Interaction of Bunyamwera Orthobunyavirus NSs Protein with Mediator Protein MED8: a Mechanism for Inhibiting the Interferon Response (original) (raw)
Abstract
The NSs protein of Bunyamwera virus (Bunyaviridae) is an antiapoptotic interferon antagonist involved in silencing host protein expression by interfering with mRNA synthesis. Here, we show that the ability to inhibit both host transcription and the interferon response is linked to interaction of NSs with the MED8 component of Mediator, a protein complex necessary for mRNA production. The interacting domain on NSs was mapped to the C-terminal region, which contains amino acids conserved among orthobunyavirus NSs proteins. A recombinant virus in which the interacting domain in NSs was deleted had strongly reduced ability to inhibit host protein expression and was unable to inhibit the interferon response. This study provides further information on the mechanisms by which bunyavirus nonstructural proteins are involved in pathogenesis.
The family Bunyaviridae is one of the largest taxonomic groupings of RNA-containing viruses, with over 300 named members (43). The family is divided into five genera, Orthobunyavirus, Phlebovirus, Hantavirus, Nairovirus, and Tospovirus. The family takes its name from Bunyamwera virus (BUNV), in the genus Orthobunyavirus, which is the prototype of the group. The genus Orthobunyavirus includes a number of important human and animal pathogens. La Crosse virus causes severe pediatric encephalitis in the United States, and about 10% of children with clinical symptoms develop long-term cognitive and behavioral defects (38). Oropouche virus has been responsible for repeated, large-scale epidemics of a debilitating febrile illness in South America, and Tahyna virus causes an influenza-like illness in Central Europe. Infection with Cache Valley virus (in North America) and Aino virus (in Africa, Asia, and Australia) results in fetal abnormalities and abortion in sheep and cattle (21).
The BUNV genome consists of three segments of single-stranded, negative-sense RNA. The largest segment (L) codes for an RNA-dependent RNA polymerase (L protein); the medium segment (M) codes for the two virion glycoproteins Gn and Gc and a nonstructural protein, NSm; and the smallest segment (S) encodes the nucleoprotein N and a second nonstructural protein, NSs. Replication occurs in the cytoplasm, and progeny virions assemble at the Golgi apparatus (43). The recovery by reverse genetics of a mutant BUNV lacking the NSs protein, BUNdelNSs, revealed that NSs is a nonessential gene that contributes to viral pathogenesis (12). This virus showed a marked reduction in its ability to shut off host cell protein synthesis in mammalian cells, in contrast to the efficient shutoff mediated by the wild-type (wt) virus. BUNdelNSs was also shown to be a strong inducer of beta interferon (IFN-β), whereas wt BUNV was not, and IFN-β-encoding mRNA was detected following infection with the mutant BUNdelNSs virus, but not with wt BUNV (12). It was further demonstrated that NSs interferes with the regulation of the cellular innate immune response (12, 51). The activation of key regulatory elements of the innate response, such as PKR (47) and IRF-3 (29), upon infection indicated that the NSs inhibitory effect was downstream of the primary signaling pathway events. Despite activation of IRF-3, BUNV NSs was also shown to inhibit IRF-3-mediated induction of apoptosis. Similarly, LaCrosse virus NSs also inhibits apoptosis in interferon-competent cells (F. Weber, University of Freiburg, personal communication), though not in cells without an intact interferon system (5).
NSs has a predominantly cytoplasmic distribution, though a proportion can also be detected in the nuclei of cells transfected with an NSs-expressing plasmid (49, 51). The presence of NSs in infected mammalian cells produced strong modifications to the phosphorylation state of the C-terminal domain (CTD) of RNA polymerase II (49). NSs, independently of other viral proteins, inhibited phosphorylation at serine 2 in the heptapeptide repeat (YSPTSPS) of the CTD of RNA polymerase II, suggesting that the elongation step of transcription and/or 3′-end processing was prevented. Thus, it was proposed that NSs inhibits host cell protein synthesis by inhibiting RNA polymerase II-mediated transcription.
To investigate the cellular target(s) of NSs that leads to host cell protein shutoff and inhibition of the interferon pathway, we performed a yeast two-hybrid screen using a HeLa cell cDNA library. Here, we demonstrate interaction between NSs and MED8, a component of the head domain of the Mediator complex, which regulates RNA polymerase II-mediated transcription (reviewed in references 6, 17, 24, and 32). The interaction was mapped on NSs, and a mutant BUNV lacking the interaction domain with MED8 was produced (BUNNSs-T83). Like BUNdelNSs, BUNNSs-T83 was impaired in its ability to induce host cell protein shutoff. BUNNSs-T83 was unable to inhibit RNA polymerase II-driven transcription from an IFN-β promoter, thus rendering the virus susceptible to interferon-based inhibition of replication. The NSs-MED8 interaction and characterization of the BUNNSs-T83 virus provide evidence for the direct targeting of the RNA polymerase II transcription machinery that leads to shutoff of host cell protein synthesis and inhibition of the host innate immune response. Notably, the interaction domain on NSs contains an amino acid motif highly conserved among orthobunyavirus NSs proteins.
MATERIALS AND METHODS
Cells and viruses.
BHK-21 cells (37) were grown in Glasgow modified Eagle's medium supplemented with 10% newborn calf serum, 10 mM l-glutamine, and 10% tryptose-phosphate broth. BSR-T7/5 cells, a BHK-21-derived cell line stably expressing T7 RNA polymerase (15) kindly provided by K. K. Conzelmann, were maintained in supplemented Glasgow modified Eagle's medium containing 1 mg/ml geneticin. Human A549 cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum. Wild-type and mutant Bunyamwera viruses were grown at 33°C and titrated by plaque assay as described previously (11). BUNdelNSs2 is a recombinant virus that does not express NSs (T. J. Hart, A. Kohl, and R. M. Elliott, submitted for publication).
Virus rescue.
Recombinant BUNV was produced using the recently described three-plasmid protocol (36). In brief, BSR-T7/5 cells were transfected with a mixture of plasmids pT7riboBUNL(+), pT7riboBUNM(+), and pT7riboBUNS(+) (11), which encode full-length cDNA copies of each of the three genome segments under the control of the bacteriophage T7 promoter and hepatitis delta ribozyme sequences. pT7riboS-T83 was constructed by site-directed mutagenesis of a pT7RiboBUNS(+) DNA template by introducing a stop codon, TAA, in the NSs open reading frame (ORF) at positions 349 to 351 on the viral S segment. This resulted in a truncated NSs ORF of 83 amino acids but did not affect the N coding sequence in this region. This construct was substituted for pT7riboBUNS(+) to recover the virus called BUNNSs-T83.
Yeast two-hybrid analysis.
