The Role of the Cyclin D1-Dependent Kinases in ErbB2-Mediated Breast Cancer (original) (raw)
Abstract
Intact cyclin D1 functions are essential for transformation by erbB2 in tissue culture and murine models. Because cyclin D1 may alter cell proliferation through a variety of mechanisms, we used transgenic models and human tumor samples to particularly address the role of cyclin D1-cyclin-dependent kinases in transformation by erbB2. The p16 tumor suppressor specifically blocks cyclin-dependent kinase 4 and 6 activity. Here we show that an MMTV-p16 transgene blocked tumorigenesis by erbB2, demonstrating that deregulation of the cyclin-dependent kinase partner of cyclin D1 is an essential target of erbB2. ErbB2 overexpression was a determining factor in deregulation of cyclin D1-cdk4/6 interactions because neither transgenic cyclin D1 nor loss of p16 accelerated tumorigenesis in MMTV-erbB2-transgenic mice. ErbB2 was also a deciding factor in deregulation of cyclin D1-cdk4/6 in human tumors because no loss of pRb or p16 was found in tumors overexpressing erbB2, although erbB2-negative invasive breast adenocarcinomas frequently lacked expression of p16 or pRb. We conclude that deregulation of cyclin D1-Cdk4/6 interactions is a critical target of erbB2 function in human and mouse breast tumors, and erbB2’s overexpression may be sufficient to deregulate cyclin D1-cdk4/6 activity in breast cancer.
Cyclin D1 functions were first shown to be downstream of transformation by erbB2 in tissue culture models.1,2 In murine models, intact cyclin D1 is required for mammary tumor formation in MMTV-erbB2 mice.3 The known functions of cyclin D1 include: 1) its phosphorylation of the retinoblastoma gene product (pRb) mediated by its interaction with cyclin-dependent kinase 4/6 (Cdk 4/6);4 2) its interactions with several transcriptional elements;5 and 3) its titration of p21.6 Using murine models and human tumor tissues we used the p16 inhibitor of cdk4/6 to evaluate whether its interaction with cdk4/6 was the cyclin D1 function that was required because p16 specifically blocks cdk4/6 activity.7 We then sought to determine whether erbB2 overexpression was a determining factor in deregulation of the cyclin D1/pRb/p16 pathway in murine models and human tumors. If erbB2 overexpression were a deciding factor in overcoming cyclin D1/pRb/p16 we would expect to find no additive effects of cyclin D1 addition or loss of p16 in the standard murine-transgenic model of erbB2 tumorigenesis. We would also expect to find loss of either p16 or pRb to be infrequent in human tumors caused by erbB2. The combination of these findings in both mouse models and in human specimens would be a stringent test for the idea that cyclin D1/cdk4/6 interactions are downstream of, and required for, tumorigenesis by erbB2.
Materials and Methods
Animals
MMTV-cyclin D18 and MMTV-p16-transgenic mice9 were described previously. MMTV-erbB2 mice were purchased from Charles River Laboratories, Wilmington, MA, and contained the mutated rat erbB2 under the control of the mouse mammary tumor virus enhancer and promoter (MMTV).10 The MMTV-cyclin D1-, erbB2-, and p16-transgenic mice were all maintained in the inbred FVB background during the breeding experiments. INK4A/ARF−/− double-null mice were generously provided by Dr. Ronald DePinho (Dana Farber Cancer Institute).11 These mice were inbreeding cross N4 in the FVB background and all crosses with the MMTV-erbB2 were therefore performed at inbreeding cross N5 into the FVB background. All animals received humane care following study guidelines established by the Massachusetts General Hospital subcommittee on laboratory animal care.
Protein Expression Studies
Protein samples were obtained from mammary tissues and tumors by crushing mammary glands frozen in liquid nitrogen using a mortar and pestle. Mammary lysates were made by passing the resulting powders through a 20-gauge needle at 4° in TNE buffer (50 mm Tris pH 8.0, 150 mm NaCl, 5 mm EDTA, 1% NP40). Protein expression was evaluated using standard immunoblots containing 50 μg of total protein. Immunoblots were successively incubated with the following antibodies: 1) a JC1 mouse monoclonal that specifically recognizes human p16INK4A12 (from Drs. Jim Koh and Ed Harlow, MGH Cancer Center); 2) an affinity-purified rabbit polyclonal anti-cyclin D1 antibody;13 3) rabbit anti-HER2 (erbB2) polyclonal antibody A0485 (DAKO, Carpinteria, CA); 4) mouse monoclonal anti-Rb antibody 3C8 (QED Bioscience, San Diego, CA); and 5) a commercial mouse monoclonal antibody against actin (Boehringer, Indianapolis, IN). Secondary antibodies used were those included in an enhanced chemiluminescence detection kit (Amersham, Arlington Heights, IL).
