Induction of Inflammatory Mediators and Microglial Activation in Mice Transgenic for Mutant Human P301S Tau Protein (original) (raw)
Abstract
Mice transgenic for human P301S tau protein exhibit many characteristics of the human tauopathies, including the formation of abundant filaments made of hyperphosphorylated tau protein and neurodegeneration leading to nerve cell loss. At 5 months of age, the pathological changes are most marked in brainstem and spinal cord. Here we show that these changes are accompanied by marked neuroinflammation. Many tau-positive nerve cells in brainstem and spinal cord were strongly immunoreactive for interleukin-1β and cyclooxygenase-2, indicating induction and overproduction of proinflammatory cytokines and enzymes. In parallel, numerous activated microglial cells were present throughout brain and spinal cord of transgenic mice, where they concentrated around tau-positive nerve cells. These findings suggest that inflammation may play a significant role in the events leading to neurodegeneration in the tauopathies and that anti-inflammatory compounds may have therapeutic potential.
The most common neurodegenerative diseases are characterized by the presence of abnormal filamentous protein inclusions in nerve cells of the brain.1 In Alzheimer’s disease (AD), these inclusions are made of hyperphosphorylated tau protein. Together with the extracellular β-amyloid deposits, they constitute the defining neuropathological characteristics of AD. Tau inclusions, in the absence of extracellular deposits, are characteristic of progressive supranuclear palsy, corticobasal degeneration, Pick’s disease, argyrophilic grain disease, and frontotemporal dementia and parkinsonism linked to chromosome 17 (FTDP-17).2 The identification of mutations in Tau in FTDP-17 has established that dysfunction of tau protein is central to the neurodegenerative process.3–5
At an experimental level, the expression of mutant tau in nerve cells is leading to improved models of neurodegeneration.6–12 We have described a line of mice transgenic for human P301S tau,10 a FTDP-17 mutation with strong functional effects and an early age of disease onset.13,14 At 5 to 6 months of age, homozygous animals from this line develop a motor phenotype dominated by a severe paraparesis. Brains and spinal cords of these animals contain abundant neuronal inclusions made of hyperphosphorylated mutant human tau protein in a filamentous state. In the spinal cord, this is accompanied by a large reduction in the number of motor neurons and a marked reactive astrocytosis.
In recent years, much work has been devoted to the study of inflammatory processes in neurodegenerative diseases, especially AD.15 Neuropathological analysis has revealed many of the hallmarks of inflammation, including microglial cell activation, expression of cytokines and complement, and invasion of immune cells. There has been debate about the relevance of anti-inflammatory drugs for the treatment of AD.16 Comparatively little is known about inflammatory processes in diseases with only tau deposits; no information is available about inflammation in experimental animal models of the tauopathies. Here we report that brains and spinal cords from 5-month-old human P301S tau transgenic mice exhibit a strong inflammatory reaction, characterized by interleukin-1β (IL-1β) production and cyclooxygenase-2 (COX-2) induction, as well as by microglial cell activation.
Materials and Methods
Animals and Antibodies
Five-month-old homozygous mice transgenic for human P301S tau10 and age-matched C57BL/6J controls were used. In some experiments, 14-month-old mice transgenic for wild-type human tau (line ALZ17)17 and age-matched controls were used. Polyclonal antibodies specific for IL-1β and COX-2 (Santa Cruz Biotechnology, Santa Cruz, CA) were used at 1:200. A monoclonal anti-actin antibody (Sigma-Aldrich, Poole, UK) was used at 1:1000. Two phosphorylation-dependent anti-tau antibodies were used. AT8 (1:750, Innogenetics, Ghent, Belgium) recognizes human and murine tau phosphorylated at S202 and T205 and AP422 (1:500) recognizes human and murine tau phosphorylated at S422.18,19 Rat monoclonal antibody OX-42 (1:250; Serotec, Oxford, UK), which binds CD11b receptors, was used to identify microglial cells. Activated microglial cells were detected using monoclonal antibodies OX-6 and M5/114.15.2 (1:200; PharMingen, San Diego, CA), which are directed against major histocompatibility complex class II antigens.
Immunohistochemistry
Mice were perfused transcardially with 4% paraformaldehyde in 0.1 mol/L of phosphate buffer, pH 7.2. Brains and spinal cords were removed, postfixed for 2 hours, followed by cryoprotection in 30% sucrose in 0.1 mol/L of phosphate buffer, pH 7.4, for 24 hours. Sagittal brain sections and transverse spinal cord sections (30 μm) were cut on a Leica SM2400 microtome (Leica Microsystems, Bucks, UK), placed in Tris-buffered saline (TBS) containing 0.1% sodium azide, and stored at 4°C. For single-labeling, sections were incubated for 20 minutes at room temperature in 0.1 mol/L of phosphate-buffered saline (PBS), pH 7.4, containing 0.3% Triton X-100, 20% methanol, and 1.5% H2O2, followed by a 30-minute incubation in blocking solution (0.1 mol/L PBS, containing 0.3% Triton X-100, 3% goat serum, and 2 g/L bovine serum albumin). This was followed by the overnight incubation at 4°C in primary antibody in blocking solution. After washing, the sections were incubated in biotinylated secondary antibody (1:1000 in blocking solution, Vectastain; Vector Laboratories, Burlingame, CA). The reaction product was visualized using avidin-biotin and 3′,3′-diaminobenzidine as the chromogen (DAB kit, Vector Laboratories). For double labeling, sections underwent a further cycle of staining and the reaction product was visualized with the Vector NovaRED kit. In control experiments, 20 μg/ml of recombinant IL-1β (Upstate Biotechnology, Lake Placid, NY) was incubated overnight at 4°C with the IL-1β antibody (1:200) before the addition to tissue sections. In additional control experiments, filamentous, sarkosyl-insoluble tau was extracted from human P301S tau transgenic mouse brain10 and the tau filaments solubilized as described.20 The dialyzed material (50 μl) was incubated overnight at 4°C with the IL-1β antibody (1:200) or anti-tau antibody AT8 (1:1000), before the addition to tissue sections.
