Relationship between peroxisome proliferator-activated receptors (PPARα and PPARγ) and endothelium-dependent relaxation in streptozotocin-induced diabetic rats (original) (raw)
Abstract
- The aim of the present study was to investigate the causal relationship between peroxisome proliferator-activated receptor (PPAR) and endothelium-dependent relaxation in streptozotocin (STZ)-induced diabetic rats.
- Acetylcholine (ACh)-induced endothelium-dependent relaxation was significantly weaker in diabetic rats than in age-matched controls. The decreased relaxation in diabetes was improved by the chronic administration of bezafibrate (30 mg kg−1, p.o., 4 weeks).
- The expressions of the mRNAs for PPAR_α_ and PPAR_γ_ were significantly decreased in STZ-induced diabetic rats (compared with the controls) and this decrease was restored partially, but not completely, by the chronic administration of bezafibrate.
- Superoxide dismutase activity in the aorta was not significantly different between diabetic rats and bezafibrate-treated diabetic rats.
- The expression of the mRNA for the p22phox subunit of NAD(P)H oxidase was significantly higher in diabetics than in controls, but it was lower in bezafibrate-treated diabetic rats than in nontreated diabetic rats. Although the expression of the mRNA for prepro ET-1 (ppET-1) was markedly increased in diabetic rats (compared with controls), this increase was prevented to a significant extent by the chronic administration of bezafibrate.
- These results suggest that downregulations of PPAR_α_ and PPAR_γ_ may lead to an increased expression of ppET-1 mRNA in diabetic states and this increment may trigger endothelial dysfunction.
Keywords: Peroxisome proliferator-activated receptor α (PPAR_α_), endothelin-1, NAD(P)H oxidase, aorta, diabetes
Introduction
Endothelial dysfunction plays a key role in the pathogenesis of diabetic vascular disease (Kamata et al., 1989; Taylor et al., 1992; Tesfamariam & Cohen, 1992; Nitenberg et al., 1993;Pieper & Peltier, 1995; Poston & Taylor, 1995; Williams et al., 1996; Koltai et al., 1997; Pieper, 1998; De Vriese et al., 2000). It has been suggested that the excessive elevations in plasma glucose, low-density lipoprotein (LDL) cholesterol and reactive oxygen species that occur in diabetes are involved in the development of this dysfunction (Kugiyama et al., 1990; Simon et al., 1990; Kamata et al., 1996; Kobayashi et al., 2000; Kobayashi & Kamata, 1999b; Kanie & Kamata, 2002).
Peroxisome proliferator-activated receptor α (PPAR_α_), which is activated by specific agonists such as fibrates and fatty acid, forms heterodimers with the retinoid X receptor (Mangelsdorf et al., 1990; Mangelsdorf & Evans, 1995) and associates with PPAR response elements in the promoter region of target genes. PPAR_α_ plays an important role in the liver by regulating the metabolism of lipoproteins and fatty acids. In addition, PPAR_α_ is widely expressed throughout the cardiovascular system, in the heart, blood vessels (endothelial cells and smooth muscle cells) and monocyte/macrophage cells (Inoue et al., 1998b; Fruchart et al., 1999; Bishop-Bailey, 2000; Buchan & Hassall, 2000).
Bezafibrate, one of the fibrate classes of drugs, has been used in the treatment of lipid disorders such as primary hypertriglyceridaemia and combined hyperlipidaemia. This lipid-lowering effect is mediated by an increase in the catabolism of triglyceride-rich lipoproteins and the resulting inhibition of hepatic very low-density lipoproteins (VLDL) via an interaction with PPAR_α_. It has been reported that bezafibrate increases the mRNA for Cu2+/Zn2+ superoxide dismutase (SOD) and decreases the mRNA for NAD(P)H oxidase in human cultured endothelial cells (Inoue et al., 1998a;2001). These results suggest that activation of PPAR_α_ may play an important role in vascular diseases such as hypertension, diabetes and coronary heart disease. It has been reported that both the activity of SOD and the expression of its mRNA are decreased in streptozotocin (STZ)-induced diabetic rats (Hattori et al., 1991; Tesfamariam & Cohen, 1992; Kamata & Kobayashi, 1996; Pieper et al., 1996). Furthermore, we have shown both that the expression of the mRNA for the p22phox subunit of NAD(P)H oxidase is increased in STZ-induced diabetic aortae (Kanie & Kamata, 2002) and that this increase is normalized by the endothelin antagonist, J-104132, suggesting that endothelin-1 (ET-1) is involved in the increased formation of superoxide anions. Thus, while PPAR_α_ activation decreases the expression of the mRNA for NAD(P)H oxidase (as implied above), ET-1 seems to increase it. However, there have been no reports concerning the relationship between PPAR_α_ and ET-1 with respect to their effects on the expression of the mRNA for NAD(P)H oxidase. Furthermore, there have been few reports concerning the relationship between PPAR_α_ and endothelium-dependent relaxation, although a change in the activity of PPAR_α_ could be related to the endothelial dysfunction seen in diabetes mellitus. In the present study, therefore, we investigated the effects of chronic bezafibrate administration on the impairment of endothelium-dependent relaxation routinely seen in aortae from rats with established STZ-induced diabetes. Since bezafibrate has a lipid-lowering effect, as well as increasing the expression of the mRNA for Cu2+/Zn2+ SOD and decreasing the expression of the mRNA for NAD(P)H oxidase, we used a dose of bezafibrate low enough not to affect the plasma lipid concentration.
Methods
Animals and experimental design
Male Wistar rats, 7 weeks old and 220–300 g in weight, received a single injection via the tail vein of STZ 75 mg kg−1, dissolved in a citrate buffer. Age-matched control rats were injected with the buffer alone. Food and water were allowed ad libitum. The rats were killed (see below) 11 weeks after the above injections. This study was conducted in accordance with the Guide for the Care and Use of Laboratory Animals adopted by the Committee on the Care and Use of Laboratory Animals of Hoshi University (which is accredited by the Ministry of Education, Science, Sports and Culture, Japan).
Bezafibrate treatment
Starting 7 weeks after the STZ injection, some STZ-induced diabetic rats were given bezafibrate (30 mg kg−1, p.o., daily) for 4 weeks. At 11 weeks after the STZ injection, these rats, like all the others, were killed by decapitation under diethyl ether anaesthesia.
Measurement of plasma cholesterol, LDL
At 11 weeks after the STZ injection, plasma total cholesterol and triglyceride were determined using a commercially available enzyme kit (Wako Chemical Company, Osaka, Japan). Plasma triglyceride level was assayed by the method described by Spayd et al. (1978). High-density lipoprotein (HDL) cholesterol was measured following phosphotungstic-MgCl2 precipitation of apolipoprotein B containing VLDL. The LDL level was derived from the above data using the Friedewald formula: LDL cholesterol=total cholesterol− HDL−1/5 triglyceride (Friedewald et al., 1972). Plasma glucose was determined using a commercially available enzyme kit (Wako Chemical Company, Osaka, Japan). This kit makes use of the _O_-toluidine method (Dubowski, 1962).
