Expression of the antiproliferative gene TIS21 at the onset of neurogenesis identifies single neuroepithelial cells that switch from proliferative to neuron-generating division (original) (raw)
Abstract
At the onset of mammalian neurogenesis, neuroepithelial (NE) cells switch from proliferative to neuron-generating divisions. Understanding the molecular basis of this switch requires the ability to distinguish between these two types of division. Here we show that in the mouse ventricular zone, expression of the mRNA of the antiproliferative gene TIS21 (PC3, BTG2) (i) starts at the onset of neurogenesis, (ii) is confined to a subpopulation of NE cells that increases in correlation with the progression of neurogenesis, and (iii) is not detected in newborn neurons. Expression of the TIS21 mRNA in the NE cells occurs transiently during the cell cycle, i.e., in the G1 phase. In contrast to the TIS21 mRNA, the TIS21 protein persists through the division of NE cells and is inherited by the neurons, where it remains detectable during neuronal migration and the initial phase of differentiation. Our observations indicate that the TIS21 gene is specifically expressed in those NE cells that, at their next division, will generate postmitotic neurons, but not in proliferating NE cells. Using TIS21 as a marker, we find that the switch from proliferative to neuron-generating divisions is initiated in single NE cells rather than in synchronized neighboring cells.
Neuroepithelial (NE) cells are the progenitors of all neurons and macroglial cells of the mammalian central nervous system (CNS). At the onset of CNS neurogenesis, NE cells are thought to switch from symmetric proliferative divisions (two NE daughter cells) to asymmetric neuron-generating divisions (one NE daughter cell, one postmitotic neuron) (1–4). Although several classes of molecules, including growth factors (5) and the products of neurogenic genes (6), have been implicated in this switch, its precise molecular mechanism is unknown. A major problem lies in the fact that during neurogenesis, proliferative and neuron-generating divisions of NE cells coexist (1–3). It is therefore essential to be able to distinguish between proliferating and neuron-generating NE cells. Here we investigate, for the early phase of neurogenesis in the mouse CNS, whether the product of the antiproliferative gene TIS21 (PC3, BTG2) (7–10), that is expressed in the neuroepithelium in correlation with neurogenesis (11), is a specific marker of neuron-generating NE cells.
METHODS
Morphology.
Cryosections. For all combined in situ hybridization (ISH)/immunoperoxidase stainings and for some immunofluorescence, cryosections (5–6 μm) were prepared from embryos (either BrdUrd-labeled or unlabeled) fixed overnight at 4°C in 4% paraformaldehyde/4% sucrose in PBS.
Polyester Sections.
In most double immunofluorescence experiments, polyester sections (6 μm) were used. Embryos were fixed overnight at 4°C in 4% paraformaldehyde/0.1% glutaraldehyde in PBS, dehydrated in ethanol, and embedded in polyester wax (BDH) (12).
Combined ISH/immunoperoxidase staining on cryosections was performed as described (13). Digoxygenin-labeled sense and antisense TIS21 riboprobes, used at 500 ng RNA per ml of hybridization solution, were prepared from a 1.5 kb TIS21 cDNA in pBluescript KS− obtained by RT-PCR of total RNA from mouse E12 brain and containing the entire coding sequence and ≈1 kb of 3′ UTR. In the case of BrdUrd staining, cryosections processed through all steps of ISH were treated with HCl (14) before to immunocytochemistry.
Immunofluorescence was performed according to standard procedures, with 3% fetal calf serum, 0.2% gelatin, and 0.1% Triton X-100 in PBS as blocking solution. Polyester sections were dewaxed in ethanol, rehydrated, permeabilized by 0.5% Triton X-100, and quenched with sodium borohydride. Cryosections were permeabilized by 0.5% Triton X-100 and quenched with 50 mM ammonium chloride. In the case of double immunofluorescence of BrdUrd-labeled embryos, cryosections after permeabilization with Triton X-100 were treated with HCl (14).
Miscellaneous.
Rabbit antibodies were raised against synthetic peptides coupled to KLH and affinity-purified by using the respective peptides coupled to Affi-Gel 10, following standard protocols.
TIS21 was expressed in COS7 cells after electroporation with the J4 plasmid (kind gift of J. P. Magaud and J. P. Rouault, Institut National de la Santé et de la Recherche Médicale, Lyon, France) containing the 1.8-kb _Sma_I–_Acc_I fragment of the mouse TIS21 gene in the pME18S vector under the control of the SRa promoter.
Immunoprecipitation was performed according to standard procedures. Because of the high proportion of nuclei in the homogenate, the starting material for each immunoprecipitation was a total protein fraction corresponding to one metabolically labeled E12 brain and obtained by phenol extraction (15); immune complexes were collected by using Protein A-Sepharose that had been preadsorbed with the total protein fraction obtained by phenol extraction from one unlabeled E12 brain. [Detailed methods are available from the authors upon request.]
RESULTS AND DISCUSSION
Two Antibodies Directed Against Distinct Epitopes of TIS21 Yield Indistinguishable Patterns of Immunoreactivity in the Developing Murine CNS.
