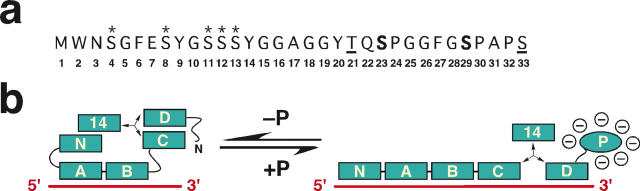A dynamic model for replication protein A (RPA) function in DNA processing pathways (original) (raw)
Abstract
Processing of DNA in replication, repair and recombination pathways in cells of all organisms requires the participation of at least one major single-stranded DNA (ssDNA)-binding protein. This protein protects ssDNA from nucleolytic damage, prevents hairpin formation and blocks DNA reannealing until the processing pathway is successfully completed. Many ssDNA-binding proteins interact physically and functionally with a variety of other DNA processing proteins. These interactions are thought to temporally order and guide the parade of proteins that ‘trade places’ on the ssDNA, a model known as ‘hand-off’, as the processing pathway progresses. How this hand-off mechanism works remains poorly understood. Recent studies of the conserved eukaryotic ssDNA-binding protein replication protein A (RPA) suggest a novel mechanism by which proteins may trade places on ssDNA by binding to RPA and mediating conformation changes that alter the ssDNA-binding properties of RPA. This article reviews the structure and function of RPA, summarizes recent studies of RPA in DNA replication and other DNA processing pathways, and proposes a general model for the role of RPA in protein-mediated hand-off.
INTRODUCTION
Replication protein A (RPA) was identified as a heterotrimeric single-stranded DNA (ssDNA)-binding protein required for replication of simian virus 40 (SV40) DNA in vitro [for reviews see (1–6)]. RPA is now known to be essential for chromosomal DNA replication, repair and recombination pathways in eukaryotic cells, and new roles in DNA damage signaling and regulation of replication origin firing frequency are emerging (7–15). RPA functions to protect ssDNA from nucleases and prevent hairpin formation in ssDNA that would interfere with DNA processing, but it also appears to actively coordinate the sequential assembly and disassembly of DNA processing proteins on ssDNA (16,17). The ability of RPA to guide DNA processing depends in large part on RPA interactions with other proteins in each pathway. Although these mechanisms are not yet well understood, we will review here several examples and discuss possible models for protein-mediated RPA conformation changes that may underlie its assembly and disassembly on ssDNA.
RPA: A MODULAR PROTEIN WITH MULTIPLE CONFORMATIONS
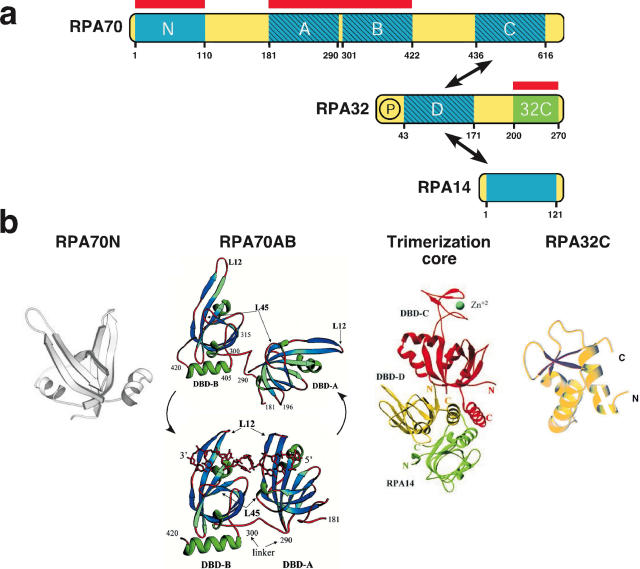
RPA is a stable complex of three subunits RPA70, RPA32 and RPA14 that are conserved among eukaryotes (Figure 1a). The 3D structures of RPA fragments reveal six domains that adopt an oligonucleotide binding (OB)-fold, a structure common to other known SSBs (13,18) (Figure 1b). Zinc binding in the C-terminal OB-fold of RPA70 is important for RPA structural stability and ssDNA binding (19). RPA32C adopts a winged-helix–turn–helix fold (20). Flexible linkers join the domains in each subunit and the two small subunits interact with RPA70C through a 3-helix bundle. However, the quaternary structure(s) of RPA remains elusive.
Figure 1.
The modular structure of RPA. (a) Schematic diagram: arrows indicate intersubunit associations; protein-binding domains are denoted by red bars; ssDNA binding domains A–D by hatching; OB-folds by blue boxes; linkers by yellow boxes; winged helix by a green box; phosphoamino acid cluster by a circled P [adapted from (29) with permission]. (b) Structural models of RPA domains. [Reprinted from (20,24,25,55) with permission.]
RPA binds tightly to ssDNA with a defined 5′→3′ polarity (21,22), and an affinity of up to ∼10−9–10−10 M (23). RPA contains four ssDNA-binding domains (A–D in order of decreasing affinities), three in the RPA70 subunit, tethered to each other through flexible linkers, and one in the RPA32 subunit. RPA binds to ssDNA in at least three different modes characterized by the length of ssDNA that it contacts (8–10, 12–23 and 28–30 nt) and the number of ssDNA-binding domains involved (24–26). In the 30 nt binding mode, the 5′ end of the binding site is occupied by RPA70A and the 3′ end by RPA32D (21,22,24,27–29). The N-terminal OB-fold of RPA70 (RPA70N) also has weak (mM) ssDNA-binding affinity and may contribute to regulation of ssDNA-binding mode under some conditions (30–32). The three ssDNA-binding modes of heterotrimeric RPA imply that the protein can adopt three different structural conformations. Indeed scanning transmission electron micrographs and gel filtration demonstrate RPA molecules in compact and extended conformations on ssDNA (33). These studies suggest that all three ssDNA-binding modes co-exist in solution, perhaps in equilibrium (33), but the intramolecular structural re-organization of RPA domains that gives rise to the three binding modes remains unknown.
A great deal of insight into RPA interactions with ssDNA has been obtained from crystal structures of RPA70AB determined in the presence and absence of dC8 (25,34) (Figure 1b). In the DNA-free state, two different relative orientations of the 70A and 70B domains were found, one of them with an unstructured linker between OB-fold A and B (25), suggesting that the linker between the A and B domains is flexible. This flexibility has been independently confirmed by NMR studies of RPA70AB in solution (29). In the presence of ssDNA, OB-fold domains A and B align in a fixed orientation with the linker parallel to the bound oligonucleotide, but on the opposite side of the protein (34). Binding of RPA70AB to ssDNA is accompanied by conformational changes, in which two extended loops in each domain close like ‘fingers’ around the DNA. The structural re-organization within each domain and the change in dynamics between the domains imply that RPA must pay a significant entropic penalty to bind ssDNA.
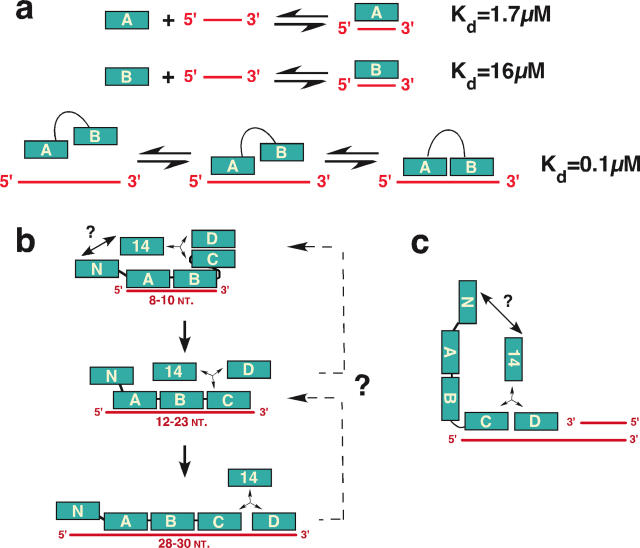
Binding to ssDNA in the 30 nt binding mode is thought to progress sequentially from 5′ to 3′, beginning with the RPA70 domains A and B in an initial 10 nt binding mode (Figure 2a). The ssDNA-binding affinity of these individual DNA-binding domains is quite weak. RPA70A binds to ssDNA with greater affinity than the other ssDNA-binding domains (_K_d ∼ 2 μM), but because only a short linker separates RPA70A and RPA70B, the local concentration of 70B is high when 70A binds to ssDNA (29). This leads to ssDNA binding of both domains. In the heterotrimeric RPA molecule, this chemical linkage between the weak individual binding domains enhances the overall affinity of RPA for an ssDNA molecule by several orders of magnitude (29), progressing to the high-affinity-binding mode that involves all four major ssDNA-binding domains (21,22,24,25,28,29) (Figure 2b). Thus RPA ‘unrolls’ readily on ssDNA, forming a stable complex that occludes ∼30 nt, stabilizes the ssDNA against nuclease digestion and stabilizes the protein against proteolytic digestion.
Figure 2.