Immunofluorescence
For co-localization experiments, sections were incubated for 30 minutes at room temperature with blocking reagent and overnight at 4°C with the primary antibody in blocking solution. After washing, the sections were incubated for 1 hour in the dark with the appropriate fluorescent secondary antibody (Green Alexa, 1:200 in blocking solution; Molecular Probes, Leiden, The Netherlands). Sections were then incubated in the dark with the second primary antibody. After washing, they were incubated in the dark with the second fluorescent secondary antibody (Red Alexa). After washing, the sections were mounted onto slides using Vectashield (Vector Laboratories).
Immunoblot Analysis
Brainstem and spinal cord (10 mg each) from human P301S tau mice and age-matched controls were homogenized in 0.5 ml of cold RIPA buffer (0.1 mol/L PBS, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 1 mmol/L phenylmethyl sulfonyl fluoride) containing the complete protease inhibitor cocktail (Roche, Basel, Switzerland). The homogenates were spun for 10 minutes at 10,000 × g and the supernatants used for immunoblotting. Protein concentrations were determined using the Bio-Rad Protein Assay (Hercules, CA) and 25 μg of protein run on 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Gels were blotted overnight at 4°C onto a polyvinylidene difluoride membrane (Pierce, Rockford, IL). Membranes were blocked for 2 hours at 25°C in TBS containing 5% milk and incubated overnight at 4°C with anti-IL-1β or anti-COX-2 antibodies (1:200) in blocking buffer. Membranes were washed in TBS and incubated for 1 hour at 25°C with peroxidase-conjugated anti-rabbit antibody (DakoCytomation, Glostrup, Denmark) (1:2000) in blocking buffer. After washing in TBS, the membranes were incubated for 1 hour at 25°C with a monoclonal anti-actin antibody (1:1000) in TBS. This was followed by washing and a 1-hour incubation at 25°C with peroxidase-conjugated anti-mouse antibody (1:2000, DakoCytomation) in blocking buffer. Blots were developed using enhanced chemiluminescence (Amersham Biosciences, Arlington Heights, IL), followed by the scanning of the bands.
Results
Staining and Immunoblotting for IL-1β and COX-2
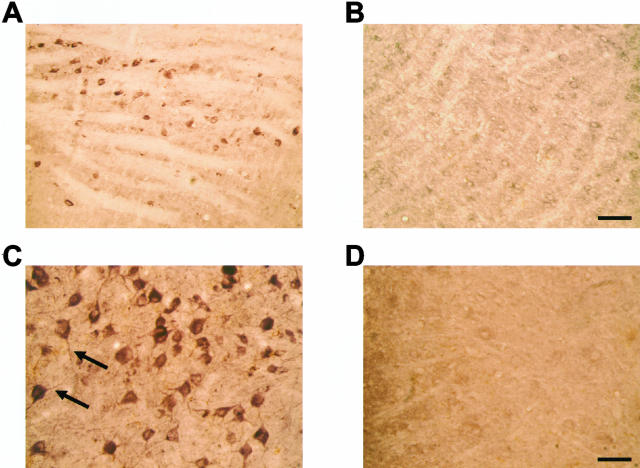
