Positive allosteric modulation of the human metabotropic glutamate receptor 4 (hmGluR4) by SIB-1893 and MPEP (original) (raw)
Abstract
We have identified 2-methyl-6-(2-phenylethenyl)pyridine (SIB-1893) and 2-methyl-6-phenylethynyl pyridine hydrochloride (MPEP) as positive allosteric modulators for the hmGluR4. SIB-1893 and MPEP enhanced the potency and efficacy of L-2-amino-4-phophonobutyrate (L-AP4) in guanosine 5′-_O_-(3-[35S]thiotriphosphate ([35S]GTPγS) binding and efficacy in cAMP studies. These effects were fully blocked by the mGluR4 competitive antagonist (RS)-α-cyclopropyl-4-phosphonophenylglycine (CPPG), indicating a dependency on receptor activation. Although SIB-1893 and MPEP had no effects alone in GTPγS binding, effects were observed in the cell-based cAMP assay due to media-derived activation as indicated by CPPG inhibition. Positive modulation of the mGluR4 was a receptor-specific effect since SIB-1893 and MPEP had neither effects on mGluR2-expressing cells nor on the parent BHK cell line. In [3H]L-AP4 binding, a two-fold decrease in _K_D but not in _B_max was observed with 100 _μ_M SIB-1893, whereas MPEP affected neither parameter. Finally, SIB-1893 and MPEP failed to displace [3H]L-AP4 binding. Taken together, these data identify positive allosteric modulators for the hmGluR4.
Keywords: Positive modulation, allosteric, mGluR4, MPEP, SIB-1893, L-AP4, [35S]GTPγS, cAMP
Introduction
Metabotropic glutamate receptors (mGluRs) belong to Family C G-protein-coupled receptors, which also include the Ca2+-sensing, GABAB, pheromone, and taste receptors. Eight subtypes (1–8) and multiple splice variants of the mGluR have been identified and grouped based on their sequence homology and pharmacological properties. In heterologous expression systems, Group I mGluRs (subtypes 1 and 5) activate the phosphatidyl inositol pathway, while Group II (2 and 3) and Group III (4, 6, 7 and 8) inhibit adenylyl cyclase (Conn & Pin, 1997). Due to their highly conserved glutamate-binding site (Kunishima et al., 2000), development of subtype-specific ligands has been difficult, obscuring the physiological importance of the individual mGluR subtypes.
Recently, a number of allosteric modulators, which all lack the _α_-amino-acidic moiety characteristic of competitive ligands and which fail to displace orthosteric ligands, have been identified for mGluRs. 7-(Hydroxyimino)cyclopropa[b]chromen-1a-carboxylate ethyl ester (CPCCOEt) and 2-methyl-6-(phenylethenyl)pyridine hydrochloride (MPEP), negative allosteric modulators for mGluR1 and mGluR5, respectively, have been shown to bind to sites distinct from glutamate within the seven-transmembrane region (Gasparini et al., 2002). Since these allosteric sites are less conserved than the glutamate-binding site, there is general consensus that ligands, which target these regions, will be key to subtype-specificity.
We show here that 2-methyl-6-(2-phenylethenyl)pyridine (SIB-1893) and MPEP are positive allosteric modulators for the hmGluR4 in addition to being mGluR5 noncompetitive antagonists as previously reported (Gasparini et al., 1999; Varney et al., 1999).
Methods
Reagents
L-2-amino-4-phosphonobutyrate (L-AP4),[3H]L-AP4 (52 Ci mmol−1), (RS)-α-cyclopropyl-4-phosphonophenylglycine (CPPG), L-serine-_O_-phosphate (L-SOP), SIB-1893, and MPEP were from Tocris Cookson (Bristol, U.K.). Phenylmethanesulphonyl fluoride (PMSF), L-glutamate, saponin, forskolin, 3-isobutyl-1-methylxanthine (IBMX), and guanosine 5′-diphosphate (GDP) were from Sigma-Aldrich (Germany). Guanosine 5′-_O_-(3-[35S]thiotriphosphate ([35S]GTPγS) (1250 Ci mmol−1) and [125I]-cAMP (2200 Ci mmol−1) were from Perkin-Elmer Life Sciences, U.S.A.
