Severe B cell hyperplasia and autoimmune disease in TALL-1 transgenic mice (original) (raw)
Abstract
TALL-1/Blys/BAFF is a member of the tumor necrosis factor (TNF) ligand superfamily that is functionally involved in B cell proliferation. Here, we describe B cell hyperplasia and autoimmune lupus-like changes in transgenic mice expressing TALL-1 under the control of a β-actin promoter. The TALL-1 transgenic mice showed severe enlargement of spleen, lymph nodes, and Peyer's patches because of an increased number of B220+ cells. The transgenic mice also had hypergammaglobulinemia contributed by elevations of serum IgM, IgG, IgA, and IgE. In addition, a phenotype similar to autoimmune lupus-like disease was also seen in TALL-1 transgenic mice, characterized by the presence of autoantibodies to nuclear antigens and immune complex deposits in the kidney. Prolonged survival and hyperactivity of transgenic B cells may contribute to the autoimmune lupus-like phenotype in these animals. Our studies further confirm TALL-1 as a stimulator of B cells that affect Ig production. Thus, TALL-1 may be a primary mediator in B cell-associated autoimmune diseases.
Keywords: TNF ligand family, B cell stimulation
The tumor necrosis factor (TNF) ligand superfamily is a group of pleiotropic cytokines involved in the regulation of cell death, proliferation, activation, and differentiation. The known family members include TNFα, LT-α, LT-β, FASL, CD40L, CD30L, CD27L, 4–1BBL, OX40L, and recently discovered TRAIL, OPGL, LIGHT, APRIL, and TALL-1 (1–6). All ligand members, with the exception of LT-α, are type-II transmembrane proteins, characterized by a conserved 150-aa region within the C-terminal extracellular domain. Though restricted to only 20–25% identity, the conserved 150-aa domain folds into a characteristic β-pleated sheet sandwich that forms homotrimers (7). This conserved region can be proteolytically released, thus generating a soluble functional form.
Many members within this ligand family are expressed in lymphoid-enriched tissues and play important roles in immune system development and modulation (1). For example, TNFα is mainly synthesized by macrophages and is an important mediator of inflammatory responses and immune defenses (8). Fas ligand (FasL), predominantly expressed in activated T cells, modulates T cell receptor (TCR)-mediated apoptosis of thymocytes (9, 10). CD40L, also expressed by activated T cells, provides an essential signal for B cell survival, proliferation, and Ig isotype switch- ing (11).
The cognate receptors for most of the TNF ligand family members have been identified. These receptors share characteristic multiple cysteine-rich repeats within their extracellular domains, and do not possess catalytic motifs within cytoplasmic regions (1). The receptors signal through direct interactions with death domain proteins (e.g., TRADD, FADD, and RIP) or with the TRAF proteins (e.g., TRAF2, TRAF3, TRAF5, and TRAF6), triggering divergent and overlapping signaling pathways, e.g., apoptosis, NF-κB activation, or JNK activation (12). These signaling events lead to cell death, proliferation, activation, or differentiation. The expression profile of each receptor member varies. For example, TNFR1 is expressed on a broad spectrum of tissues and cells (13), whereas the cell surface receptor of OPGL is mainly expressed on the osteoclasts (14).
Systemic lupus erythematosus (SLE) is an autoimmune disease associated with hyperactive B cell compartment. The disease is characterized by the presence of anti-DNA autoantibody and kidney immune complex deposits, which eventually lead to renal failure. In this report, we describe functional characterization of a TNF ligand family member TALL-1/BAFF/THANK/BLYS (6, 15, 16, 24). By using a transgenic approach, we demonstrated that TALL-1 is a potent B cell stimulatory factor. Interestingly, the TALL-1 transgenic mice also developed autoantibodies and glomerular immune complex deposits, a phenotype resembling lupus patients and lupus prone mice. Our findings suggest that TALL-1 is a potential therapeutic interference target for B cell-mediated autoimmune diseases such as SLE.
Materials and Methods
Recombinant TALL-1 Protein.
Bacteria expression plasmids were constructed to express soluble human TALL-1 protein from amino acids 128–285, preceded by an artificial methionine start codon. Escherichia coli were induced during fermentation, and the lysate was applied to Q Sepharose FF (Amersham Pharmacia) equilibrated in 10 mM Mes (pH 6.0) and eluted with 50–400 mM NaCl gradient over 30 column volumes. Fractions containing TALL-1 were pooled and loaded onto a Q Sepharose HP column (Amersham Pharmacia) equilibrated in 10 mM Tris⋅HCl (pH 8.5). TALL-1 was eluted with an increasing linear NaCl gradient (50 mM-200 mM) over 30 column volumes. Endotoxin was removed by application to Sp HiTRAP column (Amersham Pharmacia) (pH 4.8) and eluted with 100–500 mM NaCl in 10 mM sodium acetate (pH 4.8) over 25 column volumes. Final endotoxin level of the purified protein is approximately 0.2 EU/mg. The purified human TALL-1 is truncated at residue Arg133 as indicated by N-terminal sequencing and has a molecular weight of 16.5 kDa, as determined by reducing SDS/PAGE.