Potential interacting partners of NSs were sought using the yeast two-hybrid Matchmaker system 3 (Clontech, Palo Alto, CA). The full-length BUNV NSs ORF was amplified by PCR using pTM1-BUNNSs as a template (51) and cloned into the EcoRI/PstI restriction sites of pGBKT7 so that the insert was in frame with the GAL4 DNA binding domain sequence of pGBKT7, to produce pBK-NSs. Two-hybrid screening of a HeLa cell cDNA library (Clontech) was performed in Saccharomyces cerevisiae strain AH109 (26) according to the manufacturer's protocol. Following high-stringency screening in the presence of 3-amino-1,2,4 triazol, DNA from yeast colonies was extracted and library plasmids were isolated by transformation into Escherichia coli and ampicillin selection. The DNA was sequenced, and the origin of the cDNA was determined by comparison to the human genome database. One interacting partner was identified as MED8. To confirm this interaction, the full-length MED8 ORF was amplified by PCR from the library plasmid and was cloned in frame with the GAL4 activation domain sequence of pGADT7 to produce pAD-MED8. These plasmids were cotransformed into yeast, and cotransformations of each construct with the respective empty vector (pGBKT7 or pGADT7) were performed as controls. Positive and negative control plasmids (expressing simian virus 40 T antigen [pGADT7-T], p53 [pGBKT7-53], and lamin C [pGBKT7-Lam]), supplied in the Matchmaker kit, were used as directed by the manufacturer.
Analysis of proteins by radiolabeling and Western blotting.
To detect NSs, BHK cells infected at a multiplicity of infection of 5 were lysed in RIPA buffer (1% [wt/vol] Triton X-100, 50 mM Tris-HCl, pH 7.5, 300 mM NaCl, 5 mM EDTA) at 18 h postinfection, and equal amounts of cell extract were separated on a sodium dodecyl sulfate (SDS)-18% polyacrylamide gel and blotted onto a Hybond-C-pure membrane (Amersham). Rabbit anti-NSs antibody, raised against a synthetic peptide representing amino acids 71 to 90 (Hart et al., submitted), was used at a dilution of 1:300, followed by incubation with anti-rabbit horseradish peroxidase-coupled antibody (Cell Signaling Technology). Signals were revealed using a SuperSignal chemiluminescence kit (Pierce).
To monitor RNA polymerase II degradation, extracts from approximately 5 × 105 virus- or mock-infected A549 or BHK cells were prepared by lysing the cells in 200 μl lysis buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% IGEPAL-CA630 [Sigma]) containing phosphatase inhibitors (Phosphatase Inhibitor Mixture II; Calbiochem) and protease inhibitors (Complete Protease Inhibitor Mix; Roche). Twelve microliters of total extract was resolved on 6% (for RNA polymerase II) or 12% (other proteins) SDS-polyacrylamide gel electrophoresis (PAGE) and transferred onto a Hybond C membrane. RNA polymerase II was detected by using antibody PolII (N-20; Santa Cruz), actin by antibody Actin (I-19; Santa Cruz), and Bunyamwera virus N protein by a polyclonal anti-N serum. Detection was as described above.
Extracts of 106 virus- or mock-infected A549 cells labeled with 40 μCi [35S]methionine from 9 to 11 h postinfection were prepared in RIPA buffer containing Complete Protease Inhibitor Mix. Equal amounts of extract were run on 4 to 12% Bis-Tris gradient gel (Invitrogen) in SDS-MES (morpholineethanesulfonic acid) buffer. The gels were fixed in 50% methanol-10% acetic acid, dried, and exposed to X-ray film.
Coimmunoprecipitation.
CV-1 cells grown in a 35-mm-diameter dish were infected with vaccinia virus vTF7-3 (52) and transfected with pTM1-BUNNSs and/or pTM1-MED8, using 5 μg DAC-30 (Eurogentec) as a transfection reagent (30). After overnight incubation, the cells were starved for 1 h in methionine-free medium and then labeled for 4 h with 50 μCi [35S]methionine in 500 μl methionine-free medium per 35-mm-diameter dish. After being labeled, the cells were washed twice with ice-cold phosphate-buffered saline and scraped into lysis buffer (phosphate-buffered saline containing 5 mM EDTA, 0.5% Triton X-100, and a cocktail of protease inhibitors; Roche). Cell lysates were preincubated with 50 μl protein A-Sepharose beads, and the cleared lysate was incubated with the immunoprecipitation complex (protein A-Sepharose beads and anti-NSs polyclonal antibody). The precipitated complexes were washed three times in lysis buffer. Proteins were separated on an SDS-16.5% polyacrylamide gel, and the dried gel was exposed to X-ray film for 2 days.
Activation of the IFN-β promoter.
Activation of the IFN-β promoter was measured using a reporter assay as described previously (50). The plasmid for IFN-β promoter activation, p-125Luc, contains the firefly luciferase gene under the control of the IFN-β promoter (53) and was kindly provided by Takashi Fujita, Tokyo Metropolitan Institute of Medical Science, Tokyo, Japan. The internal control plasmid, phRL-CMV (Promega), contains a Renilla luciferase gene under the control of a cytomegalovirus (CMV) immediate-early promoter for RNA polymerase II-mediated transcription. For reporter gene experiments, 5 × 105 A549 cells were transfected with 1 μg of p-125Luc and 50 ng phRL-CMV using 3 μl Lipofectamine 2000 (Invitrogen). At 5 h posttransfection, the cells were infected with the various BUN viruses at 5 PFU/cell. At 16 h postinfection, the cells were lysed in 200 μl Passive Lysis Buffer, and firefly or Renilla luciferase assays were carried using a Dual Luciferase Assay kit (Promega). For determination of firefly luciferase activities, 10 μl of lysate was used, and for Renilla luciferase activities, 1 μl lysate was diluted in 50 μl H2O and 1 μl was used to measure activity.
Reporter gene assays with NSs expression plasmids.
Approximately 5 × 105 BSR-T7/5 cells, which stably express T7 RNA polymerase, were transfected with 0.1 μg phRL-CMV and 0.1 μg of either pTM1-NSs, pTM1-NS-T83, or the empty plasmid pTM1 using 1 μl Lipofectamine 2000 (Invitrogen). At 16 h posttransfection, the cells were lysed and Renilla luciferase activity was determined as described above.
Reverse transcription-PCR analyses.
A549 cells were infected with viruses at a multiplicity of infection of 1 PFU/cell, and total RNA was extracted at 12 h postinfection. The RNA was digested with DNase I prior to reverse transcription; 1 μg RNA was reverse transcribed with Moloney murine leukemia virus reverse transcriptase (Promega) using 100 ng random hexanucleotides. cDNA was amplified by 35 cycles of PCR (30 s at 94°C, 1 min at 58°C, 1 min at 72°C) using KOD polymerase (Novagen). The upstream and downstream primers were 5′-GACGCCGCATTGACCATCTA-3′ and 5′-CCTTAGGATTTCCACTCTGACT-3′ for IFN-β mRNA and 5′-CGGCTACCACATCCAAGGAA-3′ and 5′-GCTGGAATTACCGCGGCT-3′ for 45S rRNA (5). PCR products were analyzed on a 2% agarose gel.