Cloning and generation of cell line
The full-length human mGluR4 cDNA, cloned from cerebellum poly(A)+ mRNA (BD Biosciences, U.S.A.), was subcloned into pCI-neo (Promega, U.S.A.) and transfected into baby hamster kidney (BHK) cells using Lipofectamine 2000 (Gibco, U.K.). Cells were grown for 7 days in 5 mg ml−1 G418 (Gibco, U.K.) after which, clones were selected and tested in a cAMP assay. HmGluR4-expressing cells were maintained in Dulbecco's modified Eagle's medium (DMEM) containing glutamax I, 10% dialysed fetal calf serum, 1 mM sodium pyruvate, and 0.5 mg ml−1 G418 in 5% CO2 at 37°C, while cells expressing the rat mGluR2 were grown in 1 mg ml−1 G418 (Thomsen et al., 1994).
Membrane preparation
BHK cells expressing either hmGluR4 or rmGluR2 were grown to 90% of confluence and harvested as previously described (Kowal et al., 1998). On the test day, membranes were thawed, centrifuged, and resuspended in ice-cold distilled water for 1 h. Membranes were then centrifuged and resuspended in the desired assay buffer (Urwyler et al., 2001). Protein concentrations were determined using the Pierce BCA Protein Assay (Pierce Biotechnology, U.S.A.).
[35S]GTPγS binding assay
The assay was performed as described (Urwyler et al., 2001) except that 3 _μ_M GDP, 15 _μ_g per well membranes supplemented with 50 _μ_g ml−1 saponin, 1 mg per well wheat germ agglutinin-coated scintillation proximity assay (SPA) beads (RPNQ0001, Amersham), and 0.5 nM [35S]GTPγS (1250 Ci mmol−1) were used.
Inhibition of forskolin-stimulated cAMP production
Cells were seeded at 15,000 per well in 96-well plates 24 h before assaying. Prior to compound addition, the medium was replaced with 200 _μ_l/well ground buffer (DMEM, 20 mM HEPES and 0.1 mM IBMX) for 5 min. The buffer was then replaced with test compounds diluted in ground buffer with 10 _μ_M forskolin. The reaction was terminated after 15 min by aspirating and adding ice-cold 0.1 M HCl. After freezing for 30 min at −80°C, each well was neutralised with 0.15 M NaOH. cAMP levels were quantified using the Adenylyl Cyclase Flash-Plate(R) assay (NEN, Belgium) and interpolated from a standard cAMP curve.
[3H]_L_-AP4 SPA binding assay
Homologous displacement binding curves were generated on hmGluR4-expressing BHK membranes. Experiments were performed as described (Monastyrskaia et al., 1999) except that 75 _μ_g membranes, 1 mg SPA beads, and 30 nM [3H]L-AP4 (52 Ci mmol−1) per well were used and that the buffer consisted of 30 mM HEPES pH=8.0, 300 mM choline chloride, and 0.1 mM PMSF.
Data analysis
Concentration – response and homologous displacement binding curves were analysed by nonlinear regression using GraphPad Prism version 3.0 for Windows (GraphPad Software, San Diego, U.S.A.). _K_D and _B_max values were estimated using the following equations (Motulsky & Neubig, 1997), where L is the concentration of the radioactive ligand and the IC50, _B_top-plateau and _B_bottom-plateau were estimated by nonlinear regression: K_D=IC50–_L; _B_max=(_B_top-plateau−_B_bottom-plateau)/(L/IC50).