Polymerase Chain Analysis of the INK4aARF Knockout Allele in Tumor Tissues
DNA isolation from tumor tissue was accomplished by routine proteinase K digestion, phenol:chloroform extraction, and ethanol precipitation. Polymerase chain reaction (PCR) to genotype the p16 knockout allele was performed as described.11
Immunostaining
Cyclin D1 and erbB2 immunostaining was performed using the antibodies identified above. ErbB2 expression was initially characterized using the A0485 polyclonal antibody on coded specimens. The results of this analysis were then compared by the pathologist who provided us with the coded specimens to the results of clinical analyses performed by the Herceptest for further confirmation. Both results were concordant in every case analyzed. All immunostaining was evaluated and scored by at least two of the investigators involved in the study in an independent and blinded manner. Immunostaining was scored as −, no staining; +, weak positive; ++, moderate positive; and +++, strong positive. ErbB2-positives are recorded as those showing +++ staining only, and showing the classic membrane-based pattern of staining (see Figure 2N). Cyclin D1-positives included ++ and +++ nuclear staining (see Figure 2P) because those levels of intensity showed good correlation with immunoblot analysis of the same tissues in our previous study13 p16- and pRb-negatives were taken as only those that demonstrated no staining (−).
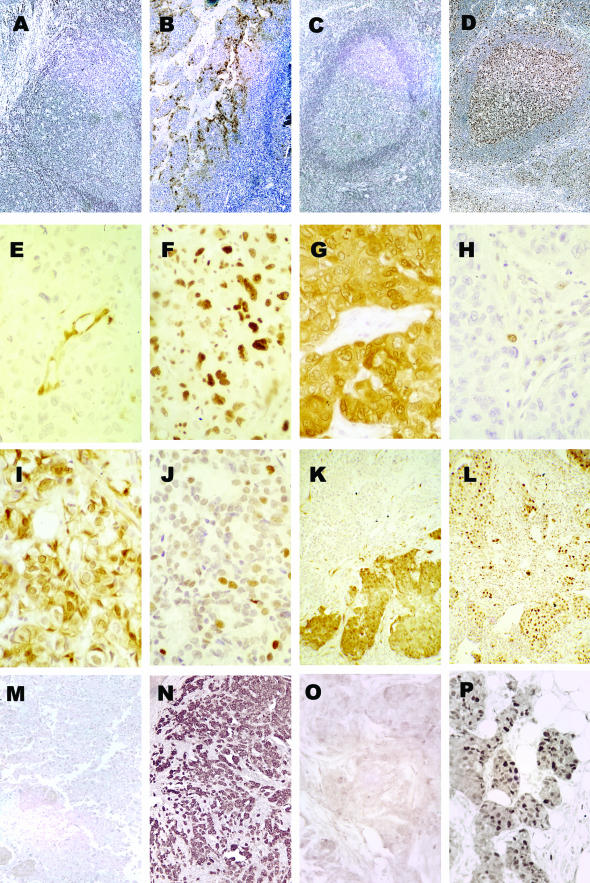
Figure 2.
p16-, pRB-, cyclin D1-, and erbB2-staining patterns in primary human breast tumors. A: Negative p16 control. Human tonsillar tissue with no primary antibody. B: Positive p16 control. Human tonsillar tissue with JC8 antibody. C: Negative pRb control. Human tonsillar tissue with no primary antibody. D: Positive pRb control. Human tonsillar tissue with G3-245 antibody. E and F: The same tumor stained for p16 (E) and pRb (F). Note that the endothelial cells stain p16+ in E, but both nuclear and cytoplasmic staining is seen in all tumor cells in F. G and H: A different tumor stained for p16 (G) and pRb (H). In G, note both nuclear and cytoplasmic staining. I and J: A third tumor stained for p16 (I) and pRb (J) as an example of low average staining for both genes in a single tumor. K and L: A fourth tumor stained for p16 (K) and pRb (L). Note the clonality of p16 loss in different portions of this tumor. M: Example of erbB2-negative staining. N: Example of erbB2-positive staining. Note the membrane localization of the erbB2 staining. O: Example of cyclin D1-negative staining. P: Example of cyclin D1-positive staining. Note the nuclear localization of the cyclin D1. Original magnifications: ×100 (A–D, K–N); ×250 (E, F); ×400 (G–J, O, P).
p16 Immunohistochemistry
The JC8 anti-pl6 mouse monoclonal IgG2a antibody recognizes an epitope in the first ankyrin repeat (amino acids 1 to 32) of the p16 protein.14 We had previously compared five noncommercial mouse monoclonal antibodies as well as two commercial antibodies. The results with the JC8 were superior to any other antibody.
Formalin-fixed, paraffin-embedded tissues were sectioned at 6 μm onto Probe-On Plus (Fisher Scientific, Pittsburgh, PA) slides. After baking at 55°C for 1 hour, the sections were deparaffinized in xylene and rehydrated in graded ethanols. Endogenous peroxidase activity was blocked by immersing the slides in 0.5% hydrogen peroxide (H2O2) in methanol for 5 minutes. An antigen retrieval step was used, consisting of microwaving the slides in 0.01 mol/L of sodium citrate (pH 6.0) for three changes of 5 minutes each, followed by cooling in phosphate-buffered saline (PBS) rinses. The sections were incubated in 10% normal horse serum in 5% milk for 20 minutes at room temperature. The JC8 anti-pl6 antibody was applied at a 1:500 dilution in 1% bovine serum albumin/PBS and incubated in a humidity chamber at room temperature for 2 hours. After the primary antibody incubation, a secondary biotinylated horse anti-mouse antibody (Vector Laboratories, Burlingame, CA) was applied at a 1:1000 dilution (in 1% bovine serum albumin in PBS) for 1 hour at room temperature, followed by the avidin-biotin complex kit (ABC Elite; Vector Laboratories, Burlingame, CA), also applied for 1 hour at room temperature. After the application of diaminobenzidine (Sigma) with H2O2 for 3 minutes, according to the manufacturer’s instructions. the slides were washed in distilled water and lightly counterstained in Hematoxylin Solution Gill No. 1 (Sigma Chemical Co., St. Louis, MO). Tonsil tissue served as a control in which nuclear and cytoplasmic staining was noted specifically in histiocytic cells in germinal centers and epithelial cells of the mucosal lining (see Figure 2B). Negative controls were performed by omitting the primary antibody and by using an irrelevant mouse monoclonal antibody (see Figure 2A).