Measurement of isometric force
After decapitation, a section of the aorta from between the aortic arch and the diaphragm was removed and placed in oxygenated, modified Krebs–Henseleit solution. The solution consisted of (mM): NaCl 118.0, KCl 4.7, NaHCO3 25.0, CaCl2 1.8, NaH2PO4 1.2, MgSO4 1.2 and dextrose 11.0. The aorta was cleaned of loosely adhering fat and connective tissue and cut into helical strips 2 mm in width and 20 mm in length. The tissue was then placed in a bath containing 10 ml of well-oxygenated (95% O2, 5% CO2) KHS at 37°C. With one end connected to a tissue holder and other to a force–displacement transducer (model TB611T; Nihon Kohden, Tokyo), the tissue was allowed to equilibrate for 60 min under a resting tension of 1.0 × g (determined to be optimal in preliminary experiments). During this period, the Krebs–Henseleit solution in the bath was replaced every 20 min. After equilibration, each aortic strip was contracted with 10−6 M noradrenaline (NA) and the presence of functional endothelial cells was confirmed by demonstrating relaxations in response to 10−5 M acetylcholine (ACh). For the relaxation studies, the aortic strips were precontracted with an equieffective concentration of NA (5 × 10−8–3 × 10−7 M). When the NA-induced contraction had reached a plateau level, ACh (10−9–10−5 M) was added in a cumulative manner.
Measurement of the expression of mRNAs
Oligonucleotides
The primers used are summarized in Table 1.
Table 1.
PCR primer sequences and PCR protocols
| DNA name | PCR primer sequences | PCR protocol |
|---|---|---|
| PPAR_α_ (495 bp) | UP; 5′-TGGCGTACGACAAGTGTGAT-3′ | 95°C min−1 |
| DP; 5′-GTTTGCAAAGCCTGGGATAG-3′ | 59°C min−1 | |
| 72°C min−1 | ||
| (30 cycles) | ||
| PPAR_γ_ (785 bp) | UP; 5′-GTCGGATCCACAAAAAGAG-3′ | 95°C min−1 |
| DP; 5′-AGCAGGTTGTCTTGGATGT-3′ | 57°C min−1 | |
| 72°C min−1 | ||
| (28 cycles) | ||
| PPAR_δ_ (740 bp) | UP; 5′-GCACATCTACAATGCCTACC-3′ | 95°C min−1 |
| DP; 5′-GGTCTCACTCTCCGTCTTCT-3′ | 57°C min−1 | |
| 72°C min−1 | ||
| (26 cycles) | ||
| p22phox (435 bp) | UP; 5′-GCTCATCTGTCTGCTGGAGTA-3′ | 95°C min−1 |
| DP; 5′-ACGACCTCATCTGTCACTGGA-3′ | 57°C min−1 | |
| 72°C min−1 | ||
| (28 cycles) | ||
| prepro ET-1 (482 bp) | UP; 5′-TCTTCTCTCTGCTGTTTGTG-3′ | 95°C min−1 |
| DP; 5′-TAGTTTTCTTCCCTCCACC-3′ | 54°C min−1 | |
| 72°C min−1 | ||
| (35 cycles) | ||
| GAPDH (308 bp) | UP; 5′-TCCCTCAAGATTGTCAGCAA-3′ | 95°C min−1 |
| DP; 5′-AGATCCACAACGGATACATT-3′ | 59 or 57 or 54°C min−1 | |
| 72°C min−1 | ||
| (20 cycles) |
RNA isolation and RT–PCR
RNA was isolated using the guanidium method (Chomczynski & Sacchi, 1987). Aortae were carefully isolated and cleaned of adhering parenchyma and connective tissue and then homogenized in RNA buffer. The RNA was quantified by ultraviolet-absorbance spectrophotometry. For the RT–PCR analysis, first-strand cDNA was synthesized from total RNA using Oligo (dT) and a cDNA Synthesis Kit (Life Sciences, Inc.). The oligonucleotides and PCR protocols are shown in Table 1. The PCR products so obtained were analysed on ethidium-bromide-stained agarose (2.0%) gel.
Competitive PCR
The amount of each mRNA under study was measured using competitive PCR techniques, with a heterogeneous DNA fragment as internal standard. A part of the heterogeneous DNA fragment (300 or 500 bp) was amplified with a protruding 20 bp in the ends specific for PPAR_α_, PPAR_γ_ and PPAR_δ_ primers, respectively. Composite primers were engineered to contain sequences that amplify the DNA fragment with gene-specific primer sequences flanking their 5′ ends. The DNA competitors were designed such that the PCR product from the cDNA could be separated from that of its competitor, and they were generated using reagents supplied in a commercial kit (Competitive DNA Construction Kit; Takara, Japan). Briefly, a 30-cycle PCR was carried out on the DNA using the relevant composite primers. A second PCR (PPAR_α_, PPAR_γ_ and PPAR_δ_: 30, 28 and 26 cycles, respectively) was then carried out on 0.5 _μ_l of the first amplicon using the corresponding primers for the target sequence. The concentrations of the DNA competitors were then measured by spectrophotometry (A260). A semiquantitative evaluation of mRNA levels was performed by comparing the various products after electrophoresis.
Measurement of SOD activity
Rat aortae were carefully isolated and cleaned of adhering parenchyma and connective tissue. The aorta was homogenized in 10 vols of 50 mM phosphate buffer, 0.1 mM ethylenediaminetetraacetic acid, pH 7.4 at 4°C for 1 min, using a glass-Teflon homogenizer. The homogenate was filtered through cheesecloth and the filtrate centrifuged at 400 × g for 5 min. The supernatant was used for the measurement of SOD activity. SOD activity was assayed by means of a previously described indirect inhibition assay, in which xanthine and xanthine oxidase serve as a superoxide generator, and nitro blue tetrazolium is used as a superoxide indicator (Loven et al., 1982; Mantha et al., 1993). The formazane produced was measured spectrophotometrically at 560 nm, the activity being expressed as U mg−1 protein. One unit inhibits the rate of reduction of cytochrome c by 50% in a coupled system with xanthine and xanthine oxidase at pH 7.8 at 25°C in a 3.0 ml reaction volume.
Enzyme immunoassay for ET-1
Plasma samples taken 11 weeks after injection of STZ or buffer were extracted using Amprep C2 columns (Amersham International plc., Buckinghamshire). The columns were equilibrated by washing with 2 ml methanol followed by 2 ml water. Each plasma sample (1 ml) was acidified with 0.25 ml 2 M HCl, centrifuged at 10,000 × g for 5 min at room temperature and then loaded onto the column. The column was washed with 5 ml of 0.1% trifluoroacetic acid (TFA) and immunoreactive ET-1 was eluted with 2 ml of 80% methanol containing 0.1% TFA. Then, the eluent was dried down under nitrogen gas, care being taken not to overdry the pellet. Measurement of the plasma ET-1 concentration was carried out using a commercially available ET-1 ELISA system (Amersham Pharmacia Biotech U.K. Ltd, England).
Drugs
STZ, bezafibrate, NA hydrochloride and sodium nitroprusside (SNP) were all purchased from Sigma Chemical Co. (St Louis, MO, U.S.A.), ACh chloride from Daiichi Pharmaceutical Co. Ltd (Tokyo, Japan) and ET-1 from Peptide Institute Inc. (Osaka, Japan). All concentrations are expressed as the final molar concentration of the base in the organ bath.
Statistical analysis
Data are expressed as the mean±s.e.m. Where appropriate, statistical differences were determined by Dunnett's test for multiple comparisons after a one-way analysis of variance, a probability level of P<0.05 being regarded as significant. Statistical comparisons between concentration–response curves were made by a two-way ANOVA, with Bonferroni's correction for multiple comparisons being performed post hoc; P<0.05 was considered significant.
Results
Plasma glucose cholesterol and triglyceride levels
As shown in Table 2, the plasma glucose, total cholesterol, LDL cholesterol and triglyceride levels were all significantly higher in STZ-induced diabetic rats than in the age-matched controls. These data are consistent with those in our previous report (Kanie & Kamata, 2002). Treatment with bezafibrate (30 mg kg−1, p.o., 4 weeks) altered none of these parameters in our established diabetic rats.
