Inflammation and Atrophy Precede Prostatic Neoplasia in a PhIP-Induced Rat Model (original) (raw)
Abstract
2-Amino-1-methyl-6-phenylimidazo(4,5-b)pyridine (PhIP) has been implicated as a major mutagenic heterocyclic amine in the human diet and is carcinogenic in the rat prostate. To validate PhIP-induced rat prostatic neoplasia as a model of human prostate cancer progression, we sought to study the earliest histologic and morphologic changes in the prostate and to follow progressive changes over time. We fed sixty-seven 5-week-old male Fischer F344 rats with PhIP (400 ppm) or control diets for 20 weeks, and then sacrificed animals for histomorphologic examination at the ages of 25, 45, and 65 weeks. Animals treated with PhIP showed significantly more inflammation (P = .002, > .001, and .016 for 25, 45, and 65 weeks, respectively) and atrophy (P = .003, > .001, and .006 for 25, 45, and 65 weeks, respectively) in their prostate glands relative to controls. Prostatic intraepithelial neoplasia (PIN) occurred only in PhIP-treated rats. PIN lesions arose in areas of glandular atrophy, most often in the ventral prostate. Atypical cells in areas of atrophy show loss of glutathione _S_-transferase π immunostaining preceding the development of PIN. None of the animals in this study developed invasive carcinomas, differing from those in previous reports. Overall, these findings suggest that the pathogenesis of prostatic neoplasia in the PhIP-treated rat prostate proceeds from inflammation to postinflammatory proliferative atrophy to PIN.
Keywords: Prostate cancer, rat model, PhIP carcinogen, prostate inflammation, prostatic intraepithelial neoplasia
Introduction
Prostate cancer is the second leading cause of cancer deaths in men in the United States. Specific etiologies remain unknown, but there have been associations with dietary factors such as the consumption of red meat and saturated fats [1]. Interestingly, prostate cancer rates are extremely low in Asia, particularly in China [2]. Although it is not known whether inherited or environmental factors play primary roles, several associations have been made. Emigrants from eastern countries to the west approach western rates for prostate cancer within one generation, potentially correlating with the adoption of a “western diet” [3] Autopsy studies of American and Chinese men show a distinct difference in prostatitis, with extremely high rates in Americans and essentially no inflammation in Chinese [4–6]. Several experimental associations between prostatic inflammation and prostate cancer risk have been made recently [7,8].
2-Amino-1-methyl-6-phenylimidazo(4,5-b)pyridine (PhIP) has been implicated as a major mutagenic heterocyclic amine in the human diet [9]. We have shown that PhIP forms DNA adducts in prostate epithelial cells and induces mutations in the rat prostate, mammary gland, and intestines when administered experimentally [10].
It has been reported that roughly 50% of Fischer F344 male rats treated with PhIP at 1 year of age develop invasive prostate carcinoma [11], and studies note a high incidence of “atypical hyperplasia.” Histologic images of PhIP-induced prostate cancers are difficult to compare to histomorphologic images of human prostate carcinoma. To characterize the progression of prostatic neoplasia and to compare the morphology and biology of PhIP-induced prostate cancers to those of human diseases, we sought to study the early and intermediate time points in these animals. We also sought to study control animals of the same ages and housed under identical conditions.
Methods
Animal Treatment
Male Fischer F344 rats (Simonsen Laboratories, Gilroy, CA) were housed in an Association for Assessment of Laboratory Animal Care (AALAC)-accredited Lawrence Livermore National Laboratory Animal Care and Use Facility and were treated according to the “Guide for the Care and Use of Laboratory Animals” (Laboratory Animal Resources, National Research Council), with the approval of the Institutional Animal Use and Care Committee. Forty rats at 5 weeks of age were fed PhIP (Toronto Research Chemicals, Ontario, Canada) in the diet for 20 weeks. Animals were weighed weekly throughout the study. A PhIP dosing schedule, as described in Shirai et al. [12], was chosen because it is the minimum dose shown to cause prostate carcinoma. For the first 13 weeks (of a planned 20 weeks), PhIP was fed to the animals at 400 ppm in a certified basal diet (18% protein rodent diet; Harlan Teklad Global, Madison, WI). However, due to weight loss and poor health in the animals, PhIP content was reduced to 200 ppm for the final 7 weeks. Forty controls received a basal diet without the addition of PhIP. At 25 weeks, 10 PhIP-dosed and 10 control animals were euthanized. Their urogenital and other grossly abnormal tissues were isolated and fixed in 10% neutral-buffered formalin for histologic examination. The remaining animals were all returned to a regular basal diet, with euthanasia and histologic examination at 40 and 60 weeks after treatment (45 and 65 weeks of age). Historically, it has been reported that over 50% of animals have invasive tumors at 60 weeks [12]. The purpose of using intermediate time points, as well as careful examination of nontumorous rats at 65 weeks, was to characterize precancerous histomorphology.