(a) Covalent linkage of RPA70 ssDNA-binding domains A and B enhances their affinity to ssDNA. Binding constants of A or B with d(CTTCA) and AB with d(CTTCA CTTCA) were determined (29). (b) Sequential 5′→3′ binding of RPA to ssDNA. Positioning of RPA70N and RPA14 relative to other domains is speculative (24,103). Dashed lines depict a potential pathway for RPA displacement from ssDNA. (c) Schematic diagram of the primer–template junction-binding mode of the RPA trimerization core (70C-32D-14) (38).
DNA processing involves not only RPA binding to ssDNA substrates as discussed above, but also to partial duplexes with a variety of structures. Photoaffinity labeling studies with partial duplex DNA uncovered a novel binding mode in which RPA32D and 70C bind to the 3′-OH of a partial duplex and to a 5′-single-stranded overhanging end (35–37) (Figure 2c). Labeling of RPA32D predominated using 3′OH photoaffinity labels with short crosslinker spacers, while RPA70C was labeled using longer spacers, indicating the relative proximity of the two domains to the 3′OH at the primer–template junction. Strikingly, the RPA trimerization core (RPA70C-32D-14) alone was sufficient for this binding mode on partial duplex with a 5′ ssDNA overhang of either 10 or 30 nt (24,38), implying that intact RPA may also use this binding mode to bind to primer–template junctions or DNA with single-stranded gaps of <30 nt. The observation that the junction-binding mode does not utilize RPA70AB raises the question of whether an RPA molecule in the 8–10 nt ssDNA-binding mode (Figure 2b) may actually have two faces able to bind different DNA structures, one composed of RPA70AB and another composed of the trimerization core. Elucidation of RPA quaternary structures will be needed to answer the question.
RPA tightly bound to ssDNA during DNA processing must somehow be displaced to allow completion of the processing pathway and restoration of the base-paired DNA. How RPA dissociation occurs is not well understood, but one possibility is that the four ssDNA-binding domains dissociate sequentially in the reverse order, i.e. from the 3′ to the 5′ end of the ssDNA (Figure 2b). Given that RPA binds tightly to ssDNA, it seems likely that its complete dissociation from ssDNA requires the participation of other proteins in each DNA processing pathway. We suggest that these proteins bind to RPA, inducing a change in its conformation to a weaker binding mode and thereby facilitating its dissociation from ssDNA.
PROTEIN-MEDIATED CONFORMATION CHANGES OF RPA
The number and variety of proteins that have been shown to bind to RPA is large and growing (10) (Table 1). Despite the variety of its binding partners, RPA appears to have only a limited number of regions available for protein interactions: three domains in RPA70 and one in RPA32 (Figure 1a). This raises the question of whether these interactions are specific, and if so, how the specificity for a given protein partner is generated. The contact surfaces in RPA have been elucidated for several of its binding partners, as discussed below, and these results are beginning to suggest that proteins from distinct processing pathways may use a small number of common patterns to bind RPA and remodel its DNA-binding mode (Table 1 and Figure 1a).
Table 1.
Physical interaction of RPA with DNA processing proteins
| Protein | RPA residuesa | Protein residuesa | Methodb | Reference |
|---|---|---|---|---|
| Activation induced Cytidine Deaminase | RPA32 | NYD | e | (104) |
| Uracil-DNA glycosylase | RPA32163–217 | 29–75, N-terminus | cd | (20,105,106) |
| Rad52 | RPA70169–326 | 221–280 | ac | (107,108) |
| RPA32224–271 | b | (20) | ||
| SV40 T antigen | RPA70181–327 | Origin binding domain 131–249 | ab | (46,109) |
| RPA32C | c | (47,48,61) | ||
| XPA | RPA70183–296 | 20–46 | be | (20,62,110) |
| RPA32C | a | (105,111–115) | ||
| p53 | RPA70N1–120 | 38–58 | ace | (30–32,57–59) |
| ATRIP | RPA70N | 1–107, other? | e | (14,73,74) |
| FACT | RPA70N | Pob3237–477 | ade | (60) |
| Rad51 | RPA70181–291 | 1–93 | ac | (63,81) |
| Werner syndrome helicase | RPA70168–308 | N-terminal acidic region, C-terminus | abd | (65,66) |
| Bloom syndrome helicase | RPA70168–308 | N-terminal acidic region, C-terminus | abd | (66) |
| Papillomavirus E1 | RPA70181–291 | NYD | ab | (46,47) |
| Parvovirus NS1 | RPA70, RPA32 | NYD | b | (116) |
| Pol-prim | RPA701–327 | Primase p48/58 | ab | (45,64,67) |
| RPA32/14? | ||||
| RFC | RPA70 | p140, p40, p28 | b | (16,117) |
| Rad9 | RPA70, RPA32 | NYD | a | (72) |
| Rad17 | RPA70, RPA32/14 | NYD | ae | (70,71) |
| p53BP1 | RPA70, RPA32 | NYD | a | (75) |
| Nucleolin | RPA14 | 645–707 | ae | (78,79) |
| BRCA2 | NYD | N-terminus | ae | (76) |
| XPG | NYD | NYD | ae | (22,118,119) |
| XPF–ERCC1 | NYD | NYD | ae | (22,119,120) |
| Mre11–Rad50–Nbs1 | NYD | NYD | e | (77) |
RPA32C interactions
Protein interactions with the C-terminal winged helix domain in RPA32 (RPA32C) have been relatively well characterized structurally. RPA32C binds to the DNA recombination protein Rad52, the base excision repair protein uracil DNA glycosylase 2 and the nucleotide excision repair protein XPA [(20) and references therein]. These interactions are weak (_K_d ∼5–10 μM) but specific. Remarkably, all three of these proteins target the same surface of RPA32C and contain an alpha helix that interacts with RPA32C (20). The conserved nature of these interactions suggests that RPA32C may serve a common function in at least three different DNA processing pathways. Consistent with biological functions for RPA32C in DNA repair pathways, an RPA32C truncation mutant in budding yeast displays mutator and hyper-recombination phenotypes (39).
Whether DNA replication proteins interact with RPA32C has been controversial. Multiple lines of evidence in the literature implicate RPA32 in SV40 DNA replication. Antibodies against RPA32 specifically inhibit SV40 replication in vitro (40,41). In the context of trimeric RPA, RPA32 can be directly cross-linked to nascent RNA–DNA primers (42) and the RPA trimerization core alone (RPA70C–RPA32D–RPA14) was shown recently to bind to a primer–template junction (38) (Figure 2c). Binding of human RPA32 to the viral replicative helicase T antigen has been reported previously (43,44), but not confirmed by others (45–47). Similarly, RPA mutants with deletions in the C-terminal domain of RPA32 supported SV40 replication poorly in one study, but displayed nearly wild-type activity in another investigation (44,45).
To resolve the controversy, the physical interaction of RPA32C with SV40 T antigen was re-examined recently in detail (48). NMR studies of RPA32C interaction with the origin DNA-binding domain of T antigen (residues 131–259) revealed that T antigen binds to virtually the same surface of RPA32C bound by DNA repair and recombination proteins (20). However, the T antigen surface involved in the interaction is composed primarily of two loops rather than the alpha helix used by the repair and recombination proteins. RPA32C-T antigen binding is weak (_K_d ∼ 60 μM) and relies in part on electrostatic interactions between acidic residues in RPA32C and basic residues in T antigen. Charge reverse mutations in either protein reduced the binding affinity by an order of magnitude. Interestingly, the charge reverse mutations in RPA32C also strongly reduced the ability of T antigen to stimulate primer synthesis on RPA-coated ssDNA, but did not inhibit primer extension. Taken together, the data confirm that RPA32C interaction with T antigen is important for primosome activity in SV40 DNA replication, provide insight into the structural interaction and suggest that T antigen may play a role in displacing RPA from ssDNA with a 3′ → 5′ polarity, permitting DNA polymerase-primase to begin replication.
RPA bound to ssDNA ahead of an elongating DNA polymerase on a primed template must also be displaced, but the mechanism is not known. It seems likely that the binding mode of RPA at a primer–template junction may be one that utilizes primarily the trimerization core of RPA, with RPA70C and RPA32D contacting the 3′OH of the primer and the ssDNA template (38). The RPA14 subunit plays a crucial role in stabilizing the trimerization core for interaction with the partial duplex, as a mutant RPA lacking the 14 kDa subunit did not properly recognize the 3′ end of the primer at the primer–template junction or support primer extension in the SV40 replication system (49). Notably, the mutant RPA also failed to support primer synthesis, implying that T antigen-mediated RPA displacement or polymerase-primase loading requires the intact trimerization core, as well as interaction between RPA32C and T antigen. Although the detailed role of the trimerization core in these events remains to be determined, it seems reasonable to speculate that it is important to form or stabilize the compact conformation of RPA (Figure 2b).