Table 2.
Levels of various plasma parameters in age-matched controls, STZ-induced diabetic and bezafibrate-treated diabetic rats
| Plasma parameter (mg dl−1) | Control (20) | Diabetic (18) | Bezafibrate-treated diabetic (16) |
|---|---|---|---|
| Glucose | 116.6±3.1 | 561.9±27.2 | 498.0±33.8 |
| Total cholesterol | 188.7±7.9*** | 316.2±103.5 | 310.3±25.5 |
| Triglyceride | 178.3±8.9*** | 571.2±57.5 | 611.5±89.8 |
| HDL | 68.1±1.3 | 66.6±14.9 | 57.2±2.3 |
| LDL | 85.0±8.1* | 135.0±16.1 | 130.8±16.1 |
Relaxation responses to ACh and SNP
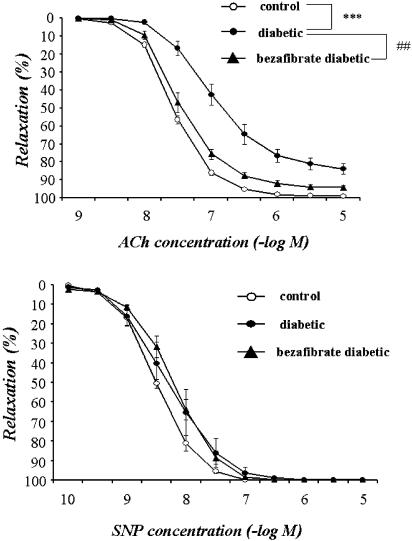
When the NA (5 × 10−8–3 × 10−7 M)-induced contraction had reached a plateau, ACh (1 × 10−9–1 × 10−5 M) or SNP (1 × 10−10–1 × 10−5 M) was added cumulatively (Figure 1). In aortic strips from age-matched control rats, ACh (1 × 10−9–1 × 10−5 M) caused a concentration-dependent relaxation, with the maximum response at 10−5 M. This relaxation was significantly weaker in strips from STZ-induced diabetic rats. By contrast, aortic strips from STZ-induced diabetic rats chronically treated with bezafibrate (30 mg kg−1, p.o., daily for 4 weeks) relaxed in a normal way to ACh (upper panel in Figure 1). The endothelium-dependent relaxation induced by ACh was not affected by bezafibrate in control rats (data not shown). The relaxation responses induced by SNP (1 × 10−10–1 × 10−5 M) did not differ significantly among the three groups (lower panel in Figure 1).
Figure 1.
Concentration–response curves for ACh-induced (upper panel) and SNP-induced (lower panel) relaxations of aortic strips obtained from age-matched controls, untreated diabetic rats and chronically bezafibrate-treated diabetic rats. The ordinate shows relaxation of aortic strips as a percentage of the contraction induced by an equieffective concentration of NA (5 × 10−8–3 × 10−7 M). Each data point represents the mean±s.e.m. of six to eight experiments; the s.e.m. is included only when it exceeds the dimension of the symbol used.
Expressions of the mRNAs for PPAR_α_, PPAR_γ_ and PPAR_δ_
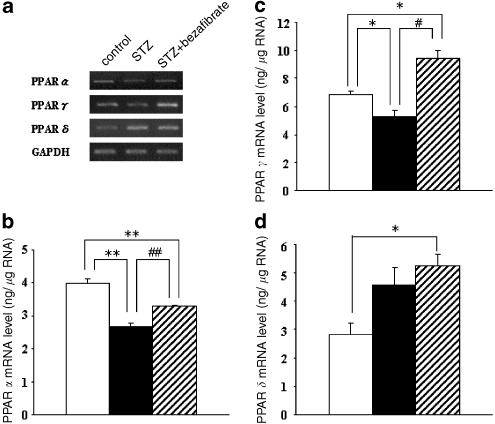
Using RT–PCR and competitive PCR method on the total RNA isolated from the aortae of age-matched controls, untreated diabetic and chronic bezafibrate-treated diabetic rats, we found that the expressions of PPAR_α_ and PPAR_γ_ mRNAs were each significantly lower in diabetic rats than in control rats (Figure 2). The lowered PPAR_α_ level was modestly but significantly raised in chronically bezafibrate-treated diabetic rats. The expression of the mRNA for PPAR_γ_ was markedly and significantly higher following chronic administration of bezafibrate than in untreated diabetic rats (Figure 2). The expression of the mRNA for PPAR_δ_ tended to be higher in the diabetic state than in the controls, although not significantly. However, it was significantly increased in bezafibrate-treated diabetic rats (as compared with the age-matched controls).
Figure 2.
RT–PCR assay of the expressions of the mRNAs for PPAR_α_, PPAR_γ_ and PPAR_δ_ in aortae from controls, STZ-induced and chronically bezafibrate-treated STZ-diabetic rats. (a) Expression of PPAR mRNAs assayed by RT–PCR. (b–d) Quantitative analysis of the expressions of PPARmRNAs (by scanning densitometry) (b, PPAR_α_; c, PPAR_γ_; d, PPAR_δ_). Control rats (open column); STZ-induced diabetic rats (closed column); bezafibrate-treated diabetic rats (hatched column). Each column represents the mean±s.e.m. of five determinations (PPAR/GAPDH). The RT–PCR assay was performed as described in Methods. Each total RNA preparation (2.0 μ_g) was reverse-transcribed and half of the cDNA product was PCR-amplified using the appropriate primers, 20 cycles (GAPDH) or 30 (PPAR_α), 28 (PPAR_γ_) or 26 (PPAR_δ_) cycles being employed. A portion of the PCR reaction product was electrophoresed on a 2.0% agarose gel containing ethidium bromide. *P<0.05, **P<0.01, vs control; #P<0.05, ##P<0.01 diabetic vs bezafibrate-treated diabetic (both, Dunnett's test).
Activity of SOD
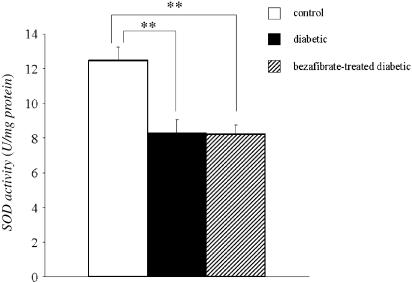
To investigate the possible mechanisms underlying the impaired ACh-induced relaxation seen in STZ-induced diabetic rats and its normalization in chronically bezafibrate-treated animals, we examined SOD activity following chronic bezafibrate treatment. As shown in Figure 3, the SOD activity in the aorta was not different between diabetic and bezafibrate-treated diabetic rats.
Figure 3.
Determination of SOD activity (by indirect inhibition) in STZ-induced diabetic and chronically bezafibrate-treated diabetic rats. Each column represents the mean±s.e.m. of eight experiments. **P<0.01 vs control.
Expression of the mRNA for NAD(P)H oxidase p22phox subunit
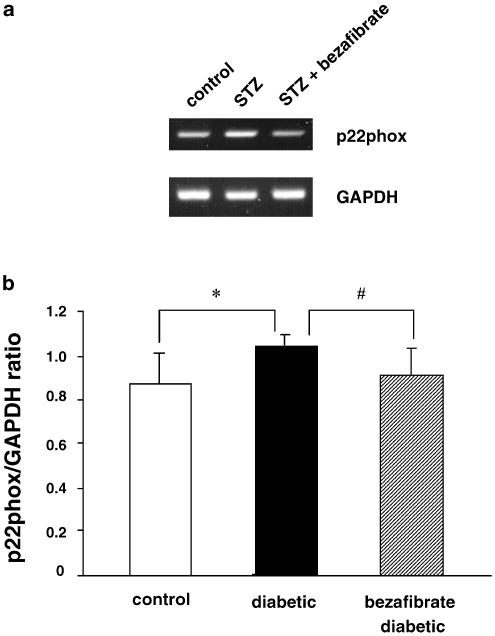
The expression of the mRNA for the NAD(P)H oxidase subunit p22phox in aortic segments was greater in diabetic rats than in the controls and this increase in expression was prevented to a significant extent by the chronic administration of bezafibrate (Figure 4).
