Borrelia burgdorferi-Induced Tolerance as a Model of Persistence via Immunosuppression (original) (raw)
Abstract
If left untreated, infection with Borrelia burgdorferi sensu lato may lead to chronic Lyme borreliosis. It is still unknown how this pathogen manages to persist in the host in the presence of competent immune cells. It was recently reported that Borrelia suppresses the host's immune response, thus perhaps preventing the elimination of the pathogen (I. Diterich, L. Härter, D. Hassler, A. Wendel, and T. Hartung, Infect. Immun. 69:687-694, 2001). Here, we further characterize _Borrelia_-induced immunomodulation in order to develop a model of this anergy. We observed that the different Borrelia preparations that we tested, i.e., live, heat-inactivated, and sonicated Borrelia, could desensitize human blood monocytes, as shown by attenuated cytokine release upon restimulation with any of the different preparations. Next, we investigated whether these _Borrelia_-specific stimuli render monocytes tolerant, i.e. hyporesponsive, towards another Toll-like receptor 2 (TLR2) agonist, such as lipoteichoic acid from gram-positive bacteria, or towards the TLR4 agonist lipopolysaccharide. Cross-tolerance towards all tested stimuli was induced. Furthermore, using primary bone marrow cells from TLR2-deficient mice and from mice with a nonfunctional TLR4 (strain C3H/HeJ), we demonstrated that the TLR2 was required for tolerance induction by Borrelia, and using neutralizing antibodies, we identified interleukin-10 as the key mediator involved. Although peripheral blood mononuclear cells tolerized by Borrelia exhibited reduced TLR2 and TLR4 mRNA levels, the expression of the respective proteins on monocytes was not decreased, ruling out the possibility that tolerance to Borrelia is attributed to a reduced TLR2 expression. In summary, we characterized tolerance induced by B. burgdorferi, describing a model of desensitization which might mirror the immunosuppression recently attributed to the persistence of Borrelia in immunocompetent hosts.
Borrelia burgdorferi sensu lato is the causative agent of Lyme borreliosis (LB), the most common vector-borne disease in the United States (32) and in many European countries (39a). If infection with this pathogen is not treated adequately with antibiotics, it may lead to a chronic multisystemic disorder which is difficult to cure. The mechanism by which Borrelia survives in its natural reservoir and in human tissues remains unclear. Although it is recognized by the host's immune defense and occasionally induces strong inflammatory reactions, Borrelia is often not eradicated. Thus, how Borrelia persists in immunologically competent hosts is a key question in understanding the pathogenesis of LB, and it remains an area of debate. It has been postulated that Borrelia interacts with the complement system, inactivating the complement regulatory proteins factor H-like protein 1/reconectin and factor H (18, 19). Others have proven by electron microscopy that Borrelia can hide in immunoprivileged sites such as the collagen fibers of the connective tissue (7) or in human synovial cells (12). The antigenic variation of the B. burgdorferi outer membrane is also discussed as a possible strategy to evade the immune response (38, 42, 44). Data from Gross et al. suggest alternatively that Borrelia induces an autoimmune process, as the authors could identify a homology between the Borrelia outer surface protein A (OspA) and the human lymphocyte function-associated antigen-1 alpha (hLFA-1) and also found cross-reactive T cells (15).
A previous study investigated whether Borrelia modulates the host's immune system in order to persist. Blood cells from patients suffering from persistent LB released significantly lower levels of proinflammatory cytokines (i.e., tumor necrosis factor alpha [TNF-α] and gamma interferon) in response to either a _Borrelia_-specific stimulus or lipopolysaccharide (LPS) than cells from healthy volunteers (8). Based on findings with patient specimens, others have also described Borrelia as an immunomodulator, supporting the hypothesis of _Borrelia_-induced immune dysregulation (28, 39). In line with this hypothesis, Borrelia seems to influence the balance between the pro- and anti-inflammatory immune responses. Previous studies have found that Borrelia not only induces proinflammatory cytokines but also leads to a strong induction of interleukin-10 (IL-10), which is an important down-regulator of the proinflammatory immune response (8, 10, 11). In addition, the modulatory and regulatory capacities of IL-10 with regard to _Borrelia_-induced cytokine release have been found in different in vitro models (24, 29). For Borrelia lipoproteins, the Toll-like receptor 2 (TLR2) has been identified as the major signal-transducing receptor (1, 3, 16, 23, 25). However, recently the existence of TLR2-independent, nonlipoprotein stimulatory Borrelia components was postulated (43). In order to address these conflicting results, we tested the role of the TLR2 and TLR4 in the recognition of different _Borrelia_-specific stimuli.
The phenomenon of endotoxin (LPS) tolerance has been investigated extensively in vitro and in vivo (for a review, see reference 21). A status of macrophage hyporesponsiveness to a high or lethal LPS dose after preexposure to low LPS doses has been described (13, 40). Recently, similar desensitization experiments were reported which demonstrated that stimuli other than LPS, e.g., highly purified lipoteichoic acid (LTA) (22) and macrophage-activating lipopeptide 2 (MALP-2) from mycoplasma (36), can also render macrophages tolerant to subsequent restimulation. Furthermore, it was shown in these same studies that tolerance can also be induced by two heterologous stimuli, independent of the receptor involved in their recognition and signaling. In this case, the appropriate term is cross-tolerance or heterotolerance. To our knowledge it has not yet been investigated whether B. burgdorferi also has the capacity to desensitize macrophages. However, _Borrelia_-induced hyporesponsiveness could represent a mechanism enabling the survival of this pathogen in the host despite the presence of immune cells. We tested this hypothesis in desensitization experiments with _Borrelia_-specific stimuli and with other well-characterized TLR2 and TLR4 agonists. Additionally, the involvement of the TLR2 and the TLR4, as well as of endogenous IL-10 formation, in tolerance and cross-tolerance induction was addressed.
(Parts of this paper were presented at the 12th European Congress of Clinical Microbiology and Infectious Diseases, Milan, Italy, April 2002, at the Conference of the International Endotoxin Society, Washington, D.C., July 2002, and at the 8th International Conference on Lyme Borreliosis and Other Emerging Tick-Borne Diseases, New York, N.Y., August 2002.)
MATERIALS AND METHODS
Borrelia cultivation and preparation of _Borrelia_-specific stimuli.
B. burgdorferi sensu stricto (strain N40, kindly provided by T. Kamradt, Berlin, Germany) was cultivated as described previously (8). Borrelia cultures passaged fewer than eight times after isolation from mice were grown to log phase (≥108 cells/ml) and differentially prepared for stimulation experiments.
For experiments with live Borrelia, B. burgdorferi cell counts were determined by microscopy by using a Thoma counting chamber with a modified depth of 0.02 mm. The culture was adjusted to a concentration of 5 × 105 cells/ml. Due to the low replication rate of Borrelia and its complex requirements with regard to culture conditions, an increase in bacterial numbers during the 24 h of subsequent incubation is improbable.
Before heat inactivation or sonication, Borrelia cultures were washed twice. Briefly, cultures were centrifuged at 10,000 × g for 30 min, the supernatant was removed, and the pellet was resuspended with pyrogen-free saline solution. Subsequently, the bacterial numbers were determined as described above. Finally, washed Borrelia was either incubated for 30 min at 56°C for heat inactivation or sonicated, yielding Borrelia lysate as described elsewhere (8). Protein concentration of the lysate was adjusted to a final concentration of 1 mg/ml. Remaining viable spirochetes were excluded visually under the microscope after 1 week of incubation under standard cultivation conditions.
The numbers of endotoxin units (EU) contained in 10 μg of protein from sonicated Borrelia cultures and from 107 heat-inactivated Borrelia cells were below the detection limit (0.1 and 0.05 EU, respectively), as assessed by a Limulus amoebocyte lysate assay (QCL-1000; Charles River Endosafe, Charleston, S.C.). The spike recoveries (0.5 EU) were 107 and 103%, respectively. Live Borrelia was used only for the first set of experiments because it constitutes a highly variable stimulus which is difficult to standardize, as the pathogen changes its surface protein expression depending on cultivation conditions (5). All the other experiments were carried out with the same batch of heat-inactivated or sonicated Borrelia.