These studies raise the question of whether RPA32C binding to other proteins (Table 1) may facilitate similar RPA displacement from ssDNA, coupled with loading of an incoming processing protein. One example of RPA involvement in displacing a DNA-bound protein and loading subsequent proteins is in nucleotide excision repair. RPA is required for global genome nucleotide excision repair (50) and cannot be substituted by other ssDNA-binding proteins, consistent with the specific interactions of RPA with excision repair proteins XPA, XPG and XPF-ERCC1 (Table 1). XPA and RPA bind and stabilize the open complex after damage-recognition by XPC-hHR23B and controlled local separation of the two strands by TFIIH (51). The joint recognition of a damage site by XPA and RPA has been suggested to serve as a ‘double-check’ before the assembly of excision endonucleases at the site (52). XPA binding to RPA at the DNA lesion is also crucial for the ability of XPA–RPA to displace XPC–hHR23B from DNA before the assembly of XPG and XPF-ERCC1 endonucleases (53,54). The polarity of RPA bound to the undamaged strand appears to spatially coordinate the assembly of XPG on the 3′ end of the damaged strand and XPF–ERCC1 on the 5′ end (22). Excision of the damaged strand leads to RPA bound to a gapped DNA, positioned such that it would interact through RPA70C and RPA32D with the 3′OH at the gap and the undamaged strand (Figure 2c) to coordinate assembly of RFC, PCNA and DNA polymerase delta to repair the gap (16).
RPA70 interactions
Mapping of protein interaction sites in RPA70 has been challenging due to its complex domain structure and the multiple linkers (Figure 1). Three protein interaction modules have been identified so far: the N-terminal domain RPA70N (residues 1–120), RPA70A and residues 168–308/327, which span domain 70A (181–290), part of domain 70B, and the short linker between them (Table 1). The 70N and 70A interacting regions are connected by a long, apparently unstructured linker (residues 120–180) (55,56), but functional interaction of the linker with other proteins has, to our knowledge, not been observed.
The 70N domain (residues 1–120) assumes an OB-fold, as determined by NMR (55) and X-ray crystallography (32). Although it binds weakly to ssDNA (30,32), it lacks the conserved aromatic residues that confer high-affinity ssDNA binding. However, RPA70N interacts physically with the tumor suppressor p53 (57–59). The structural basis of this interaction has been characterized recently in detail (32). RPA70N binds to the transactivation domain of p53 (residues 38–57), inducing the formation of two amphipathic helices. Hydrophobic residues in helix 2 of the p53 peptide bind to 70N, while the acidic residues on the opposite face of the helix are exposed to solvent. This interaction mimics those described previously for weak binding of RPA70N to ssDNA and a pseudo-phosphorylated mutant RPA32N peptide (30–32). Interestingly, these ligands compete directly with p53 for binding to 70N, leading Bochkareva et al. (32) to speculate that this competition may be one component of a threshold-sensitive response to DNA damage. Exposure of p53–RPA complex to either damage-activated protein kinases that hyperphosphorylate RPA32N or to large amounts of ssDNA would stimulate release of active p53.
RPA70N was also shown recently to interact with the nucleosome remodeling complex FACT (facilitates chromatin transcription) (60). This phylogenetically conserved complex is required for both transcription and replication of nucleosomal DNA. Genetic analysis in yeast indicates that the requirement for FACT in transcription is genetically separable from that in chromatin replication. The Pob3 subunit of yeast FACT binds specifically to the basic cleft of RPA70N based on detailed structural models, genetic and biochemical analysis. FACT interaction with RPA appears to be important in vivo for deposition of acetylated histones H3 and H4 in nucleosomes on newly replicated DNA, but the details of this process and the role that RPA plays in it remain unknown.
RPA70A alone has been reported to be sufficient to bind papillomavirus E1 helicase (47), SV40 T antigen (47,61), XPA (62) and human Rad51 recombinase (63). Binding of RPA70A to an acidic N-terminal peptide of human Rad51 (residues 1–93) has been investigated structurally in detail (63). The Rad51 peptide binds in the basic ssDNA-binding cleft of RPA70A, mimicking its interaction with ssDNA and suggesting a potential competition. Mutational analysis of Rad51 indicated that this interaction contributes to the ability of Rad51 to displace RPA from ssDNA to form a presynaptic Rad51–ssDNA filament. Mechanistically, Rad51N is proposed to capture an RPA molecule that dissociates from an overhanging 3′ ssDNA, spontaneously or mediated by another protein, thereby preventing its reassociation with ssDNA and positioning the bound Rad51 molecule to bind to the ssDNA through a separate domain. Once a few Rad51 molecules are loaded on the ssDNA, Rad51 filament formation driven by ATP hydrolysis leads to RPA displacement (63).
The largest protein interaction module of RPA70 spans domains 70A and B (residues 168–308/327) and is reported to interact with the primase subunits of DNA polymerase alpha-primase, the SV40 T antigen helicase and the RecQ family Werner and Bloom Syndrome helicases (Table 1) (45,64–67). There is evidence that these interactions have physiological importance. For example, RPA stimulated the DNA unwinding activity of Werner helicase fragments capable of binding to RPA (66). However, the RPA70 fragment 168–308/327 encompasses disordered peptides at both the N- and C-termini, raising the question of whether its physical interactions with other proteins are mediated through the structured portion of the RPA fragment, or whether physical interactions with a partner protein may structure the disordered regions of the RPA fragment.
RPA70 interactions with the primase subunits of DNA polymerase alpha-primase (45,64,67) are thought to aid polymerase-primase in primer–template binding or primer extension. Interestingly RPA enhances both the processivity and fidelity of primer extension by polymerase-primase (45,68). Since other ssDNA-binding proteins do not display this enhancement, specific RPA–primase interactions, the 3′primer–template junction-binding mode of RPA (Figure 2c) or both may be involved. RPA has thus been suggested to serve as a ‘fidelity clamp’ for polymerase-primase. The ability of RPA to facilitate primer extension by other DNA polymerases (lambda, delta) suggests that this function of RPA may be more general (16,40,69). Additional work will be required to elucidate the structural basis of RPA70 interaction with these binding partners and clarify its functional roles.
A number of other DNA damage signaling and processing proteins have been shown recently to interact directly or indirectly with RPA (Table 1): Rad17 clamp loading complex (70,71), Rad9 clamp subunit (72), ATRIP (14,73,74), 53BP1 (75), BRCA2 (76), Mre11–Rad50–Nbs1 (77) and nucleolin (78,79). Future characterization of these binding interactions with RPA and their mechanistic role in DNA processing should yield deeper understanding of DNA damage responses.
Competition and coordination among RPA-binding partners
Interestingly, a number of DNA processing proteins bind to both RPA70 and RPA32C (XPA, Rad52, SV40 T antigen) (Table 1). In Rad52 and T antigen, the same surface that binds to RPA32C also binds to RPA70. These observations raise new questions about the functional role of the dual interaction. Do these proteins interact with both RPA sites simultaneously to strengthen the complex, or do they interact with one site at a time, possibly with different functional consequences? How does ssDNA influence protein interaction with the two RPA sites?
The interaction of Rad52 with RPA in homologous recombination has been investigated in detail [for a review see (80)]. In yeast and in vertebrates, RPA assembles at double-strand breaks, preceding the association of Rad51 recombinase (81,82). Although it is not clear how the DNA at the break is resected to create 3′ ssDNA ends, RPA must be quickly loaded on the ssDNA, either through diffusion or a protein-mediated mechanism, preventing formation of secondary structures (83). Rad51 must then displace RPA to generate a recombinogenic Rad51–ssDNA filament. In yeast, Rad52 association with RPA plays an important role in mediating Rad51 assembly on RPA-coated ssDNA (84–86). The Rad52-mediated RPA–Rad51 exchange mechanism involves Rad52 binding to RPA–ssDNA to form a ternary complex (85). Since a separate domain of Rad52 binds to Rad51, it is possible that Rad52-mediated remodeling of RPA–ssDNA complexes from an extended binding mode into a compact one may facilitate Rad51 loading on the newly accessible ssDNA (for a possible model see Figure 3). Whether Rad52 binding to RPA32C or RPA70AB or both is required for Rad51 filament formation is not known. Once the Rad51–ssDNA complexes are formed, Rad51 interactions with RPA70A may accelerate filament formation as discussed above. In addition, the basic region of yeast RPA70N is essential for Rad51-mediated RPA displacement and strand invasion, perhaps by facilitating RPA remodeling (Figure 2b) (83,87). Vertebrate Rad52 also binds to RPA and can mediate Rad51 loading on RPA–ssDNA in vitro, but is not essential for Rad51 filament formation in vivo; instead, Rad51 paralogs appear to mediate Rad51 filament assembly through an unknown mechanism (80,84,85,88–90).
Figure 3.
(a–d) A model for protein-mediated RPA displacement from ssDNA in concert with loading of the next protein in the pathway [Reprinted from (48), with permission, Nature Publication Group]. For discussion see text.
In addition to mediating RPA displacement from ssDNA and loading of incoming proteins, DNA processing proteins may be capable of stimulating RPA binding to ssDNA. It has been speculated that RPA interaction with some DNA helicases may enable them to actively place RPA on ssDNA as it emerges from the helicase complex. Interaction of the Werner and Bloom syndrome helicases with RPA70 stimulates their unwinding activity on long duplexes, perhaps by facilitating RPA binding to ssDNA (65,66). If helicases do load RPA on ssDNA, the spatial orientation of RPA bound to the helicase, the polarity of helicase movement during strand displacement, and the structure or perhaps sequence of the DNA may govern whether RPA is loaded on ssDNA, the strand on which it loads, or the binding mode in which it is loaded (Figure 2b). Whether these helicases can also displace RPA from ssDNA to mediate loading of an incoming DNA processing protein (e.g. Figure 3) is not known. If so, one or more features of the protein–ssDNA complex must determine whether RPA is loaded or displaced.
