d-serine and serine racemase are present in the vertebrate retina and contribute to the physiological activation of NMDA receptors (original) (raw)
Abstract
d-serine has been proposed as an endogenous modulator of _N-_methyl-d-aspartate (NMDA) receptors in many brain regions, but its presence and function in the vertebrate retina have not been characterized. We have detected d-serine and its synthesizing enzyme, serine racemase, in the retinas of several vertebrate species, including salamanders, rats, and mice and have localized both constituents to Müller cells and astrocytes, the two major glial cell types in the retina. Physiological studies in rats and salamanders demonstrated that, in retinal ganglion cells, d-serine can enhance excitatory currents elicited by the application of NMDA, as well as the NMDA receptor component of light-evoked synaptic responses. Application of d-amino acid oxidase, which degrades d-serine, reduced the magnitude of NMDA receptor-mediated currents, raising the possibility that endogenous d-serine serves as a ligand for setting the sensitivity of NMDA receptors under physiological conditions. These observations raise exciting new questions about the role of glial cells in regulating the excitability of neurons through release of d-serine.
Although bacteria and many invertebrate species use both d- and l-enantiomers of amino acids for cellular functions, it was generally believed that higher organisms had a more restricted stereospecificity and were confined to the use of l-amino acids. Thus, the d-amino acids detected in vertebrates were generally assumed to come from ingested material or intestinal flora (1). Indeed, the enzyme d-amino acid oxidase (d-AAO), which degrades many d-amino acids, is found in vertebrate tissues, but it was assumed to exist for degrading d-amino acids from external sources. However, in the past decade, it has become clear that some d-amino acids are synthesized and used by vertebrates. The best case for a functional d-amino acid in vertebrates began with studies that demonstrated the presence of high levels of d-serine in the brain (2), with a distribution pattern similar to that of _N-_methyl-d-aspartate (NMDA) receptors (3). Schell et al. (4) localized d-serine to astrocytes in the brain and demonstrated that release of d-serine could be evoked by activation of α-amino-3-hydroxy-5-methylisoxazole-4-propionate (AMPA)/kainic acid glutamate receptors on cultured glial cells. Based on these results, as well as the observation that d-serine could function as an agonist at the glycine binding site of NMDA receptors (5), Snyder and colleagues (6) proposed that d-serine released by glia plays a regulatory role as a necessary coagonist for the glutamate activation of NMDA receptors. Thus, an expanded model for glutamate synapses emerged in which perisynaptic glial processes respond to glutamate released from the presynaptic neuron by releasing d-serine, which, in turn, facilitates the activation of NMDA receptors on the postsynaptic cell. The functional importance of d-serine was further supported by the more recent discovery that the enzyme serine racemase, which converts l-serine to d-serine, is localized to astrocytes in the nervous system (7). The discovery of this mechanism adds to the accumulating evidence that glial cells dynamically influence neuronal activity.
Although d-serine and serine racemase have been found in many regions of the brain, no study has yet been carried out in the vertebrate retina, a tissue that offers several advantages for studying the actions of d-serine. The retinal network is a well-characterized region of the central nervous system that can be studied in an intact preparation, permitting the use of light stimulation to provide natural activation of the neural circuitry and test more directly the physiological significance of d-serine. Virtually all retinal ganglion cells have AMPA and NMDA receptors, which both contribute to light-evoked excitation (8–12), and NMDA receptor currents can be enhanced through pharmacological techniques and studied in relative isolation from AMPA receptor contributions (13).
In the present study, we have used immunohistochemistry, immunoblotting, analytical chemistry (HPLC), and electrophysiology to evaluate the presence of d-serine and serine racemase in the retinas of several vertebrate species. Our findings indicate that d-serine and serine racemase are present in the retina and seem to be localized to Müller glial cells and astrocytes. In addition, we have demonstrated that exogenous d-serine can enhance NMDA receptor responses and modulate light-evoked activity in retinal ganglion cells. The enhancement of NMDA receptor function seems to involve both tonic and phasic components of glutamatergic input. When the d-serine-degrading enzyme d-AAO (14) is added to the bathing medium, currents mediated by NMDA receptors are reduced, suggesting that these responses depend on endogenous d-serine as a coagonist for the glutamate activation of NMDA receptors.
Methods
Animal Protocols. All animal experiments were performed in strict accordance with protocols approved by the Institutional Animal Care and Use Committee at the University of Minnesota.
Immunostaining. Rodent retinas. Adult C57BL/6 female mice (13 wk) and male Long–Evans rats (250–400 g) were deeply anesthetized with sodium pentobarbital (150 mg/kg) injected i.p. Animals were then transcardially perfused with oxygenated Ringer's solution composed of (in mM): KCl, 2.5; NaCl, 140.0; CaCl2, 3.0; MgCl2, 0.5; Hepes, 5.0; d-glucose, 15.0, pH 7.4. Eyes were enucleated, and the retinas were dissected into oxygenated Ringer's solution. For anti-serine racemase immunohistochemistry, retinas were immersion-fixed for 2 h in 4% paraformaldehyde in 0.1 M phosphate buffer (PB), pH 7.4, at room temperature, then rinsed extensively in PBS, pH 7.4, at room temperature. For anti-d-serine immunohistochemistry, retinas were immersion-fixed for 1 h in 2% paraformaldehyde, 2% glutaraldehyde, 0.2% sodium metabisulfite in PB, pH 7.4, at room temperature, then rinsed extensively in PBS containing 0.2% sodium metabisulfite. The retinas were then incubated for 1 h in PBS with 0.5% sodium borohydride and 0.2% sodium metabisulfite at room temperature to quench glutaraldehyde-induced fluorescence in the tissue, and rinsed 3 times for 15 min in PBS with 0.2% sodium metabisulfite. Some retinas were studied as wholemount preparations, whereas others were cryoprotected in 30% sucrose, then embedded in a 1:1 mixture of OCT compound (Miles, Elkhart, IN) and Aquamount (Lerner Laboratories, New Haven, CT), frozen and cryosectioned. Wholemounts and retinal sections were immunostained as described (15).
