Increased Angiogenic Response in Aortic Explants of Collagen XVIII/Endostatin-Null Mice (original) (raw)
Abstract
Endostatin, a proteolytic fragment of basement membrane-associated collagen XVIII, has been shown to be a potent angiogenesis inhibitor both in vivo and in vitro when given at high concentrations. The precise molecular mechanisms by which it functions and whether or not it plays a role in physiological regulation of angiogenesis are not clear. In mice with targeted null alleles of Col18a1, there appears to be no major abnormality in vascular patterns or capillary density in most organs. Furthermore, the growth of experimental tumors is not increased. However, a detailed analysis of induced angiogenesis in these mice has not been performed. Therefore, we compared the angiogenic responses induced by in vitro culture of aortic explants from collagen XVIII/endostatin-null mice (ko) to wild-type (wt) littermates. We found a twofold increase in microvessel outgrowth in explants from ko mice, relative to wt explants. This increased angiogenesis was reduced to the wt level by the addition of low levels (0.1 μg/ml) of recombinant mouse or human endostatin during the culture period. To address cellular/molecular mechanisms underlying this difference in angiogenic response between ko and wt mice, we isolated endothelial cells from both strains and compared their biological behavior. Proliferation assays showed no difference between the two types of endothelial cells. In contrast, adhesion assays showed a striking difference in their ability to adhere to fibronectin suggesting that collagen XVIII/endostatin may regulate interactions between endothelial cells and underlying basement membrane-associated components, including fibronectin, such that in the absence of collagen XVIII/endostatin, endothelial cells are more adhesive to fibronectin. In the aortic explant assay, characterized by dynamic processes of microvessel elongation and regression, this may result in stabilization of newly formed vessels, reduced regression, and a net increase in microvessel outgrowth in explants from ko mice compared to the wt littermates.
Collagen XVIII is a heparan sulfate-containing collagen and proteoglycan that is located in the basement membranes (BMs) of epithelia and vascular endothelium. Endostatin (ES), a proteolytic fragment of the C-terminal nontriple-helical (NC1) domain of collagen XVIII, has been identified as a potent angiogenesis inhibitor.1 Recombinant ES has been reported to inhibit endothelial cell proliferation1 and migration2 and to induce endothelial cell apoptosis,3,4 but it is unclear whether ES functions as a physiological regulator of angiogenesis. To gain insights into the potential physiological role of collagen XVIII_/_ES as a local regulator of angiogenesis, we generated _Col18a1_-null mice by gene targeting. Surprisingly, the mice were fertile and had a normal life span. Histological studies of embryos and adult mice have shown ocular abnormalities, including 1) delayed hyaloid vessel regression and abnormal outgrowth of the retinal vasculature;5 2) developmental defects in the iris characterized by rupture of the posterior iris pigment epithelium and flattening of the ciliary epithelium;6,7 3) age-dependent thickening of the anterior iris BM and abnormal migration of pigmented macrophage-like cells from the iris along the retina;6 and 4) age-dependent formation of abnormal deposits between the basal infoldings of the retinal pigment epithelium, resulting in deterioration of retinal pigment epithelium function and attenuation of visual function with pathological electroretinograms.8 No microscopic defects were observed in the extraocular vascular system, indicating that collagen XVIII/ES is not required for normal vasculogenesis and angiogenesis in most tissues/organs. However, this does not rule out the possibility that collagen XVIII/ES plays some role as a local, BM-associated regulator of angiogenesis in some physiological/pathological contexts. To address this possibility we used an aortic explant assay to compare in vitro angiogenesis with wild-type (wt) and _Col18a1_-null tissues. The aortic explants from _Col18a1_-null mice showed a significantly higher number of long microvessel sprouts than explants from wt littermates. This increased outgrowth of microvessels could be reduced to wt levels by addition of recombinant ES to the explant cultures. To further study the possible mechanism(s) involved in the increased aortic explant angiogenesis in ko mice, we compared the biological behavior of endothelial cells derived from lung tissues of wt and ko mice. We conclude that collagen XVIII/ES can negatively modulate angiogenic processes by mediating interactions between endothelial cells and underlying extracellular matrix components, such as fibronectin (FN).
Materials and Methods
Aortic Explant Assay
Segments of thoracic aorta, 1 to 2 cm in length, were excised in a sterile manner from 4- to 7-month-old _Col18a1_-null mice, as well as from wt littermates. The specimens were dissected carefully to remove surrounding fibroadipose tissue, rinsed extensively with phosphate-buffered saline (PBS), opened and sectioned into ∼1-mm rectangular pieces, and embedded in type I collagen gel. The gel was made by adding 1 ml of chilled collagen solution into prechilled culture inserts with 0.45-μm pore-size polyethylene terephthalate membrane of six-well plates (Becton Dickinson, Bedford, MA) and gelled at 37°C for 30 minutes. The final collagen solution was obtained by mixing 7 vol of 4.15 mg/ml rat tail type I collagen (Becton Dickinson) with 2 vol of 1.17% NaHCO3 and 1 vol of 10× minimal essential medium (Life Technologies Inc., Rockville, MD). After gelation, 2 ml of Endothelial-SFM medium (Life Technologies Inc.) was added into inserts and 2.5 ml of the same medium to the lower wells. The medium was changed every 2 days. The aortic explants, four to five explants in each well, were cultured at 37°C, 5% CO2 for a period of 15 to 21 days. Explants were treated with soluble recombinant ES at concentrations of 0.1, 0.25, 0.5, or 1 μg/ml. We used both mouse and human ES produced in 293EBNA cells and purified as described2 or human ES produced in Pichia pastoris (a generous gift from Dr. K. L. Sim, EntreMed, Inc., Rockville, MD). No differences in activities between mouse and human ESs were observed in the assays reported here. Untreated cultures were used as controls. Angiogenesis was quantitated in two different ways. One method consisted of counting the number of free tips of microvessels growing out from the two long edges of the rectangular explants. In this method we did not attempt to adjust the counts for variations in the size of the explant rectangles, but simply tried to select explants from different experiments and different animals that were similar in their lengths and widths for counting. The second method was designed to assess the number of long microvessels growing out from the long edges of the rectangular explants. In this method, we counted the number of microvessels extending to or beyond a distance of 414 μm from the long edges of the explanted tissue as illustrated in Figure 1. To control for variations in size of the explants, the number of long microvessels was expressed as number of long microvessels per μm of long explant edge (microvessel density). This method was used to combine data from three experiments, with different experiments using aortic tissue from different animals. In experiments with wt tissue, one mouse was used for each experiment; in experiments with ko tissue, two mice (littermates) were used for each experiment. In each experiment four to five explants were analyzed in each group of wt and ko explants. The Student’s _t_-test was used for statistical analysis.
