MEK kinase 1 gene disruption alters cell migration and c-Jun NH2-terminal kinase regulation but does not cause a measurable defect in NF-κB activation (original) (raw)
Abstract
MEK kinase 1 (MEKK1) is a 196-kDa mitogen-activated protein kinase (MAPK) kinase kinase that, in addition to regulating the c-Jun NH2-terminal kinase (JNK) pathway, is involved in the control of cell motility. MEKK1−/− mice are defective in eyelid closure, a TGFα-directed process involving the migration of epithelial cells. MEKK1 expression in epithelial cells stimulates lamellipodia formation, a process required for cell movement. In addition, mouse embryo fibroblasts derived from MEKK1−/− mice are inhibited in their migration relative to MEKK1+/+ fibroblasts. MEKK1 is required for JNK but not NF-κB activation in response to virus infection, microtubule disruption, and stimulation of embryonic stem cells with lysophosphatidic acid. MEKK1 is not required for TNFα or IL-1 regulation of JNK or NF-κB activation in macrophages or fibroblasts. Thus, MEKK1 senses microtubule integrity, contributes to the regulation of fibroblast and epithelial cell migration, and is required for activation of JNK but not NF-κB in response to selected stress stimuli.
Keywords: MEKK1, cell motility, JNK
Mitogen-activated protein kinase kinase kinase (MEKK1) is a 196-kDa serine-threonine kinase that is activated in response to cytokines such as epidermal growth factor (EGF) in epithelial cells (1), chemoattractants like formyl-Met-Leu-Phe in neutrophils (2), and Ag ligation of the high affinity Ig E receptor FcɛR1 in mast cells (3). MEKK1 preferentially activates the c-Jun NH2-terminal kinase (JNK) pathway and influences the activity of the extracellular signal response kinase (ERK) pathway but has little or no effect on the p38 mitogen-activated protein kinase (MAPK) pathway (4). MEKK1 has also been shown to be a transducer for the regulation of JNK activity during changes in microtubule integrity (5, 6). Microtubule-disrupting drugs, such as nocodazole and taxol, activate MEKK1, which promotes a cell survival response during changes in the cytoskeleton induced by these drugs (5, 6).
In addition to the regulation of MAPK pathways, MEKK1 has been proposed to regulate NF-κB activation (7–11). Transfection experiments have shown that MEKK1 activates NF-κB by inducing IκB degradation (7). MEKK1 has also been shown to phosphorylate and activate IKKα and -β (8–10), the kinases responsible for phosphorylating IκB, leading to its degradation. Loss of IκB allows NF-κB to translocate to the nucleus and regulate transcription. However, the role of MEKK1 in the physiological regulation of NF-κB activation is controversial (12).
We have defined the role of MEKK1 in selectively regulating the JNK pathway by using targeted gene disruption (5, 6). In addition to defining the role of MEKK1 in the regulation of MAPK pathways, MEKK1−/− cells provide the necessary cell system to define its requirement in other regulatory processes. In this report, we show that MEKK1 is involved in the control of epithelial cell and fibroblast motility. We further demonstrate the requirement of MEKK1 in the regulation of the JNK pathway but not in regulating NF-κB in cells in response to specific cytokines and stress stimuli
Methods
Generation of MEKK1 Knockout Mice.
MEKK1−/− ES cells have been characterized previously (5). Chimeric mice were generated by injection of C57BL/6 blastocysts with MEKK1+/− ES cells. MEKK1−/− mice were generated by crossing MEKK1+/− males and females. MEKK1−/− mice were identified by Southern blotting. MEKK1−/− mice represented the predicted 20%–25% of litter pups. MEKK1−/− mice are now 12–14 months old with growth and fertility characteristics similar to wild-type mice. Mouse embryo fibroblasts were generated from wild type and MEKK1−/− day 14 embryos.
Kinase, Electrophoretic Mobility Shift and Reporter Gene Assays.
JNK, ERK, and p38 kinase assays were performed as described previously (1, 4–6). Electrophoretic mobility shift assays (EMSAs) were performed using the consensus κB and NF-1 sequences (13). Nuclear extracts (8 μg for ES cells and 2 μg for macrophages and fibroblasts) were incubated with 10 fmol of radiolabeled probe and 1 μg of poly(dI-dC) in buffer containing 10 mM Tris⋅HCl (pH 7.5)/100 mM KCl/1 mM EDTA/1 mM DTT/10% glycerol. Bands were verified as NF-κB by supershift assays using anti-p65 or anti-p50 Abs. NF-κB–luciferase assays were performed by transfecting the reporter plasmid into ES cells or mouse embryo fibroblasts 24 h before manipulation of the cells (14).
Fibroblast Motility Assay.