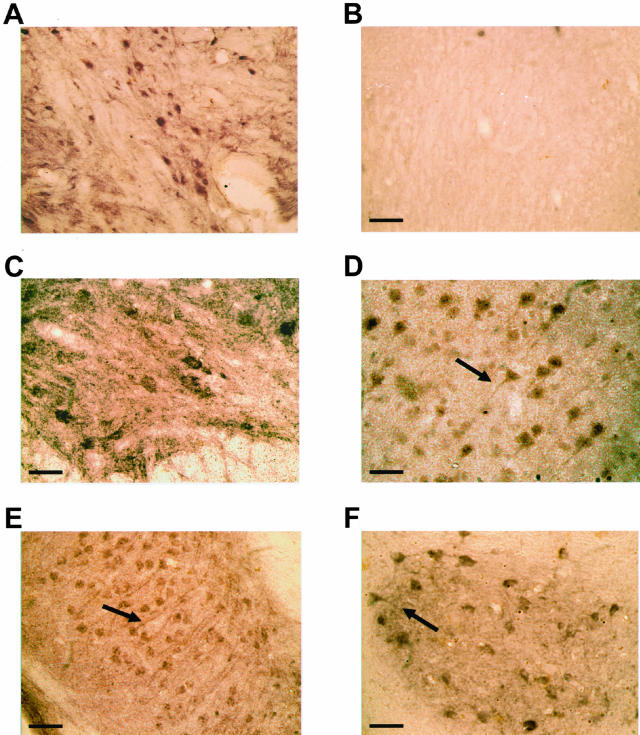
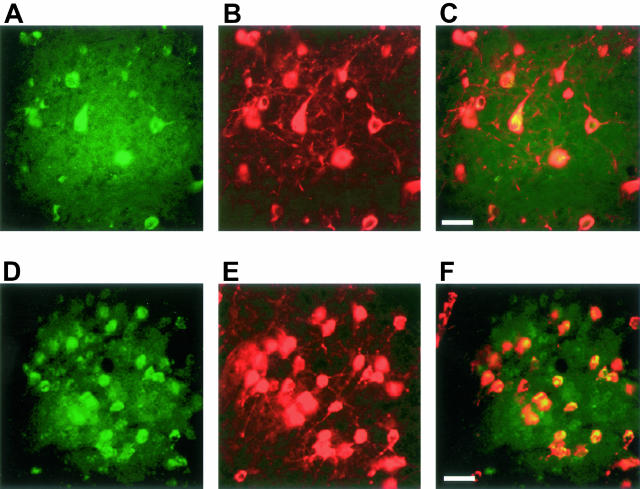
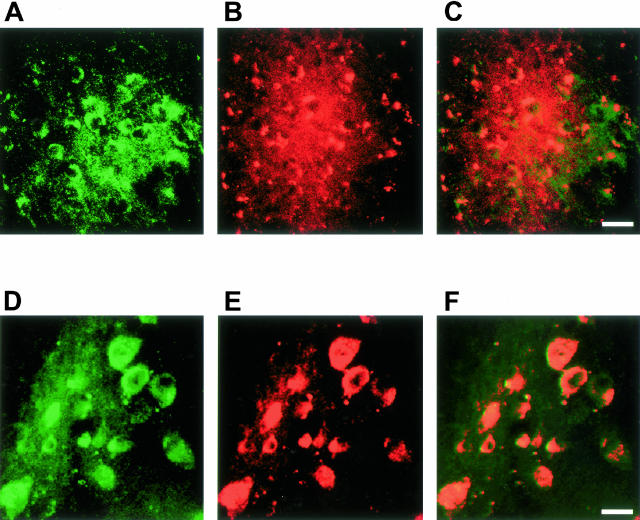
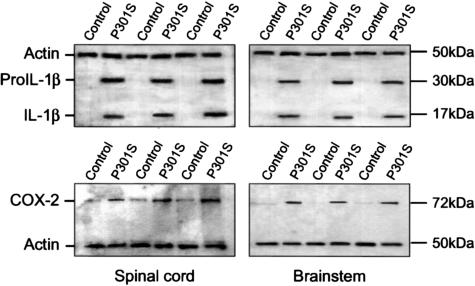
Numerous IL-1β-positive cells with a neuronal morphology were present throughout the central nervous system of 5-month-old human P301S tau transgenic mice. They were particularly abundant in brainstem and spinal cord (Figure 1, A and C). Nerve cell processes were also stained, particularly in spinal cord, where the majority of proximal dendrites was immunoreactive. In age-matched control mice, only a few nerve cells were weakly IL-1β-immunoreactive (Figure 1, B and D). In transgenic mice, numerous COX-2-immunoreactive nerve cell bodies and processes were present in cerebral cortex, hippocampus, brainstem, cerebellum, and spinal cord (Figure 2; A, C to F). In control mice, a proportion of nerve cells was positive for COX-2 (Figure 2B). Double-labeling immunofluorescence showed co-localization of IL-1β and COX-2 with staining for the phosphorylation-dependent anti-tau antibody AT8 (Figures 3 and 4). IL-1β staining was completely abolished after preincubation of the primary antibody with recombinant IL-1β. It was not changed after preincubation of the IL-1β antibody with hyperphosphorylated tau protein extracted from the brains of human P301S tau transgenic mice. Preincubation with the latter totally abolished staining with anti-tau antibody AT8. Microglial cells were not immunoreactive for IL-1β or COX-2. By immunoblotting, the levels of proIL-1β, IL-1β and COX-2 were markedly increased in brainstem and spinal cord from human P301S tau transgenic mice, when compared with age-matched controls (Figure 5). Immunoblotting with an anti-actin antibody was used to ensure equal loading.
Figure 1.
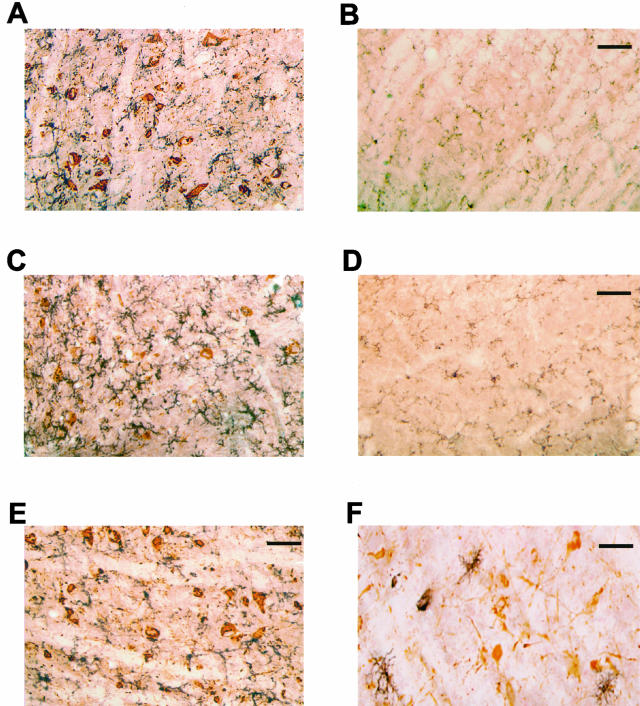
IL-1β immunoreactivity in brainstem and spinal cord of human P301S tau transgenic mice and age-matched controls. A and B: Brainstem of 5-month-old transgenic (A) and control (B) mice. C and D: Spinal cords of 5-month-old transgenic (C) and control (D) mice. Note the strong staining of some nerve cell bodies and processes (arrows in C). Scale bars: 150 μm (B, also representative for A); 71 μm (D, also representative for C).
Figure 2.