pRb Immunohistochemistry
The pRb immunohistochemical protocol was similar to the p16 assay. Blocking of endogenous peroxidase activity in H2O2/methanol was performed for 30 minutes. The slides were incubated with 10% normal horse serum in 1% bovine serum albumin/PBS for 30 minutes. The primary mouse monoclonal anti-pRb antibody (G3-245; PharMingen, La Jolla, CA) was diluted 1:2500 and applied overnight at 4°C. The secondary biotinylated horse anti-mouse antibody was diluted at 1:1000 and applied for 1 hour at room temperature. In the tonsillar tissue, there was distinct nuclear immunohistochemical expression of pRb in the germinal centers and in the basal epithelial layers (see Figure 2D). Negative controls were performed by omitting the primary antibody and by using an irrelevant mouse monoclonal antibody (see Figure 2C).
Laser Capture Microdissection, RNA Isolation, and Quantitative Real-Time (QRT) PCR Analysis of Cyclin D1 mRNA
Patient-matched normal breast epithelium and invasive ductular adenocarcinoma were microdissected from breast tissue where the normal tissue was at a minimum 0.3 cm from any premalignant or malignant lesion; total RNA was then isolated and converted to double-stranded cDNA as described.15 Details of the quantitative real-time polymerase chain reaction (QRT-PCR) method were as described previously.15 The QRT-PCR was performed using the following primers and probes: p16, forward primer: TGCCTTTTCACTGTGTTGGAGTT, reverse primer: TCGCAAGAAATGCCCACAT, probe: AGCACTCACGCCCTAAGCGCACA; Rb, forward primer: CAATGACACTAGAAAACTTGACTCCATT, reverse primer: GAACTTAGTAGCAAAAGACCCAGAAATAG, probe: CATCATTGTTTCTGCATGAATATCATACAAATCAGTTAG; cyclin D1: forward primer: GGATGCTGGAGGTCTGCGA, reverse primer: AGAGGCCACGAACATGCAAG, probe: AGGAGGTCTTCCCGCTGGCCATGAAC. Data are presented as fold changes between the tumor samples and the adjacent microdissected normal sample from the same patient.
Results
Cyclin-Dependent Kinase Partners of Cyclin D1 Are an Essential Target of ErbB2 Transformation
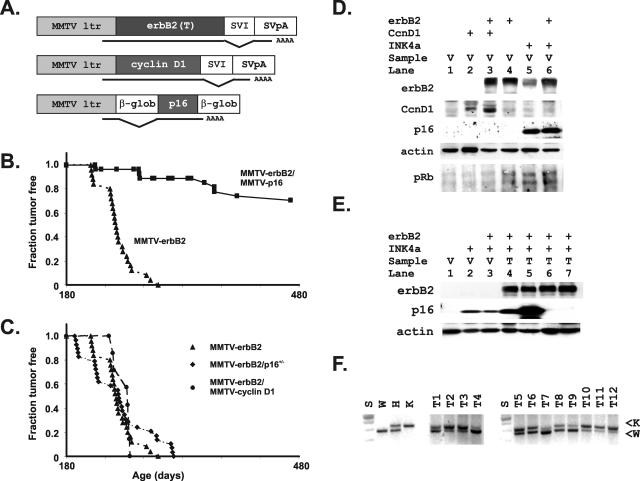
Cyclin D1 is required for transformation by erbB2 as demonstrated by the failure of an MMTV-erbB2 transgene to cause invasive adenocarcinomas in cyclin D1 knockout mice.3 We therefore bred MMTV-erbB2-transgenic mice to MMTV-p16-transgenic mice and followed the development of tumors in the crosses to determine whether cyclin D1’s interaction with cdk4/6 was its essential function (Figure 1, A and B). The p16 transgene blocked tumor formation in the double-transgenic strains (Figure 1B). Rare tumors developed after a long delay in the MMTV-erbB2-MMTVp16 double transgenics. Western blots using an antibody that only detects the human p16 in the transgene demonstrated loss of expression of the p16 transgene in two of the four tumors available for analysis (Figure 1E).
Figure 1.