Histology
Necropsy tissues, including those of the prostate, urethra, bladder, seminal vesicles, testes, and bulbourethral glands, were collected. Additional tissues were collected where possible, including tissues from the intestines, lungs, liver, kidneys, and pancreas. All tissues were fixed overnight in neutral-buffered formalin and then transferred to 70% ethanol before processing. Tissues were paraffin-embedded using standard histology protocols and sectioned at 4 mm. The resulting sections were stained with hematoxylin and eosin (H&E) and examined under light microscopy. Tissue blocks containing the majority of prostate tissues, particularly of the ventral, dorsal, and lateral glands, were identified for serial step sectioning. Additional levels through these blocks were mounted on plus-coated slides (Fischer), with five alternating levels stained with H&E for morphologic analysis.
Immunohistochemistry
Immunohistochemistry was performed on 4-µm sections mounted on plus-coated slides. For antigen retrieval, slides were incubated in 10 mM citrate at 100°C. Primary antibodies were incubated overnight at room temperature in phosphatebuffered saline. We used the following primary antibodies: rabbit monoclonal anti-Ki-67 (1:1000; LabVision, Fremont, CA), rabbit polyclonal serum anti-glutathione _S_-transferase π (GSTπ) (1:400, 354212; Calbiochem, San Diego, CA), and mouse monoclonal anti-Cox2 (1:250, 236004; Calbiochem). Secondary antibodies and detections were performed using the biotinylated secondary antibody and the EnVision HRP kit (DAKO, Carpenteria, CA), following the manufacturer's instructions. Histologic images were captured on a Zeiss Axioskop light microscope and photographed with the Zeiss AxioCam digital camera (Zeiss, Thornwood, NY).
Quantitation
The serial step sections of the ventral, dorsal, and lateral glands, and sections of the anterior prostate gland were examined by investigators blinded to the treatment group. In each, the total volume of any gland presenting with inflammation, atrophy, atypia, or prostatic intraepithelial neoplasia (PIN) was estimated by adding and averaging the slide surface areas with these characteristics. In general, the percentage of the affected part of the gland was given to the nearest 5%. Inflammation was scored if there was luminal abscess or if there was evident periepithelial or intraepithelial infiltration of any inflammatory cells (mononuclear or segmented neutrophils). Often, this was accompanied by an increased surrounding stromal thickness. Atrophy was defined as a flattened epithelial cell lining, with individual cells displaying a lateral width greater than basal-to-apical height. Areas of atypia were defined as foci of cells with increased nuclear size and hyperchromasia in the absence of inflammation. There were areas of atypia that were directly associated with inflammation, but these were not counted in quantitative analyses. The goal was to quantitate potential preneoplastic foci, rather than epithelial reaction to inflammation, which was separately quantitated. Areas of PIN were defined as multiple cell layers with loss of cellular polarity and with evidence of focality and proliferation. Areas of proliferation remaining in a single cell layer were not defined as PIN.
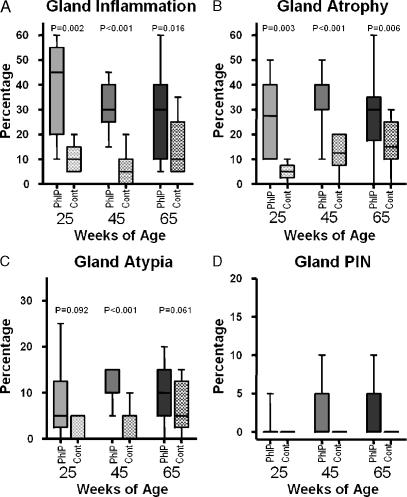
Statistical analysis was performed using Graph Pad Prism software, version 4.0.3 (Graph Pad, San Diego, CA). Unpaired nonparametric _t_-test was applied to each data set for each of the quantitated characteristics. The resulting P values are recorded above each pair of compared data sets. The data are represented in the 25th to 75th percentiles, and medians were plotted as shown in Figure 1.
Figure 1.
Quantified changes in PhIP and control prostate glands. Overview of histologic changes in PhIP-treated rats (solid bars) and control rats (hatched bars). The median (horizontal line), 25th to 75th percentiles (column boxes), and range (tail bars) of the percentages of prostate glands involved are plotted for the groups at each time point (25, 45, and 65 weeks of age). An unpaired nonparametric t-test was applied to the groups at each time point, and the resulting P values are shown above the data bars. The t-test could not be applied to the PIN data because all of the values in many of the columns were zero. The percentages of the glands showing inflammation, atrophy, atypia, and PIN are shown in (A), (B), (C), and (D), respectively.
