Ex vivo pretreatment of bone marrow mononuclear cells with endothelial NO synthase enhancer AVE9488 enhances their functional activity for cell therapy (original) (raw)
Abstract
Bone marrow mononuclear cells (BMC) from patients with ischemic cardiomyopathy (ICMP) show a reduced neovascularization capacity in vivo. NO plays an important role in neovascularization, and NO bioavailability is typically reduced in patients with ICMP. We investigated whether the impaired neovascularization capacity of ICMP patient-derived progenitor cells can be restored by pretreatment with the novel endothelial NO synthase (eNOS) transcription enhancer AVE9488 (AVE). Ex vivo pretreatment of BMC from patients with ICMP with AVE significantly increased eNOS mRNA expression by 2.1-fold (P < 0.05) and eNOS activity as assessed by ESR by >3-fold (P < 0.05). The increased eNOS expression was associated with an enhanced migratory capacity in vitro (P < 0.01) and improved neovascularization capacity of the infused BMC in an ischemic hind limb model in vivo (P < 0.001). The improvement in ischemic limb perfusion after infusion of AVE-pretreated BMC resulted in an increase in swimming time (P < 0.05). The enhancement of limb perfusion by AVE-treated BMC was abrogated by ex vivo pretreatment with the eNOS inhibitor NG-nitro-l-arginine methyl ester. Consistently, AVE showed no effect on the impaired migratory capacity of BMC derived from eNOS-deficient mice, documenting the specific involvement of NO. The reduced neovascularization capacity of BMC from patients with ICMP may limit their therapeutic potential in cell therapy studies. Here, we show that pharmacological enhancement of eNOS expression with AVE at least partially reverses the impaired functional activity of BMC from ICMP patients, highlighting the critical role of NO for progenitor cell function.
Keywords: ischemia, neovascularization, progenitor cells
The restoration of tissue vascularization after critical ischemia is a major therapeutic goal in cardiovascular medicine. Experimental studies suggest that bone marrow or peripheral blood-derived stem/progenitor cell treatment enhances neovascularization of ischemic tissue (1–3). Recently, we and others have demonstrated that intracoronary infusion of adult progenitor cells is feasible and safe in patients with peripheral arterial disease, acute myocardial infarction, and ischemic cardiomyopathy (ICMP) and may improve perfusion and regional and global left ventricular function (4–6). However, the functional activity of bone marrow mononuclear cells (BMC) and circulating blood-derived endothelial progenitor cells (EPC) is significantly impaired in patients with coronary artery disease, diabetes, or ICMP (7–9). Impaired bioavailability of NO is a hallmark of patients with atherosclerosis (10). In parallel, endothelial-derived NO is a key regulator for endothelial cell growth (11), migration (12), vascular remodeling (13), and angiogenesis (11, 14). Importantly, it was recently demonstrated that endothelial NO synthase (eNOS) plays a crucial role for the functional activity of stem and progenitor cells (15–17). Thus, impaired bioavailability of NO may significantly contribute to the functional impairment of BMC from patients with ICMP. The transcriptional enhancement of eNOS expression might be a promising pharmacological target for the pretreatment of BMC to restore their functional activity.‖ Therefore, in the present study, we investigated the effect of AVE9488 (AVE), a substance that has been shown to transcriptionally enhance eNOS expression in mature endothelial cells,‖ on the functional activity of ICMP patient-derived BMC.
Results
We have recently shown that patients with ICMP have an impaired function of their BMC, resulting in reduced homing and transmigration of these cells into ischemic tissue after i.v. administration (9). Therefore, we first investigated whether local i.m. administration of BMC from patients with ICMP might compensate for their impaired migratory activity, resulting in a greater therapeutic effect as compared with i.v. administration of the cells. However, the i.m. administration of the same numbers of BMC from identical patients with ICMP did not augment perfusion recovery as compared with the therapeutic effect observed after i.v. infusion of BMC (Fig. 5, which is published as supporting information on the PNAS web site). Based on these findings, we conclude that the functional activity of the cells is crucial for their neovascularization capacity and cannot be circumvented by local administration of the cells. Therefore, targeting the functional activity of the cells by enhanced expression and activity of NO was the purpose of the following experiments.