A mechanistic model for protein-mediated RPA dissociation from DNA
The complexity of DNA processing pathways raises questions about how the individual reaction steps are ordered, and how rapid progression through the processing pathway is achieved. In the SV40 replication pathway, RPA has been proposed to coordinate the activities of the replication proteins through competition-based switches, in which proteins ‘trade places’ on ssDNA through specific RPA-binding sites (16). In each exchange, the next protein to enter into processing has a greater affinity for RPA than the preceding one, which allows it to compete for RPA on the ssDNA when the preceding protein dissociates. This successive exchange of replication proteins, known as the hand-off mechanism, is proposed to be a general mechanism in replication, repair and recombination pathways (17,20). However, the in vivo abundance of certain incoming proteins may not be sufficient to compete with the preceding protein in the pathway. Moreover, successively increasing the affinity of protein–protein interactions as pathway progresses could limit the rapid protein exchanges necessary to complete the pathway.
An alternative model for the protein hand-off mechanism would depend on the ability of RPA-interacting proteins to remodel the conformation of RPA from an extended, stable binding mode to a more compact form with lower affinity for ssDNA (Figure 2b). Table 1 reveals a general pattern of interactions in which some processing proteins contact both RPA32C and RPA70A or AB. This common pattern suggests that these RPA-binding partners may remodel RPA conformation in a similar manner. The protein-mediated remodeling of RPA could thus be coupled to its different ssDNA-binding modes (Figure 2b). The incoming protein that binds and induces RPA to shift from an extended to a compact conformation on ssDNA could then, in concert, gain access to the ssDNA. Since the C and D domains of RPA bound to the 3′ end of ssDNA have lower affinity for ssDNA, an incoming protein that binds to RPA32C might gain access to ssDNA at the 3′ end. Binding of RPA70AB to the same incoming protein could induce a compact weaker ssDNA-binding mode, allowing the incoming protein displace RPA and load either itself or a piggy-backed protein on to the ssDNA made accessible by the partially dissociated RPA.
In the case of SV40 T antigen-mediated primer synthesis on RPA-coated ssDNA, T antigen must bind to RPA to allow primer synthesis [(48) and references therein]. We suggest a model in which T antigen binds transiently to RPA, inducing a conformation change on ssDNA, and concurrently loads DNA polymerase-alpha-primase (Figure 3) (48). RPA domains C and D bound to the 3′ end of ssDNA undergo rapid association and dissociation. In the absence of T antigen, RPA rapidly reassociates with ssDNA, preventing DNA polymerase-alpha-primase from binding to the template (29). In the presence of hexameric T antigen helicase, polymerase-primase binds to the helicase domain of T antigen (91) and RPA32C binds to the origin DNA-binding domain of T antigen (Figure 3a). We suggest that RPA32C and RPA70AB interaction with the T antigen hexamer remodels the conformation of RPA to a compact, weaker DNA-binding mode, causing the release of a short stretch of ssDNA. In concert, the polymerase-primase bound to T antigen binds to the free ssDNA and begins primer synthesis (Figure 3b and c). Dissociation of the weakly bound RPA and T antigen facilitates primer extension by the polymerase-primase (Figure 3d). Although this model is still tentative, it is consistent with the data available in the literature, provides a plausible mechanistic explanation for hand-off and is amenable to further experimental testing.
Regulation of RPA structure and function by phosphorylation
It has been known for more than a decade that RPA becomes phosphorylated, primarily in the N-terminus of RPA32 (Figure 1a), in response to cell cycle progression or DNA damaging agents, but the structural and functional significance of the modifications is still not well understood [reviewed by (9,92)].
The N-terminal 33 residues of human RPA32 contain at least 7 sites that can be modified in vivo and in vitro (Figure 4a) and give rise to 4 differentially phosphorylated forms separable by denaturing gel electrophoresis (93,94). Cyclin-dependent kinases modify serine residues 23 and 29 in mitotic cells, reducing the ability of the phosphorylated RPA to interact with purified DNA polymerase alpha-primase, ATM and DNA-PK (93,95). DNA-PK, ATM and ATR are involved in modifying threonine 21 and serine 33 in response to various DNA damaging agents in vivo, leading to hyperphosphorylated forms of RPA (96,97). The hyperphosphorylated forms of RPA also contain phosphorylated serines 4 and 8, as well as at least one phosphoserine in residues 11–13, but the kinases that modify these sites in vivo are not yet known (98). Interestingly, the kinases required and the time course of RPA phosphorylation varied depending upon the damaging agent (97). Focus formation of RPA and gammaH2AX was ATR-dependent and occurred rapidly after camptothecin exposure, but RPA32 hyperphosphorylation occurred later and required DNA-PK activity. DNA-PK was also required for RPA hyperphosphorylation in UV-treated cells (99). Both ATR-dependent RPA focus formation and hyperphosphorylation occurred more slowly after hydroxyurea exposure, but hyperphosphorylation did not require DNA-PK (97). Rad51, Rad52 and ATR were reported to preferentially co-immunoprecipitate with hyperphosphorylated RPA from extracts of UV or camptothecin-treated human cells, but it is not clear whether these interactions with phospho-RPA are direct or indirect (72).
Figure 4.
Phosphorylation of RPA32N and its potential functional roles. (a) Diagram of the phosphoamino acid cluster in RPA32N. Boldface, sites phosphorylated by CDK; underlined, likely phosphorylated by PIKKs; asterisks, phosphorylated by unknown kinases. (b) Hyperphosphorylation of RPA32N is proposed to shift the equilibrium distribution of RPA conformation states/binding modes to favor the high-affinity extended mode.
Whether the specific sites phosphorylated in RPA correlate with specific changes in RPA structure or function is an important open question. For instance, mutation of RPA32 Thr21, Ser33 or both had little effect on camptothecin-induced RPA32 hyperphosphorylation (97). It is conceivable that mere induction of massive negative charge in RPA32N is sufficient to alter RPA structure and function. Based on this rationale, some efforts to elucidate the structural and functional significance of RPA hyperphosphorylation have employed mutant forms of RPA designed to mimic the hyperphosphorylated protein, with substitution of aspartate for phosphorylatable residues in RPA32N, or of alanine as a control (31,100,101). Negatively charged mutant RPA32N was excluded from chromosomal replication centers in human in a manner proportional to the negative charge, and the alanine-substituted mutant used as a control was not excluded (101,102). Moreover, hyperphosphorylated wild-type RPA also failed to co-localize with replication centers. These results are consistent with the reduced affinity of hyperphosphorylated RPA for DNA polymerase alpha-primase (93,95).
Does the negatively charged RPA32N also alter RPA structurally and if so, how? Early work on the ssDNA-binding modes of purified human RPA using scanning transmission electron microscopy established that RPA32N in the extended 30 nt binding mode on ssDNA was preferentially hyperphosphorylated by purified DNA-PK (33), implying that RPA32N might be more accessible in the extended binding mode and sequestered in the compact mode (Figure 4b). Given the uncharged nature of unmodified RPA32N, it is easy to imagine that gross introduction of negative charge might make it difficult to reverse the extended binding mode and re-sequester RPA32N. Hyperphosphorylation would thus be expected to shift the equilibrium distribution of RPA conformations to favor the extended binding mode, which binds to ssDNA with higher affinity than the compact modes (Figure 4b). Lower levels of hyperphosphorylated RPA in the compact binding mode could explain its exclusion from replicating chromatin, since the 8–10 nt and the primer-junction-binding modes are thought to play a central role in rapidly cycling RPA on and off ssDNA during replication (Figures 2 and 3). Moreover, the greater binding affinity of the extended conformation would ensure that ssDNA in cells exposed to DNA damaging agents remains shielded from hairpin formation or nucleases until completion of repair. Counter to these predictions, binding of hyperphosphorylated RPA to 8–30 nt ssDNA and primer–junction templates in vitro was either reduced or equal to that of unmodified RPA (31,93–95). This paradox remains unresolved. Similarly, recent in vitro studies have suggested that hyperphosphorylated RPA32N may compete with ssDNA, binding to the basic cleft in RPA70N (31,32) or in RPA70B (94). These findings are consistent with the interpretation that hyperphosphorylated RPA does not localize in chromosomal replication centers (101,102), but do not explain how ssDNA remains protected in cells that have suffered DNA damage. Thus, much important work remains to understand the effects of RPA32N hyperphosphorylation on the quaternary structure of RPA.
SUMMARY AND PERSPECTIVE
RPA is the common denominator in many DNA processing pathways and is found on ssDNA from the time it is created by a DNA helicase or nuclease until the duplex DNA is restored upon completion of each processing pathway. The dynamic nature of these pathways has favored the evolution of modular proteins with multiple domains capable of interacting with multi-valent ligands. The modular interaction mechanism allows these proteins to associate and dissociate readily from each other. RPA is a prime example of such a modular protein. Its ability to adopt at least three different conformations on ssDNA and shift from one to another under the guidance of DNA processing proteins that interact with it suggests that RPA remodeling plays a major role in guaranteeing the integrity of ssDNA and its orderly processing. Future studies of RPA will need to explore the quaternary structure of the protein, the intramolecular interactions between domains and the regulation of RPA interactions by protein phosphorylation. This work may reveal in greater detail how this RPA remodeling takes place and whether general patterns of RPA remodeling may be conserved from one pathway to another.