Results
Animal Treatment
During the study, PhIP-dosed rats consistently gained less weight than control animals. After 13 weeks of dosing at 400 ppm, the rats began to lose weight. Consequently, PhIP dose was reduced to 200 ppm for the remaining 7 weeks of dosing. After PhIP treatment had been completed, the growth rate of PhIP-treated animals became the same as that of controls, but these rats remained smaller than controls. At week 20, at the end of treatment, the mean body weight of PhIP-dosed animals was 191(± 30) g compared to 413(± 25) g in controls. By week 40, treated rats had gained slightly more than controls, with a mean body weight of 287(± 42) g compared to 479(± 25) g in controls. By week 60, the PhIP-dosed rats appeared to have the same mean growth rate as that of untreated controls, although they did not “catch up” or grow at an accelerated rate [336(± 39) g for treated rats vs 525(± 37) g for controls]. In addition, 10 PhIP-dosed animals and 2 control animals died or were euthanized due to deteriorating health before the termination of the study.
Prostatic Histomorphology
Inflammation in the PhIP-treated rat prostate was the earliest and most obvious change, compared that in the control group (Figure 1_A_). In one rat that died during PhIP treatment, the prostatic epithelium was discontinuous, with areas of epithelial loss or denudation. These areas were accompanied by inflammation and luminal debris. Inflammation was found in all prostatic glands, but was least prominent in the anterior or coagulating gland. Inflammation persisted after the discontinuation of PhIP treatment, and epithelial layers at all scheduled time points were intact, even in areas of luminal microabcess. Many areas of inflammation were accompanied by reactive stromal proliferation, resulting in a distinct thickening of the thin muscular layer surrounding individual glands. These proliferations did not appear to overgrow the reactive process or to become neoplastic, as has been reported in some mouse models of prostate cancer [13]. Inflammation was seen focally in many of the control animals, but involved fewer glands and was generally mildest. Whereas the experimental animals had dense exudative luminal content, the control animals had only mild exudates with scattered luminal neutrophils. Inflammation was most persistent in the dorsal and lateral glands, and was second most persistent in the ventral prostate. Over the time course from 25 to 65 weeks, inflammation decreased as atrophy increased. This was predominantly due to decreased inflammation in the ventral prostate and the corresponding expanded zones of atrophy.
Large areas of glandular atrophy, particularly those affecting the ventral prostate, were seen in all treated animals. Nontreated animals also appeared to be prone to glandular atrophy, particularly in the ventral prostate, but with less involved areas of the prostate (Figure 1_B_). Interestingly, only in treated animals was proliferation seen to be interspersed within areas of atrophy, and PIN lesions seen in animals scheduled for late sacrifice (45 and 65 weeks) occurred in areas of atrophic ventral prostate in the majority of cases (Figure 2). A single animal in the 25-week group developed a PIN lesion. Eight (25%) PhIP-treated animals examined by histopathology had foci meeting criteria for PIN (n = 32).
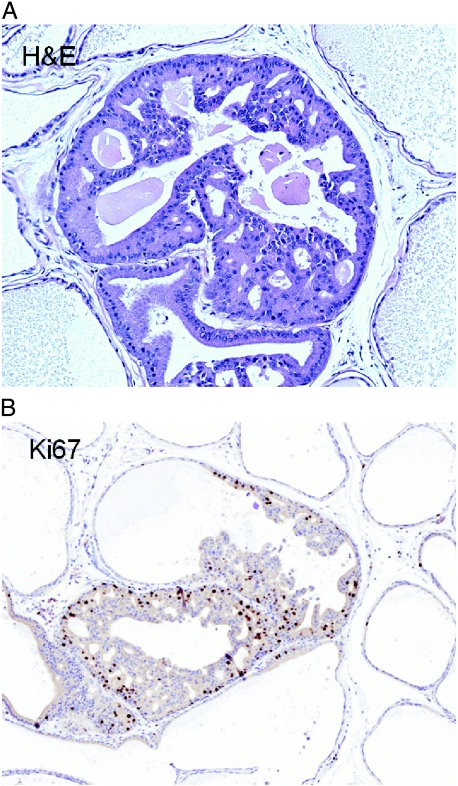
Figure 2.
PIN arises from atrophy in the PhIP-treated rat prostate. H&E stain of the ventral prostate at 65 weeks (A). Within the atrophic glands, occasional areas of proliferation with loss of polarity and cells forming cribriform spaces and Roman bridges are seen. The high proliferation of these areas is confirmed by a Ki-67 immunostain (B) showing a marked increase in the percentage of cell staining (nuclear stain) in these regions.
PIN lesions seen in PhIP-treated rats were characterized by a cribriform architecture with well-differentiated epithelial cells forming solid bridges and circular apolar lumina. The lesions filled the glandular lumen but did not show distension with foci of stromal interruption. These features would be, at least, grade 2 (of 4) and would be, at most, grade 3 (of 4) in published grading criteria for PIN in rodents [14]. There was some variation in the extent of PIN between animals, with two animals showing PIN of grades 1 to 2 (of 4). Six animals had grade 3 lesions, and three of these had more than one foci.