Increased eNOS Expression and Activity by AVE.
Ex vivo treatment for 18–24 h with AVE increased eNOS mRNA expression in peripheral blood-derived EPC, CD34+ cells, and BMC (Fig. 1). As a control, treatment of human umbilical vein endothelial cells with AVE dose-dependently increased eNOS mRNA expression (1.6- ± 0.3-fold; P < 0.05) and eNOS protein expression (1.7- ± 0.2 fold; P < 0.05) with a maximal effect at a concentration of 5 μM.
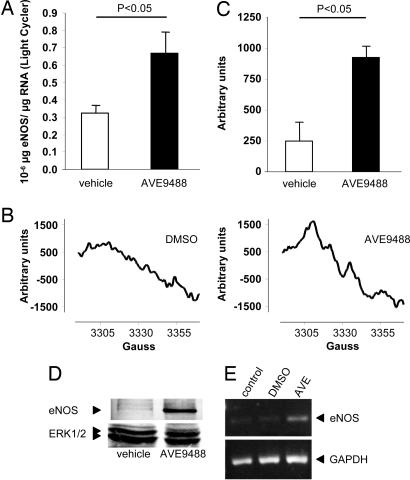
Fig. 1.
Effect of AVE on eNOS expression. (A) Expression of eNOS RNA, protein, and eNOS-derived NO is augmented in the presence of the eNOS transcription enhancer AVE. eNOS mRNA expression was detected by quantitative real-time RT-PCR of 100 ng of RNA by using a light cycler instrument in BMC derived from patients with ICMP pretreated with vehicle or AVE (5 μM) for 18 h (n = 3). (B and C) NO production of Lin−CD105+ cells was determined by ESR. (B) Representative recordings from n = 3 experiments are depicted. (C) Quantitative data are presented. (D) eNOS Western blot analysis of cells treated with either vehicle or AVE. A representative blot is shown. ERK1/2 was used as a loading control. (E) eNOS expression was measured by RT-PCR in CD34+ cells after treatment with 5 μM AVE for 24 h.
Because the detection limits of our assays were too low to detect eNOS activity in crude BMC, we isolated Lin−CD105+ bone marrow-derived cells. NO production as assessed by ESR was significantly enhanced by pretreatment of Lin−CD105+ cells by AVE (Fig. 1 B and C). Consistently, the protein expression of eNOS in Lin−CD105+ cells was markedly increased by treatment with AVE (Fig. 1D). Moreover, AVE induced the up-regulation of eNOS mRNA in CD34+ cells (Fig. 1E).
Increased Functional Activity of BMC After Pretreatment with AVE.
Next, we tested the effect of AVE on the migratory capacity of BMC. The migratory capacity of BMC in response to stromal cell-derived factor 1 (SDF-1) was significantly reduced (P < 0.01) in patients as compared with healthy controls (Fig. 2A). However, the impaired migratory capacity of BMC from patients with ICMP was significantly enhanced by pretreatment with AVE. A significantly increased SDF-1-induced migratory capacity by AVE was also detected in healthy control-derived BMC and CD34+ cells but not in total monocytic CD14+ cells (Fig. 2B). The effect of AVE on the migratory activity of BMC was inhibited by the simultaneous treatment with antibodies directed against the SDF-1 receptor CXCR4 (53 ± 17%; P < 0.05).