Results
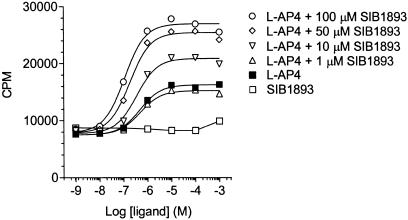
GTPγS binding has been useful in identifying positive allosteric modulators because of its level of sensitivity and lack of receptor activation from media-derived stimuli (Urwyler et al., 2001). A similar approach was taken to identify modulators for the hmGluR4. Concentration–response experiments for L-AP4 using hmGluR4-expressing BHK membranes resulted in a saturable increase in [35S]GTPγS binding with an EC50 of 0.48 _μ_M and a maximal response of 1.9-fold over basal activity (Figure 1; Table 1). Concentration – response curves for L-AP4 were generated in the presence of fixed concentrations of the mGluR5 noncompetitive antagonists SIB-1893 and MPEP, which were expected to have no effects. Surprisingly, however, they significantly potentiated L-AP4's response. In the presence of L-AP4, 100 _μ_M SIB-1893 and MPEP increased GTPγS binding by 3.2- and 2.8-folds, respectively, over basal activity (Table 1), which corresponds to 1.7- and 1.5-fold over the maximal L-AP4 response. Dimethyl sulphoxide at concentrations corresponding to those in 100 _μ_M SIB-1893 or MPEP had no effects on GTPγS binding (data not shown). Furthermore, left-shift displacements of the L-AP4 concentration–response curves were observed for SIB-1893 (3.2-fold) and MPEP (1.8-fold) (Table 1). These data show that SIB-1893 and MPEP positively modulate both the efficacy and potency of the mGluR4 orthosteric agonist L-AP4. Moreover, L-glutamate produced a maximal response comparable to that of L-AP4 and in the presence of 100 _μ_M SIB-1893 and MPEP, similar effects were observed as seen for L-AP4 (Table 1).
Figure 1.
Effect of SIB-1893 on L-AP4-induced [35S]GTPγS binding in hmGluR4 membranes. Data are given as CPM±s.e.m. from a representative experiment performed in triplicate. Nonspecific binding (∼3600 CPM) has been subtracted.
Table 1.
EC50 and maximal responses of L-AP4 and L-glutamate in the absence and presence of SIB-1893 and MPEP
| | (_μ_M) | EC 50 (_μ_M) | pD 2 | Max. response (fold over basal) | n | | | -------------- | ---------------- | -------- | ----------------------------------- | ----------- | - | | Basal | | | | 1.00±0.01 | 6 | | L-AP4 | | 0.48 | [6.32±0.01] | 1.90±0.09 | 6 | | SIB-1893 | 1 | 0.47 | [6.34±0.04] | 2.07±0.13 | 3 | | SIB-1893 | 10 | 0.32 | [6.50±0.04] | 2.53±0.10* | 4 | | SIB-1893 | 50 | 0.24 | [6.69±0.13]* | 3.01±0.13* | 4 | | SIB-1893 | 100 | 0.15 | [6.93±0.13]* | 3.18±0.12* | 5 | | MPEP | 1 | 0.55 | [6.27±0.06] | 1.97±0.14 | 3 | | MPEP | 10 | 0.44 | [6.37±0.04] | 2.13±0.09 | 4 | | MPEP | 50 | 0.39 | [6.42±0.07] | 2.53±0.11* | 4 | | MPEP | 100 | 0.26 | [6.63±0.09]* | 2.83±0.09* | 5 | | L-glutamate | | 6.41 | [5.20±0.04] | 2.13±0.09 | 3 | | SIB-1893 | 100 | 1.08 | [5.98±0.08]* | 3.03±0.12* | 3 | | MPEP | 100 | 2.15 | [5.68±0.05]* | 2.92±0.17* | 3 |
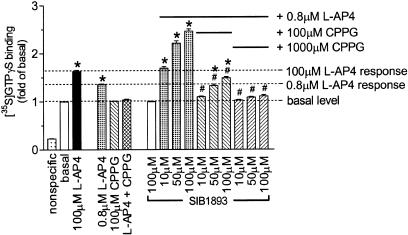
To determine whether the positive effects of SIB-1893 and MPEP were dependent on activation by L-AP4, the mGluR4 competitive antagonist CPPG was used in a series of GTPγS binding studies (Figure 2). A challenge of 0.8 _μ_M L-AP4 on hmGluR4-expressing membranes produced a submaximal response (∼EC60). This response was completely blocked with 100 _μ_M CPPG, which alone displayed no effects. Increasing the concentrations of SIB-1893 in the presence of 0.8 _μ_M L-AP4 potentiated the response to 2.5-fold above basal activity compared to that achievable by L-AP4 alone (1.6-fold in this set of experiments). In the presence of 1000 _μ_M CPPG, SIB-1893-enhanced GTPγS binding was completely blocked (Figure 2). Similar findings were made for MPEP except that it was less potent (data not shown).
Figure 2.