Isolation of PBMC.
Peripheral blood mononuclear cells (PBMC) were isolated with cell preparation tubes (Vacutainer CPT, sodium citrate; Becton Dickinson Biosciences [BD Biosciences], Heidelberg, Germany) according to the manufacturer's instructions. Briefly, blood from healthy donors (differential blood cell counts were performed with a Pentra60 [ABX Technologies, Montpellier, France] to rule out acute infections) was centrifuged in the tubes for 20 min at 680 × g. PBMC, separated from erythrocytes and neutrophils by the gel phase, were transferred into 50-ml polypropylene tubes (bio-one; Greiner, Frickenhausen, Germany) and washed twice with RPMI 1640 (Bio Whittaker, Apen, Germany) supplemented with 2.5 IU of heparin (Liquemin; Hoffmann LaRoche, Grenzach-Whylen, Germany)/ml. The overall PBMC count (i.e., monocytes and lymphocytes) was determined with a Pentra60. PBMC were adjusted to a final concentration of 107 cells/ml when stimulated in a 96-well cell culture plate (Greiner) or to 5 × 107 cells/ml when stimulated in a 24-well cell culture plate (for the flow cytometric and RNA isolation experiments). One hundred microliters of cells was pipetted per well, and 10% autologous plasma was added. Informed consent was obtained from the blood donors before investigations were carried out.
Mice.
Female C3H/HeJ mice, characterized by a nonfunctional TLR4 due to a natural point mutation in the TLR4 gene (34), and mice of the corresponding wild-type strain (C3H/HeN) were purchased from Charles River Laboratories (Sulzfeld, Germany). TLR2-deficient mice generated by homologous recombination were a generous gift from Tularik (South San Francisco, Calif.), and the corresponding wild-type mice (129Sv/B57BL/6) were bred in the animal facilities of the University of Konstanz at 24°C and 55% humidity with a 12-h day-night cycle and a diet of Altromin C 1310 (Altromin, Lage, Germany). Mice were used for the experiments at 8 to 10 weeks of age.
Isolation of primary bone marrow cells from mice.
Mice were killed by terminal pentobarbital anesthesia (Narcoren; Merial, Hallbergmoos, Germany). Femurs were subjected to lavage with 10 ml of ice-cold sterile phosphate-buffered saline (Life Technologies, Karlsruhe, Germany). The lavage fluids were transferred to siliconized glass tubes (Vacutainer; BD Biosciences) for isolation of bone marrow cells. Bone-derived debris was removed by resuspending the lavage fluid and transferring it into a new glass tube after 1 min of sedimentation. After centrifugation, primary murine bone marrow cells were resuspended in RPMI 1640 containing 10% fetal calf serum (Biochrom, Berlin, Germany) and 100 IU of penicillin-streptomycin (PAA Laboratories, Linz, Austria)/ml. Cell counts were determined with a Pentra60 (see above), and cells were plated onto 96-well culture plates (Greiner) at a density of 5 × 105 cells per well.
In vitro desensitization and restimulation experiments.
Immediately after isolation, cells (PBMC or primary murine bone marrow cells) were desensitized with different concentrations of live, heat-inactivated, or sonicated Borrelia (lysate); LTA from Staphylococcus aureus (isolated and prepared in-house as described previously [27]); and endotoxin (LPS from Salmonella enterica serovar Abortus equi; Sigma-Aldrich, Seelze, Germany) or left untreated to serve as controls. The volume was adjusted to 220 μl with medium (RPMI 1640 supplemented with 100 IU of penicillin-streptomycin/ml containing 10% fetal calf serum for the primary murine bone marrow cells or 2.5 IU of heparin/ml for the PBMC). After incubation for 24 h at 37°C in the presence of 5% CO2, the supernatants were transferred into 96-well round-bottom plates (Greiner) and stored at −70°C until cytokine amounts were measured. The remaining adherent, tolerized monocytes in the plate were washed twice with RPMI 1640 and subsequently restimulated with 1 ng of LPS/ml, 10 μg of LTA/ml, 10 μg of Borrelia lysate/ml, or 106 heat-inactivated B. burgdorferi sensu stricto cells/ml. Autologous plasma (10%) was added to the PBMC. The final volume of the incubation was adjusted to 220 μl of RPMI 1640 (supplemented as described above). After 24 h of incubation at 37°C and 5% CO2, the supernatants were stored at −70°C until cytokine measurement. To study the involvement of mediators in tolerance induction, neutralizing antibodies (anti-IL-10 [Endogen, Eching, Germany], pan-specific anti-transforming growth factor β [anti-TGF-β; R&D Systems, Wiesbaden, Germany], and polyclonal anti-mu-granulocyte colony-stimulating factor [G-CSF]-sheep immunoglobulin G raised in our laboratory [2]) or IL-10 (a kind gift from S. Narula, Schering Plough, Kenilworth, N.J.) was added for the first 24-h incubation period.
MTT assay.
MTT (3-[4,5-dimethyl-2-thiazolyl]-2,5-diphenyl-2_H_-tetrazolium bromide; Sigma) stock solution (5 mg/ml in phosphate-buffered saline) was diluted 1:5 in RPMI 1640. Two hundred microliters of this working solution was added to the adherent cells in a 96-well culture plate for 2 h at 37°C and 5% CO2. Cells with mitochondrial activity convert dissolved MTT to insoluble purple formazan. After the incubation, the supernatant was removed and the cells were lysed for 10 min with 95% isopropanol-5% formic acid. Absorbance of converted dye was measured at 555 nm, with 690 nm as the reference wavelength.
Cytokine measurement in culture supernatant by ELISA.
The concentrations of human and murine TNF-α in the supernatants were measured by in-house sandwich enzyme-linked immunosorbent assay (ELISA) by using commercially available antibody pairs and recombinant standards. Monoclonal antibody pairs against human TNF-α and IL-10 were purchased from Endogen (Perbio Science, Bonn, Germany) and Pharmingen (BD Biosciences), respectively. Recombinant human TNF-α was a gift from S. Poole (National Institute for Biological Standards and Controls, Herts, Great Britain) and was used as a standard. For IL-10, the standard was purchased from BD Biosciences. For the measurement of murine TNF-α, polyclonal antibodies from R&D Systems and a standard from Pharmingen were used. Assays were carried out in flat-bottom, Maxisorb 96-well plates (Nunc, Wiesbaden, Germany). Binding of secondary biotinylated antibodies was detected with horseradish peroxidase-conjugated streptavidin (Biosource, Camarillo, Calif.), and 3′,3′,5′,5′-tetramethylbenzidine solution (Sigma) was used as a substrate.
RNA extraction and TLR2 mRNA quantification.
PBMC (5 × 106/ml) were pipetted into 24-well culture plates in 1 ml of medium. After 24 h of incubation at 37°C and 5% CO2 with 10 μg of lysate/ml or without a stimulus, the supernatant was removed and cells were either lysed for RNA extraction or washed and restimulated with lysate (10 μg/ml) or with LPS (1 ng/ml) for another 3 h. After the removal of the supernatant, RNA was prepared from the adherent cells with a QIAmp RNA blood mini kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions, including DNA digestion with the RNase-free DNase-Set (Qiagen).
Six microliters of RNA was reverse transcribed in a sample volume of 20 μl containing 2.5 μM oligonucleotide dT16 (custom primer; Gibco BRL), MgCl2 (5 mM), deoxynucleoside triphosphates (1 mM each), RNase inhibitor (1 U/μl), and murine leukemia virus reverse transcriptase (2.5 U/μl) in PCR buffer (all from PE Applied Biosystems, Weiterstadt, Germany). Samples were incubated at 21°C for 10 min, 42°C for 15 min, 94°C for 5 min, and 5°C for 5 min in a GeneAmp PCR System 2400 (PE Applied Biosystems).