COX-2 immunoreactivity in brain and spinal cord of human P301S tau transgenic mice. Strongly stained nerve cells and processes (arrows) in brainstem (A), spinal cord (C), cerebral cortex (D), hippocampus (E), and cerebellum (F) of 5-month-old human P301S tau transgenic mice. B: COX-2 immunoreactivity in brainstem of an age-matched control mouse. Note the strong staining of some nerve cell bodies and processes in A, C, D–F. Scale bars: 150 μm (B, also representative for A); 85 μm (C); 80 μm (D); 170 μm (E); 70 μm (F).
Figure 3.
Double-labeling immunofluorescence staining for IL-1β (green) and tau phosphorylated at S202 and T205 (antibody AT8) (red) in brainstem and spinal cord of human P301S tau transgenic mice. A and B: Brainstem stained for IL-1β (A) and phosphorylated tau (B). C: Merged images shown in A and B. D and E: Spinal cord stained for IL-1β (D) and phosphorylated tau (E). F: Merged images shown in D and E. Co-localization is indicated by the yellow color. Scale bars: 60 μm (C, also representative for A, B); 125 μm (F, also representative for D, E).
Figure 4.
Double-labeling immunofluorescence staining for COX-2 (green) and tau phosphorylated at S202 and T205 (antibody AT8) (red) in brainstem and spinal cord of human P301S tau transgenic mice. A and B: Brainstem stained for COX-2 (A) and phosphorylated tau (B). C: Merged images shown in A and B. D and E: Spinal cord stained for COX-2 (D) and phosphorylated tau (E). F: Merged images shown in D and E. Co-localization is indicated by the yellow color. Scale bars: 45 μm (C, also representative for A, B); 40 μm (F, also representative for D, E).
Figure 5.
Immunoblotting for IL-1β and COX-2 of spinal cord (left) and brainstem (right) from three human P301S tau transgenic mice and three age-matched controls. Immunoblotting for actin was used to ensure equal loading. Note the increased levels of proIL-1β, IL-1β and COX-2 in tissues from the transgenic mice.
Staining for Microglial Cells
In transgenic human P301S tau mice, numerous strongly OX-42-immunoreactive microglial cells were present, particularly in close proximity to AP422-positive nerve cells in brainstem, cerebral cortex, hippocampus, and spinal cord (Figure 6; A, C, E). Some had the morphology of activated cells, in that they were characterized by a bushy appearance with swollen cell bodies and intensely stained, thickened, and branched processes. In the remainder of the brain, OX-42-immunoreactive cells were characterized by a more ramified morphology, even when showing features of activation. In brain and spinal cord from control mice, microglial cells were only weakly OX-42-immunoreactive and displayed a sessile morphology, with thin, finely branching processes extending radially from small, oblong cell bodies (Figure 6, B and D). In transgenic mice, double-labeling immunohistochemistry with OX-42 and IL-1β or COX-2 antibodies gave the same staining pattern as that obtained using OX-42 and AP422 antibodies, with OX-42-positive, activated microglial cells concentrated around anti-IL-1β and COX-2-positive cells (not shown).
Figure 6.
Double-labeling immunostaining for OX-42 or OX-6 (brown) and tau phosphorylated at S422 (antibody AP422) (red) in brainstem and spinal cord of human P301S tau transgenic mice and age-matched controls. A, B, and E: Brainstem of transgenic (A, E) and control (B) mice. C, D, and F: Spinal cord of transgenic (C, F) and control mice (D). Sections A to E were stained for OX-42 and phosphorylated tau. Section F was stained for OX-6 and phosphorylated tau. Scale bars: 70 μm (B, also representative for A); 90 μm (D, also representative for C); 60 μm (E, F).
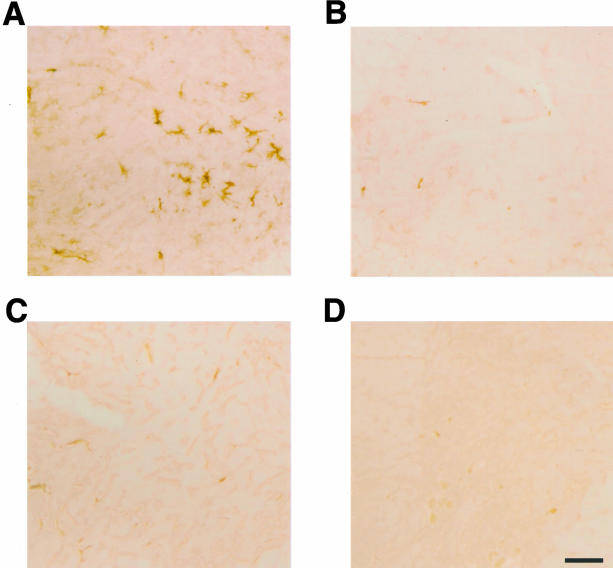
Major histocompatibility complex class II-positive microglial cells were numerous in the spinal cord of human P301S tau mice, as evidenced by staining with antibodies OX-6 and M5/114.15.2 (Figures 6F and 7A). Double-labeling immunohistochemistry showed the presence of OX-6-positive microglial cells around AP422-positive nerve cells (Figure 6F). The microglia had the morphology of activated cells, as evidenced by an amoeboid, bushy appearance with large, round cell bodies and short, thick processes. In some instances, several microglial cells clustered around a single tau-positive cell. Mice transgenic for wild-type human tau from the ALZ17 line and control mice showed no specific immunoreactivity for OX-6 (not shown) or M5/114.15.2 (Figure 7; B to D).
Figure 7.
Major histocompatibility class II-immunoreactivity (antibody M5/114.15.2) in spinal cord of mice transgenic for human wild-type tau, human P301S tau, and age-matched controls. A and B: Five-month-old mouse transgenic for human P301S tau (A) and age-matched control (B). C and D: Fourteen-month-old mouse transgenic for human wild-type tau (C) and age-matched control (D). Scale bars: 50 μm (D, also representative for A–C). Note the staining of cells with the morphology of activated microglial cells in A.
Discussion
The present findings demonstrate that the intraneuronal accumulation of human P301S tau protein is associated with the activation of inflammatory processes. The latter were characterized by increased IL-1β and COX-2 staining of nerve cells and the activation of microglial cells. In age-matched control brains, only a small number of nerve cells was weakly immunoreactive for IL-1β and COX-2, in agreement with previous findings.21,22 By immunoblotting, markedly increased levels of IL-1β and COX-2 were present in brainstem and spinal cord from human P301S tau transgenic mice. Cells strongly immunoreactive for IL-1β and COX-2 were also stained by the phosphorylation-dependent anti-tau antibodies AT8 and AP422, which recognize both soluble and filamentous tau proteins. This indicates that the accumulation of abnormal mutant human tau protein triggers the production of proinflammatory cytokines and enzymes in nerve cells.
These findings are reminiscent of work on transgenic animal models of amyotrophic lateral sclerosis (ALS) resulting from the overexpression of mutant superoxide dismutase, in which IL-1β and COX-2 were expressed at abnormally high levels in motor neurons.23,24 It suggests that the induction of proinflammatory cytokines and enzymes may be a general response of neurons to the intracellular accumulation of aggregation-prone proteins.25 IL-1β levels are elevated in AD brain26 and COX-2 expression is increased in tangle-bearing nerve cells.27,28 In addition, COX-2 protein expression is induced in nerve cells after ischemia, with COX-2 selective inhibitors preventing ischemia-induced delayed nerve cell death.29 Cytokines, such as IL-1β, are known to induce COX-2 expression in nerve cells,30,31 suggesting that IL-1β expression may have triggered COX-2 induction in the human P301S tau transgenic mice. It remains to be determined whether IL-1β was produced by neuronal or glial cells.
COX-2-derived prostanoids may play a role in the cascade of events that leads to the death of motor neurons and other nerve cells in the human P301S tau transgenic mice. Neuronal overexpression of COX-2 has been shown to lead to nerve cell death and signs of cognitive impairment.32 Conversely, inhibition of COX-2 in mice transgenic for mutant human superoxide dismutase delayed neurodegeneration and the appearance of clinical symptoms,33,34 possibly through a reduction of glutamate release.35 In the light of the present findings, inhibition of COX-2 may be a potential therapeutic target for the human tauopathies.
Brain and spinal cord of 5-month-old human P301S tau transgenic mice exhibited large numbers of OX42-immunoreactive microglial cells. They were much larger in number and more strongly immunoreactive for CD11b receptors than microglia from age-matched controls. In tissues from transgenic mice, a proportion of OX42-positive cells had the morphological appearance of activated microglia. These cells, which were concentrated around nerve cells immunoreactive for phosphorylated tau, IL-1β, and COX-2, were immunoreactive for major histocompatibility complex class II antigens. Activated microglial cells were not present in the spinal cord of mice from line ALZ17, which accumulate wild-type human tau in nerve cells, but do not develop filamentous tau deposits or nerve cell death.17 They express a four-repeat human brain tau isoform (441 amino acids) under the control of the murine Thy1 promoter. Expression levels of human tau in brainstem and spinal cord are very similar between line ALZ17 and the line transgenic for human P301S tau protein.10,17 It follows that activation of microglial cells in the human P301S tau mice was linked to tau filament formation and neurodegeneration and was not the mere consequence of the overexpression of human tau.
These findings mirror what has been described in AD,15,36,37 in other tauopathies,38,39 and in diseases with intracellular filamentous deposits, such as Parkinson’s disease,40 dementia with Lewy bodies,41 multiple system atrophy,42 and ALS.43 The lesions of AD display evidence of localized inflammation, with reactive microglia being present in neuritic plaques and in and around neurofibrillary lesions. Many of the latter are extracellular tangles,44,45 suggesting that microglia may be activated by extracellular tau deposits and involved in their attempted removal. However, activated microglial cells are also present in human tauopathies that lack extensive extracellular tangles, such as progressive supranuclear palsy and corticobasal degeneration.39 The same is also true of the human P301S tau transgenic mice, in which nerve cells degenerate without tau deposits lingering on in the extracellular space.10
Microglia have long been known to migrate to regions of nerve cell damage, where they contribute to the elimination of dead cells through their marked phagocytic activity.46 More recent work has shown that they may also cause the death of nerve cells. Thus, in the embryonic chicken retina, death of nerve cells expressing the p75 nerve growth factor receptor results from exposure to nerve growth factor released by microglia.47 Furthermore, the generation of superoxide ions and tumor necrosis factor by microglial cells has been shown to result in the death of Purkinje cells and motor neurons, respectively.48,49 In the nematode Caenorhabditis elegans, genetic analysis has established that signals released by phagocytic cells actively promote programmed nerve cell death.50,51
Inflammation is not the root cause of disease in the human P301S tau transgenic mice. However, it may well play an important role in the series of events leading to neurodegeneration. It will be interesting to determine whether anti-inflammatory compounds can have a beneficial effect on the neurodegenerative process.
Acknowledgments
We thank Professor G. Pepeu for helpful advice and insightful discussions.
Footnotes
Address reprint requests to M.G. Spillantini, Brain Repair Centre, University of Cambridge, Robinson Way, Cambridge CB2 2PY, UK. E-mail: mgs11@cam.ac.uk.
Supported by the UK Alzheimer’s Research Trust and the Medical Research Council.