Generation of TALL-1 Transgenic Mice.
A _Xba_I–_Xho_I DNA fragment encoding murine full-length TALL-1 protein was cloned into expression vector under the control of the β-actin promoter (17). A 6-kb _Cla_I fragment containing TALL-1 transgene was then injected to single-cell embryos from BDF1 × BDF1-bred mice, and transgenic mice were generated as described (14). The transgene founders were determined by genomic DNA PCR with 5′ primer (AAC AGG CTA TTT CTT CAT CTA CAG) and 3′ primer (CTC ATC AAT GTA TCT TAT CAT GTC T) residing in the TALL-1 coding region or in transgenic vector 3′ immediately after TALL-1 coding region, respectively. Transgene expression was subsequently determined by reverse transcription (RT)-PCR analysis with primers described above.
Histopathology, Immunostaining, and FACS Analysis.
Following gross dissection, tissues were removed, fixed in 10% buffered Zn-formalin, processed into paraffin blocks, and 3-μm sections were obtained. All sections were stained with hematoxylin and eosin and subjected to histologic analysis. For immunostaining, frozen sections of the lymphoid organs were stained with rat monoclonal anti-mouse B220 and CD3 (Harlan Breeders, Indianapolis), respectively. The binding was detected by biotinylated rabbit anti-rat immunoglobulins and peroxidase-conjugated streptavidin (BioGenex Laboratories, San Ramon, CA) with diaminobenzidine as chromagen (Bio-Tek, Santa Barbara, CA).
For FACS analysis, single cell suspensions were prepared by gently grinding the tissues with the flat end of a syringe against the bottom of a 100-μm nylon cell strainer (Becton Dickinson). Cells were washed and counted. Approximately 1 million cells from each tissue were stained with 0.5 μg antibody in a 100-μl volume of PBS + 0.1% bovine albumin + 0.01% sodium azide. All spleen and mesenteric lymph node samples were incubated with 0.5 μg CD16/32(FcγIII/II) Fc block in a 20-μl volume for 10 min prior to the addition of FITC- or PE-conjugated monoclonal antibodies against CD90.2 (Thy-1.2), CD45R (B220), CD11b (Mac-1), Gr-1, CD4, or CD8 (PharMingen) at 2–8°C for 30 min. The cells were washed and then analyzed by flow cytometry with a FACScan (Becton Dickinson). Thymus samples were stained with FITC-conjugated anti-Thy-1.2, FITC-conjugated anti-CD4, and PE-conjugated anti-CD8 (PharMingen).
Serum Ig and Autoantibody Analysis.
Transgenic mice and control littermates were bled at indicated ages. Serum Ig levels were quantitated by using by ELISA with Mouse Hybridoma Subtype Kit as suggested by the manufacturer (Roche Molecular Biochemicals). Presence of autoantibodies directed against nuclear antigens and dsDNA were examined in the serum by ELISA. The levels of anti-nuclear antibodies were detected by using ANA screen kit (Sigma) and anti-mouse IgG peroxidase secondary antibody. Mouse serum samples were diluted 1:200 in ANA screen ELISA. For the detection of anti-dsDNA autoantibodies in serum, high-binding ELISA plates were coated with plasmid DNA (Immunovision, Springdale, AR) as an antigen in the presence of methylated BSA. After blocking the nonspecific sites and washing, diluted mouse serum samples were added to wells in duplicate, and the binding was quantitated by using horseradish peroxidase-labeled anti-mouse IgG or anti-mouse IgM reagents (Southern Biotechnology Associates). Pooled positive serum from BWF1 mice and pooled negative serum from B6 mice was used as controls. Experiment for the detection of anti-histone antibodies was essentially done similar to anti-DNA ELISA, except that carbonate-bicarbonate (pH 9.6) buffer was used as coating buffer. Serum antibody data were compared by Mann–Whitney test using Sigmastat software (SPSS, Chicago).
B Cell Survival and Proliferation Assay.
Cells were isolated from spleens of 2- to 4-mo-old mice by negative selection. Briefly, B lymphocytes were purified by density gradient centrifugation and then passed over a B cell column (Accurate/Cedarlane, Westbury, NY). Cells isolated by this method were analyzed by flow cytometry, and >90% were found positive for B220 staining. Isolated B cells were cultured in MEM + 10% FCS at 37°C, 5%CO2. Cells were collected from triplicate wells daily on day 1 through day 9 and incubated with 5 μg/ml propidium iodide. Cells were analyzed by flow cytometry, and the percentage of dead cells was calculated. For B cell proliferation assay, purified (105) B cells from B6 mice, as described above, were cultured in MEM + 10% heat-inactivated FCS in triplicate in 96-well flat-bottom plates with/without 2 μg/ml of goat F(ab′)2 anti-mouse IgM (Jackson ImmunoResearch) and/or indicated amount of recombinant TALL-1 for a period of 4 days at 37°C, 5%CO2. Proliferation was measured by an uptake of radioactive [3H]thymidine in the last 18 h of pulse.