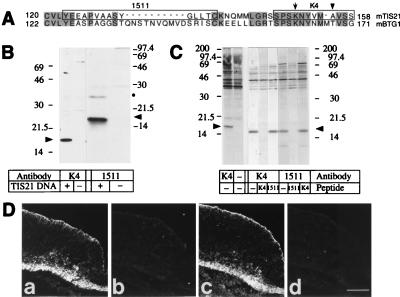
We raised two rabbit antibodies directed against distinct epitopes of mouse TIS21 (8). One antibody, referred to as K4, was raised against a synthetic peptide corresponding to the 12 C-terminal amino acid residues of TIS21 (residues 147–158, Fig. 1A), which differ from the corresponding sequence in the other members of this protein family (16). The other antibody, referred to as 1511, was raised against a synthetic peptide corresponding to amino acid residues 123–137; in BTG1, the protein family member most closely related to TIS21 (16, 17), the corresponding sequence is interrupted by a 10-amino acid residue insertion that is lacking from TIS21 (Fig. 1A).
Figure 1.
Characterization of the TIS21-antibodies. (A) Sequence comparison of the C-terminal one-third of mouse TIS21 (8) and mouse BTG1 (17). Amino acid residues identical between the two proteins are shaded. The sequences corresponding to the synthetic peptides used for immunization are boxed. The arrow indicates the single lysine residue in the K4 peptide that would be modified by glutaraldehyde-mediated coupling to keyhole limpet hemocyanin, thereby increasing the probability of the antibodies being directed against the C-terminal portion of the K4 peptide. The arrowhead indicates the alanine residue that is substituted by a threonine residue in rat TIS21 and mouse BTG1. (B) Western blot analysis of a cleared lysate of COS7 cells transfected with the TIS21 expression vector (+) or mock-transfected (−), using affinity-purified K4 antibody (1 μg/ml) or 1511 antiserum (1:2000). Arrowheads, TIS21; the dot indicates a TIS21-related 26 kDa protein detected upon overexpression of TIS21 in COS7 cells. (C) Immunoprecipitation from a total protein fraction of E12 mouse embryonic brain metabolically labeled for 1.5 h with [35S]methionine/cysteine, using affinity-purified K4 (5 μg/ml) or 1511 (9 μg/ml) antibody in the absence or presence of 50 μM K4 or 10 μM 1511 peptide as indicated. Note that in the two left lanes the background bands >20 kDa are also seen in the absence of antibody. To lower this background, the 35S-labeled total protein fraction used for immunoprecipitation in the six lanes on the right had been cleared by preadsorption to protein A-Sepharose before incubation with antibodies, and the immune complexes were eluted from protein A-Sepharose using 20 mM HCl instead of sample buffer. Arrowheads, TIS21. (D) Immunofluorescence of four adjacent (Da–Dd) 6-μm transverse cryosections through the hindbrain of an E11 mouse embryo, using the following affinity-purified primary antibodies: (Da) K4 antibody (0.5 μg/ml), (Db) K4 antibody preincubated with K4 peptide, (Dc) 1511 antibody (1.9 μg/ml), (Dd) 1511 antibody preincubated with 1511 peptide. The apical (ventricular) side of the neuroepithelium is up. Da vs. Db, and Dc vs. Dd, are the same length of exposure, and Da_–_Dd are the same magnification. [Bar = 85 μm (Dd).]
In Western blot analysis of a cleared lysate from COS7 cells transfected with a TIS21 expression vector, but not from mock-transfected cells, both antibodies recognized a band of ≈17 kDa (Fig. 1B), consistent with previous observations and the predicted molecular mass of TIS21 (8). In immunoprecipitation from metabolically labeled embryonic day 12 (E12) mouse embryonic brain, both antibodies immunoprecipitated a ≈17-kDa band (Fig. 1C). This immunoprecipitation was specific and reflected recognition of distinct epitopes on TIS21 because immunoprecipitation of the 17-kDa band, in contrast to the background bands of higher molecular mass, was blocked by the peptide against which the respective antibody had been raised. In immunofluorescence of serial sections through the hindbrain of an E11 mouse embryo, the K4 (Fig. 1Da) and the 1511 (Fig. 1Dc) antibody gave indistinguishable staining patterns. Preincubation of either antibody with the peptide used for immunization abolished staining (Fig. 1Db and Dd). Indistinguishable patterns of immunoreactivity with the K4 and the 1511 antibody were also observed in other regions of the developing CNS (e.g., spinal cord) of E11 mouse embryos, and at other developmental stages (e.g., E10 and E12) (data not shown). No immunostaining with the K4 antibody was observed in sections of the developing CNS of E13 rat embryos (data not shown), which also expresses TIS21 (11), indicating that the substitution of Ala-155 of mouse TIS21 (8) by a threonine in rat TIS21 (9) destroys the epitope. We therefore conclude that the immunoreactivity observed with the K4 antibody, which was used for the immunocytochemical analyses described below, reflected specifically the presence of TIS21 and was not caused by proteins other than TIS21.
A Subpopulation of Cells in the Neuroepithelium Expresses the TIS21 mRNA in Those Regions in Which Neurons Are Being Generated.
We compared the expression of the TIS21 mRNA with the appearance of postmitotic neurons, using ISH for TIS21 mRNA in combination with immunocytochemistry using a mAb against βIII-tubulin, a marker of young neurons (18). The results shown were obtained with E10 mouse embryos (NMRI, staging according to ref. 19), a developmental stage at which, because of the spatiotemporal gradients of neurogenesis (20–22), certain regions of the CNS do not yet show neurogenesis, others are just initiating neurogenesis, and yet others are approaching the peak of neurogenesis.