Potentiating effect of SIB-1893 in [35S]GTPγS binding on hmGluR4 membranes. The L-AP4 response enhanced by SIB-1893 at 10–100 _μ_M was completely blocked by 1000 _μ_M CPPG. SIB-1893 alone failed to increase binding above basal level. The data are mean±s.e.m from three to six independent experiments each performed in quadruplicate. Significant differences from basal: *P<0.05 and from SIB-1893 enhanced L-AP4 response; #P<0.05 (ANOVA followed by Dunnett's test).
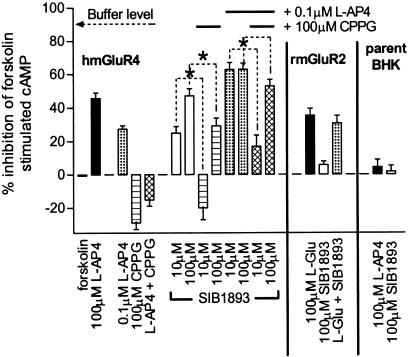
In order to examine the downstream effects of SIB-1893 and MPEP, cAMP formation in intact hmGluR4-expressing cells was measured. At 100 _μ_M, SIB-1893 and MPEP inhibited forskolin-stimulated cAMP formation on their own by 47 and 22%, respectively, compared to 45% by 100 _μ_M L-AP4 (only SIB-1893 data are shown in Figure 3). These findings were in contrast to GTPγS binding, where no effects were seen with either SIB-1893 or MPEP alone.
Figure 3.
Inhibition of forskolin-stimulated cAMP formation. In hmGluR4-expressing BHK cells, CPPG blocked the concentration-dependent effects observed with SIB-1893 alone and together with 0.1 _μ_M L-AP4. In rmGluR2-expressing BHK cells, the L-glutamate response was not augmented by SIB-1893 and no effects of SIB-1893 alone were observed. In the parent BHK cell line, L-AP4 and SIB-1893 produced no effects. The data are mean±s.e.m. of three independent experiments performed in triplicate and are expressed as % inhibition of 10 _μ_M forskolin-stimulated cAMP production. Significant differences: *P<0.05 (ANOVA followed by Dunnett's test).
To investigate if this was due to receptor preactivation by media-derived stimuli, BHK cells expressing the hmGluR4 were treated with 100 _μ_M CPPG. This alone produced an increase in cAMP of ∼25% above that induced by 10 _μ_M forskolin, indicating activation of the hmGluR4 in the absence of L-AP4. The effects of SIB-1893 and MPEP alone were sensitive to 100 _μ_M CPPG, which significantly reduced their effects. Furthermore, SIB-1893 and MPEP potentiated a submaximal effect seen with 0.1 _μ_M L-AP4, albeit not to the same extent as in GTPγS binding. CPPG similarly blocked the potentiation of SIB-1893 and MPEP as well as the effect of L-AP4 alone. These data indicate that the modulatory effects of SIB-1893 and MPEP are dependent on receptor activation in a whole-cell system. Since no effects of SIB-1893 or MPEP were observed in either the rmGluR2 or parental BHK cells at con-centrations up to 100 _μ_M (Figure 3), their effects appear to be receptor specific and not due to a nonspecific downstream effect.
In order to determine if SIB-1893 and MPEP increased affinity of L-AP4 for hmGluR4, homologous displacement experiments with [3H]L-AP4 were performed in the presence of fixed concentrations of the compounds. SIB-1893 shifted the [3H]L-AP4 curve to the left significantly albeit modestly in a dose-dependent manner (Table 2). The _K_D values of [3H]L-AP4 were decreased in the presence of 50 and 100 _μ_M SIB-1893 by a factor of 1.7 and 2.0, respectively, whereas the _B_max values were unchanged. Interestingly, 100 _μ_M MPEP altered neither the _K_D nor the _B_max of L-AP4 (data not shown). Furthermore, 100 _μ_M SIB-1893 and MPEP failed to displace [3H]L-AP4 from hmGluR4 membranes. No specific [3H]L-AP4 binding was detected in native BHK cell membranes.
Table 2.