Results
B Cell Hyperplasia and Hypergammaglobulinemia in TALL-1 Transgenic Mice.
A TNF family profile search of the GenBank dbEST database was performed. One human EST sequence (GenBank accession no. T87299) was identified as a possible member of the TNF ligand family. The full-length clone (AGP3) was subsequently isolated from screening a human spleen cDNA library. It encodes a LORF of 285 amino acids that is mostly related to human TNFα with 25% identity in the C-terminal 116-aa overlap. This ligand member was identical to the recently published TALL-1/BAFF/THANK/BlyS (6, 15, 16, 24).
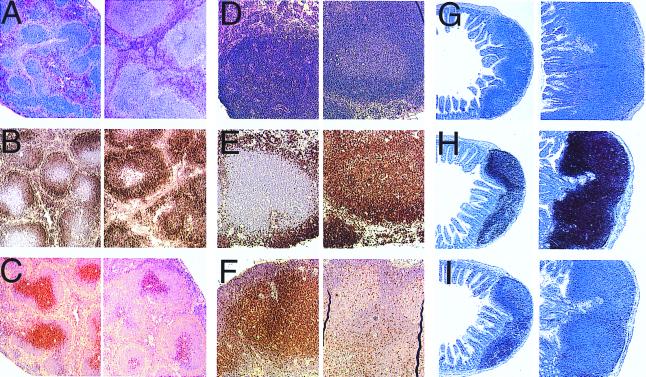
To gain insight into the biological function for TALL-1, transgenic mice were generated that expressed full-length murine TALL-1 protein driven by the ubiquitous β-actin promoter. β-actin promoter has been reported to direct widespread gene expression in transgenic mice (17, 22, 23). Founder mice harboring the TALL-1 transgene were identified by PCR analysis of genomic DNA samples (data not shown). Transgene expression was confirmed by RT-PCR from spleen total RNA (data not shown). At 8 wk of age, 10 TALL-1 transgenic mice and 5 control littermates were subject to necropsy and pathological analysis. The transgenic mice were of normal size and weight. However, the spleen weight relative to the body weight increased by approximately 45% in the TALL-1 transgenic group as compared with the control mice. The sizes of lymph nodes and Peyer's patches were also increased substantially in all of the TALL-1 transgenic mice. Histologic analysis demonstrated that the size and the number of the follicles in the spleen, lymph nodes, and Peyer's patches were increased significantly in the TALL-1 transgenic group (Fig. 1). Immunohistologic staining with B and T cell-specific markers indicated that the B cell numbers increased significantly in the spleen, lymph nodes, and Peyer's patches of the transgenic group (Fig. 1). The T cell numbers, as indicated by the anti-CD3 staining, were decreased correspondingly (Fig. 1). There were no differences in the morphology and immunostaining of thymus between the transgenic and the control groups (data not shown). No changes were observed in other organs or organ systems of the 8-wk-old transgenic mice, including kidney, liver, and hematopoietic tissues.
Figure 1.
Histology analysis of TALL-1 transgenic mice. Sections of spleen (A–C), lymph node (D–F), and Peyer's patches (G–I) from control mice (Left) and TALL-1 transgenic mice (Right) were stained with hematoxylin and eosin (A, D, and G), anti-mouse B220 antibody (B, E, and H), or anti-mouse CD3 antibody (C, F, and I). Stained sections were analyzed under microscope at ×10.
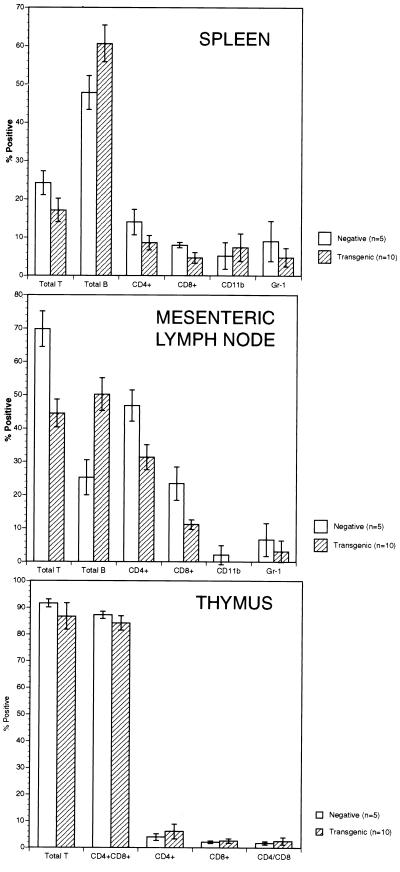
The B cell hyperplasia phenotype in the TALL-1 transgenic mice was also confirmed by flow cytometry analysis. In the mesenteric lymph nodes of the TALL-1 transgenic mice, the percentage of B220-positive B cells increased by 100% (Fig. 2). The percentage of the Thy-1.2-positive T cells decreased by approximately 36%, with similar reductions in both CD4(+) and CD8(+) T cells. Similar increase in B cell and reduction in T cell populations was also observed in the spleens of the TALL-1 transgenic mice, though to a lesser extent (Fig. 2). Of note, the total T cell numbers in the lymph node and spleen of TALL-1 transgenic mice were similar to the control littermates (data not shown). In the thymus, there were no differences in the percentages of single positive CD4 or CD8 T cells, or double positive CD4+CD8+ populations between the TALL-1 transgenic and the control mice (Fig. 2). No obvious changes in staining with anti-CD11b or anti-Gr-1 antibodies were observed in the lymph nodes and spleen between the transgenic and the control group (Fig. 2). The histological and FACS analysis, together, suggested severe B cell hyperplasia phenotype in the TALL-1 transgenic mice.