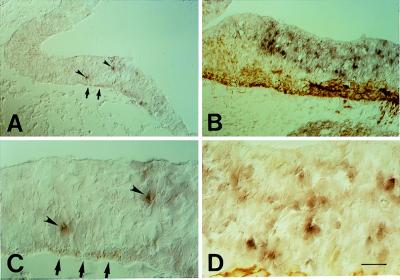
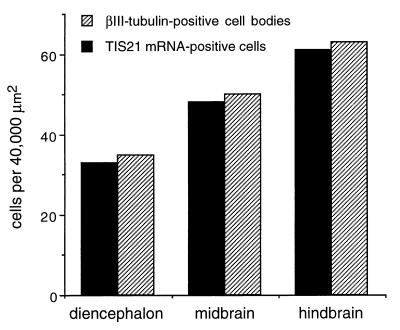
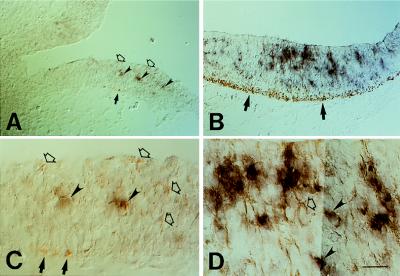
In regions of the neuroepithelium where neurogenesis is just starting (as indicated by the presence of only a few cells expressing βIII-tubulin), such as the diencephalon of the E10 mouse embryo (Fig. 2 A and C, arrows), the TIS21 mRNA was found to be expressed by only a few cells in the neuroepithelium (Fig. 2 A and C, arrowheads). In regions of the neuroepithelium where neurogenesis has not yet started (as indicated by the lack of βIII-tubulin staining), no TIS21 mRNA-positive cells were observed (Fig. 2A). In the hindbrain, where many neurons are being generated in an E10 embryo, many more cells in the neuroepithelium expressed the TIS21 mRNA (Fig. 2 B and D). Quantification of the TIS21 mRNA-positive cells in the neuroepithelium and the βIII-tubulin-positive cell bodies in the corresponding mantle zone revealed that the abundance of both cell populations increased in parallel from diencephalon to midbrain to hindbrain (Fig. 3). These data not only confirm that expression of the TIS21 mRNA correlates with the spatiotemporal gradients of neurogenesis and is restricted to the ventricular zone (11), but also show that, within the ventricular zone, the expression of the TIS21 mRNA is confined to a subpopulation of cells that is small at the onset of neurogenesis and increases as more neurons are being produced.
Figure 2.
Comparison of the cellular distribution of the TIS21 mRNA (purple, arrowheads) and the neuronal marker βIII-tubulin (brown, arrows) in the E10 mouse brain by combined ISH/immunoperoxidase staining on cryosections. (A) Transverse section of the diencephalon at the level of the optic stalk. (B) Transverse section of the hindbrain. (C) Higher magnification of a portion of the field shown in A. (D) Higher magnification of a portion of the field shown in B. [Bar = 62 μm (A and B) or 20 μm (C and D).]
Figure 3.
Correlation between the abundance of TIS21 mRNA-containing NE cells and newborn neurons in various regions of the E10 mouse brain. Sections subjected to combined ISH for TIS21 mRNA/immunoperoxidase staining for βIII-tubulin as in Fig. 2 were analyzed for the number of TIS21 mRNA-containing cells in the neuroepithelium and for the number of βIII-tubulin-positive cells in the mantle zone basal to the area of neuroepithelium analyzed. For βIII-tubulin-positive cells, only cells whose cell body showed immunoreactivity were considered. The number of βIII-tubulin-positive cells in the mantle zone is therefore underestimated, as is the number of TIS21 mRNA-containing cells in the neuroepithelium given the transient expression of the TIS21 mRNA in the G1 phase of the cell cycle (see Fig. 8 below). For each brain region, the numbers shown refer to a total area (neuroepithelium plus mantle zone) of 40,000 μm2 and were obtained by analysis of independent sections of the diencephalon (four sections), midbrain (two sections), and hindbrain (two sections).
The TIS21 Protein, Like the TIS21 mRNA, Is Expressed in Ventricular Zone Cells When Neurons Are Being Generated, but, in Contrast to the TIS21 mRNA, Is Also Found in Young Neurons.
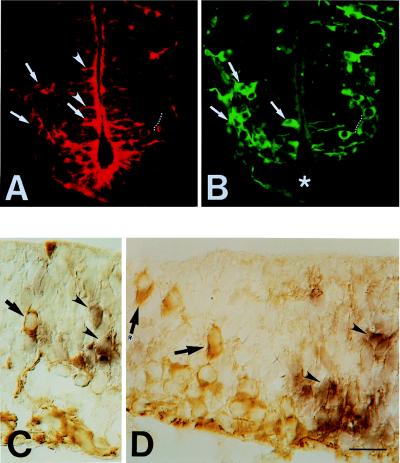
The cellular distribution of the TIS21 protein in the neural tube was analyzed and compared with that of βIII-tubulin by double immunofluorescence. In the midbrain of an E10 mouse embryo, neurogenesis is just starting dorsally and is already more advanced ventrally (21, 22), as shown by the βIII-tubulin staining of the mantle zone (Fig. 4Ab, arrows). Correspondingly, the TIS21 protein was found to be expressed at very low levels dorsally and more abundantly in the ventral region (Fig. 4Aa). Interestingly, the TIS21 protein was not only detected in the neuroepithelium (Fig. 4Aa, arrowheads), as was the case for the TIS21 mRNA (see Fig. 2), but also in neurons that had migrated to the mantle zone (Fig. 4Aa, arrows) and lacked the TIS21 mRNA (see Fig. 2). In the spinal cord, neurogenesis is even more advanced than in the ventral region of the midbrain and also follows a ventrodorsal gradient (20, 22), as shown by the βIII-tubulin staining (Fig. 4 Bb, Bd, and Bf). Correspondingly, many cells in the neuroepithelium (with a ventrodorsal gradient) and the neurons in the mantle zone contained the TIS21 protein (Fig. 4 Ba, Bc, and Be). TIS21 immunoreactivity was also detected in the floor plate (Fig. 4Ba) that is known to lack neurons (Fig. 4Bb) but whose cells also withdraw from the cell cycle (23).