RESULTS
Interaction between NSs and MED8.
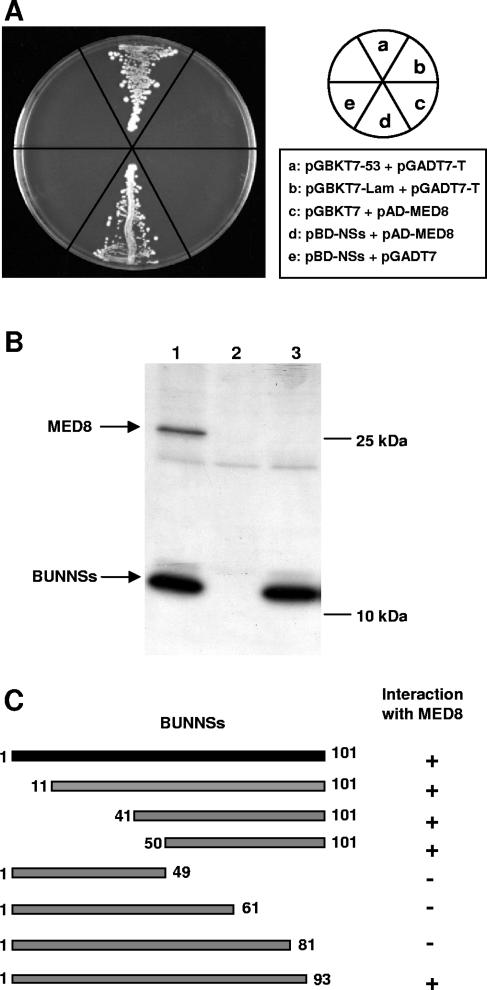
Previously, we characterized the role of Bunyamwera virus NSs protein in host cell protein shutoff and inhibition of the interferon pathway (12, 29, 47, 50). Further studies suggested that anti-interferon activity was a consequence of the ability of NSs to inhibit phosphorylation of the CTD of RNA polymerase II in mammalian cells (49). To investigate the mechanism by which NSs inhibits CTD phosphorylation, we sought to identify the cellular protein(s) targeted by NSs by performing a yeast two-hybrid screen using a HeLa cell cDNA library and the NSs protein as bait. One of the interacting clones isolated under high-stringency conditions encoded the mMed8 protein (accession number AF521562) (13), an essential component of the Mediator multiprotein complex. Hereafter, this protein will be called MED8, according to the unified nomenclature scheme for Mediator components (9). Mediator is a key element of the RNA polymerase II transcription machinery, and importantly, the Mediator subunit CDK8-cyclin C has been shown to regulate phosphorylation of the CTD of RNA polymerase II (2). Thus, based on our knowledge of NSs function, the interaction with MED8 appeared highly relevant. The full-length ORF of MED8 was cloned into the GAL4 activation domain-containing plasmid pGADT7, and its interaction with NSs was retested in the yeast two-hybrid assay, along with appropriate controls (Fig. 1A). A clear interaction between NSs and MED8, indicated by yeast colony formation, was demonstrated (sector d); the positive control for the assay also showed colony formation (sector a), whereas no growth was observed for the negative controls (sectors b, c, and e).
FIG. 1.
Characterization of the interaction between BUNNSs and MED8. (A) NSs-MED8 interaction in the yeast two-hybrid system. S. cerevisiae strain AH109 was cotransformed with plasmids as indicated, and the cotransformed clones were tested for protein-protein interaction on synthetic dropout medium lacking leucine, tryptophan, histidine, and adenine. (B) Coimmunoprecipitation of MED8 with NSs. CV-1 cells infected with vaccinia virus vTF7-3 and transfected with pTM1-BUNNSs and/or pTM1-MED8 were radiolabeled with [35S]methionine. Immunoprecipitation was performed using an anti-NSs polyclonal antibody. The cells were cotransfected with 0.5 μg pTM1-BUNNSs and 2.5 μg pTM1-MED8 (lane 1), 2.5 μg pTM1-MED8 and 0.5 μg pTM1 (lane 2), or 0.5 μg pTM1-BUNNSs and 2.5 μg pTM1 (lane 3). (C) Mapping of the NSs-MED8 interaction in the yeast two-hybrid system. Seven truncation mutants of the NSs protein were tested for interaction with MED8. Protein-protein interaction, as monitored by yeast growth, is indicated as (+) and lack of interaction by (−).
Interaction between NSs and MED8 was then examined in the more physiologically relevant situation in mammalian cells. CV-1 cells were transfected with plasmids expressing NSs and MED8, and radiolabeled cell lysates were incubated with a polyclonal anti-NSs antibody (Fig. 1B). When both proteins were expressed, the 28-kDa MED8 protein was coimmunoprecipitated with NSs (lane 1). MED8 was not precipitated by the anti-NSs antibody when expressed alone, showing the specificity of the interaction (lane 2), and no band of equivalent size was precipitated from cells expressing NSs alone (lane 3).
To map the domain on NSs responsible for the interaction with MED8, a series of truncation mutants was produced, and these were used with full-length MED8 in the yeast two-hybrid assay (Fig. 1C). The region encompassing amino acids 81 to 93 of NSs was shown to be essential for the interaction.
The C-terminal domain of NSs is essential for host cell protein shutoff.
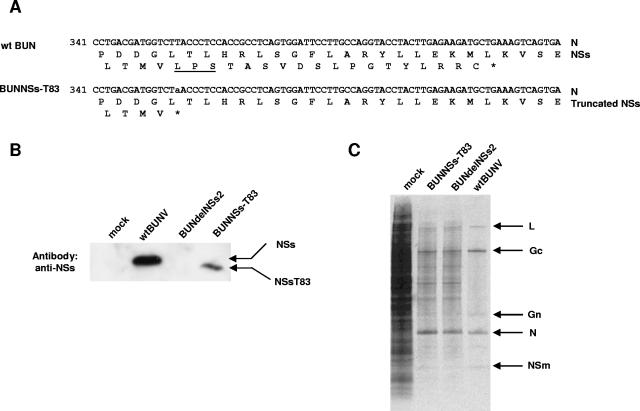
To investigate the importance of NSs-MED8 interaction during viral infection, a recombinant BUNV encoding a truncated NSs protein was constructed by reverse genetics (36). A stop codon was introduced at nucleotides 354 to 356 in the BUNV S segment cDNA by site-directed mutagenesis (TTA to TAA) (Fig. 2A), leaving the amino acid sequence of the overlapping N protein unchanged. A recombinant virus expressing an NSs protein of only 83 amino acids was rescued and designated BUNNSs-T83. Sequencing of the viral S RNA verified the presence of the truncation mutation (data not shown). To confirm the expression of the shortened NSs protein, BHK cells were infected with 5 PFU/cell of wt BUNV, BUNdelNSs2, or BUNNSs-T83 virus, and the cell lysates were analyzed by Western blotting with anti-NSs antibody. The lysate from a BUNNSs-T83-infected sample showed the truncated NSs protein, which migrated slightly faster than NSs synthesized by wt BUNV (Fig. 2B); no band was detected in the cells infected with BUNdelNSs2, in which the NSs ORF was ablated. The difference in band intensity between the full-length and truncated proteins might have occurred because the truncated protein was recognized less efficiently by the anti-NSs polyclonal antibody. The anti-NSs antibody was raised against a synthetic peptide comprising amino acids 71 to 90 of NSs, and seven of these residues were absent in the truncated protein.