Amphibian retinas. Amphibians were killed by decapitation and double pithing. Eyes were enucleated, and the retinas were isolated and immersion-fixed for 1 h at room temperature in either (i) 2% paraformaldehyde and 2% glutaraldehyde or (ii) 4% paraformaldehyde and 0.5% glutaraldehyde in PB, pH 7.4. Retinas were rinsed overnight at 4°C in PB, then washed for 1 h in PB containing 1% sodium borohydride in 3% sucrose, pH 7.4, and rinsed for 1 h in PB, then prepared for cryosectioning as described above. Sections were washed in PBS for 30 min, then incubated for 3 h at room temperature with 10% normal donkey serum diluted in antibody buffer [0.1% Triton X-100 (Sigma) in PBS]. Sections were then incubated for 1–3 days in a humid chamber at 4°C with primary antibodies. Retinal sections were then washed with PBS, incubated for 2–3 h with fluorescently conjugated secondary antibodies, washed with PBS, and coverslipped.
Dissociated cells. Tiger salamander retinas were dissociated as described (16). Cell suspensions were placed on gelatin-coated slides, and cells were allowed to settle and adhere before fixation for 5 min at room temperature with 2% paraformaldehyde/2% glutaraldehyde. Cells were rinsed for 30 min in PB, 30 min in PB with 1% sodium borohydride, and 30 min in PB, then stained by using the above protocol for amphibian retinal sections.
Antibodies. Rabbit anti-serine racemase (7) and rabbit anti-d-serine (4) antisera were diluted 1:100 and 1:300, respectively; mouse anti-glutamine synthetase (Chemicon) was diluted 1:200. Fluorescently conjugated secondary antibodies were obtained from Jackson ImmunoResearch, diluted in PBS with 0.3% Triton X-100, and used at 7 μg/ml. For anti-racemase immunohistochemistry, control samples were incubated with buffer (with or without anti-glutamine synthetase) without anti-racemase. Specificity of the anti-racemase antibody was evaluated on Western blots of rat retina proteins, prepared as described (15). For the anti-d-serine antibody, control samples were incubated with anti-d-serine preincubated with d-serine- or l-serine-conjugated to BSA (100 μg/ml) (17).
Immunofluorescence was viewed by using a conventional fluorescence microscope (Olympus IX70; Olympus America, Lake Success, NY) or a confocal microscope (Leica TCS 4D; Leica, Malvern, PA). Images were produced by using VOXBLAST (VayTek, Fairfield, IA), METAMORPH (Universal Imaging, West Chester, PA), and PHOTOSHOP (Adobe Systems, Mountain View, CA) software.
Electrophysiological Techniques. Rat retinas and whole-cell recordings (WCRs). Male Long–Evans rats (250–400 g) were deeply anesthetized with sodium pentobarbital administered i.p., and the eyes were removed. Wholemount retinas were prepared as described (18). Retinas were mounted in a recording chamber and superfused at ≈2.5 ml/min with Mg2+-free Ringer's solution at 24°C. The Ringer's solution contained, in mM: NaCl, 135.5; KCl, 3.0; CaCl2, 3.0; NaH2PO4, 0.5; d-glucose, 15.0; Hepes, 10.0; pH 7.4, equilibrated with 100% O2. Ganglion cells were viewed with infrared differential interference optics and voltage clamped in the WCR configuration. Cells were held at the resting membrane potential (0 current potential). The patch pipette solution contained, in mM: CH3NaO3S, 5.0; CH3KO3S, 128.0; MgCl2, 2.0; EGTA, 5.0; glutathione, 1.0; Hepes, 5.0; MgATP, 2.0; and NaGTP, 0.2.
Amphibian retinas and WCRs. Tiger salamanders (Ambystoma tigrinum) or mudpuppies (Necturus maculosus) were maintained in cold (4°C), aerated, water-filled tanks and exposed to a 12/12 h light/dark room light cycle. Eyes were enucleated from animals after decapitation and double-pithing. Tiger salamander retinas were isolated, mounted on filter paper, and placed in a perfusion chamber, ganglion cell layer up. The chamber was continuously perfused with Mg2+-free amphibian Ringer's solution at a rate of 1–2 ml/min. The Ringer's solution contained, in mM: NaCl, 110.0; KCl, 2.5; CaCl2, 1.8; Hepes, 10.0; d-glucose, 5.0; pH 7.8, equilibrated with 100% O2. Ganglion cells were viewed with infrared differential interference optics through a water-immersion objective. Patch electrodes (10–20 MΩ) were filled with, in mM: KCH3SO4, 98.0; NaCH3SO4, 3.5; MgSO4, 3.0; CaCl2, 1.0; EGTA, 11.0; Hepes, 5.0; d-glucose, 2.0; glutathione, 1.0; MgATP, 1.0; NaGTP, 0.5; pH 7.4. WCRs were made with an Axoclamp-2A amplifier (Axon Instruments, Foster City, CA). Proximal negative response (PNR). For PNR experiments, we used the amphibian eyecup preparation described (19); the PNR was identified and recorded with Ringer's solution-filled, beveled microelectrodes having a resistance of a few MΩ. For both WCR and PNR experiments, light stimulation was provided by a 12-V tungsten-halogen light source projected through a computer-driven liquid crystal display (LCD) panel that controlled the intensity and geometry of the light stimulus. All recordings were digitized (Digidata 1320A, Axon Instruments) and recorded with PCLAMP software (Axon Instruments).
