A Tumor Necrosis Factor Receptor 1-Dependent Conversation between Central Nervous System-Specific T Cells and the Central Nervous System Is Required for Inflammatory Infiltration of the Spinal Cord (original) (raw)
Abstract
We examined the role of tumor necrosis factor receptor 1 (TNFR1) in inflammation initiated by the adoptive transfer of central nervous system (CNS)-specific Th1 cells in experimental autoimmune encephalomyelitis, a murine model of multiple sclerosis. This adoptive transfer paradigm eliminates the confounding effects of bacterial adjuvants in the analysis of inflammation. We found that although T cells could reach the meninges and perivascular space in the absence of TNFR1, recruitment of other inflammatory cells from the blood was dramatically reduced. The reduction in the recruitment of CD11bhi cells correlated with a dramatic reduction in the production of the chemokines CCL2 (MCP-1) and CXLC2 (MIP-2) in TNFR1-deficient hosts. Bone marrow chimera experiments demonstrated that TNF can be effectively supplied by either the hematopoietic system or the CNS, but the essential TNFR1-responsive cells reside in the CNS. Previous work has demonstrated that microglia produce CCL2, and here we demonstrate that astrocytes and endothelial cells produced CXCL2 in the early stages of inflammation. Therefore, productive inflammation results from a conversation, or mutually responding signals, between the initiating T cells and cells in the parenchyma of the spinal cord.
Experimental autoimmune encephalomyelitis (EAE) is an autoimmune demyelinating disease of the central nervous system (CNS). Because of similarities in clinical symptoms and CNS histology,1–5 EAE serves as a model for multiple sclerosis and can be induced in mice by either immunization with myelin protein or peptides6 or by adoptive transfer of myelin-specific Th1 CD4+ T cells.7,8 As a result, inflammatory cells consisting mostly of CD4+ T lymphocytes and macrophages infiltrate the CNS leading to demyelination and neuronal damage.9
Tumor necrosis factor receptor 1 (TNFR1), also known as the p55 kd TNF receptor, binds to two ligands, TNF and lymphotoxin-α.10 The dominant signaling pathway for TNFR1 promotes inflammation by up-regulating inflammatory cytokines, chemokines, and adhesion molecules11–17 and also suppresses apoptosis by the induction of IAPs (inhibitors of apoptosis) through nuclear factor (NF)-κB-dependent pathways.18 TNFR1 also has the ability to induce apoptosis through a death domain in its cytoplasmic region that initiates the FADD-dependent extrinsic apoptotic pathway.19
Our previous experiments have demonstrated that TNFR1 contributes to the pathogenesis of EAE mainly by promoting CNS inflammation,20 and thereby, in the absence of TNFR1 expression, clinical disease is limited. Through our adoptive transfer experiments we have found a dramatic difference in the location of encephalitogenic T cells within the CNS of wild-type (WT) versus TNFR1-null mice. Although we observed equivalent numbers of T cells in the CNS of TNFR1-deficient mice compared to WT mice, we found that the T cells in the TNFR1-null animals were confined to the leptomeninges and perivascular spaces of the spinal cord, whereas in the WT animals an abundant number of inflammatory cells were found within the CNS parenchyma.
A critical role for macrophages in EAE has been supported by a number of studies. Monocytes along with T cells make up the majority of the inflammatory infiltrate within CNS lesions, and histological evidence of lipid-laden macrophages within multiple sclerosis and EAE lesions has been described.4,5 In addition, it has been demonstrated that depletion of peripheral macrophages using dichloromethylene diphosphonate prevents EAE and demyelination in rats and mice.21,22
In this report, we show that TNFR1 plays an important role in the production of the chemokines CXCL2 and CCL2, which are associated with inflammation and disease. Bone marrow chimera experiments demonstrate that the critical TNFR1-expressing cell(s) for induction of the inflammatory response resides in host tissue, most likely cells within the CNS. This TNFR1-dependent production of chemokines by cells in the CNS appears to play an important role in the initiation of early inflammation.
Materials and Methods
Animals
C57BL/6, congenic Thy1.1 mice (B6.PL-Thy1a/Cy), and congenic CD45.1 mice (B6.SJL-PtprcaPep3b/BoyJ) were purchased from Jackson Laboratories (Bar Harbor, ME). Breeders of the congenic TNFR1-null mutation (B6.129-Tnfrsf1atm/Mak) were purchased from the same source and maintained by brother-sister matings in our colony. All experiments were approved by the Animal Care Committee, and animals were housed in an Association for Assessment and Accreditation of Laboratory Animal Care-approved facility.
Bone Marrow Chimeras
Bone marrow was harvested from CD45.1 congenic and TNFR1-null donor mice as follows. Femurs were isolated and cut at both ends, and tibias were isolated and cut at the patellar end. Bone marrow cells were flushed from the open ends of the femur and tibia and purified with anti-CD8 and anti-CD4 antibodies plus rabbit complement followed by Ficoll 1.119 (Sigma, St. Louis, MO). Six- to eight-week-old WT, CD45.1 congenic and TNFR1-null mice were lethally irradiated (1000 R). Twenty-four hours later, 2 × 106 bone marrow cells in 300 μl of Hanks’ balanced salt solution (HBSS) were injected intravenously into the irradiated recipients.
Induction of EAE
For adoptive transfer, Thy1.1 congenic donor mice were immunized subcutaneously with 50 μg of MOG35-55 peptide (Sigma Genosys, Woodlands, TX) emulsified in Incomplete Freund’s Adjuvant supplemented with 500 μg/ml of Mycobacterium tuberculosis. These mice did not receive pertussis toxin. Spleens were removed from donor mice 14 days after immunization and passed through sterile nylon cell strainers to form single cell suspensions and passed over nylon wool to enrich for T cells. Primary CD4+ T cells were obtained by negative selection of nylon wool-enriched T cells with anti-CD8 and anti-J11d antibodies plus rabbit complement.23 Purified T cells were maintained in culture at a concentration of 1 × 106 cells/ml with 5 × 106 cells/ml of irradiated splenocytes, 10 μg/ml MOG35-55 peptide (Sigma Genosys), 10 U/ml IL-12, and 10 U/ml IL-2 for 7 days in 1.5 ml of supplemented RPMI.23 Each week cells were purified with Ficoll 1.077 (Sigma) and restimulated using the same conditions as the first stimulation. On the fourth day after the sixth stimulation, cells were harvested, washed, and resuspended in HBSS. Cells (5 × 106) in 300 μl of HBSS were injected intravenously into sublethally irradiated (450 R) 6- to 8-week-old recipients. For the bone marrow chimeras, 1 × 107 cells in 300 μl of HBSS were injected intravenously into the chimeras 6 to 8 weeks after bone marrow transplant. Mice were monitored daily for clinical signs and graded on a scale of 0 to 5 as described previously24 : 0.5, loss of the ability to curl tip of tail; 1, limp tail; 2, hind limb weakness; 3, paralysis of one hind limb; 4, paralysis of both hind limbs; 5, death or moribund mice that were sacrificed.