Figure 2.
FACS analysis splenocytes, lymph node cells, and thymocytes of TALL-1 transgenic mice. Single-cell suspensions were prepared from spleen, lymph nodes, and thymus from 10 TALL-1 transgenic mice and 5 control littermates. Cells were stained with FITC- or PE-conjugated monoclonal antibodies against Thy-1.2, B220, CD11b, Gr-1, CD4, or CD8.
We also examined B cell populations of different developmental stages by FACS analysis. No differences were observed in the percentage of the pro B (B220+IgM−), immature B (B220+IgM+), or mature B (IgM+IgD+) within the splenic B cell population of the TALL-1 transgenic mice as compared with the control littermates (data not shown). In addition, the number of the splenic CD5+ B cells in the TALL-1 transgenic mice from 1–9 mo of age was unaltered (data not shown). We also did not detect any alteration of the CD40 expression level on B cells in the transgenic mice (data not shown), suggesting that the B cell hyperplasia in the TALL-1 transgenic mice was not caused by CD40 up-regulation.
In addition to the B cell hyperplasia phenotype, the TALL-1 transgenic mice also had severe hypergammaglobulinemia. The serum globulin level in TALL-1 transgenic mice increased >100% as compared with the control group (data not shown). The total protein level also increased correspondingly in the transgenic mice, whereas the albumin level remained the same. The increased B cell numbers and high serum globulin level suggested the presence of elevated serum immunoglobulins. Thus, we examined serum levels of IgM, IgG, IgA, IgE, and IgG subclasses of 8-wk-old TALL-1 transgenic mice (Table 1). Compared with the same age-control littermates, serum IgM, IgG, IgA, and IgE were significantly increased in the TALL-1 transgenic mice. The increase found in serum IgG was not specific to any particular subclass (IgG1, IgG2a, IgG2b, and IgG3; Table 1). No significant differences in other serum chemistry or hematology parameters were observed at this age. The increased serum Ig levels are likely secondary to the increased B cell number, but may also be aggravated by increased B cell antibody production.
Table 1.
Elevation of serum immunoglobulin levels in TALL-1 transgenic mice
| IgM (μg/m) | IgA (μg/ml) | IgE (ng/ml) | IgG1 (μg/m) | IgG2a (μg/ml) | IgG2b (μg/ml) | IgG3 (μg/ml) | |
|---|---|---|---|---|---|---|---|
| TALL-1 | 7,640 ± 742 | 11,360 ± 3,045 | 634 ± 77 | 396 ± 53 | 1,132 ± 109 | 1,002 ± 190 | 1,528 ± 358 |
| Control | 475 ± 48 | 85 ± 17 | 359 ± 71 | 198 ± 40 | 302 ± 100 | 248 ± 51 | 115 ± 41 |
Autoantibodies Associated with Lupus in TALL-1 Transgenic Mice.
The presence of increased humoral immunity in TALL-1 transgenic mice warranted us to look for possible phenotypes resembling B cell-associated autoimmune diseases such as SLE. The common denominator in either lupus patients or lupus prone mice is autoantibody production, and the hallmark of this disease is the presence of elevated anti-nuclear antibodies in the serum. The emergence of anti-DNA antibodies also represents one final outcome in the different murine lupus models and patients with SLE. When sera from transgenic and nontransgenic mice at various ages were examined for the presence of autoantibodies recognizing nuclear antigens or dsDNA, two different lines of TALL-1 transgenic mice began to show presence of autoantibodies at around 8 wk of age (Table 2). The amount of anti-nuclear and anti-dsDNA antibody increased with age in the transgenic animals (Table 2). More interestingly, at 5 and 8 mo of age, TALL-1 transgenic mice showed 5–10 higher amount of anti-dsDNA antibodies compared with age matched lupus prone (NZB × NZW)F1 mice (data not shown). The presence of autoantibodies in the serum of TALL-1 transgenic mice did not discriminate between gender of mice. Both IgG and IgM antibodies to dsDNA were detected in transgenic animals. Additionally, antibody to DNA binding protein histone was also elevated in the TALL-1 transgenic mice (Table 2). Presence of such autoantibodies was undetectable in nontransgenic littermates.
Table 2.
