Expression and function of wingless and frizzled homologs in rheumatoid arthritis (original) (raw)
Abstract
Rheumatoid arthritis (RA) is accompanied by synovial inflammation, proliferation, and cartilage destruction. The reasons the activation of synovial fibroblasts often persists despite antiinflammatory therapy are not known. One possibility is that the synovial membrane becomes gradually repopulated with immature mesenchymal and bone marrow cells with altered properties. To explore this hypothesis, we have investigated the expression in RA synovial tissues of various embryonic growth factors from the wingless (wnt) and frizzled (fz) families, which have been implicated in cell-fate determination in both bone marrow progenitors and limb-bud mesenchyme. Reverse transcriptase–PCR analysis revealed expression of five wnt (wnt1, 5a,10b, 11, and 13) and three_fz_ (fz2, 5, and_7_) isoforms in RA synovial tissues. Osteoarthritis synovial tissues expressed much less wnt5a and_fz5_. Northern blotting confirmed the overexpression of_wnt5a_ and fz5 in RA synovial tissues, in comparison to a panel of normal adult tissues. Compared with normal synovial fibroblasts, cultured RA fibroblast-like synoviocytes expressed higher levels of IL-6, IL-8, and IL-15. Transfection of normal fibroblasts with a wnt5a expression vector reproduced this pattern of cytokine expression and stimulated IL-15 secretion. These results suggest that the unusual phenotypic properties of RA fibroblasts may be attributable partly to their replacement with primitive fibroblast-like synoviocytes with characteristics of immature bone marrow and mesenchymal cells. Clear delineation of the signaling pathway(s) initiated by the wnt5a/fz5 ligand–receptor pair in the RA synovium may yield new targets for therapeutic intervention.
Keywords: synovium, fibroblast, cytokine, wnt
The normal synovium contains both bone marrow-derived macrophages and mesenchymal fibroblasts. The development of rheumatoid arthritis (RA) is associated with infiltration of blood leukocytes, angiogenesis, and activation/proliferation of fibroblasts in the synovial lining and underlying connective tissue to form a pannus, which destroys articular cartilage (1–4). Inflammatory stimuli, especially tumor necrosis factor α (TNF-α) and IL-1, have been reported to activate synovial fibroblasts and induce them to produce other cytokines, chemokines, proteolytic enzymes, and inflammatory mediators (5–8). However, rheumatoid synovial fibroblast activation sometimes continues despite antiinflammatory therapy. It has been suggested that in the inflammatory stages of RA, channels between the bone marrow and the synovium might allow migration into the synovium of bone marrow stem cells with different properties (9). Cytokines can stimulate the proliferation of such immature mesenchymal cells. The gradual replacement of mature fibroblasts with more primitive cells could alter the regulation of synoviocyte activation. It is important, therefore, to know whether the fibroblast-like synoviocytes from long-standing RA express growth modulators characteristic of immature bone marrow or mesenchyme, and to determine whether such factors could play a role in synovial fibroblast activation and pannus formation.§
The wingless (wnt) and frizzled (fz) family genes were first characterized in_Drosophila_, where they specify tissue patterning and cell-fate determination during embryonic development (10, 11). The homologous wnt and fz family members in mammals have also been reported to function in tissue specification (12, 13). The secreted wnt family proteins are ligands for the_fz_ family gene products, which resemble classical G protein-coupled cell surface receptors (14, 15). All wnt and_fz_ family members, however, have not been biochemically characterized as ligand–receptor pairs. Mammals express at least 13 different wnt genes and eight or more fz genes. Besides being essential for cell-fate determination, various_wnt_ and fz family members also influence cell proliferation and the response to activating stimuli. For example,wnt1 causes stabilization of cytosolic β-catenin, which regulates both cell proliferation and cell adhesion (16).wnt10b has been suggested to influence hematopoiesis and cell-growth regulation (17, 18). Elevated expression of fz2 has been linked to tissue regeneration and hyperplasia in an animal model of atherosclerosis (19). Overexpression of fz7 has been reported to contribute to cell proliferation in esophageal cells (20). In long-standing RA, wnt5a may be of special interest because of its reported role in bone marrow stem cell development and in protein kinase C (PKC) activation (21, 22). In the present experiments, we report the expression of the wnt5a-fz5 ligand–receptor pair (23) in the rheumatoid synovium and present evidence suggesting its involvement in fibroblast activation.
Materials and Methods
Tissue Collection and Processing.
Surgically removed synovial tissue specimens were obtained frozen from the Arthritis and Rheumatism Branch, National Institutes of Health (Bethesda, MD) and the University of California at San Diego Multipurpose Arthritis and Musculoskeletal Disease Center and processed quickly for RNA extraction. Transfer of material was approved by the appropriate Human Subjects Committees.
RNA Analysis from Tissue Samples.