References
- Goedert M, Spillantini MG, Davies SW. Filamentous nerve cell inclusions in neurodegenerative diseases. Curr Opin Neurobiol. 1998;8:619–632. doi: 10.1016/s0959-4388(98)80090-1. [DOI] [PubMed] [Google Scholar]
- Lee VMY, Goedert M, Trojanowski JQ. Neurodegenerative tauopathies. Annu Rev Neurosci. 2001;24:1121–1159. doi: 10.1146/annurev.neuro.24.1.1121. [DOI] [PubMed] [Google Scholar]
- Poorkaj P, Bird TD, Wijsman E, Nemens E, Garruto RM, Anderson L, Andreadis A, Wiederholt WC, Raskind M, Schellenberg GD. Tau is a candidate gene for chromosome 17 frontotemporal dementia. Ann Neurol. 1998;43:815–825. doi: 10.1002/ana.410430617. [DOI] [PubMed] [Google Scholar]
- Hutton M, Lendon CL, Rizzu P, Baker M, Froelich S, Houlden H, Pickering-Brown S, Chakraverty S, Isaacs A, Grover A, Hackett J, Adamson J, Lincoln S, Dickson D, Davies P, Petersen RC, Stevens M, de Graaff E, Wauters E, van Baren J, Hillebrand M, Joosse M, Kwon JM, Nowotny P, Che LK, Norton J, Morris JC, Reed LA, Trojanowski JQ, Basun H, Lannfelt L, Neystat M, Fahn S, Dark F, Tannenberg T, Dodd PR, Hayward N, Kwok JBJ, Schofield PR, Andreadis A, Snowden J, Craufurd D, Neary D, Owen F, Oostra BA, Hardy J, Goate A, van Swieten J, Mann D, Lynch T, Heutink P. Association of missense and 5′-splice-site-mutations in tau with the inherited dementia FTDP-17. Nature. 1998;393:702–705. doi: 10.1038/31508. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Murrell JR, Goedert M, Farlow MR, Klug A, Ghetti B. Mutation in the tau gene in familial multiple system tauopathy with presenile dementia. Proc Natl Acad Sci USA. 1998;95:7737–7741. doi: 10.1073/pnas.95.13.7737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J, McGowan E, Rockwood J, Melrose H, Nacharaju P, van Slegtenhorst M, Gwinn-Hardy K, Murphy MP, Baker M, Yu X, Duff K, Hardy J, Corral A, Lin WL, Yen SH, Dickson DW, Davies P, Hutton M. Neurofibrillary tangles, amyotrophy and progressive motor disturbance in mice expressing mutant (P301L) tau protein. Nat Genet. 2000;25:402–405. doi: 10.1038/78078. [DOI] [PubMed] [Google Scholar]
- Götz J, Chen F, Barmettler R, Nitsch RM. Tau filament formation in transgenic mice expressing P301L tau. J Biol Chem. 2001;276:529–534. doi: 10.1074/jbc.M006531200. [DOI] [PubMed] [Google Scholar]
- Lim F, Hernandez F, Lucas JJ, Gomez-Ramos P, Moran MA, Avila J. FTDP-17 mutations in tau transgenic mice provoke lysosomal abnormalities and tau filaments in forebrain. Mol Cell Neurosci. 2001;18:702–714. doi: 10.1006/mcne.2001.1051. [DOI] [PubMed] [Google Scholar]
- Tanemura K, Murayama M, Akagi T, Hashikawa T, Tominaga T, Ichikawa M, Yamaguchi H, Taskashima A. Neurodegeneration with tau accumulation in a transgenic mouse expressing V337M human tau. J Neurosci. 2002;22:133–141. doi: 10.1523/JNEUROSCI.22-01-00133.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen B, Ingram E, Takao M, Smith MJ, Jakes R, Virdee K, Yoshida H, Holzer M, Craxton M, Emson PC, Atzori C, Migheli A, Crowther RA, Ghetti B, Spillantini MG, Goedert M. Abundant tau filaments and nonapoptotic neurodegeneration in transgenic mice expressing human P301S tau protein. J Neurosci. 2002;22:9340–9351. doi: 10.1523/JNEUROSCI.22-21-09340.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatebayashi Y, Miyasaka T, Chui DH, Akagi T, Mishima KI, Iwasaki K, Fujiwara M, Tanemura K, Murayama M, Ishiguro K, Planel E, Sato S, Hashikawa T, Takashima A. Tau filament formation and associative memory deficit in aged mice expressing mutant (R406W) human tau. Proc Natl Acad Sci USA. 2002;99:13896–13901. doi: 10.1073/pnas.202205599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular Aβ and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- Bugiani O, Murrell JR, Giaccone G, Hasegawa M, Ghigo S, Tabaton M, Morbin M, Primavera A, Carella F, Solaro C, Grisoli M, Savoiardo M, Spillantini MG, Tagliavini F, Goedert M, Ghetti B. Frontotemporal dementia and corticobasal degeneration in a family with a P301S mutation in Tau. J Neuropathol Exp Neurol. 1999;58:667–677. doi: 10.1097/00005072-199906000-00011. [DOI] [PubMed] [Google Scholar]
- Goedert M, Jakes R, Crowther RA. Effects of frontotemporal dementia FTDP-17 mutations on heparin-induced assembly of tau filaments. FEBS Lett. 1999;450:306–311. doi: 10.1016/s0014-5793(99)00508-6. [DOI] [PubMed] [Google Scholar]
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WST, Hampel H, Hull M, Landreth G, Lue LF, Mrak R, Mackenzie IR, McGeer PL, O’Banion K, Pachter J, Pasinetti G, Plata-Salaman C, Rogers J, Rydel R, Shen Y, Streit W, Strohmeyer R, Tooyoma I, van Muiswinkel FL, Veerhuis R, Walker D, Webster S, Wegrzyniak B, Wenk G, Wyss-Coray T. Inflammation and Alzheimer’s disease. Neurobiol Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer PL, Schulzer M, McGeer EG. Arthritis and anti-inflammatory agents as possible protective factors for Alzheimer’s disease: a review of 17 epidemiologic studies. Neurology. 1996;47:425–432. doi: 10.1212/wnl.47.2.425. [DOI] [PubMed] [Google Scholar]
- Probst A, Götz J, Wiederhold KH, Tolnay M, Mistl C, Jaton AL, Hong M, Ishihara T, Lee VMY, Trojanowski JQ, Jakes R, Crowther RA, Spillantini MG, Bürki K, Goedert M. Axonopathy and amyotrophy in mice transgenic for human four-repeat tau protein. Acta Neuropathol. 2000;99:469–481. doi: 10.1007/s004010051148. [DOI] [PubMed] [Google Scholar]
- Goedert M, Jakes R, Vanmechelen E. Monoclonal antibody AT8 recognises tau protein phosphorylated at both serine 202 and threonine 205. Neurosci Lett. 1995;189:167–170. [Google Scholar]
- Hasegawa M, Jakes R, Crowther RA, Lee VMY, Ihara Y, Goedert M. Characterization of mAb AP422, a novel phosphorylation-dependent monoclonal antibody against tau protein. FEBS Lett. 1996;384:25–30. doi: 10.1016/0014-5793(96)00271-2. [DOI] [PubMed] [Google Scholar]
- Goedert M, Spillantini MG, Cairns NJ, Crowther RA. Tau proteins of Alzheimer paired helical filaments: abnormal phosphorylation of all six brain isoforms. Neuron. 1992;8:159–168. doi: 10.1016/0896-6273(92)90117-v. [DOI] [PubMed] [Google Scholar]
- Lechan R, Toni R, Clark B, Cannon J, Shaw A, Dinarello C, Reichlin S. Immunoreactive interleukin-1β localization in the rat forebrain. Brain Res. 1990;514:135–140. doi: 10.1016/0006-8993(90)90445-h. [DOI] [PubMed] [Google Scholar]
- Yamagata K, Andreasson KI, Kaufmann WE, Barnes CA, Worley PF. Expression of a mitogen-inducible cyclooxygenase in brain neurons: regulation by synaptic activity and glucocorticoids. Neuron. 1993;11:371–386. doi: 10.1016/0896-6273(93)90192-t. [DOI] [PubMed] [Google Scholar]
- Li M, Ona VO, Guégan C, Chen M, Jackson-Lewis V, Andrews LJ, Olszewski AJ, Stieg PE, Lee JP, Przedborski S, Friedlander RM. Functional role of caspase-1 and caspase-3 in an ALS transgenic mouse model. Science. 2000;288:335–339. doi: 10.1126/science.288.5464.335. [DOI] [PubMed] [Google Scholar]
- Almer G, Guégan C, Teismann P, Naini A, Rosoklija G, Hays AP, Chen C, Przedborski S. Increased expression of the pro-inflammatory enzyme cyclooxygenase-2 in amyotrophic lateral sclerosis. Ann Neurol. 2001;49:176–185. [PubMed] [Google Scholar]
- Rockwell P, Yuan H, Magnusson R, Figueiredo-Pereira ME. Proteasome inhibition in neuronal cells induces a proinflammatory response manifested by upregulation of cyclooxygenase-2, its accumulation as ubiquitin conjugates, and production of the prostaglandin PGEs. Arch Biochem Biophys. 2000;374:25–33. doi: 10.1006/abbi.1999.1646. [DOI] [PubMed] [Google Scholar]
- Griffin WS, Stanley LC, Ling C, White L, MacLeod V, Perrot LJ, White CL, Araoz C. Brain interleukin 1 and S-100 immunoreactivity are elevated in Down syndrome and Alzheimer disease. Proc Natl Acad Sci USA. 1989;86:7611–7615. doi: 10.1073/pnas.86.19.7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasinetti GM, Aisen PS. Cyclooxygenase-2 expression is increased in frontal cortex of Alzheimer’s disease brain. Neuroscience. 1998;87:319–324. doi: 10.1016/s0306-4522(98)00218-8. [DOI] [PubMed] [Google Scholar]
- Oka A, Takashima A. Induction of cyclooxygenase-2 in brains of patients with Down’s syndrome and dementia of Alzheimer type: specific localization in affected neurones and axons. Neuroreport. 1997;8:1161–1164. doi: 10.1097/00001756-199703240-00020. [DOI] [PubMed] [Google Scholar]
- Nakayama M, Uchimura K, Zhu RL, Nagayama T, Rose ME, Stetler RA, Isakson PC, Chen J, Graham SH. Cyclooxygenase-2 inhibition prevents delayed death of CA1 hippocampal neurons following global ischemia. Proc Natl Acad Sci USA. 1998;95:10954–10959. doi: 10.1073/pnas.95.18.10954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serou MJ, DeCoster MA, Bazan NG. Interleukin-1 beta activates expression of cyclooxygenase-2 and inducible nitric oxide synthase in primary hippocampal neuronal culture: platelet-activating factor as a preferential mediator of cyclooxygenase-2 expression. J Neurosci Res. 1999;58:593–598. [PubMed] [Google Scholar]
- Hoozemans JJM, Veerhuis R, Janssen I, Rozemuller AJM, Eikelenboom P. Interleukin-1β induced cyclooxygenase 2 expression and prostaglandin E2 secretion by human neuroblastoma cells: implications for Alzheimer’s disease. Exp Gerontol. 2001;36:559–570. doi: 10.1016/s0531-5565(00)00226-6. [DOI] [PubMed] [Google Scholar]