Demonstration of the importance of erbB2 signaling through cyclin D1 using transgenic interactions between loss of p16/19, overexpression of cyclin D1, and inhibition by p16 in MMTV-erbB2-transgenic mice. A: Schematic diagram of transgenes used in this study. B: Shown are standard mortality curves demonstrating that expression of p16 using the MMTV-p16 transgene effectively blocks formation of tumors caused by MMTVerbB2. Fraction of mice remaining tumor-free is plotted on the y axis and compared to age in days on the x axis. Single erbB2-transgenic mice (▴) are compared to mice expressing both erbB2 and the human p16 transgene (▪). C: Shown are standard mortality curves demonstrating no acceleration of erbB2T-induced tumors (▴) by cyclin D1 (•) or heterozygous loss of p16/p19 (♦). The latter cross did result in the novel appearance of salivary tumors (not shown). D: Expression of individual transgenes used in this study was not altered by co-expression with additional transgenes. Protein lysates from mammary glands of the indicated genotypes (erbB2+ = MMTVerbB2 mice; CcnD1+ = MMTV-cyclin D1 mice; INK4a+ = MMTVp16 mice) were evaluated for expression of the erbB2 (erbB2), cyclin D1 (CcnD1), and human p16 (INK4a) proteins expressed by the various transgenes. E: Expression of the erbB2 (erbB2+) and human p16 (INK4a+) transgenes was evaluated in protein lysates from virgin (V) and tumor (T) tissues from mice of the indicated genotypes. F: Tumors that developed in the MMTVerbB2-INK4aARF+/− mice were evaluated for loss of the normal wild-type (W) compared with the knockout allele (K) using standard polymerase chain reactions.
Co-Expression of Transgenic Cyclin D1 or Loss of One Allele of Endogenous p16 Gene Does Not Accelerate ErbB2-Induced Tumorigenesis
If cyclin D1 is fully downstream of erbB2, then additional cyclin D1 should not accelerate tumor formation in the erbB2 transgenics. As predicted by the hypothesis that cyclin D1 is downstream of erbB2, an MMTV-cyclin D1 transgene failed to accelerate tumor formation by erbB2 (Figure 1C). p16 expression is sporadically lost in human breast cancers through mechanisms involving loss of expression of its mRNA, and its expression is actually lost more frequently in breast cancer than in any other tumor in these studies.16 The alternative reading frame (ARF) of the INK4a locus is less often involved in breast cancer.17 We bred MMTV-erbB2 mice to INK4aARF−/− mice to develop offspring lacking an endogenous allele of INK4aARF to simultaneously test both components of the INK4 locus. We again found no acceleration of tumor formation in these double transgenics (Figure 1C). In addition, the other allele of the endogenous p16 INK4a gene was intact in all erbB2-overexpressing tumors tested (Figure 1F). New salivary tumors appeared in the erbB2-INK4aARF+/− mice that were not seen in the erbB2-alone mice, identifying a separate tissue where erbB2 overexpression combined with p16 deficiency was required to deregulate the cyclin D1-p16-pRb pathway (not shown). The failure of both the cyclin D1 and INK4aARF+/− transgenes to collaborate with erbB2 is consistent with the view that erbB2 overexpression is a determining factor in deregulation of the cyclin D1 pathway in mammary tissues.
Loss of pRb or p16 Was Not Necessary to Deregulate Cyclin D1-cdk4/6 in Human Breast Tumors Overexpressing ErbB2
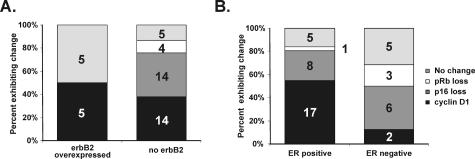
Our murine models suggest that erbB2 overexpression is a deciding factor in deregulation of cyclin D1-cdk4/6 and that this function is required for erbB2 to cause cancer. When examined in glioblastomas and a few other tumor types, typically one, and only one, mutation is found in genes regulating the cyclin D1-pRb axis.18 This phenomenon has not yet been studied in breast cancers. We examined this possibility in a previously collected series of human invasive ductular adenocarcinomas.13 We immunostained these tumors for erbB2, p16, pRb, and cyclin D1. In cancer cell lines grown in vitro, loss of pRb is typically accompanied by substantial increases in p16; loss of p16 is accompanied by substantial increases in pRb in vitro as well.19,20 The results that we describe here were obtained by immunostaining primary human tissue samples, not cultured cells, and identified a similar pattern in actual breast cancers (Figure 2). Specifically, the loss of pRb was uniformly accompanied by high p16 staining levels. The loss of p16 was accompanied by high pRb levels. We also used an affinity-purified anti-cyclin D1 antibody and a standard erbB2 antibody to evaluate their expression patterns in the same tumors (Figure 3). We found no erbB2-expressing tumors that lacked either p16 or pRb. In contrast, 9% of erbB2-negative tumors lacked pRb staining and 38% lacked p16 staining (Figure 3A). This difference was significant by Fisher’s exact test (P = 0.008). We also compared the patterns of expression changes with estrogen receptor (ER) status of the same set of tumors (Figure 3B). Although cyclin D1 positivity was clearly associated with ER-positive tumors as previously reported,13 neither loss of p16 nor loss of pRb was associated with ER status.
Figure 3.
A: ErbB2 staining is found only in tumors when p16 and pRb are intact. Paraffin-embedded specimens were immunostained and scored for erbB2, p16, and pRb expression. Total tumors, n = 47. All tumors were invasive ductular carcinomas as described previously.13 Groups of tumors losing p16 (dark gray region of the bar graph color indicated in the legend to the figure), gaining cyclin D1 (black region of the bar graph), and losing pRb (white region of the bar graph) are indicated for erbB2-positive tumors (left column graph) and erbB2-negative (right). (Light gray regions of the charts indicate that none of these changes were identified.) The numbers in the bar graphs identify the number of tumors exhibiting the indicated change. Although cyclin D1 does not correlate with erbB2 staining, erbB2 is never found in tumors lacking pRb or p16. This result is statistically significant by Fisher’s exact test (P = 0.008). B: Cyclin D1 staining is found in ER-positive tumors, but rarely in ER-negative tumors. Loss of p16 and pRb are not associated with ER-positive or -negative tumors. “No change” identifies tumors with normal D1, p16, and pRb.
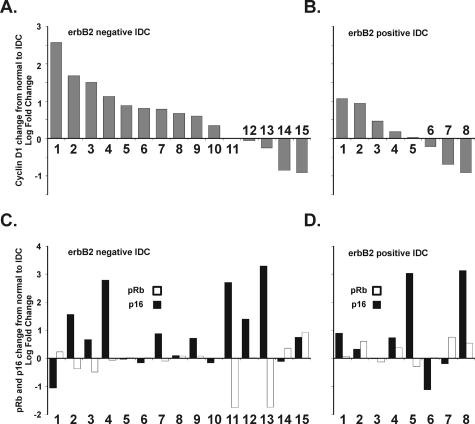
To further analyze the reported regulation of cyclin D1 by erbB2 at the mRNA level in a different set of tumors,1 we used RNA isolated from microdissected tissues from 23 matched normal and invasive tumors from the patients whose erbB2 status was known.15 Individual specimens demonstrating one or more pathological lesions [atypical ductular hyperplasia, ductular carcinoma in situ, and invasive ductular carcinoma (IDC)] were laser capture microdissected in triplicate and patient-matched normal breast epithelium was compared to the patient’s own premalignant and malignant lesions. Using amplified RNA (aRNA) samples from the laser capture microdissected-isolated tissues,15 we first measured cyclin D1 levels in the paired normal and IDC samples using QRT-PCR to analyze levels of cyclin D1 mRNA after reverse transcription of the aRNA samples (Figure 4, A and B). Although cyclin D1 mRNA levels were previously shown to increase in murine mammary tumors caused by erbB2,1 we found instead that cyclin D1 mRNA increased less frequently and to a lesser extent in the erbB2-positive human tumors than in the erbB2-negative tumors. We then examined both p16 and pRb levels in the same tumors (Figure 4, C and D). We found two tumors (nos. 11 and 13) in which pRb was strikingly decreased in association with both increased p16 and loss of cyclin D1. This combination of changes characterizes tumors lacking functional pRb.19–21 Both of these tumors were in the erbB2-negative group.
Figure 4.
A and B: Log-fold changes in cyclin D1 mRNA measured by quantitative real-time (QRT) polymerase chain reaction (PCR) are plotted for each of 15 erbB2-negative tumors (A) and 8 erbB2-positive tumors (B). All tumors were invasive ductular carcinomas as described previously.15 The mean log change for cyclin D1 in the erbB2-positive cases was 0.59 (SD = 0.94) and 0.10 (SD = 0.71) for the erbB2-negative cases (P = 0.09 by _t_-test). C and D: Log-fold changes in p16 mRNA (black columns) and pRb mRNA (white columns) are plotted for the same individual tumors as in A and B. Criteria for the loss of expression of pRb or p16 is that the CT number is 42 for the sample based on 42 cycles in QRT-PCR. Therefore samples showing a downward bar did not necessarily lose the expression of the specific message, but instead the expression of the specific message was decreased compared to their respective controls. The mean log change for p16 in the erbB2-negative cases was 0.89 (SD = 1.25) and 0.85 (SD = 1.51) for the erbB2-positive cases (P = 0.47 by _t_-test). The mean log change for pRb in the erbB2-negative cases was −0.018 (SD = 0.71) and 0.25 (SD = 0.38) for the erbB2-positive cases (P = 0.04 by _t_-test).