Acknowledgments
We thank W. Chazin, A. Bochkarev, D. Cortez and the members of the Fanning lab for discussion, and NIH (GM52948), HHMI Professors Program and Vanderbilt University for support. We apologize to our co-workers whose work could not be cited due to space limitations. The Open Access publication charges for this article were waived by Oxford University Press.
Conflict of interest statement. None declared.
REFERENCES
- 1.Fanning E., Knippers R. Structure and function of simian virus 40 large tumor antigen. Ann. Rev. Biochem. 1992;61:55–85. doi: 10.1146/annurev.bi.61.070192.000415. [DOI] [PubMed] [Google Scholar]
- 2.Bullock P.A. The initiation of simian virus 40 DNA replication in vitro. Crit. Rev. Biochem. Mol. Biol. 1997;32:503–568. doi: 10.3109/10409239709082001. [DOI] [PubMed] [Google Scholar]
- 3.Waga S., Stillman B. The DNA replication fork in eukaryotic cells. Ann. Rev. Biochem. 1998;67:721–751. doi: 10.1146/annurev.biochem.67.1.721. [DOI] [PubMed] [Google Scholar]
- 4.Simmons D.T. SV40 large T antigen functions in DNA replication and transformation. Adv. Virus Res. 2000;55:75–134. doi: 10.1016/s0065-3527(00)55002-7. [DOI] [PubMed] [Google Scholar]
- 5.Stenlund A. Initiation of DNA replication: lessons from viral initiator proteins. Nature Rev. Mol. Cell Biol. 2003;4:777–785. doi: 10.1038/nrm1226. [DOI] [PubMed] [Google Scholar]
- 6.Borowiec J.A., Dean F.B., Bullock P.A., Hurwitz J. Binding and unwinding—how T antigen engages the SV40 origin of DNA replication. Cell. 1990;60:181–184. doi: 10.1016/0092-8674(90)90730-3. [DOI] [PubMed] [Google Scholar]
- 7.Johnson A., O'Donnell M. Cellular DNA replicases: components and dynamics at the replication fork. Ann. Rev. Biochem. 2005;74:283–315. doi: 10.1146/annurev.biochem.73.011303.073859. [DOI] [PubMed] [Google Scholar]
- 8.Machida Y.J., Hamlin J.L., Dutta A. Right place, right time, and only once: replication initiation in metazoans. Cell. 2005;123:13–24. doi: 10.1016/j.cell.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 9.Binz S.K., Sheehan A.M., Wold M.S. Replication protein A phosphorylation and the cellular response to DNA damage. DNA Repair. 2004;3:1015–1024. doi: 10.1016/j.dnarep.2004.03.028. [DOI] [PubMed] [Google Scholar]
- 10.Iftode C., Daniely Y., Borowiec J.A. Replication protein A (RPA): the eukaryotic SSB. Crit. Rev. Biochem. Mol. Biol. 1999;34:141–180. doi: 10.1080/10409239991209255. [DOI] [PubMed] [Google Scholar]
- 11.Wold M.S. Replication protein A: a heterotrimeric, single-stranded DNA-binding protein required for eukaryotic DNA metabolism. Ann. Rev. Biochem. 1997;66:61–92. doi: 10.1146/annurev.biochem.66.1.61. [DOI] [PubMed] [Google Scholar]
- 12.Stauffer M.E., Chazin W.J. Structural mechanisms of DNA replication, repair, and recombination. J. Biol. Chem. 2004;279:30915–30918. doi: 10.1074/jbc.R400015200. [DOI] [PubMed] [Google Scholar]
- 13.Bochkarev A., Bochkareva E. From RPA to BRCA2: lessons from single-stranded DNA binding by the OB-fold. Curr. Opin. Struct. Biol. 2004;14:36–42. doi: 10.1016/j.sbi.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 14.Zou L., Elledge S.J. Sensing DNA damage through ATRIP recognition of RPA–ssDNA complexes. Science. 2003;300:1542–1548. doi: 10.1126/science.1083430. [DOI] [PubMed] [Google Scholar]
- 15.Shechter D., Costanzo V., Gautier J. ATR and ATM regulate the timing of DNA replication origin firing. Nature Cell Biol. 2004;6:648–655. doi: 10.1038/ncb1145. [DOI] [PubMed] [Google Scholar]
- 16.Yuzhakov A., Kelman Z., Hurwitz J., O'Donnell M. Multiple competition reactions for RPA order the assembly of the DNA polymerase delta holoenzyme. EMBO J. 1999;18:6189–6199. doi: 10.1093/emboj/18.21.6189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kowalczykowski S.C. Some assembly required. Nature Struct. Biol. 2000;7:1087–1089. doi: 10.1038/81923. [DOI] [PubMed] [Google Scholar]
- 18.Theobald D.L., Mitton-Fry R.M., Wuttke D.S. Nucleic acid recognition by OB-fold proteins. Ann. Rev. Biophys. Biomol. Struct. 2003;32:115–133. doi: 10.1146/annurev.biophys.32.110601.142506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bochkareva E., Korolev S., Bochkarev A. The role for zinc in replication protein A. J. Biol. Chem. 2000;275:27332–27338. doi: 10.1074/jbc.M000620200. [DOI] [PubMed] [Google Scholar]
- 20.Mer G., Bochkarev A., Gupta R., Bochkareva E., Frappier L., Ingles C.J., Edwards A.M., Chazin W.J. Structural basis for the recognition of DNA repair proteins UNG2, XPA, and RAD52 by replication factor RPA. Cell. 2000;103:449–456. doi: 10.1016/s0092-8674(00)00136-7. [DOI] [PubMed] [Google Scholar]
- 21.Iftode C., Borowiec J.A. 5′→3′ molecular polarity of human replication protein A (hRPA) binding to pseudo-origin DNA substrates. Biochemistry. 2000;39:11970–11981. doi: 10.1021/bi0005761. [DOI] [PubMed] [Google Scholar]
- 22.de Laat W.L., Appeldoorn E., Sugasawa K., Weterings E., Jaspers N.G.J., Hoeijmakers J.H.J. DNA-binding polarity of human replication protein A positions nucleases in nucleotide excision repair. Genes Dev. 1998;12:2598–2609. doi: 10.1101/gad.12.16.2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim C., Paulus B.F., Wold M.S. Interactions of human replication protein A with oligonucleotides. Biochemistry. 1994;33:14197–14206. doi: 10.1021/bi00251a031. [DOI] [PubMed] [Google Scholar]
- 24.Bochkareva E., Korolev S., Lees-Miller S.P., Bochkarev A. Structure of the RPA trimerization core and its role in the multistep DNA-binding mechanism of RPA. EMBO J. 2002;21:1855–1863. doi: 10.1093/emboj/21.7.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bochkareva E., Belegu V., Korolev S., Bochkarev A. Structure of the major single-stranded DNA-binding domain of replication protein A suggests a dynamic mechanism for DNA binding. EMBO J. 2001;20:612–618. doi: 10.1093/emboj/20.3.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bastin-Shanower S.A., Brill S.J. Functional analysis of the four DNA binding domains of replication protein A. The role of RPA2 in ssDNA binding. J. Biol. Chem. 2001;276:36446–36453. doi: 10.1074/jbc.M104386200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kolpashchikov D.M., Khodyreva S.N., Khlimankov D.Y., Wold M.S., Favre A., Lavrik O.I. Polarity of human replication protein A binding to DNA. Nucleic Acids Res. 2001;29:373–379. doi: 10.1093/nar/29.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wyka I.M., Dhar K., Binz S.K., Wold M.S. Replication protein A interactions with DNA: differential binding of the core domains and analysis of the DNA interaction surface. Biochemistry. 2003;42:12909–12918. doi: 10.1021/bi034930h. [DOI] [PubMed] [Google Scholar]
- 29.Arunkumar A.I., Stauffer M.E., Bochkareva E., Bochkarev A., Chazin W.J. Independent and coordinated functions of replication protein A tandem high affinity single-stranded DNA binding domains. J. Biol. Chem. 2003;278:41077–41082. doi: 10.1074/jbc.M305871200. [DOI] [PubMed] [Google Scholar]
- 30.Daughdrill G.W., Ackerman J., Isern N.G., Botuyan M.V., Arrowsmith C., Wold M.S., Lowry D.F. The weak interdomain coupling observed in the 70 kDa subunit of human replication protein A is unaffected by ssDNA binding. Nucleic Acids Res. 2001;29:3270–3276. doi: 10.1093/nar/29.15.3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Binz S.K., Lao Y., Lowry D.F., Wold M.S. The phosphorylation domain of the 32-kDa subunit of replication protein A (RPA) modulates RPA–DNA interactions: evidence for an intersubunit interaction. J. Biol. Chem. 2003;278:35584–35591. doi: 10.1074/jbc.M305388200. [DOI] [PubMed] [Google Scholar]
- 32.Bochkareva E., Kaustov L., Ayed A., Yi G.-S., Lu Y., Pineda-Lucena A., Liao J.C.C., Okorokov A.L., Milner J., Arrowsmith C.H., et al. Single-stranded DNA mimicry in the p53 transactivation domain interaction with replication protein A. Proc. Natl Acad. Sci. USA. 2005;102:15412–15417. doi: 10.1073/pnas.0504614102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blackwell L.J., Borowiec J.A., Mastrangelo I.A. Single-stranded-DNA binding alters human replication protein A structure and facilitates interaction with DNA-dependent protein kinase. Mol. Cell. Biol. 1996;16:4798–4807. doi: 10.1128/mcb.16.9.4798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bochkarev A., Pfuetzner R.A., Edwards A.M., Frappier L. Structure of the single-stranded-DNA-binding domain of the replication protein A bound to DNA. Nature. 1997;385:176–181. doi: 10.1038/385176a0. [DOI] [PubMed] [Google Scholar]