Figure 4.
Comparison of the cellular distribution of the TIS21 protein (Aa, Ba, Bc, and Be) and βIII-tubulin (Ab, Bb, Bd, and Bf) in the E10 mouse CNS by double immunofluorescence on transverse polyester sections through the midbrain (A) and spinal cord at the thoracical level (B). (A) Ventral and dorsal region, lower and upper portion of micrographs, respectively; arrows and arrow with asterisk, respectively, indicate neurons at the basal side of the neuroepithelium (Aa and Ab); arrowheads and arrowheads with asterisks, respectively, indicate TIS21 immunoreactivity in the neuroepithelium, which is concentrated toward the apical surface (Aa). White diamonds at the top of Aa and Ab, autofluorescent red blood cells. (B) White triangles at the bottom of Ba and Bb, floor plate. (Bc–Bf) the apical side of the neuroepithelium is on the left and the mantle zone is on the right. (Be and Bf) The ventral side; (Bc and Bd) more dorsal. Bar (in Bf) = 85 μm (Aa, Ab, Ba, and Bb) or 27 μm (Bc–Bf).
In the Ventricular Zone, the Subpopulation of Cells Containing the TIS21 Protein Overlaps with, and Is Greater Than, That Expressing the TIS21 mRNA.
Given that both the TIS21 mRNA and the TIS21 protein were expressed in the ventricular zone in correlation with neurogenesis, one would expect that most, if not all, ventricular zone cells positive for the TIS21 mRNA should contain the TIS21 protein. ISH for the TIS21 mRNA in combination with immunocytochemistry for the TIS21 protein showed that this was indeed the case, as illustrated for the diencephalon and hindbrain of an E10 embryo (Fig. 5, arrowheads). Significantly, however, not all ventricular zone cells containing the TIS21 protein also contained the TIS21 mRNA (Fig. 5, open arrows). Consistent with the results of immunofluorescence (see Fig. 4), immunoperoxidase staining revealed the presence of the TIS21 protein in young neurons in the mantle zone (Fig. 5, arrows), which lacked the TIS21 mRNA (compare Fig. 2).
Figure 5.
Comparison of the cellular distribution of the TIS21 mRNA (purple) and TIS21 protein (brown) in the E10 mouse brain by combined ISH/immunoperoxidase staining on cryosections. (A) Transverse section of the diencephalon at the level of the optic stalk. (B) Transverse section of the hindbrain. (C) Higher magnification of a portion of the field shown in A. (D) Higher magnification of a portion of the field shown in B. TIS21 mRNA expression is confined to the perikaryon of cells in the neuroepithelium (arrowheads); these cells also contain TIS21 protein in the perikaryon and, more prominently, in cell processes (for a comparison with cells stained for the TIS21 mRNA but not protein, see Fig. 2). Cells containing TIS21 protein but not mRNA are also observed in the neuroepithelium (open arrows). TIS21 protein but not mRNA is detected in neurons in the mantle zone (arrows). [Bar (in D) = 62 μm (A and B) or 20 μm (C and D).]
Newborn Neurons Migrating Through the Neuroepithelium Contain the TIS21 Protein but Lack the TIS21 mRNA.
The ventricular zone cells positive for the TIS21 protein but negative for the TIS21 mRNA could be NE cells (if the TIS21 mRNA is shorter-lived than the TIS21 protein) and/or newborn neurons migrating through the neuroepithelium. To investigate the latter possibility, we examined newborn neurons in the ventricular zone, identified by βIII-tubulin staining (18), for the presence of TIS21 protein and mRNA. Double immunofluorescence of the spinal cord at E10 for βIII-tubulin (Fig. 6B) and TIS21 (Fig. 6A) showed that these newborn migrating neurons, as well as the neurons accumulating in the mantle zone, contained the TIS21 protein (Fig. 6A, arrows). Analysis of individual neurons revealed that the intracellular distribution of TIS21 was not identical with that of βIII-tubulin. Whereas βIII-tubulin was detected in both the perikaryal cytoplasm and neuronal processes, TIS21 was sometimes barely detectable in the perikaryon and preferentially localized in the processes (Fig. 6 A and B, white dotted lines). This differential intracellular distribution explains the apparent difference between the βIII-tubulin and the TIS21 immunostaining pattern in the forming mantle zone. We performed a quantitative analysis, considering this differential intracellular distribution, on >100 randomly picked neurons at early neurogenesis (E10 midbrain and hindbrain), when for a given neuron the βIII-tubulin immunoreactivity can be traced from the perikaryon into the proximal segment of processes, if necessary by adjusting the focus. This revealed that 98% of the neurons stained for βIII-tubulin contained TIS21 in the perikaryon and/or proximal segment of processes.