Total RNA was extracted from fresh rapidly thawed tissue samples by using RNA-zol (GIBCO). Because it was difficult to obtain sufficient normal synovial tissue as a negative control, we compared osteoarthritis (OA) and RA surgical specimens. Different pairs of gene-specific primers based on sequences of cloned human isoforms of the wnt and fz genes were used for reverse transcriptase–PCR (RT-PCR) analysis. One microgram of RNA was used from each specimen, and 30 cycles of PCR were carried out. Expression of β-actin was used to normalize the expression of the different_wnt_ and fz isoforms. The following list summarizes the primer pairs used:
fz2: 5′-cagcgtcttgcccgaccagatcca-3′(reverse); 5′-ctagcgccgctcttcgtgtacctg-3′ (forward). fz5: 5′-ttcatgtgcctggtggtgggc-3′ (forward); 5′-tacacgtgcgacagggacacc-3′ (reverse)
wnt1: 5′-cacgacctcgtctacttcgac-3′ (forward); 5′-acagacactcgtgcagtacgc-3′ (reverse). wnt5a: 5′-acacctctttccaaacaggcc-3′ (forward); 5′-ggattgttaaactcaactctc-3′ (reverse)
wnt10b: 5′-gaatgcgaatccacaacaacag-3′ (forward); 5′-ttgcggttgtgggtatcaatgaa-3′(reverse). wnt13: 5′-aagatggtgccaacttcaccg-3′ (forward); 5′-ctgccttcttgggggctttgc-3′(reverse) β-actin: 5′-caggtgatcacgataggtaacga-3′ (forward); 5′-catccacatttgttggaacgt-3′(reverse).
The expression of the wnt5a and fz5 isoforms in RA tissues was confirmed by Northern blotting by using labeled gene-specific fragments corresponding to specific wnt and_fz_ isoforms. The probes were obtained by RT-PCR and then labeled with [32P]-dCTP by using a nick translation kit (GIBCO). The same radioactive probes were used for hybridization to a commercially available multiple tissue Northern blot (CLONTECH). The intensities of wnt and fz hybridization were compared with β-actin hybridization intensity.
Transfection of Synovial Fibroblasts with a_wnt5a_ Expression Vector.
The human wnt5a cDNA was subcloned into the expression vector pcDNA3 (Invitrogen) at the _Eco_RI and _Eco_RV restriction sites. Lipofectamine (GIBCO) was used for transfecting synovial fibroblasts with the wnt5a expression construct and the empty vector. Briefly, synovial fibroblasts were plated 1 day before transfection in 6-well tissue culture plates (≈2 × 105 cells/ml, 1 ml each well) and incubated at 370 in 5% CO2. Plasmid DNA (≈1 μg) was complexed with 6 μl of Lipofectamine in 200 μl of serum-free antibiotic-free culture medium in each sterile tube for 30° min, after which 800 μl of medium containing 5% FBS was added. The cells in each well of the 6-well plate were washed with sterile PBS and then incubated with 1 ml of the transfection mix for 7–12 h. One milliliter of culture medium with 10% FBS was added to each well after 12 h. The transfection mix was replaced by fresh culture medium containing 10% FBS after 24 h. For transient transfection, cells were collected for assay after 48 h. For obtaining stable transfectants, 48 h after transfection, cells were washed, trypsinized, suspended in culture medium with 10% serum and 400 μg/ml of G418 (geneticin; selects for neomycin resistance gene) and plated in a 24-well plate with 500 μl in each well at a density of 2–6 cells per well. These cultures were maintained for 4–6 wk, fresh medium being added every other week. Cells from two wells of a 24-well plate were subsequently passaged ≈4 times separately in selective medium containing G418. One stable cell line was subsequently used for experiments.
Cytokine Assays.
RNA was extracted from RA fibroblasts and wnt5a and empty vector transfected synovial fibroblasts, by using RNA-zol. IL-6-, IL-8-, IL-15-, and glyceraldehyde-3-phosphate dehydrogenase (G3PDH)-specific primers were used for estimating the level of expression of the corresponding messages in the different cell populations by RT-PCR. G3PDH expression was used as an internal control for all cell types. The following list summarizes the primer pairs corresponding to the IL-6, IL-8, IL-15, and G3PDH genes used for PCR analysis:
IL-6: 5′-cagatgagtacaaaagtcctga-3′(forward); 5′-ctacatttgccgaagagccc-3′(reverse). IL-8: 5′-cagttttgccaaggagtgctaa-3′(forward); 5′-aacttctccacaaccctctgc-3′(reverse)
IL-15: 5′-gagttacaagttatttcacttgag-3′(forward); 5′-caagaagtgttgatgaacatttgg-3′(reverse). G3PDH: 5′-accacagtccatgcccatcac-3′(forward); 5′-tccaccaccctgttgctgta-3′(reverse).
Analysis of wnt5a Protein [Hemagglutinin (HA)-Tagged] Expression in Transfected Cells by Western Blotting.