Fluorescence-Activated Cell Sorting (FACS) Analysis of CNS Cells
Cells were isolated from the brain and spinal cord based on earlier protocols.25–27 Briefly, the brain and spinal cord were removed after perfusion of the mouse with 30 ml of saline via cardiac puncture of the left ventricle. Single cell suspensions were prepared from brain and spinal cord by dispersion in 10 ml of cold HBSS supplemented with 0.05% collagenase D (Sigma), 0.1 μg/ml TLCK trypsin inhibitor (Sigma), 10 μg/ml DNase I (Sigma), and 10 mmol/L HEPES (pH 7.4) using sterile nylon cell strainers. The resulting suspension was gently mixed at room temperature for 1 hour followed by centrifugation at 500 × g for 10 minutes. Pelleted material was resuspended in 70% Percoll (Pharmacia Biotech, Piscataway, NJ) and additional 37% and 30% layers were added above the cells. Density gradients were centrifuged for 30 minutes at 1200 × g. The debris layer (30% Percoll) was removed and discarded. The remaining gradient was resuspended in 50 ml of HBSS and centrifuged at 500 × g for 10 minutes. Pellets were resuspended in 1 ml of HBSS supplemented with 0.2% bovine serum albumin (Sigma), 0.1% sodium azide (Sigma), and 15 mmol/L HEPES before antibody staining and flow cytometry. The following antibodies were used: anti-CD11b-FITC, anti-CD11b-APC, anti-CD45.1-PE, and anti-CD45.2-FITC (BD PharMingen, San Diego, CA). All analyses were conducted on a FACScan using CellQuest software (BD Biosciences, San Jose, CA).
FACS Analysis of Splenocytes
Animals were sacrificed and the spleen was removed and passed through sterile nylon cell strainers to form single cell suspensions. Cells were resuspended in 1 ml of HBSS supplemented with 0.2% bovine albumin serum, 0.1% sodium azide, and 15 mmol/L HEPES before antibody staining and flow cytometry using anti-CD11b-APC, anti-CD45.1-PE, and anti-CD45.2-FITC (BD PharMingen). All analyses were conducted on a FACScan using CellQuest software (BD Biosciences).
Immunohistochemistry
CNS tissues removed from mice perfused with saline were embedded and frozen in OCT. Ten-μm sections from the lumbar-sacral spinal cord were placed on Superfrost Plus slides (Fisher Scientific Co., Pittsburgh, PA), fixed in acetone for 5 minutes, immunostained for either Thy1.1, CD11b, or GR-1 (antibodies purchased from BD PharMingen) using the Tyramide signal amplification kit (Perkin-Elmer Life Sciences, Boston, MA) according to the manufacturer’s instructions, counterstained with Hoechst 33258 (Polysciences, Warrington, PA), and mounted in Fluoromount-G (Southern Biotechnology Assoc., Birmingham, AL). Control sections stained with a rat IgG isotype control antibody or no antibody were negative for Thy1.1, CD11b, and GR-1.
Immunohistochemistry for Chemokine Localization
Frozen sections were washed with phosphate-buffered saline (PBS). All tissue sections were permeabilized with 0.1% Triton X-100 (Sigma) and nonspecific antibody was blocked with 10% normal goat serum and 10% normal donkey serum (Santa Cruz Biotechnology, Santa Cruz, CA) for 1 hour at room temperature. Polyclonal antibodies specific for CXCL2 (R&D Systems, Minneapolis, MN) or monoclonal antibodies specific for glial fibrillary acidic protein (GFAP) (Zymed, South San Francisco, CA), CD11b (BD Pharmingen) or CD31 (PECAM-1) (BD Pharmingen) were applied at 5 to 15 μg/ml in PBS containing 10% donkey serum and 0.1% Triton X-100 overnight at 4°C. Primary antibodies were detected with secondary donkey anti-goat or -mouse IgG conjugated to Alexa 594 or Alexa 488 (Molecular Probes Inc., Eugene, OR) for immunofluorescence and nuclei were counterstained with 4,6-diamidino-2-phenylindole (DAPI). Staining with hematoxylin and eosin (H&E) was performed on paraffin sections from animals perfused with 10% paraformaldehyde to facilitate morphological identification of polymorphonuclear leukocytes.
RNA Isolation and Analysis
Sublethally irradiated (450 R) 6- to 8-week-old B6 WT and TNFR1-null mice were injected intravenously with either 5 × 106 Th1-skewed Thy1.1 WT CD4+ MOG-specific T cells or PBS. On days 10 and 12 after T cell transfer, WT and TNFR1-null mice were sacrificed and perfused with diethyl pyrocarbonate-treated PBS. Spinal cords were removed and homogenized with TRI Reagent (Molecular Research Center, Cincinnati, OH). RNA isolation was performed according to the manufacturer’s protocol provided with the TRI Reagent. Briefly, 1-bromo-3-chloro-propane (Sigma) at 10% the volume of TRI Reagent was added to the homogenized tissue. After centrifugation, the upper aqueous phase was isolated and precipitated with isopropyl alcohol. The RNA pellet was washed with 70% ethanol and resuspended in diethyl pyrocarbonate-treated milli-Q water. Amplification of cDNA and the real-time reverse transcriptase-polymerase chain reaction (RT-PCR) assay was performed as a service by the GeneChip Core Facility (Siteman Cancer Center, Barnes-Jewish Hospital, Washington University School of Medicine, St. Louis, MO) using the TaqMan MGB probe and primer pairs that were purchased from Applied Biosystems (ABI, Foster City, CA). Each assay was performed in duplicate and there was agreement between duplicates within one cycle. The average threshold cycle (Ct) was used for our calculations. HPRT was used as the reference gene. The relative mRNA value for the target gene was calculated as follows, taking into consideration the doubling of product at every cycle:
Ct HPRT − Ct target = Δ_Ct 2ΔCt_ = relative mRNA
Chemokine in Situ Hybridization and Immunohistochemistry
Tissue Preparation
Tissue was prepared as described for immunohistochemistry after the animals were perfused with diethyl pyrocarbonate-saline as described.
Probe Preparation
Total RNA from spinal cords of WT mice with EAE was reverse-transcribed using oligo (dT)15 primers and Stratascript reverse transcriptase (Stratagene, La Jolla, CA). A 457-bp fragment of the CXCL2 cDNA (nucleotides 274 to 731, GenBank accession no. NM 009140) was PCR-amplified using primers encoding internal _Xba_I and _Kpn_I sites: forward, 5′-AGTTTG_TCTAGA_CCCTGAAGCC-3′; reverse, 5′-ATGTAGCT_GGTACC_CAACTC-3′. The CXCL2 cDNA fragment was sequenced for verification and then cloned into a pGEM-4Z vector (Promega, Madison, WI), which contains SP6 and T7 promoters. DIG-labeled probes were generated by in vitro transcription of linearized plasmid using a DIG RNA labeling kit (Roche, Indianapolis, IN). Sense and anti-sense digoxigenin-labeled riboprobes were synthesized using plasmid linearized with _Eco_RI (sense) and _Xba_I (anti-sense) according to the manufacturer’s instructions (Boehringer Mannheim, Mannheim, Germany).
In Situ Hybridization
Frozen tissue sections were fixed in 4% paraformaldehyde in PBS for 20 minutes, washed in PBS, and then digested with 20 μg/ml of proteinase K for 5 minutes at room temperature. Sections were refixed in 4% paraformaldehyde and washed in PBS, and then in situ hybridization was conducted for 20 hours at 65°C using DIG-labeled cRNA probes in hybridization buffer (50% formamide, 5× standard saline citrate, 200 μg/ml yeast tRNA, 100 μg/ml heparin, 1 Denhardt’s, 0.1% Tween 20, 0.1% CHAPS, and 5 mmol/L ethylenediaminetetraacetic acid). The sections were washed with 0.2× standard saline citrate, 0.1% Tween 20 at 65°C and then treated with blocking reagent (20% sheep serum in buffer) and then anti-DIG antibody followed by antibody detection according to the manufacturer’s protocol (Boehringer Mannheim).