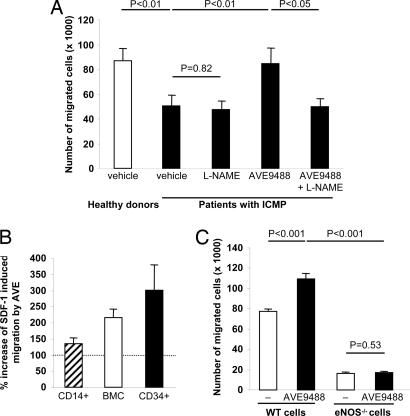
Fig. 2.
Effect of AVE on migration. (A) Shown are the numbers of migrated BMC toward the chemoattractant factor SDF-1 derived from healthy donors (n = 12) and patients with ICMP that were pretreated for 18 h with either vehicle (n = 11), l-NAME (n = 11), AVE (n = 11), or AVE plus l-NAME (n = 6). (B) Increase in SDF-1-mediated migration by AVE-pretreatment (5 μM, 24 h) in CD14+ cells, BMC, and CD34+ cells isolated from healthy volunteers (n = 3–4) is shown. (C) The number of BMC from WT and eNOS knockout (eNOS−/−) mice that migrated in response to SDF-1 to the lower chamber of a modified Boyden chamber assay after pretreatment for 18 h with either vehicle or AVE (n ≥ 8 per group) is shown.
To further define the specific role of eNOS on the AVE-enhanced migratory capacity, BMC were simultaneously treated with the eNOS inhibitor NG-nitro-l-arginine methyl ester (l-NAME). l-NAME abrogated the effect of AVE on the migratory activity of BMC (Fig. 2A). Moreover, we used murine BMC from eNOS−/− mice in comparison with WT mice. The migratory capacity in response to SDF-1 of BMC derived from eNOS−/− mice was markedly and significantly reduced as compared with WT mice (Fig. 2C). Pretreatment with AVE significantly increased the migratory capacity of isolated BMC derived from WT mice, whereas no effect on the migratory capacity of BMC derived from eNOS−/− mice was detected. Likewise, BMC lacking the NO downstream target, the cGMP-dependent protein kinase I (GKI) (18), showed a reduction in SDF-1-induced migration to 51.9 ± 2.4% of WT BMC. Moreover, SDF-1-induced migration of GKI−/− BMC was not augmented in the presence of AVE (16.3 ± 6.7% compared with WT BMC plus AVE). Thus, specific enhancement of eNOS transcription and downstream signaling is indeed critical for mediating the effects of AVE on the migratory capacity of BMC.
Increased Neovascularization Capacity of BMC After Pretreatment with AVE.
To demonstrate the in vivo relevance of the ex vivo findings, we used the murine model of hind limb ischemia. Animals i.v. infused with 5 × 105 BMC derived from patients with ICMP showed a significantly reduced recovery of limb perfusion after induction of hind limb ischemia as compared with infusion of BMC from healthy controls (Fig. 3A and B). Pharmacological enhancement of eNOS expression by pretreatment of BMC derived from patients with ICMP with AVE resulted in a significantly enhanced recovery of limb perfusion as compared with DMSO-treated BMC (Fig. 3 A and B). Likewise, AVE-pretreated, _ex vivo_-expanded EPC showed a 1.7-fold increase in functional activity to augment blood flow in vivo as compared with untreated EPC (Fig. 3C). To document an improvement in functional capacity associated with the enhanced recovery of ischemic hind limb perfusion, the physical capacity of the mice was assessed by using a swimming test. Pretreatment of infused BMC derived from patients with ICMP with AVE significantly increased the swimming time ratio as compared with mice receiving vehicle-treated BMC (Fig. 3D).
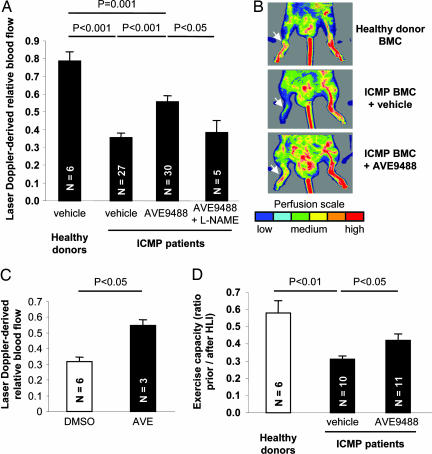
Fig. 3.
