Dual Infection with Helicobacter bilis and Helicobacter hepaticus in P-Glycoprotein-Deficient mdr1a−/− Mice Results in Colitis that Progresses to Dysplasia (original) (raw)
Abstract
Patients with inflammatory bowel disease (IBD) are at increased risk for developing high-grade dysplasia and colorectal cancer. Animal IBD models that develop dysplasia and neoplasia may help elucidate the link between inflammation and colorectal cancer. _Mdr1a_−/− mice lack the membrane efflux pump p-glycoprotein and spontaneously develop IBD that can be modulated by infection with Helicobacter sp: H. bilis accelerates development of colitis while H. hepaticus delays disease. In this study, we determined if H. hepaticus infection could prevent _H. bilis_-induced colitis. Unexpectedly, a proportion of dual-infected _mdr1a_−/− mice showed IBD with foci of low- to high-grade dysplasia. A group of dual-infected _mdr1a_−/− animals were maintained long term (39 weeks) by intermittent feeding of medicated wafers to model chronic and relapsing disease. These mice showed a higher frequency of high-grade crypt dysplasia, including invasive adenocarcinoma, possibly because H. hepaticus, in delaying the development of colitis, allows time for transformation of epithelial cells. Colonic epithelial preparations from co-infected mice showed increased expression of c-myc (5- to 12-fold) and interleukin-1α/β (600-fold) by real-time polymerase chain reaction relative to uninfected wild-type and _mdr1a_−/− animals. This animal model may have particular relevance to human IBD and colorectal cancer because certain human MDR1 polymorphisms have been linked to ulcerative colitis and increasedrisk for colorectal cancer.
Colorectal cancer is one of the most common forms of cancer in industrialized countries and is increasing in incidence.1 Although animal models have been useful in understanding the mechanisms involved in the pathogenesis of colorectal cancer, differences between these models and human disease may limit their relevance.2 For example, targeted disruption in the mouse of the APC gene, which has been implicated in human colon cancer, results primarily in cancer of the small intestine3 suggesting that additional animal models would be useful to study cancer development in the colon.
Patients with inflammatory bowel disease (IBD) have an increased risk for developing colorectal cancer.4,5 Hence, the induction of inflammation and its subsequent link to dysplasia and cancer needs further study. Animal models that display inflammation of the large bowel, which progresses to dysplasia and neoplasia would be helpful in understanding the molecular and cellular mechanisms that result in the transformation of large intestinal epithelial cells during colon carcinogenesis. Helicobacter are microaerophilic gram-negative spiral bacterial organisms6 that have been associated with gastric cancer in humans,7 and as well as hepatitis,8 hepatocellular carcinoma,9 and IBD in rodents.10 In particular, there is established literature implicating H. pylori with gastric cancer in man,11 and H. hepaticus has been associated with hepatocellular carcinoma in A/JCr mice12 and colorectal cancer in RAG2-knockout mice.13,14 We have previously used H. hepaticus and H. bilis infectionsin genetically susceptible strains of mice such as IL-10−/− and TCRα−/−15 and in _mdr1a_−/−16 to understand microbial modulation of IBD. _Mdr1a_−/− mice lack the transmembrane efflux transporter, p-glycoprotein, and develop spontaneous colitis.17 We have previously shown that H. bilis accelerates and increases the severity of inflammation and hyperplasia in _mdr1a_−/− mice, while H. hepaticus delays the development of colitis.16 Therefore, we hypothesized that H. hepaticus infection might delay or ameliorate the development of _H. bilis_-induced colitis. Notably, we found that dual-infection with both H. hepaticus and H. bilis in _mdr1a_−/− mice resulted in colitis that was characterized by dysplasia, particularly if longer survival could be attained with intermittent treatment of infected mice with medicated wafers. Dysplastic colonic tissue from dual-infected _mdr1a_−/− mice had increased numbers of CD4+ T cells, macrophages, and Cox-2+ cells. Colonic epithelial cell preparations from dual-infected _mdr1a_−/− mice had increased expression of the proinflammatory cytokines interleukin (IL)-1α and IL-1β, and the oncogene c-myc. This animal model may be useful to study factors important in the progression of chronic inflammation to dysplasia and could have relevance to human bowel disease and colorectal cancer because certain MDR1 polymorphisms in man have been linked to ulcerative colitis18 and increased risk for neoplasia19 including colorectal cancer.20
Materials and Methods
Mice
For study 1, _mdr1a_−/− (FVB.129P2-PAbcb1a tm1Bor) were obtained from Taconic Farms (Albany, NY) and 3- to 6-week-old female _mdr1a_−/− specific pathogen-free mice were used. These mice have a targeted mutation in Pgy3 and the gene name has now been changed from Pgy3 to Abcb1a, ATP-binding cassette, subfamily B (MDR/TAP), member 1A. For study 2 and study 3, colony mice were obtained from our breeder _mdr1a_−/− and FVB wild-type mouse colony (originally obtained from Taconic Farms). All mice were certified free of Helicobacter spp. by the vendor and retested on site by fecal polymerase chain reaction (PCR) before each experiment. Animals were housed in a specific pathogen-free facility at the University of Washington in polycarbonate microisolator or ventilated cages containing Bed-O-Cob (Andersons, Maumee, OH) and nestlets. Mice were fed irradiated Picolab Rodent Diet 20 no. 5053 (PMI Nutrition Int., Brentwood, MO) and autoclaved, acidified water. All supplies entering animal rooms were autoclaved and rooms were maintained at 21 to 23°C, 45 to 55% humidity, with 28 air changes/hour 12/12-hour light/dark cycle. To prevent cross contamination of uninfected mice and infected mice, cages were changed in uninfected or infected changing stations. Sentinel mice were tested quarterly for endo- and ectoparasites, mouse hepatitis virus, mouse parvovirus, and rotavirus, and annually for Mycoplasma pulmonis, pneumonia virus of mice, reovirus-3, Sendai virus, and Theiler’s murine encephalomyelitis virus. Also, quarterly fecal colon samples were screened for Citrobacter rodentium, nonlactose fermenting Escherichia coli, Salmonella spp., Klebsiella spp., and Clostridium spp. (Phoenix Laboratories, Everett, WA). All animal procedures were approved by the University of Washington Animal Care and Use Committee.
Helicobacter Isolates
H. bilis was a natural isolate and kindly provided by L. Riley (University of Missouri, Columbia, MO) and H. hepaticus was obtained from the American Tissue Culture Collection (ATCC 51448; Rockville, MD). Organisms were streaked onto Brucella blood agar plates and grown under microaerobic conditions (90% N2, 5% H2, and 5% CO2) in vented jars (Oxoid, Hampshire, UK) kept at 37°C. Bacteria were harvested and inoculated into flasks containing 150 ml of Brucella broth supplemented with 5% fetal bovine serum (Sigma Chemical Co., St. Louis, MO). The flasks were placed on a continuous shaker and incubated for 24 to 48 hours at 37°C in microaerophilic conditions. The organisms were centrifuged at 10,000 rpm at 4°C for 20 minutes. The resultant pellet was examined by gram stain and phase microscopy for purity, morphology, and motility. Organisms were confirmed to be catalase-, urease-, and oxidase-positive. The pellet was resuspended in Brucella broth and optical density was adjusted to 1.0 (OD600) for an estimated 108 CFU/ml.
Helicobacter Infection
Before infection, all mice were determined to be negative for Helicobacter spp. by fecal PCR.15 Mice were infected with 2 × 107 CFU of H. bilis or H. hepaticus in a 0.2-ml volume by oral gavage two to four times.16 Study 1 comprised 90 _mdr1a_−/− mice: H. bilis alone (n = 10), H. hepaticus alone (n = 20), H. bilis followed 5 days later by H. hepaticus (n = 20), H. hepaticus followed 5 days later by H. bilis (n = 20), or broth alone (n = 20). Study 2 used 40 _mdr1a_−/− mice: H. hepaticus alone (n = 10), H. bilis followed 5 days later by H. hepaticus (n = 20), and broth alone (n = 10). Study 3 used 20 _mdr1a_−/− mice: H. bilis followed 5 days later by H. hepaticus (n = 10), and broth alone (n = 10) and 20 FVB wild-type mice: H. bilis followed 5 days later by H. hepaticus (n = 10) and broth alone (n = 10). Confirmation of infection was done on pooled fecal cage samples taken at several points during the studies and at necropsy. Fecal samples were tested by PCR for cross-contamination with the other Helicobacter spp. in infected mice or for absence of Helicobacter infection in uninfected animals. Mice were weighed monthly and monitored weekly or bimonthly for weight loss, dehydration, and diarrhea. Mice were euthanized by CO2 when they developed severe diarrhea or 20% body weight loss and samples were taken for histopathology, immunohistochemistry, and colonic epithelial cell preparations. Study 1 mice were euthanized at 15 to 40 weeks after infection at 4 to 10 months of age and study 2 mice were euthanized at 31 to 39 weeks after infection when ∼10 months old. Study 3 animals were euthanized at 23 weeks after infection and only used for Cox-2 immunohistochemistry.
Medicated Feed to Maintain Colitic Mice
In study 2, medicated wafers containing bismuth, amoxicillin, and metronidazole (Bio-Serve, Frenchtown, NJ) were fed to _mdr1a_−/− mice to allow them to maintain body weight, decrease severity of disease, and favor the development of chronic colitis with dysplasia. To prolong survival of _mdr1a_−/− mice with chronic colitis, all uninfected broth and dual-infected (H. bilis and H. hepaticus) mice were intermittently fed medicated wafers for a 2-month period at 6 to 16 weeks after infection and for a 1-month period at 18 to 20 weeks after infection. In study 3, all uninfected broth and dual-infected (H. bilis and H. hepaticus) mdr and FVB wild-type were given a medicated wafer regimen that started at 8 weeks after infection. The regimen included feeding medicated wafers for 1 week and regular rodent chow for 3 weeks. In both study 2 and study 3, diarrhea was ameliorated to some degree while animals were on medicated wafers.