Results
Absence of TNFR1 Expression in the CNS Results in Inefficient Recruitment of Host Inflammatory Cells
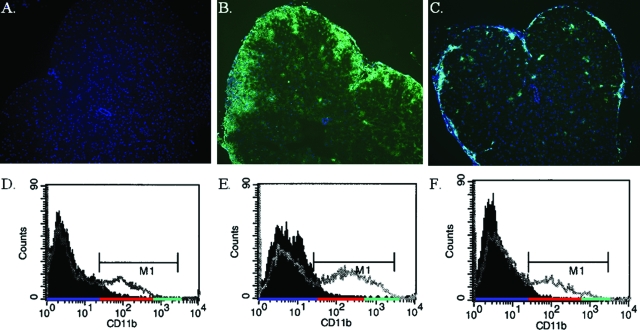
Our previous experiments with adoptively transferred EAE demonstrated a dramatic difference in the location of T cells within the CNS of WT versus TNFR1-null host mice.20 On further investigation, our findings also revealed a block in the recruitment of CD11bhi cells to the TNFR1-null CNS but abundant CD11bhi cells in the WT mice (Figure 1). Immunohistochemical analysis was performed with a concentration of anti-CD11b (Mac-1) antibody that enhanced the visualization of cells with up-regulated expression of CD11b (Figure 1B). The majority of cells that make up this CD11bhi population in the inflamed CNS are recruited monocytes from the periphery and activated resident macrophage and microglia. As for the TNFR1-null CNS, the CD11bhi cells were few in number and restricted to the leptomeninges and outer edges of the parenchyma (Figure 1C). This observation was confirmed by flow cytometry. There are no CD11bhi cells in naïve animals (Figure 1D) and an increased percentage of CD11b-positive cells and increased CD11b expression levels in the CNS of WT compared to TNFR1-null mice (Figure 1, E and F). These findings indicate that CD11bhi peripheral monocytes are efficiently recruited to the WT CNS, but are less so to the TNFR1-null CNS.
Figure 1.
Recruitment of CD11b+ cells to the CNS is attenuated in TNFR1-null mice. Th1-skewed Thy1.1 WT CD4+ MOG-specific T cells (5 × 106) were injected intravenously into sublethally irradiated (450 R) 6- to 8-week-old Thy1.2 B6 WT and TNFR1-null recipients. Frozen sections of lumbar-sacral spinal cord from naïve B6 (A); day 12 WT, clinical score = 2 (B); and day 12 TNFR1-null, clinical score = 0 (C) were immunostained for CD11b (green) and counterstained with DAPI (blue). Flow cytometry for CD11b was also performed on cells isolated from the brain and spinal cord of similar, naïve (M1 MFI = 140) (D); WT, clinical score = 2 (M1 MFI = 339) (E); and TNFR1, clinical score = 0 (M1 MFI = 187) (F), hosts day 12 after transfer. The solid histogram is staining with an isotype control antibody and represents CD11bneg cells highlighted by the blue underscore on the x axis. CD11blo cells are highlighted by the red underscore and CD11bhi cells highlighted by the green underscore. The MFIs were calculated for cells in the M1 gate. Original magnifications, ×65.
TNF Can Be Effectively Produced by Either the Hematopoietic System or the CNS
Adoptive transfer of WT T cells into WT or TNF-deficient hosts demonstrate that the majority of the TNF necessary for disease comes from the host rather than the infiltrating T cell (Table 1). Reciprocal bone marrow chimeras suggest that either the hematopoietic system or resident CNS cells can provide sufficient TNF to initiate disease (Table 1). The fact that the TNF-null → TNF-null chimeras (experiment 3) are not equivalent (some disease) to the unmanipulated TNF-null host (experiment 1, no disease) may be either an effect of irradiation or more likely is a reflection of the fact that the transferred cells were more effective in the chimera experiment. Only one WT host died in the comparison between WT and TNF-null hosts (experiment 1), whereas all three WT control hosts similar to experiment 1 (not chimeras) died in experiment 3 (data not shown). Thus the weaker disease in the TNF-null chimeras could result from the production of higher levels of lymphotoxin by the transferred cells in experiment 3.28
Table 1.
TNF Can Be Effectively Produced by Either the Hematopoietic System or the CNS
| Experiment | Host* | % Incidence, n† | Peak disease‡ |
|---|---|---|---|
| 1 | WT | 100%, 7 | 3.8 ± 0.7 |
| TNF-null | 0%, 7 | 0 ± 0 | |
| 2 | WT→WT | 100%, 5 | 4.1 ± 2.0 |
| WT→TNF-null | 100%, 4 | 5.0 ± 0 | |
| 3 | TNF-null→TNF-null | 80%, 5 | 2.3 ± 1.9 |
| TNF-null→WT | 100%, 5 | 5.0 ± 0 |
Crucial TNF-Responsive Cell(s) Are in the CNS
TNFR1 expression has been detected on various cells within the CNS including endothelial cells, astrocytes, microglia, and oligodendrocytes.29–31 Our data thus far has suggested that there is a TNFR1-expressing cell that controls trafficking of T cells into the CNS parenchyma20 and recruitment of CD11bhi cells to the CNS with subsequent development of clinical disease. Because many inflammatory cells in the blood and lymphoid compartments also express TNFR1, we sought to determine whether the essential TNFR1-expressing cell(s) were of hematogenous origin by performing reciprocal bone marrow chimera experiments.
To differentiate between the donor bone marrow cells and the host cells, we used CD45 congenic mice. CD45.1 bone marrow was used to reconstitute the bone marrow of lethally irradiated CD45.2 WT and TNFR1-null mice. In a reciprocal experiment, TNFR1-null bone marrow was used to reconstitute the bone marrow of lethally irradiated CD45.1 WT and CD45.2 TNFR1-null mice. Six to eight weeks after reconstitution, blood samples were stained for CD45 alleles and CD11b to confirm complete reconstitution of this compartment in those situations in which there was a difference in CD45 alleles between the bone marrow and the host. Staining of cells isolated from the CNS of representative animals confirmed earlier observations32–36 that microglia (CD45lo/CD11b+) expressed the recipient CD45 allele and perivascular macrophages (CD45hi/CD11b+) expressed the CD45 allele of the bone marrow donor (data not shown).
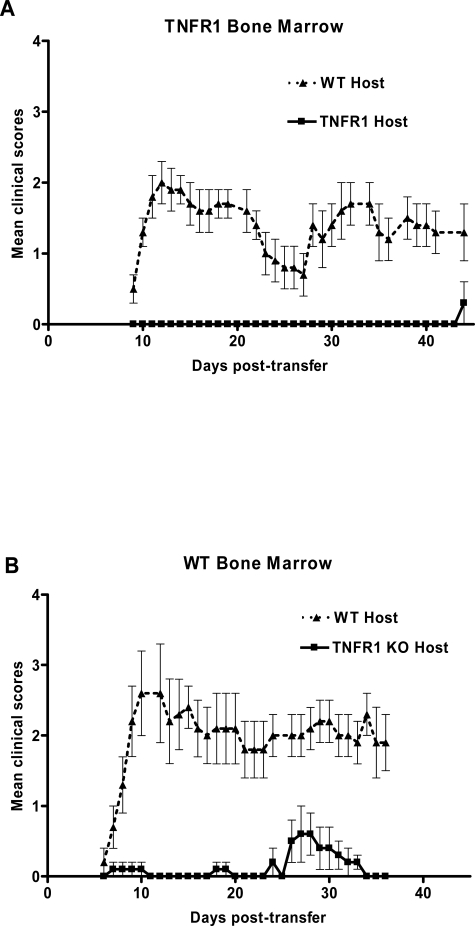
We found that Th1 cells from WT animals could not reconstitute disease in TNFR1-null hosts reconstituted with WT bone marrow (Figure 2B). In contrast, they do cause disease in bone marrow chimeras of TNFR1-null bone marrow into WT hosts (Figure 2A). This suggests that the important TNFR1-expressing cell for the initiation of clinical symptoms in EAE resides in the CNS and not in a cell of hematogenous origin, such as recruited or perivascular macrophages.