Effect of AVE on recovery after ischemia. (A) Recovery of limb perfusion after hind limb ischemia as assessed by laser Doppler blood flow analysis on day 14 after i.v. infusion of BMC derived from either healthy donors or nine patients with ICMP into nude mice. BMC from ICMP patients were pretreated with vehicle, AVE, or AVE plus l-NAME for 18 h. The number of mice in each group is indicated in the bars. (B) Representative laser Doppler images for each group are depicted. Arrows indicate ischemic limbs. Perfusion signals are subdivided into six different intervals, each displayed as a separate color. Low or no perfusion is displayed as dark blue; highest perfusion interval is displayed as red. (C) Recovery of limb perfusion by i.v. infusion of circulating blood-derived EPC isolated from patients after pretreatment with AVE for 18 h compared with controls. (D) Exercise capacity expressed as the swimming time ratio (swimming time on day 14 after hind limb ischemia/swimming time before induction of hind limb ischemia) in a murine model of hind limb ischemia. Mice were either untreated or received i.v. infusions of BMC from healthy donors or patients with ICMP.
The functional activity of AVE-treated patient-derived BMC was further confirmed in a myocardial infarction model demonstrating a significant improvement of ejection fraction (150 ± 8.8%, P < 0.05) and stroke work (188 ± 18.2%, P < 0.05) in mice injected with AVE-pretreated BMC.
Consistent with the in vitro data, the effect of AVE was abrogated by the pretreatment with the pharmacological eNOS inhibitor l-NAME (Fig. 3A). Histological analysis of the adductor muscles indicated severe necrosis in mice receiving no cell therapy and to a lesser extent in mice receiving untreated BMC from patients with ICMP (Fig. 4A). In mice receiving BMC pretreated with AVE, however, the ischemic muscle was much better preserved in line with improved blood flow recovery after induction of limb ischemia. The capillary density in mice receiving AVE-treated BMC was significantly higher as compared with vehicle-treated BMC (121 ± 5.8%, P < 0.01, versus control animals). Moreover, the number of small- and medium-sized conduit vessels in the ischemic muscles of mice receiving AVE-treated BMC was significantly higher as compared with mice receiving vehicle-treated BMC (Fig. 4 B and C). Vascular incorporation and endothelial differentiation of injected BMCs was significantly enhanced by pretreatment of infused BMC with the eNOS enhancer AVE (Fig. 4 D and E).
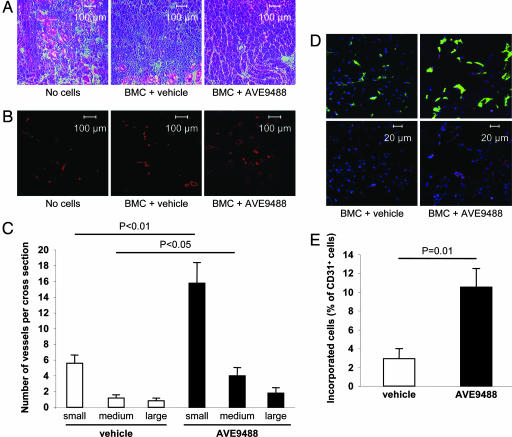
Fig. 4.
Effect of AVE on morphology, capillary density, and incorporation. (A) Histological morphology of the ischemic muscles was assessed in H&E-stained sections. (B) Conductance vessels were identified by staining for smooth muscle α-actin with a Cy3-labeled mouse mAb (red color). (C) The number of small (<50 μm), medium (50–100 μm), and large (>100 μm) vessels was counted separately (n = 5 mice per group). (D) Incorporation of human BMC (stained for HLA-ABC, red) into vessels (endothelial marker CD31, green) after pretreatment with vehicle or AVE. Sytox was used for nuclear staining (blue). Representative images are shown. (Upper) Merged pictures. (Lower) Staining for human HLA and nuclei. (E) Double positive cells were quantified from 10 sections provided by five mice per group.