Endogenous d-Serine. Reverse-phase HPLC was used to detect endogenous d-serine, by using the method of Hashimoto et al. (2) to detect amino acid enantiomers after fluorescent derivatization. Retinas of rats and tiger salamanders were homogenized. Proteins were precipitated with 10% trichloroacetic acid (TCA) and removed by centrifugation. TCA was removed with water-saturated ether, and the amino acids were rendered fluorescent by derivatization with _N-tert_-butyloxycarbonyl-L-cysteine and _o_-phthaldialdehyde. d-serine was recognized by its characteristic retention time; we validated the identification of the d-serine peak by showing that a sample of the supernatant that was exposed to d-AAO (0.3 mg/ml for 12 h at 37°C) caused a marked reduction of the d-serine peak, without affecting the other constituents in the chromatogram.
Statistics. All measurements are expressed as the mean ± SE.
Results
We examined the distribution of d-serine and serine racemase in the mouse, rat, tiger salamander, and mudpuppy retinas, and, in all cases, immunoreactivity was localized to retinal glial cells.
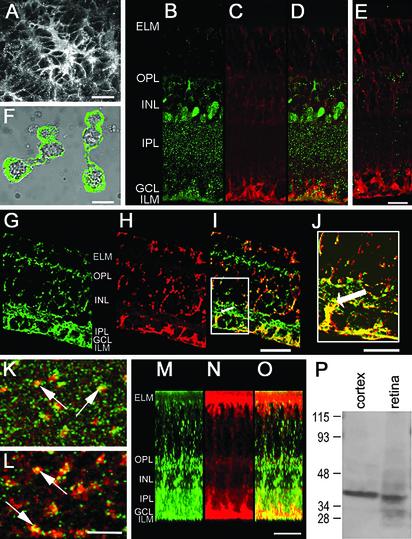
Unlike avascular amphibian retinas, vascularized mammalian retinas contain astrocytes as well as Müller cells. In rodent retinas, d-serine immunoreactivity was present in astrocytes, identified by their characteristic shape and location at the vitreal surface (Fig. 1_A_). In addition, immunoreactivities for d-serine (Fig. 1_B_, green in overlay in Fig. 1_D_) and glutamine synthetase (Fig. 1_C_, red in Fig. 1_D_), a glial marker, were observed throughout the retina in a manner consistent with the distribution of Müller cells, which extend from the internal limiting membrane (ILM) to the external limiting membrane (ELM). d-serine immunoreactivity was particularly pronounced in the inner plexiform layer (IPL) as well as the inner nuclear layer (INL), where Müller cell somata reside. Colocalization of the two labels was most apparent in Müller cell processes within the ganglion cell layer. d-serine immunoreactivity was eliminated when tissue was incubated with anti-d-serine that had been preabsorbed with d-serine-conjugated to BSA (Fig. 1_E_) but not when antibody was preabsorbed with l-serine-conjugated to BSA (compare Fig. 1 D and E). Thus, the d-serine antibody clearly recognizes d-serine over l-serine.
Fig. 1.
(A) Confocal image of the vitreal surface of a rat retina, showing an astrocyte immunoreactive for d-serine. (B_–_E) Sections of rat retina. Retinal section doubly immunostained by using the anti-d-serine antibody preabsorbed with l-serine conjugated to BSA (B, green in overlay in D) and glutamine synthetase (C, red in D). d-serine immunoreactivity extends throughout the depth of the retina, from the ILM to the ELM. Prominent staining is seen in the Müller cell endfeet that form the ILM, in the plexiform layers, and in cell bodies in the INL, where Müller cell somata are located. (E) Retinal section doubly immunostained with anti-glutamine synthetase (red) and anti-d-serine that had been preincubated with both d- and l-serine conjugated to BSA (green). Compare this section with that shown in D. (F) d-serine immunoreactivity in isolated Müller cells of tiger salamander. (G_–_J) Section of mudpuppy retina doubly immunostained for d-serine (G, green in overlays in I and J) and glutamine synthetase (H, red in I and J). (I) Note that the thick proximal processes of Müller cells, one of which is indicated by the arrow, appear yellow in this overlay, indicating colocalization of the two labels. The region within the white box in I is shown at higher magnification in J. (K_–_O) Mouse retinal wholemount doubly immunostained for serine racemase (green) and glutamine synthetase (red). (K and L) Each panel represents the superimposition of six confocal images collected at 0.2-μm intervals in a plane parallel to the retinal layers. (K) In the IPL, the proximal stalks of Müller cells, seen in cross section, stain for both serine racemase and glutamine synthetase; arrows indicate two such stalks. (L) Processes of Müller cells in the inner nuclear layer also stain for both antigens; examples are indicated by arrows. (M_–_O) “Virtual section” reconstructed from a stack of optical sections collected parallel to the retinal layers. Both serine racemase (M, green in overlay in O) and glutamine synthetase (N, red in O) are found throughout the depth of the mouse retina. (P) Western blot. Soluble proteins from rat brain and retina were separated by SDS/PAGE under reducing conditions, transferred to poly(vinylidene difluoride) (PVDF) membranes, and probed with anti-serine racemase. Positions of molecular mass markers are shown to the left of the blot. OPL, outer plexiform layer; GCL, ganglion cell layer. Calibration bars = 10 μm, A, J, and L (applies to K and L); 15 μm, O (applies to M_–_O); 20 μm, E (applies to B_–_E), F, and I (applies to G_–_I).
In the mudpuppy retina, d-serine immunoreactivity (Fig. 1_G_, green in overlays in Fig. 1 I and J) was present in Müller cells, identified by their immunoreactivity for glutamine synthetase (Fig. 1_H_, red in Fig. 1 I and J). Colocalization of d-serine and glutamine synthetase immunoreactivities is apparent throughout the retina but is particularly prominent in the large Müller cell processes (20) that separate ganglion cells (Fig. 1 I and J). Further support for this localization has come from dissociated retina preparations in which the Müller cells are easily identified and stain extensively with the d-serine antibody (Fig. 1_F_). Dissociated Müller cells sometimes showed a halo of staining around the cell membrane, whereas in other cases, more complete filling of the cytosol was observed. We did not see clear evidence of d-serine in other cell types, suggesting that it is predominantly localized to Müller cells in salamander retinas.
Rodent and amphibian retinas were also stained for serine racemase. Fig. 1 K_–_O illustrates the staining pattern observed in a mouse retinal wholemount doubly immunostained for serine racemase (green) and glutamine synthetase (red). In optical sections parallel to the retinal layers, serine racemase and glutamine synthetase immunoreactivities are seen in Müller cell profiles; the proximal stalks of Müller cells in the IPL are shown in Fig. 1_K_, and Müller cell processes in the INL are shown in Fig. 1_L_. In “virtual slices” of the retina reconstructed from stacks of confocal images, immunoreactivities for serine racemase (Fig. 1_M_, green in overlay in Fig. 1_O_) and glutamine synthetase (Fig. 1_N_, red in Fig. 1_O_) are seen from the ILM to the ELM, in a pattern consistent with localization to Müller cells. However, serine racemase immunoreactivity is especially pronounced in the plexiform layers. In Western blots of rat retina (Fig. 1_P_), anti-serine racemase stained a band of ≈37 kDa, the estimated molecular mass for mammalian serine racemase (7). We also stained amphibian retinas for serine racemase and localized this marker to Müller cells (not shown).
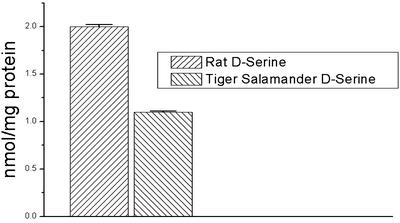
Reverse-phase HPLC revealed the presence of d-serine in homogenates of retinas of rats and tiger salamanders (Fig. 2). d-serine was identified by its retention time in chromatograms, and was significantly reduced by exposure to d-AAO. Chromatography results indicated specific d-serine concentrations of 1.1 nmol/mg total protein in tiger salamander retina, and 2.0 nmol/mg protein in rat retina.
Fig. 2.
A summary of HPLC analysis of _N-tert_-butyloxycarbonyl-l-cysteine _o_-phthaldialdehyde-derived amino acids extracted from the rat (left) and tiger salamander (right) retinas. Twelve retinas from each species were used, and three retinas were pooled for each HPLC determination. When expressed as nmol/mg protein, values for the rat were 2.0 ± 0.023, whereas those of the salamander were 1.1 ± 0.012.
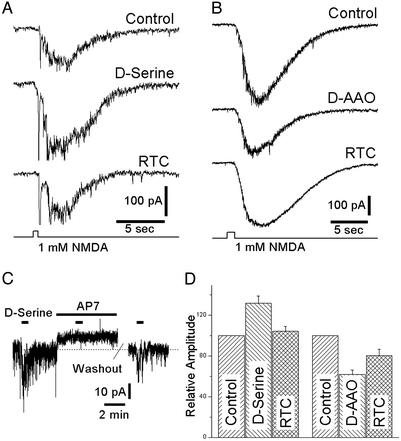
The physiological action of d-serine was investigated with WCRs from rat and tiger salamander retinas. Fig. 3_A_ illustrates a WCR from a ganglion cell studied in a perfused rat wholemount retina in which NMDA (1 mM) was applied by pressure-ejection through an extracellular pipette placed near the WCR electrode. Pressure-ejection of NMDA onto the retinal surface evoked a large inward current. Bath-applied d-serine (100 μM) reversibly enhanced the NMDA-mediated current to an average of 132 ± 7.1% of control (Fig. 3_D_, n = 10). Addition of 100 μg/ml d-AAO had the opposite effect, reducing the amplitude of NMDA-evoked inward currents to 62 ± 4.1% of control (Fig. 3_B_, n = 6), and these effects were partially reversible (Fig. 3_D_).
Fig. 3.
WCRs from ganglion cells in the isolated, perfused rat retina. (A)(Top) Pressure-ejection of NMDA (1 mM) from an extracellular pipette evoked an inward current (holding potential -61 mV) in Mg2+-free Ringer's solution. The response to NMDA was enhanced when d-serine (100 μM) was added to the bathing medium (Middle), and recovery to the control amplitude was observed after removing d-serine (Bottom). The average response in d-serine was 131.8 ± 7.1% of the control response (left bars in D). (B) Pressure-ejection of NMDA evoked an inward current (Top) that was reduced after several minutes of exposure to d-AAO (100 μg/ml, Middle). The response partially recovered after washout of d-AAO (Bottom). In 10 recordings, the average response in d-AAO was 62.0 ± 4.1% of the control response (right bars in D). (C) d-serine evoked a small inward current accompanied by a large increase in noise; the application of the NMDA antagonist AP7 induced a small net outward current associated with a significant reduction in membrane noise and virtually eliminated the response to d-serine (dashed line at zero current). The action of AP7 was reversible. (D) Summary of electrophysiological results. RTC, return to control solution.
Application of d-serine also enhanced tonically active NMDA receptor-mediated currents. When 100 μM d-serine was added to the superfusion solution, it potentiated a steady inward current in rat ganglion cells (Fig. 3_C_). Blockade of NMDA currents with 100 μM AP7, a competitive antagonist at the glutamate-binding site of the NMDA receptor, effectively abolished the inward current evoked by d-serine (response was reduced by 97.2 ± 2.8%, n = 5) and reduced the tonic inward current present before the first application of d-serine. The effect of AP7 was largely reversible (Fig. 3_D_).
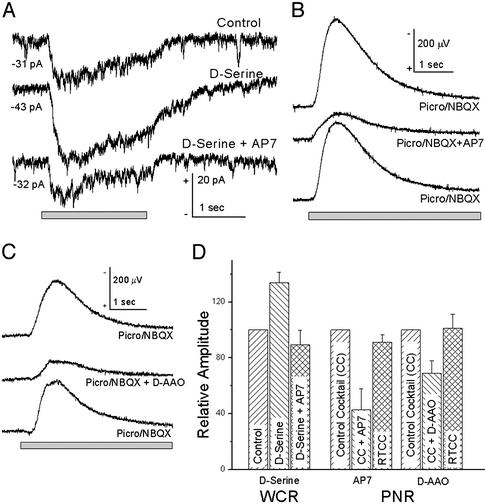
The action of d-serine on light-evoked activity of ganglion cells was studied in the isolated, perfused tiger salamander retina. Fig. 4_A_ illustrates a WCR from a sustained peak light-evoked (ON) ganglion cell, which, under voltage-clamp conditions, is characterized by an inward current that lasts for the duration of the light stimulus. When 100 μM d-serine was added to the bathing medium, large increases in both the tonic inward and light-evoked currents were apparent. These enhanced responses were diminished to values near those of control when 100 μM AP7 was added to the d-serine Ringer's solution. In two of two sustained ON cells studied, exogenous d-serine enhanced tonic inward currents and the light-evoked responses. In 8 of 12 ON-OFF cells studied, d-serine enhanced tonic inward currents and both the ON and OFF components of light-evoked responses. The tonic inward current increased by -9.74 ± 1.39 pA over control values in the presence of 100 μM d-serine, and was reduced by 2.67 ± 1.45 pA below control values in the presence of 100 μM AP7/100 μM d-serine. Fig. 4_D_ illustrates the effects of d-serine and AP7 on the peak light-evoked (ON) currents; the same trends were apparent in the peak OFF response and the integrated charge density for both ON and OFF responses. Application of 25 μM 7-chlorokynurenic acid, a competitive antagonist at the glycine binding site of NMDA receptors (21), also reduced the amplitude of light-evoked responses, and completely abolished the inward currents evoked by pressure-ejecting 1 mM NMDA (not shown). Further, whereas 7-chlorokynurenic acid blocked NMDA receptor activation in the presence of low concentrations of exogenously applied d-serine (1 μM), the competitive block was overcome by larger doses (10 μM), demonstrating that d-serine acts specifically at the coagonist site.
Fig. 4.
Light-evoked responses in the tiger salamander retina. (A) WCR from a sustained ON ganglion cell. In a Mg2+-free control Ringer's solution, presentation of a 130-μm wide bar of light evoked a relatively sustained inward current that was substantially enhanced when d-serine (100 μM) was added to the bathing medium; when 100 μM AP7 was added to the d-serine solution, the light-evoked current was reduced to a value approximately equal to the control. Numbers indicate the average inward current at -65 mV before the light stimulus. The average response in d-serine was 133.6 ± 7.5% (n = 10) of the control response; the average response in d-serine plus AP7 was 83 ± 10.4% (n = 9) of the control response (left bars in D). (B) Light-evoked PNR, an extracellularly recorded response of the inner retina (negative up). A perfusion medium containing picrotoxin (100 μM) and NBQX (100 μM) enhanced the PNR above that in Mg2+-free Ringer's solution (not shown); the enhanced PNR was reversibly reduced when d-AP7 was added to the bathing medium. d-AP7 reduced the light response to 42.9 ± 15.0% (n = 15) of the response in picrotoxin/NBQX (“control mixture,” middle bars in D). (C) d-AAO reduced the PNR in picrotoxin/NBQX; the response recovered after washout of d-AAO. d-AAO reduced the light response to 69 ± 9% (n = 10) of the response in picrotoxin/NBQX (right bars in D). (D) Summary of electrophysiological results; RTCC, return to “control mixture.”