For relative quantification, real-time PCR was performed by using a LightCycler rapid thermal cycler system (Roche Diagnostics GmbH, Mannheim, Germany). The cDNA for TLR2, TLR4, and GAPDH (glyceraldehyde-3-phosphate dehydrogenase) was amplified by using LightCycler-FastStart DNA Master SYBR-Green (Roche Diagnostics) according to the manufacturer's protocol. The sequences of the primers were GGC CAG CAA ATT ACC TGT GTG (forward) and AGG CGG ACA TCC TGA ACC T (reverse) and TGG TGG AAG TTG AAC GAA TGG (forward) and AGG ACC GAC ACA CCA ATG ATG (reverse) for TLR2 and TLR4, respectively. For GAPDH the primers were GAA GGT GAA GGT CGG AGT C (forward) and GAA GAT GGT GAT GGG ATT TC (reverse). The MgCl2 concentrations were adjusted to 2 mM for TLR2, 3 mM for TLR4, and 4 mM for GAPDH. The thermal cycling was performed according to the manufacturer's protocol (50 cycles) with annealing temperatures of 58, 55, and 65°C and elongation times of 3, 6, and 11 s for TLR2, TLR4, and GAPDH, respectively. The amplification was followed by a melting program which started at 65°C for 15 s and then increased to 95°C at 0.1°C/s. The specific melting temperatures for TLR2, TLR4, and GAPDH products were 86, 84, and 86.5°C, respectively. The size of the amplification product was checked initially on an agarose gel stained with ethidium bromide. The TLR2 and TLR4 results were normalized to the GAPDH signal.
Flow cytometry analysis of TLR expression.
PBMC were stained after 24 h of preincubation and restimulation for 4 and 6 h as described above. Cells were detached from the 24-well plate by a 30-min incubation with Accutase (PAA Laboratories) at 37°C, split into three micronic tubes (Apogent, Wehrheim, Germany), and stained with anti-CD45-peridinin chlorophyll protein, anti-CD14-fluorescein isothiocyanate (BD Biosciences), and anti-TLR2-phycoerythrin, anti-TLR4-phycoerythrin, or isotype control (eBioscience, San Diego, Calif.) at room temperature for 30 min. After the cells wre washed, fluorescence was measured in a FACSCalibur system (BD Biosciences). The median fluorescence of the isotype control in arbitrary units was subtracted from the TLR-specific median. Monocytes were identified by the height of their CD14 expression.
Statistics.
Data are shown as means ± standard errors of the mean (SEM) of results for either four different blood donors or primary cells from four to seven individual mice. To take into account the varying viabilities of the cells on day 2, TNF-α concentrations were divided by the mitochondrial activity assessed with the MTT assay. Repeated-measure analysis of variance (ANOVA) followed by Dunnett's multiple comparison test was performed by using GraphPad Prism 3.00 (GraphPad Software, San Diego, Calif.). A P value of <0.05 was considered significant.
RESULTS
Borrelia induced tolerance in human PBMC.
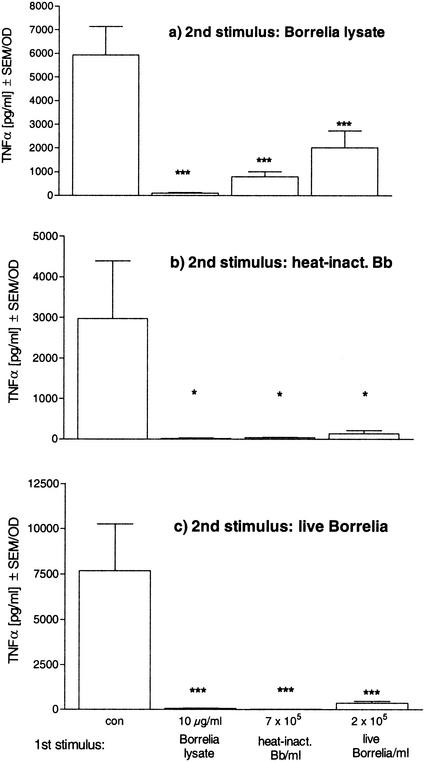
We tested whether Borrelia is able to induce a state of hyporesponsiveness in human monocytes in vitro. On the one hand, we used the same Borrelia preparations, i.e., heat-inactivated, sonicated, and live Borrelia, for prestimulation and for restimulation. On the other hand, we tested all combinations of the different Borrelia preparations in the two sequential incubation periods. When human PBMC were pretreated with either sonicated, heat-inactivated, or live Borrelia for 24 h, they showed a significantly reduced release of TNF-α in response to a secondary stimulation with the same preparation or either of the other Borrelia preparations (Fig. 1). In contrast, cells which had not been stimulated during the first incubation period were fully responsive to the second stimulus, as demonstrated by the nonpretreated control cells. In the same experiment, the release of IL-10 was also inhibited. Reductions of 52, 69, and 76% were seen after preincubation with 0.1, 1, and 10 μg of Borrelia lysate/ml, respectively, when cells were restimulated with 10 μg of lysate/ml (6.5 ± 5 ng of IL-10/ml in untolerized cells versus 3.1 ± 3, 2 ± 2, and 1.6 ± 2 ng of IL-10/ml [P of <0.001 for all] in cells pretreated with 0.1, 1, and 10 μg of lysate/ml, respectively). In summary, all _Borrelia_-specific stimuli tested could desensitize macrophages to either the same or another _Borrelia_-specific stimulus. This desensitized state is termed Borrelia tolerance.
FIG. 1.
_Borrelia_-induced tolerance in human PBMC. Human PBMC (106) from four healthy donors were incubated with culture medium (con), 10 μg of Borrelia lysate/ml, 7 × 105 heat-inactivated Borrelia cells (heat-inact. Bb), or 2 × 105 live Borrelia cells for 24 h and then washed and incubated for another 24 h in the presence of 10 μg of Borrelia lysate/ml (a), 7 × 105 heat-inactivated Borrelia cells (b), or 2 × 105 live Borrelia cells (c). The concentrations of TNF-α were measured by ELISA. Data are expressed as the means ± SEM divided by the optical density (OD) assessed by the MTT assay. The statistical significance is for comparison with the nontolerized (con) cells. * represents a P value of <0.05 and *** represents a P value of <0.001 based on ANOVA followed by Dunnett's multiple comparison test.
Comparison of TNFα-inducing potencies of different bacterial stimuli.
We next tested the TNF-α-inducing capacities of different bacterial stimuli during 24 h of incubation in vitro. Treatment of human PBMC with LPS, LTA, or two _Borrelia_-specific stimuli (heat-inactivated Borrelia and Borrelia lysate) induced dose-dependent TNF-α release. LPS (1 ng/ml), LTA (10 μg/ml), 106 heat-inactivated B. burgdorferi cells/ml, and Borrelia lysate (10 μg/ml) induced comparable amounts of TNF-α in the range of 200 to 500 pg/ml. Therefore, these concentrations were used in the subsequent restimulation experiments.
_Borrelia_-induced cross-tolerance to LTA and LPS.