Protein extracts were prepared from synovial cells transfected with the HA-tagged wnt5a expression construct, 48 h after transfection. After removal of medium and washing with PBS, 1 ml of lysis buffer (20 mM Tris⋅Hcl, pH 7.5/500 mM NaCl/1% Triton X-100/1 mM EDTA/50 mM DTT/2 mM PMSF) was added directly to the adherent cells in a 6-well tissue culture plate (150–160 μl was added to each well). The lysed mix was collected into a microfuge tube and assayed for total protein by using Bradford's reagent (Bio-Rad). Approximately 36 μg of protein from the _wnt5a_-HA transfectants and untransfected cells was used for Western blotting. A rabbit polyclonal anti-HA (Upstate Biotechnology, Lake Placid, NY) was used as a primary antibody and a horseradish peroxidase-conjugated goat anti-rabbit IgG (Transduction Laboratories, Lexington, KY) was used as a secondary antibody for the analysis, followed by visualization with a chemiluminescence system (Amersham Pharmacia Biotech).
Quantitation of IL-15 Protein Expression by ELISA.
ELISA was performed with supernatants from _wnt5a_-transfected synovial fibroblasts (stable transfectants), RA synovial fibroblasts, and normal synovial fibroblasts. About 3 μg supernatant protein was added to microtiter plates that had been coated previously with an anti-human IL-15 mAb (2 μg/ml). After overnight incubation and washing, about 1 μg/ml of a goat anti-human IL-15 polyclonal antibody was added to each well (R & D Systems). The secondary antibody used was horseradish peroxidase-conjugated donkey anti-goat IgG (The Jackson Laboratory).
Construction of wnt5a Expression Construct.
The wnt5a cDNA was amplified from human fetal brain cDNA (CLONTECH) by using the primers 5′-aaccttgaattcagttgctttggggatggctgg-3′(forward) and 5′-aaccttgatatccacccactacttgcacacaaac-3′ (reverse) for PCR. The amplified cDNA was subcloned into pcDNA3 into the _Eco_RI and_Eco_RV restriction enzyme sites. This expression vector was used for all functional studies. For the purpose of protein expression studies, an HA tag was introduced in-frame with the wnt5a coding sequence into the _Eco_RV and _Xho_I sites of a modified wnt5a expression construct. First, a modified reverse primer without the stop codon and the original forward primer were used to isolate by PCR the wnt5a cDNA from the fetal brain cDNA template. The modified wnt5a cDNA was then introduced into the _Eco_RI and _Eco_RV sites of pcDNA3. The HA tag with a stop codon incorporated at the end was then introduced in-frame with the wnt5a into the _Eco_RV and _Xho_I sites of the modified expression construct. The amino acid sequence of the HA-tag used was KAFSNCYPYDVPDYASLRS (24).
Results
Expression Pattern of wnt and fz mRNAs in RA vs. OA Tissue.
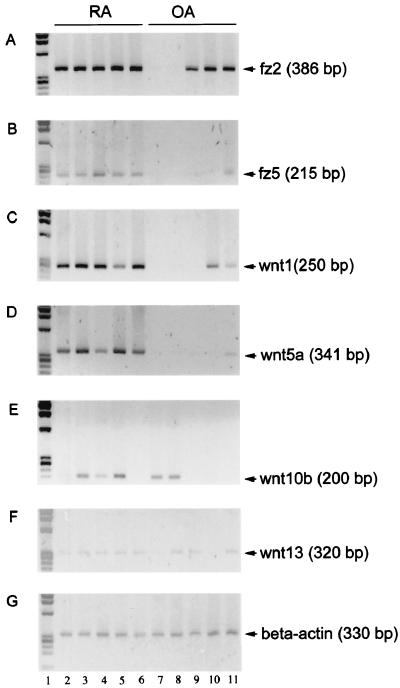
The levels of expression of different wnt and fz isoforms in RA vs. OA synovial tissues were compared first by RT-PCR. Table 1 summarizes the results, and Fig.1 depicts some of the amplified_wnt_ and fz isoform products. Of the five_fz_ isoforms tested, we detected the expression of_fz2_, fz5, and fz7 in all five RA tissue specimens. The same specimens also expressed wnt1,wnt5a, wnt11, and wnt13. wnt10b was found in three of five RA specimens. A comparison of the RA and OA tissue specimens revealed that the isoforms preferentially expressed in the RA tissues comprised the_wnt5a_ and fz5 ligand–receptor pair. All of the tissue specimens expressed comparable levels of the housekeeping gene β-actin. The wnt and fz PCR products were sequenced, confirming their identities.
Table 1.
Differential expression of wnt and_fz_ isoforms in synovial tissue specimens
| OA | RA | |
|---|---|---|
| fz2 | +/− | + |
| fz3 | nd | − |
| fz5 | −/+ | + |
| fz6 | nd | − |
| fz7 | + | + |
| wnt1 | +/− | + |
| wnt2 | nd | − |
| wnt5a | −/+ | + |
| wnt10b | +/− | +/− |
| wnt11 | + | + |
| wnt13 | +/− | + |
Figure 1.