Lupus-associated autoantibodies in the serum of TALL-1 transgenic mice
| Autoantibodies | Age (mo) | TALL-1 tg (n) | Non-tg littermates (n) | P value |
|---|---|---|---|---|
| Antinuclear (IgG)* | 2–3 | 7‡(9) | 1§ (8) | |
| 5–6 | 9 (9) | 1§ (8) | ||
| 8–9 | 8 (8) | 1§ (6) | ||
| Anti-dsDNA (IgG)† | <2 | 697 ± 284 (7) | 277 ± 67 (7) | NS |
| 3–4 | 842 ± 351 (7) | 235 ± 49 (7) | <0.005 | |
| 6–7 | 2,515 ± 428 (5) | 970 ± 344 (7) | <0.019 | |
| 8–10 | 12,293 ± 6,767 (11) | 1,070 ± 602 (12) | <0.017 | |
| Anti-dsDNA (IgM)† | <2 | 275 ± 33 (7) | 46 ± 5 (7) | <0.001 |
| 3–4 | 1,684 ± 920 (7) | 63 ± 13 (7) | <0.003 | |
| 6–7 | 6,998 ± 5,515 (5) | 98 ± 14 (7) | <0.001 | |
| 8–10 | 13,712 ± 9,147 (11) | 79 ± 14 (12) | <0.001 | |
| Anti-histone (Ig)† | <2 | 741 ± 264 (7) | 52 ± 8 (7) | <0.001 |
| 3–4 | 837 ± 436 (7) | 53 ± 14 (7) | <0.003 | |
| 6–7 | 4,220 ± 933 (5) | 60 ± 10 (7) | <0.001 | |
| 8–10 | 16,553 ± 4,618 (11) | 295 ± 173 (12) | <0.001 |
Immune Complex Deposits in the Kidney of TALL-1 Transgenic Mice.
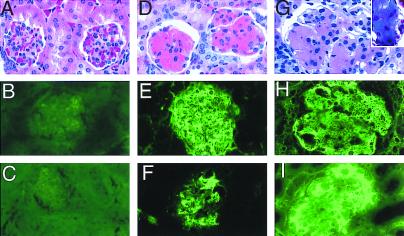
Presence of anti-DNA antibodies associated with immune complex-mediated renal disease is a classical picture seen in lupus associated nephritis. At 5 mo of age, the TALL-1 transgenic mice developed glomerular proteinaceous deposits in the kidney (Fig. 3). The deposits were seen in >60% of the glomeruli in the transgenic mice, but were absent in the control littermates. Immunohistology showed that the deposits contained moderate amounts of IgG and larger amounts of IgM (Fig. 3 E and F). The deposits also contained IgA and C3 (data not shown). The T cell number per glomerulus in the TALL-1 transgenic mice (2.4 ± 0.5) was increased as compared with the age-matched control littermates (0.32 ± 0.5). Trichrome staining showed no collagen in the glomeruli at 5 mo of age (data not shown). There is no evidence of obvious cellular proliferation (Fig. 3D). Interestingly, the kidney lesions progressed as the transgenic mice grew older. At 8 mo of age, there was obvious enlargement of glomeruli in the TALL-1 transgenic mice as compared with the age-matched control littermates (Fig. 3G). In addition, we also detected extensive connective tissue deposits in the enlarged glomeruli (Fig. 3G, Inset). Compared with the 5-mo-old mice, the 8-mo-old transgenic mice had increased IgG level in the glomeruli immune complex deposits (Fig. 3I). The majority of the glomeruli (80%–90%) in the TALL-1 transgenic mice were affected. We also performed serum and urine chemistry analysis of 5- and 8-mo-old TALL-1 transgenic along with the control littermates. No significant differences were noticed in the 5-mo-old TALL-1 transgenic mice. However, in the 8-mo-old mice, we observed increases in serum blood urea nitrogen (BUN) and calcium levels and a decrease in serum phosphate level (data not shown). In addition, the 8-mo-old TALL-1 mice also proteinuria (data not shown). Similar kidney lesions were observed in two TALL-1 transgenic lines. Together, these changes suggest the early onset of renal dysfunction in the 8-mo-old TALL-1 transgenic mice. In conclusion, the high serum autoantibodies followed by the kidney lesions in theTALL-1 transgenic mice resemble the progressive renal disease seen in SLE patients and lupus prone mice.
Figure 3.
Kidney Ig deposits in TALL-1 transgenic mice. Kidney sections of 5-mo-old control littermate (A–C), 5-mo-old TALL-1 mice (D–F), and 8-mo-old TALL-1 mice (G–I) were stained with hematoxylin and exosin (A, D, and G), anti-mouse IgM (B, E, and H), anti-mouse IgG (C, F, and I), and Trichrome (G, Inset). Stained sections were analyzed under microscope at ×60.
TALL-1 Stimulates B Cell Survival and Proliferation: a Possible Mechanism for Autoimmunity.