Discussion
The results of the present study demonstrate that pretreatment with the eNOS transcription enhancer AVE largely rescues the impaired functional activity of BMC derived from patients with ICMP. Most importantly, the improved in vivo neovascularization capacity of AVE-pretreated BMC is associated with a significant increase in physical exercise capacity after the induction of hind limb ischemia.
The functional activity of transplanted stem and progenitor cells is critical for their therapeutic effects in patients with cardiovascular diseases (9, 19). As demonstrated previously, the migratory capacity of stem and progenitor cells, which is essential for the invasion of the cells into ischemic tissue after mobilization from the bone marrow or after systemic infusion, is significantly reduced in the mononuclear fraction of bone marrow cells and circulating EPC derived from patients with ICMP or cardiovascular risk factors (7, 9). Accordingly, we initially tested whether direct i.m. injection of isolated BMC from patients with ICMP, which supposedly bypasses the impaired homing and transmigration processes of these cells, might generate a greater effect on neovascularization as opposed to the systemic infusion of BMC. However, i.m.-injected BMC did not augment the levels of blood flow recovery after hind limb ischemia compared with i.v. injections of BMC. Based on these data, we conclude that the functional activity of the cells is crucial for their neovascularization capacity even after local i.m. administration of the cells. Therefore, improving the functional activity of BMC from patients with ICMP may be a promising approach for optimizing the results after cell therapy.
Impaired NO bioavailability is a hallmark of patients with ICMP (10, 20). Mechanistically, reduced NO bioavailability in patients with ICMP is the consequence of a combination of increased inactivation of NO by elevated levels of reactive oxygen species (20), uncoupling of the eNOS itself (21), and decreased eNOS protein expression in endothelial cells (22). Thus, we hypothesized that NO deficiency might impair the functional capacity of BMC in patients with ICMP and that increasing eNOS expression may represent a promising target for improving the functional outcome after autologous transplantation of cells derived from patients with ICMP. Indeed, pretreatment of BMC derived from patients with ICMP with the pharmacological eNOS transcription enhancer AVE resulted in significant up-regulation of eNOS expression on both the mRNA and protein levels. Consistently, NO production as assessed by ESR was also increased. The enhanced NO production was associated with a significant improvement of the functional capacity of these cells. In addition, the pharmacological effect of AVE was virtually abrogated by pretreatment with the pharmacological eNOS inhibitor l-NAME or genetic knockdown of eNOS using eNOS-deficient cells, thus confirming that the enhancement of the migratory capacity of BMC to the cytokine SDF-1 by AVE is indeed related to an increased activity of eNOS. SDF-1 was recently shown to stimulate recruitment of CXCR4+ endothelial progenitor cells and hemangiocytes to augment revascularization (23, 24). In addition, retention of bone marrow-derived proangiogenic cells was shown to be mediated by SDF-1 (25). The improvement of SDF-1-mediated responses by AVE may contribute to the improved homing of patient-derived cells.
NO regulates many aspects of blood flow recovery after ischemia and exerts multiple signaling effects. In addition to the regulation of angiogenesis by its well established effects on endothelial migration, tube formation, and survival, eNOS acts as a second messenger to control arteriogenesis (26). An increased blood supply provided by NO-mediated vasodilation is believed to contribute to shear-dependent remodeling of preexisting collaterals (26). In addition, the expression of NO was shown to be essential for progenitor cell homing and progenitor cell-mediated improvement of neovascularization after ischemia (15). The augmentation of eNOS expression in BM-derived cells thus is likely to improve neovascularization on several levels. Indeed, in the present study an improved progenitor cell homing and an increase in the number of arteries documenting enhanced arteriogenesis was observed after AVE pretreatment of the infused progenitor cells.