Figure 2.
WT bone marrow does not reconstitute disease in TNFR1-null animals. EAE was passively induced into bone marrow chimeras. A: TNFR1-null bone marrow in lethally irradiated WT (n = 9, ▴), or TNFR1-null (n = 9, ▪) mice. B: TNFR1-null bone marrow in lethally irradiated WT (n = 9, ▴), or TNFR1-null (n = 9, ▪). Th1-skewed Thy1.1 WT CD4+ MOG-specific T cells (1 × 107) were injected intravenously 6 to 8 weeks after bone marrow transplantation. Clinical signs of disease were monitored daily and graded on a scale of 0 to 5 as described previously.
CXCL2 and CCL2 Are Deficient in TNFR1-Null CNS with Adoptively Transferred EAE
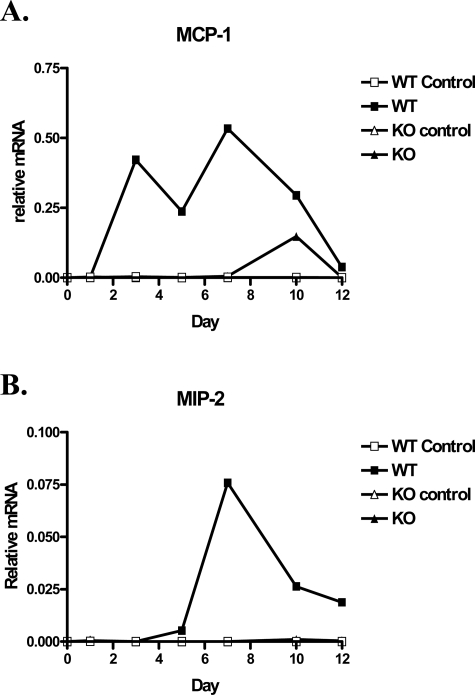
Inducible chemokines play an important role in the recruitment of various immune cells and are likely candidates for the TNFR1-dependent recruitment of CD11b+ cells observed above. Therefore we performed a preliminary PCR screen on RNA isolated from the CNS of WT and TNFR1-deficient hosts on day 10 after transfer of WT Th1 cells. We included several chemokines such as KC (CXCL3), RANTES (CCL5), IP-10 (CXCL10), MIP-1α (CCL3), and MIP-1β (CCL4) that have previously been associated with EAE,37 but only CXCL2 and CCL2 displayed dramatic differences between WT and TNFR1-null CNS (data not shown). CXCL2 is one of the major neutrophil chemotactic proteins,38 whereas CCL2 is one of the major monocyte chemotactic proteins.39 To determine whether an absence of CXCL2 or CCL2 contributed to the distinct composition of inflammatory cells within the TNFR1-null CNS, we performed real-time RT-PCR on RNA isolated from individual WT and TNFR1-null CNS on days 1, 3, 5, 7, 10, and 12 after transfer (Figure 3, A and B). We also analyzed spinal cord RNA from WT and TNFR1-null mice (three each) on day 6 after transfer for CXCL2 and CCL2 expression to allow a statistical comparison between groups early in the disease course. On day 6, CXCL2 expression in WT was >100× higher (P = 0.001) than in TNFR1-null animals, which was essentially at threshold levels. The average CCL2 expression in these day 6 samples was eightfold higher in WT than in TNFR1-null hosts, although this difference was not statistically significant (P = 0.237) because one of the three WT hosts remained at a normal TNFR1-null level. The deficiency of CXCL2 and CCL2 expression in the TNFR1-null CNS provides a molecular basis for defective recruitment of inflammatory cells, especially of neutrophils, in TNFR1-null hosts. The control RNA for these experiments was isolated from the CNS of appropriate, sublethally irradiated WT or TNFR1-null mice that had not received adoptively transferred Th1 cells.
Figure 3.
Time course of relative mRNA levels of MCP-1 (A) and MIP-2 (B) in the spinal cord of WT and TNFR1-null mice. Th1-skewed Thy1.1 WT CD4+ MOG-specific T cells (5 × 106) or PBS were injected intravenously into sublethally irradiated (450 R) 6- to 8-week-old Thy1.2 B6 WT and TNFR1-null recipients. On days 1, 3, 5, 7, 10, and 12 after transfer, total RNA was isolated from spinal cords and mRNA transcripts were analyzed by quantitative real-time RT-PCR. All samples were normalized to HPRT.
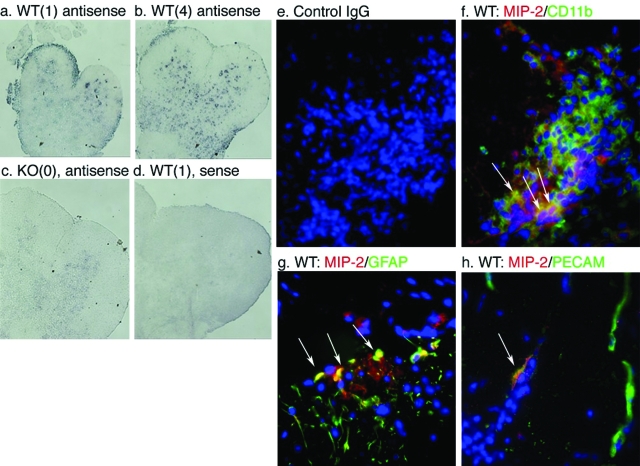
To identify the cells responsible for CXCL2 expression, we examined CXCL2 mRNA and protein expression by in situ hybridization and immunohistochemistry (Figure 4). Th1 cells were adoptively transferred into WT and TNFR1-null hosts and animals sacrificed 6 days later, just after disease onset in the WT hosts. In situ hybridization (Figure 4, a–d) indicated that the major focus of CXCL2 production was associated with an inflammatory lesion, although there may also be expression in vessels in the parenchyma (punctuate staining pattern). Immunohistochemistry (Figure 4, f and g) revealed that the principal producers of CXCL2 were CD11b+ (macrophages in the inflammatory infiltrate or activated microglia) and GFAP+ cells in the CNS (astrocytes), although there was some indication of production in PECAM+ (vascular endothelium) structures in the region of the infiltrating leukocytes (Figure 4h). Although there appears to be CXCL2 scattered in the parenchyma by in situ hybridization, the only immunoreactive material was associated with the white matter infiltration.
Figure 4.
CXCL2 is produced by astrocytes and vascular endothelium of the CNS as well as CD11b+ cells from the inflammatory infiltrate early in the disease course. In situ hybridization analysis of spinal cord sections of irradiated WT (a, b, and d) and TNFR1-null (c) mice 6 days after transfer of 5 × 106 Thy 1.1 cells using digoxigenin-labeled anti-sense (a–c) and sense (d) riboprobes. Clinical scores of animals are shown in parentheses. Immunohistochemical analyses of CXCL2 (red) expression within CD11b- (green) (f), GFAP- (green) (g), and PECAM-expressing cells (green) (h). Nuclei have been counterstained with DAPI. Arrowheads: Co-localization (yellow) of CXCL2- and CD11b- (f), GFAP- (g), and PECAM- (h) positive cells. Arrows: MIP-2-negative, PECAM-positive endothelium. e: Control IgG does not demonstrate any specific staining. Analyses performed on sections from six animals, n = three mice per group. Original magnifications, ×350.