RT-PCR analysis of various wnt and fz isoforms in RA and OA tissue specimens. Total RNA was extracted from five RA and five OA synovial tissue samples. RT-PCR was performed by using 1 μg of RNA from each sample with different primers for the specified wnt and fz isoforms as well as β-actin. (A) fz2, (B)fz5, (C) wnt1, (D) wnt5a, (E)wnt10b, (F) wnt13, and (G) β-actin. The PhiX 174 DNA standard is shown in the first lane of each gel.
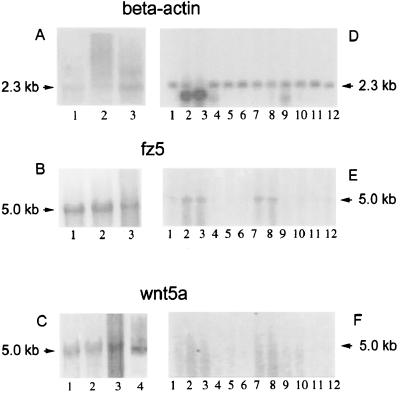
Northern blotting studies confirmed the preferential expression of_wnt5a_ and fz5 in RA synovial tissues compared with other human adult tissues (Fig. 2). Whereas three different RA tissues expressed high levels of the characteristic 5-kb fz5 and wnt5a mRNAs, 12 different human adult tissues expressed either much less or almost no detectable mRNAs of the same size. The latter results are consistent with previous data showing that wnt5a message in adult tissues is difficult to detect because of short half-life. As a positive control for wnt5a expression, we tested RNA from human fetal fibroblasts. The levels of wnt5a message were nearly equivalent in the RA synovial tissue and the embryonic cells when normalized to the housekeeping gene β-actin (Fig. 2).
Figure 2.
Expression of fz5, wnt5a, and β-actin in RA tissue samples and adult tissues by Northern analysis.A–C show the expression of β-actin,fz5, and wnt5a in RA tissue specimens.D–F show the expression of the same genes in 12 adult tissues that are peripheral blood leukocyte, lung, placenta, small intestine, liver, kidney, spleen, thymus, colon, skeletal muscle, heart, and brain (lanes 1–12, respectively, in D–F). (A) Northern blot showing 2.3-kb β-actin-specific band in three RA tissue specimens; (B) 5-kb_fz5_-specific band in three RA tissue specimens; (C) lanes 1–3, 5-kb _wnt5a_-specific band in three RA specimens; lane 4, _wnt5a_-specific band in fetal fibroblast as a positive control. (D) Northern blot showing 2.3-kb β-actin-specific band in 12 different adult tissue specimens; (E) 5-kb fz5_-specific band in the 12 adult tissue Northern blot; (F) result of_wnt5a probe hybridization by using the same Northern blot. Specific activity of the probes used for hybridization and RNA concentrations of the tissue samples was the same for each analysis. The adult multiple tissue Northern blot was stripped and reprobed during the course of the experiments, and β-actin hybridization was performed last of all.
Analysis of the Function of wnt5a in Rheumatoid Synovium.
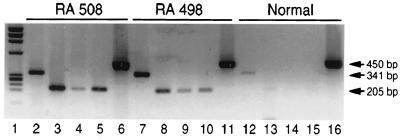
To determine whether isolated fibroblast-like cell lines established from the rheumatoid synovium continued to express wnt5a, RT-PCR studies were carried out. Compared with normal fibroblasts, the RA fibroblast cell lines expressed more wnt5a message after 6–10 passages in tissue culture (Fig.3).
Figure 3.
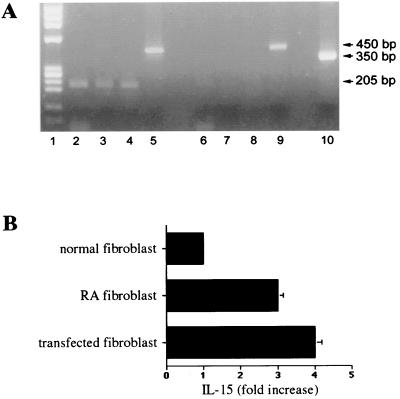
RT-PCR analysis to identify expression of wnt5a, IL-6, IL-8, and IL-15 in RA fibroblasts compared with normal synovial fibroblasts. Lane 1, DNA standard PhiX174; lane 2,_wnt5a_-specific RT-PCR product from rheumatoid synovial fibroblast (RA 508); lanes 3–5 and 6, IL-6-, IL-8-, IL-15-, and G3PDH-specific RT-PCR products from RA 508; lanes 7–11,_wnt5a_-, IL-6-, IL-8-, IL-15-, and G3PDH-specific RT-PCR products from RA 498; lanes 12–16, _wnt5a_-, IL-6-, IL-8-, IL-15-, and G3PDH-specific RT-PCR products in normal synovial fibroblasts.