MEKK1−/− and wild-type day 14 mouse embryonic fibroblasts were cultured in DMEM supplemented with 10% FCS. Four hours before the initiation of migration experiments, approximately 107 cells were plated on ΔT culture dishes (Bioptechs, Butler, PA). Immediately before the experiment, cells were switched to medium supplemented with 20 mM Hepes (pH 7.4) that had been precharged with CO2. Fibroblast migration paths were determined by tracking single cells for 18 h. Cells were observed by using a Leica DMRXA microscope with a ×10 water immersion objective. A 37°C environment was maintained by the use of a Bioptechs ΔTC3 heated stage. Visual data were acquired every 15 min by using a SensiCam charge-coupled device (CCD) (Cooke Corporation, Tonawanda, NY) controlled by slidebook software (Intelligent Imaging, Denver, CO). Cell centroid data were obtained by using National Institutes of Health image software.
Production of ES Cell-Derived Macrophages.
MEKK1−/− and MEKK1+/+ ES cells were suspended and grown as embryoid bodies for 6 days. Embryoid bodies were harvested and dissociated by trypsinization. Cells (1 × 106/ml) were cultured in macrophage differentiation medium containing Iscove's modified Dulbecco medium (IMDM), 0.15 mM monothioglycerol, 10% FCS, 10% L-cell-conditioned medium containing macrophage colony-stimulating factor (M-CSF), and 1 ng/ml IL-3 and cultured for an additional 48 h in macrophage differentiation medium. Cells were collected and cultured in macrophage differentiation medium in the absence of IL-3. Macrophages began to adhere to plastic substratum in 2–3 days of culture in differentiation medium without IL-3. Cells were fed every 2 days and characterized after 7–10 days by colony morphology and expression of macrophage markers including F4/80 and Mac-1 (CD11b/CD18). Macrophages were used for experiments 2–3 wk after initiation of the differentiation program.
Characterization of Hematopoietic Cells from MEKK1−/− Mice.
Cell surface immunofluorescence staining analyzed by flow cytometry was performed on cells isolated from lymph node, spleen, bone marrow, and peripheral blood of 8-wk-old MEKK1−/−, MEKK1+/− and wild-type mice. Staining was performed with FITC-labeled H57.597 mAB to the T cell receptor, FITC-labeled mAB to the pan B cell marker B220, phycoerythrin (PE)-labeled anti-CD4, and PE-labeled anti-CD8 mABs. Representation of hematopoietic cell types in the bone marrow of MEKK1−/− vs. wild-type animals was also analyzed. Bone marrow cells were flushed from the femur with PBS, washed, and resuspended at 107 cells/ml. Smear preparations on glass slides were stained by May-Giemsa method and morphologically examined. Three hundred bone marrow cells were counted each from three MEKK1−/− mice and one MEKK1+/+ mouse. To measure the lipopolysaccharide (LPS)-induced lung inflammatory response, mice were anaesthetized with Avertin and 300 ng O111:B4 LPS in 50 μl saline solution instilled into the lungs through a 22-gauge catheter inserted into the trachea to just above the carina to initiate the inflammatory response. After 4 h, the animals were killed with Nembutal, and the lungs were lavaged four times with 1 ml of ice cold saline containing 0.1% heparin. The numbers and composition of cells in the lavage were analyzed by direct counting after cytospin. After centrifugation to remove the cells, lavage fluids were analyzed for cytokines. ELISAs were performed by using commercially available kits for TNFα and macrophage inflammatory protein (MIP)-2, according to the manufacturer's (R&D Systems) instructions (15).
Results
MEKK1 Regulates the Motility of Epithelial Cells and Fibroblasts.
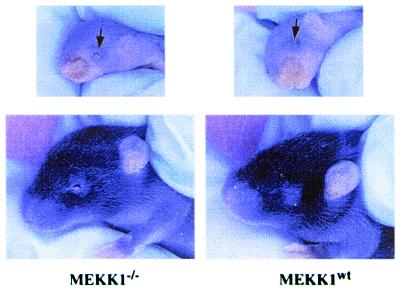
Mice that are homozygous null for the MEKK1 gene (MEKK1−/−) have open eyes at birth (Fig. 1). Eyelid fusion occurs normally between E15.5 and E16.5 of mouse embryonic development. Eyelid closure has been shown to involve the migration of periderm-derived epithelial cells over the surface of the cornea (16). The eyelid closure involves an epithelial cell migration that is regulated by transforming growth factor α (TGFα) and the α5β1 integrin extracellular matrix receptor. Targeted disruption of both TGFα alleles mimics the MEKK1 knockout in which mice are born with open eyes (17, 18). A similar open eye phenotype is observed in mice in which a transgenic α5β1 integrin under the control of the involucrin promoter is misexpressed in differentiating keratinocytes (19, 20). No other overt phenotypic change is observed in MEKK1-null mice. MEKK1−/− mice are similar to wild-type littermates in size and fertility.
