Two Ubiquitin-Conjugating Enzymes, UbcP1/Ubc4 and UbcP4/Ubc11, Have Distinct Functions for Ubiquitination of Mitotic Cyclin (original) (raw)
Abstract
Cell cycle events are regulated by sequential activation and inactivation of Cdk kinases. Mitotic exit is accomplished by the inactivation of mitotic Cdk kinase, which is mainly achieved by degradation of cyclins. The ubiquitin-proteasome system is involved in this process, requiring APC/C (anaphase-promoting complex/cyclosome) as a ubiquitin ligase. In Xenopus and clam oocytes, the ubiquitin-conjugating enzymes that function with APC/C have been identified as two proteins, UBC4 and UBCx/E2-C. Previously we reported that the fission yeast ubiquitin-conjugating enzyme UbcP4/Ubc11, a homologue of UBCx/E2-C, is required for mitotic transition. Here we show that the other fission yeast ubiquitin-conjugating enzyme, UbcP1/Ubc4, which is homologous to UBC4, is also required for mitotic transition in the same manner as UbcP4/Ubc11. Both ubiquitin-conjugating enzymes are essential for cell division and directly required for the degradation of mitotic cyclin Cdc13. They function nonredundantly in the ubiquitination of CDC13 because a defect in ubcP1/ubc4+ cannot be suppressed by high expression of UbcP4/Ubc11 and a defect in ubcP4/ubc11+ cannot be suppressed by high expression of UbcP1/Ubc4. In vivo analysis of the ubiquitinated state of Cdc13 shows that the ubiquitin chains on Cdc13 were short in ubcP1/ubc4 mutant cells while ubiquitinated Cdc13 was totally reduced in ubcP4/ubc11 mutant cells. Taken together, these results indicate that the two ubiquitin-conjugating enzymes play distinct and essential roles in the degradation of mitotic cyclin Cdc13, with the UbcP4/Ubc11-pathway initiating ubiquitination of Cdc13 and the UbcP1/Ubc4-pathway elongating the short ubiquitin chains on Cdc13.
Ubiquitin is a highly conserved peptide consisting of 76 amino acids. It is covalently attached to target proteins by multistep reactions (6, 7, 44). Ubiquitin is first activated by the formation of thioester with the ubiquitin-activating enzyme E1. Activated ubiquitin is then transferred to ubiquitin-conjugating enzyme E2 (Ubcs). In most cases, ubiquitin ligase E3 catalyzes the transfer of ubiquitin to the target proteins from Ubcs. Finally, polyubiquitinated target proteins are degraded by the 26S proteasome.
There is a subfamily of genes that encode different ubiquitin-conjugating enzymes (E2) (Ubcs). On the other hand, ubiquitin ligases (E3) are varied, depending on their structures. Recently, a growing number of ubiquitin ligases (E3) has been found. A combination of specialized ubiquitin-conjugating enzymes and ubiquitin ligases is responsible for highly specific recognition of the target proteins.
The timings of sequential activation and inactivation of Cdk kinases are important for regulation of the cell cycle (31). Synthesis of mitotic cyclin, association of mitotic cyclin to Cdk kinases, and phosphorylation-dephosphorylation of Cdk kinases achieve activation of mitotic Cdk kinases, whereas inactivation of the mitotic Cdk kinases is mainly achieved by degradation of mitotic cyclin (51). The mitotic cyclin/Cdc2 complex is a key regulator of mitosis (30). Activation of mitotic cyclin/Cdc2 kinase is important for initiation of mitotic events, i.e., nuclear envelope breakdown, chromosome condensation, and mitotic-spindle formation. On the other hand, degradation of mitotic cyclin is important for exit from mitosis (24, 29). Degradation of mitotic cyclin is regulated by a ubiquitin-proteasome system (28).
The ubiquitin ligase for ubiquitination of mitotic cyclin is a multicomponent ubiquitin ligase, APC/C (anaphase-promoting complex/cyclosome) (13, 16, 19, 39, 43). APC/C consists of at least 11 core components in budding yeast (51). APC/C activity requires a conserved subfamily of WD40 proteins called Cdc20 and Cdh1/Hct1 in budding yeast, which are fizzy and fizzy related in Drosophila and humans (22, 36, 38, 45). These proteins associate with APC/C and function as cell cycle-specific activators of APC/C. Recently, it was reported that Cdc20 and Cdh1 recognize their substrates by physically interacting with the target proteins (14, 37). The fission yeast homologues of Cdc20 and Cdh1 are Slp1 and Ste9/Srw1, respectively. Slp1 is essential for metaphase-anaphase transition and is thought to be an activator of APC/C at mitosis (25). Ste9/Srw1 is an activator of APC/C at G1 phase and is not required for the metaphase-anaphase transition (20, 46). Ste9/Srw1 associates with APC/C, and its association is regulated by phosphorylation (4, 47).
In a biochemical analysis of Xenopus and clam oocyte extracts, UBC4 and UBCx/E2-C were identified as ubiquitin-conjugating enzymes for mitotic cyclin (2, 50). Xenopus UBC4 is more processive than UBCx for ubiquitination of mitotic cyclin in a biochemical analysis (40, 50). A dominant negative form of E2-C and its human homologue UbcH10 were shown to cause mitotic arrest in mammalian cells (3, 41). Furthermore, the human and budding yeast Ubc4 proteins physically interact with a RING-H2 finger domain-containing APC/C component, Apc11, in vitro (10, 23, 40). On the other hand, human UbcH10 physically interacts with a cullin homology domain-containing APC/C component, Apc2, in vitro (40).
Fission yeast UbcP1/Ubc4, a homologue of UBC4 (Table 1), causes mitotic arrest when it is overexpressed (17). Previously, we reported that UbcP4/Ubc11, a fission yeast homologue of UBCx/E2-C, is essential for the transition of mitosis (32). Mitotic cyclin with high Cdc2 kinase activity accumulated in UbcP4/Ubc11-depleted cells. However, all of these results did not elucidate the functional difference and relationship between these two ubiquitin-conjugating enzymes in vivo.
TABLE 1.
Homologues of ubiquitin-conjugating enzymes
| Enzyme in Xenopus and clam | Homologue in: | |
|---|---|---|
| Budding yeast | Fission yeast | |
| UBC4 | Ubc4/5 | UbcP1/Ubc4 |
| UBCx/E2-C | Ubc11 | UbcP4/Ubc11 |
Here we show that ubcP1/ubc4+ is also essential for cell viability. UbcP1/Ubc4-depleted cells arrest their growth during mitosis while accumulating Cdc13. In synchronization experiments, Cdc13 is stabilized in G1-arrested UbcP1/Ubc4- or UbcP4/Ubc11-depleted cells. Disappearance of Cdc13 caused by overproduction of Ste9/Srw1 is dependent on the activity of both UbcP1/Ubc4 and UbcP4/Ubc11. Furthermore, ubiquitin chains of Cdc13 are short in ubcP1/ubc4 mutant cells. In contrast, ubiquitinated Cdc13 is totally reduced in ubcP4/ubc11 mutant cells. These results indicate that the two ubiquitin-conjugating enzymes function differently in the ubiquitination of Cdc13. We conclude that these two ubiquitin-conjugating enzymes have distinct and essential functions in the ubiquitination of Cdc13 with APC/C.
MATERIALS AND METHODS
Yeast techniques.
The yeast strains used in this study are described in Table 2. Cells were grown as described by Alfa et al. (1). Temperature-sensitive mutant strains were cultured at 25°C, while those of other strains were cultured at 30°C. For repression of the nmt1 promoter, thiamine was added at a final concentration of 5 μg/ml. The standard genetic methods used for Schizosaccharomyces pombe were described by Alfa et al. (1).