No invasive carcinoma was seen in any of the animals. There were areas of inflammation-induced stromal proliferation and high levels of “inflammatory atypia” (atypia of repair). Consistent with the low rate of prostatic neoplasia, we also observed a lower-than-expected rate of intestinal neoplasia. Not all animals were examined, but of the 20 animals examined with at least a segmental histology of the intestine, only one had a full-fledged polypoid adenoma, and only two others had areas of early adenomatous changes.
Immunohistochemistry
Ki-67 staining confirmed a high rate of proliferation in areas of PIN with a 15% to 20% nuclear positivity (Figure 2_B_). All other areas of the glands showed a nuclear positivity from 1–2% in normal noninflammed areas to 5–10% in areas with inflammation (Figure 3_B_) or in areas without inflammation but with aberrant cytologic features (Figure 4). This is consistent with the findings of mitotic figures in these foci. There was no significant increase in apoptosis in these areas.
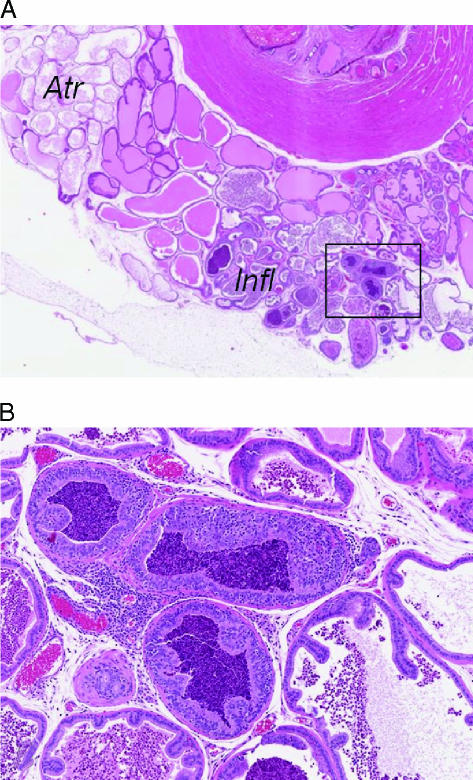
Figure 3.
Atypical proliferations arise in an inflamed prostate. Within areas of a markedly inflamed prostate, foci of the proliferative epithelium are seen. A low-power view of the dorsal and lateral prostate glands (A) shows a segmental area of inflammation (Infl) and another area of atrophy (Atr). The urethra (U) is at the top right, and the box indicates the location of high magnification seen in (B). The high magnification shows epithelial proliferation with cellular loss of polarity and cytologic atypia. These areas were not regarded as neoplastic, as similar areas in a variety of sites are known to resolve completely with resolution of the inflammation. This type of lesion is often referred to as inflammatory atypia.
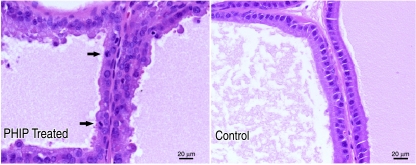
Figure 4.
PhIP induces prostate epithelial proliferation. The PhIP-treated prostate shows abnormal proliferation, with mitoses (indicated by arrows) compared to the prostate of a control vehicle-treated animal (on the right). Both panels present ventral prostate glands from rats. Images from formalin-fixed paraffin-embedded 4-µm tissue sections stained with H&E were obtained on a Zeiss Axioskop equipped with a Zeiss AxioCam digital camera using the Axiovision acquisition software. Scale bars indicate actual size (lower right of each panel).
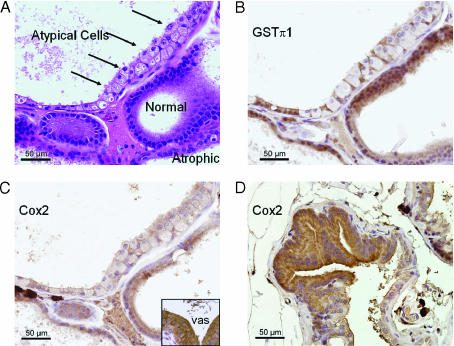
GSTπ immunostaining showed the highest expression in prostate basal cells in all glands, and moderate to low expression was seen in the epithelium. GSTπ immunostaining confirmed that PIN lesions, specific cell populations in areas of atrophy, and focal areas with atypical cells also showed loss of GSTπ (Figure 5_B_). All of the PIN lesions examined yielded negative results, although some of the PIN lesions were not present in the tissue sections obtained for immunostaining and could not be evaluated. The precise relationship between GSTπ-negative atypical cells and the development of PIN was difficult to estimate. Prostate samples did not show the two populations in continuity, nor was there a continuous spectrum of lesions between the polarized single layer of atypical cells and the fully developed PIN lesions.
Figure 5.