Effects of SIB-1893 on homologous displacement of [3H]L-AP4 from hmGluR4 membranes
| L‐AP4+ | _K_D | _B_max | n | |
|---|---|---|---|---|
| (_μ_M) | (_n_M) | (fmol/mg) | ||
| None | 0 | 196±25 | 1157±151 | 4 |
| SIB-1893 | 10 | 190±19 | 1118±112 | 4 |
| SIB-1893 | 50 | 118±8* | 926±114 | 4 |
| SIB-1893 | 100 | 98±3* | 880±85 | 4 |
Discussion
We demonstrate that SIB-1893 and MPEP positively modulate the hmGluR4 in a recombinant expression system, which to our knowledge, is the first report identifying them as positive allosteric modulators for mGluR4. In GTPγS binding, it was clearly shown that the effects of SIB-1893 and MPEP were fully dependent on the activation of the orthosteric agonist L-AP4. Moreover, the L-AP4 response and its potentiation by either SIB-1893 or MPEP were completely blocked by the competitive mGluR4 antagonist CPPG.
Similar findings were seen in whole cell-based cAMP assays. In contrast to GTPγS binding, however, the compounds activated the hmGluR4 on their own. This can be explained by the differences between the assays. In the [35S]GTPγS assay, cell membranes were water treated for 1 hr resulting in the removal of glutamate whereas the presence of glutamate could not be completely eliminated from the cAMP assays, leading to some degree of receptor activation. This was supported by the fact that CPPG produced a cAMP response above that of 10 _μ_M forskolin. The presence of glutamate in whole cell assays and its effects on mGluR pharmacology have been previously reported (Thomsen et al., 1994), posing problems in differentiating between allosteric modulators and allosteric agonists (Christopoulos & Kenakin, 2002).
It was previously reported that SIB-1893 in GTPγS binding and MPEP in cAMP assays displayed no agonistic effects on mGluR4 when tested alone (Gasparini et al., 1999; Varney et al., 1999). In cAMP experiments, modest mGluR4 agonism (50% of the L-AP4 response, EC50=26.4 _μ_M) of SIB-1893 was detected and a trend towards enhancing the L-glutamate/L-AP4 response in both GTPγS and cAMP assays was observed, albeit not significantly. The conclusions drawn that SIB-1893 and MPEP were not positive allosteric modulators for the mGluR4 are in contrast to our data, which show robust potentiating effects especially in GTPγS binding studies. An explanation for this discrepancy may lie in the different screening systems used with respect to the chosen cell lines, the expression levels of the hmGluR4, and the efficiencies of G-protein coupling. We observed that BHK cells were superior in terms of hmGluR4 membrane expression based on fluorescence microscopy and signalling compared to either HEK293 or CHO cells, the latter used by others. We were unable to compare receptor expression levels because of the absence of comparable data in the literature.
SIB-1893 and MPEP showed an increase in both efficacy and potency of the full agonist L-AP4 for the hmGluR4, a property previously observed for positive allosteric modulators of the mGluR1 and GABAB receptor (Knoflach et al., 2001; Urwyler et al., 2001). The dual effects of SIB-1893 and MPEP are consistent with a modified two-state model described by Hall (2000), which allows for allosteric modulation. According to this model, SIB-1893 and MPEP are expected to increase the intrinsic L-AP4 efficacy by increasing the affinity of L-AP4 to the activated allosteric ligand-bound state of the receptor.
Indeed, our data support this model since 50 and 100 _μ_M SIB-1893 increased L-AP4 affinity without affecting _B_max values or displacing [3H]L-AP4. An increase in orthosteric ligand affinity by an allosteric modulator has also been observed for other Family C positive allosteric modulators (Knoflach et al., 2001; Urwyler et al., 2001). Interestingly, MPEP had no effect on either the _K_D or _B_max values reflecting either its weaker potency or a difference in its mechanism of modulation.
Since antagonising Group I and agonising Groups II/III are neuroprotective (Conn & Pin, 1997), our findings may shed light on the discrepancies seen between the recombinant mGluR5 pharmacology of SIB-1893 and MPEP and their observed neuroprotective effects. SIB-1893 and MPEP exhibit equal neuroprotective effects in rat cortical cells (Bruno et al., 2000b; O'Leary et al., 2000; Movsesyan et al., 2001), despite their large differences in IC50 values of 2.3 _μ_M and 36 nM, respectively, in hmGluR5-expressing cells (Gasparini et al., 1999; Varney et al., 1999). As mGluR4 activation is neuro-protective (Bruno et al., 2000a), one cannot exclude that the observed effects of SIB-1893 and MPEP may, in part, be due to their positive allosteric effects on the mGluR4, when used at concentrations above 10 _μ_M. Their effects on the NMDA receptor is yet another possible explanation (O'leary et al., 2000; Movsesyan et al., 2001). Thus, care should be taken when considering effects of SIB-1893 and MPEP as arising solely from mGluR5 antagonism when used at concentrations above 10 _μ_M.