- 35.Lavrik O.I., Kolpashchikov D.M., Weisshart K., Nasheuer H.P., Khodyreva S.N., Favre A. RPA subunit arrangement near the 3′-end of the primer is modulated by the length of the template strand and cooperative protein interactions. Nucleic Acids Res. 1999;27:4235–4240. doi: 10.1093/nar/27.21.4235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pestryakov P.E., Weisshart K., Schlott B., Khodyreva S.N., Kremmer E., Grosse F., Lavrik O.I., Nasheuer H.-P. Human replication protein A. The C-terminal RPA70 and the central RPA32 domains are involved in the interactions with the 3′-end of a primer–template DNA. J. Biol. Chem. 2003;278:17515–17524. doi: 10.1074/jbc.M301265200. [DOI] [PubMed] [Google Scholar]
- 37.Mass G., Nethanel T., Lavrik O.I., Wold M.S., Kaufmann G. Replication protein A modulates its interface with the primed DNA template during RNA–DNA primer elongation in replicating SV40 chromosomes. Nucleic Acids Res. 2001;29:3892–3899. doi: 10.1093/nar/29.18.3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pestryakov P.E., Khlimankov D.Y., Bochkareva E., Bochkarev A., Lavrik O.I. Human replication protein A (RPA) binds a primer–template junction in the absence of its major ssDNA-binding domains. Nucleic Acids Res. 2004;32:1894–1903. doi: 10.1093/nar/gkh346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Santocanale C., Neecke H., Longhese M.P., Lucchini G., Plevani P. Mutations in the gene encoding the 34 kDa subunit of yeast replication protein A cause defective S phase progression. J. Mol. Biol. 1995;254:595–607. doi: 10.1006/jmbi.1995.0641. [DOI] [PubMed] [Google Scholar]
- 40.Kenny M.K., Schlegel U., Furneaux H., Hurwitz J. The role of human single-stranded DNA binding protein and its individual subunits in simian virus 40 DNA replication. J. Biol. Chem. 1990;265:7693–7700. [PubMed] [Google Scholar]
- 41.Erdile L.F., Wold M.S., Kelly T.J. The primary structure of the 32-kDa subunit of human replication protein A. J. Biol. Chem. 1990;265:3177–3182. [PubMed] [Google Scholar]
- 42.Mass G., Nethanel T., Kaufmann G. The middle subunit of replication protein A contacts growing RNA–DNA primers in replicating simian virus 40 chromosomes. Mol. Cell. Biol. 1998;18:6399–6407. doi: 10.1128/mcb.18.11.6399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang M., Park J.-S., Ishiai M., Hurwitz J., Lee S.-H. Species specificity of human RPA in simian virus 40 DNA replication lies in T-antigen-dependent RNA primer synthesis. Nucleic Acids Res. 2000;28:4742–4749. doi: 10.1093/nar/28.23.4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee S.-H., Kim D.K. The role of the 34-kDa subunit of human replication protein A in simian virus 40 DNA replication in vitro. J. Biol. Chem. 1995;270:12801–12807. doi: 10.1074/jbc.270.21.12801. [DOI] [PubMed] [Google Scholar]
- 45.Braun K.A., Lao Y., He Z., Ingles C.J., Wold M.S. Role of protein-protein interactions in the function of replication protein A (RPA): RPA modulates the activity of DNA polymerase alpha by multiple mechanisms. Biochemistry. 1997;36:8443–8454. doi: 10.1021/bi970473r. [DOI] [PubMed] [Google Scholar]
- 46.Han Y., Loo Y.-M., Militello K.T., Melendy T. Interactions of the papovavirus DNA replication initiator proteins, bovine papillomavirus type 1 E1 and simian virus 40 large T antigen, with human replication protein A. J. Virol. 1999;73:4899–4907. doi: 10.1128/jvi.73.6.4899-4907.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Loo Y.-M., Melendy T. Recruitment of replication protein A by the papillomavirus E1 protein and modulation by single-stranded DNA. J. Virol. 2004;78:1605–1615. doi: 10.1128/JVI.78.4.1605-1615.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arunkumar A.I., Klimovich V., Jiang X., Ott R.D., Mizoue L., Fanning E., Chazin W.J. Insights into hRPA32 C-terminal domain-mediated assembly of the simian virus 40 replisome. Nature Struct. Mol. Biol. 2005;12:332–339. doi: 10.1038/nsmbXX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weisshart K., Friedl S., Taneja P., Nasheuer H.-P., Schlott B., Grosse F., Fanning E. Partial proteolysis of simian virus 40 T antigen reveals intramolecular contacts between domains and conformation changes upon hexamer assembly. J. Biol. Chem. 2004;279:38943–38951. doi: 10.1074/jbc.M406159200. [DOI] [PubMed] [Google Scholar]
- 50.Sancar A., Lindsey-Boltz L.A., Unsal-Kacmaz K., Linn S. Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Ann. Rev. Biochemistry. 2004;73:39–85. doi: 10.1146/annurev.biochem.73.011303.073723. [DOI] [PubMed] [Google Scholar]
- 51.Tapias A., Auriol J., Forget D., Enzlin J.H., Scharer O.D., Coin F., Coulombe B., Egly J.-M. Ordered conformational changes in damaged DNA induced by nucleotide excision repair factors. J. Biol. Chem. 2004;279:19074–19083. doi: 10.1074/jbc.M312611200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Missura M., Buterin T., Hindges R., Hubscher U., Kasparkova J., Brabec V., Naegeli H. Double-check probing of DNA bending and unwinding by XPA-RPA: an architectural function in DNA repair. EMBO J. 2001;20:3554–3564. doi: 10.1093/emboj/20.13.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.You J.-S., Wang M., Lee S.-H. Biochemical analysis of the damage recognition process in nucleotide excision repair. J. Biol. Chem. 2003;278:7476–7485. doi: 10.1074/jbc.M210603200. [DOI] [PubMed] [Google Scholar]
- 54.Riedl T., Hanaoka F., Egly J.-M. The comings and goings of nucleotide excision repair factors on damaged DNA. EMBO J. 2003;22:5293–5303. doi: 10.1093/emboj/cdg489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jacobs D.M., Lipton A.S., Isern N.G., Daughdrill G.W., Lowry D.F., Gomes X., Wold M.S. Human replication protein A: global fold of the N-terminal RPA-70 domain reveals a basic cleft and flexible C-terminal linker. J. Biomol. NMR. 1999;14:321–331. doi: 10.1023/a:1008373009786. [DOI] [PubMed] [Google Scholar]
- 56.Vise P.D., Baral B., Latos A.J., Daughdrill G.W. NMR chemical shift and relaxation measurements provide evidence for the coupled folding and binding of the p53 transactivation domain. Nucleic Acids Res. 2005;33:2061–2077. doi: 10.1093/nar/gki336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dutta A., Ruppert J.M., Aster J.C., Winchester E. Inhibition of DNA replication factor RPA by p53. Nature. 1993;365:79–82. doi: 10.1038/365079a0. [DOI] [PubMed] [Google Scholar]
- 58.He Z., Brinton B.T., Greenblatt J., Hassell J.A., Ingles C.J. The transactivator proteins VP16 and GAL4 bind replication factor A. Cell. 1993;73:1223–1232. doi: 10.1016/0092-8674(93)90650-f. [DOI] [PubMed] [Google Scholar]
- 59.Li R., Botchan M.R. The acidic transcriptional activation domains of VP16 and p53 bind the cellular replication protein A and stimulate in vitro BPV-1 DNA replication. Cell. 1993;73:1207–1221. doi: 10.1016/0092-8674(93)90649-b. [DOI] [PubMed] [Google Scholar]
- 60.VanDemark A.P., Blanksma M., Ferris E., Heroux A., Hill C.P., Formosa T. The structure of the yFACT Pob3-M domain, its interaction with the DNA replication factor RPA, and a potential role in nucleosome deposition. Mol. Cell. 2006;22:363–374. doi: 10.1016/j.molcel.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 61.Park C.-J., Lee J.-H., Choi B.-S. Solution structure of the DNA-binding domain of RPA from Saccharomyces cerevisiae and its interaction with single-stranded DNA and SV40 T antigen. Nucleic Acids Res. 2005;33:4172–4181. doi: 10.1093/nar/gki736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Daughdrill G.W., Buchko G.W., Botuyan M.V., Arrowsmith C., Wold M.S., Kennedy M.A., Lowry D.F. Chemical shift changes provide evidence for overlapping single-stranded DNA- and XPA-binding sites on the 70 kDa subunit of human replication protein A. Nucleic Acids Res. 2003;31:4176–4183. doi: 10.1093/nar/gkg451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stauffer M.E., Chazin W.J. Physical interaction between replication protein A and Rad51 promotes exchange on single-stranded DNA. J. Biol. Chem. 2004;279:25638–25645. doi: 10.1074/jbc.M400029200. [DOI] [PubMed] [Google Scholar]