The variety of NO effects in vivo is mirrored by the complex downstream signaling. NO can signal by cGMP-dependent or -independent pathways (27, 28). Because cGMP-dependent protein kinase I (GKI)+/− mice show an impaired neovascularization (29) and SDF-induced BMC migration was inhibited in GKI−/− cells, one may speculate that the effects of NO are at least in part mediated by cGMP-dependent downstream signaling pathways.
Importantly, the increased migratory capacity of BMC derived from patients with ICMP pretreated with AVE not only translated into a significant improvement of their neovascularization capacity in vivo, but was also associated with a significantly improved exercise capacity as assessed by a swimming test. Matsumoto et al. (30) demonstrated that the number of hind limb kicks during swimming is strongly correlated with the water current, whereas forelimb kicks remain at a low rate even at high currents and are primarily used for body stabilization. Thus, endurance capacity assessed in a countercurrent swimming pool closely reflects the exercise capacity of hind limbs in mice (30). Of note, the swimming time ratio significantly correlated with the measured laser Doppler perfusion ratio (data not shown).
In conclusion, pretreatment of BMC derived from patients with ICMP with the pharmacological eNOS enhancer AVE resulted in a significant improvement of the impaired functional capacity and neovascularization capacity of the infused cells. These results could have major clinical implications as a safe and feasible pharmacological pretreatment of isolated stem and progenitor cells to enhance the therapeutic efficacy of autologous cell therapy in patients with chronic peripheral artery occlusive disease or ischemic heart disease.
Methods
Cell Isolation and Measurement of Migration.
Human or murine BM aspirates were collected, and BMC were isolated by density gradient centrifugation (9, 15). Subpopulations were isolated by magnetic beads. The migratory capacity toward SDF-1 was determined by using a Boyden chamber (9) (for details, see Supporting Text, which is published as supporting information on the PNAS web site).
eNOS Measurement.
eNOS expression was detected by quantitative RT-PCR and Western blot. NO production was detected by using the Fe(DETC)2 complex, which specifically traps NO (31) (see Supporting Text).
Hind Limb Ischemia Model.
The in vivo neovascularization capacity of BMC was investigated in a murine model of unilateral hind limb ischemia. For details on cell injection, limb perfusion measurements, determination of the exercise capacity, and histology see Supporting Text.
Supplementary Material
Supporting Information
Acknowledgments
We thank Tina Rasper, Marion Muhly-Reinholz, Ariane Fischer, and Tino Röxe for excellent technical assistance and Dr. Robert Feil (University of Tübingen, Tübingen, Germany) for providing the GKI−/− mice. This work was supported by the Deutsche Forschungsgemeinschaft (Forschergruppe 501:HE 3044/2-2 and Sonderforschungsbereich 553 Project B6), the Alfried Krupp-Stiftung, and the Leducq Foundation (to S.D.). K.-i.S. was in part supported by the Japan Heart Foundation/Bayer Yakuhin Research Grant Abroad.
Abbreviations
BMC
bone marrow mononuclear cells
ICMP
ischemic cardiomyopathy
eNOS
endothelial NO synthase
EPC
endothelial progenitor cells
SDF-1
stromal cellderived factor 1
l-NAME
NG-nitro-l-arginine methyl ester.
Footnotes
Conflict of interest statement: S.D., A.M., and C.H. have filed for a patent for the use of eNOS transcription enhancers in cell therapy of ischemic heart diseases (U.S. application 20050101599).
