Survivin is required for a sustained spindle checkpoint arrest in response to lack of tension (original) (raw)
Abstract
Genetic evidence is mounting that survivin plays a crucial role in mitosis, but its exact role in human cell division remains elusive. We show that mammalian cells lacking survivin are unable to align their chromosomes, fail to recruit Aurora B to kinetochores and become polyploid at a very high frequency. Survivin-depleted cells enter mitosis with normal kinetics, but are delayed in prometaphase in a BubR1/Mad2-dependent fashion. Nonetheless, these cells exit mitosis prior to completion of chromosome congression and without sister chromatid segregation, indicating that the spindle assembly checkpoint is not fully functional. Indeed, in survivin-depleted cells, BubR1 and Mad2 are prematurely displaced from kinetochores, yet no tension is generated at kinetochores. Importantly, these cells fail to respond to drugs that prevent tension, but do arrest in mitosis after depolymerization of the mitotic spindle. This demonstrates that survivin is not required for initial checkpoint activation, or for sustained checkpoint activation by loss of microtubules. However, stable association of BubR1 to kinetochores and sustained checkpoint signalling in response to lack of tension crucially depend on survivin.
Keywords: checkpoint/mitosis/survivin
Introduction
To ensure proper chromosome segregation it is essential that sister chromatids remain paired until all chromosomes have obtained bipolar attachment (Nicklas, 1997). Premature separation of sister chromatids is prevented by a complex of proteins, called the cohesin complex (Michaelis et al., 1997). Proteolysis of an essential cohesin subunit by separase at the metaphase-to-anaphase transition allows the sister chromatids to be pulled apart (Uhlmann et al., 2000; Hauf et al., 2001). Before anaphase, this separase is kept inactive by securin through direct association and by cyclin B1/cdk1-dependent phosphorylation (Nasmyth et al., 2000; Stemmann et al., 2001). Separase is activated in metaphase by degradation of securin and cyclin B1 by a ubiquitin ligase called the anaphase-promoting complex or cyclosome (APC/C) (Peters, 2002).
Timing of sister chromatid separation is controlled by the spindle assembly checkpoint (or kinetochore-attachment checkpoint) (Hoyt, 2000; Shah and Cleveland, 2000). The spindle assembly checkpoint senses unattached kinetochores and/or lack of tension on the microtubules (Nicklas, 1997). Unattached kinetochores recruit a complex of checkpoint proteins, including Mad2 and BubR1, that inhibit the APC/C accordingly (Shah and Cleveland, 2000). Attachment of microtubules to the kinetochores is essential to inactivate this checkpoint, but it is not sufficient. To ensure symmetrical transmission of chromosomes to the progeny, paired sister kinetochores need to capture microtubules coming from opposite poles. Such bipolar attachment of the sister chromatids creates tension on the kinetochores which is required to silence the checkpoint (Nicklas, 1997).
Binding of microtubules to the kinetochores is stochastic, and paired kinetochores may capture microtubules coming from the same spindle pole (syntelic attachments) (Nicklas, 1997). Misattached kinetochores are actively re-orientated, and proteins involved in this process ensure bipolar attachment (Stern, 2002; Tanaka, 2002). One of these proteins is the Ipl1/Aurora kinase of budding yeast. In Ipl1 mutants sister chromatids frequently attach to the same spindle pole (Tanaka et al., 2002) and re-orientation of kinetochore–spindle pole connections, as well as proper checkpoint signalling in the absence of tension, requires the activity of Ipl1 (Biggins and Murray, 2001; Tanaka et al., 2002). Based on these findings it has been proposed that Ipl1 functions to displace microtubules which fail to generate tension at the kinetochore (Stern, 2002; Tanaka, 2002).
Evidence from a variety of organisms indicates that Ipl/Aurora acts in concert with a BIR1/survivin protein and Sli15/INCENP (inner centromere protein) to regulate chromosome segregation (Adams et al., 2001). Aurora B, INCENP and survivin can interact directly, and interfering with the function of INCENP in vertebrates, or with the function of BIR-1 in Caenorhabditis elegans, leads to a failure to recruit Aurora B to the centromere (Adams et al., 2000; Speliotes et al., 2000; Wheatley et al., 2001; Bolton et al., 2002). Moreover, the phenotype of the survivin-deficient mice partially overlaps with that observed in mouse embryos deficient for INCENP. Both knock-out mice show dramatic polyploidy and fail to survive past E4.5 (Cutts et al., 1999; Uren et al., 2000). However, the exact role of human survivin in chromosome bi-orientation and spindle checkpoint surveillance is not clear at present.
Survivin is a member of the IAP family (inhibitor of apoptosis) of anti-apoptotic proteins. IAPs contain a BIR-domain (baculoviral IAP repeat) (Miller, 1999), essential for interaction with pro-apoptotic proteins, such as caspases (Deveraux and Reed, 1999). However, the role of survivin in apoptosis inhibition is controversial and several lines of evidence indicate it plays an important role during cell division (Fraser et al., 1999; Uren et al., 1999, 2000; Yoon and Carbon, 1999; Li et al., 2000). Survivin is specifically expressed during the G2/M phases of the cell cycle and behaves as a typical chromosome passenger protein, that associates to centromeres from late prophase to metaphase (Skoufias et al., 2000; Uren et al., 2000). Disruption of survivin function by overexpression of anti-sense survivin or dominant-negative survivin results in a failure of cell cleavage and polyploidization (Li et al., 1998, 1999).
To understand the role of human survivin in cell division, we studied human cells that were depleted of survivin by expression of specific siRNA. Our results demonstrate that survivin is essential for chromosome alignment, sister chromatid segregation and cytokinesis. Importantly, we provide evidence that survivin is required for a sustained arrest in response to lack of tension at the kinetochore, while the arrest induced through absence of microtubules is not affected by depletion of survivin.
Results
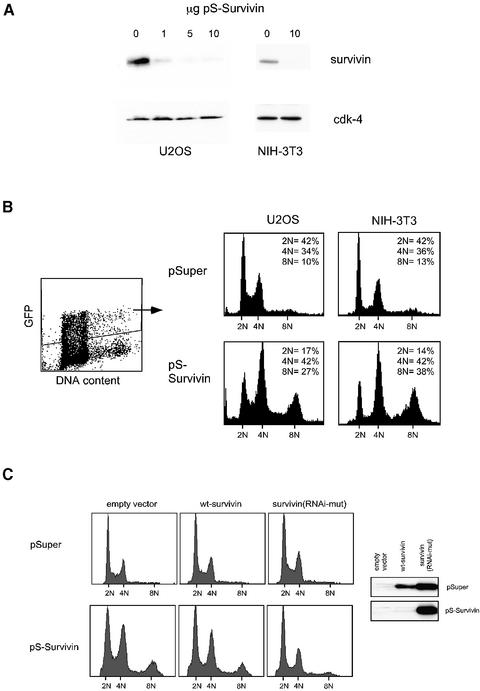
To investigate the role of survivin in cell division we knocked-down survivin expression by the use of our recently established expression vector pSuper (Brummelkamp et al., 2002) that allows stable expression of small interfering RNA (siRNA) in transfected cells. We chose a portion of the survivin mRNA that is identical in human and mouse, and tested the efficacy of our targeting construct in the mouse fibroblast cell line NIH-3T3 and the human osteosarcoma cell line U2OS. Isolation of transfected cells by magnetic activated cell sorting (MACS) demonstrated that the pS-Survivin targeting vector efficiently down-regulated expression of endogenous survivin in a dose-dependent fashion (Figure 1A).