In summary, we have shown that SIB-1893 and MPEP are positive allosteric modulators for the hmGluR4 in addition to being hmGluR5 noncompetitive antagonists as previously reported. Identification of the amino acid residues involved in mGluR4 vs mGluR5 allosteric ligand binding is anticipated to provide crucial information for the development of subtype-specific compounds.
Acknowledgments
We are grateful to Kamilla Sahl Rønne for her excellent technical assistance. J.M.M. was supported by Grant EF920 from the Danish Academy of Technical Sciences. H.B.-O. was supported by the Danish Medical Research Counsel and the Novo Nordisk Foundation.
Abbreviations
CPCCOEt
7-(Hydroxyimino)cyclopropa[b]chromen-1a-carboxylate ethyl ester
CPPG
(RS)-α-cyclopropyl-4-phosphonophenylglycine
[35S]GTPγS
Guanosine 5′-_O_-(3-[35S]thiotriphosphate)
L-AP4
L-2-amino-4-phosphonobutyrate
L-SOP
L-serine-_O_-phosphate
MPEP
2-methyl-6-(phenylethynyl)pyridine hydrochloride
SIB-1893
2-methyl-6-(2-phenylethenyl)pyridine
References
- BRUNO V., BATTAGLIA G., KSIAZEK I., VAN DER PUTTEN H., CATANIA M.V., GIUFFRIDA R., LUKIC S., LEONHARDT T., INDERBITZIN W., GASPARINI F., KUHN R., HAMPSON D.R., NICOLETTI F., FLOR P.J. Selective activation of mGlu4 metabotropic glutamate receptors is protective against excitotoxic neuronal death. J. Neurosci. 2000a;20:6413–6420. doi: 10.1523/JNEUROSCI.20-17-06413.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRUNO V., KSIAZEK I., BATTAGLIA G., LUKIC S., LEONHARDT T., SAUER D., GASPARINI F., KUHN R., NICOLETTI F., FLOR P.J. Selective blockade of metabotropic glutamate receptor subtype 5 is neuroprotective. Neuropharmacology. 2000b;39:2223–2230. doi: 10.1016/s0028-3908(00)00079-4. [DOI] [PubMed] [Google Scholar]
- CHRISTOPOULOS A., KENAKIN T. G protein-coupled receptor allosterism and complexing. Pharmacol. Rev. 2002;54:323–374. doi: 10.1124/pr.54.2.323. [DOI] [PubMed] [Google Scholar]
- CONN P.J., PIN J.P. Pharmacology and functions of metabotropic glutamate receptors. Annu. Rev. Pharmacol. Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- GASPARINI F., KUHN R., PIN J.P. Allosteric modulators of group I metabotropic glutamate receptors: novel subtype-selective ligands and therapeutic perspectives. Curr. Opin. Pharmacol. 2002;2:43–49. doi: 10.1016/s1471-4892(01)00119-9. [DOI] [PubMed] [Google Scholar]
- GASPARINI F., LINGENHOHL K., STOEHR N., FLOR P.J., HEINRICH M., VRANESIC I., BIOLLAZ M., ALLGEIER H., HECKENDORN R., URWYLER S., VARNEY M.A., JOHNSON E.C., HESS S.D., RAO S.P., SACAAN A.I., SANTORI E.M., VELICELEBI G., KUHN R. 2-Methyl-6-(phenylethynyl)-pyridine (MPEP), a potent, selective and systemically active mGlu5 receptor antagonist. Neuropharmacology. 1999;38:1493–1503. doi: 10.1016/s0028-3908(99)00082-9. [DOI] [PubMed] [Google Scholar]
- HALL D.A. Modeling the functional effects of allosteric modulators at pharmacological receptors: an extension of the two-state model of receptor activation. Mol. Pharmacol. 2000;58:1412–1423. doi: 10.1124/mol.58.6.1412. [DOI] [PubMed] [Google Scholar]