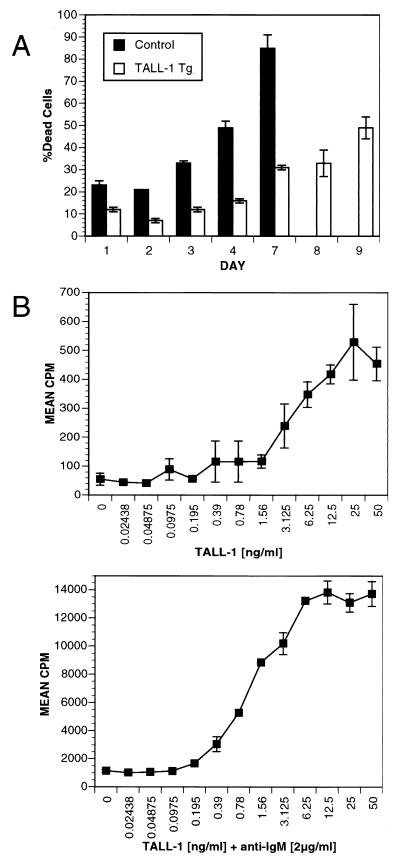
The B cell hyperplasia phenotype in the TALL-1 transgenic mice might arise from increased B cell survival and/or increased B cell proliferation. We first compared the viability of B cells from TALL-1 transgenic mice with that of the control littermates. Splenic B cells were isolated from both transgenic or control mice and incubated in MEM supplemented with 10% heat-inactivated FBS. Viability of the B cells was measured by FACS analysis for propidium iodide uptake (Fig. 4A). By day 3, 30% of B cells isolated from the control mice were dead, whereas only 10% of B cells from TALL-1 transgenic mice were dead. By day 5, 70% of B cells from TALL-1 mice were still viable, whereas only 15% of B cell from control littermates were viable. By day 9, almost 50% of the TALL-1 transgenic B cells still remained viable. Therefore, transgenic expression of TALL-1 prolonged B cell viability. It remains to be determined whether these B cell survival stimuli result directly from TALL-1 action on B cells or through its modulation of the immune system.
Figure 4.
TALL-1 stimulates B cell survival and proliferation. (A) Increased B cell viability in TALL-1 transgenic mice. B cells were isolated from spleens of 3-mo-old TALL-1 transgenic mice (n = 3) and control littermates (n = 3). A total of 2.5 × 105 B cells was aliquoted per well in a 96-well round-bottom plate and incubated for 9 days. At the indicated days, cells were incubated with 5 μg/ml propidium iodide and subjected to FACS analysis for positive staining cells. Values are expressed as mean ± SEM. (B) TALL-1 stimulates B cell proliferation. Purified B cells (105) from B6 mice were cultured in triplicates in 96-well plate with indicated amount of TALL-1 at the absence (Upper) or presence of 2 μg/ml anti-IgM antibody (Lower) for a period of 4 days. Proliferation was measured by radioactive [3H]thymidine uptake in the last 18 h of pulse. Data shown represent mean ± SD of triplicate wells.
To examine whether TALL-1 stimulates B cell growth, we generated soluble recombinant TALL-1 protein. TALL-1 alone can also stimulate B cell proliferation in a dose-dependent manner with an ED50 of approximately 3 ng/ml (Fig. 4B, Upper). A 10-fold increase of B cell proliferation was detected by TALL-1 treatment at 10 ng/ml concentration as compared with the untreated cells. We also examined B cell costimulatory activity of TALL-1 with anti-IgM. Anti-IgM alone at 2 μg/ml concentration increased B cell proliferation by 24-fold. Treatment with anti-IgM (2 μg/ml) in combination with various doses of TALL-1 led to a dose-dependent increase of B cell proliferation, with a maximal 13-fold increase as compared anti-IgM treatment alone and a total of 320-fold increase when compared with the untreated cells. Similar B cell costimulatory property was reported recently by Schneider et al. (15) and Moore et al. (16). Thus, TALL-1 is a weak stimulant and a potent costimulant of B cell growth. The increased B cell survival and proliferation may together contribute to the B cell hyperplasia and autoimmune lupus-like changes in the TALL-1 transgenic mice.
Discussion
SLE is a genetically complex prototypic autoimmune disease with a potential to involve multiple organ systems. Its clinical manifestations are exceptionally diverse (18). The initiating event causing an abnormal response in either murine or human lupus is unknown. During this complex process, helper T (Th) cells get generally activated at the beginning of autoimmune response by an unknown trigger (19, 20). The current knowledge of pathogenic mechanisms underlying the consequences of this disease involves at least the following events: (i) B cells produce pathogenic antibodies to nuclear constituents, (ii) formation and deposition of antigen–antibody (immune) complexes in the kidney, and (iii) renal failure. All three pathological changes were observed in the TALL-1 transgenic mice as they aged. Our findings demonstrate that TALL-1 is a potential mediator for autoimmune lupus-like diseases. It will be interesting to investigate whether there is any deregulation of TALL-1 expression or activity in lupus patients.