Histopathological Analysis
At necropsy, animals were sacrificed by CO2-asphixiation. The gastrointestinal tract was isolated and the colon separated from the cecum. Samples of proximal colon and cecum were placed into 10% neutral buffered formalin or frozen in OCT-embedding material. Formaldehyde-fixed tissues were routinely processed and sections stained with hematoxylin and eosin. Cecum and proximal colon sections were graded by two blinded pathologists (H.B.O. and P.T.) for degree of IBD and dysplasia. Dysplasia was graded on a 1 to 4 scale (1, no dysplasia; 2, low grade; 3, high grade; 4, high grade with invasion/adenocarcinoma) incorporating criteria recently reported by Boivin and colleagues.21 Briefly, low-grade dysplasia was characterized by thickened mucosa with branching and elongated crypts with normal epithelial differentiation including goblet cells, maintenance of polarity, and nuclear morphology. High-grade dysplasia was characterized by thickened mucosa with elongate, irregularly branching glands, cytological and nuclear atypia including loss of differentiation and polarity, bunched and enlarged nuclei, and numerous mitotic figures.
Immunohistochemistry
MHC class II, β-catenin, CD44, presence of CD4+ T cells and macrophages (F4/80) were analyzed in colon tissue from four to six animals in each experimental group from study 2 animals. Cox-2 was analyzed from two animals from each group in study 3. Immunohistochemistry was performed on tissues snap-frozen in OCT or fixed in formalin using antigen retrieval. Briefly, 5- to 6-μm frozen sections were fixed with acetone/ethanol (volume, 75%/25%) and 5-μm paraffin sections were subjected to heat-induced epitope retrieval in 0.01 mol/L citrate buffer, pH 6.0, using a microwave oven for 10 minutes as described.22 Endogenous peroxidase activity was inhibited by sodium azide with trace amounts of nascent H2O2 produced by a glucose oxidase/glucose system.23 Sections were incubated for 60 minutes with the following primary antibodies (M5/114, F4/80, RM4–5 for class II, macrophages, and CD4+ T cells, respectively; BD Biosciences, San Diego, CA; anti-β-catenin, Santa Cruz Biotechnology, Santa Cruz, CA; anti-Cox-2, Cayman Chemical, Ann Arbor, MI; anti-CD44, BD Biosciences, San Diego, CA). The secondary biotinylated antibodies were then applied for 30 minutes, followed by detection of signal with the avidin-biotin immunoperoxidase, Vectastain ABC kit (Vector Laboratories, Inc., Burlingame, CA). 3,3′-Diaminobenzidine was used as the chromogen. Negative controls included the exclusion of the primary antibody. All slides were evaluated by the pathologist blinded to experimental group.
Isolation of Colonic Epithelial Cells
Colonic epithelial preparations were done on four uninfected, four H. hepaticus- infected and seven H. bilis- and _H. hepaticus-_infected _mdr1a_−/− animals from study 2 and two uninfected FVB+/+ mice. Colon was harvested and washed with Hanks’ balanced salt solution (HBSS) without calcium, magnesium, or phenol red. Colonic epithelial cells were isolated following a minor modification of the method of Ogawa and colleagues.24 Briefly, colon was minced to 3 to 4 mm in length and incubated in HBSS with 0.01 mol/L dithiothreitol for 10 minutes at room temperature. Then tissue pieces were placed in HBSS with 1.5 mmol/L ethylenediamine tetraacetic acid at room temperature for three 15-minute periods. At the end of each period, the tissues were agitated rapidly with a glass rod, the supernatants were aspirated, centrifuged at 1000 rpm 10 minutes at 4°C, and then the cell pellets were washed twice in HBSS and used for RNA isolation.
Cytokine Analysis by Multiplex Polymerase Chain Reaction
RNA was purified using a total RNA isolation kit TRI Reagent (Sigma, St. Louis, MO). IL-6, IL-1α, IL-1Rα, IL-1β, and transforming growth factor-β1 mRNA was analyzed by using Mouse Inflammatory Cytokine Set 3 Multiplex PCR kit (Biosource Int., Inc., Camarillo, CA). Interferon-γ, IL-10, IL-13, tumor necrosis factor-α, and IL-12p40 mRNA were also analyzed by using Mouse Cytokines CytoXpress Multiplex-PCR kit (Biosource Int., Inc.). PCR reaction tubes contained 5 μl of cDNA and reagents provided by the kit. The following PCR profile was used for amplification: 95°C for 1 minute, followed by 6 cycles of 94°C for 1 minute and 60°C for 4 minutes, then 36 cycles of 94°C for 1 minute and 68°C for 2.5 minutes, and a final cycle of 70°C for 10 minutes. For Mouse Cytokines CytoXpress Multiplex-PCR kit, the following PCR profile was used for amplication: 5 cycles of 95°C for 1 minute and 60°C for 3 minutes, then 35 cycles of 94°C for 1 minute and 65°C for 2 minutes. PCR product and 1× loading buffer were loaded onto 1% agarose gel and run at 100 V. The gel was stained with ethidium bromide and photographed.
RNA and cDNA Preparation and Real-Time Quantitative PCR
Ten μg of total RNA was treated with DNAase using Absolutely RNA RT-PCR miniprep kit (Stratagene, La Jolla, CA). DNA-free cDNA was generated by taking 2 μg of total DNase-treated RNA and adding 1 μl of 10 mmol/L dNTPs, 3 μl of random hexamers (stock 1:4 diluted in depC water; Invitrogen, Carlsbad, CA), and 6 μl of water. The tubes were heated at 68°C for 5 minutes, then cooled on ice for 1 minute. Four μl of first strand cDNA buffer (Life Technologies Inc., Grand Island, NY), 2 μl of 0.1 mol/L dithiothreitol, 1 μl RNase inhibitor (40 mg/ml, Life Technologies Inc.), and 1 μl of MMLV reverse transcriptase (RT) were added to each tube. A no RT control was also made for each reaction. The reactions were incubated at room temperature for 10 minutes, followed by 42°C for 1 hour, and heat inactivated at 70°C for 15 minutes. Two μl of the RT and no RT reactions were used for PCR with eIF1α oligos to confirm the absence of DNA contamination. For real-time PCR, 1 μl of cDNA was amplified using SyBR green dye (Applied Biosystems, Foster City, CA) and PCR reactions were performed according to manufacturer’s instructions (Brilliant SYBR Green QPCR Master Mix, Applied Biosystems). Reactions were amplified using ABI 7700 Prism thermocycler and sequence detector. All PCR reactions were run in parallel with β-actin as a loading control. No cDNA controls were also run with each sample to ensure purity. The efficiency of each primer set compared to β-actin was tested with serial dilutions of cDNA to ensure that they amplify equally. We then used the comparative CT method to determine the amount of target cDNA according to the equation 2−Δ Δ CT.25 RT-PCR for H. bilis and H. hepaticus was done on fecal samples from animals in study 1 and study 2. Oligonucleotide sequences are as follows: _mdr1 5_′ CGCTCCCACTTATGATGCTGATCT; _mdr1 3_′ TAGGCGTACGTGGTCATTTCTTCC; _IL1α 3_′; GCAGGTCATTTAACCAAGTGGTGC; _IL1α 3_′; GATGAAGCTCGTCAGGCAGAAGTT; _IL1α 3_′; AAGAAGGTGCTCATGTCCTCATCC; _IL1α 5_′; CTTCAGGCAGGCAGTATCACTCAT; _p15 5_′; CCCTACCCAGTAAGACAAAGCCAA; _p15 3_′; GGCCCGGGAACTTCATACAATACT; _p21 5_′; AGTAGCAGTTGTACAAGGAGCCAG; _p21 3_′; CACCCACGGTATTCAACACTGACA; c-myc 5′; ACCAACAGGAACTATGACCTC; _c-myc 3_′ AAGGCAGTAGCGACCGCAAC; H. hepaticus and H. bilis26.
Statistical Analysis
Bonferroni’s multiple comparison test was used to detect significant differences at the P < 0.05 level between survival curves and degree of dysplasia.
Results
Co-Infection of mdr1a−/− Mice with Both H. bilis and H. hepaticus Did Not Increase Percent Survival Compared to Infection with H. hepaticus Alone
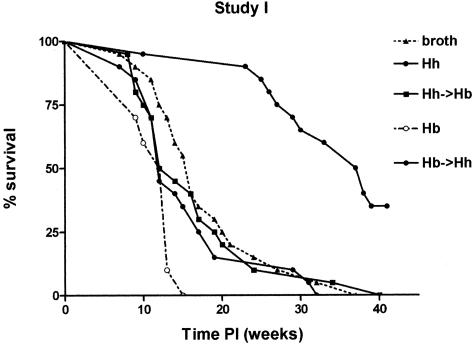
Since we had shown that H. bilis induces severe inflammation and hyperplasia in _mdr1a_−/− mice in a short period of time while H. hepaticus delays the development of colitis,16 we hypothesized that H. hepaticus infection might ameliorate (or delay) development of _H. bilis_-induced colitis and prolong survival. Therefore, the goal of study 1 was to compare colitis development and survival time in _mdr1a_−/− mice that were singly infected (H. bilis) or dual-infected (H. bilis/H. hepaticus) to _H. hepaticus_-infected and to uninfected broth animals. Animals were terminated when they displayed diarrhea and 20% body weight loss. Survival curves for the various groups are shown in Figure 1. The addition of H. hepaticus to H. bilis did not increase survival with _H. hepaticus_-infected _mdr1a_−/− mice having significantly longer (P < 0.01) survival relative to all other groups. The mean survival time was shortest in _H. bilis_-infected _mdr1a_−/− mice (11.6 weeks) and the most prolonged in _H. hepaticus_-infected _mdr1a_−/− mice (33.9 weeks) (Table 1). Survival time in broth _mdr1a_−/− mice (16.9 weeks) was similar to _H. bilis/H. hepaticus-_infected (16.4 weeks) or _H. bilis/H. hepaticus-_infected (16.7 weeks) _mdr1a_−/− mice. Hence, addition of H. hepaticus to H. bilis did not prolong survival but rather contributed to development of colitis that was characterized by dysplasia as described below.