GR1 Expression Is Abundant in the CNS of WT but Absent in TNFR1-Null Mice and Precedes T-Cell Entry into the CNS Parenchyma
To determine whether the lack of CXCL2 expression in the TNFR1-null CNS translated into an absence of neutrophil recruitment, we immunostained spinal cord sections for GR1, a marker for granulocytes. Consistent with our real-time RT-PCR findings, we observed an abundance of GR1 expression in the WT CNS parenchyma but a complete absence of GR1 expression in the TNFR1-null CNS (Figure 5, A and B). Because GR1 expression is high in both neutrophils and eosinophils, we also performed H&E staining on similar spinal cord sections to assess the prevalence of eosinophils versus neutrophils. After examining several spinal cord lesions that were positive for GR1 expression, we were able to identify numerous neutrophils but eosinophils are exceedingly rare (Figure 5, C and D), in accord with earlier reports.40
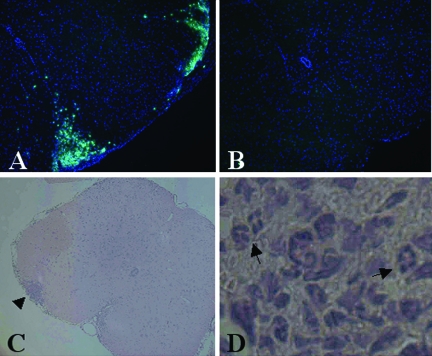
Figure 5.
Recruitment of peripheral GR1+ cells to the CNS is blocked in TNFR1-null mice. Th1-skewed Thy1.1 WT CD4+ MOG-specific T cells (5 × 106) were injected intravenously into sublethally irradiated (450 R) 6- to 8-week-old Thy1.2 B6 WT and TNFR1-null recipients. Frozen sections of lumbar-sacral spinal cord from day 6 WT, CS = 1.5 (A), and day 6 TNFR1-null, CS = 0 (B) were immunostained for GR-1 (green) and counterstained with DAPI (blue). C and D: H&E section of lumbar-sacral spinal cord from day 7 WT, CS = 1, shows a focus of inflammatory infiltrate (arrowhead) invading the parenchyma, which contains many neutrophils (arrows). Original magnifications: ×65 (A–C); and ×260 (D).
Subsequent experiments have not revealed differences in the number of GR1hi cells extracted from the CNS of WT or TNFR1-null hosts as identified by flow cytometry as neutrophils.41 Therefore, either the GR1+ cells identified by immunohistochemistry are a subset of monocytes that are GR1 intermediate, or neutrophils can be mobilized without CXCL2 but cannot localize to the lesions. As discussed above another chemokine chemotactic for neutrophils, CCL3, appears to increase qualitatively similarly in WT and TNFR1-null hosts. Our earlier experiments have clearly demonstrated that FACS analysis cannot be used to infer anatomical localization.20,42 Clearly neutrophils are in the WT but not the TNFR1-null lesions by H&E staining, but there are also fewer and perhaps less activated monocytes in which could also be reflected in an absence of GR1+ cells in the TNFR1-null hosts. It has not been possible to distinguish between these possibilities by dual-staining techniques in immunohistochemistry.
Discussion
Our adoptive transfer EAE model in TNFR1-deficient mice has provided a unique tool for examining the inflammatory response during EAE. Previously we have shown that although both WT and TNFR1-null mice with adoptively transferred EAE contain equivalent numbers of encephalitogenic T cells in the CNS (as defined by flow cytometry of extracted cells), the T cells are restrained from entering the CNS parenchyma of TNFR1-deficient mice.20 In this report we demonstrate a lack of recruited CD11b+ cells to the TNFR1-null CNS. Therefore, the composition of inflammatory cells in the TNFR1-null CNS is very unique in comparison to WT in which there is an abundance of T cells and recruited CD11bhi cells in the CNS parenchyma. As a result, TNFR1-null animals do not exhibit clinical signs of EAE whereas WT animals display severe and chronic debilitating disease.
With real-time RT-PCR we have shown that CXCL2 and CCL2 expression are more abundant in the WT than in the TNFR1-null CNS. These data are in agreement with our findings that show a lack of neutrophils and diminished peripheral macrophage recruitment to the TNFR1-null CNS. The association of CCL2 production with TNFR1 provides a critical upstream signaling mechanism for the previously described failure to recruit macrophage in CCL2-deficient animals.43 This latter study also found that cells in the CNS (vascular endothelium, astrocytes, microglia) were important sources of CCL2, providing additional evidence that the CNS plays an important role in directing the inflammatory attack initiated by Th1 cells.
A study by Matejuk and colleagues44 made similar associations between the induction of CCL2 and CXCL2 in adjuvant-induced EAE and the dependence of CCL2 and CXCL2 expression on TNF. This study examined the production of these chemokines during the peak of disease and concluded that they were produced primarily by infiltrating MAC-1+ cells, which would not be the crucial TNFR1-dependent cells in the CNS identified by the bone marrow chimeras. It is quite possible that at the peak of disease, the MAC1+ cells quantitatively produce the most CXCL2. Because the recruitment of these cells (CD11bhi) is also reduced in TNFR1-null hosts, this explains why the peak of the difference in TNFR1-dependent CXCL2 expression is after the onset of disease. Our data confirms the presence of CXCL2 in CD11b+ cells that are in the inflammatory infiltrate but demonstrate that astrocytes and endothelial cells are also important sources, which would be consistent with the observation that the crucial TNFR1+ cells identified by the bone marrow chimeras are not in the hematopoietic compartment.
Astrocytes have been shown to express TNFR1 and inflammatory mediators such as chemotactic factors and adhesion molecules.30,45–49 Luo and colleagues50 have previously reported that astrocyte production of CCL2 in response to RANTES is TNF-dependent. Therefore the reduction in early CCL2 production in TNFR1-null hosts could result from an astrocyte deficiency in TNFR1. In addition, we have previously demonstrated that astrocytes express VCAM-1 in a TNFR1-dependent manner in vivo.20 Thus, astrocytes appear to play a critical role in directing the acute inflammatory response by both recruiting inflammatory cells and providing a substrate to promote their movement and retention in the parenchyma.
Our findings also suggest an important role for neutrophils in EAE. The role of neutrophils in EAE has not been adequately addressed in the literature because they are not associated with the lesions in multiple sclerosis patients. However, they have been associated with atypical forms of EAE in a variety of models.51–54 Similarly, antibody depletion experiments have suggested their importance in the SJL model,55 although the depletions can be complicated because both monocytes and polymorphonuclear leukocytes appear to mature from GR1hi precursors.56,57 One possible explanation for the paucity of neutrophils observed in most forms of EAE and multiple sclerosis is that the more typical dominance of monocytes and lymphocytes normally associated with disease is with more established lesions and neutrophils may play a more important but transient role in the earliest stages of disease initiation.
In contrast to these reports, one recent report suggested that neutrophils may actually suppress inflammation.41 This was based on an analysis of the activity of neutrophils isolated from actively induced lesions on a variety of immunological activation assays in culture. Given our general understanding of the proinflammatory effects of neutrophils in vivo, these assays in culture may not reflect the in vivo situation in terms of the cellular ratios and activation states.