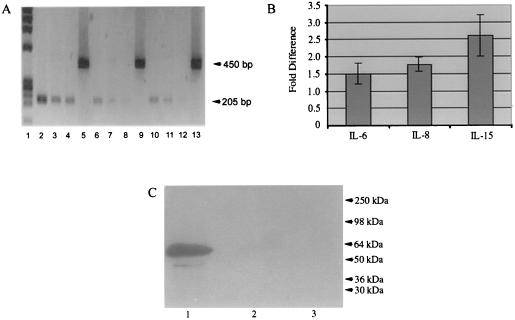
wnt5a signaling has been reported to stimulate PKC (22). The PKC pathway can modulate the transcription of NFκB-dependent genes, which include the cytokines IL-6, IL-8, and IL-15 (25–28). Indeed, the same RA synovial fibroblasts that overexpressed wnt5a also produced more IL-6, IL-8, and IL-15, compared with the normal synovial fibroblasts (Fig. 3). Moreover, transient transfection of normal synovial fibroblasts with a_wnt5a_ expression vector, but not with the pcDNA3 control vector, increased IL-6, IL-8, and IL-15 mRNA to levels approximately equivalent to those observed in the RA cells (Fig.4A). Both the_wnt5a_-transfected and empty vector-transfected synovial fibroblasts expressed similar levels of the G3PDH message (Fig.4A). In Fig. 4B, each vertical bar graphically represents the fold difference (average of five different experiments) between the intensities of a specific IL gene product (IL-6, IL-8, or IL-15) expressed in _wnt5a_- and empty vector-transfected synovial fibroblasts. Fold difference was calculated after evaluating the intensities of the different gene products by using Kodakimage analysis Software, Ver. 2.0.2, for Macintosh. The transfection of the fibroblasts was confirmed by Western blotting by using a polyclonal antibody against an HA tag fused to the carboxyl-terminal end of the wnt5a protein (Fig.4C).
Figure 4.
RT-PCR experiment to analyze IL-6, IL-8, and IL-15 expression in_wnt5a_-transfected (transient) normal synovial fibroblasts and determination of transfection efficiency.(A) Lane 1, DNA standard PhiX174; lanes 2–5, IL-6-, IL-8-, IL-15-, and G3PDH-specific RT-PCR products from_wnt5a_-pcDNA3-transfected normal synovial fibroblasts; lanes 6–9, IL-6-, IL-8-, IL-15-, and G3PDH- specific RT-PCR products from empty vector transfected normal synovial fibroblasts; lanes 10–13, IL-6-, IL-8-, IL-15-, and G3PDH-specific RT-PCR products from untransfected normal synovial fibroblasts. (B) Bar graph showing fold increase in IL-6, IL-8, and IL-15 gene expression (measured by PCR product intensity) on transfection of normal synovial fibroblasts with wnt5a expression construct. Fold increase with wnt5a is compared with the effects produced by transfection by the empty vector. This represents an average of five different experiments. For each experiment, the same amounts of RNA were used from the _wnt5a_- and empty vector-transfected cells. G3PDH expression was used an internal control. (C) Western blot of lysate from_wnt5a_-HA-transfected synovial fibroblasts. Lane 1, ≈60 kDa _wnt5a_-HA protein from_wnt5a_-HA-transfected synovial fibroblasts; lanes 2 and 3, no band observed from untransfected synovial fibroblasts.
Results obtained from transient transfection studies were reproduced by stable transfection of normal synovial fibroblasts with the wnt5a expression vector (Fig.5A). After four passages in culture, the wnt5a stable transfectants of normal synovial fibroblasts continued to express more IL-6, IL-8, and IL-15 (Fig.5A, lanes 2–4) than did the empty vector (pcDNA3) stable transfectants (Fig. 5A, lanes 6–8). Both the_wnt5a_ and empty vector stable transfectants expressed comparable levels of G3PDH (Fig. 5A, lanes 5 and 9). The specific PCR product obtained by RT-PCR on RNA of wnt5a stable transfectants by using wnt5a gene-specific and pcDNA3 vector-specific primers shows that wnt5a RNA is transcribed off the expression construct (Fig. 5A, lane 10). That_wnt5a_ protein is expressed in wnt5a stable transfectants was confirmed by Western blotting with antibodies to the HA tag fused to the carboxyl-terminal end of wnt5a (data not shown).
Figure 5.
(A) RT-PCR analysis of IL-6, IL-8, and IL-15 in_wnt5a_ and pcDNA3 stable transfectants. Lane 1, DNA standard PhiX174; lanes 2–5, IL-6-, IL-8-, IL-15-, and G3PDH-specific PCR products from wnt5a stable transfectants; lanes 6–9, IL-6-, IL-8-, IL-15-, and G3PDH-specific PCR products obtained from pcDNA3 stable transfectants; lane10, _wnt5a_-specific PCR product (using _wnt5a_-specific and pcDNA3-bovine growth hormone-specific primers) obtained from wnt5a stable transfectant. (B) IL-15 protein expression in synovial fibroblasts. Bar graph showing fold difference in IL-15 protein levels in cell supernatants of RA,_wnt5a_-transfected, and normal synovial fibroblasts. This represents the average of three experiments. The baseline level of expression was estimated to be 60 pg/μg total protein by comparison with a known standard.