FIG. 2.
Creation of BUNNSs-T83 virus. (A) Nucleotide and amino acid sequences of the S segments of wt BUNV and BUNNSs-T83 virus. The sequence is shown from nucleotides 341 to 420. Asterisks indicate stop codons. The amino acid motif LPS, conserved among orthobunyavirus NSs proteins, is underlined. (B) Western blot analysis of mock-infected cells or cells infected with different BUN viruses, as indicated. The blot was probed with an anti-NSs polyclonal antibody, and the migrations of wt NSs and a truncated NSs, NSs-T83, are indicated. (C) Inhibition of host cell protein synthesis. A549 cells were infected with the different BUN viruses or mock infected as indicated. The cells were labeled with 40 μCi [35S]methionine from 9 to 11 h postinfection, and equal amounts of total cell lysates were analyzed on a 4 to 12% Bis-Tris gradient gel. BUNV proteins are indicated by arrows.
The ability of BUNNSs-T83 virus to mediate host cell protein shutoff in mammalian cells was compared to those of wt BUNV and BUNdelNSs2. A549 cells were infected with wt BUNV, BUNdelNSs2, or BUNNSs-T83 virus at a multiplicity of infection of 5 PFU/cell and radiolabeled with [35S]methionine from 9 to 11 h postinfection, and the cell lysates were analyzed by PAGE (Fig. 2C). Cells infected with wt BUNV showed marked inhibition of host cell protein synthesis. However, shutoff of host protein synthesis was less profound in cells infected with BUNNSs-T83, which expresses the truncated NSs protein, and its extent was similar to that in cells infected with BUNdelNSs2, which lacks NSs entirely. The shutoff observed in these cells most probably resulted from the “cap-snatching” activity of the bunyavirus polymerase, whereby the 5′ ends of cellular mRNAs are cleaved to generate primers for viral mRNA transcription (43). This would render the attacked host mRNAs susceptible to degradation.
BUNNSs-T83 virus is unable to inhibit the host cell innate immune response.
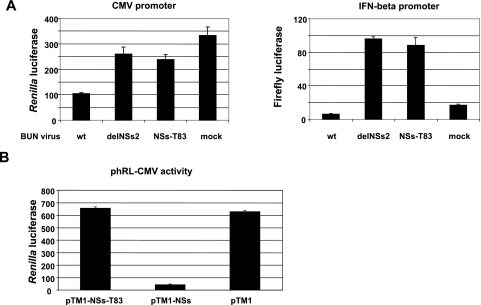
To determine if the BUNNSs-T83 virus retained the ability to inhibit the interferon pathway, A549 cells were cotransfected with p-125Luc, a plasmid containing the firefly luciferase gene under the control of the IFN-β promoter, and phRL-CMV, which contains a Renilla luciferase gene under the control of a CMV immediate-early promoter, as internal controls. The cells were then infected with wt BUNV, BUNdelNSs2, or BUNNSs-T83. Wild-type BUNV efficiently inhibited transcription from the CMV promoter, whereas BUNdelNSs2 and BUNNSs-T83 had no major effect on RNA polymerase II activity compared to mock infection (Fig. 3A, left). These results are in accord with those of the metabolic-labeling experiment shown in Fig. 2C. In cells infected with wt BUNV, activation of the IFN-β promoter was inhibited (Fig. 3A, right), as previously shown (12, 50). However, the IFN-β promoter was strongly induced following infection with either BUNNSs-T83 or BUNdelNSs2. In order to verify that the observed reduction in reporter gene expression in BUNNSs-T83-infected cells was not due to effects unrelated to the truncation of NSs, a transient-transfection assay was used. The NSs-T83 ORF was cloned into the pTM1 expression vector and cotransfected with phRL-CMV into T7 RNA polymerase-expressing BSR-T7/5 cells to monitor the effect on the RNA polymerase II-driven CMV immediate-early promoter. As shown in Fig. 3B, full-length wt NSs, as expected, strongly reduced gene expression, whereas luciferase activity in cells transfected with the NS-T83 mutant was similar to that in cells transfected with empty pTM1. This shows that the truncation of NSs in the MED8 interaction domain is indeed sufficient to abrogate the inhibitory effect of NSs on reporter gene expression.
FIG. 3.
Characterization of BUNNSs-T83 virus. (A) Inhibition of RNA polymerase II transcription and activation of the IFN-β promoter. A549 cells were cotransfected with phRL-CMV and p-125Luc and then infected with the different BUN viruses or mock infected, as indicated. Luciferase activities were measured at 16 h postinfection using a Dual Luciferase Assay kit. Renilla luciferase activity (left) indicates RNA polymerase II transcription, and firefly luciferase activity (right) indicates activation of the IFN-β promoter. The activities are presented in arbitrary light units, with the bars showing the standard errors from three experiments. (B) The MED8 interaction domain is necessary to inhibit gene expression. T7 RNA polymerase-expressing BSR-T7/5 cells were transfected with 0.1 μg of pTM1-NSs, pTM1-NSs-T83, or empty pTM1 and 0.1 μg of reporter phRL-CMV as indicated. Luciferase assays were carried out 16 h posttransfection.
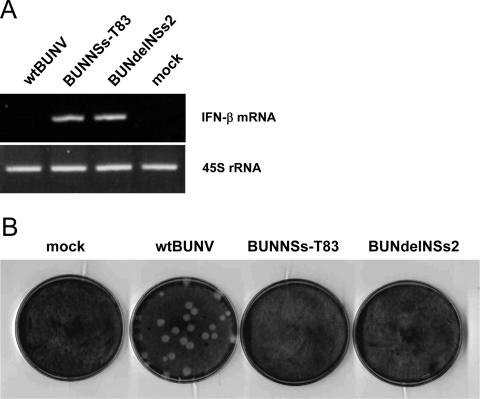
While deletion of the MED8 interaction domain has a measurable effect on the ability of NSs to shut off host protein synthesis, as shown by the results of reporter gene assays, it was necessary to examine the production of IFN-β mRNA directly to demonstrate that this interaction indeed interfered at the level of RNA polymerase II-mediated transcription. To address this, total RNA was isolated from infected A549 cells, and the presence of IFN-β mRNA was analyzed by reverse transcription (RT)-PCR. As a control, 45S rRNA (transcribed by RNA polymerase I) was also amplified. As shown in Fig. 4A, an RT-PCR signal specific for IFN-β mRNA was detected only in cells infected with BUNdelNSs or BUNNSsT83 virus, and no signal was detected in cells infected with wt BUNV or in mock-infected cells. However, the RT-PCR signals specific for 45S rRNA were equivalent in all samples. This suggests that the NSs-MED8 interaction is crucial to inhibit transcription of IFN-β mRNA.
FIG. 4.
Effect of NSs on host cell innate immune reponse. (A) Detection of IFN-β mRNA. Total RNA was isolated from virus- or mock-infected cells as indicated, and RNA polymerase II-transcribed IFN-β mRNA was detected by RT-PCR. 45S rRNA, which is transcribed by RNA polymerase I, was also amplified by RT-PCR to show that similar amounts of total RNA were used to amplify transcripts. (B) Plaque formation on A549 cells. Cells were infected with wt BUNV or mutant BUNdelNSs or BUNNSs-T83 virus as indicated, and the monolayers were fixed at 5 days postinfection and stained with Giemsa. mock, mock infected.
Neither BUNNSs-T83 nor BUNdelNSs2 replicated efficiently in the interferon-competent A549 cells, as shown by their failure to form plaques (Fig. 4B). Indeed, only wt BUNV was able to interfere with the innate immune system and replicate in these cells. Taken together, our data are consistent with the notion that the C-terminal domain of NSs is involved in the inhibition of cellular RNA polymerase II-driven transcription through interaction with the MED8 component of the Mediator complex.
BUNV infection induces degradation of RNA polymerase II.
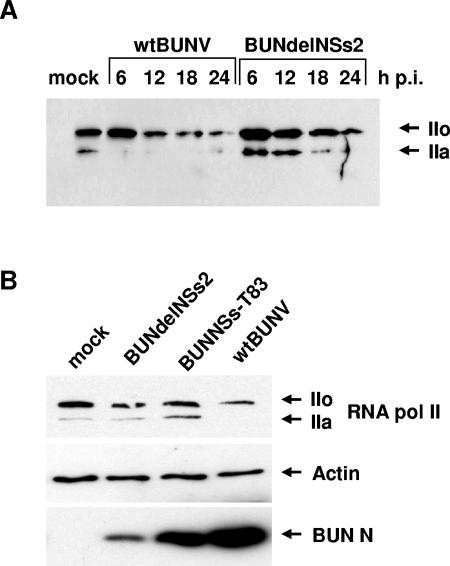
To assess the possibility that RNA polymerase II levels might be affected in infected cells, protein extracts from wt BUNV- or BUNdelNSs-infected cells were analyzed by Western blotting with RNA polymerase II antibodies. As shown in Fig. 5A, RNA polymerase II levels decreased over time in wt BUNV-infected cells, while the protein appeared considerably more stable in BUNdelNSs2-infected cells. To analyze whether the NSs-MED8 interaction might modulate the degradation of RNA polymerase II, a similar experiment was performed with the BUNNSs-T83 virus. As shown in Fig. 5B, the level of RNA polymerase II in BUNNSs-T83-infected cells was comparable to that in BUNdelNSs2-infected cells and much higher than in wt BUNV-infected cells. Detection of actin was used as a control to indicate that similar levels of protein were loaded onto the gel. The levels of the viral N protein were also measured, and less N was detected in BUNNSs-T83- and BUNdelNSs2-infected cells than in wt BUNV-infected cells. We ascribe this to the inhibition of viral replication in the former cells by the induced interferon.
FIG. 5.
Stability of RNA polymerase II in infected cells. (A) RNA polymerase II levels in BHK cells infected at a multiplicity of infection of 5 with wt BUNV or BUNdelNSs. Cell extracts were prepared at the indicated times after infection, and proteins were separated by SDS-PAGE and blotted onto a membrane, which was probed with anti-RNA polymerase II antibodies. IIo indicates the hyperphosphorylated form and IIa is the hypophosphorylated form of RNA polymerase II. (B) RNA polymerase II levels in A549 cells infected at a multiplicity of infection of 1 with wt BUNV, NUNNSs-T83, or BUNdelNSs at 12 h postinfection. Cell extracts were prepared and blotted as described previously and probed with anti-RNA polymerase II, anti-actin, or anti-N protein antibodies as indicated.
DISCUSSION
In this paper, we show that the BUNV nonstructural NSs protein interacts with the Mediator protein MED8. Based on present knowledge of Mediator and the activities of NSs in mammalian cells, we suggest that a consequence of this interaction is failure by the host cell to induce IFN after infection. Thus, the NSs-MED8 interaction appears crucial for efficient virus propagation in the face of the mammalian cell innate immune response.
Mediator is now considered a basic component of the RNA polymerase II transcriptional machinery (reference 24 and references therein), necessary for transcription of nearly all RNA polymerase II-driven genes. Mediator is a multiprotein complex that acts as a bridge between the enzyme and regulatory proteins and plays a central role in activation and repression of mRNA synthesis (32). Homologues of the Mediator subunits have been found in all eukaryotes investigated to date (7). Mediator can be divided into three modules termed head, middle (or body), and tail (or leg) (3, 6, 16, 18). The head module, which includes the MED8 protein (16, 17), is thought to be the major RNA polymerase II regulator and has extensive interactions with the enzyme. The role of the middle module is not clear, but it seems to be involved in transfer of the information from the tail to the head module; it contributes to binding to the polymerase and has contacts with the RNA polymerase II CTD. The tail module is proposed to be the sensor element of the complex, interacting with gene-specific transcriptional activators and repressors. Several activators of transcription have been shown to bind directly to the Mediator complex, such as GAL4 and GCN4 in yeast (14, 45) and the mammalian thyroid hormone receptor (22) and vitamin D receptor (46). The adenovirus E1A protein interacts with the MED23 subunit of Mediator to stimulate viral transcription (10), and the herpes simplex virus transcriptional activator VP16 interacts with MED17 (25) and MED25 (ARC92/ACID1) (39). Overexpression of the Mediator-binding domain of MED25 impaired the activation potential of VP16, highlighting a key role of Mediator in activation by VP16 (39).
RNA polymerase II activity is mainly regulated through the phosphorylation of its CTD, which in mammalian cells comprises 52 repeats of the consensus heptapeptide sequence YSPTSPS. Phosphorylation occurs on serine 2 and serine 5. Phosphorylation of serine 5 is required for initiation of transcription, while phosphorylation of serine 2 is crucial for mRNA elongation and 3′-end processing (1, 28, 31, 34, 42, 44). Accumulated evidence indicates that CTD phosphorylation is an important stage in regulation, and thus, it is not surprising that viruses could target it to facilitate their own replication. In the case of human immunodeficiency virus, the viral Tat protein stimulates serine 2 phosphorylation to increase the elongation step during mRNA production (33, 41), whereas in BUNV-infected mammalian cells, serine 2 phosphorylation is inhibited (10), contributing to the observed shutoff of host protein synthesis. In the context of silencing host gene expression, the interaction of NSs with MED8, an essential component of Mediator (40), is thus highly relevant. Mediator interacts directly with the CTD of the polymerase in its hypophosphorylated form and recruits it to the promoter-bound preinitiation complex (40). Mediator has been shown to activate CTD phosphorylation by TFIIH kinase (27) (whose major target is serine 5, though some serine 2 phosphorylation is catalyzed [28]), and Mediator is involved in the recruitment of P-TEFb (whose Cdk9 kinase activity targets serine 2) to the promoter by Brd4 (52). The NSs-MED8 interaction seems a compelling candidate to account for inhibition of serine 2 phosphorylation, though the mechanism remains elusive, mainly due to a lack of knowledge about the Mediator subunit functions. Preliminary experiments indicate that coexpression of NSs does not affect the stability of MED8, and NSs does not seem to prevent MED8 entering the Mediator complex, since it was identified in a high-molecular-weight band at the origins of SDS-polyacrylamide gels (data not shown).
Recently, MED8 was shown to interact with elongins B and C, Cul2, and Rbx1 to form a ubiquitin ligase (41). The putative target of this complex is unknown, but with MED8 as an adaptor molecule, it is suggested that ubiquitinylation by MED8 ubiquitin ligase could be involved in regulation of transcription. Once these targets are identified, it will be important to determine whether NSs affects the reconstitution of MED8 ubiquitin ligase or its activity. Intriguingly, at late times after infection, degradation of RNA polymerase II was detected in wt BUNV-infected cells, but not in BUNdelNSs2- or BUNNSs-T83-infected cells (Fig. 5), leading us to speculate whether this degradation is catalyzed by NSs-modified MED8 ubiquitin ligase activity.
The significance of the NSs-MED8 interaction during virus replication was demonstrated by creating a recombinant virus, BUNNSs-T83, in which the interaction domain of NSs was deleted. This virus displayed a phenotype similar to that of BUNdelNSs2, which does not express NSs at all, in terms of its sensitivity to IFN-mediated inhibition of viral replication and its reduced capacity to shut off host protein expression (Fig. 2 and 3). These results were confirmed by using a virus-free reporter gene assay (Fig. 3B). In addition, induction of the IFN-β mRNA was detected in both BUNNSs-T83- and BUNNSs-T83-infected cells, indicating that the transcriptional block mediated by full-length NSs was removed. Comparison of available NSs protein sequences showed them to be more variable than the corresponding N proteins, both within and between serogroups (19), displaying 40% overall amino acid similarity with few globally conserved residues. Notably, the truncation in BUNNSs-T83 occurs immediately before the amino sequence LPS (Fig. 2), which is the most C-terminally conserved motif maintained in all NSs proteins of Bunyamwera and California serogroup viruses sequenced to date (20). The extreme conservation of this motif suggests a functional role that can now be explained by involvement in the interaction with MED8.
A similar strategy for interfering with host gene expression has also been described for another bunyavirus, Rift Valley fever virus (RVFV), a member of the genus Phlebovirus. The RVFV NSs protein is considerably larger that that of orthobunyaviruses (31 kDa versus 11 kDa), shows no obvious sequence similarity, and is expressed via an ambisense coding strategy from a specific subgenomic mRNA (23). Analysis of a clonal isolate of RVFV with a large internal deletion in NSs, clone 13, showed that the RVFV NSs protein had an IFN antagonist function (8). As shown previously for BUNV NSs (29, 50), the RVFV NSs protein also antagonized the interferon pathway by inhibiting IFN-β mRNA transcription but did not prevent activation of IFN-specific transcription factors, like IRF-3 (4), suggesting that the anti-IFN effect was due to an overall suppression of host cell transcription. The NSs protein of RVFV forms filamentous structures in the nuclei of infected cells (48). The function of these structures was not clear until RVFV NSs was shown to interact with the p44 subunit of the TFIIH transcription factor (35). Confocal microscopy revealed that the NSs filaments contained p44, and in addition, the XPB subunit, thus subverting TFIIH complex assembly and leading to a reduction in the cellular pool of TFIIH and down-regulation of cellular transcription. Hence, these different bunyaviruses target the same cellular process—RNA polymerase II-mediated mRNA synthesis—by different mechanisms but with the overall goal of defeating the host innate immune response. These functional studies of bunyavirus NSs proteins will help in devising novel therapeutic intervention strategies.
Acknowledgments
V.H.J.L. was supported by a Wellcome Trust Studentship. Research in R.M.E.'s laboratory was funded by the Wellcome Trust.
REFERENCES
- 1.Ahn, S. H., M. Kim, and S. Buratowski. 2004. Phosphorylation of serine 2 within the RNA polymerase II C-terminal domain couples transcription and 3′ end processing. Mol. Cell 13**:**67-76. [DOI] [PubMed] [Google Scholar]
- 2.Akoulitchev, S., S. Chuikov, and D. Reinberg. 2000. TFIIH is negatively regulated by cdk8-containing Mediator complexes. Nature 407**:**102-106. [DOI] [PubMed] [Google Scholar]
- 3.Asturias, F. J., Y. W. Jiang, L. C. Myers, C. M. Gustafsson, and R. D. Kornberg. 1999. Conserved structures of Mediator and RNA polymerase II holoenzyme. Science 283**:**985-987. [DOI] [PubMed] [Google Scholar]
- 4.Billecocq, A., M. Spiegel, P. Vialat, A. Kohl, F. Weber, M. Bouloy, and O. Haller. 2004. NSs protein of Rift Valley fever virus blocks interferon production by inhibiting host gene transcription. J. Virol. 78**:**9798-9806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blakqori, G., and F. Weber. 2005. Efficient cDNA-based rescue of La Crosse bunyaviruses expressing or lacking the nonstructural protein NSs. J. Virol. 79**:**10420-10428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blazek, E., G. Mittler, and M. Meisterernst. 2005. The mediator of RNA polymerase II. Chromosoma 113**:**399-408. [DOI] [PubMed] [Google Scholar]
- 7.Boube, M., L. Joulia, D. L. Cribbs, and H. M. Bourbon. 2002. Evidence for a Mediator of RNA polymerase II transcriptional regulation conserved from yeast to man. Cell 110**:**143-151. [DOI] [PubMed] [Google Scholar]
- 8.Bouloy, M., C. Janzen, P. Vialat, H. Khun, J. Pavlovic, M. Huerre, and O. Haller. 2001. Genetic evidence for an interferon-antagonistic function of Rift Valley fever virus nonstructural protein NSs. J. Virol. 75**:**1371-1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bourbon, H. M., A. Aguilera, A. Z. Ansari, F. J. Asturias, A. J. Berk, S. Bjorklund, T. K. Blackwell, T. Borggrefe, M. Carey, M. Carlson, J. W. Conaway, R. C. Conaway, S. W. Emmons, J. D. Fondell, L. P. Freedman, T. Fukasawa, C. M. Gustafsson, M. Han, X. He, P. K. Herman, A. G. Hinnebusch, S. Holmberg, F. C. Holstege, J. A. Jaehning, Y. J. Kim, L. Kuras, A. Leutz, J. T. Lis, M. Meisterernest, A. M. Naar, K. Nasmyth, J. D. Parvin, M. Ptashne, D. Reinberg, H. Ronne, I. Sadowski, H. Sakurai, M. Sipiczki, P. W. Sternberg, D. J. Stillman, R. Strich, K. Struhl, J. Q. Svejstrup, S. Tuck, F. Winston, R. G. Roeder, and R. D. Kornberg. 2004. A unified nomenclature for protein subunits of Mediator complexes linking transcriptional regulators to RNA polymerase II. Mol. Cell 14**:**553-557. [DOI] [PubMed] [Google Scholar]
- 10.Boyer, T. G., M. E. Martin, E. Lees, R. P. Ricciardi, and A. J. Berk. 1999. Mammalian Srb/Mediator complex is targeted by adenovirus E1A protein. Nature 399**:**276-279. [DOI] [PubMed] [Google Scholar]
- 11.Bridgen, A., and R. M. Elliott. 1996. Rescue of a segmented negative-strand RNA virus entirely from cloned complementary DNAs. Proc. Natl. Acad. Sci. USA 93**:**15400-15404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bridgen, A., F. Weber, J. K. Fazakerley, and R. M. Elliott. 2001. Bunyamwera bunyavirus nonstructural protein NSs is a nonessential gene product that contributes to viral pathogenesis. Proc. Natl. Acad. Sci. USA 98**:**664-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brower, C. S., S. Sato, C. Tomomori-Sato, T. Kamura, A. Pause, R. Stearman, R. D. Klausner, S. Malik, W. S. Lane, I. Sorokina, R. G. Roeder, J. W. Conaway, and R. C. Conaway. 2002. Mammalian Mediator subunit mMED8 is an Elongin BC-interacting protein that can assemble with Cul2 and Rbx1 to reconstitute a ubiquitin ligase. Proc. Natl. Acad. Sci. USA 99**:**10353-10358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bryant, G. O., and M. Ptashne. 2003. Independent recruitment in vivo by Gal4 of two complexes required for transcription. Mol. Cell 11**:**1301-1309. [DOI] [PubMed] [Google Scholar]
- 15.Buchholz, U. J., S. Finke, and K. K. Conzelmann. 1999. Generation of bovine respiratory syncytial virus (BRSV) from cDNA: BRSV NS2 is not essential for virus replication in tissue culture, and the human RSV leader region acts as a functional BRSV genome promoter. J. Virol. 73**:**251-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chadick, J. Z., and F. J. Asturias. 2005. Structure of eukaryotic Mediator complexes. Trends Biochem. Sci. 30**:**264-271. [DOI] [PubMed] [Google Scholar]
- 17.Conaway, R. C., S. Sato, C. Tomomori-Sato, T. Yao, and J. W. Conaway. 2005. The mammalian Mediator complex and its role in transcriptional regulation. Trends Biochem. Sci. 30**:**250-255. [DOI] [PubMed] [Google Scholar]
- 18.Dotson, M. R., C. X. Yuan, R. G. Roeder, L. C. Myers, C. M. Gustafsson, Y. W. Jiang, Y. Li, R. D. Kornberg, and F. J. Asturias. 2000. Structural organization of yeast and mammalian Mediator complexes. Proc. Natl. Acad. Sci. USA 97**:**14307-14310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dunn, E. F., D. C. Pritlove, and R. M. Elliott. 1994. The S RNA genome segments of Batai, Cache Valley, Guaroa, Kairi, Lumbo, Main Drain and Northway bunyaviruses: sequence determination and analysis. J. Gen. Virol. 75**:**597-608. [DOI] [PubMed] [Google Scholar]
- 20.Elliott, R. M. 1996. The Bunyaviridae: concluding remarks and future prospects, p. 295-332. In R. M. Elliott (ed.), The Bunyaviridae. Plenum Press, New York, N.Y.
- 21.Elliott, R. M. 1997. Emerging viruses: the Bunyaviridae. Mol. Med. 3**:**572-577. [PMC free article] [PubMed] [Google Scholar]
- 22.Fondell, J. D., H. Ge, and R. G. Roeder. 1996. Ligand induction of a transcriptionally active thyroid hormone receptor coactivator complex. Proc. Natl. Acad. Sci. USA 93**:**8329-8333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giorgi, C. 1996. Molecular biology of phleboviruses, p. 105-128. In R. M. Elliott (ed.), The Bunyaviridae. Plenum Press, New York, N.Y.
- 24.Holstege, F. C., E. G. Jennings, J. J. Wyrick, T. I. Lee, C. J. Hengartner, M. R. Green, T. R. Golub, E. S. Lander, and R. A. Young. 1998. Dissecting the regulatory circuitry of a eukaryotic genome. Cell 95**:**717-728. [DOI] [PubMed] [Google Scholar]
- 25.Ito, M., C. X. Yuan, S. Malik, W. Gu, J. D. Fondell, S. Yamamura, Z. Y. Fu, X. Zhang, J. Qin, and R. G. Roeder. 1999. Identity between TRAP and SMCC complexes indicates novel pathways for the function of nuclear receptors and diverse mammalian activators. Mol. Cell 3**:**361-370. [DOI] [PubMed] [Google Scholar]
- 26.James, P., J. Halladay, and E. A. Craig. 1996. Genomic libraries and a host strain designed for highly efficient two-hybrid selection in yeast. Genetics 144**:**1425-1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim, Y. J., S. Bjorklund, Y. Li, M. H. Sayre, and R. D. Kornberg. 1994. A multiprotein mediator of transcriptional activation and its interaction with the C-terminal repeat domain of RNA polymerase II. Cell 77**:**599-608. [DOI] [PubMed] [Google Scholar]
- 28.Kobor, M. S., and J. Greenblatt. 2002. Regulation of transcription elongation by phosphorylation. Biochim. Biophys. Acta 1577**:**261-275. [DOI] [PubMed] [Google Scholar]
- 29.Kohl, A., R. F. Clayton, F. Weber, A. Bridgen, R. E. Randall, and R. M. Elliott. 2003. Bunyamwera virus nonstructural protein NSs counteracts interferon regulatory factor 3-mediated induction of early cell death. J. Virol. 77**:**7999-8008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kohl, A., E. F. Dunn, A. C. Lowen, and R. M. Elliott. 2004. Complementarity, sequence and structural elements within the 3′ and 5′ non-coding regions of the Bunyamwera orthobunyavirus S segment determine promoter strength. J. Gen. Virol. 85**:**3269-3278. [DOI] [PubMed] [Google Scholar]
- 31.Komarnitsky, P., E. J. Cho, and S. Buratowski. 2000. Different phosphorylated forms of RNA polymerase II and associated mRNA processing factors during transcription. Genes Dev. 14**:**2452-2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kornberg, R. D. 2005. Mediator and the mechanism of transcriptional activation. Trends Biochem. Sci. 30**:**235-239. [DOI] [PubMed] [Google Scholar]
- 33.Laspia, M. F., A. P. Rice, and M. B. Mathews. 1989. HIV-1 Tat protein increases transcriptional initiation and stabilizes elongation. Cell 59**:**283-292. [DOI] [PubMed] [Google Scholar]
- 34.Laybourn, P. J., and M. E. Dahmus. 1989. Transcription-dependent structural changes in the C-terminal domain of mammalian RNA polymerase subunit IIa/o. J. Biol. Chem. 264**:**6693-6698. [PubMed] [Google Scholar]
- 35.Le May, N., S. Dubaele, L. P. De Santis, A. Billecocq, M. Bouloy, and J. M. Egly. 2004. TFIIH transcription factor, a target for the Rift Valley hemorrhagic fever virus. Cell 116**:**541-550. [DOI] [PubMed] [Google Scholar]
- 36.Lowen, A. C., C. Noonan, A. McLees, and R. M. Elliott. 2004. Efficient bunyavirus rescue from cloned cDNA. Virology 330**:**493-500. [DOI] [PubMed] [Google Scholar]
- 37.Macpherson, I., and M. Stoker. 1962. Polyoma transformation of hamster cell clones—an investigation of genetic factors affecting cell competence. Virology 16**:**147-151. [DOI] [PubMed] [Google Scholar]
- 38.McJunkin, J. E., E. C. de los Reyes, J. E. Irazuzta, M. J. Caceres, R. R. Khan, L. L. Minnich, K. D. Fu, G. D. Lovett, T. Tsai, and A. Thompson. 2001. La Crosse encephalitis in children. N. Engl. J. Med. 344**:**801-807. [DOI] [PubMed] [Google Scholar]
- 39.Mittler, G., T. Stuhler, L. Santolin, T. Uhlmann, E. Kremmer, F. Lottspeich, L. Berti, and M. Meisterernst. 2003. A novel docking site on Mediator is critical for activation by VP16 in mammalian cells. EMBO J. 22**:**6494-6504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Myers, L. C., C. M. Gustafsson, D. A. Bushnell, M. Lui, H. Erdjument-Bromage, P. Tempst, and R. D. Kornberg. 1998. The Med proteins of yeast and their function through the RNA polymerase II carboxy-terminal domain. Genes Dev. 12**:**45-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nekhai, S., M. Zhou, A. Fernandez, W. S. Lane, N. J. Lamb, J. Brady, and A. Kumar. 2002. HIV-1 Tat-associated RNA polymerase C-terminal domain kinase, CDK2, phosphorylates CDK7 and stimulates Tat-mediated transcription. Biochem. J. 364**:**649-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ni, Z., B. E. Schwartz, J. Werner, J. R. Suarez, and J. T. Lis. 2004. Coordination of transcription, RNA processing, and surveillance by P-TEFb kinase on heat shock genes. Mol. Cell 13**:**55-65. [DOI] [PubMed] [Google Scholar]
- 43.Nichol, S. T., B. Beaty, R. M. Elliott, R. Goldbach, A. Plyusnin, A. L. Schmaljohn, and R. B. Tesh. 2005. Bunyaviridae, p. 695-716. In C. M. Fauquet, M. A. Mayo, J. Maniloff, U. Desselberger, and L. A. Ball (ed.), Virus taxonomy. Elsevier, Amsterdam, The Netherlands.
- 44.Orphanides, G., and D. Reinberg. 2002. A unified theory of gene expression. Cell 108**:**439-451. [DOI] [PubMed] [Google Scholar]
- 45.Qiu, H., C. Hu, F. Zhang, G. J. Hwang, M. J. Swanson, C. Boonchird, and A. G. Hinnebusch. 2005. Interdependent recruitment of SAGA and Srb mediator by transcriptional activator Gcn4p. Mol. Cell. Biol. 25**:**3461-3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rachez, C., B. D. Lemon, Z. Suldan, V. Bromleigh, M. Gamble, A. M. Naar, H. Erdjument-Bromage, P. Tempst, and L. P. Freedman. 1999. Ligand-dependent transcription activation by nuclear receptors requires the DRIP complex. Nature 398**:**824-828. [DOI] [PubMed] [Google Scholar]
- 47.Streitenfeld, H., A. Boyd, J. K. Fazakerley, A. Bridgen, R. M. Elliott, and F. Weber. 2003. Activation of PKR by Bunyamwera virus is independent of the viral interferon antagonist NSs. J. Virol. 77**:**5507-5511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Struthers, J. K., and R. Swanepoel. 1982. Identification of a major non-structural protein in the nuclei of Rift Valley fever virus-infected cells. J. Gen. Virol. 60**:**381-384. [DOI] [PubMed] [Google Scholar]
- 49.Thomas, D., G. Blakqori, V. Wagner, M. Banholzer, N. Kessler, R. M. Elliott, O. Haller, and F. Weber. 2004. Inhibition of RNA polymerase II phosphorylation by a viral interferon antagonist. J. Biol. Chem. 279**:**31471-31477. [DOI] [PubMed] [Google Scholar]
- 50.Weber, F., A. Bridgen, J. K. Fazakerley, H. Streitenfeld, N. Kessler, R. E. Randall, and R. M. Elliott. 2002. Bunyamwera bunyavirus nonstructural protein NSs counteracts the induction of alpha/beta interferon. J. Virol. 76**:**7949-7955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weber, F., E. F. Dunn, A. Bridgen, and R. M. Elliott. 2001. The Bunyamwera virus nonstructural protein NSs inhibits viral RNA synthesis in a minireplicon system. Virology 281**:**67-74. [DOI] [PubMed] [Google Scholar]
- 52.Yang, Z., J. H. Yik, R. Chen, N. He, M. K. Jang, K. Ozato, and Q. Zhou. 2005. Recruitment of P-TEFb for stimulation of transcriptional elongation by the bromodomain protein Brd4. Mol. Cell 19**:**535-545. [DOI] [PubMed] [Google Scholar]
- 53.Yoneyama, M., W. Suhara, Y. Fukuhara, M. Fukuda, E. Nishida, and T. Fujita. 1998. Direct triggering of the type I interferon system by virus infection: activation of a transcription factor complex containing IRF-3 and CBP/p300. EMBO J. 17**:**1087-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]