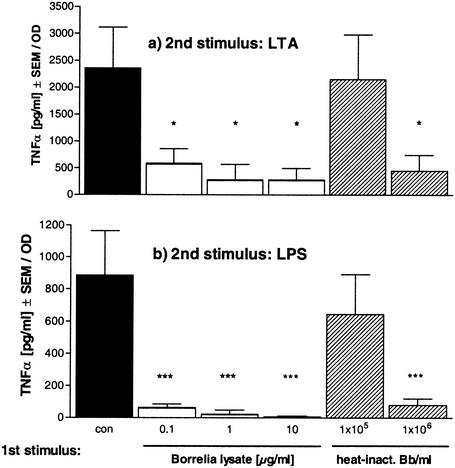
Next, we investigated whether _Borrelia_-specific stimuli—which according to the literature (1, 3, 16, 23) act via TLR2—render macrophages tolerant towards another TLR2 agonist such as LTA from gram-positive Staphylococcus aureus or towards a TLR4 agonist, e.g., LPS from Salmonella enterica serovar Abortus equi. PBMC were first treated overnight with either heat-inactivated Borrelia or Borrelia lysate. Then, the cells were restimulated with either 10 μg of LTA/ml or 1 ng of LPS/ml. Figure 2 demonstrates that prestimulation with _Borrelia_-specific stimuli led to reduced responsiveness of the PBMC to restimulation with LTA as well as with LPS, as shown by TNF-α release. Tolerance induction was concentration dependent. The stronger the stimulus during the preincubation period, the lower the TNF-α response to the second stimulus. Thus, Borrelia induced cross-tolerance towards two different heterologous stimuli, one also signaling via TLR2, the other TLR4 mediated.
FIG. 2.
Induction of Borrelia cross-tolerance to LTA or LPS in human PBMC. Human PBMC (106/well) from four different donors were incubated with culture medium (con, black bars); 0.1, 1, or 10 μg of Borrelia lysate/ml (white bars); or 105 or 106 heat-inactivated Borrelia cells (heat-inact. Bb; striped bars) for 24 h and then washed and incubated for another 24 h in the presence of 10 μg of LTA/ml (a) or 10 ng of LPS/ml (b). The concentrations of TNF-α were measured by ELISA. Data are expressed as the means ± SEM divided by the OD assessed by the MTT test. The statistical significance is for comparison with the nontolerized (con) cells. * represents a P value of <0.05 and *** represents a P value of <0.001 based on ANOVA followed by Dunnett's multiple comparison test.
LPS- and LTA-induced cross-tolerance to _Borrelia_-specific stimuli.
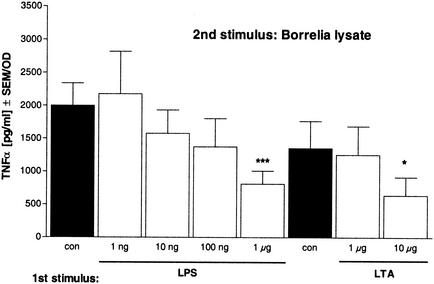
Stimulation of macrophages with LPS renders the cells tolerant to a subsequent LPS stimulation. This kind of tolerance has been known since the 1960s and 1970s (4, 14, 30). Recently, we reported tolerance induction by LTA, including cross-tolerance to LPS inducible by LTA and vice versa, indicating that cross-tolerance between TLR2 and TLR4 agonists is possible (22). Therefore, our results prompted us to test whether cells rendered tolerant by LPS or LTA were also hyporesponsive to restimulation with Borrelia lysate.
In the experiments whose results are shown in Fig. 3, LPS or LTA was used for prestimulation. For subsequent restimulation, Borrelia lysate was added to the cells. Similar to that in the previous experiments, cytokine release in response to the second stimulus was decreased in a dose-dependent fashion depending on the concentration of the first stimulus. The same results were obtained when heat-inactivated Borrelia was used for restimulation instead of Borrelia lysate (data not shown).
FIG. 3.
Effect of preincubation with LPS or LTA on restimulation with Borrelia lysate. Human PBMC (106/well) were incubated with culture medium (con, black bars); 1, 10, 100, or 1,000 ng of LPS/ml (white bars); or 1 or 10 μg of LTA/ml (white bars) for 24 h and then washed and incubated for another 24 h in the presence of 10 μg of Borrelia lysate/ml. The concentrations of TNF-α were measured by ELISA. Data are expressed as the means ± SEM divided by the OD assessed by the MTT assay. The statistical significance is for comparison with the nontolerized (con) cells. * represents a P value of <0.05 and *** represents a P value of <0.001 based on ANOVA followed by Dunnett's multiple comparison test.
IL-10 is involved in tolerance induction by Borrelia and LPS.
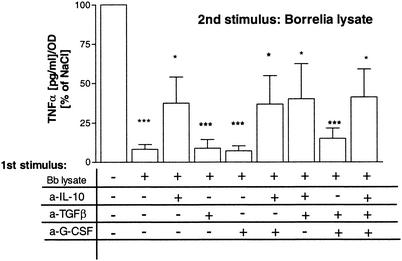
Next we were interested in the mechanism of _Borrelia_-induced tolerance. We first checked whether soluble mediators are involved. As reported previously (8), Borrelia is a relatively strong inducer of IL-10; e.g., 10 μg of lysate/ml induces 1 to 1.5 ng of IL-10/ml in human PBMC after 24 h of incubation. Furthermore, it has been shown that endogenous anti-inflammatory factors such as IL-10 and TGF-β mediate the phenomenon of LPS tolerance in human monocytes in vitro (35). In order to examine this mechanism, neutralizing antibodies were incubated simultaneously with the Borrelia lysate during the preincubation period. Pretreatment of PBMC with Borrelia and specific neutralizing antibodies against the anti-inflammatory cytokines IL-10, TGF-β, and G-CSF showed that neither antibodies against TGF-β or G-CSF alone nor the combination of both types of antibodies could block _Borrelia_-induced tolerance (Fig. 4). The neutralizing activity of the antibodies was assured in control experiments by blocking the immunosuppressive effect of recombinant cytokines on LPS-inducible cytokine release (data not shown). Anti-IL-10 antibodies alone partially prevented tolerance induction by Borrelia. Addition of anti-TGF-β or anti-G-CSF antibodies or both did not augment the inhibitory effect of anti-IL-10 antibodies.
FIG. 4.
Neutralization of IL-10, TGF-β, and G-CSF during preincubation prevents establishment of desensitization by Borrelia lysate. PBMC were untreated or were stimulated for 24 h in the presence of Borrelia lysate alone (Bb lysate) or additionally with anti-TGF-β (a-TGFβ; 1 μg/ml) and anti-IL-10 (a-IL-10; 10 μg/ml) monoclonal antibodies, and 1% anti-mu-G-CSF-sheep immunoglobulin G (a-G-CSF) or combinations of the neutralizing antibodies and serum. Controls were cultured without Borrelia lysate. After being washed, cells were restimulated with 10 μg of Borrelia lysate/ml for a further 24 h at 37°C. Cytokine amounts in supernatants were determined by ELISA. Data are expressed as the means ± SEM divided by the OD assessed by the MTT assay. The statistical significance is for comparison with the nontolerized cells. * represents a P value of <0.05 and *** represents a P value of <0.001 based on ANOVA followed by Dunnett's multiple comparison test. +, present; −, absent.
We further examined the role of IL-10 in _Borrelia_-induced tolerance and checked whether the cells could be rendered hyporesponsive to Borrelia by levels of IL-10 induced by Borrelia lysate. Preincubation of PBMC for 24 h with 1 ng of biologically active IL-10 led to a reduced TNF-α release when cells were restimulated with 10 μg of lysate/ml (control, 2 ± 0.9 ng/ml; IL-10-tolerant cells, 0.5 ± 0.4 ng/ml). The partial reduction of TNF-α by IL-10 only might indicate that further factors contribute to tolerance induction.
These findings suggest that IL-10, not G-CSF or TGF-β, is involved in tolerance induction by Borrelia in human PBMC but that other mediators are needed additionally to completely prevent the process.
TLR2 and TLR4 mRNA down-regulation by _Borrelia_-induced tolerance.