Atypical cells in the PHIP-treated rat prostate lose GSTπ expression; Cox2 expression varies. The atypical cells shown (arrows in A) have abundant cytoplasm and nuclei, with single prominent nucleoli. Basal cells can be seen interspersed among these cells and are highlighted by GST immunostain (B). Cox2 (C) is slightly reduced in the same cells, but is immunopositive. Cox2 internal positive control (vas deferens) is strongly positive (inset). In different areas (D), Cox2 is increased in atypical foci.
Cox2 immunostaining showed that there was variability in the rat prostate with high expression in the vas deferens epithelium, moderate expression in the seminal vesicle epithelium, and variable expression in the prostate gland. Consistent with previous reports, the uninflammed and nonatrophic ventral prostate had very low/negative expression [15]. Interestingly, the atrophic ventral prostate maintained a very low expression. Some areas of atypical cells, particularly those with negative GSTπ, retained a low/weak expression of Cox2 (Figure 5_C_). Other areas of atypia and areas of PIN had higher Cox2 expression (Figure 5_D_).
Leydig Cell Tumors
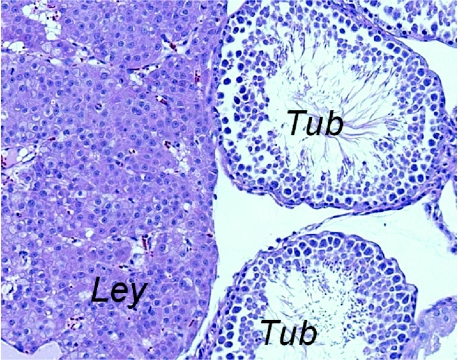
All rats in the 65-week group (both treated and controls) showed testicular Leydig cell hyperplasias, with several rats in both groups displaying very large areas that might be regarded as Leydig cell tumors (Figure 6). These were characterized by well-differentiated Leydig cells of the testicular stroma, with abundant cytoplasm and focal crystalloidlike cytoplasmic inclusions. This is a common finding in Fischer rats [16]. The testicular tubules were normal, with good maturation of germ cells. The amount of testosterone production and serum testosterone levels is not known. A few animals in both treated and control groups at 45 weeks had focal mild Leydig cell hyperplasia. Animals in the 25-week group had no evidence of testicular pathology.
Figure 6.
F344 rats develop Leydig cell hyperplasia. Rats in both control and PhIP-treated groups developed Leydig cell hyperplasia with high penetrance. Patchy areas of Leydig cell hyperplasia, varying in size, were seen in a majority of animals at the 65-week time point. This example shows a relatively large patch of Leydig cells (Ley) adjacent to two seminiferous tubules (Tub).
Discussion
For the first time, we carefully examined the prostate pathology of precancer stages in the PhIP rat model. In our study, it seems clear that PhIP is capable of inducing a rapid and persistent prostatic inflammation. The mechanism of inflammation induced by PhIP is not precisely clear, but appears to involve a specific toxicity to the prostatic epithelium. This may result in an immediate disruption of epithelial barrier and subsequent inflammation. However, this does not adequately explain the persistence of inflammation. In some areas without inflammation, atypical proliferation is seen. In areas near inflammation, it is similar to the epithelial reaction seen in any inflamed mucosa. The areas that continue to display atypia and proliferation in the absence of inflammation suggest that there is a permanent change in the epithelium induced by PhIP. It is not clear whether this is the result of specific mutations or selection based on promoter methylation induced by an inflammatory or a postinflammatory biologic niche. In our studies, we show that there are populations of cells that downregulate GSTπ, a gene known to be downregulated in the prostate epithelium by promoter methylation.
It was expected, based on previous reports, that roughly half of the 65-week PhIP-treated animals would develop invasive carcinomas. None did. It may be because the animals received an effectively decreased dose compared to those in previous publications. Storage conditions and the source and grade of the PhIP reagent were identical to those previously reported, but there may still have been unanticipated degradation of the chemical or a problem with the specific production run or lot. We believe that this is unlikely in light of an obvious effect on animal health (as indicated by animal weight and the 10 animals that became ill during treatment). Furthermore, we have found in previous high-performance liquid chromatography analyses that PhIP is very stable. In the context of histologic findings, however, additional hypotheses should be considered. Colony and housing conditions may contribute to the levels of exposure of the animals to specific bacterial flora, and these can contribute to prostatic inflammation. In the context of our experiments, perhaps a cleaner or flora-shifted colony resulted in decreased penetrance of the invasive cancer phenotype. Regardless of the absence of invasive cancer, it should be noted that several animals developed definitive PIN lesions and that PIN lesions are never seen in rats or mice as spontaneous lesions in untreated or nongenetically engineered prostates. It is possible that the effective dose of PhIP in our study was higher than those in previous reports. Mechanistically, it may be that a higher dose would result in a higher specific prostate epithelial toxicity and that this would reduce the pool of potentially neoplastic cells. This would imply that a specific subset of cells in the prostate is susceptible to both PhIP toxicity and PhIP-initiated carcinogenesis.