Association of wnt5a Overexpression with IL-15 Protein Synthesis in Synovial Fibroblasts.
Among the cytokines tested, the most pronounced effect of_wnt5a_ overexpression was on IL-15 gene transcription. Therefore, we compared IL-15 protein levels in RA and wnt5a transfected synovial fibroblasts (stable transfectants) with normal synovial fibroblasts (Fig. 5B). Initial Western blotting studies with a monoclonal anti-IL-15 antibody showed that the reactive IL-15 bands were much more intense in the RA- and_wnt5a_-transfected synovial fibroblasts than in the normal synovial fibroblasts (data not shown). Therefore we quantitated, by ELISA, the relative levels of IL-15 in the supernatants of the rheumatoid, _wnt5a_-transfected and normal synovial fibroblasts. By using equivalent amounts of culture supernatant proteins, the IL-15 levels were 3- to 4-fold higher in the RA- and_wnt5a_-transfected synovial fibroblast supernatants than in the normal fibroblast supernatant (Fig. 5B). Each horizontal bar in Fig. 5B represents an average of three different experiments. These results suggest that wnt5a overexpression can enhance IL-15 mRNA and protein expression in synovial fibroblasts.
Discussion
Various members of the wnt and fz families control tissue patterning and cell-fate determination during embryogenesis. At least 13 wnt genes and eight or more_fz_ genes have been identified. The wnt proteins are secreted glycoproteins that bind to the cell surface or the extracellular matrix and thus probably act locally in an autocrine or paracrine fashion. The fz proteins are typical G-coupled proteins that function as wnt receptors. However, the exact specificities of the wnt/fz ligand–receptor pairs have been difficult to determine, because some_wnt_ proteins can interact with multiple fz receptors. We have found that both wnt5a and fz5, which have been reported to function as a ligand–receptor pair, are overexpressed in the RA synovium in comparison to OA and normal adult tissues.
wnt5a has been reported to be an activator of the PKC signaling cascade (22), and it has been shown that activated PKC enhances NFκB activation and translocation to the nucleus (25, 26). It is also well documented that activated NFκB causes transcriptional activation of IL genes like IL-6, IL-8, and IL-15 (27, 28). Our results showed that not only wnt5a, but also IL-6, IL-8, and IL-15, are overexpressed in cultured RA fibroblast-like synoviocytes compared with normal synovial fibroblasts. Moreover, transfection of normal synovial fibroblasts with a wnt5a expression construct increased IL-6, IL-8, and IL-15 mRNAs to levels equivalent to those observed in RA fibroblast-like synoviocytes. Collectively, these data suggest that activation of the IL-6, IL-8, and IL-15 genes in RA fibroblast-like synoviocytes is influenced by_wnt5a_-dependent signaling events. Future experiments will aim to investigate whether wnt5a mediates signaling through PKC and NFκB in the RA fibroblast-like synoviocytes. It will also be investigated whether the constitutive production of the cytokines IL-6, IL-8, and IL-15 by the RA fibroblast-like synoviocytes can be blocked by inhibiting wnt5a_-mediated signaling. To carry out the relevant experiments, antisense wnt5a, a dominant negative_wnt5a, or a soluble fragment of an fz receptor that binds to the signal generating motif of wnt5a would be useful.
Yamaguchi and others (29, 30) found that wnt5a is required for limb-bud development. Austin et al. (17) reported that wnt5a could stimulate growth and colony formation of purified murine hematopoietic progenitors. Recently, Van den Berg and coworkers (21) found that wnt5a was expressed in human CD34+ bone marrow progenitors, and that infection of the cells with a _wnt5a_-expressing retrovirus enhanced mixed colony formation.
wnt5a has been shown to interact with the cell-surface receptors fz5 and fz2 (22). Detectable levels of mRNAs corresponding to both fz species were present in OA tissues (Table 1), and also in normal synovial fibroblasts. The stoichiometric levels of wnt5a and_fz5_/fz2 required to initiate signaling are not clearly known. However, a plausible mechanism by which overexpressed_wnt5a_ in the transfected synovial fibroblasts could exert its effects is via autocrine and/or paracrine loops involving cell surface fz5/fz2 receptors.
Considering that wnt5a has been shown to influence both limb-bud mesenchyme and bone marrow stem cell development, it is conceivable that it also plays a role in chronic inflammatory diseases associated with joint destruction and attempted regeneration. In long-standing RA, the fibroblast-like synoviocytes acquire an abnormal phenotype, characterized by the synthesis of inflammatory cytokines, metalloproteinases, and the up-regulation of cell-surface adhesive molecules (8, 31–33). These properties are thought to be responsible for the destruction of articular cartilage. The activated phenotype of fibroblast-like synoviocytes in RA is at least partly autonomous, because it persists after several passages of the cells in tissue culture.