Discussion
We used animal models of erbB2-induced mammary tumors and human cancers caused by erbB2 to assess the proposed erbB2 → cyclin D1 pathway in vivo. We showed that its interaction with cyclin-dependent kinase activity was the function of cyclin D1 that mediated transformation by erbB2. This was demonstrated experimentally in mouse models of human cancers as well as by the presence of nonredundant expression abnormalities in these genes in human tumor specimens. Our results are consistent with a recent report from Timms and colleagues22 who showed that cdk6 activity was essential for cell proliferation in human luminal epithelial cells overexpressing erbB2. Effective blockade of erbB2 tumorigenesis by the p16 transgene cannot be explained by a loss of erbB2 transgene expression in the presence of the p16 transgene because the erbB2 transgene was fully expressed in the presence of p16 (Figure 1E, lane 6). The notion of continuous expression of erbB2 in the presence of p16 transgene is also supported by the fact that those mice eventually developed mammary tumors after a long delay in which two tumors lost the expression of the transgene p16. The p16 levels in the remaining two tumors is actually increased, suggesting that a second site mutation may have occurred as described for the increased p16 levels found in human tumors lacking pRb.19 It is also unlikely that the failure of transgenic cyclin D1 gene to accelerate erbB2-induced tumorigenesis results from any interference of the expression of the erbB2 transgene by the cyclin D1 transgene for several reasons. First, transgenic erbB2 is fully expressed in the presence of the cyclin D1 transgene (Figure 1D, lane 3). Indeed, the transgenic cyclin D1 is somewhat increased in the presence of the erbB2 transgene. Second, both erbB2-transgenic mice and their erbB2/cyclin D1 bi-transgenic mice developed mammary tumors at ∼6 months of age and at a similar rate. If the expression of erbB2 transgene is blocked in any manner by co-expression with the cyclin D1 transgene, we would expect a prolongation of tumor onset in those mice because cyclin D1-induced tumors have a much longer latency than the erbB2 tumors.23 Instead, we see the same rate of tumor formation in the bi-transgenic mice despite the small increase in the cyclin D1 transgene. Taken together, these considerations suggest that the erbB2 transgene alone is sufficient to deregulate cyclin D1-Cdk4/6. We have previously demonstrated that the two expression constructs used in these transgenic strains collaborate in an NIH3T3 cell assay suggesting that erbB2’s direct regulation of cyclin D1/Cdk4 may be tissue-dependent.12
Our observation that Ink4a heterozygosity did not accelerate erbB2 tumorigenesis provides immediate confirmation of a recent report by D’Amico and colleagues.24 Our results put their more limited experiment in a broader context because we have looked at a wider variety of perturbations of Cdk4/6 activity in both human cells and in mouse experiments. Our additional experiments demonstrated that overexpression of cyclin D1 did not accelerate erbB2-induced tumorigenesis in erbB2 and cyclin D1 bigenic mouse model, and that forced overexpression of human p16 (Ink4a) blocked tumorigenesis induced by erbB2. In addition, we examined the expression of p16, cyclin D1, and pRb in human breast cancer samples in relation to erbB2 status of those samples by both immunohistochemistry and QRT-PCR, providing a link between data obtained in transgenic mouse models and data from human samples. Finally, we confirmed that no loss of the normal p16 allele occurs in the erbB2-INK4aARF+/− mice, further supporting the view that erbB2 overexpression can overcome the tumor suppressive effects of endogenous p16 in mammary tissues (Figure 1F).
An additional interesting observation of a reactive change in gene expression in response to the p16 transgene provides additional strong evidence that cyclin D1-Cdk4/6 interactions are downstream of erbB2 (Figure 1B, lane 5). We observed a marked increase in erbB2 levels in the virgin mammary glands of mice that overexpressed the p16 transgene. This reactive increase may provide one explanation for normal mammary development in these mice to occur despite the potent mammary-specific inhibition of proliferation caused by the MMTV-p16 transgene.
Previous immunostaining studies of human breast cancers focused on individual components of the cyclin D1-pRb axis.17,25,26 No previous study simultaneously addressed the cyclin D1-p16-pRb axis to determine whether erbB2 can be a sole determining factor of abnormal activation of the cyclin-dependent kinases. We simultaneously compared abnormalities of p16 and pRb expression in breast cancers to expression of erbB2 and found no loss of either p16 or pRb in erbB2-expressing human tumors. These results were statistically significant (P = 0.008 by Fisher’s exact test). In addition to the expected inverse correlation between p16 and pRb, we also found that loss of pRb led to loss of cyclin D1 expression as predicted by previous cyclin D1 promoter analyses.27
Our study on the expression of p16, pRb, cyclin D1, and erbB2 in breast cancer samples puts each tumor suppressor or oncogene product in a broader spectrum for analysis. Expression of each protein is analyzed relative to the expression of other proteins in the same tumor. In addition, we confirmed our protein expression data from immunostaining with our mRNA data using an advanced QRT-PCR technique. The use of laser-capture microdissection for this analysis has the obvious important advantage that the patient serves as her own control. The cyclin D1 mRNA analysis particularly benefits from this approach because the response of cyclin D1 levels to circulating hormones must certainly complicate any comparison between patient tumor samples if compared to normal breast specimens obtained from other women.9,28 On the other hand, p16 loss is known to occur in adjacent normal tissues that have not yet experienced a second oncogenic event.29,30 The use of laser capture microdissected tissues from the same patient might therefore underrepresent such a field defect in our cases. To address this concern, we found no reduction in p16 or pRb levels between our normal patient tissues and three reduction mammoplasty specimens that were included with the laser capture microdissected specimens. Furthermore, our previous comparison of global gene expression profiles from normal noncancerous mammoplasty with “phenotypic normal” from cancerous tissues revealed no consistent major transcriptional differences.15 Our observation that neither p16 nor pRb are absent from erbB2-expressing human tumors provides an independent confirmation of the results in our animal models. In this case, our mouse models and human tumors provide the same answer to this question.
Our data do not pinpoint the exact mechanism by which erbB2 deregulates the cyclin D1-dependent kinase activity. Although Pestell and colleagues earlier suggested that erbB2 regulates cyclin D1 through transcriptional mechanisms in experimental murine tumors, we found no correlation between erbB2 and cyclin D1 mRNA levels in human tumors. Thus, the mechanism by which erbB2 deregulates the cyclin D1-dependent kinase activity may not involve cyclin D1 levels in human tumors and may therefore involve effects on the p27 inhibitor of Cdk function as previously proposed.2 Further studies will be needed to clarify this mechanism. Nevertheless, these new in vivo data demonstrate that tumors caused by erbB2 may be particularly vulnerable to treatment strategies aimed at downstream cyclin D1-dependent kinase effectors of erbB2, which is an important finding for future therapeutic strategies aimed at treating erbB2-positive tumors.12,31
Acknowledgments
We thank Dr. Jim Koh of the University of Vermont for his kind provision of anti-p16 monoclonal antibodies.
Footnotes
Address reprint requests to Emmett V. Schmidt, Massachusetts General Hospital Cancer Research Center, 55 Fruit St. GRJ 904, Boston, MA 02114. E-mail: schmidt@helix.mgh.harvard.edu.
Supported by the National Cancer Institute of the National Institutes of Health (grant RO1 CA69069 to V.I.-T. and E.V.S.) and C.Y. was supported by the Harvard Breast Cancer SPORE grant (P50 CA89393 to C.Y.).
C.Y. and V.I.-T. contributed equally to this article.
References
- Lee RJ, Albanese C, Fu M, D’Amico M, Lin B, Watanabe G, Haines GK, III, Siegel PM, Hung MC, Yarden Y, Horowitz JM, Muller WJ, Pestell RG. Cyclin D1 is required for transformation by activated Neu and is induced through an E2F-dependent signaling pathway. Mol Cell Biol. 2000;20:672–683. doi: 10.1128/mcb.20.2.672-683.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane HA, Beuvink I, Motoyama AB, Daly JM, Neve RM, Hynes NE. ErbB2 potentiates breast tumor proliferation through modulation of p27(Kip1)-Cdk2 complex formation: receptor overexpression does not determine growth dependency. Mol Cell Biol. 2000;20:3210–3223. doi: 10.1128/mcb.20.9.3210-3223.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q, Geng Y, Sicinski P. Specific protection against breast cancers by cyclin D1 ablation. Nature. 2001;411:1017–1021. doi: 10.1038/35082500. [DOI] [PubMed] [Google Scholar]
- Matsushime H, Ewen ME, Strom DK, Kato JY, Hanks SK, Roussel MF, Sherr CJ. Identification and properties of an atypical catalytic subunit (p34PSK-J3/cdk4) for mammalian D type G1 cyclins. Cell. 1992;71:323–334. doi: 10.1016/0092-8674(92)90360-o. [DOI] [PubMed] [Google Scholar]
- Bernards R. CDK-independent activities of D type cyclins. Biochim Biophys Acta. 1999;1424:M17–M22. doi: 10.1016/s0304-419x(99)00024-4. [DOI] [PubMed] [Google Scholar]
- LaBaer J, Garrett MD, Stevenson LF, Slingerland JM, Sandhu C, Chou HS, Fattaey A, Harlow E. New functional activities for the p21 family of CDK inhibitors. Genes Dev. 1997;11:847–862. doi: 10.1101/gad.11.7.847. [DOI] [PubMed] [Google Scholar]
- Serrano M, Hannon GJ, Beach D. A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature. 1993;366:704–707. doi: 10.1038/366704a0. [DOI] [PubMed] [Google Scholar]
- Wang TC, Cardiff RD, Zukerberg L, Lees E, Arnold A, Schmidt EV. Mammary hyperplasia and carcinoma in MMTV-cyclin D1 transgenic mice. Nature. 1994;369:669–671. doi: 10.1038/369669a0. [DOI] [PubMed] [Google Scholar]
- Gadd M, Pisc C, Branda J, Ionescu-Tiba V, Nikolic Z, Yang C, Wang T, Shackleford GM, Cardiff RD, Schmidt EV. Regulation of cyclin D1 and p16(INK4A) is critical for growth arrest during mammary involution. Cancer Res. 2001;61:8811–8819. [PubMed] [Google Scholar]
- Muller WJ, Sinn E, Pattengale PK, Wallace R, Leder P. Single-step induction of mammary adenocarcinoma in transgenic mice bearing the activated c-neu oncogene. Cell. 1988;54:105–115. doi: 10.1016/0092-8674(88)90184-5. [DOI] [PubMed] [Google Scholar]
- Serrano M, Lee H, Chin L, Cordon-Cardo C, Beach D, DePinho RA. Role of the INK4a locus in tumor suppression and cell mortality. Cell. 1996;85:27–37. doi: 10.1016/s0092-8674(00)81079-x. [DOI] [PubMed] [Google Scholar]
- Nahta R, Iglehart JD, Kempkes B, Schmidt EV. Rate-limiting effects of cyclin D1 in transformation by ErbB2 predicts synergy between herceptin and flavopiridol. Cancer Res. 2002;62:2267–2271. [PubMed] [Google Scholar]
- Zukerberg LR, Yang WI, Gadd M, Thor AD, Koerner FC, Schmidt EV, Arnold A. Cyclin D1 (PRAD1) protein expression in breast cancer: approximately one-third of infiltrating mammary carcinomas show overexpression of the cyclin D1 oncogene. Mod Pathol. 1995;8:560–567. [PubMed] [Google Scholar]
- Burns KL, Ueki K, Jhung SL, Koh J, Louis DN. Molecular genetic correlates of p16, cdk4, and pRb immunohistochemistry in glioblastomas. J Neuropathol Exp Neurol. 1998;57:122–130. doi: 10.1097/00005072-199802000-00003. [DOI] [PubMed] [Google Scholar]
- Ma XJ, Salunga R, Tuggle JT, Gaudet J, Enright E, McQuary P, Payette T, Pistone M, Stecker K, Zhang BM, Zhou YX, Varnholt H, Smith B, Gadd M, Chatfield E, Kessler J, Baer TM, Erlander MG, Sgroi DC. Gene expression profiles of human breast cancer progression. Proc Natl Acad Sci USA. 2003;100:5974–5979. doi: 10.1073/pnas.0931261100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geradts J, Kratzke RA, Niehans GA, Lincoln CE. Immunohistochemical detection of the cyclin-dependent kinase inhibitor 2/multiple tumor suppressor gene 1 (CDKN2/MTS1) product p16INK4A in archival human solid tumors: correlation with retinoblastoma protein expression. Cancer Res. 1995;55:6006–6011. [PubMed] [Google Scholar]
- Silva J, Dominguez G, Silva JM, Garcia JM, Gallego I, Corbacho C, Provencio M, Espana P, Bonilla F. Analysis of genetic and epigenetic processes that influence p14ARF expression in breast cancer. Oncogene. 2001;20:4586–4590. doi: 10.1038/sj.onc.1204617. [DOI] [PubMed] [Google Scholar]
- Ueki K, Ono Y, Henson JW, Efird JT, von Deimling A, Louis DN. CDKN2/p16 or RB alterations occur in the majority of glioblastomas and are inversely correlated. Cancer Res. 1996;56:150–153. [PubMed] [Google Scholar]
- Parry D, Bates S, Mann DJ, Peters G. Lack of cyclin D-Cdk complexes in Rb-negative cells correlates with high levels of p16INK4/MTS1 tumour suppressor gene product. EMBO J. 1995;14:503–511. doi: 10.1002/j.1460-2075.1995.tb07026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medema RH, Herrera RE, Lam F, Weinberg RA. Growth suppression by p16ink4 requires functional retinoblastoma protein. Proc Natl Acad Sci USA. 1995;92:6289–6293. doi: 10.1073/pnas.92.14.6289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas J, Bartkova J, Rohde M, Strauss M, Bartek J. Cyclin D1 is dispensable for G1 control in retinoblastoma gene-deficient cells independently of cdk4 activity. Mol Cell Biol. 1995;15:2600–2611. doi: 10.1128/mcb.15.5.2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timms JF, White SL, O’Hare MJ, Waterfield MD. Effects of ErbB-2 overexpression on mitogenic signalling and cell cycle progression in human breast luminal epithelial cells. Oncogene. 2002;21:6573–6586. doi: 10.1038/sj.onc.1205847. [DOI] [PubMed] [Google Scholar]
- Wang TC, Cardiff RD, Zukerberg L, Lees E, Arnold A, Schmidt EV. Mammary hyperplasia and carcinoma in MMTV-cyclin D1 transgenic mice. Nature. 1994;369:669–671. doi: 10.1038/369669a0. [DOI] [PubMed] [Google Scholar]
- D’Amico M, Wu K, Di Vizio D, Reutens AT, Stahl M, Fu M, Albanese C, Russell RG, Muller WJ, White M, Negassa A, Lee HW, DePinho RA, Pestell RG. The role of Ink4a/Arf in ErbB2 mammary gland tumorigenesis. Cancer Res. 2003;63:3395–3402. [PubMed] [Google Scholar]
- Milde-Langosch K, Goemann C, Methner C, Rieck G, Bamberger AM, Loning T. Expression of Rb2/p130 in breast and endometrial cancer: correlations with hormone receptor status. Br J Cancer. 2001;85:546–551. doi: 10.1054/bjoc.2001.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geradts J, Ingram CD. Abnormal expression of cell cycle regulatory proteins in ductal and lobular carcinomas of the breast. Mod Pathol. 2000;13:945–953. doi: 10.1038/modpathol.3880172. [DOI] [PubMed] [Google Scholar]
- Muller H, Lukas J, Schneider A, Warthoe P, Bartek J, Eilers M, Strauss M. Cyclin D1 expression is regulated by the retinoblastoma protein. Proc Natl Acad Sci USA. 1994;91:2945–2949. doi: 10.1073/pnas.91.8.2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planas-Silva MD, Shang Y, Donaher JL, Brown M, Weinberg RA. AIB1 enhances estrogen-dependent induction of cyclin D1 expression. Cancer Res. 2001;61:3858–3862. [PubMed] [Google Scholar]
- Rashid A, Shen L, Morris JS, Issa JP, Hamilton SR. CpG island methylation in colorectal adenomas. Am J Pathol. 2001;159:1129–1135. doi: 10.1016/S0002-9440(10)61789-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong DJ, Paulson TG, Prevo LJ, Galipeau PC, Longton G, Blount PL, Reid BJ. p16(INK4a) lesions are common, early abnormalities that undergo clonal expansion in Barrett’s metaplastic epithelium. Cancer Res. 2001;61:8284–8289. [PubMed] [Google Scholar]
- Nahta R, Trent S, Yang C, Schmidt EV. Epidermal growth factor receptor expression is a candidate target of the synergistic combination of trastuzumab and flavopiridol in breast cancer. Cancer Res. 2003;63:3626–3631. [PubMed] [Google Scholar]