Figure 6.
Analysis of newborn, migrating neurons in the ventricular zone of the E10 mouse brain for the expression of TIS21 mRNA and protein. (A and B) Double immunofluorescence for TIS21 (A) and βIII-tubulin (B) of a transverse polyester section through the spinal cord. Arrows, newborn neurons; dotted white lines, neuronal process emerging from perikaryon; arrowheads, NE cells showing TIS21 immunoreactivity concentrated toward the apical surface (A) but lacking βIII-tubulin (B). Asterisk in B, floor plate. (C and D) Combined ISH for TIS21 mRNA (purple)/immunoperoxidase staining for βIII-tubulin (brown) of transverse cryosections through the hindbrain. The apical side of the neuroepithelium is up. Arrows, newborn neurons in the ventricular zone; arrow with asterisk in D, newborn neuron in close proximity to the apical surface; arrowheads, NE cells that express TIS21 mRNA but lack βIII-tubulin immunoreactivity (for a comparison with cells showing both, an ISH and an immunoperoxidase signal, see Fig. 5 C and D, arrowheads). [Bar (in D) = 31 μm (A and B) or 20 μm (C and D).]
Within the ventricular zone, in addition to the newborn neurons positive for both βIII-tubulin and the TIS21 protein (Fig. 6A, arrows), we also observed cellular structures negative for βIII-tubulin but positive for the TIS21 protein (Fig. 6A, arrowheads). Hence, in the ventricular zone, the newborn neurons constituted only one cell population containing the TIS21 protein; a subset of NE cells constituted the other. TIS21 in NE cells was found to be preferentially localized to their apical process and apical end (Fig. 6A, arrowheads).
Analysis of the ventricular zone by combined ISH for TIS21/immunocytochemistry for βIII-tubulin showed that the newborn neurons, identified by βIII-tubulin staining (Fig. 6 C and D, arrows), did not contain detectable levels of the TIS21 mRNA, in contrast to the NE cells (Fig. 6 C and D, arrowheads). Lack of TIS21 mRNA expression was observed not only in neurons apparently migrating through the ventricular zone (Fig. 6 C and D, arrows), but also in newborn neurons still in close proximity to the apical surface (Fig. 6D, arrow with asterisk), i.e., just after mitosis. This suggests that the presence of the TIS21 protein in these newborn neurons (Fig. 6, A and B, arrows) was not caused by a transient TIS21 mRNA expression after their birth.
A Subset of Mitotic NE Cells Contains the TIS21 Protein Whereas the TIS21 mRNA Is Not Detected in Mitotic NE Cells.
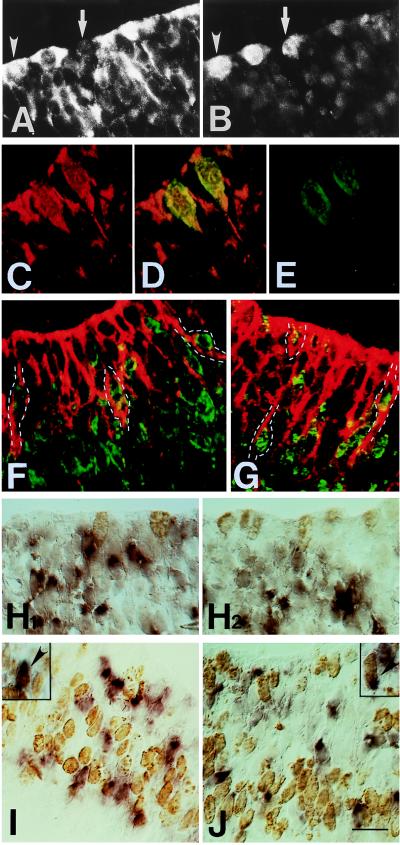
If the cells in the ventricular zone that contained both TIS21 mRNA and TIS21 protein (see Fig. 5) and lacked βIII-tubulin (see Fig. 6) were NE cells, they should be dividing. We therefore compared the staining for the TIS21 mRNA and protein with that for markers indicative of S phase and mitosis. In double immunofluorescence using anti-TIS21 antibody (Fig. 7A) and the MPM-2 mAb (Fig. 7B), a marker of mitotic cells (24), some (arrowheads) but not all (arrows) mitotic cells, found in the typical location at the apical surface of the neuroepithelium (22), were found to be immunoreactive for the TIS21 protein. Analysis of single optical sections using confocal microscopy corroborated the presence of the TIS21 protein in MPM-2-positive cells (Fig. 7 C–E), although the TIS21 staining within a given mitotic NE cell, because of the preferential localization of the TIS21 protein at the cell periphery, often appeared distinct from the perikaryal MPM-2 staining (data not shown). In contrast to the presence of the TIS21 protein in mitotic NE cells, combined ISH for the TIS21 mRNA/immunocytochemistry using the MPM-2 antibody showed that these cells lack the TIS21 mRNA (Fig. 7 H1 and H2).
Figure 7.