Possible explanations for the aggressive characteristics of RA fibroblast-like synoviocytes include, (i) genetic transformation caused by retroviral infection or somatic mutations; (ii) sorting and selection of variants from a preexisting heterogeneous cell population; and (iii) migration of new cells from the blood and bone marrow. These hypotheses are not mutually exclusive. Mutations of the p53 gene have been documented in RA and have been shown to influence the growth of RA synovial fibroblasts (34). However, the polyarticular nature of RA and the infrequent occurrence of synovial tumors argue against genetic transformation as a sole explanation for the aggressive RA fibroblast phenotype.
The major cytokines found in RA joints (TNF-α, IL-1) are potent growth stimulators of fibroblast-like synoviocytes together with osteoprotegerin (35). They may also promote the formation of osteoclast-like cells that which can enlarge channels between the bone marrow and the synovial cavity, as has been observed in a murine model of collagen-induced arthritis (9). Intense growth stimulation may cause immature mesenchymal cells, with the characteristics of RA fibroblast-like synoviocytes, to replace gradually the normal synovial lining layer of mature fibroblasts. In this context, mention should be made of pannocytes, distinctive cells observed in rheumatoid arthritis cartilage erosions, which might represent an earlier stage of mesenchymal cell differentiation (36).
The results of our investigations may have implications for the therapy of RA. Because of autocrine or paracrine signaling between_wnt_ and fz family members, the persistent activation of immature fibroblast-like synoviocytes in longstanding RA may not require continued exposure to inflammatory cells and cytokines. Thus, control of inflammation in RA should be instituted early, before the outgrowth of the immature cells. In long-standing disease refractory to inflammation inhibitors, blockade of_wnt_/fz signaling could be of therapeutic benefit. The lack of availability of recombinant mammalian proteins and specific antibodies has hampered analyses of wnt and_fz_ function in human diseases. Future studies will be needed to ascertain exactly which wnt and fz proteins are expressed in fibroblast-like synoviocytes and the effects of_wnt_/fz neutralization on the progression of arthritis.
Acknowledgments
We thank Nathan Zvaifler for helpful discussions, David Boyle for synoviocyte cultures (University of California, San Diego), and Nancy Noon for preparing the manuscript. This work was supported in part by Grants AR44850, AR07567, and AR40770 from the National Institutes of Health and by a pilot research award from Howard Hughes Medical Institute Institutional Award 76296–549809.
Abbreviations
RA
rheumatoid arthritis
TNF-α
tumor necrosis factor α
OA
osteoarthritis
RT-PCR
reverse transcriptase–PCR
HA
hemagglutinin
G3PDH
glyceraldehyde-3-phosphate dehydrogenase
PKC
protein kinase C
Footnotes
§
A preliminary report of this work was presented at the 1999 meeting of the American College of Rheumatology, at Boston.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.050574297.
References
- 1.Koch A E. Arthritis Rheum. 1998;41:951–962. doi: 10.1002/1529-0131(199806)41:6<951::AID-ART2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 2.Goronzy J J, Zettl A, Weyand C M. Int Rev Immunol. 1998;17:339–363. doi: 10.3109/08830189809054410. [DOI] [PubMed] [Google Scholar]
- 3.Ohba T, Takase Y, Ohhara M, Kasukawa R. J Rheumatol. 1996;23:1505–1511. [PubMed] [Google Scholar]
- 4.Bresnihan B. J Rheumatol. 1999;26:717–719. [PubMed] [Google Scholar]
- 5.Monier S, Reme T, Cognot C, Gao Q L, Travaglio-Encinoza A, Cuchacovich M, Gaillard J-P, Jorgensen C, Sany J, Dupuy D'Angeac A, et al. Clin Exp Rheumatol. 1994;12:595–602. [PubMed] [Google Scholar]
- 6.Badolato R, Oppenheim J J. Semin Arthritis Rheum. 1996;26:526–538. doi: 10.1016/s0049-0172(96)80041-2. [DOI] [PubMed] [Google Scholar]
- 7.Peichl P, Pursch E, Broll H, Lindley I J. Rheumatol Int. 1999;18:141–145. doi: 10.1007/s002960050073. [DOI] [PubMed] [Google Scholar]