- Andreasson KI, Savonenko A, Vidensky S, Goellner JJ, Zheng Y, Shaffer A, Kaufmann WE, Worley PF, Isakson P, Markowska AL. Age-dependent cognitive deficits and neuronal apoptosis in cyclooxygenase-2 transgenic mice. J Neurosci. 2001;21:8198–8209. doi: 10.1523/JNEUROSCI.21-20-08198.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drachman DB, Frank K, Dykes-Hoberg M, Teismann P, Almer G, Przedborski S, Rothstein JD. Cyclooxygenase-2 inhibition protects motor neurons and prolongs survival in a transgenic mouse model of ALS. Ann Neurol. 2002;52:771–778. doi: 10.1002/ana.10374. [DOI] [PubMed] [Google Scholar]
- Pompl PN, Ho L, Bianchi M, McManus T, Qin W, Pasinetti GM. A therapeutic role for cyclooxygenase-2 inhibitors in a transgenic model of amyotrophic lateral sclerosis. FASEB J. 2003;17:725–727. doi: 10.1096/fj.02-0876fje. [DOI] [PubMed] [Google Scholar]
- Drachman DB, Rothstein JD. Inhibition of cyclooxygenase-2 protects motor neurons in an organotypic model of amyotrophic lateral sclerosis. Ann Neurol. 2000;48:792–795. [PubMed] [Google Scholar]
- McGeer PL, Akiyama H, Itagaki S, McGeer EG. Immune system response in Alzheimer’s disease. Can J Neurol Sci. 1989;16:516–527. doi: 10.1017/s0317167100029863. [DOI] [PubMed] [Google Scholar]
- Haga S, Akai K, Ishii T. Demonstration of microglial cells in and around senile (neuritic) plaques in the Alzheimer brain. An immunohistochemical study using a novel monoclonal antibody. Acta Neuropathol. 1989;77:569–575. doi: 10.1007/BF00687883. [DOI] [PubMed] [Google Scholar]
- Schwab C, Steele JC, McGeer PL. Neurofibrillary tangles of Guam parkinsonism-dementia are associated with reactive microglia and complement proteins. Brain Res. 1996;707:196–205. doi: 10.1016/0006-8993(95)01257-5. [DOI] [PubMed] [Google Scholar]
- Ishizawa K, Dickson DW. Microglial activation parallels system degeneration in progressive supranuclear palsy and corticobasal degeneration. J Neuropathol Exp Neurol. 2001;60:647–657. doi: 10.1093/jnen/60.6.647. [DOI] [PubMed] [Google Scholar]
- McGeer PL, Itagaki S, Boyes BE, McGeer EG. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology. 1988;38:1285–1291. doi: 10.1212/wnl.38.8.1285. [DOI] [PubMed] [Google Scholar]
- Mackenzie IRA. Activated microglia in dementia with Lewy bodies. Neurology. 2000;55:132–134. doi: 10.1212/wnl.55.1.132. [DOI] [PubMed] [Google Scholar]
- Ishizawa K, Komori T, Sasaki S, Arai N, Mizutani T, Hirose T. Microglial activation parallels system degeneration in multiple system atrophy. J Neuropathol Exp Neurol. 2004;63:43–52. doi: 10.1093/jnen/63.1.43. [DOI] [PubMed] [Google Scholar]
- Kawatama T, Akiyama H, Yamada T, McGeer PL. Immunologic reactions in amyotrophic lateral sclerosis brain and spinal cord. Am J Pathol. 1992;140:691–707. [PMC free article] [PubMed] [Google Scholar]
- Cras P, Kawai M, Siedlak S, Perry G. Microglia are associated with the extracellular neurofibrillary tangles of Alzheimer disease. Brain Res. 1991;558:312–314. doi: 10.1016/0006-8993(91)90783-r. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Akiyama H, Haga C, Haga S. Evidence that neurofibrillary tangles undergo glial modification. Acta Neuropathol. 1992;85:101–104. doi: 10.1007/BF00304639. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Scarano F, Baltuch G. Microglia as mediators of inflammatory and degenerative diseases. Annu Rev Neurosci. 1999;22:219–240. doi: 10.1146/annurev.neuro.22.1.219. [DOI] [PubMed] [Google Scholar]
- Frade JM, Barde YA. Microglia-derived nerve growth factor causes cell death in the developing retina. Neuron. 1998;20:35–41. doi: 10.1016/s0896-6273(00)80432-8. [DOI] [PubMed] [Google Scholar]
- Marin-Teva J, Dusart I, Colin C, Gervais A, van Rooijen N, Mallat M. Microglia promote the death of developing Purkinje cells. Neuron. 2004;41:535–547. doi: 10.1016/s0896-6273(04)00069-8. [DOI] [PubMed] [Google Scholar]
- Sedel F, Béchade C, Vyas S, Triller A. Macrophage-derived tumor necrosis factor alpha, an early developmental signal for motoneuron death. J Neurosci. 2004;24:2236–2246. doi: 10.1523/JNEUROSCI.4464-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddien PW, Cameron S, Horvitz HR. Phagocytosis promotes programmed cell death in C. elegans. Nature. 2001;412:198–202. doi: 10.1038/35084096. [DOI] [PubMed] [Google Scholar]
- Hoeppner DJ, Hengartner MO, Schnabel R. Engulfment genes cooperate with ced-3 to promote cell death in Caenorhabditis elegans. Nature. 2001;412:202–206. doi: 10.1038/35084103. [DOI] [PubMed] [Google Scholar]