TABLE 2.
Strains used in this study
| Strain | Genotype |
|---|---|
| JY741 | h− leu1-32 ura4-D18 ade6-M216 |
| ΔubcP1 | h− leu1-32 ura4-D18 ade6-M216 Δ_ubcP1::ura4+_ (pREP81-ubcP1) |
| cdc10 | h− leu1-32 cdc10-129 |
| cdc10 ΔubcP1 | h− leu1-32 ura4-D18 ade6-M210 cdc10-129 Δ_ubcP1::ura4+_ (pREP81-ubcP1) |
| cdc10 ΔubcP4 | h− leu1-32 ura4-D18 ade6-M210 cdc10-129 Δ_ubcP4::ura4+_ (pREP81-ubcP4) |
| mts2 | h− leu1-32 mts2-1 |
| mts2 ΔubcP1 | h− leu1-32 ura4-D18 ade6-M210 mts2-1 Δ_ubcP1::ura4+_ (pREP81-ubcP1) |
| mts2 ΔubcP4 | h− leu1-32 ura4-D18 ade6-M210 mts2-1 Δ_ubcP4::ura4+_ (pREP81-ubcP4) |
| ubcP1-P61S | h− leu1-32 ura4-D18 ade6-M216 ubcP1-P61S::ura4+ |
| ubcP4-140 | h− leu1-32 ura4-D18 ade6-M210 ubcP4-140::ura4+ |
| mts2ubcP1-P61S | h− leu1-32 ura4-D18 ade6-M216 ubcP1-P61S::ura4+ mts2-1 |
| mts2ubcP4-140 | h− leu1-32 ura4-D18 ade6-M210 ubcP4-140::ura4+ mts2-1 |
| cut9-665 | h− leu1-32 cut9-665 |
| cdc10ste9 | h90 leu1-32 cdc10-129 ste9-B36 |
Plasmids.
pREP81-ubcP4 (32) was digested with _Nde_I and _Sal_I, and a ubcP4+ cDNA fragment was transferred into pREP41. pREP41-ubcP1 (32) was digested with _Nde_I and _Bam_HI, and a ubcP1+ cDNA fragment was transferred into pREP81. A ste9+ genomic fragment was amplified from fission yeast genomic DNA by PCR with primers ACGGATCCATGGATGAATTTGATGGGTT and ACGGATCCTTACCGTATTTTCATTGTAG (_Bam_HI sites are underlined) and cloned into a _Bam_HI site of pREP1. A cdc13+ genomic fragment was amplified from fission yeast genomic DNA by PCR with primers CGTCATATGACTACCCGTCGTTTAAC and CTTGACGTCTGCGGCCGCATTCTTCATCTTTCATG (_Nde_I and _Not_I sites, respectively, are underlined) and cloned into pINV1-spc1-HA6His (15) (a gift from F. Gaits). A fragment containing the cdc13+ open reading frame and a hemagglutinin (HA)-six-His tag (HA6His) was digested with _Nde_I and _Sac_I and transferred to pREP1.
Isolation and disruption of the ubcP1+ gene.
Fission yeast genomic DNA was partially digested with _Sau_3AI and cloned into a _Bam_HI site of pUC119. This fission yeast genomic library was screened with a ubcP1+ cDNA fragment. The isolated DNA fragment containing the ubcP1+ gene was digested with _Pst_I (this site is in the linker of pUC119) and _Eco_RI and cloned into the _Pst_I and _Eco_RI sites of pUC119. This plasmid was digested with _Bam_HI and _Eco_RV, and a fragment containing ubcP1+ was replaced with a fragment containing ura4+. A wild-type diploid strain was transformed with this fragment. Transformants were selected for uracil autotrophy, and gene disruption was confirmed by PCR and Southern blot analysis.
Construction of strain ubcP1-P61S.
The fragment of a ubcP1+ gene in pUC119 was amplified by PCR with an M13 RV primer (TaKaRa) and primer CTGAATTCATTTTACATTTCTTTC (the _Eco_RI site is underlined), cloned into the _Eco_RI and _Pst_I sites of pUC119, and mutagenized with primer GGTGGCTTGAATGAGTAGTCCG (the mutated nucleotide is underlined) as recommended in the TaKaRa construction manual for in vitro mutagenesis primers. The mutation was verified by sequencing. This allele was designated ubcP1-P61S. The mutagenized plasmid was digested with _Sna_BI, and a fragment containing ura4+ was introduced. Wild-type strain JY741 was transformed with this _ubcP1_-P61S::ura4+ fragment, and transformants with uracil autotrophy were selected at 25°C, duplicated to a fresh plate containing 5 μg of Phloxine B per ml, and incubated at 36°C. Temperature-sensitive strains were isolated on the basis of the red color of the colonies at 36°C. Homologous recombination was verified by Southern blotting, and a mutation was verified by sequencing. We also found that G was changed to A at position 5 in the second intron of the ubcP1-P61S gene. This mutation might have been introduced by misincorporation in the PCR process for site-directed mutagenesis.
Antibodies.
Anti-Cdc13 serum was described by Osaka et al. (32). Anti-Cdc2 (PSTAIRE) antibody was purchased from Santa Cruz Biotechnology. Antiubiquitin monoclonal antibody was purchased from CHEMICON International, Inc. Anti-multiubiquitinated protein monoclonal antibodies FK1 and FK2 were purchased from MBL. Anti-Ste9 antibody was a gift from Sergio Moreno.
Western blotting.
The method used to prepare total protein for sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis was described by Kaiser et al. (18). Protein samples were resolved by SDS-polyacrylamide gel electrophoresis and blotted onto nitrocellulose filters. Proteins were detected by primary antibodies, horseradish peroxidase-conjugated secondary antibodies (Amersham), and ECL Western blotting detection reagent (Amersham).
Purification of ubiquitinated Cdc13-HA6His.
Cells (10 optical density at 600 nm units) were disrupted in extraction buffer (8 M guanidine-HCl, 50 mM Tris-HCl [pH 7.5], 10 mM imidazole) with glass beads. Extracts were clarified by microcentrifugation (15,000 × g, 10 min), and the protein concentration was determined by the Bio-Rad protein assay (Bio-Rad). One hundred microliters (50%, vol/vol) of Ni-nitrilotriacetic acid (NTA) agarose (QIAGEN) was added to the 300 μg of total protein extracts. After protein binding, the Ni-NTA agarose was washed intensively with the extraction buffer and a protein buffer (50 mM Tris-HCl [pH 7.5], 10 mM imidazole, 10% glycerol). Binding proteins were eluted with SDS-gel loading buffer containing 100 mM imidazole by boiling for 5 min and analyzed by Western blotting
Other methods.
Flow cytometry was performed on a Becton Dickinson FACScan apparatus with propidium iodide staining of cells as described previously (32). For phenotypic analysis, cells were fixed with methanol and stained with 4′,6′-diamidino-2-phenylindole (DAPI). The standard DNA technique used was described by Sambrook et al. (33).
RESULTS
The ubcP1+ gene is essential for progression of mitosis.