- 64.Dornreiter I., Erdile L.F., Gilbert I.U., von Winkler D., Kelly T.J., Fanning E. Interaction of DNA polymerase alpha-primase with cellular replication protein A and SV40 T antigen. EMBO J. 1992;11:769–776. doi: 10.1002/j.1460-2075.1992.tb05110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shen J.-C., Lao Y., Kamath-Loeb A., Wold M.S., Loeb L.A. The N-terminal domain of the large subunit of human replication protein A binds to Werner syndrome protein and stimulates helicase activity. Mech. Ageing Dev. 2003;124:921–930. doi: 10.1016/s0047-6374(03)00164-7. [DOI] [PubMed] [Google Scholar]
- 66.Doherty K.M., Sommers J.A., Gray M.D., Lee J.W., von Kobbe C., Thoma N.H., Kureekattil R.P., Kenny M.K., Brosh R.M., Jr Physical and functional mapping of the replication protein A interaction domain of the Werner and Bloom syndrome helicases. J. Biol. Chem. 2005;280:29494–29505. doi: 10.1074/jbc.M500653200. [DOI] [PubMed] [Google Scholar]
- 67.Nasheuer H.P., von Winkler D., Schneider C., Dornreiter I., Gilbert I., Fanning E. Purification and functional charcterization of bovine RP-A in an in vitro SV40 DNA replication system. Chromosoma. 1992;102:S52–S59. doi: 10.1007/BF02451786. [DOI] [PubMed] [Google Scholar]
- 68.Maga G., Villani G., Tillement V., Stucki M., Locatelli G.A., Frouin I., Spadari S., Hubscher U. Okazaki fragment processing: modulation of the strand displacement activity of DNA polymerase delta by the concerted action of replication protein A, proliferating cell nuclear antigen, and flap endonuclease-1. Proc. Natl Acad. Sci. USA. 2001;98:14298–14303. doi: 10.1073/pnas.251193198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Maga G., Shevelev I., Villani G., Spadari S., Hubscher U. Human replication protein A can suppress the intrinsic in vitro mutator phenotype of human DNA polymerase lambda. Nucleic Acids Res. 2006;34:1405–1415. doi: 10.1093/nar/gkl032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ellison V., Stillman B. Biochemical characterization of DNA damage checkpoint complexes: clamp loader and clamp complexes with specificity for 5′ recessed DNA. PLoS Biol. 2003;1:e33. doi: 10.1371/journal.pbio.0000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zou L., Liu D., Elledge S.J. Replication protein A-mediated recruitment and activation of Rad17 complexes. Proc. Natl Acad. Sci. USA. 2003;100:13827–13832. doi: 10.1073/pnas.2336100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wu X., Shell S.M., Zou Y. Interaction and colocalization of Rad9//Rad1//Hus1 checkpoint complex with replication protein A in human cells. Oncogene. 2005;24:4728–4735. doi: 10.1038/sj.onc.1208674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ball H.L., Myers J.S., Cortez D. ATRIP binding to replication protein A-single-stranded DNA promotes ATR-ATRIP localization but is dispensable for Chk1 phosphorylation. Mol. Biol. Cell. 2005;16:2372–2381. doi: 10.1091/mbc.E04-11-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Namiki Y., Zou L. ATRIP associates with replication protein A-coated ssDNA through multiple interactions. Proc. Natl Acad. Sci. USA. 2006;103:580–585. doi: 10.1073/pnas.0510223103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yoo E., Kim B.U., Lee S.Y., Cho C.H., Chung J.H., Lee C.-H. 53BP1 is associated with replication protein A and is required for RPA2 hyperphosphorylation following DNA damage. Oncogene. 2005;24:5423–5430. doi: 10.1038/sj.onc.1208710. [DOI] [PubMed] [Google Scholar]
- 76.Wong J.M.S., Ionescu D., Ingles C.J. Interaction between BRCA2 and replication protein A is compromised by a cancer-predisposing mutation in BRCA2. Oncogene. 2003;22:28–33. doi: 10.1038/sj.onc.1206071. [DOI] [PubMed] [Google Scholar]
- 77.Robison J.G., Elliott J., Dixon K., Oakley G.G. Replication protein A and the Mre11/Rad50/Nbs1 complex co-localize and interact at sites of stalled replication forks. J. Biol. Chem. 2004;279:34802–34810. doi: 10.1074/jbc.M404750200. [DOI] [PubMed] [Google Scholar]
- 78.Daniely Y., Borowiec J.A. Formation of a complex between nucleolin and replication protein A after cell stress prevents initiation of DNA replication. J. Cell Biol. 2000;149:799–810. doi: 10.1083/jcb.149.4.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kim K., Dimitrova D.D., Carta K.M., Saxena A., Daras M., Borowiec J.A. Novel checkpoint response to genotoxic stress mediated by nucleolin-replication protein A complex formation. Mol. Cell. Biol. 2005;25:2463–2474. doi: 10.1128/MCB.25.6.2463-2474.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cahill D., Connor B., Carney J.P. Mechanisms of eukaryotic DNA double strand break repair. Front. Biosci. 2006;11:1958–1976. doi: 10.2741/1938. [DOI] [PubMed] [Google Scholar]
- 81.Golub E.I., Gupta R.C., Haaf T., Wold M.S., Radding C.M. Interaction of human rad51 recombination protein with single-stranded DNA binding protein, RPA. Nucleic Acids Res. 1998;26:5388–5393. doi: 10.1093/nar/26.23.5388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gasior S.L., Olivares H., Ear U., Hari D.M., Weichselbaum R., Bishop D.K. Assembly of RecA-like recombinases: distinct roles for mediator proteins in mitosis and meiosis. Proc. Natl Acad. Sci. USA. 2001;98:8411–8418. doi: 10.1073/pnas.121046198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang X., Haber J.E. Role of Saccharomyces single-stranded DNA-binding protein RPA in the strand invasion step of double-strand break repair. PLoS Biol. 2004;2:e21. doi: 10.1371/journal.pbio.0020021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Song B., Sung P. Functional interactions among yeast Rad51 recombinase, Rad52 mediator, and replication protein A in DNA strand exchange. J. Biol. Chem. 2000;275:15895–15904. doi: 10.1074/jbc.M910244199. [DOI] [PubMed] [Google Scholar]
- 85.Sugiyama T., Kowalczykowski S.C. Rad52 protein associates with replication protein A (RPA)-single-stranded DNA to accelerate Rad51-mediated displacement of RPA and presynaptic complex formation. J. Biol. Chem. 2002;277:31663–31672. doi: 10.1074/jbc.M203494200. [DOI] [PubMed] [Google Scholar]
- 86.Symington L.S. Role of RAD52 epistasis group genes in homologous recombination and double-strand break repair. Microbiol. Mol. Biol. Rev. 2002;66:630–670. doi: 10.1128/MMBR.66.4.630-670.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kantake N., Sugiyama T., Kolodner R.D., Kowalczykowski S.C. The recombination-deficient mutant RPA (rfa1-t11) is displaced slowly from single-stranded DNA by Rad51 protein. J. Biol. Chem. 2003;278:23410–23417. doi: 10.1074/jbc.M302995200. [DOI] [PubMed] [Google Scholar]
- 88.Sigurdsson S., Van Komen S., Bussen W., Schild D., Albala J.S., Sung P. Mediator function of the human Rad51B-Rad51C complex in Rad51/RPA-catalyzed DNA strand exchange. Genes Dev. 2001;15:3308–3318. doi: 10.1101/gad.935501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sung P. Function of yeast Rad52 protein as a mediator between replication protein A and the Rad51 recombinase. J. Biol. Chem. 1997;272:28194–28197. doi: 10.1074/jbc.272.45.28194. [DOI] [PubMed] [Google Scholar]
- 90.McIlwraith M.J., Van Dyck E., Masson J.-Y., Stasiak A.Z., Stasiak A., West S.C. Reconstitution of the strand invasion step of double-strand break repair using human Rad51 Rad52 and RPA proteins. J. Mol. Biol. 2000;304:151–164. doi: 10.1006/jmbi.2000.4180. [DOI] [PubMed] [Google Scholar]
- 91.Huang S.G., Weisshart K., Gilbert I., Fanning E. Stoichiometry and mechanism of assembly of SV40 T antigen complexes with the viral origin of DNA replication and DNA polymerase alpha-primase. Biochemistry. 1998;37:15345–15352. doi: 10.1021/bi9810959. [DOI] [PubMed] [Google Scholar]
