228:358–370. [PubMed] [Google Scholar]
- KONG W., MCCONALOGUE K., KHITIN L.M., HOLLENBERG M.D., PAYAN D.G., BÖHM S.K., BUNNETT N.W. Luminal trypsin may regulate enterocytes through proteinase-activated receptor 2. Proc. Natl. Acad. Sci. 1997;94:8884–8889. doi: 10.1073/pnas.94.16.8884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACFARLANE S.R., SEATTER M.J., KANKE T., HUNTER G., PLEVIN R. Proteinase-activated receptors. Pharmacol. Rev. 2001;53:245–282. [PubMed] [Google Scholar]
- MOLINO M., BARNATHAN E.S., NUMEROF R., CLARK J., DREYER M., CUMASHI A., HOXIE J.A., SCHECTER N., WOOLKALIS M., BRASS L.F. Interactions of mast cell tryptase with thrombin receptors and PAR-2. J. Biol. Chem. 1997;272:4043–4049. doi: 10.1074/jbc.272.7.4043. [DOI] [PubMed] [Google Scholar]
- MULÈ F., D'ANGELO S., AMATO A., CONTINO I., SERIO R. Modulation by nitric oxide of spontaneous mechanical activity in rat proximal colon. J. Auton. Pharmacol. 1999a;19:1–6. doi: 10.1046/j.1365-2680.1999.00108.x. [DOI] [PubMed] [Google Scholar]
- MULÈ F., D'ANGELO S., SERIO R. Tonic inhibitory action by nitric oxide on spontaneous activity in rat proximal colon: involvement of cyclic GMP and apamin-sensitive K+ channels. Br. J. Pharmacol. 1999b;127:514–520. doi: 10.1038/sj.bjp.0702537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MULÈ F., POSTORINO A., GERACI A., SERIO R. Neurotensin: dual effect on the motor activity of rat duodenum. Eur. J. Pharmacol. 1992;212:215–224. doi: 10.1016/0014-2999(92)90332-x. [DOI] [PubMed] [Google Scholar]
- MULÈ F., SERIO R. Inhibition of mechanical activity by neurotensin in rat proximal colon: involvement of nitric oxide. Am. J. Physiol. 1997;273:G491–G497. doi: 10.1152/ajpgi.1997.273.2.G491. [DOI] [PubMed] [Google Scholar]
- MULÈ F., SERIO R., POSTORINO A. Motility pattern of isolated proximal colon and excitatory action of neurotensin. Eur. J. Pharmacol. 1995;275:131–137. doi: 10.1016/0014-2999(94)00760-5. [DOI] [PubMed] [Google Scholar]
- NYSTEDT S., EMILSSON K., WAHLESTEDT C., SUNDELIN J. Molecular cloning of a potential proteinase activated receptor. Proc. Natl. Acad. Sci. 1994;91:9208–9212. doi: 10.1073/pnas.91.20.9208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAIFEDDINE M., AL-ANI B., CHENG C.H., WANG L., HOLLENBERG M.D. Rat proteinase-activated receptor-2 (PAR-2): cDNA sequence and activity of receptor-derived peptides in gastric and vascular tissue. Br. J. Pharmacol. 1996;118:521–530. doi: 10.1111/j.1476-5381.1996.tb15433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SERIO R., MULÈ F., POSTORINO A., VETRI T., BONVISSUTO F. Apamin-sensitive and -insensitive components of inhibitory junction potentials in rat caecum: role of nitric oxide. J. Auton. Pharmacol. 1996;16:183–189. doi: 10.1111/j.1474-8673.1996.tb00421.x. [DOI] [PubMed] [Google Scholar]
- TOGNETTO M., TREVISANI M., MAGGIORE B., NAVARRA G., TURINI A., GUERRINI R., BUNNETT N.W., GEPPETTI P., HARRISON S. Evidence that PAR-1 and PAR-2 mediate prostanoid-dependent contraction in isolated guinea-pig gallbladder. Br. J. Pharmacol. 2000;131:689–694. doi: 10.1038/sj.bjp.0703618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VERGNOLLE N. Proteinase-activated receptors – novel signals for gastrointestinal pathophysiology. Aliment. Pharmacol. Ther. 2000;14:257–266. doi: 10.1046/j.1365-2036.2000.00690.x. [DOI] [PubMed] [Google Scholar]
- VERGNOLLE N., MACNAUGHTON W.K., AL-ANI B., SAIFEDDINE M., WALLACE J.L., HOLLENBERG M.D. Proteinase-activated receptor-2-activating peptides: identification of a receptor that regulates intestinal transport. Proc. Natl. Acad. Sci. 1998;95:7766–7777. doi: 10.1073/pnas.95.13.7766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VERGNOLLE N., WALLACE J.L., BUNNETT N.W., HOLLENBERG M.D. Protease-activated receptors in inflammation, neuronal signaling and pain. Trends Pharmacol. Sci. 2001;22:146–152. doi: 10.1016/s0165-6147(00)01634-5. [DOI] [PubMed] [Google Scholar]
- VU T.K., HUNG D.T., WHEATON V.I., COUGHLIN S.R. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991;64:1057–1068. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- WOOD J.D.Enteric neuropathobiology Functional disorders of the gut 1998London: Churchill Livingstone; 19–42.ed. Phillips, S.F. & Wingate, D.L. pp [Google Scholar]
- XU W.F., ANDERSEN H., WHITMORE T.E., PRESNELL S.R., YEE D.P., CHING A., GILBERT T., DAVIE E.W., FOSTER D.C. Cloning and characterization of human protease-activated receptor 4. Proc. Natl. Acad. Sci. 1998;95:6642–6646. doi: 10.1073/pnas.95.12.6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHENG X.L., RENAUX B., HOLLENBERG M.D. Parallel contractile signal transduction pathways activated by receptors for thrombin and epidermal growth factor-urogastrone in guinea pig gastric smooth muscle: blockade by inhibitors of mitogen-activated protein kinase-kinase and phosphatidyl inositol 3′-kinase. J. Pharmacol. Exp. Therap. 1998;285:325–334. [PubMed] [Google Scholar]