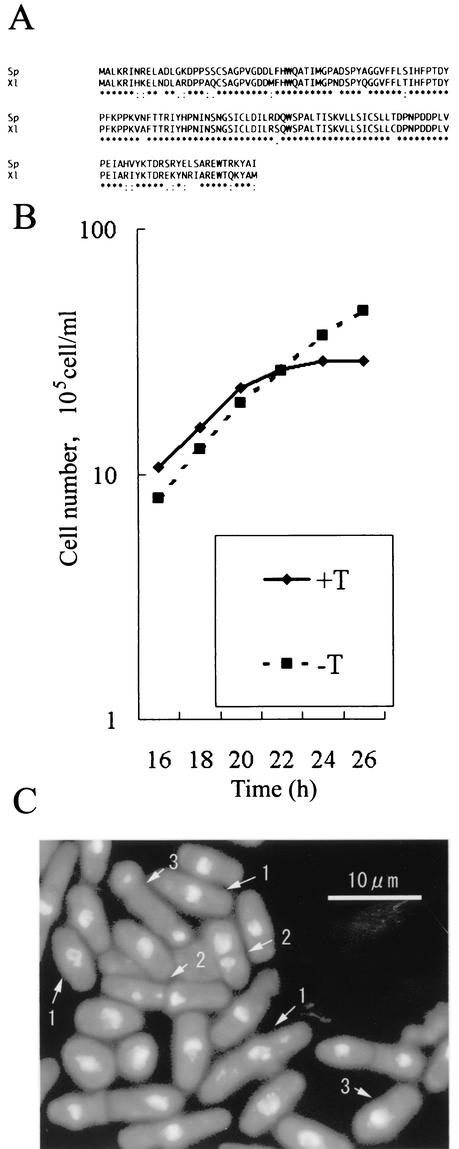
We previously reported the isolation of four ubiquitin-conjugating enzymes from fission yeast by in vitro analysis (32). Two of these ubiquitin-conjugating enzymes, UbcP1/Ubc4 and UbcP4/Ubc11, are homologous to Xenopus UBC4 and UBCx, respectively. The amino acid sequence of fission yeast UbcP1/Ubc4 is highly homologous to that of Xenopus UBC4 through its entire region (Fig. 1A). To elucidate the function(s) of UbcP1 in fission yeast, we constructed a mutant with ubcP1+ disrupted. In this mutant, approximately half of the N-terminal, Cys residue-containing portion (amino acids 1 to 87) of UbcP1, which contributes to thioester formation with a ubiquitin molecule, is disrupted. In the diploid strain, ubcP1+ was disrupted by one-step gene replacement (see Materials and Methods). Conventional tetrad analysis showed that ubcP1+ is essential for cell viability (data not shown). To examine the phenotype of _ubcP1_-deficient cells, we constructed a strain in which chromosomal ubcP1+ was replaced with ura4+ and kept alive by conditionally expressed UbcP1 from an nmt1 promoter on pREP81 (26). This strain, designated ΔubcP1, was first grown in medium without thiamine, and then thiamine was added to the medium to repress the expression of UbcP1. About 20 h after the addition of thiamine, the ΔubcP1 mutant stopped cell division (Fig. 1B). At 24 h after addition of thiamine, cells were fixed and their chromosomes were stained with DAPI (Fig. 1C). The cells exhibited abnormal mitosis with highly condensed chromosomes. About 40% of the cells had condensed chromosomes that were not separated, about 15% of the cells had condensed and displaced chromosomes, and about 10% of the cells had the cut (cell untimely tone) phenotype, in which the condensed chromosomes were disrupted by septation. The DNA content of the arrested cells was 2c (data not shown). These results indicate that ubcP1+ is required for progression of mitosis.
FIG. 1.
ubcP1+ function is required for transition of mitosis. (A) An alignment of the amino acid sequences of S. pombe UbcP1 (Sp) and Xenopus laevis UBC4 (Xl) is shown. Asterisks indicate identical amino acids. Dots indicate chemically conserved amino acids. (B) Strain ΔubcP1 cells were grown in minimal medium. Thiamine (T) was added at the time point at which the cell density was 105/ml, and cells were counted at the indicated times with a hemacytometer. (C) At 24 h after addition of thiamine, cells were fixed with methanol and stained with DAPI. Arrowheads indicate cells with the typical phenotype. 1, cells with condensed chromosomes that were not separated; 2, cells with the cut phenotype; 3, cells with highly condensed chromosomes that were displaced
Cdc13 accumulates in UbcP1-depleted cells.
The phenotype of the UbcP1-depleted cells suggests that UbcP1 is involved in the degradation of mitotic cyclin Cdc13. Therefore, we examined the amount of Cdc13 in UbcP1-depleted cells. Thiamine was added to a culture of ΔubcP1 cells to repress the expression of UbcP1. At the indicated times after addition of thiamine, protein samples were prepared. The level of Cdc13 was analyzed by Western blotting with anti-Cdc13 serum (Fig. 2). At about 12 h after the addition of thiamine, Cdc13 began to accumulate in the UbcP1-depleted cells. The accumulation of Cdc13 began before the growth arrest (compare Fig. 1B and 2), suggesting that Cdc13 accumulation did not result from the cell cycle arrest at mitosis.
FIG. 2.
Cdc13 accumulates in UbcP1-depleted cells. After addition of thiamine (T), protein samples were prepared from cultures at the indicated times and analyzed by Western blotting with anti-Cdc13 serum. As a control, the amount of Cdc2 was also measured.
Cdc13 is stabilized in UbcP1- or UbcP4-depleted cells at G1 phase.
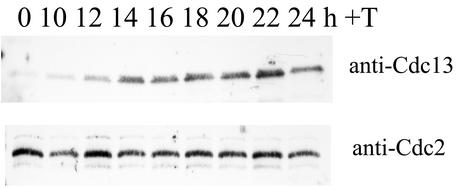
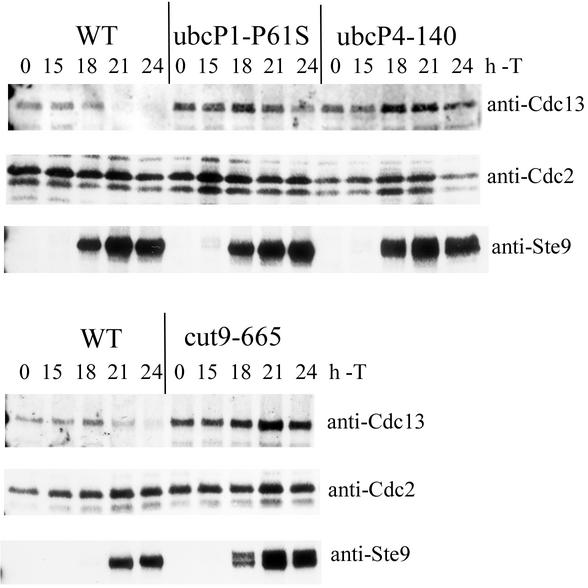
The above results suggest two possibilities. One is that the mitotic arrest before the degradation of Cdc13 causes Cdc13 accumulation. The other is that Cdc13 is actually stabilized. To distinguish between these possibilities, cells were synchronized at the G1 phase and the stability of Cdc13 was determined. Cdc13 disappeared in strain cdc10 cells at 4 h after a shift to 36°C as previously described (Fig. 3A and C) (21, 48). In contrast, Cdc13 remained at a significant level in cdc10ΔubcP1 cells at 4 h after the shift to 36°C (Fig. 2A). At this time point, although the DNA content of a small number of cells remained at 2c, a significant number of strain cdc10ΔubcP1 cells were arrested at the G1 phase (Fig. 2B). These results indicate that Cdc13 is stabilized in UbcP1-depleted cells even at the G1 phase. We previously reported that Cdc13 also accumulated in UbcP4-depleted cells (32). When similar synchronization experiments were done with strain cdc10ΔubcP4 cells, essentially the same results were obtained when UbcP4 was depleted in G1-arrested cells (Fig. 3C and D). In these G1 cells, Cdc13 was degraded by the APC/CSte9 pathway (4, 20, 46). As previously reported, Cdc13 was stabilized when Ste9 was absent in G1-arrested cells (Fig. 3E and F) (20). These results indicate that Cdc13 is stabilized in UbcP1- or UbcP4-depleted cells at the G1 phase. Thus, we concluded that two ubiquitin-conjugating enzymes, UbcP1 and UbcP4, are directly required for the degradation of Cdc13.
FIG. 3.
Cdc13 stabilization in UbcP1- or UbcP4-depleted cells. (A) Strain cdc10 and cdc10ΔubcP1 cells were cultured in minimal medium with thiamine for 10 h at 25°C, and the temperature was shifted to 36°C. At the indicated times, SDS samples were prepared and analyzed with anti-Cdc13 serum. As a control, the amount of Cdc2 was also measured. (B) At the same time points, DNA contents were analyzed by flow cytometry. (C and D) Same as panels A and B. Strain cdc10 and cdc10ΔubcP4 cells were cultured in minimal medium with thiamine for 7 h at 25°C, and the temperature was shifted to 36°C. The cells were analyzed by Western blotting and flow cytometry. (E and F) Same as panels A and B. Strain cdc10 and cdc10ste9 cells were cultured at 25°C, and the temperature was shifted to 36°C. The cells were analyzed by Western blotting and flow cytometry.
Construction of a temperature-sensitive ubcP1 mutant strain.
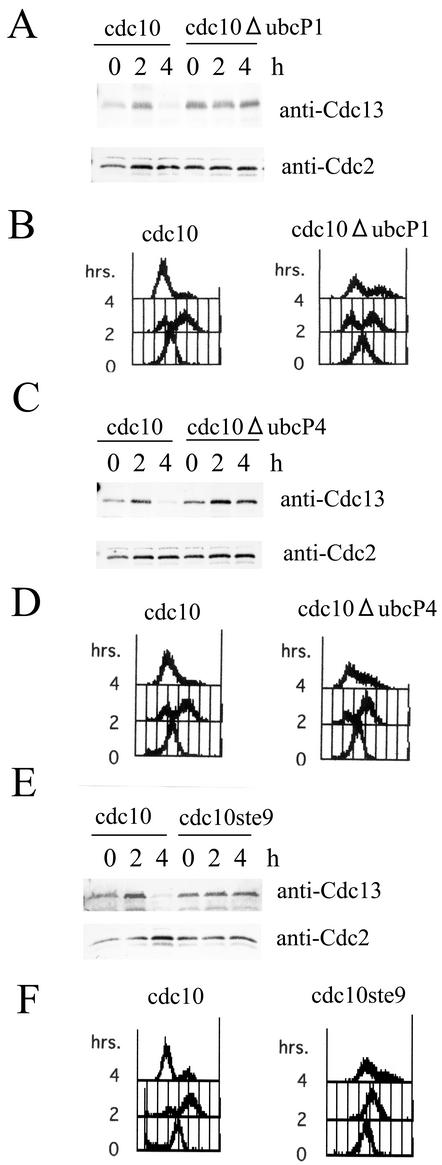
To analyze the function(s) of UbcP1 in detail, we constructed a temperature-sensitive ubcP1 mutant strain. We changed Pro-61 to Ser in the ubcP1+ gene in analogy with the same change at position 71 in cdc34-1, a temperature-sensitive mutation of the budding yeast gene encoding one of the ubiquitin-conjugating enzymes, Ubc3/Cdc34 (8). This allele was designated ubcP1-P61S. To replace the chromosomal region of wild-type ubcP1 with the ubcP1-P61S fragment, ubcP1-P61S was tagged with a ura4+ fragment and transformed into wild-type strain JY741. Some of the transformants, which were selected as a uracil autotroph, showed temperature-sensitive growth (Fig. 4A). Homologous recombination was verified by Southern blotting (data not shown). Backcrossing experiments resulted in the cosegregation of uracil autotrophy and a temperature-sensitive phenotype (data not shown). The temperature sensitivity was rescued by introduction of pREP81-ubcP1 (Fig. 4B). Thus, we designated this mutant strain ubcP1-P61S. The phenotype of this strain at a nonpermissive temperature resembled that of UbcP1-depleted cells (data not shown).
FIG. 4.
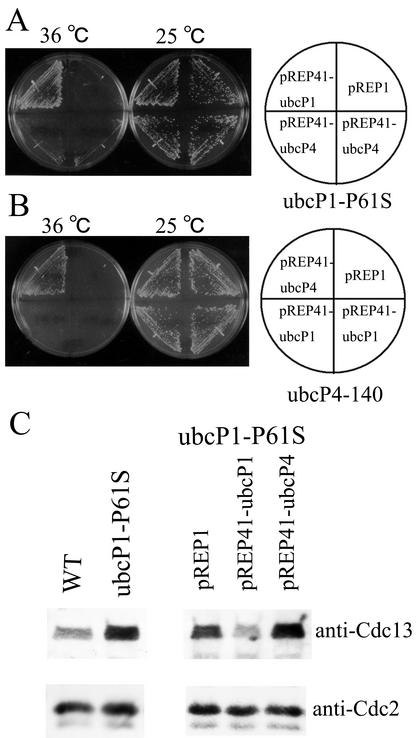
Temperature-sensitive strain ubcP1-P61S. (A) Parental and ubcP1-P61S mutant strains were streaked on yeast extract plates and incubated at 25 and 36°C. (B) Strain ubcP1-P61S mutant cells harboring pREP81 and pREP81-UbcP1 were streaked onto minimal medium plates containing appropriate supplements and incubated at 25 and 36°C. WT, wild type.
Independent functions of UbcP1 and UbcP4.
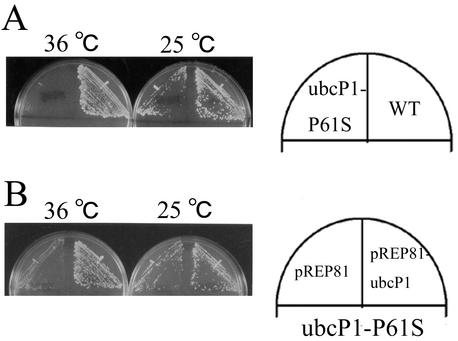
The fission yeast mutants lacking UbcP1 or UbcP4 activity showed Cdc13 stabilization. One reason for this may be that the defect in one of these two ubiquitin-conjugating enzymes led to a decrease in the total activity of ubiquitin conjugation to Cdc13. Another reason may be that the two ubiquitin-conjugating enzymes have distinct functions in the degradation of Cdc13. To test these two possibilities, we investigated whether overexpression of UbcP4 rescued the temperature sensitivity of the ubcP1-P61S mutant and whether overexpression of UbcP1 rescued the temperature sensitivity of the ubcP4-140 mutant (27). Ubiquitin-conjugating enzymes expressed from the strongest nmt1 promoter on pREP1 were somewhat toxic for cells (data not shown) (17). Thus, we used pREP41 harboring the modest nmt1 promoter for expression of ubiquitin-conjugating enzymes. As shown in Fig. 5A and B, expression of UbcP4 did not rescue the temperature-sensitive phenotype of the ubcP1-P61S mutant and overexpression of UbcP1 did not rescue the temperature-sensitive phenotype of the ubcP4-140 mutant. Furthermore, the accumulation of Cdc13 in strain ubcP1-P61S cells was not repressed by overexpression of UbcP4 (Fig. 5C). These results suggest that the functions of UbcP1 and UbcP4 in the degradation of Cdc13 are different.
FIG. 5.
Independent functions of UbcP1 and UbcP4. (A) pREP41-ubcP1, pREP41-ubcP4, and pREP1 were introduced into a temperature-sensitive mutant strain ubcP1-P61S, and transformants were streaked onto appropriate minimal medium plates and incubated at 25 and 36°C. (B) Same as panel A. The same plasmids were introduced into strain ubcP4-140, and transformants were streaked and incubated at 25 and 36°C. (C) The indicated strains were incubated at 36°C for 6 h, and the amount of Cdc13 was examined. As a control, the amount of Cdc2 was also measured. WT, wild type.
The functions of both UbcP1 and UbcP4 are required for the activity of APC/CSte9.
The next question is whether these two ubiquitin-conjugating enzymes function with APC/C. To clarify this question, we examined the requirements of two ubiquitin-conjugating enzymes for the degradation of Cdc13 by overexpression of Ste9. Overexpression of Ste9 caused unregulated activation of APC/CSte9 and degradation of Cdc13 (4, 20). As shown in Fig. 6, in wild-type cells, Cdc13 disappeared at 18 to 21 h concomitantly with accumulation of Ste9. In contrast, Cdc13 remained at significant levels in ubcP1-P61S and ubcP4-140 mutant cells although Ste9 accumulated at the same rate as in wild-type cells. As a control, we examined the requirement of APC/C component Cut9 for the degradation of Cdc13 by overexpression of Ste9. Cdc13 remained at significant levels in cut9-665 mutant cells although Ste9 accumulated. These results suggest that the functions of both UbcP1 and UbcP4 are required for the activity of APC/CSte9.
FIG. 6.
The functions of both UbcP1 and UbcP4 are required for the activity of APC/CSte9. Ste9 was overexpressed from pREP1 in minimal medium containing appropriate supplements. At 15 h after induction of Ste9, cultures were shifted to 36°C. At the indicated times after induction of Ste9, SDS samples were prepared and analyzed by Western blotting with anti-Cdc13 serum. As a control, the amount of Cdc2 was also measured. Induction of Ste9 was also verified by anti-Ste9 antibody. WT, wild type; T, thiamine.
The two ubiquitin-conjugating enzymes have distinct functions in the ubiquitination of Cdc13.
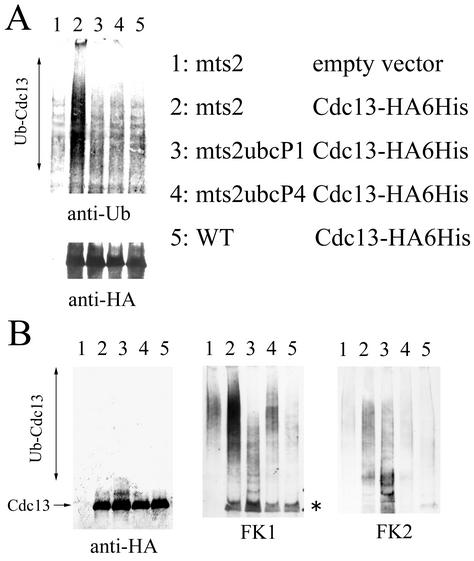
To assess the functional difference between these two ubiquitin-conjugating enzymes, we examined the in vivo ubiquitination level of Cdc13 in each mutant cell. For this purpose, we used an mts2-1 mutation in which ubiquitinated proteins were stable owing to the defective proteasome subunit (11). Cdc13-HA6His was expressed in mts2, mts2ubcP1-P61S, and mts2ubcP4-140 mutant cells and in wild-type cells. At 4 h after a shift to 36°C, Cdc13-HA6His was purified in a denatured condition. The purified proteins were analyzed by Western blotting (Fig. 7). When blotted with an antiubiquitin monoclonal antibody, the signal of ubiquitinated Cdc13 was extremely reduced in mts2ubcP1-P61S and mts2ubcP4-140 mutant cells compared with that in mts2 mutant cells (Fig. 7A). This result indicates that the number of ubiquitin molecules on Cdc13 decreases in mts2ubcP1-P61S and mts2ubcP4-140 mutant cells. Thus, UbcP1 and UbcP4 are involved in the ubiquitination of Cdc13 in vivo. When analyzed with an anti-HA antibody (Fig. 7B, left side), almost the same amount of Cdc13 was expressed; notably, in the mts2ubcP1-P61S mutant strain, a slowly migrating form of Cdc13 was more clearly detected than in other cells.
FIG. 7.
The two ubiquitin-conjugating enzymes have distinct functions in the ubiquitination of Cdc13. (A) Strains harboring pREP1-cdc13-HA6His were cultured in minimal medium without thiamine for 16 h at 25°C and shifted to 36°C. At 4 h after the shift to 36°C, cells were disrupted under denaturing conditions and Cdc13-HA6His was purified from extracts with Ni-NTA agarose. Released proteins were analyzed by Western blotting with antiubiquitin (anti-Ub) and anti-HA antibodies. WT, wild type. (B) Same as panel A. Samples were analyzed with the indicated antibodies. The asterisk indicates Cdc13 that was cross-reacted to by FK1.
To analyze the ubiquitinated state of Cdc13 in these mutants in more detail, the same samples were analyzed with monoclonal antibodies FK1 and FK2, each of which recognizes a specific structure of ubiquitinated proteins (9). FK1 was originally isolated as the monoclonal antibody that reacted to polyubiquitinated lysozyme but did not react to monoubiquitinated lysozyme and free ubiquitin molecules. Thus, this antibody has been thought to recognize the molecular structure of the isopeptide bond between a C terminus of the ubiquitin molecule and the lysine residue of another ubiquitin molecule (9). Thus, the intensity of the signal recognized by FK1 reflects the number and length of ubiquitin chains and reflects a multiply ubiquitinated state of target proteins. When the same samples were blotted with the FK1 antibody (Fig. 7B, middle), in mts2 mutant cells, multiubiquitinated proteins were detected at significant levels. In contrast, in mts2ubcP4-140 mutant cells, the multiubiquitinated protein signal was decreased to the level detected in mts2 mutant cells with the empty vector (compare lane 4 with lane 1 [mts2 empty vector]). We think that these background signals in lanes 1 and 4 are polyubiquitinated proteins that were nonspecifically purified by Ni-NTA beads. In mts2ubcP1-P61S mutant cells, the multiubiquitinated protein signal was significantly reduced. Interestingly, in this strain, the small molecular size of the ubiquitinated signal increased. The reason that the background signals were not seen in this lane is that multiubiquitinated proteins were totally eliminated from _ubcP1_-deficient cells (see Fig. 8). These results suggest that in _ubcP1_-deficient cells, the ubiquitin chains on Cdc13 are short; on the other hand, in _ubcP4_-deficient cells, ubiquitination of Cdc13 is totally defective. To address this idea, we used another monoclonal antibody, FK2 (Fig. 7B, right side). FK2 was isolated as a monoclonal antibody that detected both mono- and polyubiquitinated lysozymes but did not react to free ubiquitin molecules. As judged by the recognition pattern of this antibody, FK2 has been thought to recognize the structure around the isopeptide bond between the C terminus of a ubiquitin molecule and the lysine residue of the substrate (9). Thus, the intensity of the signal recognized by FK2 reflects only the number of ubiquitin chains formed on the substrate. The fast-migrating (low-molecular-weight) ubiquitinated proteins accumulated more in mts2ubcP1-P61S cells than in mts2 cells. On the other hand, ubiquitinated proteins were totally reduced in mts2ubcP4-140 cells. These results indicate that the two ubiquitin-conjugating enzymes have distinct functions in the ubiquitination of Cdc13.
FIG. 8.
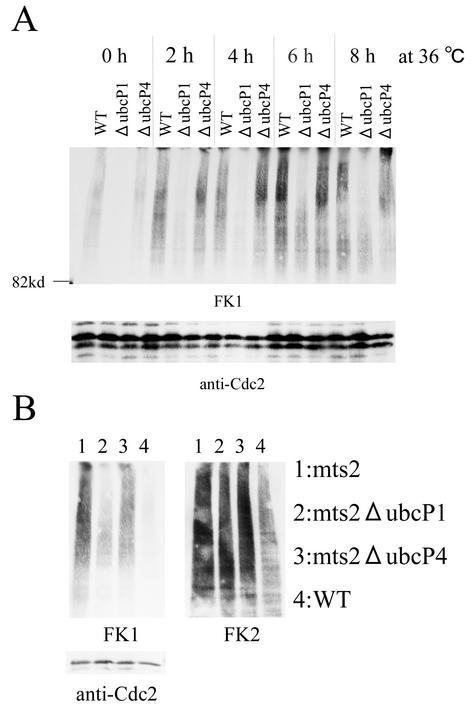
UbcP1 is required for elongation of ubiquitin chains on most ubiquitinated proteins. (A) Ubiquitin-conjugating enzymes were depleted in the indicated strains for 24 h in minimal medium with thiamine, and cultures were shifted to 36°C. At the times indicated after the shift to 36°C, total proteins were prepared from cells and analyzed by Western blotting with FK1. As a control, the amount of Cdc2 was also measured. (B) Same as panel A. Total protein samples were prepared at 6 h after the shift to 36°C and analyzed by Western blotting with FK1 and FK2. As a control, the amount of Cdc2 was also measured. WT, wild type.
To address the distinct roles of UbcP1 and UbcP4 further, we examined the ubiquitinated state in total protein samples (Fig. 8). Whole-cell extract was prepared from mts2 cells completely depleted of each ubiquitin-conjugating enzyme. As analyzed with FK1, the amount of multiubiquitinated proteins was reduced in UbcP1-depleted cells (Fig. 8A). In contrast, the number of ubiquitin chains detected by FK2 was not reduced in the same mutant cells (Fig. 8B). These results indicate that UbcP1 is required for the multiubiquitination of most target proteins and that in the absence of UbcP1, ubiquitin chains are formed but most of them are short. In UbcP4-depleted cells, the pattern of ubiquitinated proteins detected by FK1 and FK2 is almost the same as that in mts2 cells. Thus, these results suggest that, in contrast to UbcP1, UbcP4 is highly specific for certain proteins, including Cdc13, since the ubiquitination defect of a few proteins has no effect on the amount of total ubiquitinated proteins.
DISCUSSION
In a biochemical analysis of Xenopus and clam oocyte extracts, the ubiquitin-conjugating enzymes involved in the ubiquitination of mitotic cyclin were revealed to be UBC4 and UBCx/E2-C (2, 50). However, the functional difference and relationship between these two ubiquitin-conjugating enzymes have not been elucidated. This is the first report indicating that the homologues of these two ubiquitin-conjugating enzymes have distinct biological functions in the ubiquitination of mitotic cyclin.
We previously reported that ubcP4+ is essential for progression of mitosis (32). Cdc13 accumulates in UbcP4-depleted cells. Overexpression of UbcP4 partially rescues cut9-665, a temperature-sensitive mutation in one of the APC/C components (34). Cells harboring both the ubcP4-140 and cut9-665 mutations exhibit a synergistic effect on temperature sensitivity (our unpublished data). These results suggest a functional relationship between UbcP4 and APC/C. However, we did not show that UbcP4 is directly involved in the ubiquitination of Cdc13.
Here we show that ubcP1+ is also essential for progression of mitosis. Cdc13 is stabilized in UbcP1- or UbcP4-depleted cells arrested at the G1 phase. Cdc13 degradation mediated by APC/CSte9 activity requires the functions of these two ubiquitin-conjugating enzymes. The patterns of ubiquitination of Cdc13 are different in the mutant cells of these two ubiquitin-conjugating enzymes. These results suggest that UbcP1 and UbcP4 are directly involved in the ubiquitination of Cdc13 with APC/C in a nonredundant fashion in vivo.
We show that the activity of APC/CSte9 depends on the functions of UbcP1 and UbcP4. These results indicate that these two ubiquitin-conjugating enzymes function with APC/CSte9. However, Ste9/Srw1 functions as an activator of APC/C for Cdc13 ubiquitination during the G1 phase and its function is not required during the metaphase-anaphase transition (4, 20, 46, 47). On the other hand, Slp1 is thought to be an activator of APC/C for ubiquitination of Cdc13 during the mitotic transition (25). _slp1_-deficient cells exhibit abnormal mitosis, which resembles the phenotype of _ubcP1_- or _ubcP4_-deficient cells. We found that the functions of both UbcP1 and UbcP4 are required at mitosis in exponentially growing cells. Furthermore, we found that Cdc13 accumulated in exponentially growing asynchronous UbcP1-depleted (Fig. 2) or UbcP4-depleted (32) cells. Our findings suggest that these two ubiquitin-conjugating enzymes are also required for the ubiquitination of Cdc13 by the APC/CSlp1 pathway.
What, then, is the functional difference between UbcP1 and UbcP4 in Cdc13 degradation? In both _ubcP1_- and _ubcP4_-deficient cells, polyubiquitination and degradation of Cdc13 are defective. The ubiquitin chains on Cdc13 are short in _ubcP1_-deficient (and _ubcP4_-functional) cells. This low-molecular-weight ubiquitination is achieved by the UbcP4 pathway because ubiquitinated Cdc13 is totally reduced in UbcP4-deficient cells. A possible explanation for these results is that the polyubiquitination of Cdc13 is a two-step reaction; i.e., the UbcP4 pathway initiates ubiquitination of Cdc13, and the UbcP1-pathway elongates the short ubiquitin chains on Cdc13. This idea is consistent with the finding that UBC4 is more processive than UBCx for ubiquitination of mitotic cyclin in a biochemical analysis of Xenopus oocyte extract (40, 50). In Xenopus oocyte extract, two ubiquitin-conjugating enzymes, UBC4 and UBCx, were found to function with ubiquitin-activating enzyme E1 and APC/C for ubiquitination of the mitotic cyclin. Furthermore, a ubiquitination reaction involving UBC4 lengthens the ubiquitin chains on mitotic cyclin more than does the reaction involving UBCx. However, in this system, only one ubiquitin-conjugating enzyme, UBC4 or UBCx, is required for the ubiquitination of mitotic cyclin. In contrast, according to our findings, efficient polyubiquitination of mitotic cyclin requires both ubiquitin-conjugating enzymes, UbcP1 and UbcP4, at least in fission yeast. One possible mechanism is that Cdc13 is initially ubiquitinated by a complex containing APC/C and UbcP4, ubiquitinated Cdc13 is transferred to another complex containing APC/C and UbcP1, and ubiquitin chains are elongated. Another possibility is that APC/C, UbcP4, and UbcP1 exist in the same complex. Thus, the initiation and elongation reactions are achieved by the same complex by UbcP4 and UbcP1, respectively. Whether the two ubiquitin-conjugating enzymes exist in the same complex or not is unknown because we could not identify the interaction between the ubiquitin-conjugating enzymes and the APC/C component in vivo in fission yeast. We cannot rule out the possibility that UbcP1 interacts and functions with another ubiquitin ligase (for example, elongation E3). However, our results do not rule out the possibility that the two ubiquitin-conjugating enzymes cooperate in the ubiquitination of mitotic cyclin. Another possibility is that the localizations of these two ubiquitin-conjugating enzymes are different and that differently localized Cdc13 proteins differ in accessibility as well.
What is the biological significance of a two-step reaction model of the ubiquitination of mitotic cyclin? The timing of the degradation of mitotic cyclin is important for genomic stability. For example, unregulated inactivation of Cdc2 kinase in fission yeast causes overreplication (5, 12). In budding yeast, unregulated inactivation of Cdk kinase activity by overproduction of Sic1 and Rum1 also causes unregulated replication (35). In mammals, polyploidization of megakaryocytic cell lines is associated with reduced levels of cyclin B1 protein (52). Thus, tight regulation of mitotic cyclin degradation is required for the prevention of unregulated degradation of mitotic cyclin and overreplication of DNA.
In the case of securin protein Cut2, the timing of degradation is also important for accurate segregation of chromosomes (49). UbcP1 and UbcP4 may be required for the degradation of Cut2 because chromosome segregation is defective in both _ubcP1_- and _ubcP4_-deficient cells. It is important to examine whether the two ubiquitin-conjugating enzymes are also required in the ubiquitination of Cut2 as they are in the ubiquitination of Cdc13.
Budding yeast UBC4/5 and UBC11 are homologues of ubcP1+ and ubcP4+, respectively. UBC4/_5_- and/or _UBC11_-deficient strains of budding yeast did not have significant phenotypes. In these cells, mitotic cyclin Clb2 also does not stabilize (42). We suppose that this difference reflects the difference of functional redundancy of ubiquitin-conjugating enzymes between budding yeast and fission yeast. It is not clear whether other organisms, such as mammals, have adopted the functional redundancy of fission yeast. However, our results strongly suggest that research related to the functions of ubiquitin-conjugating enzymes in fission yeast is strongly advantageous for studying the ubiquitin-proteasome system.
Acknowledgments
We thank Sergio Moreno for providing the anti-Ste9 antibody, Frederique Gaits for the plasmid pINV1-spc1-HA6His, Colin Gordon for strain mts2, Mitsuhiro Yanagida for strain cut9-665, and Kenji Kitamura for strain cdc10ste9. We also thank Akira Ishihama, Susumu Hirose, Shigeo Hayashi, and Hitoshi Ueda for their discussion.
Part of this workk was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
REFERENCES
- 1.Alfa, C., P. Fantes, J. Hyman, M. Mcleod, and E. Warbrick. 1993. Experiments with fission yeast: a laboratory course manual Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 2.Aristarkhov, A., E. Eytan, A. Moghe, A. Admon, A. Hershko, and J. V. Ruderman. 1996. E2-C, a cyclin-selective ubiquitin carrier protein required for the destruction of mitotic cyclins. Proc. Natl. Acad. Sci. USA 93**:**4294-4299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bastians, H., L. M. Topper, G. L. Gorbsky, and J. V. Ruderman. 1999. Cell cycle-regulated proteolysis of mitotic target proteins. Mol. Biol. Cell 10**:**3927-3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blanco, M. A., Sanchez Diaz, A., J. M. de Prada, and S. Moreno. 2000. APCste9/srw1 promotes degradation of mitotic cyclins in G1 and is inhibited by cdc2 phosphorylation. EMBO J. 19**:**3945-3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Broek, D., R. Bartlett, K. Crawford, and P. Nurse. 1991. Involvement of p34cdc2 in establishing the dependency of S phase on mitosis. Nature 349**:**388-393. [DOI] [PubMed] [Google Scholar]
- 6.Ciechanover, A. 1998. The ubiquitin-proteasome pathway: on protein death and cell life. EMBO J. 17**:**7151-7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ciechanover, A., A. Orian, and A. L. Schwartz. 2000. Ubiquitin-mediated proteolysis: biological regulation via destruction. Bioessays 22**:**442-451. [DOI] [PubMed] [Google Scholar]
- 8.Ellison, K. S., T. Gwozd, J. A. Prendergast, M. C. Paterson, and M. J. Ellison. 1991. A site-directed approach for constructing temperature-sensitive ubiquitin-conjugating enzymes reveals a cell cycle function and growth function for RAD6. J. Biol. Chem. 266**:**24116-24120. [PubMed] [Google Scholar]
- 9.Fujimuro, M., H. Sawada, and H. Yokosawa. 1994. Production and characterization of monoclonal antibodies specific to multi-ubiquitin chains of polyubiquitinated proteins. FEBS Lett. 349**:**173-180. [DOI] [PubMed] [Google Scholar]
- 10.Gmachl, M., C. Gieffers, A. V. Podtelejnikov, M. Mann, and J. M. Peters. 2000. The RING-H2 finger protein APC11 and the E2 enzyme UBC4 are sufficient to ubiquitinate substrates of the anaphase-promoting complex. Proc. Natl. Acad. Sci. USA 97**:**8973-8978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gordon, C., G. McGurk, P. Dillon, C. Rosen, and N. D. Hastie. 1993. Defective mitosis due to a mutation in the gene for a fission yeast 26S protease subunit. Nature 366**:**355-357. [DOI] [PubMed] [Google Scholar]
- 12.Hayles, J., D. Fisher, A. Woollard, and P. Nurse. 1994. Temporal order of S phase and mitosis in fission yeast is determined by the state of the p34cdc2-mitotic B cyclin complex. Cell 78**:**813-822. [DOI] [PubMed] [Google Scholar]
- 13.Hershko, A., D. Ganoth, V. Sudakin, A. Dahan, L. H. Cohen, F. C. Luca, J. V. Ruderman, and E. Eytan. 1994. Components of a system that ligates cyclin to ubiquitin and their regulation by the protein kinase cdc2. J. Biol. Chem. 269**:**4940-4946. [PubMed] [Google Scholar]
- 14.Hilioti, Z., Y. S. Chung, Y. Mochizuki, C. F. Hardy, and O. Cohen Fix. 2001. The anaphase inhibitor Pds1 binds to the APC/C-associated protein Cdc20 in a destruction box-dependent manner. Curr. Biol. 11**:**1347-1352. [DOI] [PubMed] [Google Scholar]
- 15.Iacovoni, J. S., P. Russell, and F. Gaits. 1999. A new inducible protein expression system in fission yeast based on the glucose-repressed inv1 promoter. Gene 232**:**53-58. [DOI] [PubMed] [Google Scholar]
- 16.Irniger, S., S. Piatti, C. Michaelis, and K. Nasmyth. 1995. Genes involved in sister chromatid separation are needed for B-type cyclin proteolysis in budding yeast. Cell 81**:**269-278. [DOI] [PubMed] [Google Scholar]
- 17.Javerzat, J. P., G. Cranston, and R. C. Allshire. 1996. Fission yeast genes which disrupt mitotic chromosome segregation when overexpressed. Nucleic Acids Res. 24**:**4676-4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaiser, C., S. Michaelis, and A. Mitchel. 1994. Methods in yeast genetics, 1994 edition. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 19.King, R. W., J. M. Peters, S. Tugendreich, M. Rolfe, P. Hieter, and M. W. Kirschner. 1995. A 20S complex containing CDC27 and CDC16 catalyzes the mitosis-specific conjugation of ubiquitin to cyclin B. Cell 81**:**279-288. [DOI] [PubMed] [Google Scholar]
- 20.Kitamura, K., H. Maekawa, and C. Shimoda. 1998. Fission yeast Ste9, a homolog of Hct1/Cdh1 and Fizzy-related, is a novel negative regulator of cell cycle progression during G1 phase. Mol. Biol. Cell 9**:**1065-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kominami, K., H. Seth Smith, and T. Toda. 1998. Apc10 and Ste9/Srw1, two regulators of the APC-cyclosome, as well as the CDK inhibitor Rum1 are required for G1 cell-cycle arrest in fission yeast. EMBO J. 17**:**5388-5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kramer, E. R., C. Gieffers, G. Holzl, M. Hengstschlager, and J. M. Peters. 1998. Activation of the human anaphase-promoting complex by proteins of the CDC20/Fizzy family. Curr. Biol. 8**:**1207-1210. [DOI] [PubMed] [Google Scholar]
- 23.Leverson, J. D., C. A. Joazeiro, A. M. Page, H. Huang, P. Hieter, and T. Hunter. 2000. The APC11 RING-H2 finger mediates E2-dependent ubiquitination. Mol. Biol. Cell 11**:**2315-2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luca, F. C., and J. V. Ruderman. 1989. Control of programmed cyclin destruction in a cell-free system. J. Cell Biol. 109**:**1895-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsumoto, T. 1997. A fission yeast homolog of CDC20/p55CDC/Fizzy is required for recovery from DNA damage and genetically interacts with p34cdc2. Mol. Cell. Biol. 17**:**742-750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maundrell, K. 1993. Thiamine-repressible expression vectors pREP and pRIP for fission yeast. Gene 123**:**127-130. [DOI] [PubMed] [Google Scholar]
- 27.Mitsuzawa, H., H. Seino, F. Yamao, and A. Ishihama. 2001. Two WD repeat-containing TATA-binding protein-associated factors in fission yeast that suppress defects in the anaphase-promoting complex. J. Biol. Chem. 276**:**17117-17124. [DOI] [PubMed] [Google Scholar]
- 28.Murray, A. W. 1995. Cyclin ubiquitination: the destructive end of mitosis. Cell 81**:**149-152. [DOI] [PubMed] [Google Scholar]
- 29.Murray, A. W., M. J. Solomon, and M. W. Kirschner. 1989. The role of cyclin synthesis and degradation in the control of maturation promoting factor activity. Nature 339**:**280-286. [DOI] [PubMed] [Google Scholar]
- 30.Nurse, P. 1990. Universal control mechanism regulating onset of M phase. Nature 344**:**503-508. [DOI] [PubMed] [Google Scholar]
- 31.Nurse, P. 1994. Ordering S phase and M phase in the cell cycle. Cell 79**:**547-550. [DOI] [PubMed] [Google Scholar]
- 32.Osaka, F., H. Seino, T. Seno, and F. Yamao. 1997. A ubiquitin-conjugating enzyme in fission yeast that is essential for the onset of anaphase in mitosis. Mol. Cell. Biol. 17**:**3388-3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1990. Molecular cloning: a laboratory manual, second edition. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 34.Samejima, I., and M. Yanagida. 1994. Bypassing anaphase by fission yeast cut9 mutation: requirement of cut9+ to initiate anaphase. J. Cell Biol. 127**:**1655-1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sanchez Diaz, A., I. Gonzalez, M. Arellano, and S. Moreno. 1998. The Cdk inhibitors p25rum1 and p40SIC1 are functional homologues that play similar roles in the regulation of the cell cycle in fission and budding yeast. J. Cell Sci. 111**:**843-851. [DOI] [PubMed] [Google Scholar]
- 36.Schwab, M., A. S. Lutum, and W. Seufert. 1997. Yeast Hct1 is a regulator of Clb2 cyclin proteolysis. Cell 90**:**683-693. [DOI] [PubMed] [Google Scholar]
- 37.Schwab, M., M. Neutzner, D. Mocker, and W. Seufert. 2001. Yeast Hct1 recognizes the mitotic cyclin Clb2 and other substrates of the ubiquitin ligase APC. EMBO J. 20**:**5165-5175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sigrist, S. J., and C. F. Lehner. 1997. Drosophila fizzy-related down-regulates mitotic cyclins and is required for cell proliferation arrest and entry into endocycles. Cell 90**:**671-681. [DOI] [PubMed] [Google Scholar]
- 39.Sudakin, V., D. Ganoth, A. Dahan, H. Heller, J. Hershko, F. C. Luca, J. V. Ruderman, and A. Hershko. 1995. The cyclosome, a large complex containing cyclin-selective ubiquitin ligase activity, targets cyclins for destruction at the end of mitosis. Mol. Biol. Cell 6**:**185-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tang, Z., B. Li, R. Bharadwaj, H. Zhu, E. Ozkan, K. Hakala, J. Deisenhofer, and H. Yu. 2001. APC2 Cullin protein and APC11 RING protein comprise the minimal ubiquitin ligase module of the anaphase-promoting complex. Mol. Biol. Cell 12**:**3839-3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Townsley, F. M., A. Aristarkhov, S. Beck, A. Hershko, and J. V. Ruderman. 1997. Dominant-negative cyclin-selective ubiquitin carrier protein E2-C/UbcH10 blocks cells in metaphase. Proc. Natl. Acad. Sci. USA 94**:**2362-2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Townsley, F. M., and J. V. Ruderman. 1998. Functional analysis of the Saccharomyces cerevisiae UBC11 gene. Yeast 14**:**747-757. [DOI] [PubMed] [Google Scholar]
- 43.Tugendreich, S., J. Tomkiel, W. Earnshaw, and P. Hieter. 1995. CDC27Hs colocalizes with CDC16Hs to the centrosome and mitotic spindle and is essential for the metaphase to anaphase transition. Cell 81**:**261-268. [DOI] [PubMed] [Google Scholar]
- 44.Varshavsky, A. 1997. The ubiquitin system. Trends Biochem. Sci. 22**:**383-387. [DOI] [PubMed] [Google Scholar]
- 45.Visintin, R., S. Prinz, and A. Amon. 1997. CDC20 and CDH1: a family of substrate-specific activators of APC-dependent proteolysis. Science 278**:**460-463. [DOI] [PubMed] [Google Scholar]
- 46.Yamaguchi, S., H. Murakami, and H. Okayama. 1997. A WD repeat protein controls the cell cycle and differentiation by negatively regulating Cdc2/B-type cyclin complexes. Mol. Biol. Cell 8**:**2475-2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamaguchi, S., H. Okayama, and P. Nurse. 2000. Fission yeast Fizzy-related protein srw1p is a G1-specific promoter of mitotic cyclin B degradation. EMBO J. 19**:**3968-3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yamano, H., J. Gannon, and T. Hunt. 1996. The role of proteolysis in cell cycle progression in Schizosaccharomyces pombe. EMBO J. 15**:**5268-5279. [PMC free article] [PubMed] [Google Scholar]
- 49.Yanagida, M. 2000. Cell cycle mechanisms of sister chromatid separation; roles of Cut1/separin and Cut2/securin. Genes Cells 5**:**1-8. [DOI] [PubMed] [Google Scholar]
- 50.Yu, H., R. W. King, J. M. Peters, and M. W. Kirschner. 1996. Identification of a novel ubiquitin-conjugating enzyme involved in mitotic cyclin degradation. Curr. Biol. 6**:**455-466. [DOI] [PubMed] [Google Scholar]
- 51.Zachariae, W., and K. Nasmyth. 1999. Whose end is destruction: cell division and the anaphase-promoting complex. Genes Dev. 13**:**2039-2058. [DOI] [PubMed] [Google Scholar]
- 52.Zhang, Y., Z. Wang, D. X. Liu, M. Pagano, and K. Ravid. 1998. Ubiquitin-dependent degradation of cyclin B is accelerated in polyploid megakaryocytes. J. Biol. Chem. 273**:**1387-1392. [DOI] [PubMed] [Google Scholar]