Signaling mechanism of thrombin-induced gingival fibroblast-populated collagen gel contraction (original) (raw)
Abstract
- Thrombin is activated during gingival tissue injury and inflammation. Thrombin (platelet)-rich plasma has been used for periodontal regeneration with success. Thrombin and other bacterial proteases also affect the functions of adjacent periodontal cells via stimulation of protease-activated receptors (PARs).
- We noted that thrombin (0.1–2 U ml−1), human, and frog PAR-1 agonist peptide (20–240 _μ_M) induced the gingival fibroblast (GF)-populated collagen gel contraction within 2 h of exposure. However, PAR-2, PAR-3, and PAR-4 agonist peptide (20–240 _μ_M) showed little effect on collagen gel contraction. U73122 (phospholipase C inhibitor) and 2-APB (IP3 antagonist) were effective in inhibition of GF contraction.
- Thrombin-induced GF contraction was inhibited by 5 mM EGTA (an extracellular calcium chelator) and verapamil (an L-type calcium channel blocker). In addition, W7 (10 and 25 _μ_M, a calcium/calmodulin (CaM) inhibitor), ML-7 (50 _μ_M, myosin light chain kinase (MLCK) inhibitor), and HA1077 (100 _μ_M, Rho kinase inhibitor) completely inhibited the thrombin-induced collagen gel contraction. Thrombin also induced the phosphorylation of ERK1/ERK2 and elevated the Rho-GTP levels in GF.
- However, U0126 only partially inhibited the thrombin-induced GF contraction. Similarly, wortmannin (100 nM), LY294002 (20 _μ_M) (two PI3K inhibitor) and genistein also showed partial inhibition. Moreover, NAC was not able to suppress the GF contraction, as supported by the slight decrease in reactive oxygen species production in GF by thrombin.
- Thrombin also stimulated metalloproteinase-2 (MMP-2) and MMP-3 production in GF. But addition of GM6001 or 1,10-phenanthroline, two MMP inhibitors, could not inhibit the thrombin-induced GF contraction.
- These results indicate that thrombin is crucial in the periodontal inflammation and wound healing by promoting GF contraction. This event is mainly mediated via PAR-1 activation, PLC activation, extracellular calcium influx via L-type calcium channel, and the calcium/CaM–MLCK and Rho kinase activation pathway.
Keywords: Calcium/calmodulin, calcium, L-type calcium channel, gingival fibroblast, myosin light chain kinase, protease-activated receptors, signal transduction, thrombin
Introduction
It has been estimated that there are about 5–15% of Europeans aged 40 or higher suffering from moderate to severe periodontitis (Pilot & Miyazaki, 1991). There are approximately 116 million Americans with adult periodontitis (Slade & Beck, 1999). Clinically, the patients always suffer from gingival inflammation and bleeding during tooth brushing, gingival swelling with pain, and evident tooth mobility. Nonsurgical periodontal treatment and even periodontal surgery are often conducted to improve the health of the periodontium. During gingival bleeding after toothbrushing, or after periodontal therapy, thrombin is generated from prothrombin to promote blood coagulation and cease bleeding (Tatakis, 1992). Moreover, thrombin can be released from fibrin clot (Liu et al., 1979) and the receptor can be activated by gingipains, a cysteine proteinase from periodontal pathogen, such as Porphyromonas gingivalis (Imamura et al., 2001). Thrombin and other proteases generated by periodontal pathogens are suggested to be responsible for the increased risks of cardiovascular diseases for periodontitis patients (Imamura et al., 2001; Lourbakos et al., 2001). Recently, thrombin-rich plasma and platelet-rich plasma, which contain thrombin and other growth factors, have been used for periodontal regenerative surgery with success (Kassolis et al., 2000; Tozum & Demiralp, 2004). Furthermore, thrombin can be released from fibrin clot and induces a wide array of biological effects such as cell proliferation, collagen synthesis, prostaglandin, IL-6 production, etc., on adjacent cells (Liu et al., 1979; Sundqvist et al., 1995; Hou et al., 1998; Coughlin & Camerer, 2003). However, the precise roles of thrombin in the pathobiological processes of periodontium are not fully clear.
The major goals of periodontal therapy are to decrease gum inflammation as well as promote the periodontal tissue repair and regeneration (Takata, 1994). The wound-healing procedure usually consists of inflammation, followed by granulation tissue development and tissue reorganization. Fibroblast contraction is a crucial aspect of both normal wound healing and fibrosis. Fibroblast contraction is also believed to contribute to the contraction that characterizes scars and fibrotic tissues, as well as the granulation tissue resolution (Grinnell, 1994; 2000; Sumiyoshi et al., 2003). Periodontal ligament (PDL) fibroblasts and gingival fibroblasts (GF) are shown to express PAR-1 receptor, which mediates the growth and clustering, and promotes collagen gel contraction of these cells by thrombin and PAR-1 agonist peptide (Chan et al., 1998; Chang et al., 2001), revealing the role of PAR activation in regulating gingival tissue healing and inflammation after tissue wounding. We interestingly noted the rapid induction of GF-populated collagen gel contraction following protease activation of PARs by thrombin. GF contraction is associated with by actin filament polymerization (Chang et al., 2001). Proteolytic cleavage of four isoforms of PARs by thrombin or other proteases exposes the ‘tethered ligand' and provokes various cellular responses, such as human platelet aggregation (Vu et al., 1991), fibroblast [Ca2+]i increase, and proliferation (Rasmussen et al., 1991; Chan et al., 1998; Chang et al., 2001). GF express mainly PAR-1 and PAR-3 mRNA, which may potentially mediate the calcium signaling, mitogenic, and protein synthetic effects of thrombin, which exposes the tether ligands via its serine proteinase activity (Chang et al., 2001; Tanaka et al., 2003).
We recently noted that thrombin and PAR-1 agonist peptide induce calcium mobilization of GF via calcium/calmodulin (Ca–CaM), phospholipase C (PLC) activation, IP3 production, intracellular release of calcium store, and the subsequent capacitative calcium entry (Jeng et al., 2004a). However, Tanaka et al. (2004) found that PAR-1 activation is responsible for thrombin-induced IL-6 production in GF via tyrosine kinase/p38 MAPK pathway rather than calcium signaling. We therefore further investigated the role of reactive oxygen species and metalloproteinase (MMP) production, as well as the signal transduction pathway responsible for thrombin-induced GF-populated collagen gel contraction, a crucial event in periodontal wound closure or tissue fibrosis.
Methods
Culture of human GF
Human GF were cultured and passaged as described previously (Chang et al., 1998; 2001; Jeng et al., 1999; 2003). Briefly, healthy gingival tissues (with a gingivitis index <1) were obtained during surgical crown-lengthening procedures with adequate informed consent. Gingival tissues were cut into small pieces by a surgical knife and cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum (FCS) and penicillin/streptomycin by using the tissue explant technique. When the outgrowth of GF was near confluence, they were passaged at a ratio of 1 : 3. The passage numbers from 3 to 8 were used through these studies. Four GF strains were established and used through these studies with similar results.
Preparation of GF-populated collagen gels
Contraction of GF-populated collagen gel contraction was measured as described previously (Chang et al., 2001). Briefly, GF were detached from culture plate by a brief exposure to 0.05% trypsin/EDTA and collected. After centrifugation, GF were resuspended in serum-free DMEM to a concentration of 4 × 105 cells ml−1. Thereafter, equal volumes of suspended GF and cold collagen solution (2.4 mg ml−1, prepared by 8 : 1 : 1 (v v−1) of Vitrogen 100/10 × DMEM/0.1 N NaOH, pH 7.2) were mixed together, and then 2.5 ml of this collagen–GF mixture was added into six-well culture plate. The final concentration of collagen was 1.2 mg ml−1. Collagen was allowed to gel for 3 h at 37°C and placed into the cell culture incubator. Before the experiment, the gels were covered with 2.5 ml of serum-free DMEM.
Effects of thrombin and PAR agonist peptides on GF-populated collagen gel contraction
GF-populated collagen gels were detached from the culture plate and exposed to thrombin (0.1, 0.5, 1, and 2 U ml−1) or PAR-1 (SFLLRN), PAR-2 (SLIGKV), PAR-3 (TFRGAP), and PAR-4 (GYPGQV) agonist peptides, as well as the more specific frog PAR-1 agonist peptide (TFLLRN) and control peptide (FTLLRN) (20, 80, 160, and 240 _μ_M). The diameter of collagen gels was measured with a transparent ruler (millimeters) at 0.5, 1, 2, and 4 h (Chang et al., 2001). The maximal and minimal diameters of the gels were averaged and calculated for analysis. Culture medium was collected for measuring the production of MMP-2 and MMP-3 by GF using ELISA kits.