Figure 1.
Percent survival of _Helicobacter_-infected and uninfected _mdr1a_−/− mice. The addition of H. hepaticus to H. bilis did not increase survival. _H. hepaticus_-infected _mdr1a_−/− mice have significantly increased (P < 0.01) survival relative to all other groups.
Table 1.
Survival Time (Weeks after Infection) in _mdr1a_−/− Mice in Study 1
| Infection | Mean survival time | Range of survival time | n |
|---|---|---|---|
| Broth | 17.1 ± 6.4 | 7–32 | 20 |
| H. bilis | 11.6 ± 2.2 | 9–15 | 10 |
| H. hepaticus | 33.9 ± 8.4 | 10–41 | 20 |
| H. bilis->H. hepaticus | 16.4 ± 8.3 | 7–32 | 20 |
| H. hepaticus->H. bilis | 16.4 ± 8.5 | 8–40 | 20 |
Colitis Was Characterized by Dysplasia More Frequently in mdr1_a_−/− Mice Infected with H. bilis Alone or Dual-Infected with Both H. bilis and H. hepaticus
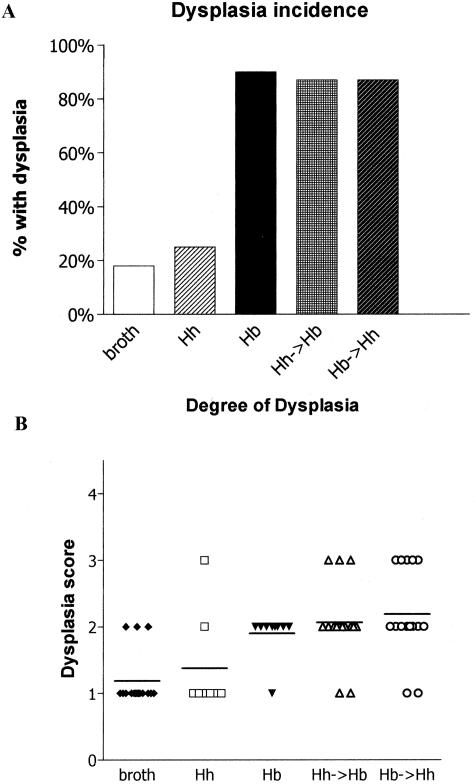
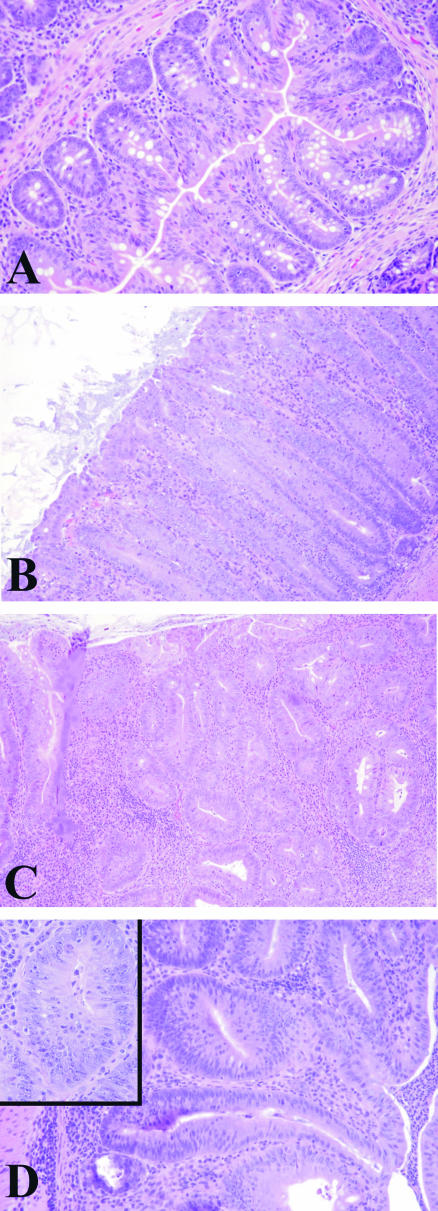
Although survival time differed among the groups, there were important histopathological differences in the degree of dysplasia. _Mdr1a_−/− mice infected with H. bilis alone or dual-infected with H. bilis and H. hepaticus had the highest incidence of dysplasia (dysplasia score, >1; Figure 2A) but dual-infected _mdr1a_−/− mice exhibited the most severe dysplasia relative to broth mice (P < 0.001) or singly infected mice (P < 0.05) (Figure 2B). Figure 3 shows the spectrum of mucosal lesions noted. Hyperplastic mucosa was a feature seen in all _Helicobacter_-infected groups and was characterized by diffusely thickened mucosa with elongate glands, reduction in goblet cells, and increased mitotic figures (Figure 3B). Low-grade dysplasia was characterized by gland proliferation with branching, hyperchromasia of the epithelial cell nuclei, and loss of a cell maturation gradient toward the lumen (Figure 3C). High-grade dysplastic mucosa was characterized by thickened irregularly branching, elongated glands with nuclear atypia, loss of goblet cells, and epithelial polarity (Figure 3D). No evidence of invasive colonic carcinoma was seen in study 1 animals.
Figure 2.
Incidence and degree of dysplasia in uninfected and _Helicobacter_-infected _mdr1a_−/− mice (study 1). A: Infection of _mdr1a_−/− mice with H. bilis alone or both H. bilis and H. hepaticus had the highest incidence of dysplasia. B: Dual-infected _mdr1a_−/− mice exhibited the most severe dysplasia relative to uninfected mice (P < 0.001) or singly infected mice (P < 0.05); 1, no dysplasia; 2, low grade; 3, high grade; 4, high grade with invasion/adenocarcinoma).
Figure 3.
H&E-stained paraffin-embedded sections of colons from _mdr1a_−/− mice demonstrating the spectrum of mucosal lesions observed in study 1. A: Relatively normal mucosa from a nonclinically colitic _mdr1a_−/− mouse. Note low-grade inflammation in the lamina propria confined to the mucosa. B: Hyperplastic colitis characterized by a diffuse moderately thickened mucosa with elongated glands, reduction in goblet cells, and increased mitotic figures. C: Low-grade dysplasia. Note irregularly branching glands, hyperchromatic nuclei, and loss of maturation gradient toward the lumen. D: High-grade dysplasia. Note irregular glands lined by crowded epithelial cells. The nuclei are pleomorphic, hyperchromatic, and stratified (inset). Original magnifications: ×10; ×40 (inset).
Alterations in MHC Class II, CD4, Macrophages, and Cox-2 in Colons from Uninfected and _Helicobacter_-Infected FVB Wild-Type and mdr1_a_−/− Mice
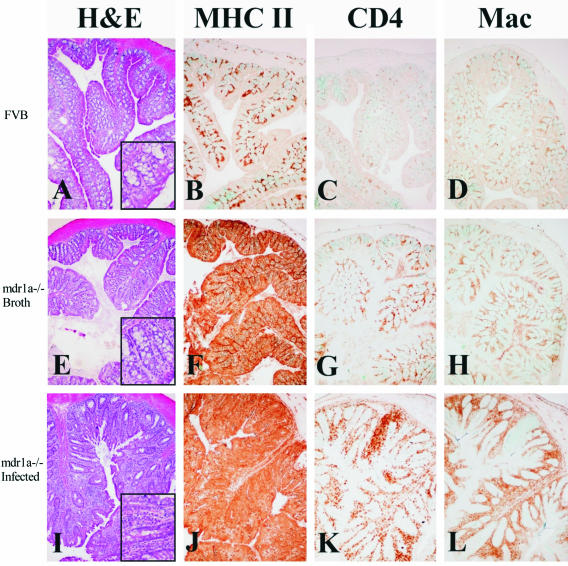
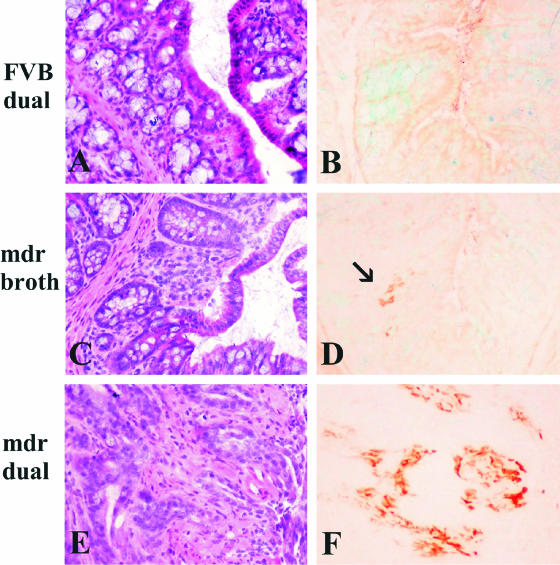
_Mdr1a_−/− mice showed increased expression of MHC class II in colonic epithelial cells independent of colitis or Helicobacter infection. Proinflammatory cytokines such as interferon-γ are increased in IBD27,28 and potentially responsible for enhanced expression of MHC class II in intestinal epithelial cells. As we previously reported in _Helicobacter_-infected IL-10−/− mice,15 we noted increased expression of MHC class II in epithelial cells from proximal colon of _mdr1a_−/− mice dual-infected with both H. bilis and H. hepaticus compared with proximal colon from FVB mice in which only lamina propria cells expressed MHC class II (Figure 4B). Notably, however, we also saw increased MHC class II expression in colonic tissues from uninfected _mdr1a_−/− mice before there was minimal if any colonic inflammation (Figure 4F) suggesting these animals are primed to develop enteric disease. _Mdr1a_−/− mice showed diffuse, very intense expression of MHC class II antigen in the enterocytes as well as lamina propria cells, which became more marked after _Helicobacter_-induced colitis and dysplasia (Figure 4J). Additionally, uninfected, noncolitic _mdr1a_−/− mice had more CD4- and F4/80-positive cells in the proximal colon compared with uninfected FVB mice (Figure 4, G and H). The number of these cells increased in inflammatory infiltrates in dual-infected mice with colitis and dysplasia (Figure 4, K and L). Dual-infected _mdr1a_−/− mice had large numbers of Cox-2-positive cells in the lamina propria of the proximal colon (Figure 5F) and cecum (data not shown). Uninfected _mdr1a_−/− mice showed only rare faintly positive Cox-2-positive cells (Figure 5D, arrow) and dual-infected FVB wild-type mice had no Cox-2-positive cells (Figure 5B). No differences in intensity or distribution of β-catenin or CD44 expression were detected between dysplastic and nondysplastic crypts in individual animals, nor did there appear to be any differences in the expression of these two antigens between groups (data not shown).