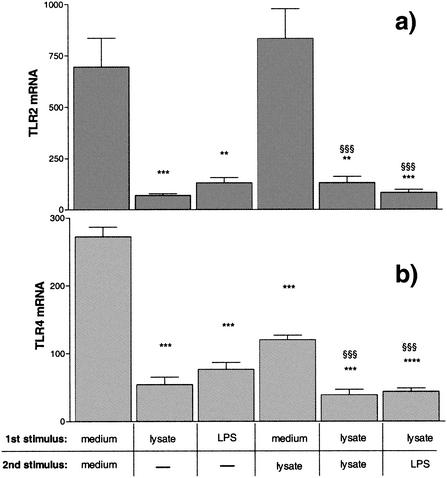
Recently published results of experiments by Wang et al. suggest that TLR2 is down-regulated in the case of synthetic bacterial lipopeptide tolerance (41). We therefore measured the TLR2 mRNA expression of human PBMC with _Borrelia_-induced tolerance. Stimulation of PBMC for 24 h in the presence of Borrelia lysate (10 μg/ml) or LPS (1 ng/ml) led to significantly reduced TLR2 mRNA expression, which remained attenuated after restimulation of the tolerized cells (Fig. 5a).
FIG. 5.
TLR2 and TLR4 mRNA expression in human PBMC. PBMC (5 × 106) from four different donors were incubated with culture medium, 1 ng of LPS/ml, or 10 μg of Borrelia lysate/ml for 24 h and either lysed or incubated for another 3 h in the presence of medium, 10 μg of Borrelia lysate/ml, or 1 ng of LPS/ml. The cDNA for TLR2 (a), TLR4 (b), and GAPDH (a and b) was amplified, and relative quantification was performed by using a LightCycler. The TLR results were normalized to GAPDH. ** and §§ represent a P value of <0.01 and *** and §§§ represent a P value of <0.001 based on ANOVA followed by Bonferroni's multiple comparison test. *, versus medium and medium; §, versus medium and lysate.
We further determined the expression of TLR4 mRNA in the same samples and similarly observed a significant down-regulation compared to baseline levels by stimulation with Borrelia lysate or LPS for 24 h. TLR4 mRNA levels remained reduced after a second stimulation of tolerized cells. These data suggested that in the case of _Borrelia_-induced tolerance, down-regulation of the TLR2 and TLR4 contributes to suppression of TNF-α formation upon restimulation with either a TLR2 or a TLR4 agonist.
TLR2 and TLR4 surface expression in _Borrelia_-induced tolerance.
Since mRNA levels may not necessarily reflect protein levels, we additionally assessed TLR2 and TLR4 expression on the surface of monocytes, which are the TNF-α-producing cells in our test system, by flow cytometry. Stimulation of PBMC overnight with Borrelia lysate led to an increase of TLR2 expression on the surface of monocytes (median fluorescence of the control in arbitrary units was 101 ± 18; that of lysate-stimulated cells was 208 ± 52 [not significant]). When the tolerant cells were restimulated for another 4 h with the same stimulus, the TLR2 expression returned to control levels (108 ± 44). With the use of LPS as the second stimulus, i.e., in the case of cross-tolerance, TLR2 expression was down-regulated below the expression level of the control cells (75 ± 17). No major differences in TLR4 expression were seen, neither with stimulation with LPS or lysate nor with lysate-induced tolerance and cross-tolerance. The median fluorescence ranged between 31 ± 9 on unstimulated controls and 34 ± 4 on lysate-stimulated tolerized or nontolerized PBMC.
TLR2 but not TLR4 is required for tolerance and cross-tolerance induction by Borrelia.
To further investigate the role of TLR2 and TLR4 in _Borrelia_-induced tolerance, we carried out desensitization experiments using primary murine bone marrow cells from TLR2 knockout mice and C3H/HeJ mice (with a nonfunctional TLR4) and corresponding wild-type cells. As expected, there was no measurable TNF-α or IL-10 release in TLR2−/− bone marrow macrophages after stimulation with either heat-inactivated Borrelia or Borrelia lysate after 8 or 24 h (data not shown). Hereby, we confirmed data from others (1, 3, 16, 23) showing that _Borrelia_-specific stimuli employ TLR2. Cells from TLR2−/− mice responded to LPS to the same extent as did cells from wild-type mice, reflecting the normal responsivity to a TLR4 agonist (data not shown). Cells from TLR2- and TLR4-defective mice, obtained by crossing both strains, did not release TNF-α upon stimulation with either Borrelia or LPS or LTA (data not shown). We checked that the cells were still responsive by activating them with other stimuli such as CpG oligonucleotides, a TLR9-mediated stimulus modeling bacterial DNA, and the phorbol ester phorbol myristate acetate, a receptor-independent stimulus (data not shown).
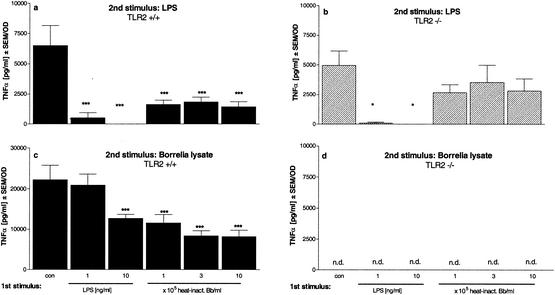
Preincubation of cells from TLR2−/− mice with a _Borrelia_-specific stimulus had no significant effect on the responsiveness of the cells to LPS (Fig. 6b). Their behavior was similar to that of saline-pretreated cells. This result indicates that the cells had not been desensitized by heat-inactivated Borrelia and demonstrates that TLR2 is required for _Borrelia_-induced tolerance. Furthermore, as expected, no TNF-α release could be measured upon restimulation of the TLR2-deficient cells with either lysate (Fig. 6d) or heat-inactivated Borrelia (data not shown), independent of the preincubation stimulus. In contrast, restimulating wild-type and TLR2−/− cells with LPS after LPS pretreatment resulted in strongly reduced TNF-α release, showing that the cells had been rendered tolerant (Fig. 6a). Thus, the TLR2 was not required for LPS-induced tolerance. In line with our PBMC results (Fig. 1 and 3), this experiment shows that prestimulation of primary murine wild-type cells with LPS or Borrelia lysate tolerized the cells to Borrelia lysate.
FIG. 6.
Induction of tolerance and cross-tolerance in primary bone marrow cells from TLR2+/+ and TLR2−/− mice. Bone marrow cells (5 × 105/well) from TLR2+/+ mice (black bars) and TLR2−/− mice (striped bars) were incubated with culture medium (con), 1 and 10 ng of LPS/ml, or 105, 3 × 105, or 106 heat-inactivated Borrelia cells (heat-inact. Bb) for 24 h and then washed and incubated for another 24 h in the presence of 1 ng of LPS/ml (a and b) or 10 μg of Borrelia lysate/ml (c and d). The concentrations of TNF-α were measured by ELISA. Data are expressed as the means ± SEM divided by the viability assessed by the MTT assay. The statistical significance is for comparison with the nontolerized cells. * represents a P value of <0.05 and *** represents a P value of <0.001 based on ANOVA followed by Dunnett's multiple comparison test. n.d., not detectable.
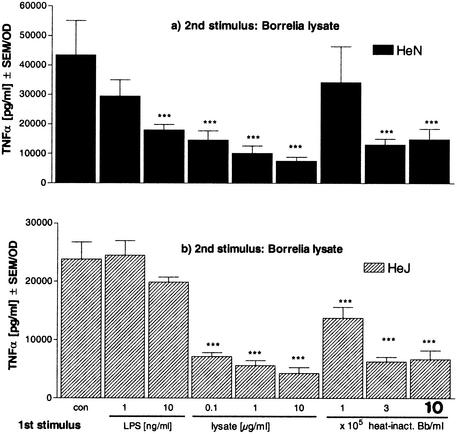
The same experiments were also conducted with primary murine bone marrow cells from C3H/HeJ mice, which lack a functional TLR4, and cells from the corresponding wild-type mice. Cells from both mouse strains responded similarly to _Borrelia_-specific stimuli during the preincubation period (data not shown), confirming that TLR4 is not required for signaling of the _Borrelia_-specific stimuli. Consequently, cells from C3H/HeJ mice behaved like wild-type cells, showing a reduced TNF-α release in response to restimulation with heat-inactivated Borrelia after pretreatment with the same _Borrelia_-specific stimulus (Fig. 7a). Furthermore, in line with our expectations, neither tolerance to Borrelia (Fig. 7b) nor tolerance to LPS (data not shown) could be induced by LPS pretreatment in cells without a functional TLR4.
FIG. 7.
Induction of tolerance and cross-tolerance in primary bone marrow cells from C3H/HeN and C3H/HeJ mice. Bone marrow cells (5 × 105/well) from C3H/HeN mice (black bars) and C3H/HeJ mice (striped bars) were incubated with culture medium (con), 1 or 10 ng of LPS/ml, or 105, 3 × 105, or 106 heat-inactivated Borrelia cells (heat-inact. Bb) for 24 h and then washed and incubated for another 24 h in the presence of 10 μg of Borrelia lysate/ml. The concentrations of TNF-α were measured by ELISA. Data are expressed as the means ± SEM divided by the viability assessed by the MTT assay. The statistical significance is for comparison with the nontolerized cells. *** represents a P value of <0.001 based on ANOVA followed by Dunnett's multiple comparison test.
Hence, in the absence of TLR2, no cytokine release could be induced by Borrelia and no state of hyporesponsiveness for restimulation with LPS was achieved. In line with these findings, no desensitization to Borrelia stimuli could be induced by LPS in the absence of a functional TLR4.
DISCUSSION
Understanding the immunopathology of LB is still a major challenge. Although it induces strong immune activation, e.g., in phases of arthritis, the causative agent of LB persists and leads to a chronic pathology in the immunocompetent host. Of note, the inflammatory episodes associated with LB are typically self-limiting and the site of manifestation often changes, e.g., between different joints. These phenomena suggest counterregulatory anti-inflammatory mechanisms, and the long phases of latency indicate phases of immune evasion.
A possible mechanism for survival of Borrelia in the immunologically competent host was recently proposed: since ex vivo experiments with whole blood from patients with LB revealed a significantly reduced capacity to release TNF-α and gamma interferon in comparison to that of blood cells from healthy controls, it was postulated that Borrelia modulates the host's immune system in order to evade immune clearance (8). There are a few reports about Borrelia acting as an immunomodulator (28, 33, 39). Further evidence was found for _Borrelia_-induced immunomodulation by demonstrating that Borrelia induces a stronger anti-inflammatory cytokine response than do endotoxins from various other gram-negative bacteria (8). These data are in line with findings from other authors indicating that B. burgdorferi is a potent inducer of the anti-inflammatory cytokine IL-10 (9-11). In addition, the modulatory and regulatory capacity of IL-10 with regard to _Borrelia_-induced cytokine release has been found in different in vitro models (24, 29).
In the present study, we examined whether tolerance could represent a possible model for _Borrelia_-induced immunomodulation. First, we checked whether Borrelia can render cells hyporesponsive, and second, we investigated some of the underlying mechanisms involved in this immunomodulation. Borrelia could indeed render human PBMC tolerant, i.e., unable to react to a second stimulation with Borrelia, as shown by a reduced capacity to release TNF-α and IL-10. All the settings tested led to a significantly reduced TNF-α release as a result of preincubation with Borrelia compared to that of saline controls. As no major differences between live and killed Borrelia with respect to the pathogen's ability to render the monocyte hyporesponsive could be observed, we selected the more standardized stimuli (heat-inactivated Borrelia and Borrelia lysate) for the subsequent experiments. Based on these experiments, we demonstrated that Borrelia is able to modulate the monocytic immune response. Next, assuming that Borrelia can induce a more general desensitization in the cell, we investigated whether a monocyte rendered tolerant by Borrelia was also hyporesponsive to a different stimulus. Differences in the potency of TNF-α induction among the bacterial stimuli (Borrelia, LPS, and LTA) were excluded by choosing equipotent concentrations, i.e., concentrations which induced comparable amounts of TNF-α release during 24 h of incubation.
According to the literature, Borrelia signal via the TLR2 (1, 3, 16, 23). This notion could be confirmed in our experiments with primary murine bone marrow cells from mice lacking either a functional TLR2 or a TLR4 or both. The findings shown in Fig. 2 demonstrate that Borrelia desensitizes monocytes in a more general manner, because it also rendered PBMC hyporesponsive to subsequent stimulation with heterologous stimuli such as the TLR2 agonist LTA or the TLR4 agonist LPS. The extent of desensitization was concentration dependent. Using LPS or LTA to tolerize the cells similarly led to hyporesponsiveness to Borrelia (Fig. 3). We did not observe any difference in levels of cross-tolerance induction of TNF-α release by the tested stimuli. These findings confirm our data regarding cross-tolerance between LTA and LPS, showing that the degree of hyporesponsiveness induced by the TLR2 or the TLR4 agonist does not vary (22). Opposing results have been published by others, who postulate that LPS pretreatment is less effective in other cross-tolerance models. Sato et al., for example, describe poor tolerance induction by LPS in response to MALP restimulation (36).
Results of tolerance experiments presented here suggest that the underlying mechanisms which lead to Borrelia tolerance seem to be very similar to those involved in tolerance induced by cell wall components from other bacteria, since no difference could be observed between the levels of tolerance and cross-tolerance induced by the tested combinations. Furthermore, cross-tolerance data indicate that signal pathways shared by LPS and TLR2 agonists seem to be impaired. Similar heterologous tolerance phenomena have recently also been described for MALPs from mycoplasma (36), bacterial DNA (CpG) (37), Staphylococcus aureus (20), LTA (22), arabinose-capped lipoarabinomannan (26), and LPS. There is accumulating evidence that tolerance induced by different TLR2 and TLR4 agonists shares common intracellular signal transduction pathways (36, 41). However, recently Jacinto et al. suggested the involvement of unique TLR2 signaling components downstream of TLR2 and upstream of MyD88/IL-1 receptor-associated kinase (IRAK) in LTA tolerance by demonstrating that IRAK expression and IRAK activity differ with LPS and LTA tolerance (17). Controversial data are reported regarding the involvement of the TLR in LPS and non-LPS tolerance and cross-tolerance. Our findings with primary murine bone marrow cells show that in the absence of the Borrelia recognition receptor, TLR2, no tolerance could be induced (Fig. 6b). Similarly, the TLR4 was required for LPS-induced tolerance (Fig. 7b), indicating that these two receptors are essential in _Borrelia_- and LPS-induced tolerance, respectively.
Differing results regarding the regulation of the TLR in the case of tolerance have been published (26, 31, 36). Wang et al. showed down-regulation of TLR2 protein on THP-1 cells with tolerance induced by synthetic bacterial lipopeptide (41). Our results regarding the TLR2 are partially in line with these observations, since we could reproduce the down-regulation of TLR2 mRNA in PBMC tolerized by Borrelia lysate upon restimulation with the same stimulus. However, as a major difference from the results in the cited paper, no significant changes in the expression of the respective protein on the surface of the desensitized monocytes were measurable by flow cytometry. The different results could be ascribed to the use of a monocytic cell line versus primary cells and to other stimuli employed for their experiments. Of note, a down-regulation of TLR2 mRNA was not reflected at the protein level. Since we could not observe any significant changes in TLR2 or TLR4 protein expression, with either _Borrelia_-induced tolerance or cross-tolerance, our results suggest that tolerance is induced independently of down-regulation of cell surface expression of the receptor.
We also addressed the involvement of soluble mediators. Data from different authors demonstrate that LPS-induced tolerance is mediated by endogenous cytokines such as IL-10 and TGF-β (6, 35). Our results with regard to tolerance induced by Borrelia in the presence of IL-10-, TGF-β-, and G-CSF-neutralizing antibodies (Fig. 4) indicate that IL-10 is indeed involved in induction of Borrelia tolerance. However, tolerance induction could not be completely prevented by blockage of this mediator, nor could recombinant IL-10 alone induce complete tolerance to Borrelia lysate, suggesting that other mediators also play a role in this process. TGF-β, which also contributed to LPS tolerance in human PBMC (35), was apparently not required for Borrelia tolerance, nor was G-CSF. Further studies will be necessary to definitely settle this point, since our data oppose data from others who showed that peritoneal macrophages from wild-type and IL-10−/− mice could be similarly tolerized by LPS and MALP, suggesting that IL-10 was not involved (36). Results from coculture experiments with TLR2- and TLR4-deficient primary murine cells and the corresponding wild-type cells stimulated with LTA and LPS also argue against soluble factors' being responsible for suppression of TNF-α upon secondary stimulation (22).
Our experiments comparing LPS- and _Borrelia_-induced tolerance cannot provide final evidence regarding differences and similarities of these two phenomena, since our observations were restricted to TNF-α release and neglected other mediators induced by bacteria. So far, except for TLR involvement, there was little difference between the phenomena. In this study, we characterized _Borrelia_-induced desensitization in human monocytes, which is cross-reactive with LPS and LTA and dependent on IL-10 and TLR2. Based on this ability of Borrelia to render cells hyporesponsive, we propose that the model described here might mirror the mechanism by which this human pathogen avoids elimination and persists in the host, leading to chronic disease.
Acknowledgments
The excellent technical assistance of Margarete Kreuer-Ullmann and Petra Krause is gratefully acknowledged.
REFERENCES
- 1.Aliprantis, A. O., R. B. Yang, M. R. Mark, S. Suggett, B. Devaux, J. D. Radolf, G. R. Klimpel, P. Godowski, and A. Zychlinsky. 1999. Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science 285**:**736-739. [DOI] [PubMed] [Google Scholar]
- 2.Barsig, J., D. S. Bundschuh, T. Hartung, A. Bauhofer, A. Sauer, and A. Wendel. 1996. Control of fecal peritoneal infection in mice by colony-stimulating factors. J. Infect. Dis. 174**:**790-799. [DOI] [PubMed] [Google Scholar]
- 3.Brightbill, H. D., D. H. Libraty, S. R. Krutzik, R. B. Yang, J. T. Belisle, J. R. Bleharski, M. Maitland, M. V. Norgard, S. E. Plevy, S. T. Smale, P. J. Brennan, B. R. Bloom, P. J. Godowski, and R. L. Modlin. 1999. Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science 285**:**732-736. [DOI] [PubMed] [Google Scholar]
- 4.Brooke, M. S. 1965. Conversion of immunological paralysis to immunity by endotoxin. Nature 206**:**635-636. [DOI] [PubMed] [Google Scholar]
- 5.Carroll, J. A., C. F. Garon, and T. G. Schwan. 1999. Effects of environmental pH on membrane proteins in Borrelia burgdorferi. Infect. Immun. 67**:**3181-3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cavaillon, J. M., C. Pitton, and C. Fitting. 1994. Endotoxin tolerance is not a LPS-specific phenomenon: partial mimicry with IL-1, IL-10, and TGFb. J. Endotoxin Res. 1**:**21-29. [Google Scholar]
- 7.de Koning, J., D. J. Tazelaar, J. A. Hoogkamp-Korstanje, and J. D. Elema. 1995. Acrodermatitis chronica atrophicans: a light and electron microscopic study. J. Cutan. Pathol. 22**:**23-32. [DOI] [PubMed] [Google Scholar]
- 8.Diterich, I., L. Härter, D. Hassler, A. Wendel, and T. Hartung. 2001. Modulation of cytokine release in ex vivo-stimulated blood from borreliosis patients. Infect. Immun. 69**:**687-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giambartolomei, G. H., V. A. Dennis, B. L. Lasater, P. K. Murthy, and M. T. Philipp. 2002. Autocrine and exocrine regulation of interleukin-10 production in THP-1 cells stimulated with Borrelia burgdorferi lipoproteins. Infect. Immun. 70**:**1881-1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giambartolomei, G. H., V. A. Dennis, B. L. Lasater, and M. T. Philipp. 1999. Induction of pro- and anti-inflammatory cytokines by Borrelia burgdorferi lipoproteins in monocytes is mediated by CD14. Infect. Immun. 67**:**140-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giambartolomei, G. H., V. A. Dennis, and M. T. Philipp. 1998. Borrelia burgdorferi stimulates the production of interleukin-10 in peripheral blood mononuclear cells from uninfected humans and rhesus monkeys. Infect. Immun. 66**:**2691-2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Girschick, H. J., H. I. Huppertz, H. Russmann, V. Krenn, and H. Karch. 1996. Intracellular persistence of Borrelia burgdorferi in human synovial cells. Rheumatol. Int. 16**:**125-132. [DOI] [PubMed] [Google Scholar]
- 13.Granowitz, E. V., R. Porat, J. W. Mier, S. F. Orencole, G. Kaplanski, E. A. Lynch, K. Ye, E. Vannier, S. M. Wolff, and C. A. Dinarello. 1993. Intravenous endotoxin suppresses the cytokine response of peripheral blood mononuclear cells of healthy humans. J. Immunol. 151**:**1637-1645. [PubMed] [Google Scholar]
- 14.Greisman, S. E., E. J. Young, and F. A. Carozza, Jr. 1969. Mechanisms of endotoxin tolerance. V. Specificity of the early and late phases of pyrogenic tolerance. J. Immunol. 103**:**1223-1236. [PubMed] [Google Scholar]
- 15.Gross, D. M., T. Forsthuber, M. Tary-Lehmann, C. Etling, K. Ito, Z. A. Nagy, J. A. Field, A. C. Steere, and B. T. Huber. 1998. Identification of LFA-1 as a candidate autoantigen in treatment-resistant Lyme arthritis. Science 281**:**703-706. [DOI] [PubMed] [Google Scholar]
- 16.Hirschfeld, M., C. J. Kirschning, R. Schwandner, H. Wesche, J. H. Weis, R. M. Wooten, and J. J. Weis. 1999. Cutting edge: inflammatory signaling by Borrelia burgdorferi lipoproteins is mediated by toll-like receptor 2. J. Immunol. 163**:**2382-2386. [PubMed] [Google Scholar]
- 17.Jacinto, R., T. Hartung, C. McCall, and L. Li. 2002. Lipopolysaccharide- and lipoteichoic acid-induced tolerance and cross-tolerance: distinct alterations in IL-1 receptor-associated kinase. J. Immunol. 168**:**6136-6141. [DOI] [PubMed] [Google Scholar]
- 18.Kraiczy, P., C. Skerka, M. Kirschfink, P. F. Zipfel, and V. Brade. 2002. Immune evasion of Borrelia burgdorferi: insufficient killing of the pathogens by complement and antibody. Int. J. Med. Microbiol. (Suppl.) 29133**:**141-146. [DOI] [PubMed] [Google Scholar]
- 19.Kraiczy, P., C. Skerka, M. Kirschfink, P. F. Zipfel, and V. Brade. 2001. Mechanism of complement resistance of pathogenic Borrelia burgdorferi isolates. Int. Immunopharmacol. 1**:**393-401. [DOI] [PubMed] [Google Scholar]
- 20.Kreutz, M., U. Ackermann, S. Hauschildt, S. W. Krause, D. Riedel, W. Bessler, and R. Andreesen. 1997. A comparative analysis of cytokine production and tolerance induction by bacterial lipopeptides, lipopolysaccharides and Staphylococcus aureus in human monocytes. Immunology 92**:**396-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lehner, M. D., and T. Hartung. 2002. Endotoxin tolerance—mechanisms and beneficial effects in bacterial infection. Rev. Physiol. Biochem. Pharmacol. 144**:**96-141. [DOI] [PubMed] [Google Scholar]
- 22.Lehner, M. D., S. Morath, K. S. Michelsen, R. R. Schumann, and T. Hartung. 2001. Induction of cross-tolerance by lipopolysaccharide and highly purified lipoteichoic acid via different toll-like receptors independent of paracrine mediators. J. Immunol. 166**:**5161-5167. [DOI] [PubMed] [Google Scholar]
- 23.Lien, E., T. J. Sellati, A. Yoshimura, T. H. Flo, G. Rawadi, R. W. Finberg, J. D. Carroll, T. Espevik, R. R. Ingalls, J. D. Radolf, and D. T. Golenbock. 1999. Toll-like receptor 2 functions as a pattern recognition receptor for diverse bacterial products. J. Biol. Chem. 274**:**33419-33425. [DOI] [PubMed] [Google Scholar]
- 24.Lisinski, T. J., and M. B. Furie. 2002. Interleukin-10 inhibits proinflammatory activation of endothelium in response to Borrelia burgdorferi or lipopolysaccharide but not interleukin-1beta or tumor necrosis factor alpha. J. Leukoc. Biol. 72**:**503-511. [PubMed] [Google Scholar]
- 25.Lorenz, E., J. P. Mira, K. L. Cornish, N. C. Arbour, and D. A. Schwartz. 2000. A novel polymorphism in the toll-like receptor 2 gene and its potential association with staphylococcal infection. Infect. Immun. 68**:**6398-6401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Medvedev, A. E., P. Henneke, A. Schromm, E. Lien, R. Ingalls, M. J. Fenton, D. T. Golenbock, and S. N. Vogel. 2001. Induction of tolerance to lipopolysaccharide and mycobacterial components in Chinese hamster ovary/CD14 cells is not affected by overexpression of Toll-like receptors 2 or 4. J. Immunol. 167**:**2257-2267. [DOI] [PubMed] [Google Scholar]
- 27.Morath, S., A. Geyer, and T. Hartung. 2001. Structure-function relationship of cytokine induction by lipoteichoic acid from Staphylococcus aureus. J. Exp. Med. 193**:**393-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mullegger, R. R., G. McHugh, R. Ruthazer, B. Binder, H. Kerl, and A. C. Steere. 2000. Differential expression of cytokine mRNA in skin specimens from patients with erythema migrans or acrodermatitis chronica atrophicans. J. Investig. Dermatol. 115**:**1115-1123. [DOI] [PubMed] [Google Scholar]
- 29.Murthy, P. K., V. A. Dennis, B. L. Lasater, and M. T. Philipp. 2000. Interleukin-10 modulates proinflammatory cytokines in the human monocytic cell line THP-1 stimulated with Borrelia burgdorferi lipoproteins. Infect. Immun. 68**:**6663-6669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neter, E. 1969. Endotoxins and the immune response. Curr. Top. Microbiol. Immunol. 47**:**82-124. [DOI] [PubMed] [Google Scholar]
- 31.Nomura, F., S. Akashi, Y. Sakao, S. Sato, T. Kawai, M. Matsumoto, K. Nakanishi, M. Kimoto, K. Miyake, K. Takeda, and S. Akira. 2000. Cutting edge: endotoxin tolerance in mouse peritoneal macrophages correlates with down-regulation of surface toll-like receptor 4 expression. J. Immunol. 164**:**3476-3479. [DOI] [PubMed] [Google Scholar]
- 32.Orloski, K. A., E. B. Hayes, G. L. Campbell, and D. T. Dennis. 2000. Surveillance for Lyme disease—United States, 1992-1998. Morb. Mortal. Wkly. Rep. 49**:**1-11. [PubMed] [Google Scholar]
- 33.Pohl-Koppe, A., K. E. Balashov, A. C. Steere, E. L. Logigian, and D. A. Hafler. 1998. Identification of a T cell subset capable of both IFN-gamma and IL-10 secretion in patients with chronic Borrelia burgdorferi infection. J. Immunol. 160**:**1804-1810. [PubMed] [Google Scholar]
- 34.Poltorak, A., X. He, I. Smirnova, M. Y. Liu, C. V. Huffel, X. Du, D. Birdwell, E. Alejos, M. Silva, C. Galanos, M. Freudenberg, P. Ricciardi-Castagnoli, B. Layton, and B. Beutler. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282**:**2085-2088. [DOI] [PubMed] [Google Scholar]
- 35.Randow, F., U. Syrbe, C. Meisel, D. Krausch, H. Zuckermann, C. Platzer, and H. D. Volk. 1995. Mechanism of endotoxin desensitization: involvement of interleukin 10 and transforming growth factor beta. J. Exp. Med. 181**:**1887-1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sato, S., F. Nomura, T. Kawai, O. Takeuchi, P. F. Muhlradt, K. Takeda, and S. Akira. 2000. Synergy and cross-tolerance between toll-like receptor (TLR) 2- and TLR4-mediated signaling pathways. J. Immunol. 165**:**7096-7101. [DOI] [PubMed] [Google Scholar]
- 37.Schwartz, D. A., C. L. Wohlford-Lenane, T. J. Quinn, and A. M. Krieg. 1999. Bacterial DNA or oligonucleotides containing unmethylated CpG motifs can minimize lipopolysaccharide-induced inflammation in the lower respiratory tract through an IL-12-dependent pathway. J. Immunol. 163**:**224-231. [PubMed] [Google Scholar]
- 38.Seiler, K. P., and J. J. Weis. 1996. Immunity to Lyme disease: protection, pathology and persistence. Curr. Opin. Immunol. 8**:**503-509. [DOI] [PubMed] [Google Scholar]
- 39.Silberer, M., F. Koszik, G. Stingl, and E. Aberer. 2000. Downregulation of class II molecules on epidermal Langerhans cells in lyme borreliosis. Br. J. Dermatol. 143**:**786-794. [DOI] [PubMed] [Google Scholar]
- 39a.Stanek, G., N. Satz, F. Strle, and B. Wilske. 1993. Epidemiology of Lyme borreliosis, p. 358-370. In K. Weber and W. Burgdorfer (ed.), Aspects of Lyme borreliosis. Springer, New York, N.Y.
- 40.Takasuka, N., T. Tokunaga, and K. S. Akagawa. 1991. Preexposure of macrophages to low doses of lipopolysaccharide inhibits the expression of tumor necrosis factor-alpha mRNA but not of IL-1 beta mRNA. J. Immunol. 146**:**3824-3830. [PubMed] [Google Scholar]
- 41.Wang, J. H., M. Doyle, B. J. Manning, Q. D. Wu, S. Blankson, and H. P. Redmond. 2002. Induction of bacterial lipoprotein tolerance is associated with suppression of toll-like receptor 2 expression. J. Biol. Chem. **277:**36068-36075. [DOI] [PubMed]
- 42.Wilske, B., U. Busch, V. Fingerle, S. Jauris-Heipke, V. Preac Mursic, D. Rossler, and G. Will. 1996. Immunological and molecular variability of OspA and OspC. Implications for Borrelia vaccine development. Infection 24**:**208-212. [DOI] [PubMed] [Google Scholar]
- 43.Wooten, R. M., Y. Ma, R. A. Yoder, J. P. Brown, J. H. Weis, J. F. Zachary, C. J. Kirschning, and J. J. Weis. 2002. Toll-like receptor 2 is required for innate, but not acquired, host defense to Borrelia burgdorferi. J. Immunol. 168**:**348-355. [DOI] [PubMed] [Google Scholar]
- 44.Zhang, J. R., J. M. Hardham, A. G. Barbour, and S. J. Norris. 1997. Antigenic variation in Lyme disease borreliae by promiscuous recombination of VMP-like sequence cassettes. Cell 89**:**275-285. [DOI] [PubMed] [Google Scholar]