Neutrophils provide an immediate source of proteinases that can degrade the extracellular matrix and may also contribute to myelin degradation or produce a variety of mediators that could affect axonal function.58,59 Our histological findings show that T-cell entry of the CNS parenchyma is temporally close to the appearance of neutrophils in the CNS. In the TNFR1-deficient CNS, we demonstrate an overall lack of neutrophil recruitment and T-cell entry into the CNS parenchyma. These findings imply that neutrophils may help to directly break down the blood brain barrier or participate in the recruitment of other cells for inflammatory cell entry into the white matter.
Our data indicate that the critical TNFR1-expressing cells are in the CNS and not part of the blood or lymphoid compartments. Previous reports have demonstrated that perivascular macrophages are bone marrow-derived, whereas microglia residing in the parenchyma, although developmentally derived from a similar lineage, undergo little turnover with the bone marrow compartment.32–36
As a whole, our data supports the following model for CNS inflammation in initiating disease. Encephalitogenic T cells gain access to the perivascular spaces of the CNS producing RANTES, TNF, and lymphotoxin. The adoptive transfer and bone marrow chimera experiments suggest that the levels of TNF and lymphotoxin produced by the T cells must be enhanced by their stimulation of TNF from either cells derived from hematopoietic or CNS origins. This is in contrast to earlier experiments in which hematopoietic cells were the required source of TNF.60 However, these experiments involved active induction, including the use of adjuvants, that have been demonstrated to obscure TNF effects on disease.61
Astrocytes, endothelial cells, and/or microglia in the CNS respond to TNF by recruiting monocytes and polymorphonuclear leukocytes to the CNS. These recruited cells may be a source of metalloproteases, additional chemokines, TNF, or other mediators of acute pathogenesis. Degradation of the extracellular matrix in combination with up-regulation of VCAM-1 on astrocytes allows further encephalitogenic T cells, polymorphonuclear leukocytes, and macrophages into the CNS parenchyma. Alternatively, recruited polymorphonuclear leukocytes may also participate in the recruitment of monocytes through the production of CCL2, also reportedly enhanced by TNF.62 The presence of this inflammatory infiltrate in the parenchyma leads to clinical disease.
The expression of TNFR1 on oligodendrocytes and/or neurons may also contribute to pathogenesis.63,64 However, our data suggests that the primary role of TNFR1 is in the early events associated with inflammation and invasion of the CNS parenchyma. This dominant role of TNF in the initiation of disease rather than in disease progression is in accord with other experiments65 and may explain the anomalous findings of exacerbation of established disease in patients.66 The data here provides evidence that TNFR1-dependent chemokine production by cells in the CNS is an important mechanism of T cell-mediated inflammation.
Acknowledgments
We thank Kira Moore and Eugene Lin for technical assistance and Dr. Jason Lees for a critical discussion of the manuscript.
Footnotes
Address reprint requests to John H. Russell, Dept. of Molecular Biology and Pharmacology, Washington University School of Medicine, 660 S. Euclid Ave., St. Louis, MO 63110. E-mail: jrussell@wustl.edu.
Supported by the National Institutes of Health (grants AI45861 to J.H.R. and NS045607 to R.S.K.), the National Multiple Sclerosis Society (grants RG 3314 to J.H.R. and RG 3450 to R.S.K.), and Pfizer (J.H.R.).
References
- Goverman J, Brabb T. Rodent models of experimental allergic encephalomyelitis applied to the study of multiple sclerosis. Lab Anim Sci. 1996;46:482–492. [PubMed] [Google Scholar]
- Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey. National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology. 1996;46:907–911. doi: 10.1212/wnl.46.4.907. [DOI] [PubMed] [Google Scholar]
- Lucchinetti CF, Brueck W, Rodriguez M, Lassmann H. Multiple sclerosis: lessons from neuropathology. Semin Neurol. 1998;18:337–349. doi: 10.1055/s-2008-1040885. [DOI] [PubMed] [Google Scholar]
- Prineas JW. The Neuropathology of Multiple Sclerosis. Koetsier JC, editor. New York: Elsevier,; 1985:p 213. [Google Scholar]
- Raine CS. Biology of disease. Analysis of autoimmune demyelination: its impact upon multiple sclerosis. Lab Invest. 1984;50:608–635. [PubMed] [Google Scholar]
- Zamvil SS, Steinman L. The T lymphocyte in experimental allergic encephalomyelitis. Annu Rev Immunol. 1990;8:579–621. doi: 10.1146/annurev.iy.08.040190.003051. [DOI] [PubMed] [Google Scholar]
- Mokhtarian F, McFarlin DE, Raine CS. Adoptive transfer of myelin basic protein-sensitized T cells produces chronic relapsing demyelinating disease in mice. Nature. 1984;309:356–358. doi: 10.1038/309356a0. [DOI] [PubMed] [Google Scholar]
- Panitch HS, McFarlin DE. Experimental allergic encephalomyelitis: enhancement of cell-mediated transfer by concanavalin A. J Immunol. 1977;119:1134–1137. [PubMed] [Google Scholar]
- Raine CS, Barnett LB, Brown A, Behar T, McFarlin DE. Neuropathology of experimental allergic encephalomyelitis in inbred strains of mice. Lab Invest. 1980;43:150–157. [PubMed] [Google Scholar]
- Hochman PS, Majeau GR, Mackay F, Browning JL. Proinflammatory responses are efficiently induced by homotrimeric but not heterotrimeric lymphotoxin ligands. J Inflamm. 1995;46:220–234. [PubMed] [Google Scholar]
- Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- Paul NL, Ruddle NH. Lymphotoxin. Annu Rev Immunol. 1988;6:407–438. doi: 10.1146/annurev.iy.06.040188.002203. [DOI] [PubMed] [Google Scholar]
- Vassalli P. The pathophysiology of tumor necrosis factor. Annu Rev Immunol. 1992;10:411–452. doi: 10.1146/annurev.iy.10.040192.002211. [DOI] [PubMed] [Google Scholar]
- Barten DM, Ruddle NH. Vascular cell adhesion molecule-1 modulation by tumor necrosis factor in experimental allergic encephalomyelitis. J Neuroimmunol. 1994;51:123–133. doi: 10.1016/0165-5728(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Bevilacqua MP. Endothelial-leukocyte adhesion molecules. Annu Rev Immunol. 1993;11:767–804. doi: 10.1146/annurev.iy.11.040193.004003. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Benveniste EN. Adhesion molecule expression and regulation on cells of the central nervous system. J Neuroimmunol. 1999;98:77–88. doi: 10.1016/s0165-5728(99)00084-3. [DOI] [PubMed] [Google Scholar]
- Neumann B, Machleidt T, Lifka A, Pfeffer K, Vestweber D, Mak TW, Holzmann B, Kronke M. Crucial role of 55-kilodalton TNF receptor in TNF-induced adhesion molecule expression and leukocyte organ infiltration. J Immunol. 1996;156:1587–1593. [PubMed] [Google Scholar]