- 92.Zou Y., Liu Y., Wu X., Shell S.M. Functions of human replication protein A (RPA): From DNA replication to DNA damage and stress responses. J. Cell. Physiol. 2006;208:267–273. doi: 10.1002/jcp.20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Oakley G.G., Patrick S.M., Yao J., Carty M.P., Turchi J.J., Dixon K. RPA phosphorylation in mitosis alters DNA binding and protein–protein interactions. Biochemistry. 2003;42:3255–3264. doi: 10.1021/bi026377u. [DOI] [PubMed] [Google Scholar]
- 94.Liu Y., Kvaratskhelia M., Hess S., Qu Y., Zou Y. Modulation of replication protein A function by its hyperphosphorylation-induced conformational change involving DNA binding domain B. J. Biol. Chem. 2005;280:32775–32783. doi: 10.1074/jbc.M505705200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Patrick S.M., Oakley G.G., Dixon K., Turchi J.J. DNA damage induced hyperphosphorylation of replication protein A. 2. Characterization of DNA binding activity, protein interactions, and activity in DNA replication and repair. Biochemistry. 2005;44:8438–8448. doi: 10.1021/bi048057b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Block W.D., Yu Y., Lees-Miller S.P. Phosphatidyl inositol 3-kinase-like serine/threonine protein kinases (PIKKs) are required for DNA damage-induced phosphorylation of the 32 kDa subunit of replication protein A at threonine 21. Nucleic Acids Res. 2004;32:997–1005. doi: 10.1093/nar/gkh265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sakasai R., Shinohe K., Ichijima Y., Okita N., Shibata A., Asahina K., Teraoka H. Differential involvement of phosphatidylinositol 3-kinase-related protein kinases in hyperphosphorylation of replication protein A2 in response to replication-mediated DNA double-strand breaks. Genes Cells. 2006;11:237–246. doi: 10.1111/j.1365-2443.2006.00942.x. [DOI] [PubMed] [Google Scholar]
- 98.Zernik-Kobak M., Vasunia K., Connelly M., Anderson C.W., Dixon K. Sites of UV-induced phosphorylation of the p34 subunit of replication protein A from HeLa cells. J. Biol. Chem. 1997;272:23896–23904. doi: 10.1074/jbc.272.38.23896. [DOI] [PubMed] [Google Scholar]
- 99.Cruet-Hennequart S., Coyne S., Glynn M.T., Oakley G.G., Carty M.P. UV-induced RPA phosphorylation is increased in the absence of DNA polymerase [eta] and requires DNA-PK. DNA Repair. 2006;5:491–504. doi: 10.1016/j.dnarep.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 100.Henricksen L.A., Carter T., Dutta A., Wold M.S. Phosphorylation of human replication protein A by the DNA-dependent protein kinase is involved in the modulation of DNA replication. Nucleic Acids Res. 1996;24:3107–3112. doi: 10.1093/nar/24.15.3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vassin V.M., Wold M.S., Borowiec J.A. Replication protein A (RPA) phosphorylation prevents RPA association with replication centers. Mol. Cell. Biol. 2004;24:1930–1943. doi: 10.1128/MCB.24.5.1930-1943.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Francon P., Lemaitre J.M., Dreyer C., Maiorano D., Cuvier O., Mechali M. A hypophosphorylated form of RPA34 is a specific component of pre-replication centers. J. Cell Sci. 2004;117:4909–4920. doi: 10.1242/jcs.01361. [DOI] [PubMed] [Google Scholar]
- 103.Alani E., Thresher R., Griffith J.D., Kolodner R.D. Characterization of DNA-binding and strand-exchange stimulation properties of y-RPA, a yeast single-strand-DNA-binding protein. J. Mol. Biol. 1992;227:54–71. doi: 10.1016/0022-2836(92)90681-9. [DOI] [PubMed] [Google Scholar]
- 104.Chaudhuri J., Khuong C., Alt F.W. Replication protein A interacts with AID to promote deamination of somatic hypermutation targets. Nature. 2004;430:992–998. doi: 10.1038/nature02821. [DOI] [PubMed] [Google Scholar]
- 105.Nagelhus T.A., Haug T., Singh K.K., Keshav K.F., Skorpen F., Otterlei M., Bharati S., Lindmo T., Benichou S., Benarous R., et al. A sequence in the N-terminal region of human uracil-DNA glycosylase with homology to XPA interacts with the C-terminal part of the 34-kDa subunit of replication protein A. J. Biol. Chem. 1997;272:6561–6566. doi: 10.1074/jbc.272.10.6561. [DOI] [PubMed] [Google Scholar]
- 106.Otterlei M., Warbrick E., Nagelhus T.A., Haug T., Slupphaug G., Akbari M., Aas P.A., Steinsbekk K., Bakke O., Krokan H.E. Post-replicative base excision repair in replication foci. EMBO J. 1999;18:3834–3844. doi: 10.1093/emboj/18.13.3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Davis A.P., Symington L.S. The Rad52-Rad59 complex interacts with Rad51 and replication protein A. DNA Repair. 2003;2:1127–1134. doi: 10.1016/s1568-7864(03)00121-6. [DOI] [PubMed] [Google Scholar]
- 108.Jackson D., Dhar K., Wahl J.K., Wold M.S., Borgstahl G.E.O. Analysis of the human replication protein A:Rad52 complex: evidence for crosstalk between RPA32, RPA70, Rad52 and DNA. J. Mol. Biol. 2002;321:133–148. doi: 10.1016/s0022-2836(02)00541-7. [DOI] [PubMed] [Google Scholar]
- 109.Weisshart K., Taneja P., Fanning E. The replication protein A binding site in simian virus 40 (SV40) T antigen and its role in the initial steps of SV40 DNA replication. J. Virol. 1998;72:9771–9781. doi: 10.1128/jvi.72.12.9771-9781.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yang Z.G., Liu Y., Mao L.Y., Zhang J.T., Zou Y. Dimerization of human XPA and formation of XPA2–RPA protein complex. Biochemistry. 2002;41:13012–13020. doi: 10.1021/bi026064z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Stigger E., Drissi R., Lee S.-H. Functional analysis of human replication protein A in nucleotide excision repair. J. Biol. Chem. 1998;273:9337–9343. doi: 10.1074/jbc.273.15.9337. [DOI] [PubMed] [Google Scholar]
- 112.Mer G., Bochkarev A., Chazin W.J., Edwards A.M. Three-dimensional structure and function of replication protein A. Cold Spring Harb. Symp. Quant. Biol. 2000;65:193–200. doi: 10.1101/sqb.2000.65.193. [DOI] [PubMed] [Google Scholar]
- 113.Saijo M., Matsuda T., Kuraoka I., Tanaka K. Inhibition of nucleotide excision repair by anti-XPA monoclonal antibodies which interfere with binding to RPA, ERCC1, and TFIIH. Biochem. Biophys. Res. Commun. 2004;321:815–822. doi: 10.1016/j.bbrc.2004.07.030. [DOI] [PubMed] [Google Scholar]
- 114.Saijo M., Kuraoka I., Masutani C., Hanaoka F., Tanaka K. Sequential binding of DNA repair proteins RPA and ERCC1 to XPA in vitro. Nucleic Acids Res. 1996;24:4719–4724. doi: 10.1093/nar/24.23.4719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Li L., Lu X., Peterson C.A., Legerski R.J. An interaction between the DNA repair factor XPA and replication protein A appears essential for nucleotide excision repair. Mol. Cell. Biol. 1995;15:5396–5402. doi: 10.1128/mcb.15.10.5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Christensen J., Tattersall P. Parvovirus initiator protein NS1 and RPA coordinate replication fork progression in a reconstituted DNA replication system. J. Virol. 2002;76:6518–6531. doi: 10.1128/JVI.76.13.6518-6531.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kim H.-S., Brill S.J. Rfc4 interacts with Rpa1 and is required for both DNA replication and DNA damage checkpoints in Saccharomyces cerevisiae. Mol. Cell. Biol. 2001;21:3725–3737. doi: 10.1128/MCB.21.11.3725-3737.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.He Z., Henricksen L.A., Wold M.S., Ingles C.J. RPA involvement in the damage-recognition and incision steps of nucleotide excision repair. Nature. 1995;374:566–569. doi: 10.1038/374566a0. [DOI] [PubMed] [Google Scholar]
- 119.Matsunaga T., Park C.-H., Bessho T., Mu D., Sancar A. Replication protein A confers structure-specific endonuclease activities to the XPF-ERCC1 and XPG subunits of human DNA repair excision nuclease. J. Biol. Chem. 1996;271:11047–11050. doi: 10.1074/jbc.271.19.11047. [DOI] [PubMed] [Google Scholar]
- 120.Bessho T., Sancar A., Thompson L.H., Thelen M.P. Reconstitution of human excision nuclease with recombinant XPF–ERCC1 complex. J. Biol. Chem. 1997;272:3833–3837. doi: 10.1074/jbc.272.6.3833. [DOI] [PubMed] [Google Scholar]