A complementary method for studying the action of d-serine on light-evoked activity is to use extracellular recording techniques similar to those used for studies of long-term potentiation (22). Burkhardt (23) described the PNR in the frog retina, a localized, extracellularly recorded signal of the inner retina that seems to be a population response postsynaptic to bipolar cells and distinct from the electroretinogram. Fig. 4_B_ illustrates a PNR recording (negative up) from a mudpuppy eyecup evoked by a 130-μm spot of light centered over the recording electrode. In a Mg2+-free Ringer's solution, the NMDA receptor contribution to the PNR was minimal, because the PNR showed little change when 50 μM d-AP7 was added to the bathing medium (not shown). We used picrotoxin (100 μM) to enhance the PNR (23) and added 1,2,3,4-tetrahydro-6-nitro-2,3-dioxo-benzol[_f_]quinoxaline-7-sulfonamide disodium (NBQX; 100 μM) to block AMPA/kainic acid receptors (13). When d-AP7 was added to the picrotoxin/NBQX mixture, the PNR was reduced in amplitude (Fig. 4_C_), demonstrating an NMDA receptor component to the PNR under these conditions. In 15 experiments, d-AP7 reduced the PNR by 57.1 ± 3.8%, showing a strong NMDA receptor contribution to the response. Good recovery was observed in all experiments when the bathing solution was returned to the picrotoxin/NBQX bathing solution (339 ± 54 μV control; 307 ± 46 μV recovery; Fig. 4_D_).
We evaluated the actions of d-serine on the picrotoxin/NBQX-enhanced PNR. Although d-serine increased the amplitude of the PNR in most cases (not shown), the magnitude of this effect was smaller than that observed in WCRs from tiger salamander ganglion cells. In contrast to the actions of d-serine on the PNR, the action of exogenous d-AAO was more pronounced. d-AAO (Fig. 4_C_) added to the picrotoxin/NBQX Ringer's reduced the amplitude of the PNR by 31 ± 9.0% (n = 13). These effects were reversible (mean amplitude for control = 306 ± 51 μV and 301 ± 51 μV for return to control). Our studies of the PNR were confined to the ON response, because a component of the OFF pathway is blocked by NBQX (24–27).
Discussion
The observations of this study indicate that d-serine and its synthesizing enzyme, serine racemase, are present in retinal glial cells (Müller cells and astrocytes), and that d-serine modulates NMDA receptor sensitivity in retinal neurons. We established the presence of d-serine through immunohistochemistry and HPLC, whereas serine racemase was revealed by immunohistochemistry and Western blot analysis. WCRs demonstrated that bath-applied d-serine enhanced the inward currents evoked by exogenously applied NMDA, as well as the NMDA receptor-mediated component of light-evoked responses. These results indicate that d-serine can act as a coagonist at the NMDA receptor and that this site is not normally saturated in retinal ganglion cells. In addition, we have clearly demonstrated that endogenous d-serine is a necessary component of full NMDA receptor activation in the retina because d-AAO added to the bathing medium significantly decreased currents evoked by exogenously applied NMDA, and the NMDA receptor-mediated component of the PNR.
The physiological observations of this study placed a strong emphasis on evaluating the effects of d-serine on light-evoked synaptic currents of ganglion cells. d-serine typically enhanced the portion of the light response mediated by NMDA receptors. d-serine facilitation of NMDA receptors could come about by transient d-serine release during light stimulation (4), or by the presence of a continuous background level of d-serine. Previous studies have suggested that d-serine release from astrocytes can be induced by activation of AMPA receptors intrinsic to these glial cells (4, 28). AMPA receptor subunits have been identified in Müller cells (29). However, in our studies of the PNR, the transient, AMPA receptor-mediated release of d-serine from glial cells should have been eliminated by the application of NBQX, a powerful AMPA receptor antagonist (30).
Several observations suggest that, in the retina, ganglion cells show a tonic, background activation of NMDA receptors. Gottesman and Miller (31) provided evidence from retinal slice experiments that the background noise and a steady, inward current in ganglion cells were generated by continuous activation of NMDA receptors. A more complete study of the tonic NMDA-mediated current has been done by using noise analysis, which confirms and strengthens this conclusion (J. Gottesman and R.F.M., unpublished observations). Consistent with these reports, most ganglion cells examined in this study revealed a tonic inward current (Figs. 3_C_ and 4_A_) that was enhanced by exogenous d-serine and was often reduced below the control values when NMDA receptors were blocked. If d-serine contributes to this continuous background activation of NMDA receptors, then a steady background level of d-serine is required in addition to the continuous presence of glutamate. Measurements of d-serine from the perfused salamander retina using capillary electrophoresis indicate that background levels of d-serine are at least in the low μM range (44), sufficient to activate NMDA receptors in isolated cell preparations (5). This continuous background level of d-serine may obviate the need for a light-evoked, transient release of d-serine from glial cells.
A maintained level of d-serine seems necessary to account for the full range of our experimental observations. The cellular mechanisms that regulate the extracellular levels of d-serine have not been established. If AMPA receptor-mediated release from glial cells is blocked by the NBQX in our PNR experiments, it raises some questions as to how a continuous background level of d-serine can be maintained. One possible explanation for this apparent discrepancy is that the slow uptake of d-serine (32) allows significant levels to remain in the extracellular spaces long after d-serine release from the glial cells is blocked by NBQX. However, another possibility has been revealed by more recent studies in which a Na+-dependent transport system for d-serine has been described in astrocytes (28). This uptake system not only sequesters d-serine in astrocytes, but d-serine can be released into the extracellular environment by exchange with other amino acids, including l-serine. A better understanding of d-serine release and regulation is required before these issues can be fully addressed.
An immunohistochemical analysis of the distribution of d-serine, glycine, and NMDA receptors in the rat brain has suggested that d-serine may serve as the coagonist for NMDA receptors in some brain regions, whereas in others, glycine is a more likely candidate (32). The retina presents an atypical site in which both glycine and d-serine levels are high. Glycine is a prominent inhibitory neurotransmitter in the retina (33–37), and has been assumed to be the sole endogenous coagonist of retinal NMDA receptors (31, 38). Although we have clearly demonstrated that d-serine is an endogenous coagonist of NMDA receptors in the retina, it remains to be demonstrated that reductions in endogenous glycine levels can influence NMDA receptor function. Lukasiewicz and Roeder (38) showed that high concentrations of exogenously applied glycine did not potentiate NMDA receptor responses of ganglion cells in a retinal slice preparation of the tiger salamander. In contrast, 5,7-dichlorokynurenic acid, an antagonist to the glycine binding site of NMDA receptors, reduced ganglion cell responses evoked by application of NMDA. This result led to the conclusion that the glycine binding site of ganglion cell NMDA receptors was normally saturated. In the present study, we observed a consistent increase in NMDA receptor currents when d-serine was added to the bathing medium, suggesting that the glycine binding sites of NMDA receptors are not saturated under our experimental conditions. The presence of high-affinity glycine uptake sites, which may be important in clearing glycine from the extracellular space, could prevent one from adequately testing the actions of exogenous glycine on NMDA receptor sensitivity (39). The absence of a high-affinity uptake mechanism for d-serine leads to the speculation that it remains in the extracellular space for longer periods than glycine (32) and is presumably less restricted in its ability to distribute to synaptic sites. In addition, it seems that NMDA receptors are more sensitive to d-serine than to glycine when studied in isolated cells using an expression system (40).
NMDA receptors are important synaptic receptors that are intrinsic to all ganglion cells and most amacrine cells (41) (as well as horizontal cells in some species) (42). However, NMDA receptors have also been described in Müller cells (43). Thus, d-serine may function as a coagonist at NMDA receptors of neurons and glial cells. A direct feedback influence on glial cells may play an important role in the regulatory mechanisms of d-serine synthesis and release.
The results of this study suggest that d-serine and serine racemase in the retina are found predominantly and perhaps exclusively in glial cells, including Müller cells and astrocytes. This study has also extended previous results by demonstrating that d-serine and d-AAO can affect responses evoked by a natural physiological stimulus. We conclude from our studies that d-serine subserves synaptic transmission in the retinas of diverse vertebrate species.
Acknowledgments
We thank Terry Doerr for excellent technical assistance with the amphibian immunohistochemistry and we appreciate conversations about this work with Jon Gottesman. We thank Derek Miller for reading the manuscript and contributing to the illustrations. This work was supported by National Eye Institute Grant EY03014 (to R.F.M.), National Institute of Mental Health Grant MH18501 (to S.H.S.), and Research Scientist Award DA-00074 (to S.H.S.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: NMDA, _N_-methyl-d-aspartate; d-AAO, d-amino acid oxidase; AMPA, α-amino-3-hydroxy-5-methylisoxazole-4-propionate; WCR, whole-cell recording; PNR, proximal negative response; NBQX, 1,2,3,4-tetrahydro-6-nitro-2,3-dioxo-benzol[_f_]quinoxaline-7-sulfonamide disodium; AP7, 2-amino-7-phosphonoheptanoic acid; ILM, internal limiting membrane; ELM, external limiting membrane; IPL, inner plexiform layer; INL, inner nuclear layer; PB, phosphate buffer.
References
- 1.Corrigan, J. J. (1969) Science 164**,** 142-149. [DOI] [PubMed] [Google Scholar]
- 2.Hashimoto, A., Nishikawa, T., Oka, T., Takahashi, K. & Hayashi, T. (1992) J. Chromatogr. 582**,** 41-48. [DOI] [PubMed] [Google Scholar]
- 3.Hashimoto, A., Nishikawa, T., Oka, T. & Takahashi, K. (1993) J. Neurochem. 60**,** 783-786. [DOI] [PubMed] [Google Scholar]
- 4.Schell, M. J., Molliver, M. E. & Snyder, S. H. (1995) Proc. Natl. Acad. Sci. USA 92**,** 3948-3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kleckner, N. W. & Dingledine, R. (1988) Science 241**,** 835-837. [DOI] [PubMed] [Google Scholar]
- 6.Baranano, D. E., Ferris, C. D. & Snyder, S. H. (2001) Trends Neurosci. 24**,** 99-106. [DOI] [PubMed] [Google Scholar]
- 7.Wolosker, H., Blackshaw, S. & Snyder, S. H. (1999) Proc. Natl. Acad. Sci. USA 96**,** 13409-13414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller, R. F. & Slaughter, M. M. (1986) Trends Neurosci. 9**,** 211-218. [Google Scholar]
- 9.Slaughter, M. M. & Miller, R. F. (1983) J. Neurosci. 3**,** 1701-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Slaughter, M. M. & Miller, R. F. (1983) Nature 303**,** 537-538. [DOI] [PubMed] [Google Scholar]
- 11.Diamond, J. S. & Copenhagen, D. R. (1993) Neuron 11**,** 725-738. [DOI] [PubMed] [Google Scholar]
- 12.Diamond, J. S. & Copenhagen, D. R. (1995) J. Physiol. (London) 487**,** 711-725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu, W. & Miller, R. F. (1996) Brain Res. 709**,** 184-196. [DOI] [PubMed] [Google Scholar]
- 14.Meister, A. & Wellner, D. (1963) in The Enzymes, ed. Boyer, P. D. (Academic, New York), pp. 634-648.