Effects of various modulators on the thrombin-induced GF-populated collagen gel contraction
GF-populated collagen gels were pretreated with various inhibitors, including EGTA (5 mM), verapamil (25 and 50 _μ_M), W7 (10, 25, and 50 _μ_M), U73122 (50 _μ_M), 2-APB (50 and 100 _μ_M), ML-7 (25 _μ_M), HA1077 (100 _μ_M), genistein (50 _μ_M), LY294002 (20 _μ_M), Wortmannin (100 nM), U0126 (50 _μ_M), _N_-acetyl-L-cysteine (NAC, 3 mM), GM6001 (10 and 40 _μ_M), or 1,10-phenanthroline (100 and 200 _μ_M) for 15 min. Therefore, GF-populated collagen gels were detached from the culture plate and exposed to thrombin (2 U ml−1). The diameter of collagen gels was measured with a transparent ruler (millimeters) at 0.5, 1, 2, and 4 h.
Western blotting
To evaluate whether thrombin could induce ERK1/ERK2 phosphorylation, GF were serum-starved for 24 h and then exposed to thrombin (2 U ml−1) for various time periods (1, 2.5, 5, 10, 20, and 40 min). Cell lysates were prepared as described previously (Jeng et al., 2003; Chang et al., 2004) and 50 _μ_g of proteins was subjected to 12% polyacrylamide gel electrophoresis and transferred to a PVDF membrane. The membrane was blocked for 30 min at room temperature in a blocking reagent (20 mM Tris, pH 7.4; 125 mM NaCl; 0.2% Tween 20; 5% nonfat dry milk; and 0.1% sodium azide), and then incubated for 2 h with mouse anti-human p-ERK1/ERK2, Rho A, ROCK-1, and G3PDH antibodies. After incubation with secondary antibody and final membrane rinsing, the immunoreactive bands were developed on a Fuji X-ray film by Amersham enhanced chemiluminescence (ECL) reagents.
Rho activation assay
Rho activation in GF by thrombin was evaluated by Pierce EZ-Detect™ Rho activation assay kits. Briefly, GF was serum-starved for 24 h and then exposed to thrombin (2 U ml−1) for 1, 2.5, 5, 10, 20, and 40 min. Cell lysates were collected and 500 _μ_g of proteins was incubated with 400 _μ_g GST-Rhotekin-PBD and SwellGel Immobilized Glutathione Disc in a rotor at 4°C for 1 h. Cells lysates pretreated with 10 mM GTP_γ_S were used as positive control. The active Rho-GTP was pulled down from the cell lysates by centrifugation at 10,000 r.p.m. for 1 min and subjected to Western blot analysis as described above using mouse-anti-Rho antibody (1 : 250) for probing.
Measurement of cellular ROS and glutathione (GSH) levels
For clarifying whether redox changes may affect thrombin-induced GF contraction, ROS and GSH levels in GF were measured by analysis of single-cell dichlorofluorescein (DCF) and 5-chloromethyl fluorescein (CMF) fluorescence as described previously (Chang et al., 2002; Jeng et al., 2004b). In brief, 5 × 105 GF were plated into 10-cm culture dishes and then starved in serum-free DMEM for 24 h. They were exposed to various concentrations of thrombin or changed to full medium (DMEM with 10% FBS). After 24 h of exposure, GF were labeled for dichlorofluorescein-diacetate (DCFH-DA) (10 _μ_M) or CMF-DA (25 _μ_M) for 30 min. Cells were therefore collected in PBS and immediately subjected for flow-cytometric analysis (Becton Dickinson, CA, U.S.A.).
Materials and reagents
DMEM, FCS, and penicillin/streptomycin were from Gibco (Life Technologies, Grand Island, NY, U.S.A.). Vitrogen 100 was purchased from Collagen Corporation (Fremont, CA, U.S.A.). It is a sterile solution of purified, pepsin-solubilized dermal collagen dissolved in 0.012 N HCl. About 95–98% of the content is type I collagen, with the remainder being comprised of type III collagen. Human _α_-thrombin, EGTA, NAC, 1,10-phenanthroline, DCFH-DA, 5-chloromethyl fluorescein diacetate (CMF-DA), ML-7, HA1077, and genistein were obtained from Sigma (Sigma Chemical Company, St Louis, MO, U.S.A.). Human PAR-1, PAR-2, PAR-3, and PAR-4 agonist peptides were purchased from Bachem (Bubendorf, Switzerland). The frog PAR-1 agonist peptide as well as the control peptide (Asokananthan et al., 2002) were synthesized by Blossom Biotechnologies Inc. (Taipei, Taiwan) with a purity higher than 95%. 2-amino-ethoxydiphenyl borate (2-APB), verapamil, U73122, U0126, LY294003, wortmannin and W7 (_N_-(6-aminohexyl)-5-chloro-1-naphthalene-sulfonamide) were from Tocris (Tocris Cookson Ltd, Northpoint Fourth Way Avonmouth, U.K.). Protein assay kits were obtained from Bio-Rad (Bio-Rad Labs, Hercules, CA, U.S.A.). GM6001 (Ilomastat) was purchased from Calbiochem (San Diego, CA, U.S.A.). MMP2 and MMP-3 ELISA kits were obtained from R&D (Minneapolis, MN, U.S.A.). Flow cytometric reagents were obtained from Becton-Dickinson (San Jose, CA, U.S.A.). Mouse anti-human antibodies for G3PDH and p-ERK1/ERK2 were from Santa Cruz (CA, U.S.A.). EZ-Detect™ Rho activation assay kits were obtained from Pierce (Rockfrod, IL, U.S.A.).
Statistical analysis
Four or more separate experiments were conducted for each test. Results were expressed as the diameter of collagen gels (mm, mean±s.e.). Kruskal–Wallis test and Mann–Whitney _U_-test were used for statistical analysis. A _P_-value <0.05 was considered to be statistically significant difference.
Results
Effect of thrombin and PAR agonist peptides on GF-populated collagen gel contraction
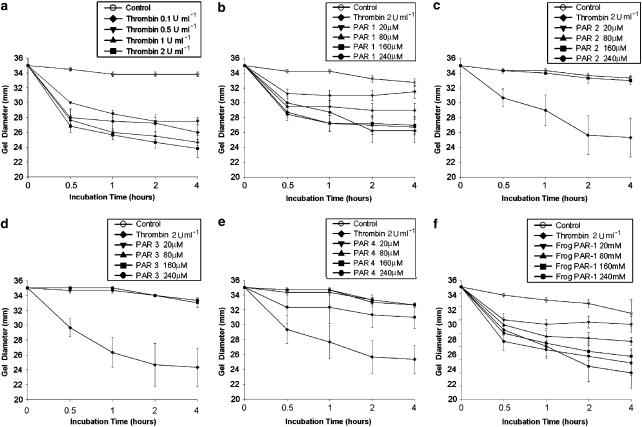
Thrombin (0.1, 0.5, 1, and 2 U ml−1) evidently stimulated the GF contraction even at a concentration of 0.1 U ml−1 and reached about maximal at a concentration of 2 U ml−1 (Figure 1a). Human PAR-1 agonist peptide also induced the GF-populated collagen gel contraction within 1 h of exposure in a dose-dependent manner at concentrations ranging from 20–240 _μ_M (Figure 1b) and sustained for more than 4 h. However, PAR-2, PAR-3, and PAR-4 agonist peptides (20–240 _μ_M) show little effect on GF-populated collagen gel contraction (Figure 1c–e). Specific frog PAR-1 agonist peptide also induced GF contraction at concentrations ranging from 20 to 240 _μ_M (Figure 1f), whereas control frog peptide had little effect on GF contraction (data not shown).
Figure 1.