The lupus-like disease seen in TALL-1 transgenic mice can be related to other murine models of lupus. These include animals with (i) targeted mutation of specific gene, such as FcRII, C4, Lynn, TGF-b, IL-2, PD-1 gene or (ii) spontaneous mutations in motheaten, MRL-lpr/lpr, BXSB, and F1s between (NZB × NZW) and (NZB × SWR) crosses (18). These murine models of lupus have defective immunological function leading to development of autoimmunity. Immunologically, lupus seen in TALL-1 transgenic mice can be correlated with BCL-2 protein-expressing transgenic animals (21). Both of these transgenic animals have prolonged survival of B cells. In BCL-2 transgenic animals, prolonged survival of lymphocyte is not restricted to B cells. One common feature shared in these animal models and in patients with SLE is the hyperactivity of B cells, which may be because of a factor such as TALL-1. Most of the lupus animal models develop disease after 4 mo of age. We found that TALL-1 could also accelerate the production anti-DNA antibody in the lupus prone BWF1 mice (our unpublished results). Further investigation is needed to confirm the role of TALL-1 as a common factor stimulating the B cells in murine and human lupus.
Previous studies (15, 16) showed TALL-1 as a costimulator of B cells in the presence of primary stimulation by anti-IgM. Our results confirm these findings. In addition, we also found that TALL-1 costimulates B cell proliferation with IL-4 (data not shown). Of note, contrary to the previous reports, we found that TALL-1 alone can also directly stimulate B cell proliferation by 10-fold. This stimulatory activity is TALL-1-specific in consideration of the low ED50 (approximately 3 ng/ml) and low endotoxin level of TALL-1 preparation (0.2 EU/mg). The cause of the discrepancy remains to be determined. Our findings suggest that, like CD40L, TALL-1 is a weak stimulant and a potent costimulant of B cells. Interestingly, transgenic overexpression of TALL-1 also increased B cell viability. This survival effect probably results from the direct TALL-1 induction on B cells because of the B cell-specific expression of TALL-1 receptor. We believe that both hyperstimulation and prolonged survival of B cells induced by TALL-1 contribute to the autoimmune phenotype in the TALL-1 transgenic mice.
Our studies in the TALL-1 transgenic mice demonstrated increases in serum IgM, IgG, IgA and IgE and IgG subclasses, suggesting a non-specific B cell proliferation effect mediated through TALL-1. The elevation of serum Ig levels not only sustained but also increased as the mice aged (data not shown). Moore et al. (16) recently reported that mice injected with TALL-1/BLyS protein had increased serum IgM and IgA, but not IgG. The discrepancy might be because of the shorter exposure time of mice to the injected recombinant BlyS protein in their study. The increased serum Ig levels are likely secondary to the increased B cell number, but may also be aggravated by increased B cell antibody production.
The receptor for TALL-1 is expressed specifically on B cells, as suggested by the FACS analysis of peripheral blood-nucleated cells and leukemic cell lines by our lab (data not shown) and others (15, 16). This notion is further supported by the specific B cell phenotype in the TALL-1 transgenic mice. B cell activation induced by TALL-1 is independent of the CD40L/CD40 pathway. First, TALL-1 did not bind directly to CD40 (data not shown). Second, TALL-1 did not affect CD40 expression level on B cells from the TALL-1 transgenic mice (data not shown). In addition, TALL-1 and CD40L in combination had additive B cell stimulatory activity as compared with each stimulant alone, supporting the presence of two different receptors (data not shown). As reported previously, we failed to detect TALL-1 binding to any known TNFR receptor family member (e.g., TNFR1, TNFR2, CD40, RANK, OPG, ATAR, GITR, FAS, DR3, DR4, DR5, and DR6). Thus, the identity of the TALL-1 receptor remains to be determined.
In summary, our results from TALL-1 transgenic mice demonstrate that TALL-1 is a driving force toward the development of B cell hyperplasia and autoimmune lupus-like disease. Considering universal features as hyperactive B cells in lupus, TALL-1 may be an important target in B cell-mediated autoimmune diseases. Studies done in our laboratory and by others clearly indicate that the receptor of TALL-1 is located on B cells. A soluble receptor of TALL-1 or neutralizing anti-TALL-1 antibody could have a significant potential in the treatment of such diseases.
Acknowledgments
We thank James McCabe, Kathy Christensen, and Carl Whitely for the maintenance of TALL-1 transgenic mice. We also thank Dave Hill and Sylvia Copon for their help with the pathology analysis, and all members of the Amgen Genome Program for their collective contributions to this work.