- KNOFLACH F., MUTEL V., JOLIDON S., KEW J.N., MALHERBE P., VIEIRA E., WICHMANN J., KEMP J.A. Positive allosteric modulators of metabotropic glutamate 1 receptor: characterization, mechanism of action, and binding site. Proc. Natl. Acad. Sci. U.S.A. 2001;98:13402–13407. doi: 10.1073/pnas.231358298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOWAL D., HSIAO C.L., GE A., WARDWELL-SWANSON J., GHOSH K., TASSE R. A [35S]GTPgammaS binding assessment of metabotropic glutamate receptor standards in Chinese hamster ovary cell lines expressing the human metabotropic receptor subtypes 2 and 4. Neuropharmacology. 1998;37:179–187. doi: 10.1016/s0028-3908(98)00011-2. [DOI] [PubMed] [Google Scholar]
- KUNISHIMA N., SHIMADA Y., TSUJI Y., SATO T., YAMAMOTO M., KUMASAKA T., NAKANISHI S., JINGAMI H., MORIKAWA K. Structural basis of glutamaterecognition by a dimeric metabotropic glutamate receptor. Nature. 2000;407:971–977. doi: 10.1038/35039564. [DOI] [PubMed] [Google Scholar]
- MONASTYRSKAIA K., LUNDSTROM K., PLAHL D., ACUNA G., SCHWEITZER C., MALHERBE P., MUTEL V. Effect of the umami peptides on the ligand binding and function of rat mGlu4a receptor might implicate this receptor in the monosodium glutamate taste transduction. Br. J. Pharmacol. 1999;128:1027–1034. doi: 10.1038/sj.bjp.0702885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOTULSKY H., NEUBIG R.Analyzing radioligand binding data Current Protocols in Neuroscience 1997New York: John Wiley & Sons, Inc; 7.5.1–7.5.56.ed. Crawley, J., Gerfen, C., Mckay, R., Roogawski, MM., Sibley, D., & Skolnick, P. pp [DOI] [PubMed] [Google Scholar]
- MOVSESYAN V.A., O'LEARY D.M., FAN L., BAO W., MULLINS P.G., KNOBLACH S.M., FADEN A.I. mGluR5 antagonists 2-methyl-6-(phenylethynyl)-pyridine and (E)-2-methyl-6-(2-phenylethenyl)-pyridine reduce traumatic neuronal injury in vitro and in vivo by antagonizing N-methyl-D-aspartate receptors. J. Pharmacol. Exp. Ther. 2001;296:41–47. [PubMed] [Google Scholar]
- O'LEARY D.M., MOVSESYAN V., VICINI S., FADEN A.I. Selective mGluR5 antagonists MPEP and SIB-1893 decrease NMDA or glutamate-mediated neuronal toxicity through actions that reflect NMDA receptor antagonism. Br. J. Pharmacol. 2000;131:1429–1437. doi: 10.1038/sj.bjp.0703715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THOMSEN C., HANSEN L., SUZDAK P.D. L-glutamate uptake inhibitors may stimulate phosphoinositide hydrolysis in baby hamster kidney cells expressing mGluR1a via heteroexchange with L-glutamate without direct activation of mGluR1a. J. Neurochem. 1994;63:2038–2047. doi: 10.1046/j.1471-4159.1994.63062038.x. [DOI] [PubMed] [Google Scholar]
- URWYLER S., MOSBACHER J., LINGENHOEHL K., HEID J., HOFSTETTER K., FROESTL W., BETTLER B., KAUPMANN K. Positive allosteric modulation of native and recombinant gamma-aminobutyric acid(B) receptors by 2,6-Di-tert-butyl-4-(3-hydroxy-2,2-dimethyl-propyl)-phenol (CGP7930) and its aldehyde analog CGP13501. Mol. Pharmacol. 2001;60:963–971. [PubMed] [Google Scholar]
- VARNEY M.A., COSFORD N.D., JACHEC C., RAO S.P., SACAAN A., LIN F.F., BLEICHER L., SANTORI E.M., FLOR P.J., ALLGEIER H., GASPARINI F., KUHN R., HESS S.D., VELICELEBI G., JOHNSON E.C. SIB-1757 and SIB-1893: selective, noncompetitive antagonists of metabotropic glutamate receptor type 5. J Pharmacol. Exp. Ther. 1999;290:170–181. [PubMed] [Google Scholar]