Effect of (a) thrombin, (b) human PAR-1, (c) PAR-2, (d) PAR-3, and (e) PAR-4 as well as (f) frog PAR-1 agonist peptides on the GF-populated collagen gel contraction. GF-populated collagen gels were detached and the thrombin (0.1, 0.5, 1 and 2 U ml−1) or human PAR-1, PAR-2 PAR-3 and PAR-4 and frog PAR-1 agonist peptides (20, 80, 160 and 240 _μ_M) exposed for 0.5–4 h. The diameter of collagen gels during exposure for 0.5–4 h was measured. Results were the mean diameter (mm) of collagen gels (_n_=4). Thrombin was regularly used as a positive control during testing of contractile effect by PARs.
Role of PLC on thrombin-induced collagen gel contraction
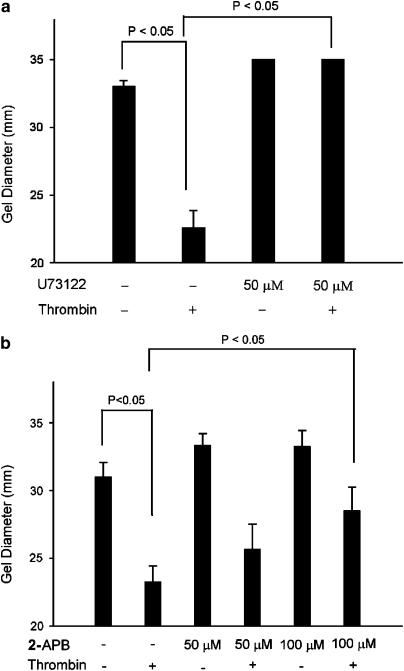
Thrombin induced evident contraction of GF-population collagen gels and decreased the gel diameter from 33 mm (control) to 23 mm. Pretreatment with U73122 (50 _μ_M), a PLC inhibitor, almost completely prevented the thrombin-induced GF contraction (Figure 2a). Since previous study has found the expression of IP3 receptor isoforms in human GF (Jeng et al., 2004a), we further checked whether activation of these receptors by inositol-(1,4,5)-trisphosphate (IP3) was crucial for fibroblast contraction. Interestingly, 2-APB (50, 100 _μ_M), an IP3 receptor antagonist, also inhibited the thrombin-induced collagen gel contraction. The diameter of thrombin-induced collagen gels (23 mm) was decreased by 50 and 100 _μ_M 2-APB and the diameter of collagen gels increased to 25 and 28.5 mm, respectively (Figure 2b).
Figure 2.
Effect of U73122 and 2-APB on thrombin-induced GF-populated collagen gel contraction. GF-populated collagen gels were pretreated with (a) U73122 (50 _μ_M) (_n_=5) or (b) 2-APB (50 and 100 _μ_M) (_n_=5) for 15 min. Gels were therefore detached and then exposed to thrombin (2 Uml−1) for 0.5–4 h. The diameter of collagen gels after 2 h of exposure were used for comparison. P<0.05 indicates difference between groups.
Role of calcium mobilization on thrombin-induced collagen gel contraction
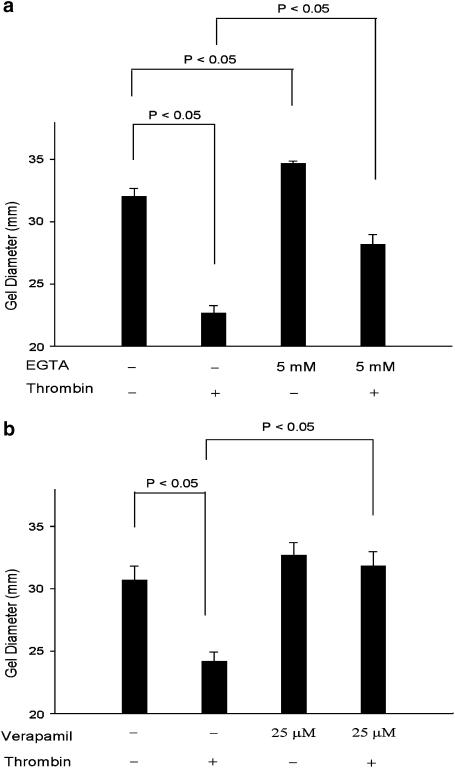
The intrinsic GF contractile response was inhibited by 5 mM EGTA (an extracellular calcium chelator). The thrombin-induced fibroblast contraction was inhibited by 60% by EGTA. The diameter of thrombin-induced collagen gel was 23 mm, whereas when pretreated with EGTA it increased to 28 mm (Figure 3a). Thrombin-induced GF contraction was almost completely inhibited by pretreatment with verapamil (25 _μ_M, an L-type calcium channel blocker), with a gel diameter of 32 mm (Figure 3b).
Figure 3.
Effect of EGTA and verapamil on thrombin-induced GF-populated collagen gel contraction. GF-populated collagen gels were pretreated with (a) EGTA (5 mM) or (b) verapamil (25 _μ_M) for 15 min. Gels were therefore detached and then exposed to thrombin (2 U ml−1) for 0.5–4 h. The diameter of collagen gels after 2 h of exposure was used for comparison. P<0.05 indicates difference between groups (_n_=6).
Role of calcium/CaM and MLCK on GF-populated collagen gel contraction
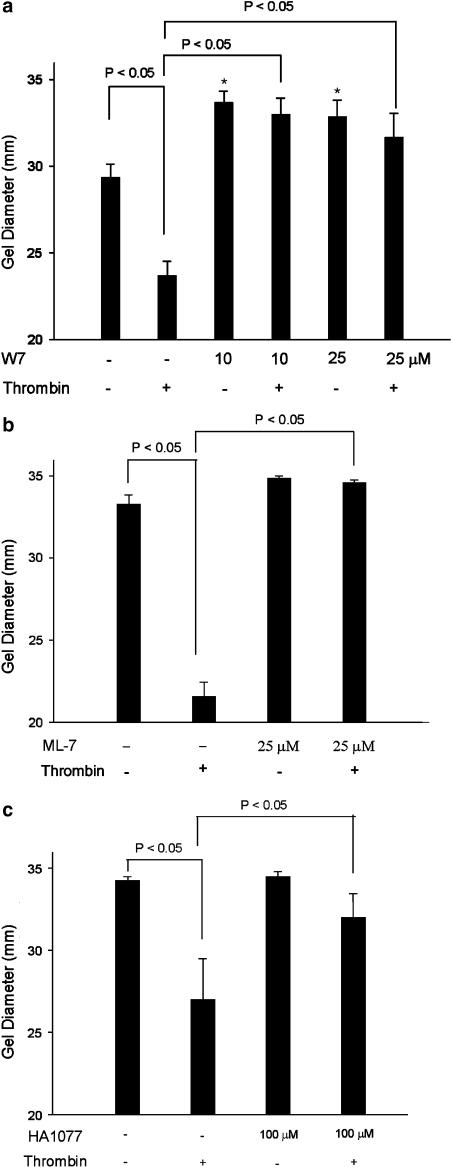
W7 (10 and 25 _μ_M), a calcium/CaM inhibitor, effectively inhibited the intrinsic contractile activity of fibroblasts. In addition, thrombin-induced GF-populated collagen gel contraction was also completely inhibited by W7 (10, 25 _μ_M) with an increase in gel diameter to 32–33 mm (Figure 4a). ML-7 (25 _μ_M), an MLCK inhibitor, entirely inhibited the thrombin-induced GF-populated collagen gel contraction (Figure 4b). Interestingly, HA1077 (100 _μ_M), a Rho kinase inhibitor, was able to suppress the thrombin-induced GF contraction by 75%, as revealed by an increase in gel diameter to 32 mm (Figure 4c).
Figure 4.
Effect of W7, ML-7, and HA1077 on thrombin-induced GF-populated collagen gel contraction. GF-populated collagen gels were pretreated with (a) W7 (10 and 25 _μ_M), (b) ML-7 (25 M), or (c) HA1077 (100 _μ_M) for 15 min. Gels were therefore detached from the periphery of the well and then exposed to thrombin (2 U ml−1) for 0.5–4 h. The diameter of collagen gels after 2 h of exposure was used for comparison. (_n_=6). P<0.05 indicates difference between groups. *Denotes marked difference (P<0.05) when compared with untreated gel.