Abbreviations
TNF
tumor necrosis factor
TCR
T cell receptor
L
ligand
SLE
systemic lupus erythematosus
RT
reverse transcription
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.050580697.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.050580697
References
- 1.Smith C A, Farrah T, Goodwin R G. Cell. 1994;76:959–962. doi: 10.1016/0092-8674(94)90372-7. [DOI] [PubMed] [Google Scholar]
- 2.Lacey D L, Timms E, Tan H-L, Kelley M J, Dunstan C R, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, et al. Cell. 1998;93:165–176. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- 3.Chichepotiche Y, Bourdon P R, Xu J, Hsu Y M, Scott H, Hession C, Garcia I, Browning J L. J Biol Chem. 1997;272:32401–32410. doi: 10.1074/jbc.272.51.32401. [DOI] [PubMed] [Google Scholar]
- 4.Mauri D N, Ebner R, Montgomery R I, Kochel R I, Cheung T C, Yu G L, Ruben S, Murphy M, Eisenberg R J, Cohen G H, et al. Immunity. 1998;8:21–30. doi: 10.1016/s1074-7613(00)80455-0. [DOI] [PubMed] [Google Scholar]
- 5.Hahne M, Kataoka T, Schroter M, Hofmann K, Irmler M, Bodmer I, Schneider P, Bornard T, Holler N, French L E, et al. J Exp Med. 1998;188:1185–1190. doi: 10.1084/jem.188.6.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shu H B, Hu W H, Johnson H. J Leukocyte Biol. 1999;65:680–683. [PubMed] [Google Scholar]
- 7.Banner D W, D'Arcy A, Janes W, Gentz R, Schoenfeld H-J, Broger C, Loetscher H, Lesslauer W. Cell. 1993;73:431–445. doi: 10.1016/0092-8674(93)90132-a. [DOI] [PubMed] [Google Scholar]
- 8.Tracey K J, Cerami A. Annu Rev Med. 1994;45:491–503. doi: 10.1146/annurev.med.45.1.491. [DOI] [PubMed] [Google Scholar]
- 9.Nagata S, Suda T. Immunol Today. 1995;16:39–43. doi: 10.1016/0167-5699(95)80069-7. [DOI] [PubMed] [Google Scholar]
- 10.Castrim J, Listman J A, Jacobson B A, Wang Y, Lopez P A, Ju S, Finn P W, Perkins D L. Immunity. 1996;5:617–627. doi: 10.1016/s1074-7613(00)80275-7. [DOI] [PubMed] [Google Scholar]
- 11.Noelle R. Immunity. 1996;4:415–419. doi: 10.1016/s1074-7613(00)80408-2. [DOI] [PubMed] [Google Scholar]
- 12.Wallach D, Varfolomeev E E, Malinin N L, Goltsev Y V, Kovalenko A V, Boldin M P. Annu Rev Immunol. 1999;17:331–367. doi: 10.1146/annurev.immunol.17.1.331. [DOI] [PubMed] [Google Scholar]
- 13.Schall T J, Lewis M, Koller K J, Lee A, Rice G C, Wong G H W, Gatanaga T, Granger G A, Lentz R, Raab H, et al. Cell. 1990;61:361–370. doi: 10.1016/0092-8674(90)90816-w. [DOI] [PubMed] [Google Scholar]
- 14.Hsu H, Lacey D L, Dunstan C R, Solovyev I, Colombero A, Timms E, Tan H L, Elliott G, Kelley M J, Sarosi I, et al. Proc Natl Acad Sci USA. 1999;96:3540–3545. doi: 10.1073/pnas.96.7.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schneier P, Mackay F, Steiner V, Hofmann K, Bodmer J L, Holler N, Ambrose C, Lawton P, Bixler S, Acha-Orbea H, et al. J Exp Med. 1999;189:1747–1756. doi: 10.1084/jem.189.11.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moore P A, Belvedere O, Orr A, Pieri K, LaFleur D W, Feng P, Soppet D, Charters M, Gentz R, Parmelee D, et al. Science. 1999;285:260–263. doi: 10.1126/science.285.5425.260. [DOI] [PubMed] [Google Scholar]
- 17.Graham M, Shutter J, Sarmiento U, Sarosi I, Stark K. Nat Genet. 1997;17:273–274. doi: 10.1038/ng1197-273. [DOI] [PubMed] [Google Scholar]
- 18.Vyse T J, Kotzin B L. Annu Rev Immunol. 1998;16:261–292. doi: 10.1146/annurev.immunol.16.1.261. [DOI] [PubMed] [Google Scholar]
- 19.Mohan C, Datta S K. Clin Immunol Immunopathol. 1995;77:209–220. doi: 10.1006/clin.1995.1146. [DOI] [PubMed] [Google Scholar]
- 20.Kotzin B L. Cell. 1998;85:303–306. doi: 10.1016/s0092-8674(00)81108-3. [DOI] [PubMed] [Google Scholar]
- 21.Strasser A, Whittingham S, Vaux D L, Bath M L, Adams J M, Cory S, Harris A W. Proc Natl Acad Sci USA. 1991;88:8661–8665. doi: 10.1073/pnas.88.19.8661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klebig M L, Wilkinson J E, Geisler J G, Woychik R P. Proc Natl Acad Sci USA. 1995;92:4728–4732. doi: 10.1073/pnas.92.11.4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ray P, Higgins K M, Tan J C, Chu T Y, Yee N S, Nguyen H, Lacy E, Besmer P. Genes Dev. 1991;5:2265–2273. doi: 10.1101/gad.5.12a.2265. [DOI] [PubMed] [Google Scholar]
- 24.Mukhopadhyay A, Ni J, Zhai Y, Yu G L, Aggarwal B B. J Biol Chem. 1999;274:15978–15981. doi: 10.1074/jbc.274.23.15978. [DOI] [PubMed] [Google Scholar]