‖
Wohlfart, P., Tiemann, M., Strobel, H., Suzuki, T., Li, H., Foerstermann, U., Ruetten, H., Scientific Sessions 2002, Nov. 17–20, 2002, Chicago, abstr. 1074.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Kocher AA, Schuster MD, Szabolcs MJ, Takuma S, Burkhoff D, Wang J, Homma S, Edwards NM, Itescu S. Nat Med. 2001;7:430–436. doi: 10.1038/86498. [DOI] [PubMed] [Google Scholar]
- 2.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, et al. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 3.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 4.Assmus B, Schachinger V, Teupe C, Britten M, Lehmann R, Dobert N, Grunwald F, Aicher A, Urbich C, Martin H, et al. Circulation. 2002;106:3009–3017. doi: 10.1161/01.cir.0000043246.74879.cd. [DOI] [PubMed] [Google Scholar]
- 5.Perin EC, Dohmann HF, Borojevic R, Silva SA, Sousa AL, Mesquita CT, Rossi MI, Carvalho AC, Dutra HS, Dohmann HJ, et al. Circulation. 2003;107:2294–2302. doi: 10.1161/01.CIR.0000070596.30552.8B. [DOI] [PubMed] [Google Scholar]
- 6.Tateishi-Yuyama E, Matsubara H, Murohara T, Ikeda U, Shintani S, Masaki H, Amano K, Kishimoto Y, Yoshimoto K, Akashi H, et al. Lancet. 2002;360:427–435. doi: 10.1016/S0140-6736(02)09670-8. [DOI] [PubMed] [Google Scholar]
- Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, Zeiher AM, Dimmeler S. Circ Res. 2001;89:E1–E7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- 8.Tepper OM, Galiano RD, Capla JM, Kalka C, Gagne PJ, Jacobowitz GR, Levine JP, Gurtner GC. Circulation. 2002;106:2781–2786. doi: 10.1161/01.cir.0000039526.42991.93. [DOI] [PubMed] [Google Scholar]
- 9.Heeschen C, Lehmann R, Honold J, Assmus B, Aicher A, Walter DH, Martin H, Zeiher AM, Dimmeler S. Circulation. 2004;109:1615–1622. doi: 10.1161/01.CIR.0000124476.32871.E3. [DOI] [PubMed] [Google Scholar]
- 10.Zeiher AM, Drexler H, Saurbier B, Just H. J Clin Invest. 1993;92:652–662. doi: 10.1172/JCI116634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ziche M, Morbidelli L, Masini E, Amerini S, Granger HJ, Maggi CA, Geppetti P, Ledda F. J Clin Invest. 1994;94:2036–2044. doi: 10.1172/JCI117557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murohara T, Witzenbichler B, Spyridopoulos I, Asahara T, Ding B, Sullivan A, Losordo DW, Isner JM. Arterioscler Thromb Vasc Biol. 1999;19:1156–1161. doi: 10.1161/01.atv.19.5.1156. [DOI] [PubMed] [Google Scholar]
- 13.Rudic RD, Shesely EG, Maeda N, Smithies O, Segal SS, Sessa WC. J Clin Invest. 1998;101:731–736. doi: 10.1172/JCI1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murohara T, Asahara T, Silver M, Bauters C, Masuda H, Kalka C, Kearney M, Chen D, Symes JF, Fishman MC, et al. J Clin Invest. 1998;101:2567–2578. doi: 10.1172/JCI1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aicher A, Heeschen C, Mildner-Rihm C, Urbich U, Ihling C, Technau-Ihling K, Zeiher AM, Dimmeler S. Nat Med. 2003;9:1370–1376. doi: 10.1038/nm948. [DOI] [PubMed] [Google Scholar]
- 16.Iwakura A, Luedemann C, Shastry S, Hanley A, Kearney M, Aikawa R, Isner JM, Asahara T, Losordo DW. Circulation. 2003;108:3115–3121. doi: 10.1161/01.CIR.0000106906.56972.83. [DOI] [PubMed] [Google Scholar]