- 8.Harada S, Yamamura M, Okamoto H, Morita Y, Kawashima M, Aita T, Makino H. Arthritis Rheum. 1999;42:1508–1618. doi: 10.1002/1529-0131(199907)42:7<1508::AID-ANR26>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki Y, Nishikaku F, Nakatuka M, Koga Y. J Rheumatol. 1998;25:1154–1160. [PubMed] [Google Scholar]
- 10.Tomlinson A, Strapps W R, Heemskerk J. Development (Cambridge, UK) 1997;124:4515–4521. doi: 10.1242/dev.124.22.4515. [DOI] [PubMed] [Google Scholar]
- 11.Zhang J, Carthew R W. Development (Cambridge, UK) 1998;125:3075–3085. doi: 10.1242/dev.125.16.3075. [DOI] [PubMed] [Google Scholar]
- 12.Wodarz A, Nusse R. Annu Rev Cell Dev Biol. 1998;14:59–88. doi: 10.1146/annurev.cellbio.14.1.59. [DOI] [PubMed] [Google Scholar]
- 13.Cadigan K M, Nusse R. Genes Dev. 1997;11:3286–3305. doi: 10.1101/gad.11.24.3286. [DOI] [PubMed] [Google Scholar]
- 14.Slusarski D C, Corces V G, Moon R T. Nature (London) 1997;390:410–413. doi: 10.1038/37138. [DOI] [PubMed] [Google Scholar]
- 15.Barnes M R, Duckworth D M, Beeley L J. Trends Pharmacol Sci. 1998;19:399–400. doi: 10.1016/s0165-6147(98)01246-2. [DOI] [PubMed] [Google Scholar]
- 16.Giarre M, Samenov M V, Brown A M C. Ann NY Acad Sci. 1998;857:43–55. doi: 10.1111/j.1749-6632.1998.tb10106.x. [DOI] [PubMed] [Google Scholar]
- 17.Austin T W, Solar G P, Ziegler F C, Liem L, Matthews W. Blood. 1997;89:3624–3635. [PubMed] [Google Scholar]
- 18.Lane T F, Leder P. Oncogene. 1997;15:2133–2144. doi: 10.1038/sj.onc.1201593. [DOI] [PubMed] [Google Scholar]
- 19.Blankesteijn W M, Essers-Janssen Y P, Ulrich M M, Smits J F. J Mol Cell Cardiol. 1996;28:1187–1191. doi: 10.1006/jmcc.1996.0109. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka S, Akiyoshi T, Mori M, Wands J R, Sugimachi K. Proc Natl Acad Sci USA. 1998;95:10164–10169. doi: 10.1073/pnas.95.17.10164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Den Berg D J, Sharma A K, Bruno E, Hoffman R. Blood. 1998;92:3189–3202. [PubMed] [Google Scholar]
- 22.Sheldahl L C, Park M, Malbon C C, Moon R T. Curr Biol. 1999;9:695–698. doi: 10.1016/s0960-9822(99)80310-8. [DOI] [PubMed] [Google Scholar]
- 23.He X, Saint-Jeannet J-P, Wang Y, Nathans J, Dawid I, Varmus H. Science. 1997;275:1652–1654. doi: 10.1126/science.275.5306.1652. [DOI] [PubMed] [Google Scholar]
- 24.Field J. J Mol Cell Biol. 1998;8:2159–2165. doi: 10.1128/mcb.8.5.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilson L, Szabo C, Salzman A L. Gastroenterology. 1999;117:106–114. doi: 10.1016/s0016-5085(99)70556-1. [DOI] [PubMed] [Google Scholar]
- 26.Anrather J, Csizmadia V, Soares M P, Winkler H. J Biol Chem. 1999;274:13594–13603. doi: 10.1074/jbc.274.19.13594. [DOI] [PubMed] [Google Scholar]
- 27.Ghosh S, May M J, Kopp E B. Annu Rev Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 28.Washizu J, Nishimura H, Nakamura N, Nimura Y, Yoshikai Y. Immunogenetics. 1998;48:1–7. doi: 10.1007/s002510050393. [DOI] [PubMed] [Google Scholar]
- 29.Kawakami Y, Wada N, Nishimatsu S I, Ishikawa T, Noji S, Nohno T. Dev Growth Differ. 1999;41:29–40. doi: 10.1046/j.1440-169x.1999.00402.x. [DOI] [PubMed] [Google Scholar]
- 30.Yamaguchi T P, Bradley A, McMahon A P, Jones S. Development (Cambridge, UK) 1999;126:1211–1223. doi: 10.1242/dev.126.6.1211. [DOI] [PubMed] [Google Scholar]
- 31.Miyazawa K, Mori A, Okudaira H. J Allergy Clin Immunol. 1999;103:S437–S444. doi: 10.1016/s0091-6749(99)70159-4. [DOI] [PubMed] [Google Scholar]
- 32.Case J P, Lafyatis R, Remmers E F, Kumkumian G K, Wilder R L. Am J Pathol. 1989;135:1055–1064. [PMC free article] [PubMed] [Google Scholar]
- 33.Sarkissan M, Lafyatis R. J Immunol. 1999;162:1772–1779. [PubMed] [Google Scholar]
- 34.Firestein G S, Echeverri F, Yeo M, Zvaifler N J, Green D R. Proc Natl Acad Sci USA. 1997;94:10895–10900. doi: 10.1073/pnas.94.20.10895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kong Y-Y, Feige U, Sarosi I, Bolon B, Tafuri A, Morony S, Capparelli C, Li J, Elliott R, McCabe S, et al. Nature (London) 1999;402:304–308. doi: 10.1038/46303. [DOI] [PubMed] [Google Scholar]
- 36.Zvaifler N J, Tsai V, Alsalameh S, von Kempis J, Firestein G S, Lotz M. Am J Pathol. 1997;150:1125–1138. [PMC free article] [PubMed] [Google Scholar]