Comparison of the expression of the TIS21 mRNA and protein to markers of the cell cycle in the ventricular zone of the E10 mouse brain. (A and B) Double immunofluorescence for TIS21 (A) and the mitotic marker MPM-2 (B) of a transverse polyester section through the hindbrain. Some mitotic cells stained by the MPM-2 antibody also contain TIS21 (arrowheads), whereas other mitotic cells do not (arrows). (C–E) Double immunofluorescence confocal microscopy for TIS21 (C, red) and MPM-2 (E, green) of a transverse cryosection through the hindbrain. A single optical section is shown. (D) overlay. (F and G) Double immunofluorescence confocal microscopy for TIS21 (red) and BrdUrd (green) of transverse cryosections through the hindbrain of embryos labeled with BrdUrd for either 30 min (F) or 2 h (G). Single optical sections are shown. BrdUrd is localized to the nucleus, whereas the TIS21 antibody stains the cytoplasm. Examples of double-positive cells are outlined in white. Because the TIS21 protein is localized to the apical processes and ends of NE cells, BrdUrd-labeled nuclei could only be assigned to TIS21-positive cells when the former were sufficiently close to the apical surface, which is the case in late S phase and G2 phase because of interkinetic nuclear migration (22). For this reason, we could not quantitate the proportion of TIS21 protein-positive NE cells that contain S phase and G2 phase BrdUrd-labeled nuclei. (H1 and H2) Both panels show combined ISH for TIS21 mRNA (purple)/ immunoperoxidase staining for MPM-2 (brown) of transverse cryosections through the hindbrain. (I and J) Combined ISH for TIS21 mRNA (purple)/immunoperoxidase staining for BrdUrd (brown) of transverse cryosections through the hindbrain (I) or the midbrain (J) of embryos labeled with BrdUrd for either 30 min (I) or 2 h (J). The insets show double-positive cells (arrowheads) that are occasionally observed; in these cells, the brown outline of the nucleus is visible through the purple staining of the cell body. (A–J) The apical side of the neuroepithelium is up. [Bar (in J) = 17 μm (A and B), 13 μm (C–E), 25 μm (G), and 20 μm (F and H–J).]
The TIS21 mRNA is Transiently Expressed During the G1 Phase of the Cell Cycle.
To determine the phase of the cell cycle during which NE cells synthesize the TIS21 mRNA, we administered a single dose of BrdUrd (1.25 mg per pregnant mouse) to E10 mouse embryos 30 min and 2 h before analysis. This should result in the labeling of nuclei that at the time point of analysis were mostly in S phase and in S plus G2 phase, respectively, given that at this developmental stage the S phase and G2 phase lasts ≈4.6 h and ≈0.6 h, respectively (25, 26). We then compared the anti-BrdUrd mAb staining with that for the TIS21 protein by double immunofluorescence confocal microscopy, bearing in mind that the nuclear BrdUrd and the cytoplasmic TIS21 staining are mutually exclusive within a given NE cell (see Fig. 7 legend). This showed that NE cells containing the TIS21 protein included cells in S phase (Fig. 7F) and G2 phase (Fig. 7G). In contrast, combined ISH for TIS21 mRNA/immunocytochemistry for BrdUrd showed that the vast majority of TIS21 mRNA-containing NE cells were neither in S phase (Fig. 7I) nor G2 phase (Fig. 7J). Only 3–4% of the TIS21 mRNA-containing NE cells (≈1000 cells counted for 30 min BrdUrd, >600 cells counted for 2 h BrdUrd; E10–10.5, various brain regions, various embryos) showed BrdUrd-labeled nuclei (Fig. 7 I and J, Insets and arrowheads). This proportion of cells did not increase when embryos received BrdUrd 2 h (3.4%) rather than 30 min (3.8%) before analysis, a condition that should increase the probability of detecting nuclei in G2 phase. However, most nuclei of TIS21 mRNA-positive NE cells were found to be labeled after 10 h of BrdUrd labeling (injections at 2 h intervals, data not shown). Hence, the TIS21 mRNA is apparently synthesized during the G1 phase of the cell cycle and degraded at the G1/S transition, a conclusion in agreement with previous data obtained with synchronized cell cultures (10, 27). The transient presence of TIS21 mRNA in the G1 phase is consistent with the observation that TIS21 protein is detected in S phase cells (Fig. 7F).
The TIS21 mRNA, a Marker of Neuron-Generating NE Cells.
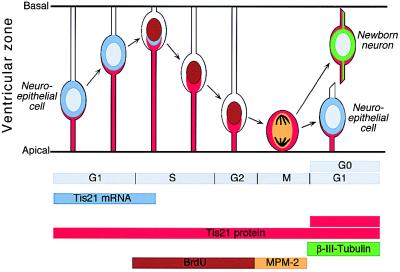
Considering the results described so far, we conclude that in the developing mouse CNS (i) the synthesis of the TIS21 mRNA and protein, which shows a temporal and spatial correlation with neurogenesis, is confined to a subpopulation of NE cells of the ventricular zone; (ii) this synthesis occurs in the G1 phase; and (iii) the TIS21 mRNA is degraded at the beginning of S phase (i.e., before mitosis), whereas the TIS21 protein persists through mitosis. Given that newborn neurons, which originate from the division of NE cells, contain the TIS21 protein but not the TIS21 mRNA, the TIS21 protein in the neuronal daughter cell is presumably inherited from the TIS21-positive NE mother cell (Fig. 8). This implies that the NE cells that synthesize the TIS21 mRNA and protein are those that generate neurons in the subsequent division. Moreover, the TIS21 mRNA is not expressed in proliferating NE cells, because it is not detected before neurogenesis, nor does it appear in the vast majority of NE cells concomitant with the onset of neurogenesis but rather in an increasing population of NE cells as more neurons are being generated. Hence, the TIS21 mRNA (and, with regard to the βIII-tubulin-negative ventricular zone cells, also the TIS21 protein) is a marker to identify neuron-generating NE cells and to distinguish these from proliferating NE cells.