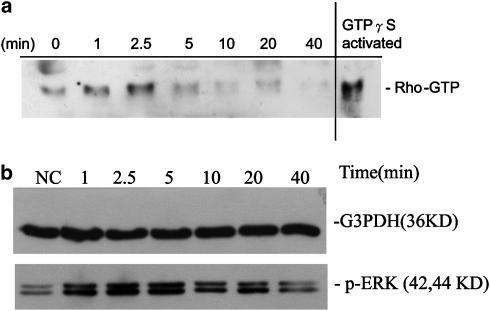
Activation of Rho and ERK in GF by thrombin
Western blotting of GF exposure to thrombin showed little changes in protein levels of Rho A and ROCK-1 (data not shown). Interestingly, thrombin elevated the Rho-GTP levels of GF within 1–2.5 min of exposure, indicating a rapid response (Figure 5a). Thrombin (2 U ml−1) also induced the ERK1/ERK2 phosphorylation in GF within 2.5–10 min of exposure with a peak activation at 5 min of exposure. Phosphorylation of ERK1/ERK2 in GF decreased to near basal level after 20 min of exposure to thrombin (Figure 5b). Thrombin also stimulated the phosphorylation of cAMP-responsive element binding protein (CREB) (data not shown).
Figure 5.
Activation of Rho and ERK1/ERK2 by thrombin in GF. (a) One representative Western blotting picture of Rho activation following exposure of GF to thrombin. Elevated Rho-GTP level in GF was noted after 1 and 2.5 min of exposure to thrombin. (b) GF were exposed to thrombin (2 U ml−1) and cell lysates (50 _μ_g protein) were used for Western blotting using G3PDH- and p-ERK1/pERK2-specific antibodies.
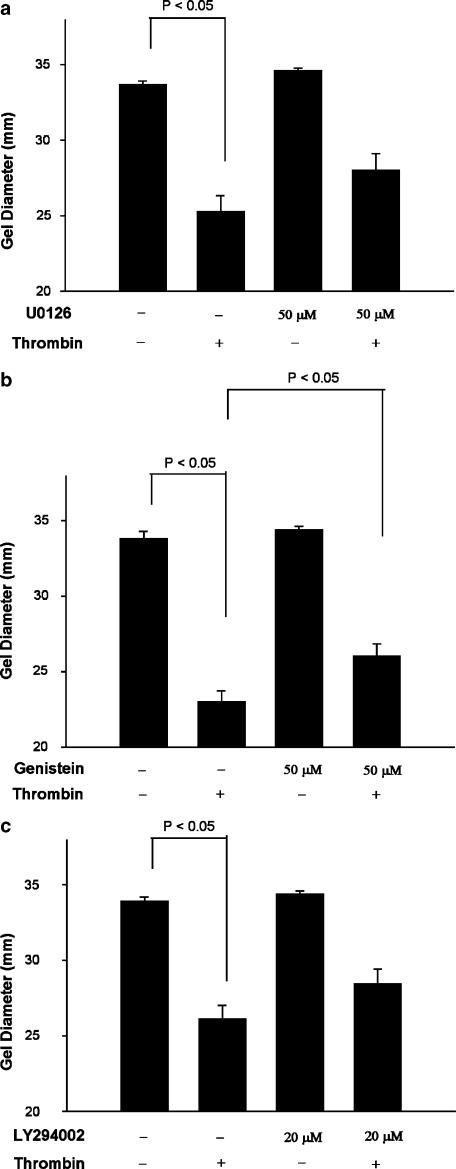
Role of some kinase cascade on thrombin-induced GF-populated collagen gel contraction
U0126 (50 _μ_M), a MEK1 inhibitor, which inhibits the downstream ERK1/ERK2 signaling, partially suppressed the thrombin-induced contraction of GF by 32% (_P_=0.091) (Figure 6a). Genistein (50 _μ_M) (Figure 6b), LY294002 (20 _μ_M) (Figure 6c) and wortmannin (100 nM) (data not shown) only partially inhibited the thrombin-induced collagen gel contraction with an inhibition of 27, 30 and 20%, respectively.
Figure 6.
Effect of MEK/ERK, tyrosine kinase, and PI3K activation on thrombin-induced GF-populated collagen gel contraction. GF-populated collagen gels were pretreated with (a) U0126 (50 _μ_M) (_n_=15), a MEK inhibitor, or (b) genistein (50 _μ_M) (_n_=5), or (c) LY294002 (20 _μ_M) (_n_=11) for 15 min. Gels were therefore detached from the periphery of the well and then exposed to thrombin (2 U ml−1) for 0.5–4 h. The diameter of collagen gels after 2 h of exposure was used for comparison. P<0.05 indicates difference between groups.
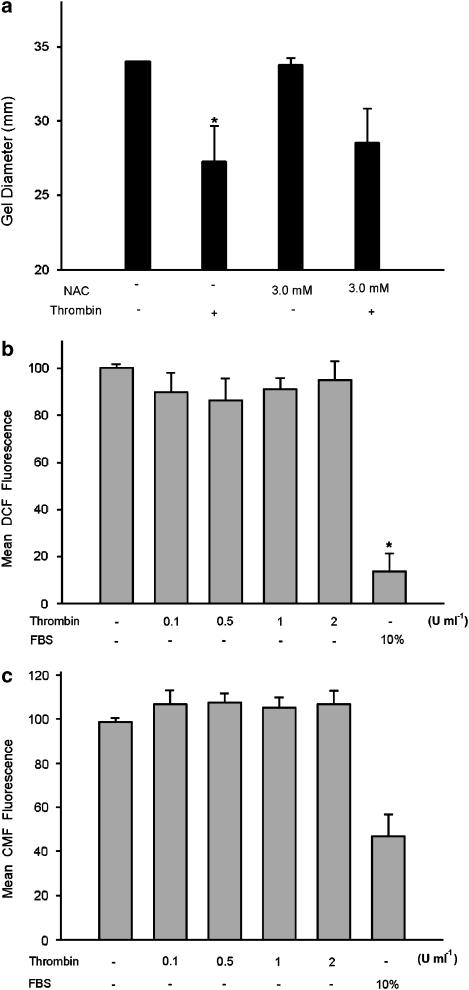
Role of redox changes in thrombin-induced collagen gel contraction
Since induction of ROS production by thrombin has been observed and is important for vascular smooth muscle cells and platelet functions (Wachowicz et al., 2002; Wang et al., 2004), we therefore checked whether thrombin-induced GF contraction was related to ROS production. Unexpectedly, NAC (3 mM), a wide-spectrum antioxidant with scavenging properties toward superoxide radicals, hydrogen peroxide, etc. (Dekhuijzen, 2004), showed little effect on thrombin-induced GF-populated collagen gel contraction (Figure 7a). GF in serum-starved condition evidently increased the level of intracellular ROS, as indicated by elevation in DCF fluorescence. However, exposure to thrombin only slightly decreased the intracellular ROS levels in GF (_P_>0.05) (Figure 7b). Moreover, serum-starved GF obviously elevated the intracellular CMF fluorescence, whereas exposure to thrombin slightly increased the cellular CMF fluorescence (_P_>0.05) (Figure 7c).
Figure 7.
Role of ROS in thrombin-induced GF-populated collagen gel contraction. (a) GF-populated collagen gels were pretreated with NAC (3 mM) for 15 min. Gels were therefore detached from the periphery of the well and then exposed to thrombin (2 U ml−1) for 0.5–4 h. The diameter of collagen gels after 2 h of exposure was used for comparison (_n_=5). P<0.05 indicates difference between groups. In addition, GF in serum-free condition were exposed to various concentrations of thrombin or in full medium for 24 h. (b) Cellular DCF fluorescence of GF was measured (_n_=4). *Denotes difference when compared with control. (c) Cellular CMF fluorescence of GF was measured (_n_=4).