Figure 4.
OCT-embedded frozen colon section with immunohistochemical detection of MHC class II (B, F, J), CD4+ cells (C, G, K), and F4/80+ macrophages (D, H, L) in the colon of FVB wild-type (B, C, D), broth control (F, G, H), and dual-infected (J, K, L) _mdr1a_−/− mice. In the normal, noninflamed colon of FVB mice (A), MHC class II expression is restricted to small numbers of leukocytes in the lamina propria (B) and numbers of CD4+ cells and macrophages are low (C, D). In contrast, there are subtle proliferative and inflammatory changes in broth-uninfected _mdr1a_−/− mice (E), which is accompanied by pronounced up-regulation of MHC class II expression in enterocytes (F) and moderately increased numbers of CD4+ cells and macrophages in lamina propria (G, H). In animals dual-infected with H. bilis and H. hepaticus, there are severe proliferative and inflammatory changes in the colon (I), accompanied by significant up-regulation of MHC class II on enterocytes and infiltrating leukocytes (J) and marked infiltration of CD4+ T cells (K) and macrophages (L) in lamina propria. Original magnifications, ×10.
Figure 5.
OCT-embedded frozen colon sections from dual-infected FVB (A and B), uninfected _mdr1a_−/− (C and D), and dual-infected _mdr1a_−/− mice (E and F). B: Dual-infected FVB wild-type mice remain lesion-free and no Cox-2-positive cells are found in the colonic mucosa. D: Uninfected _mdr1a_−/− mice show rare Cox-2-positive cells (arrow) in the mild, spontaneous inflammatory infiltrates of the colon. F: In contrast, dual-infected _mdr1a_−/− mice have large numbers of cells in the lamina propria of colon (and cecum, data not shown) that express high levels of Cox-2. Note diffuse light background staining of intestinal contents, which is especially prominent in B and D. Original magnifications, ×20.
Medicated Feed Was Used to Prolong Chronic Colitis and Contributed to the Development of High-Grade Dysplasia and Invasive Adenocarcinoma in Dual-Infected mdr1a−/− Mice
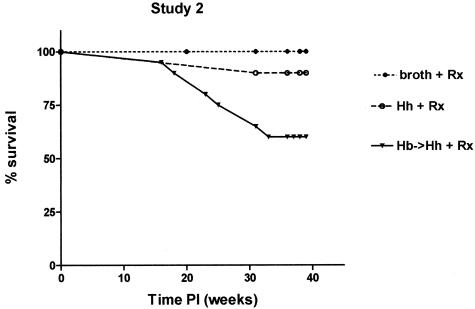
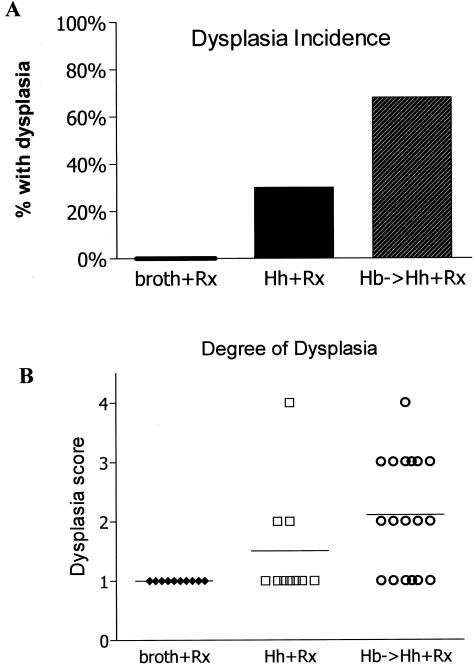
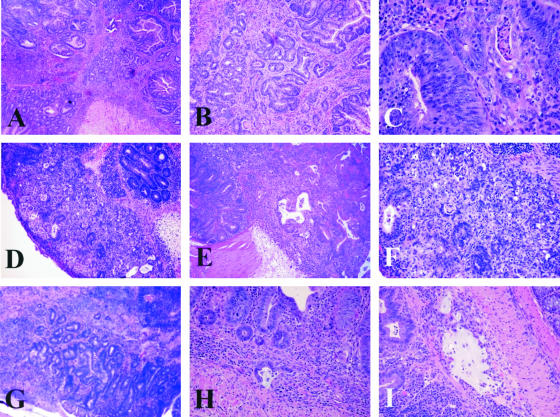
The goal of study 2 and study 3 was to model chronic and relapsing IBD by allowing animals with chronic colitis to survive longer with the use of medicated wafers. Hence, in study 2, all uninfected and dual-infected mice were intermittently fed medicated wafers for a 2-month period at 6 to 16 weeks after infection and for a 1-month period at 18 to 20 weeks after infection. In study 3, all uninfected and dual-infected mice were intermittently fed medicated wafers (1 week on and 3 weeks off). Prolonged survival of uninfected and dual-infected _mdr1a_−/− mice was achieved with the medicated wafers with a higher percentage (>50%) of dual-infected animals still alive by 39 weeks after infection (Figure 6) as compared with ∼25% survival of dual-infected animals at 39 weeks after infection in study 1 (Figure 1). Nonetheless dual-infected _mdr1a_−/− mice in study 2 had decreased survival (P < 0.05) relative to uninfected _mdr1a_−/− mice and they also exhibited a high incidence of dysplasia (Figure 7A). However, more dual-infected _mdr1a_−/− mice in study 2 exhibited a higher degree of dysplasia compared with uninfected _mdr1a_−/− mice (P < 0.01) as shown in Figure 7B. Interestingly, singly infected _H. hepaticus mdr1a_−/− had prolonged survival and a percentage of those animals also exhibited low- to high-grade dysplasia (Figure 7B). High-grade dysplastic mucosa was thickened by irregularly branching to cribiform glands with reduction in intraglandular stroma and goblet cells, increased nuclear and cytological atypia, loss of nuclear and epithelial polarity, and numerous mitoses. High-grade dysplastic lesions included two instances of medullary carcinoma in situ as recently described by Erdman and colleagues14 (Figure 8; D to F), and two instances of moderately differentiated adenocarcinoma with invasion of the muscularis mucosa by neoplastic glands (Figure 8; G to I).
Figure 6.
Percent survival of _Helicobacter_-infected and uninfected _mdr1a_−/− mice in study 2. Prolonged survival of uninfected and dual-infected (H. bilis and H. hepaticus) _mdr1a_−/− mice was achieved by feeding medicated wafers. Dual-infected _mdr1a_−/− mice had decreased survival (P < 0.05) relative to uninfected _mdr1a_−/− mice and exhibited a high incidence of dysplasia.
Figure 7.
Incidence and degree of dysplasia in uninfected and _Helicobacter-_infected _mdr1a_−/− mice (study 2). Dual-infected _mdr1a_−/− mice had the highest incidence of dysplasia (A) and exhibited the most severe dysplasia relative to uninfected mice (P < 0.001) or singly infected mice (P < 0.05) (B). 1, no dysplasia; 2, low grade; 3, high grade; 4, high grade with invasion/adenocarcinoma.
Figure 8.
Histopathology of high-grade dysplasia and invasive adenocarcinoma in dual-infected _mdr1a_−/− mice. A: Colonic high-grade dysplasia (grade 3). B: Higher power of A. C: Cecal high-grade dysplasia (grade 3). Note haphazard stratification of hyperchromatic nuclei. D: Cecal high-grade dysplasia/medullary carcinoma in situ. E: Deeper serial section of D. F: Higher magnification of D. G: Invasive colonic adenocarcinoma. Note irregular glands present at the submucosa junction muscularis externa. H: Invasive colonic mucinous adenocarcinoma. I: Additional section of H demonstrating lake of mucin within a dilated submucosal lymphatic. Original magnifications: ×4 (A, G); ×10 (B, D, E); ×20 (F, H, I); ×40 (C).
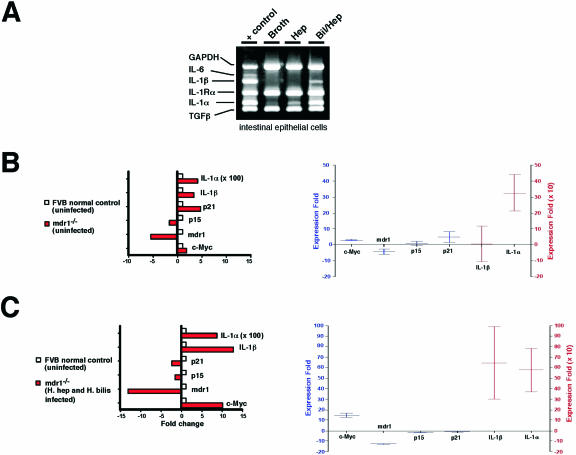
Dual-Infected mdr1a−/− Mice Have Increased Expression of IL-1α/β in Colonic Epithelial Cells
We examined the expression of multiple cytokines between uninfected and _Helicobacter_-infected _mdr1a_−/− mice and noted differences in the message for IL-1α/β. There was increased expression of IL-1α and IL-1β mRNA in colonic epithelial cells from dual-infected _mdr1a_−/− mice compared to uninfected _mdr1a_−/− mice (Figure 9A). Changes in IL-1α and IL-1β expression were further quantitated by real-time PCR. First, we compared the expression of IL-1α and IL-1β mRNA in epithelial cell preparations from uninfected _mdr1a_−/− mice compared to uninfected FVB control mice. We found that loss of mdr1a expression alone was sufficient to increase expression of IL-1α, but not IL-1β, an average of 300-fold when compared to FVB control mice. In contrast, expression of both IL-1α and IL-1β mRNA were increased an average of 600-fold in dual-_Helicobacter-_infected _mdr1a_−/− mice relative to uninfected _mdr1a_−/− mice (Figure 9C). These results suggest that loss of MDR1 expression is sufficient to result in increased local expression of IL-1α, while infection with Helicobacter spp. are required for consistent elevation of both IL-1α and IL-1β mRNA levels. No differences were noted in the message for IL-6, transforming growth factor-β, interferon-γ, IL-10, IL-13, tumor necrosis factor-α, and IL-12p40 in epithelial cells from uninfected and _Helicobacter_-infected _mdr1a_−/− and FVB mice (data not shown).
Figure 9.
A: Multiplex PCR for multiple cytokines in colonic epithelial cells from uninfected and infected _mdr1a_−/− mice. Increased expression of IL-1α and IL-1β was only noted in colonic epithelial cells of _mdr1a_−/− mice infected with both H. bilis and H. hepaticus and not in epithelial cells from H. hepaticus or uninfected broth animals. Real-time PCR showing expression of c-myc and IL-1α and IL-1β in colonic epithelial cells from uninfected _mdr1a_−/− and FVB+/+ mice (B) and from uninfected FVB+/+ and dual-infected _mdr1a_−/− mice (C).
c-myc Is Increased in Colonic Epithelial Cells of Uninfected and Dual-Infected mdr1a−/− Mice
To address how loss of MDR1 expression may result in dysplasia, we measured the expression of c-myc, an oncogene known to be deregulated in many cancers in humans, including colon cancer.29 We found that loss of MDR1a expression alone was sufficient to result in a twofold to fourfold increase in c-myc expression in epithelial cell preparations from uninfected _mdr1a_−/− versus FVB mice (Figure 9B). Infection of _mdr1a_−/− mice with H. bilis and H. hepaticus resulted in a further increase in c-myc expression to 5- to 12-fold (Figure 9C).
H. bilis Organisms Predominate in Dual-Infected mdr1a−/− Mice
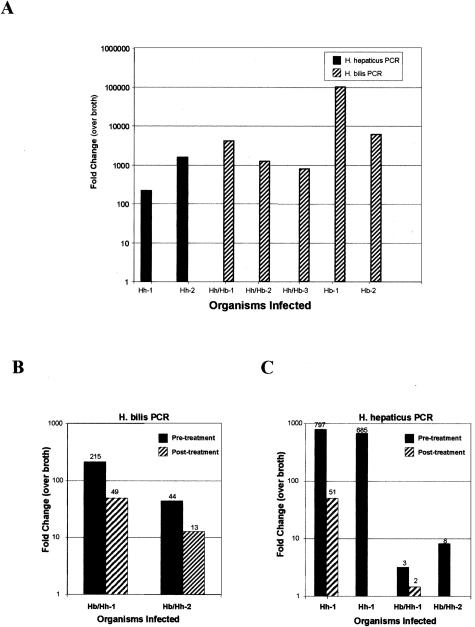
To understand how two species of Helicobacter could be interacting to induce colonic epithelial cell dysplasia, we used real-time PCR to quantitate H. bilis and H. hepaticus organisms in fecal samples of _Helicobacter_-infected mice (Figure 10).26 In study 1, we found that oral gavage with H. hepaticus resulted in a significant infection at 20 weeks after infection, as measured by 100- to 1000-fold increased expression by real-time PCR over background (Figure 10A; Hh-1 and Hh-2). However, dual-infection of _mdr1a_−/− mice with both H. hepaticus and H. bilis resulted in apparent competition between Helicobacter spp. with overgrowth of H. bilis relative to H. hepaticus, as measured by the nearly complete obliteration of H. hepaticus expression in dual-infected animals (Figure 10A, Hb/Hh1–3). Interestingly, we found that animals infected with H. bilis alone still retained higher levels of H. bilis expression than dual-infected animals suggesting that H. hepaticus may somehow compete with H. bilis for nutrients or niches required to sustain maximal infection (Figure 10A, Hb1–2). Although the expression of H. hepaticus was below background in study 1, we were able to detect H. hepaticus in dual-infected animals in study 2 (Figure 10, B and C). In study 2, we found that medicated feed decreased H. bilis infection up to fourfold (Figure 10B) while medication decreased H. hepaticus up 16-fold or greater (Figure 10C). These results would suggest that medicated wafers were effective in significantly reducing or eliminating some Helicobacter organisms allowing the animals to live longer due to a reduction in clinical gastrointestinal disease.
Figure 10.
Real-time PCR for H. bilis and H. hepaticus from fecal samples from _mdr1a_−/− mice. A: H. bilis is the predominant organism in dual-infected animals in study 1. B: Medicated feed has reduced numbers of H. bilis in dual-infected animals in study 2. C: Medicated feed has dramatically reduced H. hepaticus in dual-infected animals in study 2. Fold-expression was generated by the CT method, whereby we compare the amount of Helicobacter DNA relative to β-actin DNA in the stool, and then comparing the normalized values to values generated in stool from broth-only animals.
Discussion
Human patients afflicted with IBD and chronic ulcerative colitis (UC) are at increased risk of developing colorectal cancer. The relative risk for colorectal cancer in UC patients is 10-fold greater compared to the general population.30 Such patients are routinely screened for the degree of dysplasia with low-grade dysplasia associated with an ∼10% risk of invasive carcinoma and high-grade dysplasia associated with an ∼40% risk of carcinoma.31 Animal models used to study the transitions from hyperplasia to dysplasia, and dysplasia to neoplasia should ideally have phenotypic features that are comparable to the human disorders. An important risk factor for colorectal cancer in patients with IBD is the extent of colitis with increasing risk associated with more colonic surface involvement.32 _Helicobacter_-infected _mdr1a_−/− mice develop chronic inflammatory disease that involves all of the cecum, and proximal, mid, and distal colon,16 and is characterized by hyperplasia with varying levels of dysplasia depending on the Helicobacter spp. This murine model may prove to be more useful to study the role of inflammation in colorectal cancer, since other reported models with intestinal polyposis33–35 develop more focal lesions with minimal inflammation. Also, the absence of p-glycoprotein in the intestinal epithelial cells of _mdr1a_−/− mice36 and its propensity for large bowel disease could have particular relevance for the study of gastrointestinal disease and cancer in humans. Although the MDR1 gene was first identified in multidrug-resistant tumor cells,37 it is also expressed in intestinal epithelial cells38,39 although its physiological roles are unclear. There is accumulating evidence that p-glycoprotein is important in host-bacterial interactions in the gastrointestinal tract and maintenance of homeostasis.40 Certain MDR1 gene polymorphisms have been suggested to confer susceptibility for ulcerative colitis18 and colorectal cancer20,41 and other epithelial tumors.19 In addition, a potential role of genetic polymorphisms of enzymes involved in metabolism of xenobiotics, is emerging as a risk factor in other tumors such as renal cell carcinoma.42 Studies in _mdr1a_−/− mice suggest that the absence of the transporter alone is not sufficient to induce disease,16 but rather the presence of a particular bacterial trigger such as Helicobacter or members of the colonic flora are needed to initiate and sustain prolonged intestinal inflammation. Studies by Yamada and colleagues43 and Mochida and colleagues44 in _mdr1a_−/−, Apc Min+ mice showed that the absence of p-glycoprotein suppressed intestinal polyp formation. However, this tumor model43 differs from ours in that intestinal lesions do not show inflammation.
The relationship between chronic intestinal inflammation and the development of dysplasia and neoplasia is complex. In human patients with IBD, the degree of inflammation has been reported to correlate with dysplasia and cancer,45 yet not all patients with chronic inflammation develop dysplasia or neoplasia suggesting the involvement of other factors. Based on experiments described here and previous reports by others, we propose that intestinal bacterial infections are one important predisposing factor in the induction of colon cancer. Others have shown that bacterial infection with Citrobacter rodentium can promote colonic tumors in ApcMin+ mice in sites of bacterial-induced hyperplasia.46 Our studies show that _mdr1a_−/− mice infected with Helicobacter will eventually develop large bowel inflammation and dysplasia. In addition, we find that _Helicobacter_-induced dysplastic changes in colonic epithelial cells of _mdr1a_−/− mice correlated with increased expression of the inflammation markers/mediators, MHC class II, Cox-2, CD4, F4/80, and the proinflammatory cytokines IL-1β and IL-1α. Importantly, we also find increased expression of the oncogene c-myc, a potent transforming gene in many human cancers. Human population-based studies suggest that the susceptibility to cancer increases when tissues are chronically inflamed, and the long-term use of nonsteroidal anti-inflammatory drugs reduces the risk of several cancers including colorectal cancer.47 Here, we find that the absence of p-glycoprotein in the intestinal epithelial cell of uninfected _mdr1a_−/− mice is a priori associated with a twofold to fourfold increase in c-myc and a 300-fold increase in IL-1α. After dual infection of _mdr1a_−/− mice with two Helicobacter spp., colonic crypts undergo dysplastic changes that are associated with a further increase (5- to 12-fold) in c-myc and a 600-fold increase in expression of IL-1β and IL-1α, and cyclooxygenase-2 (Cox-2), all of which have been associated with dysplasia and cancer in humans. For example, c-myc is expressed in epithelial cells of patients with IBD48 and increased expression has been reported in patients with IBD,49 colon cancer,50 and in Barrett’s esophageal metaplasia and neoplasia.51 High c-myc protein expression correlates with _H. pylori-_associated gastric dysplasia and carcinoma,52 as well as with the degree of dysplasia and worsening prognosis in human patients with colorectal lesions.53 C-myc expression has been correlated with increased apoptosis in colorectal cancer54 and IL-1 protects transformed cells from apoptosis,55 suggesting that IL-1 and c-myc collaborate during transformation. Consistent with this notion, certain IL-1β alleles are associated with an increased risk for high-grade dysplasia or gastric cancer in human patients.56–58 Additionally, advanced dysplasia and cervical carcinoma in women is associated with increases in IL-1β and IL-1α59 and mucosal levels of IL-1β increased before the development of cancer in a mouse model of _H. pylori_-induced gastric tumors.60 The prostaglandin-synthesis enzyme Cox-2 is also highly associated with colorectal cancer and is expressed early in the development of colitis-associated tumors.61 Cox-2 expression is elevated in 85% of human colorectal cancers, and ∼50% of colorectal adenomas62,63 and proposed mechanisms involve inhibition of apoptosis and increased cell migration and angiogenesis in tumors. C-myc has been proposed to bind the cox-2 promoter via association with the transcriptional regulators TIP49 and TATA-binding protein, resulting in increased expression of Cox-2.64 Nonsteroidal anti-inflammatory agents that inhibit Cox-1 and -2 reduce the size and number of colonic polyps in humans that harbor germ-line mutations in the tumor suppressor gene adenomatous polyposis coli (APC).65
Although the relationship between p-glycoprotein, c-myc, Cox-2, and IL-1 are not proven in this animal model of colonic dysplasia and neoplasia, our data provide significant leads toward a mechanism of bacterial-induced colon carcinogenesis. From our studies, we propose a model whereby loss of MDR1 results in enhanced c-myc and IL-1 expression, which results in increased cell division and survival. Infection with Helicobacter results in further increases in expression of c-_my_c, IL-1, and Cox-2, which further increases the propensity for epithelial cells to divide and survive. Finally, increased inflammation associated with Helicobacter infection increases oxidative damage to cells, further increasing the chance for additional transforming events. In our _mdr1a_−/− model, further studies using siRNA, anti-sense oligonucleotide, or gene targeting would be required to more formally test the roles of c-myc, IL-1, and Cox-2 in modulating the development of epithelial cell dysplasia.
Helicobacter spp. may be a good prototype bacterial organism to study the mechanisms of bacterial-induced intestinal hyperplasia, dysplasia, and neoplasia and studies using both human and animal isolates have explored the pathogenesis. It is well documented that H. pylori is linked to gastritis, gastric adenocarcinoma, and malignant lymphoma in human patients.66 Using HT29-19A intestinal epithelial cells, H. pylori has been shown to increase the passage of antigens across the epithelium and alone or in concert with IL-1β decrease epithelial cell integrity.67 In animal models of gastritis and gastric lymphoma, Helicobacter heilmannii isolates have shown varying capacity for antigen-driven inflammation and associated disease.68 H. hepaticus has been shown to induce hepatocellular carcinomas in susceptible strains of mice12 that are not associated with mutations in p53 or Ras,69,70 and colorectal cancer has been induced in RAG2-deficient mice by infection with H. hepaticus.71 H. bilis has been associated with biliary tract malignancies in Japanese and Thai patients but a cause and effect has not been established.72 In this study, we found that the combination of infection with both H. hepaticus and H. bilis in _mdr1a_−/− mice was most effective in producing dysplasia particularly if the chronic and relapsing disease process of human IBD was modeled with intermittent treatment with medicated wafers. This may be due in part to qualitative differences in the host immune response to these two Helicobacter species. We have previously shown that H. bilis induces severe inflammation and hyperplasia in _mdr1a_−/− mice in a short period of time while H. hepaticus can delay the development of IBD in this mouse strain.16 The effect of H. hepaticus may be due to its ability to induce regulatory T cells (unpublished data)73 and ameliorate the severe inflammation caused by the H. bilis thus allowing time for eventual transformation of epithelial cells. Additionally, other undefined bacterial species may compete with one another in the gastrointestinal tract and provide the necessary environment for progression to neoplastic lesions. Ongoing inflammation induced by certain bacterial species may result in oxidative stress-induced cell damage74 increasing the frequency of genetic or epigenetic changes contributing to the promotion phase of carcinogenesis.75 The _mdr1a_−/− mouse with a propensity for IBD that can be modulated with Helicobacter spp. may be a useful murine model to determine critical factors involved in the progression of large bowel inflammation to colorectal cancer.
Acknowledgments
We thank Tania Habib, Loida Torres, Mingzu Lei, Tristan Root, and Bettty Yue for technical assistance and care and monitoring of mice; and Denny Liggitt for his assistance in classifying dysplastic lesions.
Footnotes
Address reprint requests to Lillian Maggio-Price, Department of Comparative Medicine, School of Medicine, Box 357190, Seattle, WA. 98195. E-mail: lmprice@u.washington.edu.
Supported in part by the Broad Medical Research Program of the Eli and Edythe L. Broad Foundation (to L.M.P.) and the National Institutes of Health (grant 1RO1 AI053568-01 to B.M.I.).
References
- Dancourt V, Faivre J. Epidemiology and screening of colorectal cancer. Rev Prat. 2004;54:135–142. [PubMed] [Google Scholar]
- Kobaek-Larsen M, Thorup I, Diederichsen A, Fenger C, Hoitinga MR. Review of colorectal cancer and its metastases in rodent models: comparative aspects with those in humans. Comp Med. 2000;50:16–26. [PubMed] [Google Scholar]
- Galvez JJ, Cardiff RD, Munn RJ, Borowsky AD, Boivin GP, Groden J, Longnecker DS, Shmidt EN, Nikitin AY, Connolly DC, Hamilton TC. Mouse models of human cancers (part 2). Comp Med. 2004;54:13–28. [PubMed] [Google Scholar]
- Karlen P, Lofberg R, Brostrom O, Leijonmarck CE, Hellers G, Persson PG. Increased risk of cancer in ulcerative colitis: a population-based cohort study. Am J Gastroenterol. 1999;94:1047–1052. doi: 10.1111/j.1572-0241.1999.01012.x. [DOI] [PubMed] [Google Scholar]
- Bachwich DR, Lichtenstein GR, Traber PG. Cancer in inflammatory bowel disease. Med Clin North Am. 1994;78:1399–1412. doi: 10.1016/s0025-7125(16)30107-9. [DOI] [PubMed] [Google Scholar]