It also is hard to compare the reported invasive phenotypes in the literature because few histologic images are available. In our study, there were areas of profound inflammation with stromal reaction, sclerosis, and significant epithelial cytologic atypia (atypia of repair). Some of these might mimic invasion, out of the context of the glandular architecture. Further study is needed to determine if the PIN lesions seen in fact progress to invasive carcinomas. It will be useful to characterize the histomorphology of invasive carcinomas occurring in PhIP-treated rats and to compare their morphology to human prostate cancers. This comparative pathology is an essential part of validating the model for further study, including preclinical trials.
Aging F344 rats develop PIN lesions identical to those seen in this study, although at much lower rates and at older ages [17]. F344 rats, studied at 2 years of age, showed the same kind of cribriform proliferative PIN lesions in areas of atrophy that we saw in this study. Four percent of the rats in the study developed “adenomas or carcinomas,” with rats from 12 different potential carcinogen treatment groups and untreated control groups having the same rates. Similarly, another strain, the ACI/seg HapBR rat, which was examined between 2 and 3.5 years of age, had a high incidence of PIN-like lesions and invasive carcinomas [18]. These studies suggest that, first, inflammation and atrophy precede PIN, even in the absence of a mutagen. Second, these PIN lesions likely can progress to invasive carcinoma (although no evidence of metastasis was found). Finally, these data support a hypothesis that PhIP acts primarily by increasing inflammation and subsequent atrophy in the rat prostate. Increased inflammation and atrophy, as documented in our data, result in increased rates and decreased latency of neoplasia.
The finding that F344 rats have Leydig cell hyperplasia and Leydig cell testicular tumors may explain the overall susceptibility of this strain to prostate tumorigenesis. One group compared susceptibility across common laboratory strains and measured testosterone and estradiol levels. Interestingly, F344 rats had the second highest testosterone levels at 54 weeks and the highest estradiol levels. Only spontaneously hypertensive rats had testosterone levels significantly higher than those of other strains, and these and ACI rats appeared to be most sensitive to prostatic neoplasia [19]. This remains an important consideration when modeling endocrine-responsive tissues and cancers.
We and others have shown that PhIP is capable of forming DNA adducts and inducing DNA mutations in the prostate of F344 rats [10,11,20,21]. It has generally been proposed that low levels (relative to the experimental treatment in rats) of PhIP in the western diet might induce low levels of mutations in the human prostate epithelium, which might accumulate and confer a selective advantage on epithelial cells, ultimately resulting in cancer. Other groups have proposed that PhIP is a potent endocrine hormone analog [22,23]. Several groups have used the rat model to test the effect of potential adduct-preventing and mutation-preventing agents [10,24–26]. This is a highly useful surrogate endpoint that can be quantified with high specificity at early time points, before morphologic changes arise. It is not precisely known whether these adducts and mutations will lead to the progressive acquisition of cancer cell characteristics. In part, it was the goal of this study to try to more clearly make this association. Instead, the data suggest that adduct mutations are either unrelated to neoplastic progression or, alternatively, may cause or contribute primarily to inflammation, with neoplasia occurring secondary to inflammation rather than to mutation.
Increasing evidence suggests that prostatic inflammation and the resulting reactive processes are highly associated with cancer risk. The concept of prostatic inflammatory atrophy has been proposed to be a prostate cancer precursor, or at least to increase susceptibility to further changes, resulting in prostate cancer [5,8]. A number of molecular correlates with postinflammatory atrophic morphology have been established, including promoter methylation and gene silencing for GSTπ and Cox2 [7,25,27,28]. We have shown that benign prostate lesions are sometimes accompanied by changes in apoptosis mediator proteins [28].
Our interest in PhIP administration in rodents as a model of human prostate carcinogenesis is chiefly based on clinical epidemiology. The high meat and fat content in the western diet, particularly in African Americans, may influence both the development and the progression of prostate cancer. We have previously demonstrated the exquisite sensitivity of the prostate to PhIP. At a dose equivalent to one overcooked hamburger fed to F344 rats, DNA adducts can be detected in the prostate [10]. Our data suggest that PhIP-treated rats will also help to characterize the role of prostate inflammation in cancer initiation. In summary, we have shown that the PhIP-treated rat is a useful model of human disease that is suitable for testing prevention strategies targeting either DNA adduct formation or prostate inflammation.
Acknowledgements
The authors would like to acknowledge the support of the UC Davis Mouse Biology Program. The core Mutant Mouse Pathology Laboratory prepared all of the slides and immunohistochemical studies, and Katie Bell and Lisa DillardTelm are specifically acknowledged. Robert Munn helped with photomicroscopy.
Footnotes
1
This work was performed by the University of California and the Lawrence Livermore National Laboratory under the auspices of the US Department of Energy (contract no. W-7405-Eng-48) and was partially supported by National Cancer Institute grant CA5586. Additional support was obtained from Department of Defense PC040947 (R.D.V.-W. and K.D.) and National Institutes of Health RO3 CA097474 (K.T. and K.D.).
References
- 1.Cross AJ, Peters U, Kirsh VA, Andriole GL, Reding D, Hayes RB, Sinha R. A prospective study of meat and meat mutagens and prostate cancer risk. Cancer Res. 2005;65:11779–11784. doi: 10.1158/0008-5472.CAN-05-2191. [DOI] [PubMed] [Google Scholar]
- 2.Cook LS, Goldoft M, Schwartz SM, Weiss NS. Incidence of adenocarcinoma of the prostate in Asian immigrants to the United States and their descendants. J Urol. 1999;161:152–155. [PubMed] [Google Scholar]
- 3.Shirai T, Asamoto M, Takahashi S, Imaida K. Diet and prostate cancer. Toxicology. 2002;181–182:89–94. doi: 10.1016/s0300-483x(02)00260-3. [DOI] [PubMed] [Google Scholar]
- 4.Gardner WA. Hypothesis: the prenatal origins of prostate cancer. Hum Pathol. 1995;26:1291–1292. doi: 10.1016/0046-8177(95)90291-0. [DOI] [PubMed] [Google Scholar]
- 5.Gardner WA, Jr, Culberson DE. Atrophy and proliferation in the young adult prostate. J Urol. 1987;137:53–56. doi: 10.1016/s0022-5347(17)43869-9. [DOI] [PubMed] [Google Scholar]
- 6.Smith CJ, Gardner WA., Jr Inflammation-proliferation: possible relationships in the prostate. Prog Clin Biol Res. 1987;239:317–325. [PubMed] [Google Scholar]
- 7.Nelson WG, De Marzo AM, DeWeese TL. The molecular pathogenesis of prostate cancer: implications for prostate cancer prevention. Urology. 2001;57:39–45. doi: 10.1016/s0090-4295(00)00939-0. [DOI] [PubMed] [Google Scholar]
- 8.De Marzo AM, Marchi VL, Epstein JI, Nelson WG. Proliferative inflammatory atrophy of the prostate: implications for prostatic carcinogenesis. Am J Pathol. 1999;155:1985–1992. doi: 10.1016/S0002-9440(10)65517-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bogen KT, Keating GA. US dietary exposures to heterocyclic amines. J Expo Anal Environ Epidemiol. 2001;11:155–168. doi: 10.1038/sj.jea.7500158. [DOI] [PubMed] [Google Scholar]
- 10.Dingley KH, Ubick EA, Chiarappa-Zucca ML, Nowell S, Abel S, Ebeler SE, Mitchell AE, Burns SA, Steinberg FM, Clifford AJ. Effect of dietary constituents with chemopreventive potential on adduct formation of a low dose of the heterocyclic amines PhIP and IQ and phase II hepatic enzymes. Nutr Cancer. 2003;46:212–221. doi: 10.1207/S15327914NC4602_15. [DOI] [PubMed] [Google Scholar]
- 11.Shirai T, Sano M, Tamano S, Takahashi S, Hirose M, Futakuchi M, Hasegawa R, Imaida K, Matsumoto K, Wakabayashi K, et al. The prostate: a target for carcinogenicity of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) derived from cooked foods. Cancer Res. 1997;57:195–198. [PubMed] [Google Scholar]
- 12.Shirai T, Cui L, Takahashi S, Futakuchi M, Asamoto M, Kato K, Ito N. Carcinogenicity of 2-amino-1-methyl-6-phenylimidazo [4,5-b]pyridine (PhIP) in the rat prostate and induction of invasive carcinomas by subsequent treatment with testosterone propionate. Cancer Lett. 1999;143:217–221. doi: 10.1016/s0304-3835(99)00128-7. [DOI] [PubMed] [Google Scholar]
- 13.Shappell SB, Thomas GV, Roberts RL, Herbert R, Ittmann MM, Rubin MA, Humphrey PA, Sundberg JP, Rozengurt N, Barrios R, et al. Prostate pathology of genetically engineered mice: definitions and classification. The consensus report from the Bar Harbor meeting of the Mouse Models of Human Cancer Consortium Prostate Pathology Committee. Cancer Res. 2004;64:2270–2305. doi: 10.1158/0008-5472.can-03-0946. [DOI] [PubMed] [Google Scholar]