- Yang YL, Li XM. The IAP family: endogenous caspase inhibitors with multiple biological activities. Cell Res. 2000;10:169–177. doi: 10.1038/sj.cr.7290046. [DOI] [PubMed] [Google Scholar]
- Trauth BC, Klas C, Peters AM, Matzku S, Moller P, Falk W, Debatin KM, Krammer PH. Monoclonal antibody-mediated tumor regression by induction of apoptosis. Science. 1989;245:301–305. doi: 10.1126/science.2787530. [DOI] [PubMed] [Google Scholar]
- Gimenez MA, Sim JE, Russell JH. TNFR1-dependent VCAM-1 expression by astrocytes exposes the CNS to destructive inflammation. J Neuroimmunol. 2004;151:116–125. doi: 10.1016/j.jneuroim.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Huitinga I, van Rooijen N, de Groot CJ, Uitdehaag BM, Dijkstra CD. Suppression of experimental allergic encephalomyelitis in Lewis rats after elimination of macrophages. J Exp Med. 1990;172:1025–1033. doi: 10.1084/jem.172.4.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran EH, Hoekstra K, van Rooijen N, Dijkstra CD, Owens T. Immune invasion of the central nervous system parenchyma and experimental allergic encephalomyelitis, but not leukocyte extravasation from blood, are prevented in macrophage-depleted mice. J Immunol. 1998;161:3767–3775. [PubMed] [Google Scholar]
- Abrams SI, Russell JH. CD4+ T lymphocyte-induced target cell detachment. A model for T cell-mediated lytic and nonlytic inflammatory processes. J Immunol. 1991;146:405–413. [PubMed] [Google Scholar]
- Sabelko KA, Kelly KA, Nahm MH, Cross AH, Russell JH. Fas and Fas ligand enhance the pathogenesis of experimental allergic encephalomyelitis, but are not essential for immune privilege in the central nervous system. J Immunol. 1997;159:3096–3099. [PubMed] [Google Scholar]
- Cohen JA, Essayan DM, Zweiman B, Lisak RP. Limiting dilution analysis of the frequency of antigen-reactive lymphocytes isolated from the central nervous system of Lewis rats with experimental allergic encephalomyelitis. Cell Immunol. 1987;108:203–213. doi: 10.1016/0008-8749(87)90204-8. [DOI] [PubMed] [Google Scholar]
- Irani DN, Griffin DE. Isolation of brain parenchymal lymphocytes for flow cytometric analysis. Application to acute viral encephalitis. J Immunol Methods. 1991;139:223–231. doi: 10.1016/0022-1759(91)90192-i. [DOI] [PubMed] [Google Scholar]
- Vandenbark AA, Vainiene M, Celnik B, Hashim G, Offner H. TCR peptide therapy decreases the frequency of encephalitogenic T cells in the periphery and the central nervous system. J Neuroimmunol. 1992;39:251–260. doi: 10.1016/0165-5728(92)90259-n. [DOI] [PubMed] [Google Scholar]
- Suen WE, Bergman CM, Hjelmstrom P, Ruddle NH. A critical role for lymphotoxin in experimental allergic encephalomyelitis. J Exp Med. 1997;186:1233–1240. doi: 10.1084/jem.186.8.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bebo BF, Jr, Linthicum DS. Expression of mRNA for 55-kDa and 75-kDa tumor necrosis factor (TNF) receptors in mouse cerebrovascular endothelium: effects of interleukin-1 beta, interferon-gamma and TNF-alpha on cultured cells. J Neuroimmunol. 1995;62:161–167. doi: 10.1016/0165-5728(95)00113-5. [DOI] [PubMed] [Google Scholar]
- Dopp JM, Mackenzie-Graham A, Otero GC, Merrill JE. Differential expression, cytokine modulation, and specific functions of type-1 and type-2 tumor necrosis factor receptors in rat glia. J Neuroimmunol. 1997;75:104–112. doi: 10.1016/s0165-5728(97)00009-x. [DOI] [PubMed] [Google Scholar]
- Kahn MA, Dopp JM, Liva S, MacKenzie-Graham AJ, Chang R, Huang A, Nazarian R, Dell’Albani P, Condorelli D, Voskuhl RR, de Vellis J. Temporal kinetics and cellular phenotype of TNF p55/p75 receptors in experimental allergic encephalomyelitis. J Neuroimmunol. 1999;95:19–34. doi: 10.1016/s0165-5728(98)00258-6. [DOI] [PubMed] [Google Scholar]
- Hickey WF, Kimura H. Perivascular microglial cells of the CNS are bone marrow-derived and present antigen in vivo. Science. 1988;239:290–292. doi: 10.1126/science.3276004. [DOI] [PubMed] [Google Scholar]
- Hickey WF, Vass K, Lassmann H. Bone marrow-derived elements in the central nervous system: an immunohistochemical and ultrastructural survey of rat chimeras. J Neuropathol Exp Neurol. 1992;51:246–256. doi: 10.1097/00005072-199205000-00002. [DOI] [PubMed] [Google Scholar]
- Lassmann H, Schmied M, Vass K, Hickey WF. Bone marrow derived elements and resident microglia in brain inflammation. Glia. 1993;7:19–24. doi: 10.1002/glia.440070106. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y, Fujiwara M. Absence of donor-type major histocompatibility complex class I antigen-bearing microglia in the rat central nervous system of radiation bone marrow chimeras. J Neuroimmunol. 1987;17:71–82. doi: 10.1016/0165-5728(87)90032-4. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y, Hara N, Tanaka R, Fujiwara M. Immunohistochemical analysis of the rat central nervous system during experimental allergic encephalomyelitis, with special reference to Ia-positive cells with dendritic morphology. J Immunol. 1986;136:3668–3676. [PubMed] [Google Scholar]
- Karpus WJ, Ransohoff RM. Chemokine regulation of experimental autoimmune encephalomyelitis: temporal and spatial expression patterns govern disease pathogenesis. J Immunol. 1998;161:2667–2671. [PubMed] [Google Scholar]
- Bell MD, Taub DD, Kunkel SJ, Strieter RM, Foley R, Gauldie J, Perry VH. Recombinant human adenovirus with rat MIP-2 gene insertion causes prolonged PMN recruitment to the murine brain. Eur J Neurosci. 1996;8:1803–1811. doi: 10.1111/j.1460-9568.1996.tb01324.x. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM. Chemokines in neurological disease models: correlation between chemokine expression patterns and inflammatory pathology. J Leukoc Biol. 1997;62:645–652. doi: 10.1002/jlb.62.5.645. [DOI] [PubMed] [Google Scholar]
- Cross AH, Raine CS. Central nervous system endothelial cell-polymorphonuclear cell interactions during autoimmune demyelination. Am J Pathol. 1991;139:1401–1409. [PMC free article] [PubMed] [Google Scholar]
- Zehntner SP, Brickman C, Bourbonniere L, Remington L, Caruso M, Owens T. Neutrophils that infiltrate the central nervous system regulate T cell responses. J Immunol. 2005;174:5124–5131. doi: 10.4049/jimmunol.174.8.5124. [DOI] [PubMed] [Google Scholar]
- Archambault AS, Sim J, Gimenez MA, Russell JH. Defining antigen-dependent stages of T cell migration from the blood to the central nervous system parenchyma. Eur J Immunol. 2005;35:1076–1085. doi: 10.1002/eji.200425864. [DOI] [PubMed] [Google Scholar]