Fig. 1. pS-Survivin efficiently down-regulates endogenous survivin expression resulting in polyploidy. U2OS and NIH-3T3 cells were co-transfected with the indicated amounts of pS-Survivin or pSuper and 1 µg of a CD20 (A) or spectrin–GFP (B) expression vector. (A) At 48 h after transfection, transfected cells were collected by MACS and analysed by western blotting. Blots were probed with anti-survivin pAb and subsequently reprobed with anti-cdk-4 to check for equal loading. (B) At 48 h after transfection, DNA profiles of the GFP-positive cells were determined by FACS analysis. (C) Cells were transfected with 10 µg of pS-Survivin or empty pSuper vector in combination with 0.2 µg of an expression plasmid for FLAG-tagged wild-type survivin (wt-survivin) or a survivin mutant carrying two silent mutations in the RNAi target sequence (survivin-RNAi/mut). At 48 h after transfection, some of the cells were analysed by western blotting (anti-FLAG), while the remaining cells were fixed and DNA profiles of the transfected cells were determined by FACS analysis.
Cell cycle analysis showed that survivin-depleted cells accumulate with a 4N DNA content and a significant proportion (27 ± 9% compared with 8 ± 2% in control cells, n = 12) becomes polyploid (Figure 1B). This is consistent with observations made in survivin-deficient embryos (Uren et al., 2000) and in experiments using antisense RNA or dominant-negative mutants of survivin (Li et al., 1998, 1999). Less than 20% of the cells had a 2N DNA content 48 h after transfection of pS-Survivin, indicating that mitotic exit is severely affected. Depleting survivin did not result in apoptosis as judged by the low percentage of sub-G1 cells (Figure 1B). Thus, survivin is essential for cell division, but no primary defect in cell survival is observed in these experiments.
To provide proof that our results are indeed due to specific down-regulation of survivin, we inserted two silent mutations within the survivin sequence recognised by the siRNA, to render it insensitive to our pS-Survivin construct. Expression of the RNAi-mutant survivin was not affected by pS-Survivin (Figure 1C, inset), and caused a reversion of the pS-Survivin-induced effects on cell cycle progression and polyploidization (Figure 1C), indicating that these effects were indeed specific to survivin down-regulation.
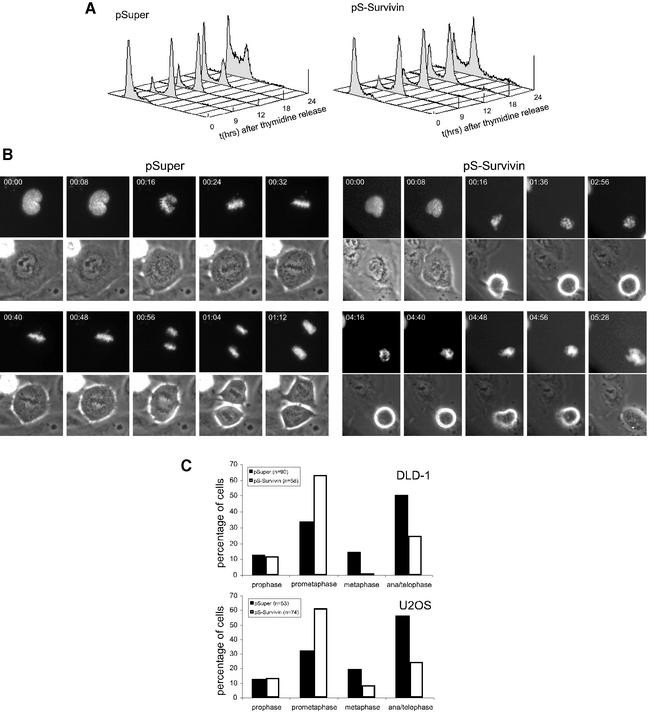
We next synchronized cells at the G1/S transition directly after transfection, before cell cycle progression was affected by survivin knock-down. Cells were then released and followed as they progressed through the cell cycle. Control cultures progress to the subsequent G1 phase ∼15 h after release from a thymidine block (Figure 2A). Cells depleted of survivin progress normally from G1/S to G2/M, but the majority of cells fail to complete the cell cycle and remain arrested with a 4N DNA content. In addition, tetraploid cells appear to enter a new round of DNA replication, as evidenced from the appearance of cells with a >4N DNA content at 24 h after release (Figure 2A, and also Figure 1B).
Fig. 2. Survivin knock-down interferes with normal mitotic progression. U2OS cells were transfected with 10 µg of pS-Survivin or empty pSuper vector as indicated. Immediately following transfection, cells were synchronized with thymidine for 24 h and subsequently released from this block. (A) Cells were fixed at the indicated time points after release and DNA profiles of the transfected cell populations were determined by FACS analysis. (B) Cells were grown on glass-bottom culture dishes and cotransfected with H2B–GFP and the indicated plasmids. Cells were followed by time-lapse imaging 12 h after release from the thymidine block. H2B–GFP fluorescence is shown on top, while the corresponding phase-contrast image is below. Time indicated in the upper left corner refers to the elapsed time (h:min) from the start of the time-lapse. (C) Mitotic stages were scored in DLD-1 and U2OS cells co-transfected with H2B–GFP and pSuper or pS-Survivin.
To monitor mitotic progression more closely, we followed survivin knock-down cells by time-lapse microscopy. Cells were transfected with the survivin targeting vector in combination with a histone H2B–GFP construct to analyse chromosomal behaviour. Typically, control U2OS cells start to enter mitosis 12 h after release from the thymidine block, and cells take 0:36 ± 0:07 h:min (n = 8) from nuclear envelope breakdown to complete chromosome alignment in metaphase (Figure 2B, left panels). Cells depleted of survivin enter mitosis with similar kinetics, and timing from nuclear envelope breakdown to prometaphase is similar to control cells (Figure 2A; data not shown). However, survivin-depleted cells spend 5:18 ± 1:55 h:min (n = 8) in a prometaphase state (Figure 2B). Chromosome condensation occurs normally, but cells were unable to align their chromosomes at the metaphase plate (Figure 2B, right panels; Supplementary figures 1 and 2, available at The EMBO Journal Online). In line with this, we clearly observed an increase in the number of prometaphases and a decrease in the number of metaphases, anaphases and telophases when survivin was depleted from U2OS or DLD-1 cells (Figure 2C). Strikingly, after this long lag time in prometaphase, cells exit mitosis without attaining chromosome alignment. This is evidenced by chromosome decondensation, flattening of the cells and reappearance of the nuclear envelope without chromosome segregation (Figure 2B, right panels; Supplementary figures 1 and 2). In almost all cells analysed, a single tetraploid nucleus is formed upon exit from mitosis (Figure 2B, right panels). Thus, survivin is not required for entry into mitosis, but is essential for chromosome congression and cytokinesis, as well as maintenance of a proper spindle checkpoint arrest.
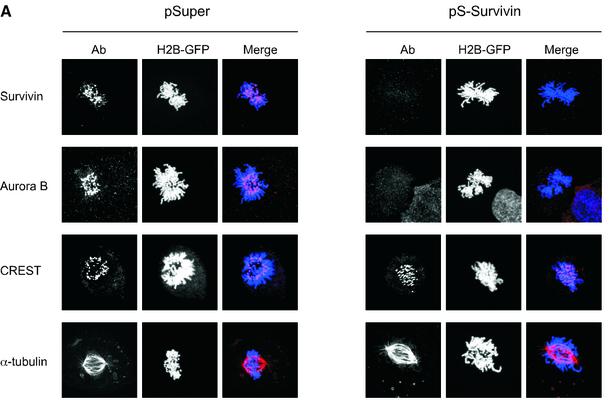
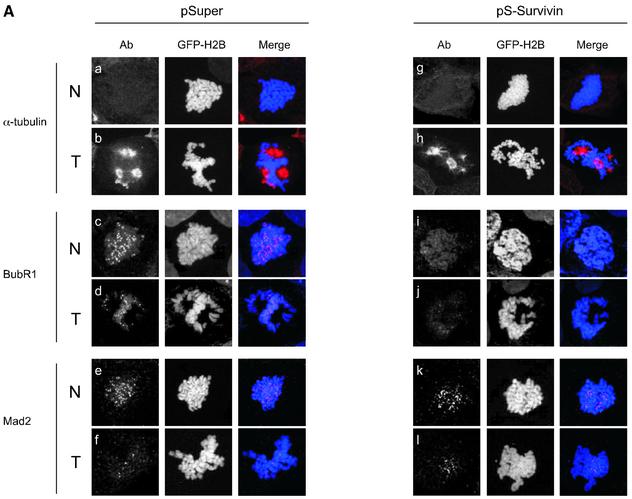
Since survivin and Aurora B have been demonstrated to interact in vitro (Wheatley et al., 2001), we analysed if survivin is directly involved in the recruitment of Aurora B to centromeres. Indeed, Aurora B was completely absent from centromeres in cells that do not express survivin (Figure 3A), consistent with a recent report using antisense survivin (Chen et al., 2003). Another centromere-specific antiserum, CREST, showed identical staining patterns in pSuper- and pS-Survivin-transfected cells (Figure 3A), indicating that the absence of Aurora B from centromeres is not simply due to loss of centromere structure. Endogenous protein expression levels of Aurora B were not affected by survivin-depletion, and ectopic expression of Aurora B was unable to restore normal cell cycle progression in cells lacking survivin (data not shown). Interestingly, spindle formation appeared normal, as judged by α-tubulin staining (Figure 3A). This is in apparent contrast to a previous report where it was described that blocking the function of survivin in HeLa cells leads to the formation of multipolar spindles (Li et al., 1999). However, it should be noted that we analyse spindle formation in the first mitosis following survivin depletion, and it is possible that multipolar spindles will be formed at later stages as a consequence of a failed mitosis. Nevertheless, the primary cell cycle defect we observe upon depletion of survivin cannot be a consequence of a defect in spindle formation, but may be due to a failure to recruit Aurora B to centromeres.