- 15.Zahs, K. R., Kofuji, P., Meier, C. & Dermietzel, R. (2003) J. Comp. Neurol. 455**,** 531-546. [DOI] [PubMed] [Google Scholar]
- 16.Esguerra, M. & Miller, R. F. (2002) Glia 39**,** 314-319. [DOI] [PubMed] [Google Scholar]
- 17.Pow, D. V. (1997) Methods Mol. Biol. 72**,** 103-123. [DOI] [PubMed] [Google Scholar]
- 18.Zahs, K. R. & Newman, E. A. (1997) Glia 20**,** 10-22. [PubMed] [Google Scholar]
- 19.Miller, R. F. & Dacheux, R. F. (1976) J. Gen. Physiol. 67**,** 639-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller, R. F. & Dowling, J. E. (1970) J. Neurophysiol. 33**,** 323-341. [DOI] [PubMed] [Google Scholar]
- 21.Kemp, J. A., Foster, A. C., Leeson, P. D., Priestley, T., Tridgett, R., Iversen, L. L. & Woodruff, G. N. (1988) Proc. Natl. Acad. Sci. USA 85**,** 6547-6550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bliss, T. V. & Collingridge, G. L. (1993) Nature 361**,** 31-39. [DOI] [PubMed] [Google Scholar]
- 23.Burkhardt, D. A. (1970) J. Neurophysiol. 33**,** 405-420. [DOI] [PubMed] [Google Scholar]
- 24.Yu, W. & Miller, R. F. (1995) Brain Res. 692**,** 190-194. [DOI] [PubMed] [Google Scholar]
- 25.Cohen, E. D. & Miller, R. F. (1995) Proc. Natl. Acad. Sci. USA 92**,** 1127-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cohen, E. D. & Miller, R. F. (1994) Visual Neurosci. 11**,** 317-332. [DOI] [PubMed] [Google Scholar]
- 27.Cohen, E. D. & Miller, R. F. (1999) Brain Res. 831**,** 206-228. [DOI] [PubMed] [Google Scholar]
- 28.Ribeiro, C. S., Reis, M., Panizzutti, R., de Miranda, J. & Wolosker, H. (2002) Brain Res. 929**,** 202-209. [DOI] [PubMed] [Google Scholar]
- 29.Peng, Y. W., Blackstone, C. D., Huganir, R. L. & Yau, K. W. (1995) Neuroscience 66**,** 483-497. [DOI] [PubMed] [Google Scholar]
- 30.Watkins, J. C., Krogsgaard-Larsen, P. & Honoré, T. (1990) Trends Pharmacol. Sci. 11**,** 25-33. [DOI] [PubMed] [Google Scholar]
- 31.Gottesman, J. & Miller, R. F. (1992) J. Neurophysiol. 68**,** 596-604. [DOI] [PubMed] [Google Scholar]
- 32.Schell, M. J., Brady, R. O., Jr., Molliver, M. E. & Snyder, S. H. (1997) J. Neurosci. 17**,** 1604-1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller, R. F., Dacheux, R. F. & Frumkes, T. E. (1977) Science 198**,** 748-750. [DOI] [PubMed] [Google Scholar]
- 34.Koontz, M. A., Hendrickson, L. E., Brace, S. T. & Hendrickson, A. E. (1993) Vision Res. 33**,** 2617-2628. [DOI] [PubMed] [Google Scholar]
- 35.Marc, R. E. (1989) Prog. Ret. Res. 8**,** 67-107. [Google Scholar]
- 36.Bolz, J., Thier, P., Voigt, T. & Wassle, H. (1985) J. Physiol. (London) 362**,** 395-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wassle, H., Koulen, P., Brandstatter, J. H., Fletcher, E. L. & Becker, C. M. (1998) Vision Res. 38**,** 1411-1430. [DOI] [PubMed] [Google Scholar]
- 38.Lukasiewicz, P. D. & Roeder, R. C. (1995) J. Neurosci. 15**,** 4592-4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pow, D. V. & Hendrickson, A. E. (1999) Visual Neurosci. 16**,** 231-239. [DOI] [PubMed] [Google Scholar]
- 40.Matsui, T., Sekiguchi, M., Hashimoto, A., Tomita, U., Nishikawa, T. & Wada, K. (1995) J. Neurochem. 65**,** 454-458. [DOI] [PubMed] [Google Scholar]
- 41.Dixon, D. B. & Copenhagen, D. R. (1992) J. Physiol. (London) 449**,** 589-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O'Dell, T. J. & Christensen, B. N. (1989) J. Neurophysiol. 61**,** 1097-1109. [DOI] [PubMed] [Google Scholar]
- 43.Uchihori, Y. & Puro, D. G. (1993) Brain Res. 613**,** 212-220. [DOI] [PubMed] [Google Scholar]
- 44.O'Brien, K. B., Esguerra, M., Klug, C. T., Miller, R. F. & Bowser, M. T. (2003) Electrophoresis 24**,** 1227-1235. [DOI] [PubMed] [Google Scholar]