- 17.Landmesser U, Engberding N, Bahlmann FH, Schaefer A, Wiencke A, Heineke A, Spiekermann S, Hilfiker-Kleiner D, Templin C, Kotlarz D, et al. Circulation. 2004;110:1933–1939. doi: 10.1161/01.CIR.0000143232.67642.7A. [DOI] [PubMed] [Google Scholar]
- 18.Wegener JW, Nawrath H, Wolfsgruber W, Kuhbandner S, Werner C, Hofmann F, Feil R. Circ Res. 2002;90:18–20. doi: 10.1161/hh0102.103222. [DOI] [PubMed] [Google Scholar]
- 19.Britten MB, Abolmaali ND, Assmus B, Lehmann R, Honold J, Schmitt J, Vogl TJ, Martin H, Schachinger V, Dimmeler S, Zeiher AM. Circulation. 2003;108:2212–2218. doi: 10.1161/01.CIR.0000095788.78169.AF. [DOI] [PubMed] [Google Scholar]
- 20.Hornig B, Arakawa N, Kohler C, Drexler H. Circulation. 1998;97:363–368. doi: 10.1161/01.cir.97.4.363. [DOI] [PubMed] [Google Scholar]
- 21.Munzel T, Daiber A, Ullrich V, Mulsch A. Arterioscler Thromb Vasc Biol. 2005;25:1551–1557. doi: 10.1161/01.ATV.0000168896.64927.bb. [DOI] [PubMed] [Google Scholar]
- 22.Oemar BS, Tschudi MR, Godoy N, Brovkovich V, Malinski T, Luscher TF. Circulation. 1998;97:2494–2498. doi: 10.1161/01.cir.97.25.2494. [DOI] [PubMed] [Google Scholar]
- 23.Jin DK, Shido K, Kopp HG, Petit I, Shmelkov SV, Young LM, Hooper AT, Amano H, Avecilla ST, Heissig B, et al. Nat Med. 2006;12:557–567. doi: 10.1038/nm1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamaguchi J, Kusano KF, Masuo O, Kawamoto A, Silver M, Murasawa S, Bosch-Marce M, Masuda H, Losordo DW, Isner JM, Asahara T. Circulation. 2003;107:1322–1328. doi: 10.1161/01.cir.0000055313.77510.22. [DOI] [PubMed] [Google Scholar]
- 25.Grunewald M, Avraham I, Dor Y, Bachar-Lustig E, Itin A, Yung S, Chimenti S, Landsman L, Abramovitch R, Keshet E. Cell. 2006;124:175–189. doi: 10.1016/j.cell.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 26.Yu J, deMuinck ED, Zhuang Z, Drinane M, Kauser K, Rubanyi GM, Qian HS, Murata T, Escalante B, Sessa WC. Proc Natl Acad Sci USA. 2005;102:10999–1004. doi: 10.1073/pnas.0501444102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arnold WP, Mittal CK, Katsuki S, Murad F. Proc Natl Acad Sci USA. 1977;74:3203–3207. doi: 10.1073/pnas.74.8.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lopez-Rivera E, Lizarbe TR, Martinez-Moreno M, Lopez-Novoa JM, Rodriguez-Barbero A, Rodrigo J, Fernandez AP, Alvarez-Barrientos A, Lamas S, Zaragoza C. Proc Natl Acad Sci USA. 2005;102:3685–3690. doi: 10.1073/pnas.0408217102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamahara K, Itoh H, Chun TH, Ogawa Y, Yamashita J, Sawada N, Fukunaga Y, Sone M, Yurugi-Kobayashi T, Miyashita K, et al. Proc Natl Acad Sci USA. 2003;100:3404–3409. doi: 10.1073/pnas.0538059100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsumoto K, Ishihara K, Tanaka K, Inoue K, Fushiki T. J Appl Physiol. 1996;81:1843–1849. doi: 10.1152/jappl.1996.81.4.1843. [DOI] [PubMed] [Google Scholar]
- 31.Mulsch A, Bara A, Mordvintcev P, Vanin A, Busse R. Br J Pharmacol. 1995;116:2743–2749. doi: 10.1111/j.1476-5381.1995.tb17236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information