Figure 1.
Failure of eyelid closure in MEKK1−/− mice. Mice that are MEKK1−/− are born with open eyelids. Wild-type littermates are born with closed eyes that remain closed for approximately 2 wk after birth. Two MEKK1−/− and two wild-type pups from different litters are shown. The open eyelid phenotype has a 95% penetrance in MEKK1−/− animals.
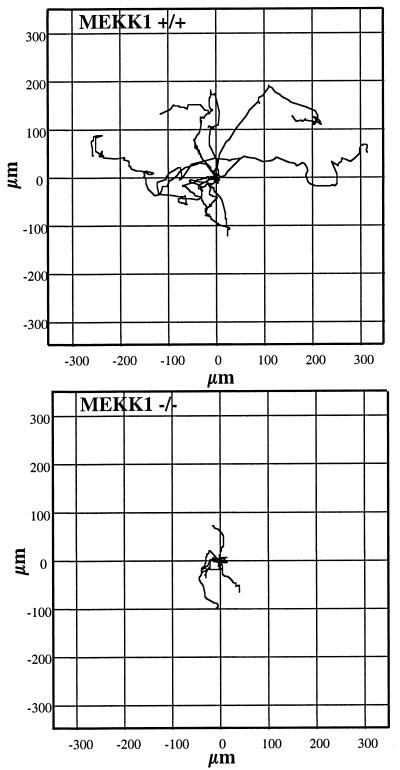
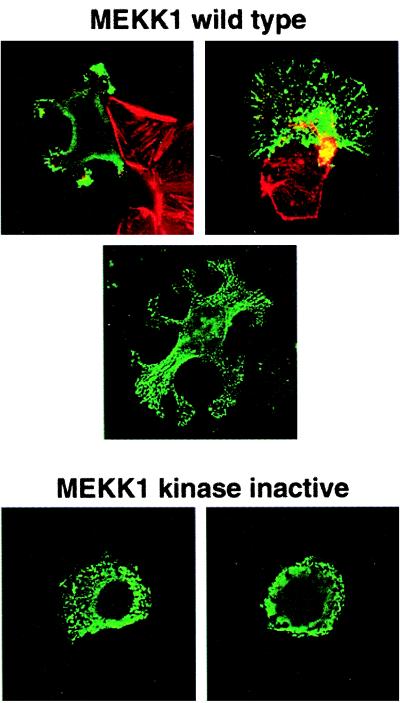
The failure of eyelid closure in MEKK1−/− mice is characteristic of a defect in cell migration. Migration of eyelid epithelial cells is difficult to quantitate in the animal. The most definitive studies have used transgenic animals with a β-galactosidase reporter gene regulated by the involucrin promoter to monitor cell migration during eyelid closure (19). As an alternative, we analyzed the migration properties of primary fibroblasts isolated from MEKK1−/− and wild-type 14-day embryos. MEKK1−/− and MEKK1+/+ mouse embryo fibroblasts were monitored for their motility in the presence of 5% serum (Fig. 2). Single cells were tracked for 18 h with visual data acquired every 15 min. The plots in Fig. 2 show the tracks for nine cells of each phenotype, with their origins superimposed at 0,0 in the X and Y coordinates. The results clearly demonstrate that MEKK1−/− cells are defective in cell motility. This result with mouse embryo fibroblasts is consistent with the failure of eyelid closure in MEKK1-null mice, a phenotype that has been characterized to involve defective epithelial cell migration (19, 20). When overexpressed in epithelial cells, MEKK1 induces large lamellipodia-like structures (Fig. 3). Lamellipodia are involved in cell migration (21), and their production is stimulated by the low molecular weight GTP-binding protein, Rac (22). MEKK1 binds Rac1 in a GTP-dependent manner consistent with MEKK1 contributing to Rac-mediated lamellipodia formation and cell motility. However, the defect in cell motility observed in MEKK1−/− cells is not global in MEKK1-null animals because the mice are apparently developmentally normal except for a loss of eyelid closure. There appears to be a significant redundancy in the pathways that control cell motility. The eyelid closure involves a single layer of cells whose migration is extremely sensitive to disruption of EGF receptor signaling and is easily observed in the MEKK1−/− mouse. To observe the defect in fibroblast migration cells had to be studied in vitro. The mechanism by which MEKK1 influences cell motility is currently unclear. Unlike ES cells, MEKK1−/− mouse embryonic fibroblasts respond to serum with activation of JNK (not shown); therefore, it is unlikely that JNK is involved in the MEKK1 control of cell motility. MEKK1−/− cells are, however, morphologically different from wild-type MEKK1 cells isolated from embryos of identical age (not shown). Wild-type fibroblasts in serum show a polarized morphology, generally indicative of cell movement. MEKK1−/− fibroblasts have a more isoformly spread morphology, without a defined cell front. Fluorescent staining of actin fibers and focal adhesions did not show a difference in their content or distribution between MEKK1-null and wild-type cells. The kinase activity of MEKK1 was required for the morphological changes observed in the transfection studies (Fig. 3), and the morphological differences observed in MEKK1−/− fibroblasts is probably related to loss of MEKK1 kinase activity. Substrates for MEKK1 involved in cell migration are not identified, but MEKK1 has been reported to localize to both actin fibers and focal adhesions (23). The findings are consistent with MEKK1 activation contributing to the regulation of cell movement that might involve the formation of a leading edge and/or adherence of fibroblasts.
Figure 2.
Loss of MEKK1 expression inhibits mouse embryonic fibroblast migratory behavior in the presence of 5% serum. Each plot presents representative cell tracks for nine cells, with the origins of migration superimposed at 0,0 for clarity of presentation. Tracks were acquired over 18 h at 15-min intervals.
Figure 3.
Human T47D breast adenocarcinoma cells were transfected with pcDNA3 encoding full length MEKK1 or kinase inactive MEKK1, which were tagged at their NH2 terminus with the hemagglutinin (HA) epitope. Cells were stained with anti-HA mouse mAb followed by FITC-labeled rabbit anti-mouse Ab. (Top) Cells were costained with rhodamine-phalloidin for labeling of the actin cytoskeleton and anti-HA for MEKK1. (Middle) A T47D cell with dramatic lamellipodia-like structures stained only for HA-MEKK1. This cell shows the association of transfected MEKK1 with cytoskeletal structures. (Bottom) Two representative cells stained for kinase-inactive HA-MEKK1. The formation of lamellipodia-like structures was observed in greater than 65% of wild-type MEKK1-transfected cells and less than 5% of cells expressing kinase-inactive MEKK1. One hundred transfected cells expressing wild-type and 100 cells expressing kinase-inactive MEKK1 were scored for lamellipodia-like structures.
MEKK1 Regulates JNK but Not NF-κB Activation.
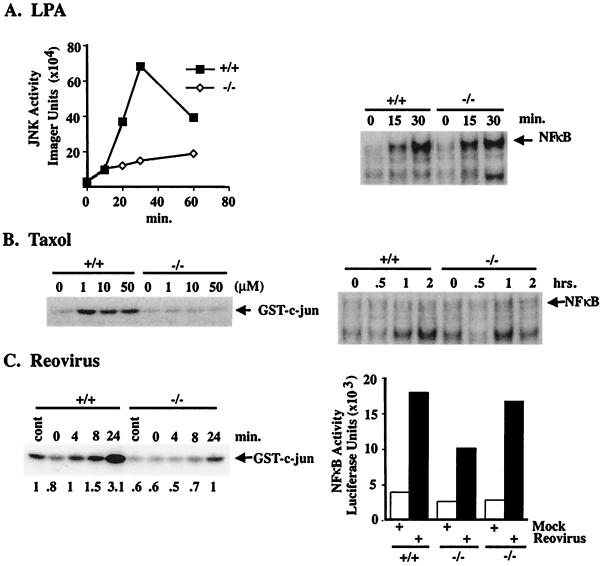
Findings shown in Fig. 4 demonstrate that MEKK1 expression in ES cells is required for activation of the JNK pathway in response to lysophosphatidic acid (LPA), taxol-induced microtubule disruption, and virus infection. With each stimulus, the MEKK1−/− cells have a diminished or absent JNK response, compared with MEKK1+/+ cells. The regulation of NF-κB activation is markedly different in response to these stimuli. LPA and infection of cells with reovirus activates NF-κB in MEKK1−/− and wild-type cells (Fig. 4 A and C, respectively). Taxol does not measurably activate NF-κB assayed by EMSA (Fig. 4B) or by NF-κB promoter luciferase assay (not shown) in either wild-type or MEKK1−/− ES cells. Thus, there is discordance in the regulation of MEKK1 and NF-κB. Two stimuli, LPA and reovirus infection, required MEKK1 for JNK activation but had normal NF-κB stimulation in the absence of MEKK1 expression. MEKK1 is activated in response to taxol (5, 6), and taxol-stimulated JNK activation in ES cells requires MEKK1. However, taxol does not stimulate NF-κB in ES cells. This result demonstrates that MEKK1 activation is dissociated from NF-κB activation in taxol treated ES cells. Cumulatively, the findings indicate that MEKK1 is selectively involved in JNK activation under conditions where it is not required for NF-κB activation.
Figure 4.
JNK and NF-κB regulation in MEKK1−/− and wild-type ES cells. (A) Cells were removed from serum for 5 h and then challenged with 10 μM LPA for the indicated times. JNK activity was assayed by using glutathione _S_-transferase (GST)-c-Jun as substrate by in vitro kinase assay. NF-κB was assayed by EMSA. (B) Cells were challenged with different concentrations of taxol for 30 min for JNK assay and with 10 μM taxol for the indicated times for NF-κB assay. (C) Cells were infected with the Abney strain of reovirus (multiplicity of infection = 4) or mock infected in the absence of virus. At the indicated times after infection, cells were assayed for JNK or NK-κB activity. JNK activity was quantitated by PhosphorImager (Molecular Dynamics) analysis, with control activity given a value of 1.0 for the MEKK1+/+ cells (shown as numbers under the autoradiograph). NF-κB activity was assayed by electroporation of an NF-κB-regulated luciferase reporter gene 24 h before virus infection. Two independent MEKK1−/− ES clones are shown for the NF-κB assay after reovirus infection. Each experiment is representative of two to four independent experiments for each stimulus.
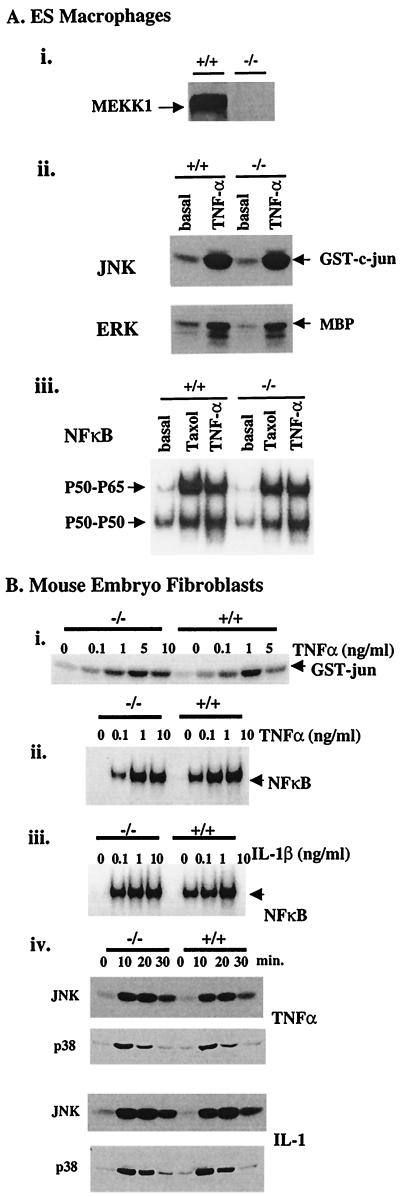
ES cells do not respond to cytokines such as TNFα. Therefore, to further define the requirement of MEKK1 in cytokine signaling, macrophages were derived by in vitro differentiation of MEKK1−/− and MEKK1+/+ ES cells (Fig. 5A). Macrophages were challenged with TNFα and assayed for JNK and ERK activity. TNFα stimulation of JNK and ERK are similar in MEKK1−/− and MEKK1+/+ macrophages. Thus, MEKK1 is not required for JNK or ERK activation in macrophages challenged with TNFα. Similarly, the activation of NF-κB is similar in TNFα-stimulated macrophages independent of MEKK1 expression status. Unlike ES cells, NF-κB is activated in macrophages in response to taxol treatment. Fig. 5A, panel iii, shows that NF-κB activation in response to taxol is similar in MEKK1−/− and MEKK1+/+ macrophages. There is no apparent requirement for MEKK1 in the regulation of NF-κB activity in macrophage responses to either TNFα or taxol.
Figure 5.
JNK and NF-κB are regulated by TNFα and IL-1β similarly in MEKK1+/+ and MEKK1−/− macrophages and fibroblasts. (A) ES-derived macrophages. (i) Immunoblot of MEKK1 protein expression in MEKK1−/− and MEKK1+/+ macrophages. (ii) JNK and ERK activation in response to a 10-min exposure of ES cell-derived macrophages to 10 ng/ml TNFα. JNK activity was measured by using GST-c-Jun as substrate, and ERK was assayed after immunoprecipitation using myelin basic protein (MBP) as substrate in in vitro kinase assays. (iii) EMSA analysis of NF-κB activation was determined after exposure of macrophages to 5 μM taxol for 2 h or 10 ng/ml TNFα for 30 min. (B) Mouse embryo fibroblasts. (i) Dose-response curve for TNFα activation of JNK activity in MEKK1−/− and MEKK1+/+ fibroblasts. JNK was assayed in cell lysates prepared after a 20-min exposure of cells to TNFα. (ii and iii) NF-κB activity after a 30-min exposure of MEKK1−/− and MEKK1+/+ cells to the indicated concentrations of TNFα or IL-1β, respectively. (iv) Time course of JNK and p38 activation in response to 10 ng/ml TNFα or 10 ng/ml IL-1β. JNK activity was assayed by using GST-c-Jun as substrate. p38 activity was assayed after SDS/PAGE of cell lysates by immunoblotting with an anti-phospho-p38 Ab. The results are representative of two to five independent experiments for both macrophages and fibroblasts.
Fig. 5B shows the response of MEKK1−/− and MEKK1+/+ mouse embryo fibroblasts to TNFα and IL-1. Stimulation of JNK, p38, and NF-κB is similar in MEKK1−/− and wild-type fibroblasts in response to both TNFα and IL-1. Similarly, the IL-1 stimulation of JNK and p38 activity is similar in the MEKK1−/− and wild-type fibroblasts. Thus, the loss of MEKK1 expression does not influence the TNFα or IL-1 regulation of JNK, p38, or NF-κB activity in macrophages and fibroblasts.
Immune Cell Differentiation Appears Normal in MEKK1−/− Cells.
JNK is known to influence the differentiation of T cells (24). Therefore, an analysis of bone marrow-derived cells was performed for MEKK1−/−, MEKK1+/−, and wild-type mice (not shown). The number of T cell receptor-positive cells, CD4+ and CD8+ T cells, B220+ B cells, neutrophils, monocytes, and erythroid series cells was similar in MEKK1−/−, MEKK1+/−, and MEKK1+/+ cells, demonstrating that hematopoietic cell differentiation is not significantly altered in MEKK1-null mice. To further define the phenotype of MEKK1−/− cells, an LPS-stimulated inflammatory response was characterized for MEKK1−/− and wild-type mice. Bacterial LPS stimulates a strong inflammatory response that activates both JNK and NF-κB in several cell types, including monocytes and neutrophils (2, 25). The lungs of MEKK1−/− and wild-type mice were instilled with 300 ng of LPS to induce an inflammatory response that recruits stimulated neutrophils and monocytes to the lung. Four hours after LPS instillation, the levels of two cytokines, MIP-2 and TNFα, were measured in the bronchial alveolar lavage fluid. Infiltrating monocytes produce both MIP-2 and TNFα. The LPS stimulation of MIP-2 and TNFα production in the lungs of MEKK1−/− and wild-type mice was similar (not shown). The results show that the production of two cytokines produced by monocytes that have infiltrated the lung in response to LPS does not require MEKK1 expression. The promoters controlling MIP-2 and TNFα gene expression both contain AP-1 and NF-κB regulatory elements (25) but do not require MEKK1 for regulation of their expression.
Discussion
As characterized to date, the MEKK1-null mouse is phenotypically normal except for the failure of eyelid closure. Eyelid closure has been previously demonstrated to involve a population of cells derived from the periderm that is the outermost layer of the developing epidermis. Eyelid tip cells migrate from the margins of the eyelid to cover the corneal epithelium. The targeted disruption of the EGF receptor and TGFα has been shown to cause failure of eyelid closure (17, 18, 26, 27). TGFα is expressed in the advancing eyelid tip epithelium and appears to be involved in controlling migration of these cells (28). MEKK1 is activated in response to EGF receptor stimulation (1). Our results are consistent with MEKK1 being in the signaling pathway required for EGF receptor control of eyelid tip cell migration.
To directly demonstrate a role for MEKK1 in cell migration, fibroblasts were characterized in vitro. MEKK1−/− mouse embryo fibroblasts are significantly impaired in migration relative to wild-type fibroblasts. A rather subtle morphological difference is also seen in MEKK1−/− fibroblasts relative to MEKK1+/+ fibroblasts. The MEKK1−/− cells have a flattened “pancake”-like morphology whereas the wild-type cells have a more fusiform morphology, with a characteristic leading edge and tail. It is likely that MEKK1 signaling is involved in regulating this morphological feature of mouse embryo fibroblasts. MEKK1 overexpression stimulates pronounced lamellipodia-like structures in a kinase-dependent mechanism, a morphological characteristic that is diminished in MEKK1−/− fibroblasts. The findings are consistent with the observations of Christerson et al. (23) that MEKK1 interacts with α-actinin, actin stress fibers and can be localized in focal adhesions, suggesting a role for MEKK1 in adhesion and/or migration. Because MEKK1-null mice are visibly normal except for an eyelid closure defect, it is obvious that most regulatory processes involving cell migration are compensated for in MEKK1−/− animals. The pathological consequence of the MEKK1-null phenotype is postnatal inflammation of the eye and blindness. These ocular pathologies generally occur in mice with failure of prenatal eyelid closure (17, 18, 20).
Our findings also begin to address the controversial role of MEKK1 in the regulation of JNK and NF-κB pathways. The loss of MEKK1 expression clearly can cause the loss of JNK activation in response to a specific stress stimulus in specific cell types. In no case where JNK activation was deficient in MEKK1−/− cells was there a corresponding loss of NF-κB activation. In ES cells, there is a clear dissociation of MEKK1 regulation of the JNK pathway and the control of NF-κB. In the ES cell response to taxol, MEKK1 and JNK are activated, but NF-κB is not. This finding indicates that there are conditions in specific cell types where MEKK1 is activated without measurable NF-κB activation. It is possible that taxol poisoning of microtubules uncouples MEKK1 from NF-κB but not JNK activation in mouse ES cells. We also did not find a dependence of MEKK1 for NF-κB activation in response to TNFα in macrophages or TNFα and IL-1 stimulation of fibroblasts. These results conflict with the finding of others using transfection and overexpression of wild-type and kinase-inactive MEKK1. In MEKK1 overexpression studies using transient transfection, it is clear that activated MEKK1 can stimulate IKKα and -β (7–11). Furthermore, kinase-inactive MEKK1 can suppress NF-κB activation. We have searched for a stimulus, including cytokines and multiple stress insults, to find an NF-κB response that depends on MEKK1. MEKK1−/− cells provide the appropriate model for this analysis. Whereas we can clearly demonstrate in specific cell types the role of MEKK1 in JNK activation and cell motility in response to specific stimuli, we have failed to find a stimulus that requires MEKK1 for NF-κB activation. Thus, if MEKK1 is physiologically capable of regulating NF-κB activation, it is neither the dominant kinase nor required for NF-κB activation in response to TNFα, IL-1, taxol, LPA, or virus infection in the three cell types we have screened.
Our studies have indicated that MEKK1 regulation has three primary characteristics. First, MEKK1 is activated by changes in cell shape and microtubule integrity. Second, MEKK1 can be involved in the control of cell motility. Third, MEKK1 regulates the JNK pathway and can influence the activity of the ERK pathway. We propose that a primary function of MEKK1 is to sense cell changes that are reflected in the architecture of the cytoskeleton. The three functions clearly defined for the full-length 196-kDa MEKK1 protein defined by MEKK1 gene disruption are: control of JNK activation in response to specific stimuli in specific cell types (5), suppression of apoptosis in response to microtubule-disrupting drugs (5, 6), and control of cell motility. The last two functions, cell survival after microtubule poisoning and cell motility, are most likely overlapping regulatory functions of MEKK1. The requirement of MEKK1 for JNK activation is variable in different cell types in response to a common stimulus, indicating the potential of redundant functions for different MAPK kinase kinases. A similar scenario is probably true for the regulation of NF-κB, where multiple kinases and/or other regulatory mechanisms are able to regulate the activation of IKKs.
Acknowledgments
This work was supported by National Institutes of Health Grants DK37871, DK48845, and GM30324.
Abbreviations
MAPK
mitogen-activated protein kinase
MEK
MAPK kinase
MEKK
MEK kinase
JNK
c-Jun NH2-terminal kinase
EGF
epidermal growth factor
ERK
extracellular signal response kinase
EMSA
electrophoretic mobility-shift assay
MIP
macrophage inflammatory protein
TGF
transforming growth factor
ES
embryonic stem
LPS
lipopolysaccharide
LPA
lysophosphatidic acid
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.130176697.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.130176697
References
- 1.Fanger G R, Johnson N L, Johnson G L. EMBO J. 1997;16:4961–4972. doi: 10.1093/emboj/16.16.4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Avdi N J, Winston B W, Russel M, Young S K, Johnson G L, Worthen G S. J Biol Chem. 1996;271:33598–606. doi: 10.1074/jbc.271.52.33598. [DOI] [PubMed] [Google Scholar]
- 3.Ishizuka T, Oshiba A, Sakata N, Terada N, Johnson G L, Gelfand E W. J Biol Chem. 1996;271:12762–12766. doi: 10.1074/jbc.271.22.12762. [DOI] [PubMed] [Google Scholar]
- 4.Lange-Carter C A, Pleiman C M, Gardner A M, Blumer K J, Johnson G L. Science. 1993;260:315–319. doi: 10.1126/science.8385802. [DOI] [PubMed] [Google Scholar]
- 5.Yujiri T, Sather S, Fanger G R, Johnson G L. Science. 1998;282:1911–1914. doi: 10.1126/science.282.5395.1911. [DOI] [PubMed] [Google Scholar]
- 6.Yujiri T, Fanger G R, Garrington T P, Schlesinger T K, Gibson S, Johnson G L. J Biol Chem. 1999;274:12605–12610. doi: 10.1074/jbc.274.18.12605. [DOI] [PubMed] [Google Scholar]
- 7.Hirano M, Osada S, Aoki T, Hirai S, Hosaka M, Inoue J, Ohno S. J Biol Chem. 1996;271:13234–13238. doi: 10.1074/jbc.271.22.13234. [DOI] [PubMed] [Google Scholar]
- 8.Lee F S, Hagler J, Chen Z J, Maniatis T. Cell. 1997;88:213–222. doi: 10.1016/s0092-8674(00)81842-5. [DOI] [PubMed] [Google Scholar]
- 9.Lee F S, Peters R T, Dang L C, Maniatis T. Proc Natl Acad Sci USA. 1998;95:9319–9324. doi: 10.1073/pnas.95.16.9319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nemoto S, DiDonato J A, Lin A. Mol Cell Biol. 1998;18:7336–7343. doi: 10.1128/mcb.18.12.7336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meyer C F, Wang X, Chang C, Templeton D, Tan T H. J Biol Chem. 1996;271:8971–8976. doi: 10.1074/jbc.271.15.8971. [DOI] [PubMed] [Google Scholar]
- 12.Karin M, Delhase M. Proc Natl Acad Sci USA. 1998;95:9067–9069. doi: 10.1073/pnas.95.16.9067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dobrzanski P, Ryseck R P, Bravo R. Mol Cell Biol. 1993;13:1572–1582. doi: 10.1128/mcb.13.3.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brasier A R, Ron D, Tate J E, Habener J F. Mol Endocrinol. 1990;4:1921–1933. doi: 10.1210/mend-4-12-1921. [DOI] [PubMed] [Google Scholar]
- 15.Worthen G S, Haslett C, Smedly L A, Rees A J, Gumbay R S, Henson J E, Henson P M. Fed Proc. 1986;45:7–12. [PubMed] [Google Scholar]
- 16.Findlater G S, McDougall R D, Kaufman M H. J Anat. 1993;183:121–129. [PMC free article] [PubMed] [Google Scholar]
- 17.Luetteke N C, Qiu T H, Peiffer R L, Oliver P, Smithies O, Lee D C. Cell. 1993;73:263–278. doi: 10.1016/0092-8674(93)90228-i. [DOI] [PubMed] [Google Scholar]
- 18.Mann G B, Fowler K J, Gabriel A, Nice E C, Williams R L, Dunn A R. Cell. 1993;73:249–261. doi: 10.1016/0092-8674(93)90227-h. [DOI] [PubMed] [Google Scholar]
- 19.Carroll J M, Luetteke N C, Lee D C, Watt F M. Mech Dev. 1998;78:37–45. doi: 10.1016/s0925-4773(98)00145-2. [DOI] [PubMed] [Google Scholar]
- 20.Carroll J M, Romero M R, Watt F M. Cell. 1995;83:957–968. doi: 10.1016/0092-8674(95)90211-2. [DOI] [PubMed] [Google Scholar]
- 21.Jones G E, Allen W E, Ridley A J. Cell Adhes Commun. 1998;6:237–245. doi: 10.3109/15419069809004479. [DOI] [PubMed] [Google Scholar]
- 22.Nobes C D, Hall A. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- 23.Christerson L B, Vanderbilt C A, Cobb M H. Cell Motil Cytoskeleton. 1999;43:186–198. doi: 10.1002/(SICI)1097-0169(1999)43:3<186::AID-CM2>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 24.Yang D D, Conze D, Whitmarsh A J, Barrett T, Davis R J, Rincon M, Flavell R A. Immunity. 1998;9:575–585. doi: 10.1016/s1074-7613(00)80640-8. [DOI] [PubMed] [Google Scholar]
- 25.Parry G C, Mackman N. J Biol Chem. 1994;269:20823–20825. [PubMed] [Google Scholar]
- 26.Threadgill D W, Dlugosz A A, Hansen L A, Tennenbaum T, Lichti U, Yee D, LaMantia C, Mourton T, Herrup K, Harris R C, et al. Science. 1995;269:230–234. doi: 10.1126/science.7618084. [DOI] [PubMed] [Google Scholar]
- 27.Miettinen P J, Berger J E, Meneses J, Phung Y, Pedersen R A, Werb Z, Derynck R. Nature (London) 1995;376:337–341. doi: 10.1038/376337a0. [DOI] [PubMed] [Google Scholar]
- 28.Berkowitz E A, Seroogy K B, Schroeder J A, Russell W E, Evans E P, Riedel R F, Phillips H K, Harrison C A, Lee D C, Luetteke N C. Cell Growth Differ. 1996;7:1271–1282. [PubMed] [Google Scholar]