- 14.Park JH, Walls JE, Galvez JJ, Kim M, Abate-Shen C, Shen MM, Cardiff RD. Prostatic intraepithelial neoplasia in genetically engineered mice. Am J Pathol. 2002;161:727–735. doi: 10.1016/S0002-9440(10)64228-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shappell SB, Olson SJ, Hannah SE, Manning S, Roberts RL, Masumori N, Jisaka M, Boeglin WE, Vader V, Dave DS, et al. Elevated expression of 12/15-lipoxygenase and cyclooxygenase-2 in a transgenic mouse model of prostate carcinoma. Cancer Res. 2003;63:2256–2267. [PubMed] [Google Scholar]
- 16.Weisburger JH, Rivenson A, Reinhardt J, Braley J, Pittman B, Zang E. On the occurrence of Leydig cell tumors in the F344 rat. Cancer Lett. 2002;182:213–216. doi: 10.1016/s0304-3835(01)00865-5. [DOI] [PubMed] [Google Scholar]
- 17.Reznik G, Hamlin MH, II, Ward JM, Stinson SF. Prostatic hyperplasia and neoplasia in aging F344 rats. Prostate. 1981;2:261–268. doi: 10.1002/pros.2990020304. [DOI] [PubMed] [Google Scholar]
- 18.Ward JM, Reznik G, Stinson SF, Lattuada CP, Longfellow DG, Cameron TP. Histogenesis and morphology of naturally occurring prostatic carcinoma in the ACI/segHapBR rat. Lab Invest. 1980;43:517–522. [PubMed] [Google Scholar]
- 19.Inaguma S, Takahashi S, Ohnishi H, Suzuki S, Cho YM, Shirai T. High susceptibility of the ACI and spontaneously hypertensive rat (SHR) strains to 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) prostate carcinogenesis. Cancer Sci. 2003;94:974–979. doi: 10.1111/j.1349-7006.2003.tb01387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Archer CL, Morse P, Jones RF, Shirai T, Haas GP, Wang CY. Carcinogenicity of the N-hydroxy derivative of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine, 2-amino-3,8-dimethyl-imidazo[4,5-f]quiinoxaline and 3,2′-dimethyl-4-aminobiphenyl in the rat. Cancer Lett. 2000;155:55–60. doi: 10.1016/s0304-3835(00)00413-4. [DOI] [PubMed] [Google Scholar]
- 21.Stuart GR, Holcroft J, de Boer JG, Glickman BW. Prostate mutations in rats induced by the suspected human carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine. Cancer Res. 2000;60:266–268. [PubMed] [Google Scholar]
- 22.Gooderham NJ, Zhu H, Lauber S, Boyce A, Creton S. Molecular and genetic toxicology of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) Mutat Res. 2002:506–507. 91–99. doi: 10.1016/s0027-5107(02)00155-0. [DOI] [PubMed] [Google Scholar]
- 23.Lauber SN, Ali S, Gooderham NJ. The cooked food derived carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b] pyridine is a potent oestrogen: a mechanistic basis for its tissue-specific carcinogenicity. Carcinogenesis. 2004;25:2509–2517. doi: 10.1093/carcin/bgh268. [DOI] [PubMed] [Google Scholar]
- 24.Imaida K, Tamano S, Kato K, Ikeda Y, Asamoto M, Takahashi S, Nir Z, Murakoshi M, Nishino H, Shirai T. Lack of chemopreventive effects of lycopene and curcumin on experimental rat prostate carcinogenesis. Carcinogenesis. 2001;22:467–472. doi: 10.1093/carcin/22.3.467. [DOI] [PubMed] [Google Scholar]
- 25.Nelson CP, Kidd LC, Sauvageot J, Isaacs WB, DeMarzo AM, Groopman JD, Nelson WG, Kensler TW. Protection against 2-hydroxyamino-1-methyl-6-phenylimidazo[4,5-b]pyridine cytotoxicity and DNA adduct formation in human prostate by glutathione S-transferase π. Cancer Res. 2001;61:103–109. [PubMed] [Google Scholar]
- 26.Schut HA, Yao R. Tea as a potential chemopreventive agent in PhIP carcinogenesis: effects of green tea and black tea on PhIP-DNA adduct formation in female F-344 rats. Nutr Cancer. 2000;36:52–58. doi: 10.1207/S15327914NC3601_8. [DOI] [PubMed] [Google Scholar]
- 27.Lodygin D, Epanchintsev A, Menssen A, Diebold J, Hermeking H. Functional epigenomics identifies genes frequently silenced in prostate cancer. Cancer Res. 2005;65:4218–4227. doi: 10.1158/0008-5472.CAN-04-4407. [DOI] [PubMed] [Google Scholar]
- 28.Gandour-Edwards R, Mack PC, Devere-White RW, Gumerlock PH. Abnormalities of apoptotic and cell cycle regulatory proteins in distinct histopathologic components of benign prostatic hyperplasia. Prostate Cancer Prostatic Dis. 2004;7:321–326. doi: 10.1038/sj.pcan.4500749. [DOI] [PubMed] [Google Scholar]