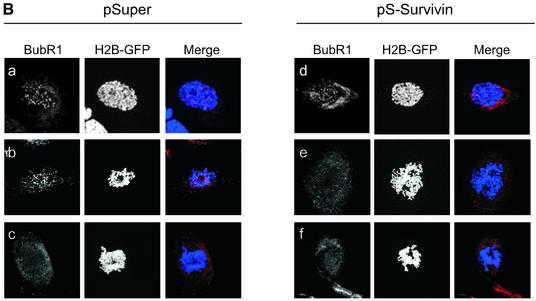
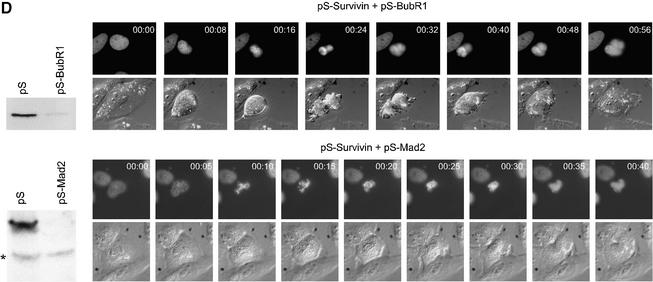
Fig. 3. Effects of survivin depletion on spindle formation and the localization of Aurora B, BubR1 and Mad2. U2OS cells cotransfected with H2B–GFP (1 µg) and 10 µg pSuper (left panels) or pS-Survivin (right panels) were fixed and stained with antibodies against the indicated proteins. Transfected, mitotic cells were imaged by confocal microscopy, by analysis of H2B–GFP staining of DNA (column labelled H2B–GFP). For better colour contrast, H2B–GFP is depicted in blue. (A) Confocal images of transfected, mitotic U2OS cells stained with antibodies against survivin (top row), Aurora B (second row), CREST (third row) or α-tubulin (bottom row). (B) BubR1 localization in mitotic U2OS cells. In control cells (left panels) BubR1 is localized at kinetochores during prophase (a) and prometaphase (b), but is absent from kinetochores during anaphase (c). In survivin knock-down cells (right panels), BubR1 is present on kinetochores during prophase (d), but is no longer localized at kinetochores during prometaphase (e and f). (C) Mad2 localization in mitotic U2OS cells. In pSuper-transfected cells (left panels) Mad2 is localized to unattached kinetochores during prophase (a) and prometaphase (b and c) and absent from kinetochores of cells that have entered anaphase (d). In pS-Survivin-transfected cells (right panels) Mad2 is present on kinetochores during prophase (e), but is no longer localized at kinetochores during prometaphase (f–h). (D) Knock-down of BubR1 and Mad2 overcomes the prometaphase delay in survivin knock-down cells. U2OS cells cotransfected with 1 µg H2B–GFP, 7.5 µg pS-Survivin plus 7.5 μg pS-BubR1 (upper panels) or with 7.5 µg pS-Survivin plus 7.5 μg pS-Mad2 (lower panels), were synchronized and life imaging was started 12 h after release from a thymidine block. Numbers indicated in the upper right corner indicate the elapsed time (h:min) from the start of the time-lapse. H2B–GFP fluorescence is shown on top, while the corresponding DIC image is below. Protein levels (western blot) of pS-BubR1- and pS-Mad2-transfected cells are shown on the left hand side of the corresponding time-lapse. Asterisk indicates non-specific band.
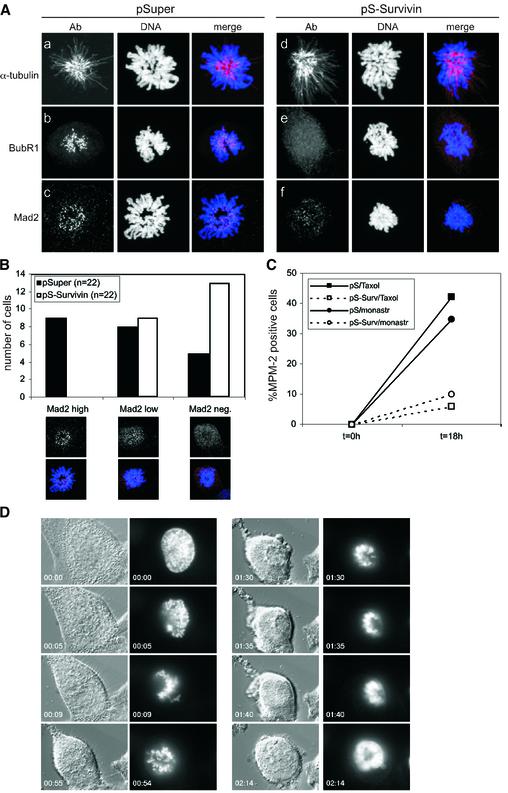
Our time-lapse experiments suggested that depletion of survivin leads to an impaired spindle checkpoint. Therefore, we decided to investigate the behaviour of two crucial spindle checkpoint components, BubR1 and Mad2. Both BubR1 and Mad2 were localized to kinetochores during prophase and prometaphase in control cells, and absent from the chromosomes in anaphase (Figure 3B, a–c and C, a–d). In survivin-deficient cells, BubR1 and Mad2 were normally recruited to the kinetochores in prophase, but could not be detected on kinetochores in prometaphase (Figure 3B, d–f and C, e–h). This shows that survivin is not required for the initial recruitment of BubR1 and Mad2, but appears to be essential for the maintenance of these proteins at the kinetochore. Nonetheless, survivin-deficient cells are delayed in prometaphase, suggesting that the spindle checkpoint is not completely abrogated.
To examine if the prometaphase delay seen in the absence of survivin was indeed due to spindle checkpoint activation, we combined the survivin RNAi with targeting vectors for Mad2 or BubR1. Expression of Mad2 and BubR1 was effectively down-regulated upon transfection of pS-Mad2 or pS-BubR1, respectively (Figure 3D). Moreover, knocking down either expression of Mad2 or BubR1 was sufficient to decrease the duration of mitosis from ∼60 to ∼35 min, and cells would exit mitosis before full congression of the chromosomes (data not shown). More importantly, time-lapse analysis of cells in which the function of both survivin and Mad2 or BubR1 was compromised, demonstrated that the prometaphase delay seen with pS-Survivin alone was completely abrogated when Mad2 or BubR1 were co-depleted (Figure 3D). Thus, checkpoint activation does occur in the absence of survivin, and only sustained activation of the spindle checkpoint appears to be affected by depletion of survivin. However, cell division was still impaired in these cells, in accordance with a proposed role for survivin in cytokinesis (Uren et al., 2000).
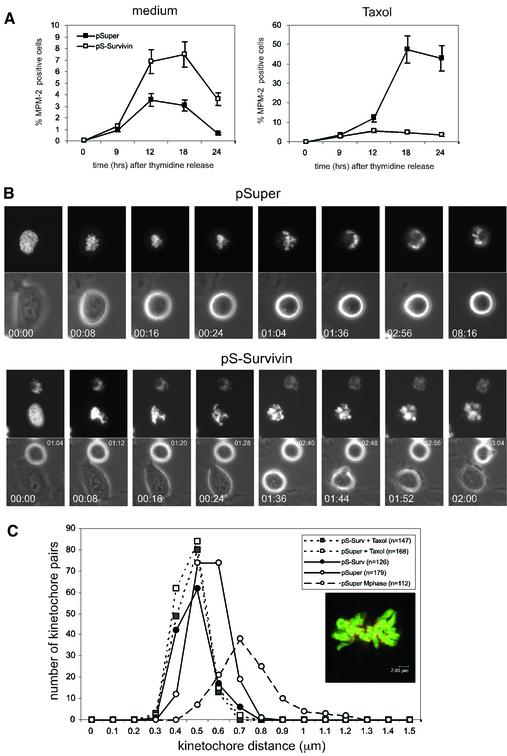
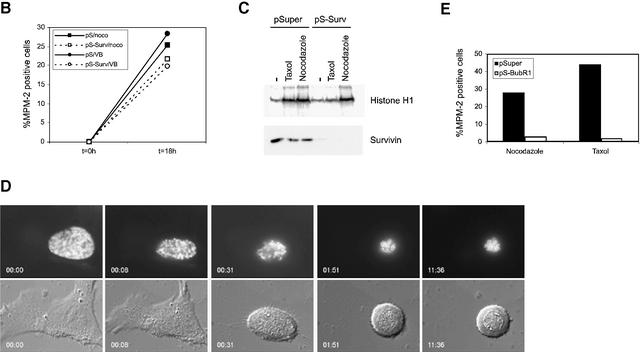
To further elucidate the role of survivin in spindle checkpoint maintenance, we next examined the effects of the microtubule-stabilizing agent taxol on survivin-depleted cells. In the presence of taxol, microtubules can attach to the kinetochores, but due to stabilization of microtubules no tension can be generated (McEwen et al., 1997; Waters et al., 1998). Cells transfected with pSuper or pS-Survivin were followed after release from a thymidine block and appearance of mitotic cells in time was monitored by MPM-2-reactivity, an established marker of mitotic cells. As shown in Figure 4A (left panel), both the control and the survivin-depleted cultures become MPM-2-positive at similar time points after release from thymidine. The somewhat higher MPM-2-reactivity seen in the pS-Survivin-transfected cells correlates with the delay in prometaphase observed in the time-lapse analysis (Figure 2B). Addition of taxol to control cultures released from a thymidine-block resulted in progressive accumulation of mitotic cells (Figure 4A, right panel). However, upon knock-down of survivin, the fraction of MPM-2-positive cells remained low (Figure 4A, right panel). Since entry into mitosis was not affected in these cultures (Figures 2 and 4B), this indicated that they escaped a taxol-induced arrest. This was confirmed by time-lapse microscopy. Taxol could elicit a very efficient arrest in mitosis (>16 h) in the pSuper-transfected cultures (Figure 4B, upper panel), but knocking down survivin expression abrogated the mitotic arrest induced by taxol (Figure 4B, lower panel). Remarkably, the duration of prometaphase in survivin-depleted cultures (see Figure 2) was significantly shortened by the presence of taxol (5:18 ± 1:55 h:min, n = 8 in pS-Survivin compared with 2:27 ± 0:40 h:min, n = 6 in pS-Survivin + taxol), indicating that stabilization of microtubules can promote mitotic exit in survivin-deficient cells.
Fig. 4. Survivin is required for a taxol-induced spindle checkpoint arrest. (A) U2OS cells cotransfected with 1 µg spectrin–GFP and 10 µg pSuper (closed squares) or pS-Survivin (open squares) were synchronized with thymidine and harvested at the indicated time points after release from the block (left panel). Taxol was added immediately after thymidine release (right panel). PI staining was combined with intracellular staining for MPM-2 to quantify mitotic cells. Note the difference in scale between the two panels, since mitotic exit is not blocked in the absence of taxol (left panel). Average ± SD of three independent experiments is shown. (B) U2OS cells cotransfected with 1 µg H2B–GFP and 10 µg pSuper (upper panels) or pS-Survivin (lower panels) were synchronized and life imaging was started 12 h after release in the presence of taxol. H2B–GFP fluorescence is shown on top, while the corresponding phase-contrast image is below. Numbers indicated in the lower left corner indicate the elapsed time (h:min) from the start of mitosis. In the pS-Survivin panel, numbers (h:min) in the upper right corner indicate the elapsed time of a second cell that had entered mitosis 64 min earlier. Total duration of mitosis of this particular cell was 3:20. (C) Histograms of sister kinetochore distances in pSuper- (open symbols) and pS-Survivin-transfected (closed symbols) cells, treated with (dashed line) or without (solid line) taxol. Inset shows an example of one focal plane of GFP–H2B-labelled chromosomes stained with CREST antiserum to reveal the kinetochores. Bar = 2 µm, Mphase = metaphase.
To determine if depletion of survivin affected tension at kinetochores, we stained kinetochores with CREST serum and measured the distance between the two kinetochores belonging to one pair of sister chromatids (Figure 4C, inset). As kinetochores become attached in a bipolar fashion, the tension generated by pulling forces from the opposing spindle poles will create a larger distance between the kinetochores of two sister chromatids (Zhou et al., 2002). Indeed, average kinetochore distance in pSuper-transfected U2OS cells was 0.55 ± 0.08 µm (n = 179 pairs in eight cells) in prometaphase, compared with 0.74 ± 0.14 µm (n = 112 pairs in five cells) in metaphase. Moreover, control cells that are in metaphase reach larger intra-kinetochore distances (maximum 1.2 µm) than cells in prometaphase (maximum 0.7 µm; Figure 4C). In contrast, the average intra-kinetochore distance in survivin-depleted cells was 0.48 ± 0.07 µm (n = 252 pairs in 10 cells), which was similar to the average distances in the presence of taxol, both in control cells (0.46 ± 0.06 µm, n = 168 pairs in six cells) and in pS-Survivin-transfected cells (0.47 ± 0.06 µm, n = 147 pairs in six cells). Thus, lack of survivin affects the build-up of tension at the kinetochores.
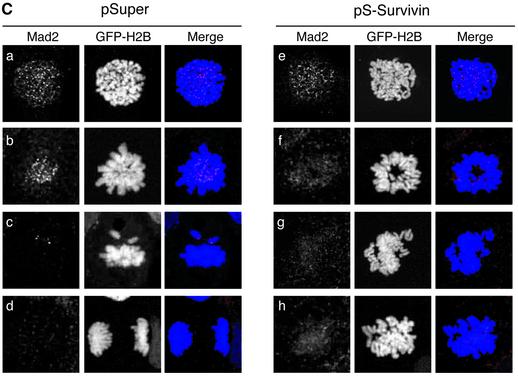
We next wanted to know what happens to survivin-depleted cells when microtubule attachment is blocked. Thus, we compared the efficacy of nocodazole and taxol in recruiting spindle checkpoint proteins in survivin-deficient cells. Spindle formation was severely inhibited in all cells by the addition of nocodazole (Figure 5A, a and g), while treatment with taxol caused the formation of abundant microtubule fibers that formed an aberrant spindle, which appeared to establish close contacts with the chromosomes (Figure 5A, b and h). As has been described previously (Waters et al., 1998; Skoufias et al., 2001), Mad2 is retained on kinetochores in the presence of nocodazole (Figure 5A, e and k), reflecting the lack of microtubule attachment at the kinetochore. In the presence of taxol, most kinetochores are devoid of Mad2, indicating that attachments can be established under these conditions (Figure 5A, f and l) (Waters et al., 1998; Skoufias et al., 2001). Interestingly, kinetochore-association of Mad2 in the presence of nocodazole was not affected by depletion of survivin (Figure 5A, k). This indicates that stable recruitment of Mad2 to kinetochores does not depend on survivin, as long as microtubule attachment is blocked. Consistent with this notion, we found that addition of nocodazole caused a mitotic arrest in pS-Survivin-transfected cells that was very comparable to the arrest seen in pSuper-transfected cells (Figure 5B). Similar results were obtained when vinblastine was used at concentrations described to block microtubule attachment (Skoufias et al., 2001) (Figure 5B). Also, cyclin B1-associated kinase activity was high in pSuper- and pS-Survivin-transfected cells blocked with nocodazole, while taxol was unable to stabilize cyclin B1 in cells depleted of survivin (Figure 5C). Time-lapse analysis demonstrated that survivin-deficient cells remained arrested in mitosis with condensed DNA after addition of nocodazole in prometaphase, confirming that they can execute a proper checkpoint arrest (Figure 5D).
Fig. 5. (A) In contrast to Mad2, retention of BubR1 at kinetochores depends on the presence of survivin. U2OS cells cotransfected with H2B–GFP (1 µg) and 10 µg pSuper (left panels) or pS-Survivin (right panels) were treated with nocodazole (N) or taxol (T) for 2 h and stained with antibodies against α-tubulin (a and b, g and h), BubR1 (c and d, i and j) or Mad2 (e and f, k and l). Transfected, mitotic cells were imaged by confocal microscopy, by analysis of H2B–GFP staining of DNA (column labelled H2B–GFP). For better colour contrast, H2B–GFP is depicted in blue (column labelled merge). (B) Survivin-depleted cells are blocked in mitosis when treated with spindle depolymerizing drugs. U2OS cells cotransfected with 1 µg spectrin–GFP and 10 µg pSuper (closed symbols) or pS-Survivin (open symbols) were synchronized with thymidine and harvested at the indicated time points after release from the block. Nocodazole (=noco) and vinblastine (=VB) were added immediately after thymidine release. PI staining was combined with intracellular staining for MPM-2 to quantify mitotic cells. One representative out of three independent experiments is shown. (C) U2OS cells cotransfected with 1 µg pBabe-puro and 10 µg pSuper or 10 µg pS-Survivin were synchronized with thymidine for 24 h in the presence of puromycin (2 µg/ml). Puromycin-selected cells were released and harvested 18 h after release from the block. Taxol and nocodazole were added immediately after release. Cyclin B/cdk1 kinase assays were performed using histone H1 as a substrate. Cell lysates were subjected to western blotting and blots were probed with an anti-survivin pAb. (D) U2OS cells cotransfected with 1 µg H2B–GFP and 10 µg pS-Survivin were synchronized and life imaging was started 12 h after release in the presence of nocodazole. H2B–GFP fluorescence is shown on top, while the corresponding DIC image is below. Numbers indicated in the lower left corner indicate the elapsed time (h:min) from the start of mitosis. (E) U2OS cells cotransfected with 1 µg spectrin–GFP and 10 µg pSuper or pS-BubR1 were synchronized with thymidine and harvested 18 h after release from the block. Taxol or nocodazole was added immediately after thymidine release. PI staining was combined with intracellular staining for MPM-2 to quantify mitotic cells.
The behaviour of BubR1 was clearly different in survivin-depleted cells. In control cells, BubR1 remained associated to kinetochores in the presence of nocodazole, as well as taxol (Figure 5A, c and d). However, after depletion of survivin, BubR1 was no longer associated to kinetochores, regardless of the type of drug that was used (Figure 5A, i and j). These data demonstrate that sustained association of BubR1 to the kinetochore crucially depends on the presence of survivin, even when microtubule attachment is inhibited. Importantly, we found a crucial difference between the depletion of BubR1 by RNAi-mediated knock-down and the failure to maintain BubR1 on kinetochores in survivin-deficient cells. Inactivation of BubR1 by siRNA-mediated depletion causes cells to become non-responsive to nocodazole as well as taxol (Figure 5E), while we clearly show that cells lacking survivin do arrest in response to nocodazole. Thus, survivin is not essential for initial activation of the spindle assembly checkpoint, but sustained association of BubR1 specifically depends on the presence of survivin.
Our data show that survivin-depleted kinetochores fail to come under maximum tension, but that microtubule attachment is still required to silence the spindle assembly checkpoint in survivin-depleted cells. Indeed, as judged by the progressive loss of Mad2 from kinetochores of cells lacking survivin, attachments appear to be established. This would suggest that microtubule attachments that fail to generate tension at the kinetochore are more stable in survivin-deficient cells. To address this possibility, we treated cells with monastrol, a drug known to cause the formation of a monopolar spindle by blocking the separation of the centrosomes (Mayer et al., 1999). Microtubules can attach to the kinetochores in the presence of monastrol, but the attachment cannot be stabilized by tension. Indeed, as a reflection of this dynamic instability, we found that cells that entered mitosis during a short treatment with monastrol (1 h) either retained Mad2 on almost all kinetochores (Mad2 high), while others showed intermediate staining for Mad2 (Mad2 low), and some scored Mad2-negative on nearly all kinetochores (Mad2 neg; Figure 6B). This is similar to what has been reported previously by others (Kapoor et al., 2000). However, in survivin knock-down cells that entered mitosis in the presence of monastrol, this dynamic instability was clearly affected as the majority of kinetochores were Mad2-negative (Figure 6B). This indicates that syntelic attachments are stabilized in the absence of survivin. Similar to taxol-treated cells, BubR1 was absent from kinetochores in all the cells that lacked survivin, while it was retained on kinetochores in monastrol-treated control cells (Figure 6A). Importantly, in contrast to control cells in which monastrol could block the cells in mitosis for >11 h (data not shown), monastrol failed to block pS-Survivin-transfected cells in mitosis (Figure 6C and D). Therefore, whereas the spindle assembly checkpoint is normally activated in cells lacking survivin, its premature silencing appears to be a consequence of a failure to displace misattached microtubules.
Fig. 6. Survivin is required for a monastrol-induced spindle checkpoint arrest. (A and B) U2OS cells, grown on coverslips, were cotransfected with 1 µg GFP–H2B and 10 µg pSuper (left panel) or pS-Survivin (right panel), were synchronized with thymidine, released for 15 h while monastrol was added during the final hour of the release. (A) Coverslips were fixed and stained with the indicated Abs. (B) Number of cells with Mad2 localized to all kinetochores (Mad2 high), Mad2 localized to a few kinetochores (Mad2 low) or no Mad2 (Mad2 neg.). (C) U2OS cells cotransfected with 1 µg spectrin–GFP and 10 µg pSuper (closed symbols) or pS-Survivin (open symbols) were synchronized with thymidine and harvested at the indicated time points after release from the block. Taxol (=tax) or monastrol (=monastr) were added immediately after thymidine release. PI staining was combined with intracellular staining for MPM-2 to quantify mitotic cells. (D) Time-lapse analysis of survivin knockdown cells as described in Figure 5D, but now in the presence of monastrol.
Discussion
In this study we found that survivin is essential for chromosome alignment and checkpoint responses elicited by drugs that interfere with tension. Cells lacking survivin entered mitosis with normal kinetics and formed bipolar spindles, but were delayed in a prometaphase state. This prometaphase delay is due to activation of the spindle assembly checkpoint, as it depends on the function of Mad2 and BubR1. Moreover, we could show that kinetochores lacking survivin fail to come under (maximum) tension, despite the extensive time spent in a prometaphase state. However, survivin-deficient cells do exit mitosis prior to completion of chromosome congression, indicating that they are unable to sustain this spindle checkpoint arrest. Consistent with this notion, we demonstrate that both Mad2 and BubR1 are lost from kinetochores in prometaphase cells lacking survivin. This is not due to a failure to recruit Mad2 or BubR1 to kinetochores in the first place, as we could show that these proteins properly localize at kinetochores in prophase cells that are depleted of survivin.
The spindle checkpoint defect of survivin-depleted cells was further confirmed in experiments with different spindle poisons. These experiments showed that survivin is essential to sustain a checkpoint arrest induced by drugs that affect tension, but survivin is dispensable for maintenance of a mitotic arrest when microtubule–kinetochore attachments are absent. Indeed, we could show that after nocodazole treatment Mad2 is retained at the kinetochores in the absence of survivin. In contrast, kinetochore retention of BubR1 appears to depend more directly on the presence of survivin, since BubR1 is displaced from kinetochores in nocodazole-, taxol- or monastrol-treated cells when survivin is absent. Importantly, while depletion of survivin selectively interferes with the response to taxol, siRNA-mediated depletion of BubR1 causes cells to become non-responsive to nocodazole as well as taxol. This difference is explained by the fact that BubR1 is recruited to kinetochores during prophase in cells lacking survivin, while BubR1 depletion will prevent this altogether. Apparently, Mad2 and BubR1 are both required for spindle checkpoint initiation, but their affinity for the kinetochore is regulated differentially. Continued recruitment of Mad2 does not directly depend on survivin, and Mad2 is permanently displaced when microtubules become stably attached (an event that requires tension in an unperturbed mitosis). In contrast, we show that sustained association of BubR1 critically depends on the presence of survivin and this appears crucial for sustained checkpoint activation in response to lack of tension.
Based on our data we would like to propose the following model for the function of survivin. As cells enter mitosis, survivin recruits Aurora B to the inner centromere, while a survivin-independent mechanism leads to recruitment of Mad2 and BubR1 to the kinetochore. During prometaphase, microtubule attachment occurs randomly with a high chance of syntelic or merotelic attachments. Although these types of attachments can result in transient displacement of Mad2, tension fails to be generated on the kinetochore and displacement of the microtubules by the survivin/Aurora B complex will result in renewed recruitment of Mad2 (Biggins and Murray, 2001; Tanaka et al., 2002). In the absence of survivin, Mad2 and BubR1 are normally recruited to the kinetochores in prophase, but Aurora B is not on the inner centromere and the signal coming from survivin/Aurora B that normally retains BubR1 at the kinetochore, is absent. Now, microtubules that fail to generate tension are not efficiently displaced and kinetochores will permanently loose Mad2, even if attachment is syntelic or merotelic. Under conditions that block microtubule attachment the function of survivin is no longer critical, since Mad2 will not be displaced. However, BubR1 is lost from kinetochores in survivin-depleted cells treated with nocodazole, indicating that the affinity of BubR1 for the kinetochore is determined by survivin rather than microtubule attachment. In this model, Aurora B is responsible for the displacement of misattached microtubules, but based on our observations it is equally possible that this is mediated through BubR1. At present, we do not know how survivin regulates the affinity of BubR1 for kinetochores. Preliminary pull-down experiments infer that survivin and BubR1 do not interact directly (our unpublished observation), but the observation that BubR1 is hyperphosphorylated on unattached chromosomes (Chen, 2002) makes it tempting to speculate that BubR1 is a direct substrate of Aurora B.
Although our data show that sustained checkpoint signalling in response to lack of tension is survivin-dependent, it should not be taken as evidence that tension and attachment activate two distinct branches of the spindle checkpoint. Tension and attachment tightly regulate one another to communicate proper spindle assembly to the checkpoint. Microtubule attachment is essential to generate tension at the kinetochore, but at the same time tension is required to stabilize microtubule attachment (Nicklas, 1997). Indeed, a recent report showed that Mad2 and BubR1 act in a single pathway to activate the checkpoint in response to lack of tension (Shannon et al., 2002). Our data suggest that lack of survivin will stabilize attachments, regardless of their capacity to generate tension at the kinetochore. As a result, depletion of survivin can circumvent the need for tension to silence the checkpoint. Therefore, survivin does not appear to be a direct effector of the spindle checkpoint, but renders a cell capable of displacing misattached microtubules to ensure bi-orientation of the chromosomes.
Finally, it should be noted that survivin-deficient cells spend a considerable amount of time in prometaphase while Mad2 and BubR1 have been displaced from kinetochores. Apparently, the time it takes from displacement of Mad2 and BubR1 from the kinetochores to silencing of the checkpoint is significantly extended in survivin-deficient cells. This suggests that Mad2 continues to inhibit the APC/C for a considerable amount of time after its displacement from kinetochores in these cells. Interestingly, recent findings support this notion, as it was demonstrated that cells lacking Hec1 arrest in a Mad2-dependent fashion whilst Mad2 is absent from kinetochores (Martin-Lluesma et al., 2002). Indeed, based on the structure of the Mad2/Cdc20 complex, it has been proposed that the spindle checkpoint needs to be actively silenced once the criteria of attachment and tension have been met (Musacchio and Hardwick, 2002). Thus, it is possible that active silencing of the spindle checkpoint is delayed in cells that lack survivin.
Taken together, depletion of survivin has allowed us to separate the function of BubR1 in initial checkpoint activation from its role in maintaining the spindle checkpoint. This latter function of BubR1 critically depends on the presence of survivin and is essential for monitoring tension at the kinetochores. Thus far, we have been unable to detect a prominent role for survivin in inhibition of apoptosis. Clearly, our approach to rescuing the siRNA-induced effects by co-expressing a silent mutant of survivin now allows us to analyse the functionality of different survivin protein domains and splice variants with respect to cell cycle regulation and spindle checkpoint function. These studies may help us separate out the proposed functions of survivin and shed more light on the controversies surrounding this protein.
Materials and methods
Plasmids
The survivin, BubR1 and Mad2 targeting vectors were based on a 19-mer sequence present in the coding sequence of both human and mouse survivin (GAGGCTGGCTTCATCCACT), human BubR1 (AGATCCT GGCTAACTGTTC) and human Mad2 (GGAAGAGTCGGGACCAC AG). 64-mer synthetic oligonucleotides for cloning into pSuper were synthesized, annealed and ligated into the pSuper construct as described (Brummelkamp et al., 2002). To obtain a survivin mutant that is non-responsive to our RNAi-targeting vector we changed the coding sequence within the targeted sequence at two wobble bases by PCR-based mutagenesis, without affecting the encoded amino acids (GAGGCC GGCTTTATCCACT). PCR fragments were cloned into _Eco_RI/_Xho_I-digested pCR3 containing a FLAG-epitope tag. Spectrin–GFP (Kalejta et al., 1997), histone H2B–GFP (Kanda et al., 1998), CMV-CD20 (van den Heuvel and Harlow, 1993) and pBabe-puro (Brummelkamp et al., 2002) have all been described.
Antibodies and dyes
The following antibodies were used: mouse anti-MPM-2 (Upstate Biotech), rabbit anti-Mad2 (Babco), rabbit anti-Survivin (R&D Systems), mouse anti-α-tubulin, mouse anti-FLAG, biotinylated goat anti-human antiserum (Sigma), peroxidase-conjugated goat anti-rabbit antiserum (DAKO), donkey anti-mouse/Cy5, donkey anti-rabbit/Cy5 (Jackson Immunoresearch), streptavidine/Alexa568, goat anti-rabbit/Alexa568 and goat anti-mouse/Alexa568 (Molecular Probes), human anti-CREST (gift of J.Kuijpers, UMC Utrecht, The Netherlands), sheep anti-BubR1 (gift of S.Taylor, University of Manchester, UK; Taylor et al., 2001). Propidium iodide was from Sigma and TOPRO-III from Molecular Probes.
Cells, synchronization and checkpoint activation
U2OS, NIH-3T3 and DLD-1 cells were grown in DMEM supplemented with 6% FCS and antibiotics. For cell synchronization at the G1/S transition, cells were incubated with 2.5 mM thymidine for 24 h. Checkpoint activation was performed using the following drugs; nocodazole (250 ng/ml), taxol (paclitaxel 1 µM), vinblastine (100 nM) or monastrol (200 µM). All drugs were obtained from Sigma.
Transfection
Cells were transfected by the standard calcium phosphate transfection protocol. Where indicated, the cells were synchronized by a thymidine-block directly after washing away the calcium phosphate precipitate. Cells were maintained in thymidine for 24 h, after which they were released from the block, and harvested at different time points after release for analysis by flow cytometry or immunofluorescence. Alternatively, transfected cells were monitored by time-lapse analysis.
Time-lapse analysis
Cells plated on 35 mm glass-bottom culture dishes (Willco-dish, Amsterdam, The Netherlands) were transfected the following day with pSuper or pS-Survivin, in combination with H2B–GFP or GFP–spectrin. Cells were followed by time-lapse microscopy starting at ∼10 h after release from the thymidine block. More than 80% of the cells in the culture are in late G2 at this point, as determined by flow cytometry. Dishes were supplied with CO2-independent medium (Gibco), covered with prewarmed mineral oil and transferred to a heated stage (37°C) on a Zeiss Axiovert 200M microscope equipped with a 0.55 numerical aperture (N.A.) condenser and a 40× Achroplan phase contrast objective (N.A. = 0.60). 12 bits phase contrast images (100 msec exposures to halogen light) and green fluorescence (100 msec exposures to blue light) were captured every 6 or 8 min using a Photometrics Coolsnap HQ charged-coupled device (CCD) camera set at gain 1.0 (Scientific, Tucson, AZ) and a GFP filter cube (Chroma Technology Corp.) to select specific fluorescence. Images were processed using Metamorph software (Universal Imaging, Downington, PA).
Magnetic activated cell sorting (MACS), flow cytometry and immunoflourescence
MACS-based isolation of transfected cells was performed as described (Medema et al., 2000). MPM-2-postivity of transfected cells was determined by flow cytometry as described (Smits et al., 2000). For immunofluorescence, cells were grown on coverslips coated with 10% poly-l-lysine (Sigma). Cells were transfected the next day, and synchronized using thymidine as described. At the indicated time points, coverslips were washed twice with PBS, fixed for 10 min in 3.7% formaldehyde, washed with PBS and stored in ice-cold methanol until staining with the appropriate antibodies. For α-tubulin staining, coverslips were permeabilized with 0.5% Triton X-100 for 0.5 min in PHEM buffer (45 mM Pipes, 45 mM Hepes, 10 mM EGTA, 5 mM MgCl2, 1 mM PMSF pH 6.8) prior to fixation. For staining, cells were washed with PBS and PBS containing 0.02% Tween-20, and subsequently incubated with the appropriate primary/secondary antibody combinations diluted in PBS/0.02% Tween-20 and 2% BSA. To visualize Mad2, cells were fixed and stained using paraformaldehyde/Triton as described (Pines, 1997). Cells were counterstained with TO-PRO III and analysed by confocal microscopy using a Leica TCS NT (Leica Microsystems) confocal system, equipped with an Ar/Kr laser. Images were taken using a 63× N.A. 1.3 objective. Standard filter combination(s) and Kalman averaging was used. To determine inter-kinetochore distances, coverslips were stained with CREST serum and the center-to-center distances between sister-kinetochores were measured from confocal image stacks (100× N.A. 1.4 objective) as described (Zhou et al., 2002).
Western blotting, immunoprecipitation and in vitro kinase reactions
Western blotting, immunoprecipitation and in vitro kinase assays were performed as described (Smits et al., 2000).
Supplementary data
Supplementary data are available at The EMBO Journal Online.
Acknowledgments
Acknowledgements
We would like to thank the other members of the lab for discussions; L.Oomen and L.Brocks for support with confocal microscopy; S.Taylor for comments on the manuscript and antibodies; J.Kuijpers for antibodies and J.Rodriguez for the survivin silent mutant. This work was supported by grants from the Dutch Cancer Society (NKB 2002-2764 and NKB 2003-2967).
Note added in proof
The data presented in this article are supported by recent data from A.Carvalho, M.Carmena, C.Sambade, W.C.Earnshaw and S.P.Wheatley (J. Cell Sci., in press).
References
- Adams R.R., Wheatley,S.P., Gouldsworthy,A.M., Kandels-Lewis,S.E., Carmena,M., Smythe,C., Gerloff,D.L. and Earnshaw,W.C. (2000) INCENP binds the Aurora-related kinase AIRK2 and is required to target it to chromosomes, the central spindle and cleavage furrow. Curr. Biol., 10, 1075–1078. [DOI] [PubMed] [Google Scholar]
- Adams R.R., Carmena,M. and Earnshaw,W.C. (2001) Chromosomal passengers and the (aurora) ABCs of mitosis. Trends Cell Biol., 11, 49–54. [DOI] [PubMed] [Google Scholar]
- Biggins S. and Murray,A.W. (2001) The budding yeast protein kinase Ipl1/Aurora allows the absence of tension to activate the spindle checkpoint. Genes Dev., 15, 3118–3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton M.A., Lan,W., Powers,S.E., McCleland,M.L., Kuang,J. and Stukenberg,P.T. (2002) Aurora B kinase exists in a complex with survivin and INCENP and its kinase activity is stimulated by survivin binding and phosphorylation. Mol. Biol. Cell, 13, 3064–3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummelkamp T.R., Bernards,R. and Agami,R. (2002) A system for stable expression of short interfering RNAs in mammalian cells. Science, 296, 550–553. [DOI] [PubMed] [Google Scholar]
- Chen J. et al. (2003) Survivin enhances Aurora-B kinase activity and localizes Aurora-B in human cells. J. Biol. Chem., 278, 486–490. [DOI] [PubMed] [Google Scholar]
- Chen R.H. (2002) BubR1 is essential for kinetochore localization of other spindle checkpoint proteins and its phosphorylation requires Mad1. J. Cell Biol., 158, 487–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutts S.M. et al. (1999) Defective chromosome segregation, microtubule bundling and nuclear bridging in inner centromere protein gene (Incenp)-disrupted mice. Hum. Mol. Genet., 8, 1145–1155. [DOI] [PubMed] [Google Scholar]
- Deveraux Q.L. and Reed,J.C. (1999) IAP family proteins—suppressors of apoptosis. Genes Dev., 13, 239–252. [DOI] [PubMed] [Google Scholar]
- Fraser A.G., James,C., Evan,G.I. and Hengartner,M.O. (1999) Caenorhabditis elegans inhibitor of apoptosis protein (IAP) homologue BIR-1 plays a conserved role in cytokinesis. Curr. Biol., 9, 292–301. [DOI] [PubMed] [Google Scholar]
- Hauf S., Waizenegger,I.C. and Peters,J.M. (2001) Cohesin cleavage by separase required for anaphase and cytokinesis in human cells. Science, 293, 1320–1323. [DOI] [PubMed] [Google Scholar]
- Hoyt M.A. (2000) Exit from mitosis: spindle pole power. Cell, 102, 267–270. [DOI] [PubMed] [Google Scholar]
- Kalejta R.F., Shenk,T. and Beavis,A.J. (1997) Use of a membrane-localized green fluorescent protein allows simultaneous identification of transfected cells and cell cycle analysis by flow cytometry. Cytometry, 29, 286–291. [DOI] [PubMed] [Google Scholar]
- Kanda T., Sullivan,K.F. and Wahl,G.M. (1998) Histone–GFP fusion protein enables sensitive analysis of chromosome dynamics in living mammalian cells. Curr. Biol., 8, 377–385. [DOI] [PubMed] [Google Scholar]