- Owen RJ. Helicobacter—species classification and identification. Br Med Bull. 1998;54:17–30. doi: 10.1093/oxfordjournals.bmb.a011667. [DOI] [PubMed] [Google Scholar]
- Rothenbacher D, Brenner H. Helicobacter pylori and gastric cancer. Gastroenterology. 2004;126:1927–1928. doi: 10.1053/j.gastro.2004.04.047. [DOI] [PubMed] [Google Scholar]
- Li X, Fox JG, Whary MT, Yan L, Shames B, Zhao Z. SCID/NCr mice naturally infected with Helicobacter hepaticus develop progressive hepatitis, proliferative typhlitis, and colitis. Infect Immun. 1998;66:5477–5484. doi: 10.1128/iai.66.11.5477-5484.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward JM, Fox JG, Anver MR, Haines DC, George CV, Collins MJ, Jr, Gorelick PL, Nagashima K, Gonda MA, Gilden RV, Tully JG, Russell RJ, Benueniste RE, Paster BJ, Dewhirst RE, Donovan JC, Anderson LM, Rice JM. Chronic active hepatitis and associated liver tumors in mice caused by a persistent bacterial infection with a novel Helicobacter species. J Natl Cancer Inst. 1994;86:1222–1227. doi: 10.1093/jnci/86.16.1222. [DOI] [PubMed] [Google Scholar]
- Ward JM, Anver MR, Haines DC, Melhorn JM, Gorelick P, Yan L, Fox JG. Inflammatory large bowel disease in immunodeficient mice naturally infected with Helicobacter hepaticus. Lab Anim Sci. 1996;46:15–20. [PubMed] [Google Scholar]
- Correa P. The biological model of gastric carcinogenesis. IARC Sci Publ. 2004;157:301–310. [PubMed] [Google Scholar]
- Fox JG, Li X, Yan L, Cahill RJ, Hurley R, Lewis R, Murphy JC. Chronic proliferative hepatitis in A/JCr mice associated with persistent Helicobacter hepaticus infection: a model of helicobacter-induced carcinogenesis. Infect Immun. 1996;64:1548–1558. doi: 10.1128/iai.64.5.1548-1558.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdman SE, Poutahidis T, Tomczak M, Rogers AB, Cormier K, Plank B, Horwitz BH, Fox JG. CD4+ CD25+ regulatory T lymphocytes inhibit microbially induced colon cancer in Rag2-deficient mice. Am J Pathol. 2003;162:691–702. doi: 10.1016/S0002-9440(10)63863-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdman SE, Rao VP, Poutahidis T, Ihrig MM, Ge Z, Feng Y, Tomczak M, Rogers AB, Horwitz BH, Fox JG. CD4(+)CD25(+) regulatory lymphocytes require interleukin 10 to interrupt colon carcinogenesis in mice. Cancer Res. 2003;63:6042–6050. [PubMed] [Google Scholar]
- Burich A, Hershberg R, Waggie K, Zeng W, Brabb T, Westrich G, Viney JL, Maggio-Price L. Helicobacter-induced inflammatory bowel disease in IL-10- and T cell-deficient mice. Am J Physiol. 2001;281:G764–G778. doi: 10.1152/ajpgi.2001.281.3.G764. [DOI] [PubMed] [Google Scholar]
- Maggio-Price L, Shows D, Waggie K, Burich A, Zeng W, Escobar S, Morrissey P, Viney JL. Helicobacter bilis infection accelerates and H. hepaticus infection delays the development of colitis in multiple drug resistance-deficient (mdr1a−/−) mice. Am J Pathol. 2002;160:739–751. doi: 10.1016/S0002-9440(10)64894-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panwala CM, Jones JC, Viney JL. A novel model of inflammatory bowel disease: mice deficient for the multiple drug resistance gene, mdr1a, spontaneously develop colitis. J Immunol. 1998;161:5733–5744. [PubMed] [Google Scholar]
- Schwab M, Schaeffeler E, Marx C, Fromm MF, Kaskas B, Metzler J, Stange E, Herfarth H, Schoelmerich J, Gregor M, Walker S, Cascorbi I, Roots I, Brinkmann U, Zanger UM, Eichelbaum M. Association between the C3435T MDR1 gene polymorphism and susceptibility for ulcerative colitis. Gastroenterology. 2003;124:26–33. doi: 10.1053/gast.2003.50010. [DOI] [PubMed] [Google Scholar]
- Siegsmund M, Brinkmann U, Schaffeler E, Weirich G, Schwab M, Eichelbaum M, Fritz P, Burk O, Decker J, Alken P, Rothenpieler U, Kerb R, Hoffmeyer S, Brauch H. Association of the P-glycoprotein transporter MDR1(C3435T) polymorphism with the susceptibility to renal epithelial tumors. J Am Soc Nephrol. 2002;13:1847–1854. doi: 10.1097/01.asn.0000019412.87412.bc. [DOI] [PubMed] [Google Scholar]
- Potocnik U, Glavac MR, Golouh R, Glavac D. The role of P-glycoprotein (MDR1) polymorphisms and mutations in colorectal cancer. Pflugers Arch. 2001;442:R182–R183. doi: 10.1007/s004240100017. [DOI] [PubMed] [Google Scholar]
- Boivin GP, Washington K, Yang K, Ward JM, Pretlow TP, Russell R, Besselsen DG, Godfrey VL, Doetschman T, Dove WF, Pitot HC, Halberg RB, Itzkowitz SH, Groden J, Coffey RJ. Pathology of mouse models of intestinal cancer: consensus report and recommendations. Gastroenterology. 2003;124:762–777. doi: 10.1053/gast.2003.50094. [DOI] [PubMed] [Google Scholar]
- Goff BA, Ries JA, Els LP, Coltrera MD, Gown AM. Immunophenotype of ovarian cancer as predictor of clinical outcome: evaluation at primary surgery and second-look procedure. Gynecol Oncol. 1998;70:378–385. doi: 10.1006/gyno.1998.5094. [DOI] [PubMed] [Google Scholar]
- Andrew SM, Jasani B. An improved method for the inhibition of endogenous peroxidase non-deleterious to lymphocyte surface markers. Application to immunoperoxidase studies on eosinophil-rich tissue preparations. Histochem J. 1987;19:426–430. doi: 10.1007/BF01675753. [DOI] [PubMed] [Google Scholar]
- Ogawa H, Fukushima K, Sasaki I, Matsuno S. Identification of genes involved in mucosal defense and inflammation associated with normal enteric bacteria. Am J Physiol. 2000;279:G492–G499. doi: 10.1152/ajpgi.2000.279.3.G492. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drazenovich NL, Franklin CL, Livingston RS, Besselsen DG. Detection of rodent Helicobacter spp. by use of fluorogenic nuclease polymerase chain reaction assays. Comp Med. 2002;52:347–353. [PubMed] [Google Scholar]
- Salomon P, Pizzimenti A, Panja A, Reisman A, Mayer L. The expression and regulation of class II antigens in normal and inflammatory bowel disease peripheral blood monocytes and intestinal epithelium. Autoimmunity. 1991;9:141–149. doi: 10.3109/08916939109006750. [DOI] [PubMed] [Google Scholar]
- Bisping G, Lugering N, Lutke-Brintrup S, Pauels HG, Schurmann G, Domschke W, Kucharzik T. Patients with inflammatory bowel disease (IBD) reveal increased induction capacity of intracellular interferon-gamma (IFN-gamma) in peripheral CD8+ lymphocytes co-cultured with intestinal epithelial cells. Clin Exp Immunol. 2001;123:15–22. doi: 10.1046/j.1365-2249.2001.01443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schraml P, Kononen J, Bubendorf L, Moch H, Bissig H, Nocito A, Mihatsch MJ, Kallioniemi OP, Sauter G. Tissue microarrays for gene amplification surveys in many different tumor types. Clin Cancer Res. 1999;5:1966–1975. [PubMed] [Google Scholar]
- Brostrom O. Ulcerative colitis and colon cancer: the role of surveillance. Ann Med. 1989;21:309–311. doi: 10.3109/07853898909149212. [DOI] [PubMed] [Google Scholar]
- Guindi M, Riddell RH. The pathology of epithelial pre-malignancy of the gastrointestinal tract. Best Pract Res Clin Gastroenterol. 2001;15:191–210. doi: 10.1053/bega.2001.0169. [DOI] [PubMed] [Google Scholar]
- Itzkowitz SH, Harpaz N. Diagnosis and management of dysplasia in patients with inflammatory bowel diseases. Gastroenterology. 2004;126:1634–1648. doi: 10.1053/j.gastro.2004.03.025. [DOI] [PubMed] [Google Scholar]
- Harada N, Tamai Y, Ishikawa T, Sauer B, Takaku K, Oshima M, Taketo MM. Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene. EMBO J. 1999;18:5931–5942. doi: 10.1093/emboj/18.21.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermiston ML, Gordon JI. Inflammatory bowel disease and adenomas in mice expressing a dominant negative N-cadherin. Science. 1995;270:1203–1207. doi: 10.1126/science.270.5239.1203. [DOI] [PubMed] [Google Scholar]
- Williams BO, Jacks T. Mechanisms of carcinogenesis and the mutant mouse. Curr Opin Genet Dev. 1996;6:65–70. doi: 10.1016/s0959-437x(96)90012-x. [DOI] [PubMed] [Google Scholar]
- Schinkel AH, Mol CA, Wagenaar E, van Deemter L, Smit JJ, Borst P. Multidrug resistance and the role of P-glycoprotein knockout mice. Eur J Cancer. 1995;31A:1295–1298. doi: 10.1016/0959-8049(95)00130-b. [DOI] [PubMed] [Google Scholar]
- Ambudkar SV, Kimchi-Sarfaty C, Sauna ZE, Gottesman MM. P-glycoprotein: from genomics to mechanism. Oncogene. 2003;22:7468–7485. doi: 10.1038/sj.onc.1206948. [DOI] [PubMed] [Google Scholar]
- Mahmood B, Daood MJ, Hart C, Hansen TW, Watchko JF. Ontogeny of P-glycoprotein in mouse intestine, liver, and kidney. J Invest Med. 2001;49:250–257. doi: 10.2310/6650.2001.33969. [DOI] [PubMed] [Google Scholar]
- Hsing S, Gatmaitan Z, Arias IM. The function of Gp170, the multidrug-resistance gene product, in the brush border of rat intestinal mucosa. Gastroenterology. 1992;102:879–885. doi: 10.1016/0016-5085(92)90173-v. [DOI] [PubMed] [Google Scholar]
- Ho GT, Moodie FM, Satsangi J. Multidrug resistance 1 gene (P-glycoprotein 170): an important determinant in gastrointestinal disease? Gut. 2003;52:759–766. doi: 10.1136/gut.52.5.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potocnik U, Ravnik-Glavac M, Glavac D. Functional MDR1 polymorphisms (G2677T and C3435T) and TCF4 mutations in colorectal tumors with high microsatellite instability. Cell Mol Biol Lett. 2002;7:92–95. [PubMed] [Google Scholar]