- Huang DR, Wang J, Kivisakk P, Rollins BJ, Ransohoff RM. Absence of monocyte chemoattractant protein 1 in mice leads to decreased local macrophage recruitment and antigen-specific T helper cell type 1 immune response in experimental autoimmune encephalomyelitis. J Exp Med. 2001;193:713–726. doi: 10.1084/jem.193.6.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matejuk A, Dwyer J, Ito A, Bruender Z, Vandenbark AA, Offner H. Effects of cytokine deficiency on chemokine expression in CNS of mice with EAE. J Neurosci Res. 2002;67:680–688. doi: 10.1002/jnr.10156. [DOI] [PubMed] [Google Scholar]
- Glabinski AR, Tani M, Strieter RM, Tuohy VK, Ransohoff RM. Synchronous synthesis of alpha- and beta-chemokines by cells of diverse lineage in the central nervous system of mice with relapses of chronic experimental autoimmune encephalomyelitis. Am J Pathol. 1997;150:617–630. [PMC free article] [PubMed] [Google Scholar]
- Hayashi M, Luo Y, Laning J, Strieter RM, Dorf ME. Production and function of monocyte chemoattractant protein-1 and other beta-chemokines in murine glial cells. J Neuroimmunol. 1995;60:143–150. doi: 10.1016/0165-5728(95)00064-9. [DOI] [PubMed] [Google Scholar]
- Hurwitz AA, Lyman WD, Guida MP, Calderon TM, Berman JW. Tumor necrosis factor alpha induces adhesion molecule expression on human fetal astrocytes. J Exp Med. 1992;176:1631–1636. doi: 10.1084/jem.176.6.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff RM, Hamilton TA, Tani M, Stoler MH, Shick HE, Major JA, Estes ML, Thomas DM, Tuohy VK. Astrocyte expression of mRNA encoding cytokines IP-10 and JE/MCP-1 in experimental autoimmune encephalomyelitis. FASEB J. 1993;7:592–600. doi: 10.1096/fasebj.7.6.8472896. [DOI] [PubMed] [Google Scholar]
- Rosenman SJ, Shrikant P, Dubb L, Benveniste EN, Ransohoff RM. Cytokine-induced expression of vascular cell adhesion molecule-1 (VCAM-1) by astrocytes and astrocytoma cell lines. J Immunol. 1995;154:1888–1899. [PubMed] [Google Scholar]
- Luo Y, Berman MA, Abromson-Leeman SR, Dorf ME. Tumor necrosis factor is required for RANTES-induced astrocyte monocyte chemoattractant protein-1 production. Glia. 2003;43:119–127. doi: 10.1002/glia.10231. [DOI] [PubMed] [Google Scholar]
- Ferber IA, Brocke S, Tayloredwards C, Ridgway W, Dinisco C, Steinman L, Dalton D, Fathman CG. Mice with a disrupted IFN-gamma gene are susceptible to the induction of experimental autoimmune encephalomyelitis (EAE). J Immunol. 1996;156:5–7. [PubMed] [Google Scholar]
- Klein RS, Izikson L, Means T, Gibson HD, Lin E, Sobel RA, Weiner HL, Luster AD. IFN-inducible protein 10/CXC chemokine ligand 10-independent induction of experimental autoimmune encephalomyelitis. J Immunol. 2004;172:550–559. doi: 10.4049/jimmunol.172.1.550. [DOI] [PubMed] [Google Scholar]
- Lafaille JJ, Keere FV, Hsu AL, Baron JL, Haas W, Raine CS, Tonegawa S. Myelin basic protein-specific T helper 2 (Th2) cells cause experimental autoimmune encephalomyelitis in immunodeficient hosts rather than protect them from the disease. J Exp Med. 1997;186:307–312. doi: 10.1084/jem.186.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maatta JA, Sjoholm UR, Nygardas PT, Salmi AA, Hinkkanen AE. Neutrophils secreting tumor necrosis factor alpha infiltrate the central nervous system of BALB/c mice with experimental autoimmune encephalomyelitis. J Neuroimmunol. 1998;90:162–175. doi: 10.1016/s0165-5728(98)00135-0. [DOI] [PubMed] [Google Scholar]
- McColl SR, Staykova MA, Wozniak A, Fordham S, Bruce J, Willenborg DO. Treatment with anti-granulocyte antibodies inhibits the effector phase of experimental autoimmune encephalomyelitis. J Immunol. 1998;161:6421–6426. [PubMed] [Google Scholar]
- Lagasse E, Weissman IL. Flow cytometric identification of murine neutrophils and monocytes. J Immunol Methods. 1996;197:139–150. doi: 10.1016/0022-1759(96)00138-x. [DOI] [PubMed] [Google Scholar]
- Sunderkotter C, Nikolic T, Dillon MJ, Van Rooijen N, Stehling M, Drevets DA, Leenen PJ. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J Immunol. 2004;172:4410–4417. doi: 10.4049/jimmunol.172.7.4410. [DOI] [PubMed] [Google Scholar]
- Kjeldsen L, Bjerrum OW, Askaa J, Borregaard N. Subcellular localization and release of human neutrophil gelatinase, confirming the existence of separate gelatinase-containing granules [erratum appears in Biochem J 1993 Feb 1;289(Pt 3):927]. Biochem J. 1992;287:603–610. doi: 10.1042/bj2870603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masure S, Proost P, Van Damme J, Opdenakker G. Purification and identification of 91-kDa neutrophil gelatinase. Release by the activating peptide interleukin-8. Eur J Biochem. 1991;198:391–398. doi: 10.1111/j.1432-1033.1991.tb16027.x. [DOI] [PubMed] [Google Scholar]
- Murphy CA, Hoek RM, Wiekowski MT, Lira SA, Sedgwick JD. Interactions between hemopoietically derived TNF and central nervous system-resident glial chemokines underlie initiation of autoimmune inflammation in the brain. J Immunol. 2002;169:7054–7062. doi: 10.4049/jimmunol.169.12.7054. [DOI] [PubMed] [Google Scholar]
- Ruuls SR, Hoek RM, Ngo VN, McNeil T, Lucian LA, Janatpour MJ, Korner H, Scheerens H, Hessel EM, Cyster JG, McEvoy LM, Sedgwick JD. Membrane-bound TNF supports secondary lymphoid organ structure but is subservient to secreted TNF in driving autoimmune inflammation. Immunity. 2001;15:533–543. doi: 10.1016/s1074-7613(01)00215-1. [DOI] [PubMed] [Google Scholar]
- Yamashiro S, Kamohara H, Yoshimura T. MCP-1 is selectively expressed in the late phase by cytokine-stimulated human neutrophils: TNF-alpha plays a role in maximal MCP-1 mRNA expression. J Leukoc Biol. 1999;65:671–679. doi: 10.1002/jlb.65.5.671. [DOI] [PubMed] [Google Scholar]
- Akassoglou K, Bauer J, Kassiotis G, Pasparakis M, Lassmann H, Kollias G, Probert L. Oligodendrocyte apoptosis and primary demyelination induced by local TNF/p55TNF receptor signaling in the central nervous system of transgenic mice: models for multiple sclerosis with primary oligodendrogliopathy. Am J Pathol. 1998;153:801–813. doi: 10.1016/S0002-9440(10)65622-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selmaj KW, Raine CS. Tumor necrosis factor mediates myelin and oligodendrocyte damage in vitro. Ann Neurol. 1988;23:339–346. doi: 10.1002/ana.410230405. [DOI] [PubMed] [Google Scholar]
- Korner H, Riminton DS, Strickland DH, Lemckert FA, Pollard JD, Sedgwick JD. Critical points of tumor necrosis factor action in central nervous system autoimmune inflammation defined by gene targeting. J Exp Med. 1997;186:1585–1590. doi: 10.1084/jem.186.9.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anonymous TNF neutralization in MS: results of a randomized, placebo-controlled multicenter study. The Lenercept Multiple Sclerosis Study Group and The University of British Columbia MS/MRI Analysis Group. Neurology. 1999;53:457–465. [PubMed] [Google Scholar]