- Kapoor T.M., Mayer,T.U., Coughlin,M.L. and Mitchison,T.J. (2000) Probing spindle assembly mechanisms with monastrol, a small molecule inhibitor of the mitotic kinesin, Eg5. J. Cell Biol., 150, 975–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F., Ambrosini,G., Chu,E.Y., Plescia,J., Tognin,S., Marchisio,P.C. and Altieri,D.C. (1998) Control of apoptosis and mitotic spindle checkpoint by survivin. Nature, 396, 580–584. [DOI] [PubMed] [Google Scholar]
- Li F., Ackermann,E.J., Bennett,C.F., Rothermel,A.L., Plescia,J., Tognin,S., Villa,A., Marchisio,P.C. and Altieri,D.C. (1999) Pleiotropic cell-division defects and apoptosis induced by interference with survivin function. Nat. Cell Biol., 1, 461–466. [DOI] [PubMed] [Google Scholar]
- Li F., Flanary,P.L., Altieri,D.C. and Dohlman,H.G. (2000) Cell division regulation by BIR1, a member of the inhibitor of apoptosis family in yeast. J. Biol. Chem., 275, 6707–6711. [DOI] [PubMed] [Google Scholar]
- Martin-Lluesma S., Stucke,V.M. and Nigg,E.A. (2002) Role of Hec1 in spindle checkpoint signaling and kinetochore recruitment of Mad1/Mad2. Science, 297, 2267–2270. [DOI] [PubMed] [Google Scholar]
- Mayer T.U., Kapoor,T.M., Haggarty,S.J., King,R.W., Schreiber,S.L. and Mitchison,T.J. (1999) Small molecule inhibitor of mitotic spindle bipolarity identified in a phenotype-based screen. Science, 286, 971–974. [DOI] [PubMed] [Google Scholar]
- McEwen B.F., Heagle,A.B., Cassels,G.O., Buttle,K.F. and Rieder,C.L. (1997) Kinetochore fiber maturation in PtK1 cells and its implications for the mechanisms of chromosome congression and anaphase onset. J. Cell Biol., 137, 1567–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medema R.H., Kops,G.J., Bos,J.L. and Burgering,B.M. (2000) AFX-like Forkhead transcription factors mediate cell-cycle regulation by Ras and PKB through p27kip1. Nature, 404, 782–787. [DOI] [PubMed] [Google Scholar]
- Michaelis C., Ciosk,R. and Nasmyth,K. (1997) Cohesins: chromosomal proteins that prevent premature separation of sister chromatids. Cell, 91, 35–45. [DOI] [PubMed] [Google Scholar]
- Miller L.K. (1999) An exegesis of IAPs: salvation and surprises from BIR motifs. Trends Cell Biol., 9, 323–328. [DOI] [PubMed] [Google Scholar]
- Musacchio A. and Hardwick,K.G. (2002) The spindle checkpoint: structural insights into dynamic signalling. Nat. Rev. Mol. Cell Biol., 3, 731–741. [DOI] [PubMed] [Google Scholar]
- Nasmyth K., Peters,J.M. and Uhlmann,F. (2000) Splitting the chromosome: cutting the ties that bind sister chromatids. Science, 288, 1379–1385. [DOI] [PubMed] [Google Scholar]
- Nicklas R.B. (1997) How cells get the right chromosomes. Science, 275, 632–637. [DOI] [PubMed] [Google Scholar]
- Peters J.M. (2002) The anaphase-promoting complex: proteolysis in mitosis and beyond. Mol. Cell, 9, 931–943. [DOI] [PubMed] [Google Scholar]
- Pines J. (1997) Localization of cell cycle regulators by immunofluorescence. Methods Enzymol., 283, 99–113. [DOI] [PubMed] [Google Scholar]
- Shah J.V. and Cleveland,D.W. (2000) Waiting for anaphase: Mad2 and the spindle assembly checkpoint. Cell, 103, 997–1000. [DOI] [PubMed] [Google Scholar]
- Shannon K.B., Canman,J.C. and Salmon,E.D. (2002) Mad2 and BubR1 function in a single checkpoint pathway that responds to a loss of tension. Mol. Biol. Cell, 13, 3706–3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoufias D.A., Mollinari,C., Lacroix,F.B. and Margolis,R.L. (2000) Human survivin is a kinetochore-associated passenger protein. J. Cell Biol., 151, 1575–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoufias D.A., Andreassen,P.R., Lacroix,F.B., Wilson,L. and Margolis,R.L. (2001) Mammalian mad2 and bub1/bubR1 recognize distinct spindle-attachment and kinetochore-tension checkpoints. Proc. Natl Acad. Sci. USA, 98, 4492–4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits V.A., Klompmaker,R., Arnaud,L., Rijksen,G., Nigg,E.A. and Medema,R.H. (2000) Polo-like kinase-1 is a target of the DNA damage checkpoint. Nat. Cell Biol., 2, 672–676. [DOI] [PubMed] [Google Scholar]
- Speliotes E.K., Uren,A., Vaux,D. and Horvitz,H.R. (2000) The survivin-like C. elegans BIR-1 protein acts with the Aurora-like kinase AIR-2 to affect chromosomes and the spindle midzone. Mol. Cell, 6, 211–223. [DOI] [PubMed] [Google Scholar]
- Stemmann O., Zou,H., Gerber,S.A., Gygi,S.P. and Kirschner,M.W. (2001) Dual inhibition of sister chromatid separation at metaphase. Cell, 107, 715–726. [DOI] [PubMed] [Google Scholar]
- Stern B.M. (2002) Mitosis: aurora gives chromosomes a healthy stretch. Curr. Biol., 12, R316–R318. [DOI] [PubMed] [Google Scholar]
- Tanaka T.U. (2002) Bi-orienting chromosomes on the mitotic spindle. Curr. Opin. Cell Biol., 14, 365–371. [DOI] [PubMed] [Google Scholar]
- Tanaka T.U., Rachidi,N., Janke,C., Pereira,G., Galova,M., Schiebel,E., Stark,M.J. and Nasmyth,K. (2002) Evidence that the Ipl1-Sli15 (Aurora kinase-INCENP) complex promotes chromosome bi-orientation by altering kinetochore-spindle pole connections. Cell, 108, 317–329. [DOI] [PubMed] [Google Scholar]
- Taylor S.S., Hussein,D., Wang,Y., Elderkin,S. and Morrow,C.J. (2001) Kinetochore localisation and phosphorylation of the mitotic checkpoint components Bub1 and BubR1 are differentially regulated by spindle events in human cells. J. Cell Sci., 114, 4385–4395. [DOI] [PubMed] [Google Scholar]
- Uhlmann F., Wernic,D., Poupart,M.A., Koonin,E.V. and Nasmyth,K. (2000) Cleavage of cohesin by the CD clan protease separin triggers anaphase in yeast. Cell, 103, 375–386. [DOI] [PubMed] [Google Scholar]
- Uren A.G., Beilharz,T., O’Connell,M.J., Bugg,S.J., van Driel,R., Vaux,D.L. and Lithgow,T. (1999) Role for yeast inhibitor of apoptosis (IAP)-like proteins in cell division. Proc. Natl Acad. Sci. USA, 96, 10170–10175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uren A.G., Wong,L., Pakusch,M., Fowler,K.J., Burrows,F.J., Vaux,D.L. and Choo,K.H. (2000) Survivin and the inner centromere protein INCENP show similar cell-cycle localization and gene knockout phenotype. Curr. Biol., 10, 1319–1328. [DOI] [PubMed] [Google Scholar]
- van den Heuvel S. and Harlow,E. (1993) Distinct roles for cyclin-dependent kinases in cell cycle control. Science, 262, 2050–2054. [DOI] [PubMed] [Google Scholar]
- Waters J.C., Chen,R.H., Murray,A.W. and Salmon,E.D. (1998) Localization of Mad2 to kinetochores depends on microtubule attachment, not tension. J. Cell Biol., 141, 1181–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheatley S.P., Carvalho,A., Vagnarelli,P. and Earnshaw,W.C. (2001) INCENP is required for proper targeting of Survivin to the centromeres and the anaphase spindle during mitosis. Curr. Biol., 11, 886–890. [DOI] [PubMed] [Google Scholar]
- Yoon H.J. and Carbon,J. (1999) Participation of Bir1p, a member of the inhibitor of apoptosis family, in yeast chromosome segregation events. Proc. Natl Acad. Sci. USA, 96, 13208–13213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Panda,D., Landen,J.W., Wilson,L. and Joshi,H.C. (2002) Minor alteration of microtubule dynamics causes loss of tension across kinetochore pairs and activates the spindle checkpoint. J. Biol. Chem., 277, 17200–17208. [DOI] [PubMed] [Google Scholar]