Figure 8.
Expression of the TIS21 gene in neuron-generating NE cells. See Discussion.
Additional evidence corroborates this conclusion. First, consistent with the previously reported expression of the TIS21 mRNA in correlation with neurogenesis during development (11), TIS21 immunoreactivity was detected in both NE cells and neurons also at later stages of embryonic development. (See the supplemental material on the PNAS web site, www.pnas.org.) Second, TIS21 immunoreactivity was found in the superficial zone of the external granular layer of the P2 mouse cerebellum, which contains the dividing precursor cells, and in the deeper zones containing the migrating granule cell neurons. Third, both TIS21 mRNA and protein was observed in the basal precursor cells of the P2 mouse olfactory epithelium, and TIS21 protein but not mRNA was found in the olfactory neurons originating from these precursor cells. Fourth, after a 20-h in vitro culture of E9.5 and E10.5 telencephalic NE cells under low- and high-serum conditions, the extent of neuron generation correlated with the abundance of the TIS21-positive precursor cells (data not shown).
The Switch from Proliferative to Neuron-Generating Cell Divisions Is Initiated in Single NE Cells.
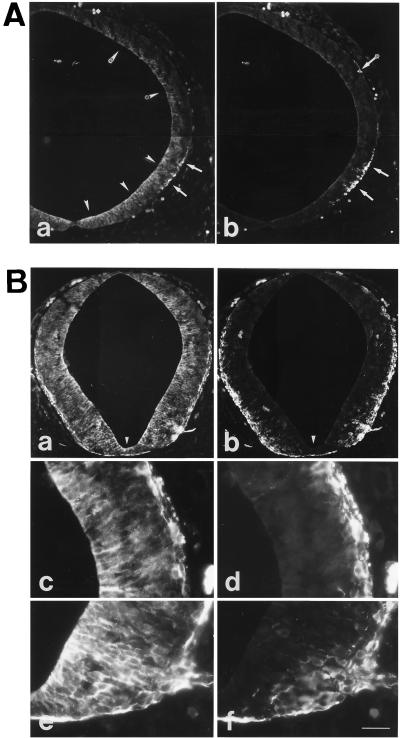
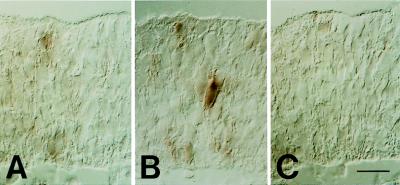
Retroviral lineage tracing studies with mammalian embryos indicate that, during advanced phases of neurogenesis, groups of neurons can arise simultaneously from clonally related NE cells (28–31). Early phases of neurogenesis have not been investigated using retroviral lineage tracing. It was therefore of interest to use the specific expression of the TIS21 mRNA in neuron-generating NE cells to investigate whether at the onset of neurogenesis, the switch of NE cells from proliferative to neuron-generating divisions also occurs in a synchronous fashion in clusters of neighboring cells. Interestingly, however, analysis of serial 5-μm thick sections of the diencephalon of an E10 embryo by ISH for the TIS21 mRNA showed that when a NE cell switches on the expression of the TIS21 mRNA (Fig. 9B), none of the adjacent cells expresses the TIS21 mRNA (Fig. 9, A–C). Hence, the switch of NE cells from proliferative to neuron-generating divisions is initiated in single NE cells. The specific expression of the TIS21 gene in neuron-generating NE cells provides a new tool for investigating the molecular basis of this switch. In this regard, it will be interesting to investigate the role of the recently identified TIS21-interacting proteins, the protein arginine _N_-methyltransferase 1 (32) and the CCR4-associated factor 1 (33).
Figure 9.
Analysis of TIS21 mRNA expression at the onset of neurogenesis by in situ hybridization on three serial (A–C), 5-μm transverse cryosections through the diencephalon of an E10 embryo. The example shown is representative of six independent observations. (Bar = 20 μm.)
Supplementary Material
Supplemental Figure
Acknowledgments
P.I. was supported by fellowships from European Molecular Biology Organization, the Boehringer-Ingelheim Fonds and the Training and Mobility of Researchers Programme of the European Community. M.M. was supported by a fellowship from the Graduiertenkolleg Neurobiologie, and W.B.H. was supported by a grant from the Deutsche Forschungsgemeinschaft (SFB 317).