Figure 4.
RT–PCR assay of expression of the mRNA for p22phox in aortae from controls, STZ-induced and chronically bezafibrate-treated STZ-diabetic rats. (a) Expression of p22phox mRNA assayed by RT–PCR. (b) Quantitative analysis of the expression of p22phox mRNA (by scanning densitometry). Control rats (_n_=6, open column); STZ-induced diabetic rats (_n_=6, closed column); bezafibrate-treated diabetic rats (_n_=5, hatched column). The RT–PCR assay was performed as described in Methods. Each total RNA preparation (2.0 _μ_g) was reverse-transcribed and half of the cDNA product was PCR-amplified using the appropriate primers, 20 cycles (GAPDH) or 28 cycles (p22phox) being employed. A portion of the PCR reaction product was electrophoresed on a 2.0% agarose gel containing ethidium bromide. Each column represents the mean±s.e.m. (p22phox/GAPDH). *P<0.05, diabetic vs control; #P<0.05, diabetic vs bezafibrate-treated diabetic (both, Dunnett's test).
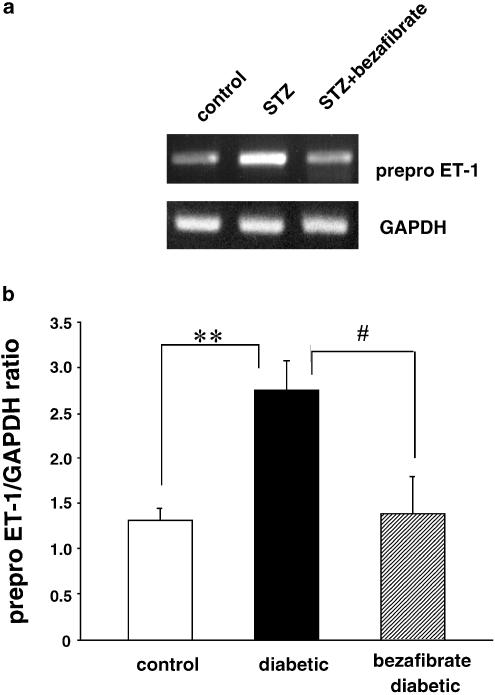
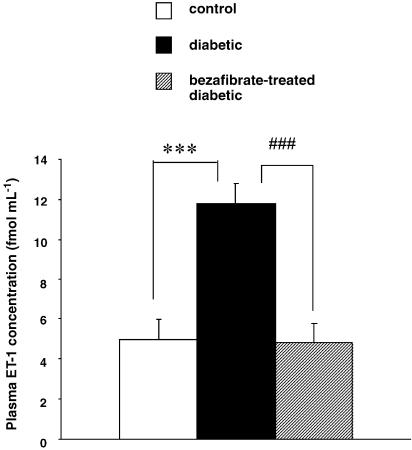
Expression of the mRNA for prepro ET-1 and plasma ET-1 levels
To help determine whether there might be a relationship between PPAR_α_ ET-1 and the increase in p22phox, we investigated the expression of prepro ET-1 (ppET-1) mRNA as well as plasma ET-1 levels after the chronic administration of bezafibrate (30 mg kg−1, p.o., 4 weeks). The expression of ppET-1 mRNA was significantly increased in STZ-diabetic rats and this increase was completely normalized by chronic bezafibrate (Figure 5). In line with these results, the plasma ET-1 level was greater in diabetic rats than in the controls and chronic administration of bezafibrate completely normalized this (Figure 6).
Figure 5.
RT–PCR assay of expression of the mRNA for ppET-1 in aortae from controls, STZ-induced and chronically bezafibrate-treated STZ-diabetic rats. (a) Expression of ppET-1 mRNA assayed by RT–PCR. (b) Quantitative analysis of the expression of ppET-1 mRNA (by scanning densitometry). Control rats (_n_=7, open column); STZ-induced diabetic rats (_n_=6, closed column); bezafibrate-treated diabetic rats (_n_=6, hatched column). The RT–PCR assay was performed as described in Methods. Each total RNA preparation (2.0 _μ_g) was reverse-transcribed and half of the cDNA product was PCR-amplified using the appropriate primers, 20 cycles (GAPDH) or 35 cycles (ppET-1) being employed. A portion of the PCR reaction product was electrophoresed on a 2.0% agarose gel containing ethidium bromide. Each column represents the mean±s.e.m. (ppET-1/GAPDH). **P<0.01, diabetic vs control; #P<0.05, diabetic vs bezafibrate-treated diabetic (both, Dunnett's test).
Figure 6.
Plasma levels of ET-1 in controls, STZ-induced diabetic and chronically bezafibrate-treated STZ-diabetic rats. Each column represents the mean±s.e.m. of six experiments (controls, open column; diabetic rats, closed column; bezafibrate-treated diabetic rats, hatched column). ***P<0.01, diabetic vs control; ###P<0.05, diabetic vs bezafibrate-treated diabetic (both, Dunnett's test).
Discussion
The main conclusion to be drawn from the present study is that in rats with established STZ-induced diabetes, chronic administration of bezafibrate improves endothelium-dependent relaxation (which is impaired in these animals) without changing the plasma levels of cholesterols and triglyceride. The mechanism underlying this improved endothelial function in bezafibrate-treated diabetic rats may be related to increased expressions of the mRNAs for PPAR_α_ and PPAR_γ_. This may lead to a decrease in the expression of prepro ET-1, and the consequent decrease in plasma ET-1 may cause a decline in the expression of NAD(P)H oxidase (p22phox), thereby resulting in a decrease in superoxide anion and a normalization of the endothelial dysfunction. This idea is discussed in more detail below.
An accumulating body of evidence suggests that the impairment of endothelium-dependent relaxation seen in diabetes and atherosclerosis may involve inactivation of NO by oxygen-derived free radicals (Meraji et al., 1987; Hattori et al., 1991; Pieper et al., 1992; 1996; Kamata & Kobayashi, 1996; Ooboshi et al., 1997; Pagano et al., 1998; Kobayashi & Kamata, 1999a;2001; Lund et al., 1999). Production of superoxide anion inactivates NO (Rubanyi & Vanhoutte, 1986; Marshall et al., 1988; Kobayashi & Kamata, 2001) and dismutation of free radicals has generally (Hattori et al., 1991; Kamata & Kobayashi, 1996; Pieper et al., 1996) but not always (Heygate et al., 1995) been found to improve impaired endothelium-dependent relaxation in experimental models of diabetes. We recently found that chronic administration of the endothelin antagonist J-104132 improves the endothelial dysfunction seen in the aorta in rats with established STZ-induced diabetes and we suggested that this effect of J-104132 may be due to a decrease in aortic superoxide anions via an inhibitory effect of this agent on the induction of NAD(P)H oxidase (Kanie & Kamata, 2002).
The presence of a high triglyceride level in the plasma is also thought to be an important factor in cardiovascular diseases. In our previous studies, fructose-fed animals (a model of triglyceride-rich insulin-resistant diabetes) were found to exhibit a markedly increased plasma triglyceride level as well as an impaired endothelium-dependent relaxation, suggesting that an increased plasma triglyceride level may be a risk factor for vascular disease (Kamata & Yamashita, 1999; Kamata et al., 2001). Furthermore, it has been reported that elevated triglyceride levels are associated with an increased risk of mortality in coronary heart disease (Haim et al., 1999). In the present study, the plasma triglyceride was not affected by the chronic administration of a low dose of bezafibrate, suggesting that the improvement effect on endothelium-dependent relaxation we saw with a relatively low dose of this drug is not related to a lowering of plasma LDL or triglyceride.
In the present study, we found that the expressions of the mRNAs for PPAR_α_ and PPAR_γ_ were decreased in STZ-diabetic rats and that the expression level was modestly but significantly restored by the chronic administration of bezafibrate. Since bezafibrate also increases the expression of the mRNA for Cu2+/Zn2+ SOD (Inoue et al., 1998a; 2001), we measured SOD activity in aortae from STZ-diabetic and bezafibrate-treated diabetic rats. Unexpectedly, SOD activity was found not to differ between these two groups. The reason may be that in the present study, we used a relatively low dose of bezafibrate.
Recent studies have underscored the importance of NAD(P)H-oxidase-derived reactive oxygen species in vascular biology. Many components of the leucocyte-NADPH-oxidase complex – including p22phox, p47phox, p67phox and gp91phox (or a related homologue) – have been identified in endothelial cells or vascular smooth muscle cells (Jones et al., 1996; Ushio-Fukai et al., 1996; Bayraktutan et al., 1998; Patterson et al., 1999; Gorlach et al., 2000). Reactive oxygen species from sources other than NADPH oxidase, such as xanthine oxidase (Adkins & Taylor, 1990) or cytochrome _P_-450 (Bysani et al., 1990), may also play a role in blood vessels. In the present study, we focused on NADPH oxidase as a source of reactive oxygen species because (i) the mRNA for the gp91phox NAD(P)H oxidase subunit is upregulated in the steady state in the aorta in STZ-induced diabetic rats (Hink et al., 2001), (ii) ET-1 increases the expression of gp91phox mRNA in human endothelial cells (Duerrschmidt et al., 2000) and (iii) the expression of the mRNA for the p22phox NADH/NADPH oxidase subunit is significantly increased in STZ-induced diabetic rats and this increase can be completely prevented by chronic administration of the endothelin-receptor antagonist J-104132 (Kanie & Kamata, 2002). This suggests that in STZ-induced diabetic rats, ET-1 may be directly involved in impairing endothelium-dependent relaxation via increased superoxide-anion production. In the present study, we provided evidence that the increase in the expression of the mRNA for the NAD(P)H oxidase subunit p22phox that is seen in the aorta in STZ-induced diabetic rats is also prevented by the chronic administration of bezafibrate. This effect may be involved in the restoration by bezafibrate of normal endothelial function in diabetic animals. However, these results raised a question: is the effect of bezafibrate on the expression of the mRNA for NAD(P)H oxidase subunit p22phox due to a direct effect on this expression or to an effect on ET-1 synthesis? To address this question, we examined the expression of the mRNA for ppET-1 as well as the plasma ET-1 level.
We have previously reported (i) that the plasma ET-1 concentration is increased in STZ-induced diabetic rats and that this increase may be due to an overexpression of the mRNA for ppET-1 (Makino & Kamata, 1998; Makino et al., 2001), (ii) that the overproduction of ET-1 seen in STZ-induced diabetes is a result of hyperglycaemia, not of an increase in LDL cholesterol or triglyceride (Makino & Kamata, 2000) and (iii) that the expression of the mRNA for the p22phox NADH/NADPH oxidase subunit is significantly increased in STZ-induced diabetic rats, an increase that was completely prevented by the chronic administration of J-104132. This suggested that ET-1 is involved in the synthesis of superoxide anion and that this effect may impair endothelium-dependent relaxation in diabetes (Kanie & Kamata, 2002). If so, an increase in ET-1 might play an important role in the endothelial dysfunction seen in diabetic states. If this is indeed the case, we should expect the chronic administration of bezafibrate to affect the expression of ET-1 and/or its plasma concentration.
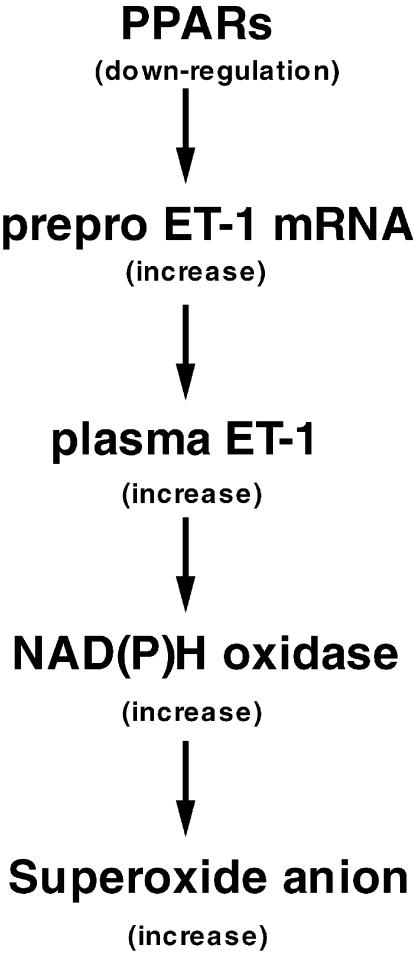
In fact, in the present study chronic administration of bezafibrate normalized both the expression of the mRNA for ppET-1 and the plasma concentration of ET-1. Furthermore, we also demonstrated that the expressions of the mRNAs for PPAR_α_ and PPAR_γ_ in aortic segments showed downregulation in STZ-induced diabetic rats. These results, which seem to be supported by previous findings showing that PPAR_α_ and PPAR_γ_ ligands regulate ppET-1 gene expression and transcription through activator protein-1 (AP-1) and nuclear factor-kappa B (NF-κ_B) (Delerive et al., 1999; Ohkita et al., 2002), suggest that downregulation of PPAR_α and PPAR_γ_ in the aorta may contribute to endothelial dysfunction in diabetes mellitus through the ET-1 system. Consideration of the evidence outlined above suggests that the improvement effect of bezafibrate on the endothelial dysfunction seen in STZ-induced diabetic rats may be explained by the following sequence of events (Figure 7): (i) in the diabetic aorta, PPAR_α_ and PPAR_γ_ are downregulated by several mechanisms and this may subsequently increase the expression of the mRNA for ppET-1 and thus increase the plasma ET-1 level; (ii) a chronically increased ET-1 level in the aorta may stimulate NAD(P)H oxidase, which produces superoxide anion in the vascular smooth muscle cell or endothelium; (iii) the expressions of PPAR_α_ and PPAR_γ_ are increased when bezafibrate is chronically administered to STZ-induced diabetic rats and AP-1 and/or NF-κ_B activity is suppressed by these increases in PPAR_α and PPAR_γ_; (iv) by this means, an increase in the expression of the mRNA for ppET-1 may be prevented, thereby resulting in a restoration of normal endothelial function in diabetes by bezafibrate. Although the cause of endothelial dysfunction seen in the diabetes is due to the existence of high levels of AGEs, lipid peroxidation and an alteration of the production of nitric oxide and prostanoids, increased plasma ET-1 may be a key role of endothelial dysfunction in the STZ-induced diabetic rats.
Figure 7.
Scheme showing the sequence described in the text between downregulation of PPAR_α_ and increased superoxide anion production (leading to endothelial dysfunction) in diabetes.
In the present study, bezafibrate induced an increased expression not only of PPAR_α_ but also of PPAR_γ_. However, we will need to perform further studies to determine the exact mechanisms underlying the bezafibrate-induced increases in the expressions of these mRNAs. Especially, the expression of the mRNA for PPAR_δ_ tended to be higher in the diabetic state than in the controls, although not significantly. It has been reported that PPAR_γ_ gene expression is under the control of PPAR_δ_ activated by fatty acid in 3T3C2 fibroblasts (Bastie et al., 1999). Furthermore, it has also been reported that troglitazone, a PPAR_γ_ agonist, inhibits ET-1 mRNA expression and secretion in bovine vascular endothelial cells possibely through activation of PPAR_γ_ (Satoh et al., 1999). These reports strongly support our hypothesis that downregulation of PPAR_γ_ may be responsible for increase in ET-1 production. Further investigations are required on these points.
In conclusion, we found that chronic administration of a relatively low dose of bezafibrate, which did not change the levels of plasma lipids (including total cholesterol, LDL cholesterol, HDL cholesterol and triglyceride), exerts an improvement effect on the endothelial dysfunction seen in the aorta in rats with established STZ-induced diabetes. This effect of bezafibrate may result from a reduction in the NAD(P)H oxidase p22phox subunit through an inhibitory effect on ET-1 synthesis. In addition, we demonstrated a downregulation of PPAR_α_ and PPAR_γ_ in the aorta and this may be responsible for the endothelial dysfunction seen in diabetic states.
Acknowledgments
This work was supported in part by the Ministry of Education, Science, Sports, and Culture, Japan, and the Promotion and Mutual Aid Cooperation for Private Schools of Japan.
Abbreviations
ACh
acetylcholine
ET-1
endothelin-1
GAPDH
glyceraldehydes-3-phosphate dehydrogenase
HDL
high-density lipoprotein
KHS
Krebs–Henseleit solution
LDL
low-density lipoprotein
NA
noradrenaline
NAD(P)H
nicotinamide adenine dinucleotide (phosphate)
PPAR_α_
peroxisome proliferator-activated receptor α
ppET-1
prepro ET-1
RT–PCR
reverse transcription–polymerase chain reaction
SNP
sodium nitroprusside
SOD
superoxide dismutase
STZ
streptozotocin
VLDL
very low-density lipoprotein
References
- ADKINS W.K., TAYLOR A.E. Role of xanthine oxidase and neutrophils in ischemia–reperfusion injury in rabbit lung. J. Appl. Physiol. 1990;69:2012–2018. doi: 10.1152/jappl.1990.69.6.2012. [DOI] [PubMed] [Google Scholar]
- BASTIE C., HOLST D., GAILLARD D., JEHL-PIETRI C., GRIMALDI P.A. Expression of peroxisome proliferator-activated receptor PPARδ promotes induction of PPARγ and adipocyte differentiation in 3T3C2 fibroblasts. J. Biol. Chem. 1999;274:21920–21926. doi: 10.1074/jbc.274.31.21920. [DOI] [PubMed] [Google Scholar]
- BAYRAKTUTAN U., DRAPER N., LANG D., SHAH A.M. Expression of functional neutrophil-type NADPH oxidase in cultured rat coronary microvascular endothelial cells. Cardiovasc. Res. 1998;38:256–262. doi: 10.1016/s0008-6363(98)00003-0. [DOI] [PubMed] [Google Scholar]
- BISHOP-BAILEY D. Peroxisome proliferator-activated receptors in the cardiovascular system. Br. J. Pharmacol. 2000;129:823–864. doi: 10.1038/sj.bjp.0703149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUCHAN K.W., HASSALL D.G. PPAR agonists as direct modulators of the vessel wall in cardiovascular disease. Med. Res. Rev. 2000;20:350–366. doi: 10.1002/1098-1128(200009)20:5<350::aid-med2>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- BYSANI G.K., KENNEDY T.P., KY N, RAO N.V., BLAZE C.A., HOIDAL J.R. Role of cytochrome P-450 in reperfusion injury of the rabbit lung. J. Clin. Invest. 1990;86:1434–1441. doi: 10.1172/JCI114859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHOMCZYNSKI P., SACCHI N. Single-step method of RNA isolation by acid guanidinium thiocyanate–phenol–chloroform extraction. Anal. Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- DELERIVE P., MARTIN-NIZARD F., CHINETTI G., TROTTEIN F., FRUCHART J.C., NAJIB J., DURIEZ P., STAELS B. Peroxisome proliferator-activated receptor activators inhibit thrombin-induced endothelin-1 production in human vascular endothelial cells by inhibiting the activator protein-1 signaling pathway. Circ. Res. 1999;85:394–402. doi: 10.1161/01.res.85.5.394. [DOI] [PubMed] [Google Scholar]
- DE VRIESE A.S., VERBEUREN T.J., DE VOORDE J.V., LAMEIRE N.H., VANHOUTTE P.M. Endothelial dysfunction in diabetes. Br. J. Pharmacol. 2000;130:963–974. doi: 10.1038/sj.bjp.0703393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUBOWSKI K.M. An O-toluidine method for body-fluid glucose determination. Clin. Chem. 1962;8:215–235. [PubMed] [Google Scholar]
- DUERRSCHMIDT N., WIPPICH N., GOETTSH W., BROEMME H.J., MORAWIETZ H. Endothelin-1 induces NAD(P)H oxidase in human endothelial cells. Biochem. Biophys. Res. Commun. 2000;269:713–717. doi: 10.1006/bbrc.2000.2354. [DOI] [PubMed] [Google Scholar]
- FRIEDEWALD W.T., LEVY R.I., FREDRICKSON D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- FRUCHART J.C., DURIEZ P., STAELS B. Peroxisome proliferator-activated receptor-α activators regulate genes governing lipoprotein metabolism, vascular inflammation and atherosclerosis. Curr. Opin. Lipidol. 1999;10:245–257. doi: 10.1097/00041433-199906000-00007. [DOI] [PubMed] [Google Scholar]
- GORLACH A., BRANDES R.P., NGUYEN K., AMIDI M., DEHGHANI F., BUSSE R. A gp91phox containing NADPH oxidase selectively expressed in endothelial cells is a major source of oxygen radical generation in the arterial wall. Circ. Res. 2000;87:26–32. doi: 10.1161/01.res.87.1.26. [DOI] [PubMed] [Google Scholar]
- HAIM M., BENDERLY M., BRUNNER D., BEHAR S., GRAFF E., REICHER-REISS H., GOLDBOURT U. Elevated serum triglyceride levels and long-term mortality in patients with coronary heart disease: the bezafibrate infarction prevention (BIP) registry. Circulation. 1999;100:475–482. doi: 10.1161/01.cir.100.5.475. [DOI] [PubMed] [Google Scholar]
- HATTORI Y., KAWASAKI H., ABE K., KANNO M. Superoxide dismutase recovers altered endothelium-dependent relaxation in diabetic rat aorta. Am. J. Physiol. 1991;261:H1086–H1094. doi: 10.1152/ajpheart.1991.261.4.H1086. [DOI] [PubMed] [Google Scholar]
- HEYGATE K.M., LAWRENCE I.G., BENNETT M.A., THURSTON H. Impaired endothelium-dependent relaxation in isolated resistance arteries of spontaneously diabetic rats. Br. J. Pharmacol. 1995;116:3251–3259. doi: 10.1111/j.1476-5381.1995.tb15132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HINK U., LI H., MOLLNAU H., OELZE M., MATHEIS E., HARTMANN M., SKATCHKOV M., THAISS F., STAHL R.A., WARNHOLTZ A., MEINERTZ T., GRIENDLING K., HARRISON D.G., FORSTERMANN U., MUNZEL T. Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circ. Res. 2001;88:e14–e22. doi: 10.1161/01.res.88.2.e14. [DOI] [PubMed] [Google Scholar]
- INOUE I., GOTO S., MATSUNAGA T., NAKAJIMA T., AWATA T., HOKARI S., KOMODA T., KATAYAMA S. The ligands/activators for peroxisome proliferator-activated receptor α (PPARα) and PPARγ increase Cu2+, Zn2+-superoxide dismutase and decrease p22phox message expressions in primary endothelial cells. Metabolism. 2001;50:3–11. doi: 10.1053/meta.2001.19415. [DOI] [PubMed] [Google Scholar]
- INOUE I., NOJI S., AWATA T., TAKAHASHI K., NAKAJIMA T., SONODA M., KOMADA T., KATAYAMA S. Bezafibrate has an antioxidant effect: peroxisome proliferator-activated receptor α is associated with Cu2+, Zn2+-superoxide dismutase in the liver. Life Sci. 1998a;63:135–144. doi: 10.1016/s0024-3205(98)00249-5. [DOI] [PubMed] [Google Scholar]
- INOUE I., SHINO K., NOJI S., AWATA T., KATAYAMA S. Expression of peroxisome proliferator-activated receptor α (PPARα) in primary cultures of human vascular endothelial cells. Biochem. Biophys. Res. Commun. 1998b;246:370–374. doi: 10.1006/bbrc.1998.8622. [DOI] [PubMed] [Google Scholar]
- JONES S.A., O'DONNELL V.B., WOOD J.D., BROUGHTON J.P., HUGHES E.J. Expression of phagocyte NADPH oxidase components in human endothelial cells. Am. J. Physiol. 1996;271:H1626–H1634. doi: 10.1152/ajpheart.1996.271.4.H1626. [DOI] [PubMed] [Google Scholar]
- KAMATA K., KANIE N., INOSE A. Mechanisms underlying attenuated contractile response of aortic rings to noradrenaline in fructose-fed mice. Eur. Pharmacol. 2001;428:241–249. doi: 10.1016/s0014-2999(01)01262-6. [DOI] [PubMed] [Google Scholar]
- KAMATA K, KOBAYASHI T. Changes in superoxide dismutase mRNA expression by streptozotocin-induced diabetes. Br. J. Pharmacol. 1996;119:583–589. doi: 10.1111/j.1476-5381.1996.tb15712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAMATA K., MIYATA N., KASUYA Y. Impairment of endothelium-dependent relaxation and changes in levels of cyclic GMP in aorta from streptozotocin-induced diabetic rats. Br. J. Pharmacol. 1989;97:614–618. doi: 10.1111/j.1476-5381.1989.tb11993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAMATA K., SUGIURA M., KOJIMA S., KASUYA Y. Preservation of endothelium-dependent relaxation in cholesterol-fed and streptozotocin-induced diabetic mice by the chronic administration of cholestyramine. Br. J. Pharmacol. 1996;118:385–391. doi: 10.1111/j.1476-5381.1996.tb15414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAMATA K., YAMASHITA K. Insulin resistance and impaired endothelium-dependent renal vasodilation in fructose-fed hypertensive rats. Res. Commun. Mol. Pathol. Pharmacol. 1999;103:195–210. [PubMed] [Google Scholar]
- KANIE N, KAMATA K. Effect of chronic administration of the novel endothelin antagonist J-104132 on endothelial dysfunction in streptozotocin-induced diabetic rat. Br. J. Pharmacol. 2002;135:1935–1942. doi: 10.1038/sj.bjp.0704659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOBAYASHI T., KAMATA K. Effect of insulin treatment on smooth muscle contractility and endothelium-dependent relaxation in rat aortae from established STZ-induced diabetes. Br. J. Pharmacol. 1999a;127:835–842. doi: 10.1038/sj.bjp.0702554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOBAYASHI T., KAMATA K. Relationship among cholesterol, superoxide anion and endothelium-dependent relaxation in diabetic rats. Eur. J. Pharmacol. 1999b;367:213–222. doi: 10.1016/s0014-2999(98)00971-6. [DOI] [PubMed] [Google Scholar]
- KOBAYASHI T., KAMATA K. Effect of chronic insulin treatment on NO production and endothelium-dependent relaxation in aortas from established STZ-induced diabetic rats. Atherosclerosis. 2001;155:313–320. doi: 10.1016/s0021-9150(00)00583-9. [DOI] [PubMed] [Google Scholar]
- KOBAYASHI T., MATSUMOTO T., KAMATA K. Mechanisms underlying the chronic pravastatin treatment-induced improvement in the impaired endothelium-dependent aortic relaxation seen in streptozotocin-induced diabetic rats. Br. J. Pharmacol. 2000;131:231–238. doi: 10.1038/sj.bjp.0703572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOLTAI M.Z., HADHAZY P., POSA I., KOCSIS E., WINKLER G., ROSEN P., POGATSA G. Characteristics of coronary endothelial dysfunction in experimental diabetes. Cardiovasc. Res. 1997;34:157–163. doi: 10.1016/s0008-6363(97)00050-3. [DOI] [PubMed] [Google Scholar]
- KUGIYAMA K., KERNS S.A., MORRISETT J.D., ROBERTS R., HENRY P.D. Impairment of endothelium-dependent arterial relaxation by lysolecithin in codified low-density lipoprotein. Nature. 1990;344:160–162. doi: 10.1038/344160a0. [DOI] [PubMed] [Google Scholar]
- LOVEN D.P., SCHEDL H.P., OBERLEY L.W., WILSON H.D., BRUCH L., NIEHAUS C.L. Superoxide dismutase activity in the intestine of the streptozotocin-diabetic rat. Endocrinology. 1982;111:737–742. doi: 10.1210/endo-111-3-737. [DOI] [PubMed] [Google Scholar]
- LUND D.D., FARACI F.M., OOBOSHI H., DAVIDSON B.L., HEISTAD D.D. Adenovirus-mediated gene transfer is augmented in basilar and carotid arteries of heritable hyperlipidemic rabbits. Stroke. 1999;30:120–125. doi: 10.1161/01.str.30.1.120. [DOI] [PubMed] [Google Scholar]
- MAKINO A., KAMATA K. Elevated plasma endothelin-1 level in streptozotocin-induced diabetic rats and responsiveness of the mesenteric arterial bed to endothelin-1. Br. J. Pharmacol. 1998;123:1065–1072. doi: 10.1038/sj.bjp.0701704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAKINO A, KAMATA K. Time-course changes in plasma endothelin-1 and its effects on the mesenteric arterial bed in streptozotocin-induced diabetic rats. Diabetes Obes. Metab. 2000;2:47–55. doi: 10.1046/j.1463-1326.2000.00024.x. [DOI] [PubMed] [Google Scholar]
- MAKINO A., ODA S., KAMATA K. Mechanisms underlying increased release of endothelin-1 from aorta in diabetic rats. Peptide. 2001;22:639–645. doi: 10.1016/s0196-9781(01)00374-6. [DOI] [PubMed] [Google Scholar]
- MANGELSDORF D.J., EVANS R.M. The RXR heterodimers and orphan receptors. Cell. 1995;83:841–850. doi: 10.1016/0092-8674(95)90200-7. [DOI] [PubMed] [Google Scholar]
- MANGELSDORF D.J., ONG E.S., DYCK J.A., EVANS R.M. Nuclear receptor that identifies a novel retinoic acid response pathway. Nature. 1990;345:224–229. doi: 10.1038/345224a0. [DOI] [PubMed] [Google Scholar]
- MANTHA S.V., PRASAD M., KALRA J., PRASAD K. Antioxidant enzymes in hypercholesterolemia and effects of vitamin E in rabbits. Atherosclerosis. 1993;101:135–144. doi: 10.1016/0021-9150(93)90110-g. [DOI] [PubMed] [Google Scholar]
- MARSHALL J.J., WEI E.P., KONTOS H.A. Independent blockade of cerebral vasodilation from acetylcholine and nitric oxide. Am. J. Physiol. 1988;255:H847–H854. doi: 10.1152/ajpheart.1988.255.4.H847. [DOI] [PubMed] [Google Scholar]
- MERAJI S., JAYAKODY L., SENARATNE M.P., THOMSON A.B., KAPPAGODA T. Endothelium-dependent relaxation in aorta of BB rat. Diabetes. 1987;36:978–981. doi: 10.2337/diab.36.8.978. [DOI] [PubMed] [Google Scholar]
- NITENBERG A., VALENSI P., SACHS R., DALI M., APTECAR E., ATTALI J. Impairment of coronary vascular reserve and ACh-induced coronary vasodilation in diabetic patients with angiographically normal coronary arteries and normal left ventricular systolic function. Diabetes. 1993;42:1017–1025. doi: 10.2337/diab.42.7.1017. [DOI] [PubMed] [Google Scholar]
- OHKITA M., TAKANO M., SHIOTA Y., NOJIRI R., SUGII M., MATSUMURA Y. A nuclear-κB inhibitor BAY 11-7082 suppresses endothelin-1 production in cultured vascular endothelial cells. Jpn. J. Pharmacol. 2002;89:81–84. doi: 10.1254/jjp.89.81. [DOI] [PubMed] [Google Scholar]
- OOBOSHI H., RIOS C.D., CHU Y., CHRISTENSEN S.D., FARACI F.M., DAVIDSON B.L., HEISTAD D.D. Augmented adenovirus-mediated gene transfer in atherosclerotic vessels. Arterioscler. Thromb. Vasc. Biol. 1997;17:1786–1792. doi: 10.1161/01.atv.17.9.1786. [DOI] [PubMed] [Google Scholar]
- PAGANO P.J., GRISWOLD M.C., RAVEL D., COHEN R.A. Vascular action of the hypoglycaemic agent gliclazide in diabetic rabbits. Diabetologia. 1998;41:9–15. doi: 10.1007/s001250050860. [DOI] [PubMed] [Google Scholar]
- PATTERSON C., RUEF J., MADAMANCHI N.R., BARRY-LANE P., HU Z., HORAIST C., BALLINGER C.A., BRASIER A.R., BODE C., RUNGE M.S. Stimulation of a vascular smooth muscle cell NAD(P)H oxidase by thrombin. Evidence that p47(phox) may participate in forming this oxidase in vitro and in vivo. J. Biol. Chem. 1999;274:19814–19822. doi: 10.1074/jbc.274.28.19814. [DOI] [PubMed] [Google Scholar]
- PIEPER G.M. Review of alterations in endothelial nitric oxide production in diabetes: protective role of arginine on endothelial dysfunction. Hypertension. 1998;31:1047–1060. doi: 10.1161/01.hyp.31.5.1047. [DOI] [PubMed] [Google Scholar]
- PIEPER G.M., MEI D.A., LANGENSTROER P., O'ROURKE S.T. Bioassay of endothelium-derived relaxing factor in diabetic rat aorta. Am. J. Physiol. 1992;263:H676–H680. doi: 10.1152/ajpheart.1992.263.3.H676. [DOI] [PubMed] [Google Scholar]
- PIEPER G.M., PELTIER B.A. Amelioration by L-arginine of a dysfunctional arginine/nitric oxide pathway in diabetic endothelium. J. Cardiovasc. Pharmacol. 1995;25:397–403. doi: 10.1097/00005344-199503000-00008. [DOI] [PubMed] [Google Scholar]
- PIEPER G.M., SIEBENEICH W., ROZA A.M., JORDAN M., ADAMS M.B. Chronic treatment in vivo with dimethylthiourea, a hydroxyl radical scavenger, prevents diabetes-induced endothelial dysfunction. J. Cardiovasc. Pharmacol. 1996;28:741–745. doi: 10.1097/00005344-199612000-00002. [DOI] [PubMed] [Google Scholar]
- POSTON L., TAYLOR P.D. Glaxo/MRS young investigator prize. Endothelium-mediated vascular function in insulin-dependent diabetes mellitus. Clin. Sci. 1995;88:245–255. doi: 10.1042/cs0880245. [DOI] [PubMed] [Google Scholar]
- RUBANYI G.M., VANHOUTTE P.M. Oxygen-derived free radicals, endothelium and responsiveness of vascular smooth muscle. Am. J. Pharmacol. 1986;250:H815–H821. doi: 10.1152/ajpheart.1986.250.5.H815. [DOI] [PubMed] [Google Scholar]
- SATOH H., TSUKAMOTO K., HASHIMOTO Y., HASHIMOTO N., TOGO M., HARA M., MAEKAWA H., ISOO N., KIMURA S., WATANABE T. Thiazolidinediones suppress endothelin-1 secretion from bovine vascular endothelial cells: a new possible role of PPARγ on vascular endothelial function. Biochim. Biophys. Res. Commun. 1999;254:757–763. doi: 10.1006/bbrc.1998.0126. [DOI] [PubMed] [Google Scholar]
- SIMON B.C., CUNNINGHAM L.D., COHEN R.A. Oxidized low-density lipoproteins cause contraction and inhibit endothelium-dependent relaxation in the pig coronary artery. J. Clin. Invest. 1990;86:75–79. doi: 10.1172/JCI114718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPAYD R.W., BRUSCHI B., BURDICK B.A., DAPPEN G.M., EIKENBERRY J.N., ESDERS T.W., FIGUERAS J., GOODHUE C.T., LAROSSA D.D., NELSON R.W., RAND R.N., WU T.W. Multilayer film elements for clinical analysis: applications to representative chemical determinations. Clin. Chem. 1978;24:1343–1350. [PubMed] [Google Scholar]
- TAYLOR P.D., MCCARTHY A.L., THOMAS C.R., POSTON L. Endothelium-dependent relaxation and noradrenaline sensitivity in mesenteric resistance arteries of streptozotocin-induced diabetic rats. Br. J. Pharmacol. 1992;107:393–399. doi: 10.1111/j.1476-5381.1992.tb12757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TESFAMARIAM B., COHEN R.A. Free radicals mediate endothelial cell dysfunction caused by elevated glucose. Am. J. Physiol. 1992;263:H321–H326. doi: 10.1152/ajpheart.1992.263.2.H321. [DOI] [PubMed] [Google Scholar]
- USHIO-FUKAI M., ZAFARI A.M., FUKUI T., ISHIZAKA N. p22phox is a critical component of the superoxide-generating NADH/NADPH oxidase system and regulates angiotensin II-induced hypertrophy in vascular smooth muscle cells. J. Biol. Chem. 1996;271:23317–23321. doi: 10.1074/jbc.271.38.23317. [DOI] [PubMed] [Google Scholar]
- WILLIAMS S.B., CUSCO J.A., RODDY M., JOHNSTONE M.T., CREAGER M.A. Impaired nitric oxide-mediated vasodilation in patients with non-insulin-dependent diabetes mellitus. J. Am. Coll. Cardiol. 1996;27:567–574. doi: 10.1016/0735-1097(95)00522-6. [DOI] [PubMed] [Google Scholar]