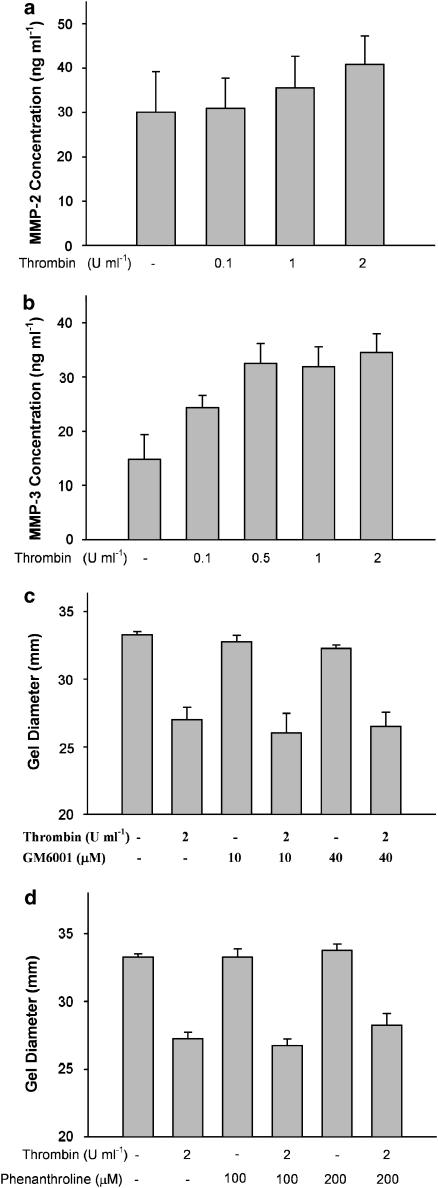
Role of thrombin-induced MMP production on GF contraction
Thrombin elevated the MMP-2 concentration in culture medium of GF from 30 ng ml−1 in control to 35.6 and 40.9 ng ml−1, respectively by 1 and 2 U ml−1 of thrombin (Figure 8a). Accordingly, thrombin also elevated the total MMP-3 levels in the culture medium from 14.8 (control) to 24.4 and 32.5 ng ml−1 by 0.1 and 0.5 U ml−1 of thrombin, respectively (Figure 8b). However, GM6001 (10 and 40 _μ_M) and 1,10-phenanthroline (100 and 200 _μ_M) were not able to prevent the thrombin-induced GF contraction (Figure 8c and d).
Figure 8.
Stimulation of MMP-2 and MMP-3 production of GF by thrombin. GF in collagen gels were exposed to thrombin for 24 h and culture medium was collected for (a) MMP-2 (_n_=6), (b) MMP-3 (_n_=8) production by ELISA. GF in collagen gels were exposed to (c) GM6001 (_n_=4) or (d) 1,10-phenanthroline (_n_=4) for 15 min and then incubated with thrombin. The diameter of collagen gels after 2 h of exposure was used for comparison.
Discussion
Healing of the periodontium showed marked differences from those of other tissues, which usually heal by primary closure of opposed surgical wound edges. During wound healing, contraction may decrease the wound size and eventually reduce scar tissue formation during healing. Thrombin has been shown to induce contraction of GF (Chang et al., 2001) and chick embryo fibroblasts (Kolodney et al., 1999) via its protease activity. In the present study, thrombin and human PAR-1 agonist peptide induced marked contraction of GF-populated collagen gels, whereas PAR-2, PAR-3, and PAR-4 agonist peptides showed little effect on this event. This indicates that thrombin-induced GF contraction is mainly due to PAR-1 activation. Since human PAR-1 agonist peptide also activates PAR-2 (Blackhart et al., 1996), more specific frog PAR-1 agonist peptide (Asokananthan et al., 2002) is also tested and it is found that activation of PAR-1 really mediates GF contraction. Accordingly, trypsin, as a PAR-2 activator, could not induce GF contraction (Chang et al., 2001). Some PAR-3 mRNA expression has been reported in GF (Chang et al., 2001; Tanaka et al., 2003), but PAR-3 agonist peptide was not able to induce GF contraction. Similarly, PAR-3 agonist peptide also shows little effect on IL-6 and IL-8 production in human bronchial epithelial cells, although these cells express PAR-3 receptor (Asokananthan et al., 2002). Since PAR-3 agonist peptide has been reported to be not functionally active, further studies are necessary to clarify whether PAR-3 activation contributed to fibroblast contraction.
Activation of PARs has been linked to G-protein-coupled signal transduction pathways including G_α_q/PLC/CaM–MLCK, pertussis toxin (PTX)-insensitive G_α_12/13/Rho/Rho kinase pathway, or the PTX-sensitive G_α_o/i/Rho/Rho kinase pathways (Nguyen et al., 2004). Recently, we have found that GF expresses all three isoforms of IP3 receptors and thrombin induces IP3 production of GF within 30 s of exposure possibly via activation of PLC (Jeng et al., 2004a). U73122, as a PLC inhibitor, suppressed the thrombin-induced GF contraction. 2-APB, as an IP3 receptor antagonist, also inhibited the thrombin-induced GF-populated collagen contraction, supporting the activation of PLC/IP3 pathways for GF contraction.
Binding of IP3–IP3 receptors on plasma membrane and intracellular organelles has been shown to regulate the cytosolic calcium levels (Taylor & Broad, 1998). Thrombin may induce extracellular calcium influx and intracellular calcium release in human GF within 30–40 s of exposure (Tanaka et al., 2003; Jeng et al., 2004a). Cytosolic Ca2+ ions are important second messengers that modulate membrane excitability, ion channel gating, gene expression, and muscle contraction (Hoesch et al., 2002). Although PAR-1-mediated Ca2+ mobilization undergoes rapid desensitization (Tanaka et al., 2003), PGD2-induced contraction of human fetal lung fibroblasts has been shown to associate with calcium-independent PKC-ɛ pathway, but unrelated to PKA, PKG, PLC, and extracellular Ca2+ (Kohyama et al., 2002). Recently, we have found that thrombin-induced calcium mobilization in GF was mediated by PLC activation and IP3 production (Jeng et al., 2004a). Intriguingly, thrombin-induced GF-populated collagen gel contraction was inhibited by EGTA and verapamil, indicating that the GF contraction was possibly mediated by binding of IP3–IP3 receptors followed by release of intracellular Ca2+ store and subsequent capacitative Ca2+ entry via L-type calcium channel (Jeng et al., 2004a). Both intrinsic GF contraction and the thrombin-induced GF-populated collagen gel contraction were associated with extracellular Ca2+ influx.
Cell contraction has been reported to be regulated by the Ca2+/CaM–MLCK system and terminated by myosin phosphatase (Katoh et al., 2001a, 2001b; Emmert et al., 2003). Phosphorylation of human myosin phosphatase target subunit (MYPT1) site, Thr696, by Rho kinase results in inhibition of its activity (Ito et al., 2004). Serum-induced sustained contraction of NIH fibroblasts may depend on Rho-kinase activation, but is unrelated to CaM–MLCK pathway (Nobe et al., 2003). However, thrombin induces the myosin II regulatory light chain phosphorylation and contraction by the Rho-kinase-mediated pathway in rat embryonic fibroblast (Emmert et al., 2003). CaM has been shown to mediate the effect of thrombin-induced calcium mobilization (Jeng et al., 2004a). In this study, W7 effectively inhibited the thrombin-induced GF contraction and the intrinsic GF contraction, indicating that Ca2+/CaM was necessary for these events. Accordingly, W7 also inhibits the serum-induced contraction of human dermal fibroblasts by inhibition of MLCK (Ehrlich et al., 1989; 1991). From these results, Ca–CaM/MLCK pathway may be related to thrombin-induced GF contraction. Recently, three possible signal transduction pathways are suggested to mediate the contraction of nonmuscle cells. The first pathway was mediated by the Ca2+/CaM–MLCK system and terminated by myosin phosphatase. The second type of contraction, if regulated by Rho-kinase, regulated myosin phosphatase. The third type of contraction is the production of the general cytoplasmic tension (Katoh et al., 2001). Supporting this hypothesis, we found that ML-7 inhibited the thrombin-induced GF contraction. In addition, thrombin was able to activate Rho-GTPase activity in GF within 1–2.5 min of exposure and HA 1077, a Rho kinase inhibitor, also prevented the thrombin-induced GF contraction. Rho kinase inhibits MLC phosphatase, which dephosphorylates pMLC (Nguyen et al., 2004). Our results suggest that both MLCK and Rho kinase activation were important for GF contraction, possibly by differential phosphorylation of Ser19 and Thr18 of MLC (Totsukawa et al., 2004). In addition, Rho activation promotes actin stress fiber assembly and the formation of focal adhesion. Inhibition of actin filament polymerization by cytochalasin B can therefore effectively inhibit the thrombin-induced GF contraction (Chang et al., 2001).
TGF-_β_-mediated collagen gel contraction of human dermal fibroblasts is not mediated by MAPK activation (Sumiyoshi et al., 2003). However, activation of ERK and p38 MAPK was noted in human fibroblasts following 10 min of contraction (Lee et al., 2000). Actin stress fiber assembly has been reported to require synergistic activation of ERK and Rho kinases (Sabri et al., 2004). In our study, thrombin stimulated the phosphorylation of ERK1/ERK2 in GF within 5–10 min of exposure. However, inhibition of the upstream MEK-1 by U0126 was partially effective in suppressing the thrombin-induced GF contraction. This indicates that MEK-1/ERK activation was not the major signaling pathway of thrombin-induced GF contraction. This is further supported by the comparable inhibitory effect of genistein, a tyrosine kinase inhibitor, on thrombin-induced GF contraction. Similarly, thrombin-induced IL-6 production in GF is also partially attenuated by blocking the MEK/ERK pathway. Thrombin has been found to stimulate PI3K/Akt pathway in Chinese hamster embryonic fibroblasts (Goel et al., 2004). PI3K/Akt is responsible for beta-1 integrin viability signaling of fibroblasts in collagen matrices (Xia et al., 2004). PI3K inhibitors also partly inhibited the thrombin-induced GF contraction. Although the activation of MEK/ERK and PI3K by thrombin may link to LIM kinase/cofilin and WASP(profilin)/Arp2/3-dependent activation as described in fibroblasts (Prichard et al., 2004; Schwartz, 2004), which regulates the actin assembly (Levinson et al., 2004), these events do not seem to be the major pathway for GF contraction.
Thrombin-stimulated migration of vascular smooth muscle cells has been linked to ROS production and MAPK activation (Wachowicz et al., 2002; Wang et al., 2004). Platelet aggregation induced by thrombin and other agonists is also associated with ROS production (Wachowicz et al., 2002). We found that NAC was not effective in prevention of thrombin-induced GF contraction. On the contrary, exposure of GF to serum-free DMEM elevated ROS levels in GF and thrombin only slightly declined the ROS production. Accordingly, serum withdrawal is responsible for killing of U937 blood cells in rat hepatoma cells by elevating cellular ROS levels (Pandey et al., 2003; Lee et al., 2005). This indicates that thrombin-induced GF contraction was not mediated by cellular ROS production. Thrombin is also shown to stimulate metalloproteinase (MMP) production in vascular smooth muscle cells and human umbilical vein endothelial cells (Maragoudakis et al., 2001; Oak et al., 2004). Some MMPs were reported to be able to cleave cell membrane receptors (Conant et al., 2002) and thereby activate. Similarly, thrombin also stimulates total MMP-2 and MMP-3 production in GF. However, thrombin-induced GF contraction does not seem to be directly mediated by MMP enzymatic cleavage of cell surface PARs, because GM6001 and 1,10-phenanthroline show little effect on thrombin-induced GF contraction.
In summary, thrombin stimulates the proliferation, contraction, and IL-6 production of human GF via activation of PAR-1 by its serine protease activity (Chang et al., 2001; Tanaka et al., 2004). Thrombin induces the calcium mobilization of GF mainly via the activation of PAR-1, PLC, and CaM (Tanaka et al., 2003; Jeng et al., 2004a). In this study, we further found that thrombin-induced GF contraction was mainly associated with PLC activation, IP3 production, and the induction of Ca2+ release from intracellular store. Subsequent capacitative Ca2+ influx and binding to CaM result in calcium-saturated CaM that binds and activate MLCK. Phosphorylation of MLC by MLCK then optimizes myosin ATPase activity, which causes cytoskeleton actin–myosin contraction in GF. In addition, activation Rho kinase signaling pathway by thrombin may directly phosphorylate MLC or indirectly inactivate MYPT. Thrombin and other bacterial proteases therefore may affect the inflammation, healing, and fibrosis of the periodontium. Whether thrombin and its related derivatives may have potential use for periodontal regeneration should be further addressed in future.
Acknowledgments
We thank Miss H.F. Jeng for technical assistance. The study is supported by a grant from the National Science Council, Taiwan (NSC88-2314-B255-002) and Chang Gung Memorial Hospital (CMRP-F32002).
Abbreviations
BAPTA/AM
1,2-bis(2-aminophenoxy)ethane-N,N,_N_′,_N_′-tetraacetic acid/acetoxy methylester
CaM
calmodulin
CMF-DA
5-chloromethylfluorescein diacetate
DCFH-DA
dichlorofluorescein-diacetate
DMEM
Dulbecco's modified Eagle's medium
DMSO
dimethylsulfoxide
EGTA
ethylene-glycol-bis-(_β_-aminoethylether)-N,N,_N_′,_N_′-tetraacetic acid
FCS
fetal calf serum
GF
gingival fibroblasts
IP3
inositol trisphosphate
MLCK
myosin light chain kinase
NAC
_N_-acetyl-L-cysteine
PAR
protease-activated receptor
PBS
phosphate-buffered saline
PDL
periodontal ligament
PKC
protein kinase C
PLC
phospholipase C
References
- ASOKANANTHAN N., GRAHAM P.T., FINK J., KNIGHT D.A., BAKKER A.J., MCWILLIAM A.S., THOMPSON P.J., STEWART G.A. Activation of protease-activated receptor (PAR)-1, PAR-2, and PAR-4 stimulates IL-6, IL-8, and prostaglandin E2 release from human respiratory epithelial cells. J. Immunol. 2002;168:3577–3585. doi: 10.4049/jimmunol.168.7.3577. [DOI] [PubMed] [Google Scholar]
- BLACKHART B.D., EMILSSON K., NGUYEN D., TENG W., MARTELLI A.J., NYSTEDT S., SUNDELIN J., SCARBOROUGH R.M. Ligand cross-reactivity within the protease-activated receptor family. J. Biol. Chem. 1996;271:16466–16471. doi: 10.1074/jbc.271.28.16466. [DOI] [PubMed] [Google Scholar]
- CHAN C.P., LIN C.P., CHANG M.C., HSIEH C.C., HSU C.C., LIN C.L., JENG J.H. Effects of thrombin on the growth, protein synthesis, attachment, clustering and alkaline phosphatase activity of cultured human periodontal ligament fibroblasts. Proc. Natl. Sci. Council ROC. 1998;22:137–143. [PubMed] [Google Scholar]
- CHANG M.C., CHAN C.P., WU H.L., CHEN R.S., LAN W.H., CHEN Y.J., JENG J.H. Thrombin-stimulated growth, clustering and collagen lattice contraction of human gingival fibroblasts is associated with its protease activity. J. Periodontol. 2001;72:303–313. doi: 10.1902/jop.2001.72.3.303. [DOI] [PubMed] [Google Scholar]
- CHANG M.C., KUO M.Y.P., HAHN L.J., HSIEH C.C., LIN S.K., JENG J.H. Areca nut extract inhibit the growth, attachment and matrix protein synthesis of cultured human gingival fibroblasts. J. Periodontol. 1998;69:1092–1097. doi: 10.1902/jop.1998.69.10.1092. [DOI] [PubMed] [Google Scholar]
- CHANG M.C., UANG B.J., WU H.L., LEE J.J., HAHN L.J., JENG J.H. Inducing the cell cycle arrest and apoptosis of oral KB carcinoma cells by hydroxychavicol: roles of glutathione and reactive oxygen species. Br. J. Pharmacol. 2002;135:619–630. doi: 10.1038/sj.bjp.0704492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANG M.C., WU H.L., LEE J.J., LEE P.H., CHANG H.H., HAHN L.J., LIN B.R., CHEN Y.J., JENG J.H. The induction of PGE2 production, IL-6 production, cell cycle arrest and cytotoxicity in primary oral keratinocytes and KB cancer cells by areca nut ingredients is differentially regulated by MEK/ERK activation. J. Biol. Chem. 2004;279:50676–50683. doi: 10.1074/jbc.M404465200. [DOI] [PubMed] [Google Scholar]
- CONANT K., HAUGHLEY N., NATH A., ST HILLAIRE C., GARY D.S., PARDO C.A., WAHL L.M., BILAK M., MILWARD E., MATTSON M.P. Matrix metalloproteinase-1 activates a pertissis toxin-sensitive signaling pathway that stimulates the release of matrix metalloproteinase-9. J. Neurochem. 2002;82:885–893. doi: 10.1046/j.1471-4159.2002.01038.x. [DOI] [PubMed] [Google Scholar]
- COUGHLIN S.R., CAMERER E. Participation in inflammation. J. Clin. Invest. 2003;111:25–27. doi: 10.1172/JCI17564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEKHUIJZEN P.N. Antioxidant properties of N-acetylcysteine: their relevance in relation to chronic obstructive pulmonary disease. Eur. Respir. J. 2004;23:629–636. doi: 10.1183/09031936.04.00016804. [DOI] [PubMed] [Google Scholar]
- EHRLICH H.P., BUTTLE D.J., BERNANKE D.H. Physiological variables affecting collagen lattice contraction by human dermal fibroblasts. Exp. Mol. Pathol. 1989;50:220–229. doi: 10.1016/0014-4800(89)90033-6. [DOI] [PubMed] [Google Scholar]
- EHRLICH H.P., ROCKWELL W.B., CORNWELL T.L., TAJARATNAM J.B. Demonstration of a direct role for myosin light chain kinase in fibroblast populated collagen lattice contraction. J. Cell Physiol. 1991;146:1–7. doi: 10.1002/jcp.1041460102. [DOI] [PubMed] [Google Scholar]
- EMMERT D.A., FEE J.A., GOECKELER Z.M., GROJEAN J.M., WAKATSUKI T., ELSON E.L., HERRING B.P., GALLAGHER P.J., WYSOLMERSKI R.B. Rho-kinase-mediated Ca2+-independent contraction in rat embryo fibroblasts. Am. J. Physiol. 2003;286:C8–C21. doi: 10.1152/ajpcell.00428.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOEL R., PHILLIPS-MASSON P.J., GARDNER A., RABEN D.M., BALDASSARE J.J. Alpha-thrombin-mediated phosphatidyl inositol 3-kinase activation through release of Gbetagamma dimmers from Galphaq and Galphai2. J. Biol. Chem. 2004;279:6701–6710. doi: 10.1074/jbc.M308753200. [DOI] [PubMed] [Google Scholar]
- GRINNELL F. Fibroblasts, myofibroblasts and wound contraction. J. Cell Biol. 1994;124:401–404. doi: 10.1083/jcb.124.4.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRINNELL F. Fibroblast-collagen-matrix contraction: growth-factor signalling and mechanical loading. Trends Cell Biol. 2000;10:362–365. doi: 10.1016/s0962-8924(00)01802-x. [DOI] [PubMed] [Google Scholar]
- HOESCH R.E., YIENGER K., WEINREICH D., KAO J.P.Y. Co-existence of functional IP3 and ryanodine receptors in vagal sensory neurons and their activation by ATP. J. Neurophysiol. 2002;88:1212–1219. doi: 10.1152/jn.2002.88.3.1212. [DOI] [PubMed] [Google Scholar]
- HOU L., ROVENALL S., MACEY M.G., HARRIOTT P., KAPAS S., HOWELLS G.L. Protease-activated receptors and their role in IL-6 and NF-IL-6 expression in human gingival fibroblasts. J. Periodont. Res. 1998;33:205–211. doi: 10.1111/j.1600-0765.1998.tb02192.x. [DOI] [PubMed] [Google Scholar]
- IMAMURA T., BANBULA A., PEREIRA P.J., TRAVIS J., POTEMPA J. Activation of human prothrombin by arginine-specific cysteins proteinases (Gingipains R) from Porphyromonas gingivalis. J. Biol. Chem. 2001;276:18984–18991. doi: 10.1074/jbc.M006760200. [DOI] [PubMed] [Google Scholar]
- ITO M., NAKANO T., ERDODI F., HARTSHORNE D.J. Myosin phosphatase: structure, regulation and function. Mol. Cell. Biochem. 2004;259:197–209. doi: 10.1023/b:mcbi.0000021373.14288.00. [DOI] [PubMed] [Google Scholar]
- JENG J.H., CHAN C.P., HO Y.S., LAN W.H., HSIEH C.C., CHANG M.C. Effects of butyrate and propionate on the adhesion, growth, cell cycle kinetics and protein synthesis of cultured human gingival fibroblasts. J. Periodontol. 1999;70:1435–1442. doi: 10.1902/jop.1999.70.12.1435. [DOI] [PubMed] [Google Scholar]
- JENG J.H., CHAN C.P., WU H.L., HO Y.S., LEE J.J., LIAO C.H., CHANG Y.K., CHANG H.H., CHEN Y.J., CHANG M.C. Protease-activated receptor-1-induced calcium signaling in gingival fibroblasts is mediated by sarcoplasmic reticulum calcium release and extracellular calcium influx. Cell. Signal. 2004a;16:731–740. doi: 10.1016/j.cellsig.2003.11.008. [DOI] [PubMed] [Google Scholar]
- JENG J.H., WANG Y.J., CHANG W.H., WU H.L., LI C.H., UANG B.J., KANG J.J., LEE J.J., HAHN L.J., LIN B.R., CHANG M.C. Reactive oxygen species are crucial for hydroxychavicol toxicity toward KB epithelial cells. Cell. Mol. Life Sci. 2004b;61:83–96. doi: 10.1007/s00018-003-3272-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENG J.H., WANG Y.J., CHIANG B.L., LEE P.H., CHAN C.P., HO Y.S., WANG T.M., LEE J.J., HAHN L.J., CHANG M.C. Role of keratinocytes inflammation in oral cancer: regulating the prostaglandin E2, interleukin-6 and TNF-alpha production of oral epithelial cells by areca nut extract and arecoline. Carcinogenesis. 2003;28:1301–1315. doi: 10.1093/carcin/bgg083. [DOI] [PubMed] [Google Scholar]
- KASSOLIS J.D., ROSEN P.S., REYNOLDS M.A. Alveolar ridge and sinus sugmentation utilizing platelet-rich plasma in combination with freeze-dried bone allograft: case series. J. Periodontol. 2000;71:1654–1661. doi: 10.1902/jop.2000.71.10.1654. [DOI] [PubMed] [Google Scholar]
- KATOH K., KANO Y., AMANO M., KAIBUCHI K., FUJIWARA K. Stress fiber organization regulated by MLCK and Rho-kinase in cultured human fibroblasts. Am. J. Physiol. 2001a;280:C1669–C1679. doi: 10.1152/ajpcell.2001.280.6.C1669. [DOI] [PubMed] [Google Scholar]
- KATOH K., KANO Y., AMANO M., ONISHI H., KAIBUCHI K., FUJIWARA K. Rho-kinase-mediated contraction of isolated stress fibers. J. Cell Biol. 2001b;153:569–584. doi: 10.1083/jcb.153.3.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOHYAMA T., WYATT T.A., LIU X., WEN F.Q., KOBAYASHI T., FANG Q., KIM H.J., RENNARD S.I. PGD2 modulates fibroblast-mediated native collagen gel contraction. Am. J. Respir. Cell. Mol. Biol. 2002;27:375–381. doi: 10.1165/rcmb.4830. [DOI] [PubMed] [Google Scholar]
- KOLODNEY M.S., THIMGAN M.S., HONDA H.M., TSAI G., YEE H.F. Ca2+-independent myosin II phosphorylation and contraction in chicken embryo fibroblasts. J. Physiol. 1999;515:87–92. doi: 10.1111/j.1469-7793.1999.087ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOTAH K., KANO Y., AMANO M., ONISHI H., KAIBUCHI K., FUJIWARA K. Rho-kinase-mediated contraction of isolated stress fibers. J. Cell Biol. 2001;153:569–583. doi: 10.1083/jcb.153.3.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE D.J., ROSENFELDT H., GRINNELL F. Activation of ERK and p38 MAP kinases in human fibroblasts during collagen matrix contraction. Exp. Cell Res. 2000;257:190–197. doi: 10.1006/excr.2000.4866. [DOI] [PubMed] [Google Scholar]
- LEE S.B., CHO E.S., YANG H.S., KIM H., UM H.D. Serum withdrawal kills U937 cells by inducing a positive mutual interaction between reactive oxygen species and phosphoinositide 3-kinase. Cell. Signal. 2005;17:197–204. doi: 10.1016/j.cellsig.2004.07.001. [DOI] [PubMed] [Google Scholar]
- LEVINSON H., MOYER K.E., SAGGERS G.C., EHRLICH H.P. Calmodulin–myosin light chain kinase inhibition changes fibroblast-populated collagen lattice contraction, cell migration, focal adhesion formation and wound contraction. Wound Repair Regen. 2004;12:505–511. doi: 10.1111/j.1067-1927.2004.012502.x. [DOI] [PubMed] [Google Scholar]
- LIU C.Y., MOSSEL H.L., KAPLAN K.L. The binding of thrombin by fibrin. J. Biol. Chem. 1979;254:10421–10425. [PubMed] [Google Scholar]
- LOURBAKOS A., YUAN Y.P., JENKINS A.L., TRAVIS J., ANDRADE-GORDON P., SANTULLI R., POTEMPA J., PIKE R.N. Activation of protease-activated receptors by gingipains from Prophyromonas gingivalis leads to platelet aggregation: a new trait in mivrobial pathogenicity. Blood. 2001;97:3790–3797. doi: 10.1182/blood.v97.12.3790. [DOI] [PubMed] [Google Scholar]
- MARAGOUDAKIS M.E., KRANITI N., GIANNOPOULOU E., ALEXOPOULOS K., MATSOUKAS J. Modulation of angiogenesis and progelatinase A by thrombin receptor mimetics and antagonist. Endothelium. 2001;8:195–205. doi: 10.1080/10623320109051565. [DOI] [PubMed] [Google Scholar]
- NGUYEN Q., FAIVRE S., BRUYNEEL E., RIVAT C., SETO M., ENDO T., MAREEL M., EMAMI S., GESPACH C. RhoA- and RhoD-dependent regulatory switch of G subunit signaling by PAR-1 receptors in cellular invasion. FASEB J. 2004;16:565–576. doi: 10.1096/fj.01-0525com. [DOI] [PubMed] [Google Scholar]
- NOBE H., NOBE K., FAZAL F., DE LANEROLLE P., PAUL R.J. Rho kinase mediates serum-induced contraction in fibroblast fibers independent of myosin LC20 phosphorylation. Am. J. Physiol. 2003;284:C599–C606. doi: 10.1152/ajpcell.00188.2002. [DOI] [PubMed] [Google Scholar]
- OAK M.H., EL BEDOUI J., ANGLARD P., SCHINI-KERTH V.B. Red wine polyphenolic compounds strongly inhibit pro-matrix metalloproteinase-2 expression and its activation in response to thrombin via direct inhibition of membrane type 1-matrix metalloproteinase in vascular smooth muscle cells. Circulation. 2004;110:1861–1867. doi: 10.1161/01.CIR.0000142617.52881.F4. [DOI] [PubMed] [Google Scholar]
- PANDEY S., LOPEZ C., JAMMU A. Oxidative stress and activation of proteasome protease during serum deprivation-induced apotosis in rat hepatoma cells: inhibition of cell death by melatonin. Apoptosis. 2003;8:497–508. doi: 10.1023/a:1025542424986. [DOI] [PubMed] [Google Scholar]
- PILOT T., MIYAZAKI H. Periodontal conditions in Europe. J. Clin. Periodontol. 1991;18:353–357. doi: 10.1111/j.1600-051x.1991.tb02300.x. [DOI] [PubMed] [Google Scholar]
- PRICHARD C.A., HAYES L., WOJNOWSKI L., ZIMMER A., MARAIS R.M., NORMAN J.C. B-Rad acts via ROCKII/LIMK/cofilin pathway to maintain actin stress fibers in fibroblasts. Mol. Cell. Biol. 2004;24:5937–5952. doi: 10.1128/MCB.24.13.5937-5952.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RASMUSSEN U.B., VOURET-CRAVIARI V., JALLAT S., SCHLESINGER Y., PAGES G., PAVIRANI A., LECOQ J.P., POUYSSEGUR J., VAN OBBERGHEN-SCHILLING E. cDNA cloning and expression of a hamster α-thrombin receptor coupled to Ca2+ mobilization. FEBS Lett. 1991;288:123–128. doi: 10.1016/0014-5793(91)81017-3. [DOI] [PubMed] [Google Scholar]
- SABRI S., JANDROT-PERRUS M., BERTOGLIO J., FARNDALE R.W., MAS V.M., DEBILI N., VAINCHENKER W. Differential regulation of actin stress fiber assembly and proplatelet formation by alpha2beta1 integrin and GPVI in human megakaryocytes. Blood. 2004;104:3117–3125. doi: 10.1182/blood-2003-12-4398. [DOI] [PubMed] [Google Scholar]
- SCHWARTZ M. Rho signaling at a glance. J. Cell Sci. 2004;117:5457–5458. doi: 10.1242/jcs.01582. [DOI] [PubMed] [Google Scholar]
- SLADE G.D., BECK J.D. Plausibility of periodontal disease estimates from NHANES III. J. Public Health Dent. 1999;59:67–72. doi: 10.1111/j.1752-7325.1999.tb03237.x. [DOI] [PubMed] [Google Scholar]
- SUMIYOSHI K., NAKAO A., SETOGUCHI Y., OKUMURA K., TSUBOI R., OGAWA H. Smads regulate collagen gel contraction by human dermal fibroblasts. Br. J. Dermatol. 2003;149:464–470. doi: 10.1046/j.1365-2133.2003.05490.x. [DOI] [PubMed] [Google Scholar]
- SUNDQVIST G., ROSENQUIST J.B., LERNER U.H. Effects of bradykinin and thrombin on prostaglandin formation, cell proliferation and collagen biosynthesis in human dental pulp fibroblasts. Arch. Oral Biol. 1995;40:247–256. doi: 10.1016/0003-9969(95)98813-e. [DOI] [PubMed] [Google Scholar]
- TAKATA T. Oral wound healing concepts in periodontology. Curr. Opin. Periodontol. 1994;3:119–127. [PubMed] [Google Scholar]
- TANAKA N., MORITA T., NEZU A., TANIMURA A., MIZOGUCHI I., TOJYO Y. Thrombin-induced Ca2+ mobilization in human gingival fibroblasts is mediated by protease-activated receptor-1 (PAR-1) Life Sci. 2003;73:301–310. doi: 10.1016/s0024-3205(03)00269-8. [DOI] [PubMed] [Google Scholar]
- TANAKA N., MORITA T., NEZU A., TANIMURA A., MIZOGUCHI I., TOJYO Y. Signaling mechanisms involved in protease-activated receptor-1-mediated interleukin-6 production by human gingival fibroblasts. J. Pharmacol. Exp. Ther. 2004;311:778–786. doi: 10.1124/jpet.104.068569. [DOI] [PubMed] [Google Scholar]
- TATAKIS D.N. Blood coagulation factors in periodontal patho-physiology: a review with emphasis on the role of thrombin. Semin. Thromb. Hemostas. 1992;18:28–33. doi: 10.1055/s-2007-1002407. [DOI] [PubMed] [Google Scholar]
- TAYLOR C.W., BROAD L.M. Pharmacological analysis of intracellular Ca2+ signaling: problems and pitfalls. Trends Pharmacol. Sci. 1998;19:370–375. doi: 10.1016/s0165-6147(98)01243-7. [DOI] [PubMed] [Google Scholar]
- TOTSUKAWA G., WU Y., SASAKI Y., HARTSHORNE D.J., YAMAKITA Y., TAMASHIRO S., MATSUMURA F. Distinct roles of MLCK and ROCK in the regulation of membrane protrusions and focal adhesion dynamics during cell migration of fibroblasts. J. Cell Biol. 2004;164:427–439. doi: 10.1083/jcb.200306172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOZUM T.F., DEMIRALP B. Platelet-rich plasma: a promising innovation in dentistry. J. Can. Dent. Assoc. 2004;69:664. [PubMed] [Google Scholar]
- VU T.-K.H., HUNG D.T., WHEATON V.I., COUGHLIN S.R. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991;64:1057–1068. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- WACHOWICZ B., OLAS B., ZHIKOWSKA H.M., BUCZYNSKI A. Generation of reactive oxygen species in blood platelets. Platelets. 2002;13:175–182. doi: 10.1080/09533710022149395. [DOI] [PubMed] [Google Scholar]
- WANG Z., CASTRESANA M.R., NEWMAN W.H. Reactive oxygen species-sensitive p38 MAPK controls thrombin-induced migration of vascular smooth muscle cells. J. Mol. Cell Cardiol. 2004;36:49–56. doi: 10.1016/j.yjmcc.2003.09.014. [DOI] [PubMed] [Google Scholar]
- XIA H., NHO R.S., KAHM J., KLEIDON J., HENKE C.A. Focal adhesion kinase is upstream of phosphatidylinositol 3-kinase/Akt in regulating fibroblast survival in response to contraction of type I collagen matrices via a beta 1 integrin viability signaling pathway. J. Biol. Chem. 2004;279:33024–33034. doi: 10.1074/jbc.M313265200. [DOI] [PubMed] [Google Scholar]