- Longuemaux S, Delomenie C, Gallou C, Mejean A, Vincent-Viry M, Bouvier R, Droz D, Krishnamoorthy R, Galteau MM, Junien C, Beroud C, Dupret JM. Candidate genetic modifiers of individual susceptibility to renal cell carcinoma: a study of polymorphic human xenobiotic-metabolizing enzymes. Cancer Res. 1999;59:2903–2908. [PubMed] [Google Scholar]
- Yamada T, Mori Y, Hayashi R, Takada M, Ino Y, Naishiro Y, Kondo T, Hirohashi S. Suppression of intestinal polyposis in Mdr1-deficient ApcMin/+ mice. Cancer Res. 2003;63:895–901. [PubMed] [Google Scholar]
- Mochida Y, Taguchi K, Taniguchi S, Tsuneyoshi M, Kuwano H, Tsuzuki T, Kuwano M, Wada M. The role of P-glycoprotein in intestinal tumorigenesis: disruption of mdr1a suppresses polyp formation in Apc(Min/+) mice. Carcinogenesis. 2003;24:1219–1224. doi: 10.1093/carcin/bgg073. [DOI] [PubMed] [Google Scholar]
- Seril DN, Liao J, Ho KL, Yang CS, Yang GY. Inhibition of chronic ulcerative colitis-associated colorectal adenocarcinoma development in a murine model by N-acetylcysteine. Carcinogenesis. 2002;23:993–1001. doi: 10.1093/carcin/23.6.993. [DOI] [PubMed] [Google Scholar]
- Newman JV, Kosaka T, Sheppard BJ, Fox JG, Schauer DB. Bacterial infection promotes colon tumorigenesis in Apc(Min/+) mice. J Infect Dis. 2001;184:227–230. doi: 10.1086/321998. [DOI] [PubMed] [Google Scholar]
- Gupta RA, Dubois RN. Colorectal cancer prevention and treatment by inhibition of cyclooxygenase-2. Nat Rev Cancer. 2001;1:11–21. doi: 10.1038/35094017. [DOI] [PubMed] [Google Scholar]
- Alexander RJ, Panja A, Kaplan-Liss E, Mayer L, Raicht RF. Expression of protooncogene-encoded mRNA by colonic epithelial cells in inflammatory bowel disease. Dig Dis Sci. 1996;41:660–669. doi: 10.1007/BF02213120. [DOI] [PubMed] [Google Scholar]
- Macpherson AJ, Chester KA, Robson L, Bjarnason I, Malcolm AD, Peters TJ. Increased expression of c-myc proto-oncogene in biopsies of ulcerative colitis and Crohn’s colitis. Gut. 1992;33:651–656. doi: 10.1136/gut.33.5.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillem JG, Levy MF, Hsieh LL, Johnson MD, LoGerfo P, Forde KA, Weinstein IB. Increased levels of phorbin, c-myc, and ornithine decarboxylase RNAs in human colon cancer. Mol Carcinog. 1990;3:68–74. doi: 10.1002/mc.2940030204. [DOI] [PubMed] [Google Scholar]
- Tselepis C, Morris CD, Wakelin D, Hardy R, Perry I, Luong QT, Harper E, Harrison R, Attwood SE, Jankowski JA. Upregulation of the oncogene c-myc in Barrett’s adenocarcinoma: induction of c-myc by acidified bile acid in vitro. Gut. 2003;52:174–180. doi: 10.1136/gut.52.2.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan N, Xiong YY, Lan J, Wang BC, Tian SF, Yu SP. Relationship between Helicobacter pylori infection and expression of c-myc, Bcl-2, and Bax protein in different gastric mucosa lesions. Ai Zheng. 2003;22:1034–1037. [PubMed] [Google Scholar]
- Pavelic ZP, Pavelic L, Kuvelkar R, Gapany SR. High c-myc protein expression in benign colorectal lesions correlates with the degree of dysplasia. Anticancer Res. 1992;12:171–175. [PubMed] [Google Scholar]
- Sugao Y, Koji T, Yao T, Ueki T, Tsuneyoshi M. The incidence of apoptosis during colorectal tumorigenesis. Int J Surg Pathol. 2000;8:123–132. doi: 10.1177/106689690000800207. [DOI] [PubMed] [Google Scholar]
- Kothny-Wilkes G, Kulms D, Luger TA, Kubin M, Schwarz T. Interleukin-1 protects transformed keratinocytes from tumor necrosis factor-related apoptosis-inducing ligand- and CD95-induced apoptosis but not from ultraviolet radiation-induced apoptosis. J Biol Chem. 1999;274:28916–28921. doi: 10.1074/jbc.274.41.28916. [DOI] [PubMed] [Google Scholar]
- Garza-Gonzalez E, Hold G, Perez-Perez GI, Bosques-Padilla FJ, Tijerina-Menchaca R, Maldonado-Garza HJ, El-Omar E. Role of polymorphism of certain cytokines in gastric cancer in Mexico. Preliminary results. Rev Gastroenterol Mex. 2003;68:107–112. [PubMed] [Google Scholar]
- El-Omar EM, Carrington M, Chow WH, McColl KE, Bream JH, Young HA, Herrera J, Lissowska J, Yuan CC, Rothman N, Lanyon G, Martin M, Fraumeni JF, Jr, Rabkin CS. The role of interleukin-1 polymorphisms in the pathogenesis of gastric cancer. Nature. 2000;404:398–402. doi: 10.1038/35006081. [DOI] [PubMed] [Google Scholar]
- Machado JC, Pharoah P, Sousa S, Carvalho R, Oliveira C, Figueiredo C, Amorim A, Seruca R, Caldas C, Carneiro F, Sobrinho-Simoes M. Interleukin 1B and interleukin 1RN polymorphisms are associated with increased risk of gastric carcinoma. Gastroenterology. 2001;121:823–829. doi: 10.1053/gast.2001.28000. [DOI] [PubMed] [Google Scholar]
- Belokrinitskaia TE, Vitkovskii Iu A, Ponomareva Iu N. Role of cytokines in the development of immunologic and homeostatic disorders in advanced dysplasia and carcinoma of the uterine cervix. Vopr Onkol. 2003;49:51–54. [PubMed] [Google Scholar]
- Fox JG, Wang TC, Rogers AB, Poutahidis T, Ge Z, Taylor N, Dangler CA, Israel DA, Krishna U, Gaus K, Peek RM., Jr Host and microbial constituents influence Helicobacter pylori-induced cancer in a murine model of hypergastrinemia. Gastroenterology. 2003;124:1879–1890. doi: 10.1016/s0016-5085(03)00406-2. [DOI] [PubMed] [Google Scholar]
- Shattuck-Brandt RL, Varilek GW, Radhika A, Yang F, Washington MK, DuBois RN. Cyclooxygenase 2 expression is increased in the stroma of colon carcinomas from IL-10(−/−) mice. Gastroenterology. 2000;118:337–345. doi: 10.1016/s0016-5085(00)70216-2. [DOI] [PubMed] [Google Scholar]
- Sano H, Kawahito Y, Wilder RL, Hashiramoto A, Mukai S, Asai K, Kimura S, Kato H, Kondo M, Hla T. Expression of cyclooxygenase-1 and -2 in human colorectal cancer. Cancer Res. 1995;55:3785–3789. [PubMed] [Google Scholar]
- Williams CS, Smalley W, DuBois RN. Aspirin use and potential mechanisms for colorectal cancer prevention. J Clin Invest. 1997;100:1325–1329. doi: 10.1172/JCI119651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson ML, Wilson ET, Prescott SM. Regulation of COX-2 transcription in a colon cancer cell line by Pontin52/TIP49a. Mol Cancer. 2003;2:42. doi: 10.1186/1476-4598-2-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuBois RN, Giardiello FM, Smalley WE. Nonsteroidal anti-inflammatory drugs, eicosanoids, and colorectal cancer prevention. Gastroenterol Clin North Am. 1996;25:773–791. doi: 10.1016/s0889-8553(05)70274-0. [DOI] [PubMed] [Google Scholar]
- Yuasa Y. Control of gut differentiation and intestinal-type gastric carcinogenesis. Nat Rev Cancer. 2003;3:592–600. doi: 10.1038/nrc1141. [DOI] [PubMed] [Google Scholar]
- Matysiak-Budnik T, Thomas-Collignon A, Megraud F, Heyman M. Alterations of epithelial permeability by Helicobacter and IL-1beta in vitro: protective effect of rebamipide. Dig Dis Sci. 2001;46:1558–1566. doi: 10.1023/a:1010664626431. [DOI] [PubMed] [Google Scholar]
- O’Rourke J, Dixon M, Jack A, Enno A, Lee A. Gastric B-cell mucosa-associated lymphoid tissue (MALT) lymphoma in an animal model of ‘Helicobacter heilmannii’ infection. J Pathol. 2004;203:896–903. doi: 10.1002/path.1593. [DOI] [PubMed] [Google Scholar]
- Sipowicz MA, Weghorst CM, Shiao YH, Buzard GS, Calvert RJ, Anver MR, Anderson LM, Rice JM. Lack of p53 and ras mutations in Helicobacter hepaticus-induced liver tumors in A/JCr mice. Carcinogenesis. 1997;18:233–236. doi: 10.1093/carcin/18.1.233. [DOI] [PubMed] [Google Scholar]
- Canella KA, Diwan BA, Gorelick PL, Donovan PJ, Sipowicz MA, Kasprzak KS, Weghorst CM, Snyderwine EG, Davis CD, Keefer LK, Kyrtopoulos SA, Hecht SS, Wang M, Anderson LM, Rice JM. Liver tumorigenesis by Helicobacter hepaticus: considerations of mechanism. In Vivo. 1996;10:285–292. [PubMed] [Google Scholar]
- Erdman SE, Poutahidis T, Tomczak M, Rogers AB, Cormier K, Plank B, Horwitz BH, Fox JG. CD4(+) CD25(+) regulatory T lymphocytes inhibit microbially induced colon cancer in Rag2-deficient mice. Am J Pathol. 2003;162:691–702. doi: 10.1016/S0002-9440(10)63863-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsukura N, Yokomuro S, Yamada S, Tajiri T, Sundo T, Hadama T, Kamiya S, Naito Z, Fox JG. Association between Helicobacter bilis in bile and biliary tract malignancies: H. bilis in bile from Japanese and Thai patients with benign and malignant diseases in the biliary tract. Jpn J Cancer Res. 2002;93:842–847. doi: 10.1111/j.1349-7006.2002.tb01327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullberg MC, Jankovic D, Gorelick PL, Caspar P, Letterio JJ, Cheever AW, Sher A. Bacteria-triggered CD4(+) T regulatory cells suppress Helicobacter hepaticus-induced colitis. J Exp Med. 2002;196:505–515. doi: 10.1084/jem.20020556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seril DN, Liao J, Yang GY, Yang CS. Oxidative stress and ulcerative colitis-associated carcinogenesis: studies in humans and animal models. Carcinogenesis. 2003;24:353–362. doi: 10.1093/carcin/24.3.353. [DOI] [PubMed] [Google Scholar]
- Parsonnet J. Molecular mechanisms for inflammation-promoted pathogenesis of cancer—The Sixteenth International Symposium of the Sapporo Cancer Seminar. Cancer Res. 1997;57:3620–3624. [PubMed] [Google Scholar]