Figure 1.
Diagram for quantification of long microvessel sprouts in aortic explant assay. During real-time imaging of the rectangular explants the number of long vessels that emerged from the long explant edges and reached or crossed a virtual line (L) drawn at a distance of 414 μm (chosen for convenience based on the physical parameters of the imaging setup) from each edge were counted. The number of vessels (N) reaching or crossing line L over length B were divided by the length (in μm) of the corresponding explant edge (C) to give the density of microvessels along that edge.
Immunohistochemical and Immunofluorescent Analysis
Immunostaining was performed on whole mounts of aortic explant cultures. Gels containing aortic explants were fixed with 4% paraformaldehyde in PBS for 4 hours at 4°C, washed briefly in PBS, treated with 0.25% Triton X-100 in PBS for 1 hour, and blocked with 1.5% blocking reagent (BioGenex, San Ramon, CA) or 1% bovine serum albumin (BSA)/PBS (Sigma, St. Louis, MO) for 2 hours at room temperature. Anti-mouse CD31 (PECAM, 1:800; BD PharMingen, San Diego, CA), anti-α-smooth muscle actin (α-SMA, 1:500; Sigma), anti-collagen IV (1:1000, Chemicon Int., Inc., Temecula, CA) and anti-FN monoclonal antibody (1: 400, Sigma) were used as primary antibodies and gels were incubated at 4°C overnight with gentle shaking. The gels were washed for 2 hours with three changes of PBS. For immunohistochemical staining, cultures were incubated with biotinylated secondary antibodies for 3 hours at room temperature with gentle shaking. After 2 hours of washing, the gels were incubated with ABC reagent for 1 hour, and the bound antibodies were detected by Vector VIP or diaminobenzidine substrate (Vector Laboratories, Inc., Burlingame, CA). For immunofluorescent staining, gels were incubated with fluorescein isothiocyanate (FITC)-conjugated secondary antibodies (Vector Laboratories, Inc.) for 3 hours. Images were analyzed using Nikon E800 upright microscope (Nikon, Melville, NY). Controls for immunostaining included incubations with species-matched immunoglobulin and incubations in which the primary antibody was omitted.
Electron Microscopy
Aortic explants embedded in collagen gels were fixed at room temperature for 1 hour with 4% glutaraldehyde and 4% paraformaldehyde in 0.1 mol/L cacodylate buffer, pH 7.4, rinsed and postfixed in cold 1% osmium tetroxide in the same buffer for 1 hour, and stained en bloc for 30 minutes with saturated uranyl acetate in distilled water. They were dehydrated through graded ethanols, cleared in propylene oxide, and embedded in Epon/Araldite. Thin sections were obtained and examined by transmission electron microscopy.
Isolation of Mouse Lung Endothelial Cells
The lungs of 2- to 3-month-old ko and wt mice (more than 10 mice in each group) were perfused with PBS-heparin (1 U/ml, Sigma) and collected into 40 ml of Dulbecco’s modified Eagle’s medium/F12. The tissue was minced into small pieces, and digested in 20 ml of 0.2% collagenase I (Sigma) at 37°C for 1 hour with occasional shaking. The solution was passed through a nylon gauze filter (two layers, 200 mesh) and centrifuged. The pellet was collected and resuspended in 1% BSA/PBS. Dynabeads M-450 with sheep anti-rat IgG (Dynal Biotech Inc., Lake Success, NY) were conjugated with rat anti-mouse CD31 (MEC13.3 no azide/low endotoxin, PharMingen) and added into the cell suspension. After incubating for 30 minutes with rotation, the cells bound to the beads were isolated by placing the tubes on a magnetic device (MPC, Dynal Biotech Inc.). The beads were removed by incubation with 0.25% trypsin/ethylenediaminetetraacetic acid (Irvine Scientific, Santa Ana, CA) for 5 to 10 minutes, and the isolated mouse lung endothelial cells (mLECs) were plated on a gelatin-coated (Cascade Biologics, Inc., Portland, OR) 10-cm-diameter culture dish and cultured in Dulbecco’s modified Eagle’s medium/F12 medium (Mediatech, Inc., Herndon, VA) supplemented with 10% fetal bovine serum (Paragon Biotech, Inc., Baltimore, MD), 20 mmol/L HEPES (Sigma), 100 μg/ml streptomycin, 100 U/ml penicillin (Irvine Scientific, Santa Ana, CA), 2 mmol/L sodium pyruvate, 20 U/ml heparin (Sigma), 100 μg/ml endothelial cell growth supplement (Becton Dickinson). The medium was changed every 2 days, and cells from passage 3 to 10 were used for the experiments.
Indirect Immunofluorescence of Endothelial Cells
ECs were cultured on gelatin-coated glass coverslips and fixed in acetone/methanol at 1:1 ratio for 15 minutes at −20°C. The cells were blocked in 1% BSA/PBS for 1 hour and probed with primary antibodies against mouse CD31 (1:400), Tie2 (1:200), mouse recombinant ES (1:400; Medical & Biological Laboratories Co., Ima-City, Japan), Von Willebrand Factor (vWF, 1:400; DAKO Corp., Carpinteria, CA) or α-SMA (1:400). Secondary antibodies, conjugated with FITC, were used for detection by conventional fluorescence microscopy.
Proliferation Assay
Trypsinized ECs suspended in 5% fetal bovine serum-containing medium were seeded into gelatin-coated 96-well plates at a density of 2000 cells/well with or without human ES (0.5 μg/ml). At each time point of 0, 12, 24, 48, 72, 96, and 120 hours, CellTiter nonradioactive cell proliferation assay (Promega Corp., Madison, WI) was performed according to the manufacturer’s instructions. The amount of 490-nm absorbance, proportional to the number of living cells, was measured using an enzyme-linked immunosorbent assay plate reader (Molecular Devices, Sunnyvale, CA).
Adhesion Assay
The cell adhesion assay was performed as previously described9 with slight modifications. Briefly, 96-well plates were coated overnight at 4°C with collagen I (50 μg/ml, Becton Dickinson), laminin (LN, 20 μg/ml), collagen IV (10 μg/ml), or FN (10 μg/ml) diluted in PBS. PBS alone was used as control. All of the above reagents were purchased from Sigma unless otherwise indicated. Wells were washed with PBS and blocked with 0.1% BSA/PBS for 1 hour at room temperature. mLECs were harvested by trypsin treatment, labeled with 5 μmol/L Calcein AM (Molecular Probes, Inc., Eugene, OR), collected in Dulbecco’s modified Eagle’s medium/F12 containing 0.1% BSA, and washed three times with the same medium. Subsequently, 2 × 104 cells in 100 μl of medium/BSA were added to each well and incubated for 45 minutes at 37°C in 5% CO2. Nonadherent cells were removed with four vigorous PBS washes. The relative number of adherent cells was determined by fluorescent intensity measured by a fluorescence microplate reader (LJL Biosystem, Analyst).
To study the effect of exogenous ES on cell adhesion to FN, mLECs were either preincubated with 0.5 μg/ml human ES for 30 minutes at 37°C and washed two times in medium/BSA before seeding or ES of indicated concentrations was added to the cell suspensions immediately before seeding. The absence of ES was used as control.
For heparin treatment studies, 96-well plates were coated with FN overnight at 4°C, and 9 μg/ml of heparin either alone or together with 0.5 μg/ml of ES were added to cell suspensions. The omission of both heparin and ES was used as control.
For studies of the effects of RGD-containing peptides, 96-well plates were coated with FN overnight at 4°C, and the peptide GRGDS (Sigma) added at concentrations of 0.01, 0.05, 0.1, or 0.2 mmol/L to the cell suspensions, followed by a 20-minute incubation at 37°C before the cells were added to the FN-coated wells. The absence of GRGDS or the addition of SDGRG peptide (Sigma) at a concentration of 0.1 mmol/L were used as controls.
To block synthesis of endogenous collagen XVIII/ES, confluent cells of both ko (control) and wt were incubated with 2.5 μg/ml cycloheximide (Sigma) for 12 hours at 37°C, 5% CO2. To completely eliminate collagen XVIII-associated ES from cell surfaces, cells were first treated with cycloheximide for 12 hours and then treated with 0.5 μg/ml Cathepsin L (Cat L, Calbiochem Corp., La Jolla, CA) in 50 mmol/L Tris-acetate, pH 5.5, containing 5 mmol/L dithiothreitol at 37°C for 8 minutes. The treatment with Cat L was based on previous studies of proteolytic release of ES10 and on experiments in which a brief Cat L treatment was shown to release ES from mLECs (see below). The subsequent adhesion assays to FN were performed as described above. For all adhesion assays, the results are presented as mean values from three individual experiments. In each experiment eight wells were analyzed for each treatment group of cells. Student’s _t_-test was used for statistical analysis.
Western Blotting
Confluent wt mLECs were washed two times with PBS and incubated with 0.5 μg/ml Cat L in 50 mmol/L Tris-acetate, pH 5.5, containing 5 mmol/L dithiothreitol at 37°C for 5, 10, and 30 minutes. Cathepsin inhibitor E64 (2 μmol/L) (Sigma) was added at the end of each incubation. Supernatants were collected and concentrated approximately sixfold using Centricon (Millipore Corp., Bedford, MA). The treated cells, as well as control wt cells without Cat L treatment and untreated ko mLECs were solubilized for 30 minutes at 4°C with rotation in a lysis buffer, containing 10 mmol/L Tris-HCl, pH 7.5, 1% Nonidet P-40, 0.25% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 0.15 mol/L NaCl, 6 mmol/L ethylenediaminetetraacetic acid, a cocktail of protease inhibitors (Sigma) and 2 μmol/L E64. Lysates were centrifuged at 14,000 rpm for 10 minutes and supernatants were concentrated as described above. Samples were separated on 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis under reducing condition (4% β-mercaptoethanol) and proteins were transferred onto nitrocellulose membranes. Membranes were probed for 1 hour with anti-mouse ES polyclonal antibody and 30 minutes with horseradish peroxidase anti-rabbit IgG (1:5000; Santa Cruz Biotechnology, Santa Cruz, CA). The blots were visualized with chemiluminescent substrate (Pierce, Rockford, IL).
Tube Formation
The assay for endothelial cell tube formation was performed as previously described11 with few modifications. Briefly, 45 μl of reduced Matrigel (Becton Dickinson) was added to prechilled 96-well plates and allowed to gel at 37°C for 30 minutes. mLECs from collagen XVIII ko and wt mice (15,000 cells/well) were seeded with or without exogenous human ES (0.5 μg/ml). Cells were incubated for 8 hours at 37°C, 5% CO2. The formation of capillary-like tubes was studied by phase contrast microscopy.
Results
Microvessel Formation in Mouse Aortic Explants
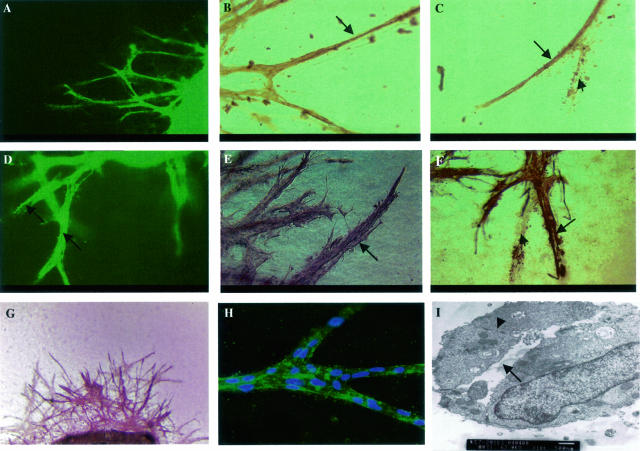
Aortic explants embedded in collagen gels and cultured in serum-free medium gave rise to microvessels that resemble capillary structures. The cells at the core of the sprouts were immunoreactive with the endothelial cell-specific anti-CD31 antibody as shown in Figure 2, A to C (arrows), whereas peri-endothelial cells were immunonegative. The sprouts showed positive staining with anti-α-SMA antibody in the outer layer of sprouts (Figure 2; D to F, arrows). We also demonstrated positive staining for other endothelial cell markers, such as Tie2, Flk1, and endoglin (data not shown). The microvessel sprouts showed immunoreactivity with anti-BM component antibodies, as shown in Figure 2G for collagen IV and Figure 2H for FN. Electron microscopy revealed the presence of a lumen (Figure 2I, arrow) in the sprouting vessels and the presence of typical endothelial cell-cell junctions (Figure 2I, arrowhead). The growth of microvessels in this culture system is a self-regulated, highly dynamic process, consisting of capillary growth and regression that recapitulates vascular remodeling during angiogenesis. In some regions of the cultures, regression resembling in vivo vascular remodeling could be observed (Figure 2, C and F; arrowheads). These cellular processes continued during a 2- to 3-week or longer period of culture.
Figure 2.
Whole mount immunostaining of microvessel sprouts from mouse aortic explants embedded in collagen gels and cultured for 15 to 21 days. A to C: Staining for endothelial cell-specific marker, CD31. A: CD31 staining is visualized by FITC-conjugated secondary antibody. B and C: Staining pattern (diaminobenzidine substrate) shows specific immunoreactivity in the core of sprouts, as indicated by arrows. D to F: Staining for smooth muscle cell marker, α-SMA. D: Anti-α-SMA antibody detected by FITC-conjugated secondary antibody. Note that the diameter of the sprout is much bigger than when the sprouts are stained for CD31 (A). A lumen can be seen in the middle of sprouts, as indicated by arrows. E and F: Outer cell layer of sprouts is α-SMA-positive (VIP substrate for E and diaminobenzidine substrate for F), arrows. C and F: Images of vessel regression (arrowheads) during outgrowth demonstrate cell debris and silhouettes of previously existing vessels. G: Sprouts show positive staining (VIP substrate) for collagen IV. H: Fluorescent staining for FN. Secondary antibody conjugated with FITC. Nuclei labeled with DAPI. I: Sprouts, examined by EM, reveal lumen (arrow) and cell-cell junction (arrowhead). Original magnifications: ×8 (A, D); ×20 (B, C, E, F); ×5 (G); ×40 (H); ×10,000 (I).
Quantitative Analysis of Angiogenesis in Aortic Explant Assay
Previous studies have shown that collagen XVIII/ES is present at relatively high levels in the aortic wall.12 We used the aortic explant assay to test whether these levels are functionally sufficient for local regulation of induced angiogenesis. We cultured aortic explants from homozygous mutant and wt mice and examined the outgrowth of microvessels. We used serum-free media without adding exogenous angiogenic factors. The results showed that explants from _Col18a1_-null mice had more than a twofold increase in the number of microvessel ends, as compared to explants from wt littermates (Figure 3, A and B). The number of sprout ends in Figure 3C represent the average number of tips of microvessels growing out from the long edges of explants derived from ko and wt mice. The numbers in Figure 3D represent the average value of long vessel number per unit length of explant edge (see Materials and Methods). The addition of recombinant ES to the cultures at a concentration as low as 0.1 μg/ml reduced vascular outgrowth in the mutant cultures to the wt control level (Figure 3, C and D), but had no effects on the wt cultures. Further inhibition of outgrowth in both wt and ko explants required a much higher concentration (>2 μg/ml, data not shown).
Figure 3.
Microvessel outgrowth in aortic explants from _Col18 a1_-null mice and wt littermates. A and B: Cultures (incubated for 17 days) stained with anti-CD31 antibody (VIP substrate) (A, ko; B, wt). C: Quantitation of microvessel outgrowth in ko and wt explants by counting the number of ends of vessel sprouts that had grown out from the two long edges of the rectangular explants. The cultures were incubated in the absence or presence of various concentrations of recombinant ES. The heights of the columns in the histogram show the arithmetic mean number of vessel ends in the various groups; the standard deviations are indicated by the vertical error bars. Except for the groups of wt explants treated with 0.1 μg/ml or 0.5 μg/ml of ES in which three explants were used for counting, all other groups show the results of counting vessel ends in four to seven explants. The difference in number of vessel ends between ko and wt explants in the absence of ES was highly significant (*, P < 0.005). D: Quantitation of long microvessel sprouts in aortic explants shows that the arithmetic mean number of long vessels growing from the two long edges of explants (expressed as vessel density, see Materials and Methods) was significantly higher (*, P < 0.005) in explants from ko mice than from wt mice. The results shown are derived from several different explants, experiments, and mice as described in Materials and Methods. The increased outgrowth in ko explants was inhibited by the addition of exogenous ES at a concentration as low as 0.1 μg/ml (*, P < 0.005 when comparing ko sample without ES and ko samples with ES). Standard deviations are indicated by the vertical error bars. Original magnifications, ×4 (A, B).
Proliferative Activities of mLECs
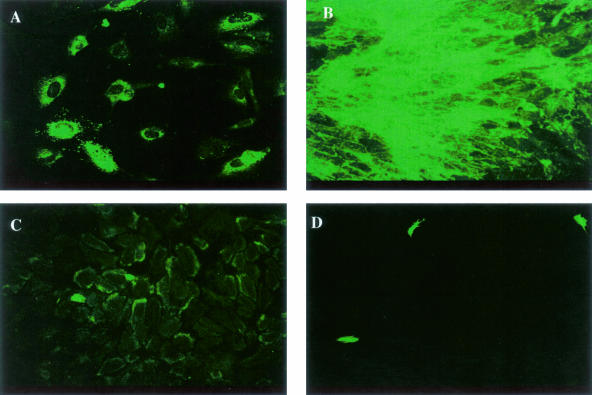
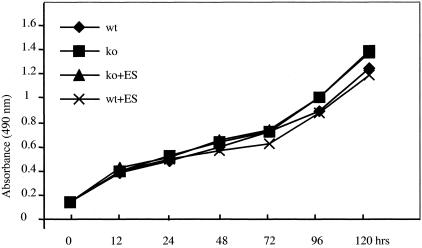
To address the question of what mechanism(s) contribute to the increased in vitro angiogenic response in aortic explants from _Col18a1_-null mice, we isolated endothelial cells (mLECs) from mouse lungs of both ko and wt mice. The endothelial cells were characterized by staining for endothelial-specific markers. Both mutant and wt mLECs were immunoreactive for vWF (Figure 4A), CD31 (Figure 4B), and Tie 2 (Figure 4C). There were few α-SMA-positive cells (Figure 4D). First we tested whether there was a difference in proliferative activity between mutant and wt mLECs; however, the results showed no significant differences in the growth rates (Figure 5). There was no inhibitory effect of exogenous ES (0.5 μg/ml) on proliferation of both cell types. In addition, mutant and wt cells showed similar stimulatory responses to vascular endothelial growth factor and basic fibroblast growth factor and inhibitory responses to transforming growth factor-β1 (data not shown).
Figure 4.
Lung endothelial cells isolated from ko mice stained with antibodies against endothelial and smooth muscle cell markers. A: Staining for von Willebrand factor VIII; B: CD31; C: Tie2; D: α-SMA.
Figure 5.
Proliferation assay of mLECs derived from ko and wt mice. Cells cultured in 5% fetal bovine serum containing medium with or without ES (0.5 μg/ml) for 12 to 120 hours. The results shown at each time point are the arithmetic means of the absorbance values measured in 8 wells of a 96-well plate. No significant differences between mutant and wt cells and no effect of ES.
Adhesion Analysis of mLECs to Extracellular Matrix Proteins
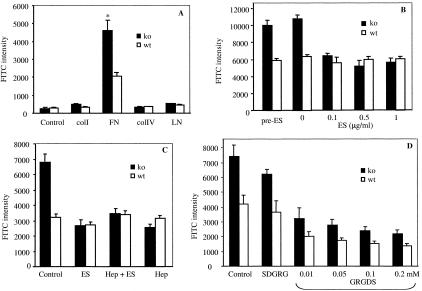
Because collagen XVIII is a heparan sulfate proteoglycan and component of BMs,13 it is possible that it may regulate angiogenesis by modulating interactions between endothelial cells and the underlying matrix. To examine this possibility, we evaluated the ability of mLECs to adhere to different components of BM. As shown in Figure 6A, mutant cells had strikingly higher adherence to FN compared to wt mLECs. In contrast, there were no significant differences in the adhesion to LN, collagen I, or collagen IV between the two cell types. Exogenous ES added during the assay inhibited adhesion of mutant mLECs to FN, but had no effect on wt cells as seen in Figure 6B. Furthermore, the addition of heparin at 9 μg/ml, either alone or together with ES, inhibited the increased adhesion of mutant mLECs to FN in a similar manner (Figure 6C). We did not observe significant inhibitory effect of ES when mutant cells were preincubated with ES for 30 minutes and washed before seeding (Figure 6B). Addition of RGD peptide reduced the adhesion of both ko and wt cells to FN proportionally; even at a peptide concentration of 0.2 mmol/L the mutant cells adhered better to FN than wt cells (Figure 6D).
Figure 6.
Adhesion analysis of mLECs. A: Cell adhesion to substrates collagen I (colI), FN, collagen IV (colIV), and LN. Endothelial cells derived from ko mice showed significantly higher adherence to FN than wt cells (*, P < 0.003 comparing ko and wt cells). B: Effect of exogenous ES on ko and wt mLEC adhesion to FN. No significant inhibition in both cell types when the cells were preincubated with ES and ES was washed away before adhesion assay (pre-ES, see Materials and Methods). Significant inhibition of adhesion obtained with ko cells when ES was added at 0.1 (or higher) μg/ml when plating cells (P < 0.005 comparing ko cell without and with 0.1 μg/ml of ES). ES did not inhibit wt mLEC adhesion at concentrations up to 1 μg/ml. C: Effect of heparin and ES on mLEC adhesion to FN. The adhesion of ko cells was reduced to the levels of wt cell adhesion by either ES (0.5 μg/ml) or heparin (Hep, 9 μg/ml) alone. Addition of both Hep and ES did not further inhibit adhesion of ko cells below the levels seen with either Hep or ES alone. The adhesion of wt cells to FN was not affected by addition of ES or Hep at the indicated concentrations. D: Effect of GRGDS peptide on adhesion of wt and ko mLECs to FN. The adhesion of both cell types was reduced with increasing concentrations of GRGDS peptide; however, at all concentrations tested the ko cells adhered better to FN than the wt cells. The addition of SDGRG peptide (at 0.1 mmol/L concentration) had no significant effect on the adhesion of either ko or wt cells to FN. Standard deviations are indicated by vertical error bars.
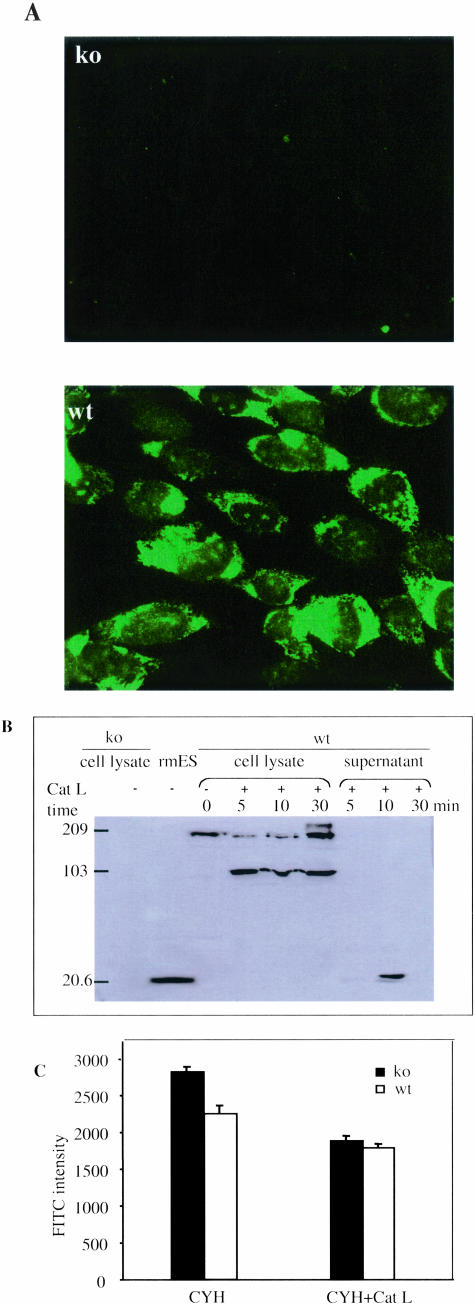
It has been shown that collagen XVIII is expressed in several major internal organs and in several cell types, including fibroblasts and endothelial cells.14 With an antibody against mouse recombinant ES, we demonstrated endogenous ES expression at the protein level in the cultured wt mLECs by both immunofluorescent staining and Western blots (Figure 7, A and B). Mutant cells were immunonegative. Cathepsin L is responsible for the generation of ES in hemangioendothelioma cell culture and can generate ES from recombinant NC1 protein.10 To evaluate the role of endogenous collagen XVIII/ES in the low degree of wt endothelial cell adhesion to FN (compared to ko cells), we first tested whether collagen XVIII on the cell surface could be cleaved by Cat L. As shown in Figure 7B, ES was released into cell-free supernatants after treatment of wt mLECs with Cat L for 10 minutes. Treatment for 30 minutes resulted in further degradation of the released ES, consistent with previous studies.10 In an attempt to eliminate endogenous ES from the cell surface of mLECs, we therefore treated cells with Cat L for 8 minutes after incubation for 12 hours with cycloheximide to block protein synthesis. When such wt and ko mLECs, treated with cycloheximide and Cat L, were used in adhesion assays, they both showed reduced adhesion and there was no difference in their adhesion to FN (Figure 7C).
Figure 7.
Detection of endogenous collagen XVIII/ES in cultured mLECs. A: Wt cells were immunoreactive to ES antibody as demonstrated by immunofluorescent staining (A, bottom panel); ko cells were negative (A, top panel). B: Western blot of cell lysates from ko and wt mLECs probed with anti-ES antibody. Molecular weight markers are indicated on the left. No bands are seen in lane containing lysate from ko cells (left). Lane containing recombinant mouse ES (rmES) shows band at 20 kd. Lane containing wt cell lysate shows band of intact collagen XVIII at 200 kd. Treatment of wt cell cultures with cathepsin L (Cat L) for 5 to 30 minutes before preparing the lysate results in generation of an ES-containing fragment of 100 kd. Supernatant of wt cells treated with Cat L for 10 minutes shows band of released ES; treatment for only 5 minutes shows a very faint ES band and treatment for 30 minutes appears to further degrade the released ES, consistent with previous findings that ES is sensitive to degradation by Cat L.10 C: Wt and ko mLECs treated with cycloheximide (CYH) or cycloheximide followed by treatment with cathepsin L (Cat L) show almost the same adhesion to FN.
Capillary-Like Tube Formation on Matrigel
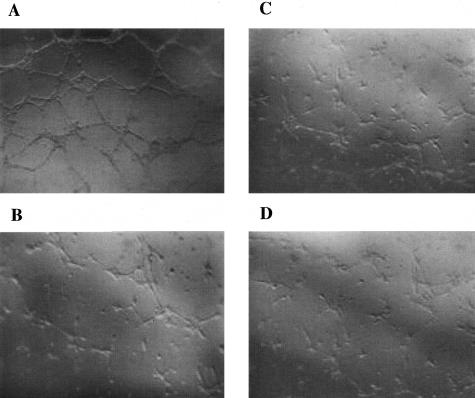
To compare the ability of mutant and wt mLECs to form capillary-like tubes in vitro, mLECs were plated on reduced Matrigel. This assay reflects cell-extracellular matrix interactions. As shown in Figure 8, under the same conditions, mutant mLECs formed more continuous capillary-like structures than wt cells. The addition of exogenous ES inhibited tube formation in the ko mLEC cultures so that the extent of tube formation appeared similar with mutant and wt cells.
Figure 8.
Capillary-like tube formation on Matrigel. Mutant mLECs formed more continuous networks (A) than wt mLECs (C). In the presence of ES (0.5 μg/ml) tube formation was similar in mutant (B) and wt mLECs (D).
Discussion
Angiogenesis is a complex biological process that begins with degradation of BM components, continues with endothelial cell migration and proliferation, and is followed by tube morphogenesis, synthesis, and assembly of new subendothelial BM along with investment of supporting smooth muscle cells and pericytes. We have taken advantage of a modified aortic explant assay, which comprises all of the steps involved in angiogenesis in vivo, to evaluate the role of both endogenous and exogenous collagen XVIII/ES as regulators of angiogenesis. The assay was modified based on the rat aortic ring culture initially described by Nicosia and colleagues.15,16 After injury by dissection, quiescent endothelial cells switch their phenotype and begin to migrate and proliferate in response to cytokines and growth factors released from the aortic tissue. All of the steps of angiogenesis, including a dynamic remodeling process during formation of vessels, occur in this assay and can be studied by whole mount immunohistochemical staining, as shown in Figure 2. The assay allowed the function of collagen XVIII/ES in induced angiogenesis to be examined independently of or in the presence of exogenous ES without interference by other unknown serum factors and without the complication of endothelial cell differentiation from circulating progenitor cells. Microvessel sprouting continues at a high level during the 2- to 3-week culture period. This suggests that continuous synthesis and secretion of local soluble autocrine and paracrine factors from aortic tissue or newly formed microvessels, sustains or amplifies the overall angiogenic sprouting. This behavior differs from the rat aortic ring assay in which vessel sprouting reaches a peak after 1 week of culture and begins to regress from the second week in the absence of exogenous vascular endothelial growth factor or basic fibroblast growth factor.
Using the aortic explant assay, we observed a twofold increase in the number and length of microvessels in explants from _Col18a1_-null mice. This suggests that aortic tissue is more angiogenic in the absence of endogenous collagen XVIII/ES. The underlying mechanism could be increased proliferation or migration of endothelial cells in the mutant explants or increased vascular stabilization by altered cell-matrix interactions during remodeling. We measured the length of the outgrowing vessels on a daily basis and did not observe any differences in the rate of sprout elongation between mutant and wt explants (data not shown). Hence, a difference in endothelial cell migration seems to be an unlikely explanation for the increased vessel density in the mutant explants. Furthermore, there was no significant difference in the proliferative activity of mLECs isolated from ko and wt mice and addition of exogenous ES did not significantly inhibit cell proliferation in both cell types. In addition, the cells also showed similar responses to growth factors; both were stimulated by basic fibroblast growth factor and to a lesser extent by vascular endothelial growth factor and inhibited by transforming growth factor-β1 (data now shown).
The most striking difference in biological behavior between wt and mutant ECs was in their ability to adhere to FN. Exogenous ES (at a concentration of 100 ng/ml) suppressed the elevated adhesion of mutant ECs to the wt level. The molecular basis for the adhesion of endothelial cells to extracellular matrix components involves two major families of cell-surface molecules, integrins, and heparan sulfate proteoglycans. FN contains several discrete domains that are involved in the binding to cell surfaces, including the C-terminal Hep II domain,17,18 the alternatively spliced type III connecting segment,19 and the central cell-binding domain.20 EC adhesion to FN is primarily mediated by α5β1,21 but also by proteoglycans on the cell surface. A chondroitin sulfate proteoglycan with weak affinity for FN reduced binding of FN to both cells and the purified FN receptor (α5β1), probably by steric hindrance.22 Because the adhesion of both mutant and wt cells to FN was inhibited proportionally by the addition of RGD peptide (the number of adhered cells was reduced to ∼35% in both cell types with 0.1 mmol/L RGD peptide added to the assay), we believe that differential binding to α5β1 integrin is unlikely to explain the difference in adhesion to FN of ko and wt mLECs.
Several lines of evidence point to an important role for intercalated heparan sulfate proteoglycan on cell surfaces in interactions with FN via its heparin binding sites.23,24 The ES domain of intact collagen XVIII, as well as proteolytically cleaved ES, can bind to heparan sulfate, and the affinity for binding to heparan sulfate is higher when ES is a trimeric domain within intact collagen XVIII than when it is proteolytically cleaved and released as a monomer.25,26 Thus, it is possible that endogenous collagen XVIII/ES affects cell-matrix interactions by occupying cell surface heparan sulfate sites, resulting in fewer binding sites for FN in wt cells than in collagen _Col18a1_-null cells. In support of this hypothesis is the finding that when heparin was added to the mutant and wt cell suspensions, either alone or together with ES before seeding, the cells exhibited similar adhesion to FN. This observation suggests that ES and heparin inhibit cell adhesion to FN through the same pathway, and that the difference in adhesion to FN between ko and wt endothelial cells is because of a heparan sulfate-dependent mechanism. The hypothesis implies that collagenXVIII/ES produced by wt mLECs before and/or during the adhesion assay inhibits cell-FN interactions. Consistent with this idea, we found that treatment of the cells with cycloheximide to inhibit protein synthesis followed by treatment with cathepsin L to release ES from cell surface-bound collagen XVIII, eliminated the difference in adhesion of wt and ko cells to FN. It has been shown that ES inhibits basic fibroblast growth factor-induced angiogenesis in the chorio-allantoic membrane assay, and that this inhibitory activity is abolished when two arginine residues in the heparin-binding site are converted to alanine.25 This suggests that in situations in which heparin-binding of ES is required for its anti-angiogenic activity, for example during inhibition of tumor-induced angiogenesis by exogenous ES, the effect of ES may be based on a mechanism similar to the one described here for the aortic explant angiogenesis model.
Tube formation during angiogenesis involves complex cell-cell and cell-matrix interactions. The increased capillary-like tube formation in mutant mLECs and its inhibition by exogenous ES further support the hypothesis that collagen XVIII/ES modulates cell-matrix interactions. On the basis of the above data, we conclude that collagen XVIII/ES can act as a local negative regulator of angiogenesis by modulating endothelial cell-extracellular matrix interactions, such as those involving FN. This modulation can lead to destabilization of newly formed vessels and cause regression.
Acknowledgments
We thank Dr. Dan Sun for help with the electron microscopy; Dr. Naomi Fukai for providing _Col18 a1-_null mice; and Dr. Masao Shibata at Medical and Biological Laboratories Co., Ltd. in Japan for the anti-ES antibody.
Footnotes
Address reprint requests to Bjorn R. Olsen, Department of Cell Biology, Harvard Medical School, 240 Longwood Ave., Boston, MA 02115. E-mail: bjorn_olsen@hms.harvard.edu.
Supported by the National Institutes of Health (grants AR 36820 and AR 048564).
References
- O’Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR, Folkman J. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277–285. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- Yamaguchi N, Anand-Apte B, Lee M, Sasaki T, Fukai N, Shapiro R, Que I, Lowik C, Timpl R, Olsen BR. Endostatin inhibits VEGF-induced endothelial cell migration and tumor growth independently of zinc binding. EMBO J. 1999;18:4414–4423. doi: 10.1093/emboj/18.16.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhanabal M, Ramchandran R, Waterman MJ, Lu H, Knebelmann B, Segal M, Sukhatme VP. Endostatin induces endothelial cell apoptosis. J Biol Chem. 1999;274:11721–11726. doi: 10.1074/jbc.274.17.11721. [DOI] [PubMed] [Google Scholar]
- Dixelius J, Larsson H, Sasaki T, Holmqvist K, Lu L, Engstrom A, Timpl R, Welsh M, Claesson-Welsh L. Endostatin-induced tyrosine kinase signaling through the Shb adaptor protein regulates endothelial cell apoptosis. Blood. 2000;95:3403–3411. [PubMed] [Google Scholar]
- Fukai N, Eklund L, Marneros AG, Oh SP, Keene DR, Tamarkin L, Niemela M, Ilves M, Li E, Pihlajaniemi T, Olsen BR. Lack of collagen XVIII/endostatin results in eye abnormalities. EMBO J. 2002;21:1535–1544. doi: 10.1093/emboj/21.7.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marneros AG, Olsen BR. Age-dependent iris abnormalities in collagen XVIII/endostatin deficient mice with similarities to human pigment dispersion syndrome. Invest Ophthalmol Vis Sci. 2003;44:2367–2372. doi: 10.1167/iovs.02-1180. [DOI] [PubMed] [Google Scholar]
- Ylikarppa R, Eklund L, Sormunen R, Kontiola AI, Utriainen A, Maatta M, Fukai N, Olsen BR, Pihlajaniemi T. Lack of type XVIII collagen results in anterior ocular defects. EMBO J. 2003;17:2257–2259. doi: 10.1096/fj.02-1001fje. [DOI] [PubMed] [Google Scholar]
- Marneros AG, Keene DR, Hansen U, Fukai N, Moulton K, Goeltz PL, Moiseyev G, Pawlyk BS, Halfter W, Dong S, Shibata M, Li T, Crouch RK, Bruckner P, Olsen BR. Collagen XVIII/endostatin is essential for vision and retinal pigment epithelial function. EMBO J. 2004;23:89–99. doi: 10.1038/sj.emboj.7600014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens CL, Cwirla SE, Lee RY, Whitehorn E, Chen EY, Bakker A, Martin EL, Wagstrom C, Gopalan P, Smith CW, Tate E, Koller KJ, Schatz PJ, Dower WJ, Barrett RW. Peptides which bind to E-selectin and block neutrophil adhesion. J Biol Chem. 1995;270:21129–21136. doi: 10.1074/jbc.270.36.21129. [DOI] [PubMed] [Google Scholar]
- Felbor U, Dreier L, Bryant RA, Ploegh HL, Olsen BR, Mothes W. Secreted cathepsin L generates endostatin from collagen XVIII. EMBO J. 2000;19:1187–1194. doi: 10.1093/emboj/19.6.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinda KM, Nomizu M, Chung M, Delgado M, Kuratomi Y, Yamada Y, Kleinman HK, Ponce ML. Identification of laminin alpha1 and beta1 chain peptides active for endothelial cell adhesion, tube formation, and aortic sprouting. EMBO J. 1999;13:53–62. [PubMed] [Google Scholar]
- Miosge N, Sasaki T, Timpl R. Angiogenesis inhibitor endostatin is a distinct component of elastic fibers in vessel walls. EMBO J. 1999;13:1743–1750. doi: 10.1096/fasebj.13.13.1743. [DOI] [PubMed] [Google Scholar]
- Halfter W, Dong S, Schurer B, Cole GJ. Collagen XVIII is a basement membrane heparan sulfate proteoglycan. J Biol Chem. 1998;273:25404–25412. doi: 10.1074/jbc.273.39.25404. [DOI] [PubMed] [Google Scholar]
- Saarela J, Rehn M, Oikarinen A, Autio-Harmainen H, Pihlajaniemi T. The short and long forms of type XVIII collagen show clear tissue specificities in their expression and location in basement membrane zones in humans. Am J Pathol. 1998;153:611–626. doi: 10.1016/S0002-9440(10)65603-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicosia RF, Ottinetti A. Growth of microvessels in serum-free matrix culture of rat aorta. A quantitative assay of angiogenesis in vitro. Lab Invest. 1990;63:115–122. [PubMed] [Google Scholar]
- Nicosia RF, Villaschi S. Rat aortic smooth muscle cells become pericytes during angiogenesis in vitro. Lab Invest. 1995;73:658–666. [PubMed] [Google Scholar]
- McCarthy JB, Chelberg MK, Mickelson DJ, Furcht LT. Localization and chemical synthesis of fibronectin peptides with melanoma adhesion and heparin binding activities. Biochemistry. 1988;27:1380–1388. doi: 10.1021/bi00404a044. [DOI] [PubMed] [Google Scholar]
- McCarthy JB, Hagen ST, Furcht LT. Human fibronectin contains distinct adhesion- and motility-promoting domains for metastatic melanoma cells. J Cell Biol. 1986;102:179–188. doi: 10.1083/jcb.102.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries MJ, Komoriya A, Akiyama SK, Olden K, Yamada KM. Identification of two distinct regions of the type III connecting segment of human plasma fibronectin that promote cell type-specific adhesion. J Biol Chem. 1987;262:6886–6892. [PubMed] [Google Scholar]
- Danen EH, Aota S, van Kraats AA, Yamada KM, Ruiter DJ, van Muijen GN. Requirement for the synergy site for cell adhesion to fibronectin depends on the activation state of integrin alpha 5 beta 1. J Biol Chem. 1995;270:21612–21618. doi: 10.1074/jbc.270.37.21612. [DOI] [PubMed] [Google Scholar]
- Dejana E, Colella S, Conforti G, Abbadini M, Gaboli M, Marchisio PC. Fibronectin and vitronectin regulate the organization of their respective Arg-Gly-Asp adhesion receptors in cultured human endothelial cells. J Cell Biol. 1988;107:1215–1223. doi: 10.1083/jcb.107.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hautanen A, Gailit J, Mann DM, Ruoslahti E. Effects of modifications of the RGD sequence and its context on recognition by the fibronectin receptor. J Biol Chem. 1989;264:1437–1442. [PubMed] [Google Scholar]
- Bernfield ME, Banerjee SD, Koda JE, Repraeger AC. Remodeling of the basement membrane: morphogenesis and maturation. CIBA Symp. 1984;108:179–196. doi: 10.1002/9780470720899.ch12. [DOI] [PubMed] [Google Scholar]
- Hook MKL, Johansson S, Robinson J. Cell-surface glycosaminoglycans. Annu Rev Biochem. 1984;53:847–896. doi: 10.1146/annurev.bi.53.070184.004215. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Larsson H, Kreuger J, Salmivirta M, Claesson-Welsh L, Lindahl U, Hohenester E, Timpl R. Structural basis and potential role of heparin/heparan sulfate binding to the angiogenesis inhibitor endostatin. EMBO J. 1999;18:6240–6248. doi: 10.1093/emboj/18.22.6240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T, Fukai N, Mann K, Gohring W, Olsen BR, Timpl R. Structure, function and tissue forms of the C-terminal globular domain of collagen XVIII containing the angiogenesis inhibitor endostatin. EMBO J. 1998;17:4249–4256. doi: 10.1093/emboj/17.15.4249. [DOI] [PMC free article] [PubMed] [Google Scholar]