ABBREVIATIONS
NE
neuroepithelial
CNS
central nervous system
ISH
in situ hybridization
BrdUrd
bromodeoxyuridine
E_n_
embryonic day n
References
- 1.Rakic P. Science. 1988;241:170–176. doi: 10.1126/science.3291116. [DOI] [PubMed] [Google Scholar]
- 2.McConnell S K. Neuron. 1995;15:761–768. doi: 10.1016/0896-6273(95)90168-x. [DOI] [PubMed] [Google Scholar]
- 3.Takahashi T, Nowakowski R S, Caviness V S., Jr J Neurosci. 1996;16:6183–6196. doi: 10.1523/JNEUROSCI.16-19-06183.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huttner W B, Brand M. Curr Opin Neurobiol. 1997;7:29–39. doi: 10.1016/s0959-4388(97)80117-1. [DOI] [PubMed] [Google Scholar]
- 5.Cameron H A, Hazel T G, McKay R D G. J Neurobiol. 1998;36:287–306. [PubMed] [Google Scholar]
- 6.Lewis J. Curr Opin Neurobiol. 1996;6:3–10. doi: 10.1016/s0959-4388(96)80002-x. [DOI] [PubMed] [Google Scholar]
- 7.Lim R W, Varnum B C, Herschmann H R. Oncogene. 1987;1:263–270. [PubMed] [Google Scholar]
- 8.Fletcher B S, Lim R W, Varnum B C, Kujubu D A, Koski R A, Herschman H R. J Biol Chem. 1991;266:14511–14518. [PubMed] [Google Scholar]
- 9.Bradbury A, Possenti R, Shooter E M, Tirone F. Proc Natl Acad Sci USA. 1991;88:3353–3357. doi: 10.1073/pnas.88.8.3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rouault J P, Falette N, Guéhenneux F, Guillot C, Rimokh R, Wang Q, Berthet C, Moyret-Lalle C, Savatier P, Pain B, Shaw P, et al. Nat Genet. 1996;14:482–486. doi: 10.1038/ng1296-482. [DOI] [PubMed] [Google Scholar]
- 11.Iacopetti P, Barsacchi G, Tirone F, Maffei L, Cremisi F. Mech Dev. 1994;47:127–137. doi: 10.1016/0925-4773(94)90085-x. [DOI] [PubMed] [Google Scholar]
- 12.Kent A P. Microsc Anal. 1991;24:37. [Google Scholar]
- 13.Tiveron M C, Hirsch M R, Brunet J F. J Neurosci. 1996;16:7649–7660. doi: 10.1523/JNEUROSCI.16-23-07649.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soriano E, Del Rio J A. J Histochem Cytochem. 1991;39:255–263. doi: 10.1177/39.3.1671576. [DOI] [PubMed] [Google Scholar]
- 15.Huttner W B. Methods Enzymol. 1984;107:200–223. doi: 10.1016/0076-6879(84)07013-0. [DOI] [PubMed] [Google Scholar]
- 16.Guéhenneux F, Duret L, Callanan M B, Bouhas R, Hayette S, Berthet C, Samarut C, Rimokh R, Birot A M, Wang Q, et al. Leukemia. 1997;11:370–375. doi: 10.1038/sj.leu.2400599. [DOI] [PubMed] [Google Scholar]
- 17.Rouault J P, Samarut C, Duret L, Tessa C, Samarut J, Magaud J P. Gene. 1993;129:303–306. doi: 10.1016/0378-1119(93)90284-a. [DOI] [PubMed] [Google Scholar]
- 18.Lee M K, Tuttle J B, Rebhuhn L I, Cleveland D W, Frankfurter A. Cell Motil Cytoskeleton. 1990;17:118–132. doi: 10.1002/cm.970170207. [DOI] [PubMed] [Google Scholar]
- 19.Kaufman M H. The Atlas of Mouse Development. London: Academic; 1992. [Google Scholar]
- 20.Nornes H O, Carry M. Brain Res. 1978;159:1–16. doi: 10.1016/0006-8993(78)90105-1. [DOI] [PubMed] [Google Scholar]
- 21.Gardette R, Courtois M, Bisconte J-C. J Hirnforschung. 1982;23:415–431. [PubMed] [Google Scholar]
- 22.Bayer S A, Altman J. Neocortical Development. New York: Raven; 1991. [Google Scholar]
- 23.Altman J, Bayer S A. Adv Anat Embryol Cell Biol. 1984;85:1–164. doi: 10.1007/978-3-642-69537-7. [DOI] [PubMed] [Google Scholar]
- 24.Davis F M, Tsao T Y, Fowler S K, Rao P N. Proc Natl Acad Sci USA. 1983;80:2926–2930. doi: 10.1073/pnas.80.10.2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kauffman S L. Exp Cell Res. 1968;49:420–424. doi: 10.1016/0014-4827(68)90191-2. [DOI] [PubMed] [Google Scholar]
- 26.Miller M W, Nowakowski R S. Brain Res. 1988;457:44–52. doi: 10.1016/0006-8993(88)90055-8. [DOI] [PubMed] [Google Scholar]
- 27.Montagnoli A, Guardavaccaro D, Starace G, Tirone F. Cell Growth Differ. 1996;7:1327–1336. [PubMed] [Google Scholar]
- 28.Walsh C, Cepko C. Science. 1992;255:434–440. doi: 10.1126/science.1734520. [DOI] [PubMed] [Google Scholar]
- 29.Kornack D R, Rakic P. Neuron. 1995;15:311–321. doi: 10.1016/0896-6273(95)90036-5. [DOI] [PubMed] [Google Scholar]
- 30.Cai L, Hayes N L, Nowakowski R S. J Neurosci. 1997;17:2088–2100. doi: 10.1523/JNEUROSCI.17-06-02088.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mione M C, Cavanagh J F R, Harris B, Parnavelas J G. J Neurosci. 1997;17:2018–2029. doi: 10.1523/JNEUROSCI.17-06-02018.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin W-J, Gary J D, Yang M C, Clarke S, Herschman H R. J Biol Chem. 1996;271:15034–15044. doi: 10.1074/jbc.271.25.15034. [DOI] [PubMed] [Google Scholar]
- 33.Rouault J-P, Prévôt D, Berthet C, Birot A-M, Billaud M, Magaud J-P, Corbo L. J Biol Chem. 1998;273:22563–22569. doi: 10.1074/jbc.273.35.22563. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure