slalom encodes an adenosine 3′-phosphate 5′-phosphosulfate transporter essential for development in Drosophila (original) (raw)
Abstract
Sulfation of all macromolecules entering the secretory pathway in higher organisms occurs in the Golgi and requires the high-energy sulfate donor adenosine 3′-phosphate 5′-phosphosulfate. Here we report the first molecular identification of a gene that encodes a transmembrane protein required to transport adenosine 3′-phosphate 5′-phosphosulfate from the cytosol into the Golgi lumen. Mutations in this gene, which we call slalom, display defects in Wg and Hh signaling, which are likely due to the lack of sulfation of glycos aminoglycans by the sulfotransferase sulfateless. Analysis of mosaic mutant ovaries shows that sll function is also essential for dorsal–ventral axis determination, suggesting that sll transports the sulfate donor required for sulfotransferase activity of the dorsal–ventral determinant pipe.
Keywords: glycosaminoglycan/Hedgehog/Pipe/sulfation/Wingless
Introduction
Secreted signaling molecules of the FGF, Hh, TGF-β and WNT families rely on proteoglycans (PGs) for efficient activation of their respective signaling pathways (reviewed in Perrimon and Bernfield, 2000). PGs consist of secreted or transmembrane core proteins to which glycosaminoglycan (GAG) side chains are attached at specific consensus sites. In Drosophila, the secreted PG perlecan (Park et al., 2003), the transmembrane PG syndecan (Spring et al., 1994) and two members of the glypican family of glycosylphosphatidylinositol (GPI) anchored PGs have been identified. Phenotypes associated with loss of function mutations of the glypican-encoding genes dally (Nakato et al., 1995) and dally-like (dlp) (Khare and Baumgartner, 2000; Baeg et al., 2001) have revealed the requirement of these PGs for efficient activation of several signal transduction pathways.
The function of PGs is critically dependent on the integrity of the attached GAGs. GAGs are unbranched polysaccharide chains, which are synthesized on proteoglycan core proteins in the Golgi and undergo complex modification reactions before the PG to which they are attached is transported to the cell surface. In the case of glypican, heparan sulfate (HS) chains, which consist of a sugar backbone of alternating units of _N_-acetylglucosamine (GlcNAc) and glucuronic acid (GlcA), are synthesized on the core protein. The nucleotide sugar substrates for this reaction are synthesized in the cytoplasm and must be transported into the Golgi. In Drosophila, the activated precursor UDP-GlcA is synthesized from UDP-glucose by the enzymatic activity of the sugarless (sgl) gene product (Binari et al., 1997; Häcker et al., 1997; Haerry et al., 1997), a homolog of mammalian UDP-glucose dehydrogenases. The gene product of fringe connection (frc), a predicted ER/Golgi multipass transmembrane protein, has been shown to transport UDP-GlcA and UDP-GlcNAc from the cytosol into the Golgi (Goto et al., 2001; Selva et al., 2001). Mutations in either gene severely affect the Wg and FGF signaling pathways. Elongation of the HS chains requires the activity of HS polymerases. tout-velu (ttv) encodes a protein with homology to the mammalian HS co-polymerase EXT1 and has been demonstrated to be required specifically for Hh signaling (Bellaiche et al., 1998). Subsequent to their synthesis, GAGs undergo multiple modifications such as epimerization and sulfation. Mutations in sulfateless (sfl), a homolog of vertebrate _N_-deacetylase/_N_-sulfotransferases, lead to a severe reduction in the activity of the Wg, Hh and FGF signaling pathways (Lin and Perrimon, 1999; Lin et al., 1999). A characteristic feature of all mutations in genes involved in GAG biosynthesis is that their segment polarity phenotypes can be rescued by ectopic expression of wg or hh, suggesting that GAGs are not essential components of the respective signaling cascades but accessory factors most likely required for the proper distribution of extracellular signaling molecules throughout morphogenetically active tissues in vivo.
GAGs have also been proposed to play a role in the determination of the dorsal–ventral (D/V) axis of the Drosophila embryo (Sen et al., 1998). The D/V polarity of the embryo is established during oogenesis by asymmetric expression of the key D/V determinant pipe (pip) in the follicle cell epithelium. pip expression in the ventral follicle cell layer is necessary and sufficient to trigger a serine–protease cascade in the perivitelline space, which leads to the generation of an active ligand for the transmembrane receptor Toll (Tl). Activation of Tl on the ventral side of the embryo results in a gradient of nuclear localization of the transcription factor Dorsal, which patterns the D/V axis (reviewed in Amiri and Stein, 2002). Based on sequence similarity to a family of vertebrate enzymes and its localization in the Golgi apparatus, pip has been hypothesized to encode a heparan sulfate 2-_O_-sulfotransferase. However, neither the enzymatic activity nor the substrate specificity of pip have been demonstrated directly.
Sulfation of secreted molecules occurs in the Golgi and requires the high-energy sulfate donor adenosine 3′-phosphate 5′-phosphosulfate (PAPS) to be present within that organelle. In Drosophila, PAPS is synthesized in the cytoplasm by PAPS-synthetase (Jullien et al., 1997), which incorporates both ATP-sulfurylase and adenosine 5′-phosphosulfate-kinase (APS-kinase) activity. PAPS must be transported into the Golgi thereafter (Lyle et al., 1994) to serve as a substrate for sulfotransferases. Here, we report the first molecular identification and functional characterization of a PAPS transporter. Mutations in this gene, which we call slalom (sll), are associated with defects in multiple signaling pathways, including Wg and Hh signaling. A phenotypic analysis suggests that the effects of sll on signal transduction are caused by its requirement for GAG modification. We present evidence that sll is also required to supply PAPS to the machinery initiating the establishment of embryonic D/V polarity, supporting the view that Pipe protein is a sulfotransferase.
Results
Identification of slalom
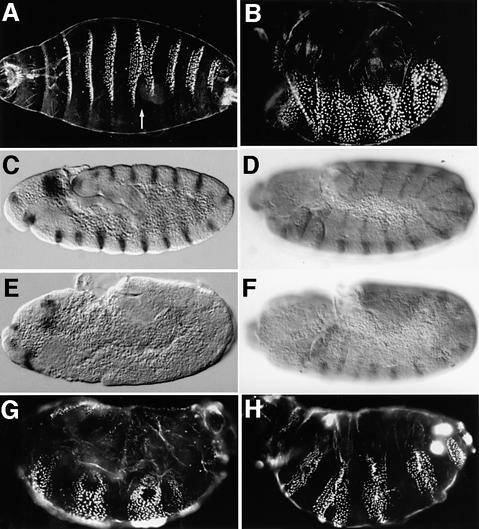
The sll mutant was identified in a genetic screen for zygotic lethal mutations associated with maternal effect phenotypes. Homozygous _sll_7E18 larvae, derived from heterozygous parents, do not hatch and produce cuticles with occasional fusions of denticle belts (Figure 1A). Homozygous _sll_7E18 larvae derived from mothers carrying _sll_7E18 germ-line clones (referred to as sll null mutants throughout the text) exhibit a fully penetrant segment polarity phenotype (Figure 1B) similar to that seen in wg or hh mutants, suggesting that sll may have a function in the Wg and/or Hh signaling pathways.
Fig. 1. Embryonic phenotypes of sll mutants. (A) Cuticle phenotype of a _sll_7E18 homozygous embryo derived from heterozygous parents. Note occasional fusion of denticle belts (arrow). (B) Cuticle phenotype of a _sll_7E18 homozygous embryo derived from a female carrying _sll_7E18 germ-line clones (referred to as sll null mutant hereafter). Note the complete absence of smooth cuticle on the ventral side. (C) Wg expression in a wild-type embryo at stage 10. (D) En expression in a wild-type embryo at stage 11. (E) Wg expression in thoracic and abdominal segments of a _sll_7E18 null mutant is completely abolished at stage 10. (F) En expression in thoracic and abdominal segments of a _sll_7E18 null mutant is dramatically decreased at stage 11. (G) Expression of a UAS-wg transgene using a prd-Gal4 driver line in _sll_7E18 null mutants. Note the development of smooth cuticle in alternating segments. (H) Expression of a UAS-hh transgene using a prd-Gal4 driver line in _sll_7E18 null mutants. Note the development of smooth cuticle in alternating segments. Anterior is to the left and dorsal side up in all panels, except (A), which is a ventral view.
In wild-type embryos, pair-rule genes initiate the expression of wg and its target gene, the homeodomain transcription factor engrailed (en), at the cellular blastoderm stage. After germ-band extension, the expression of wg and en is maintained by a positive feedback loop between the Wg and Hh signaling pathways. In mutants of segment polarity genes essential for Wg signal transduction, the expression of wg and en fades at stage 10. To confirm that sll is involved in segment polarity determination we stained _sll_7E18 null mutants for Wg (Figure 1E) or En (Figure 1F) protein. The expression of both genes is initiated normally in _sll_7E18 null mutants (not shown) but fades prematurely compared with wild type. However, in contrast to null mutations in other segment polarity genes, such as dishevelled (dsh) or armadillo (arm) (Peifer et al., 1991), en expression is maintained significantly longer in _sll_7E18 mutants, until stage 12. This phenotype is also seen in mutations that disrupt enzymes required for the biosynthesis of heparan sulfate proteoglycans (HSPGs), such as sugarless (sgl), sulfateless (sfl) or fringe connection (frc) (Binari et al., 1997; Häcker et al., 1997; Haerry et al., 1997; Selva et al., 2001).
In contrast to the segment polarity defects caused by mutations in core components of the Wg signaling pathway, such as dsh, the phenotype of sgl, sfl or frc mutants can be rescued by overexpression of either wg or hh. To test whether this is the case for sll, we expressed UAS-wg or UAS-hh transgenes in sll null mutants using a prd-Gal4 driver. Larval cuticles from embryos expressing wg (Figure 1G) or hh (Figure 1H) in a pair-rule pattern show rescue of the segment polarity phenotype in alternating segments, corresponding to the domains of prd expression. These observations show that the Wg and Hh signaling pathways can be activated in the absence of sll function, and suggest that sll may play a role in the biosynthesis of GAGs.
sll is required for Wg and Hh signaling
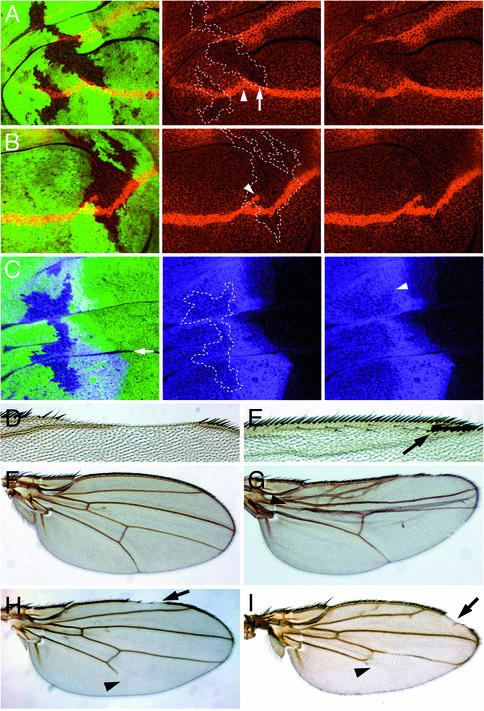
The Wg and Hh signaling pathways are engaged in a positive feedback loop during patterning of the ventral embryonic epidermis (reviewed in Perrimon, 1994), which makes it difficult to distinguish which of the pathways requires sll activity. Therefore, the question of whether sll function is required for Wg or Hh signaling was addressed during wing imaginal disc development, where the two pathways act in clearly distinguishable patterning processes. In the wing imaginal disc, wg is expressed in a stripe of cells along the D/V boundary of the wing. A Wg protein gradient emanating from _wg_-expressing cells is required to pattern the wing margin (reviewed in Irvine, 1999). When clones of _sll_7E18 mutant cells crossing the D/V boundary were stained for Wg protein (Figure 2A and B), no change in Wg protein levels was observed in _wg_-expressing cells. However, despite their close proximity to the Wg source, _sll_7E18 mutant cells immediately adjacent to the D/V boundary showed a significant reduction in Wg protein levels, leading to a disruption of the gradient (Figure 2A). The wings of adults carrying _sll_7E18 mutant clones frequently exhibited gaps in the anterior margin (Figure 2D). Similar gaps were also observed in _sll_L(3)142214/_sll_7E18 transheterozygous adults, which are viable but show patterning defects due to reduced sll activity (arrow in Figure 2I). These observations suggest a role for sll in Wg distribution and are consistent with an involvement of sll in GAG metabolism.
Fig. 2. Role of sll during wing development. (A and B) Examples of Wg distribution (red) in wing imaginal discs carrying _sll_7E18 mutant clones. (A, center and right panel) Same as left panel showing only Wg expression. Compare Wg levels adjacent to the D/V boundary inside (arrow) and outside the clone. Note the reduction of Wg protein levels in _sll_7E18 mutant cells. The D/V boundary often shows a change in direction associated with _sll_7E18 mutant clones (arrowhead in center panel). (B, center and right panel) Same as left panel showing only Wg expression. Note bifurcation of the D/V boundary inside the _sll_7E18 mutant clone (arrowhead in center panel). Homozygous _sll_7E18 mutant cells are marked by the absence of GFP (green) expression in left panels and by the dashed outlines in center panels of (A), (B) and (C). (C) Ci distribution (purple) in wing imaginal discs carrying _sll_7E18 mutant clones. The center and right panels are the same as the left panel showing only Ci expression. Note that Ci expression is moderately reduced in _sll_7E18 mutant cells receiving the Hh signal. The arrowhead in the right panel indicates the region where strongest reduction is seen. (D–G) Wing phenotypes associated with _sll_7E18 mutant clones. (D) Wing exhibiting a gap in the anterior margin. (E) Bifurcation of the anterior wing margin (arrow). (F) Wild-type wing. (G) Wing showing reduction in region between wing veins L3 and L4 (arrowhead). (H) Wing of dally_527/dally_gem transheterozygote. Note truncation of wing vein L5 (arrowhead). (I) Wing of _sll_l(3)142214/_sll_7E18 transheterozygote showing gaps in the margin (arrow) and truncation of wing vein L5 (arrowhead) very similar to (H).
Furthermore, wg expression in wing imaginal discs frequently exhibited changes of direction or bifurcations of the D/V boundary (Figure 2A and B, arrowheads in center panels) in association with _sll_7E18 mutant clones, suggesting a role for sll during establishment of compartment boundaries at an early stage of imaginal disc development. In adult wings, these abnormalities in wg expression resulted in the production of extra margin bristles inside the wing blade (Figure 2E, arrow).
We also stained wing discs carrying _sll_7E18 mutant clones for the protein product of the Hh target gene Cubitus interruptus (Ci) (Motzny and Holmgren, 1995) (Figure 2C). _sll_7E18 mutant clones located in the anterior compartments of wing discs showed a moderate reduction in Ci protein levels, indicating reduced Hh signaling activity. Consistent with this observation, adult wings carrying _sll_7E18 mutant clones frequently showed a reduction in the region between longitudinal veins L3 and L4, which has been demonstrated to be sensitive to Hh signaling (Figure 2G, arrowhead) (Mullor et al., 1997; Strigini and Cohen, 1997). In addition, wings carrying _sll_7E18 mutant clones, or _sll_L(3)142214/_sll_7E18 wings, form ectopic vein tissue associated with wing vein L2 (Figure 2G). This phenotype cannot be explained by defects in Wg or Hh signaling and suggests that sll is also required for the activity of other signal transductions pathways, such as the TGF-β pathway. These results suggest that, similar to genes encoding proteoglycan core proteins, such as dally, or genes involved in GAG biosynthesis, such as sgl, frc or sfl, sll function is required for the activity of several signaling pathways.
Molecular identification of sll
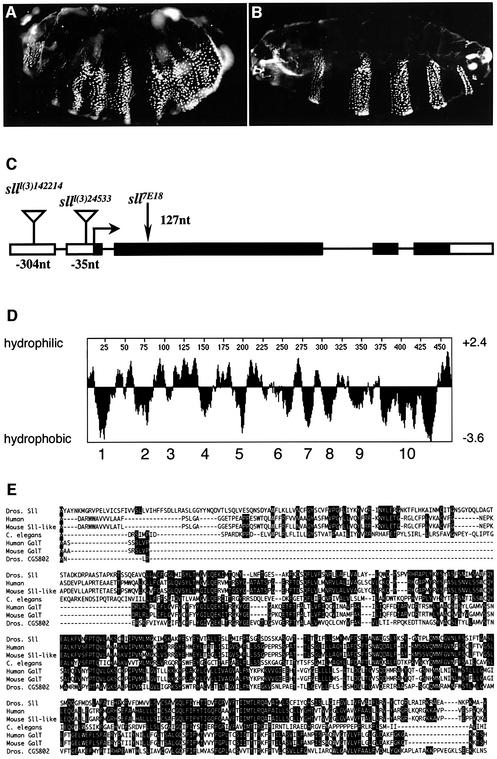
The original ethyl methane sulfonate-induced allele _sll_7E18 was used to identify a non-complementing P-element insertion allele _sll_l(3)24533. Embryos maternally and zygoti cally mutant for _sll_l(3)24533 showed a cuticle phenotype similar to, though weaker than, _sll_7E18 (Figure 3A). The P-element in _sll_l(3)24533 is inserted in the 5′-UTR of a putative protein-encoding transcription unit. In another, independently identified P-element-induced allele, _sll_l(3)142214, the transposon was also found inserted in the 5′-UTR of the same transcription unit (Figure 3C). Furthermore, we determined the nucleotide sequence of this transcription unit in the _sll_7E18 allele and detected a nonsense mutation which leads to termination of the open reading frame (ORF) after 42 amino acids. The truncated ORF contains none of the predicted transmembrane regions of the encoded protein, suggesting that _sll_7E18 is a null allele (Figure 3C).
Fig. 3. Molecular identification of sll. (A) Cuticle phenotype of _sll_l(3)24533 maternal and zygotic mutant larva. Note the occasional development of smooth cuticle in some segments, indicating that _sll_l(3)24533 is not a null allele. (B) Pair-rule segmentation phenotype of _sll_7E18 null mutant larvae expressing a UAS-sll transgene under the control of a prd_-Gal_4 driver line. (C) Gene structure of sll. The P-element insertions in the sll transcript are located 304 nucleotides [_sll_l(3)142214] and 35 nucleotides [_sll_l(3)24533], respectively, upstream of the start codon. The _sll_7E18 allele has a C to T transition creating a nonsense mutation in the 42nd codon (nucleotide 127) of the reading frame. (D) The sll cDNA encodes a putative hydrophobic protein of 465 amino acids (52 kDa) with at least 10 predicted transmembrane spanning regions. (E) Homology alignment of Sll with a putative human protein (39.4%) (Protein Data Bank accession No. AK075456), a putative mouse Sll-like protein (41.1%) (BC036992), Caenorhabditis elegans gene M03F8.2 (28.8%) (NM072147), a putative human galactose transporter UGTREL1 (28.6%) (NM005827), a putative mouse galactose transporter (29.2%) (NM016752) and the Drosophila gene CG5802 (21.3%). Amino acid identity to Sll is indicated by percentage values in brackets. Protein accession numbers are in brackets.
To demonstrate that this transcription unit represents the sll gene, a putative UAS-sll transgene was introduced into the genome and expressed in sll mutants. Expression of UAS-sll in _sll_7E18 null mutants using a prd-Gal4 driver resulted in larval cuticles with a pair-rule-like segmentation pattern (Figure 3B), indicating that the UAS-sll transgene was responsible for the rescuing activity. Embryos homozygous for _sll_7E18, carrying an actin-Gal4 driver and a UAS-sll transgene, developed into viable and fertile adults with no phenotypic abnormalities, showing that UAS-sll identifies the sll gene. This result further suggests that sll can be ubiquitously expressed throughout development with no deleterious effects for the organism, and that sll function is largely dosage independent.
sll encodes a PAPS transporter located in the Golgi apparatus
A hydrophobicity analysis of the putative Sll protein predicted a hydrophobic polypeptide with at least 10 transmembrane regions, which is a structural characteristic of many nucleotide–sugar transporters (Figure 3D) (reviewed in Gerardy-Schahn et al., 2001). A BLAST search of non-redundant protein databases, available at NCBI, with the Sll sequence revealed that Sll is conserved throughout the animal kingdom (Figure 3D), as well as in plants, and shares nearly 40% of amino acid sequence identity with predicted mouse and human proteins of unknown function. Sll is also similar to mammalian proteins that have been classified as nucleotide–sugar transporters on the basis of their homology (Ishida et al., 1996) to the human UDP-galactose transporter hUGT (Miura et al., 1996). However, Sll itself has no significant sequence similarity to hUGT. While these data suggest that Sll encodes a transmembrane transporter, they leave open what substrate Sll may be transporting.
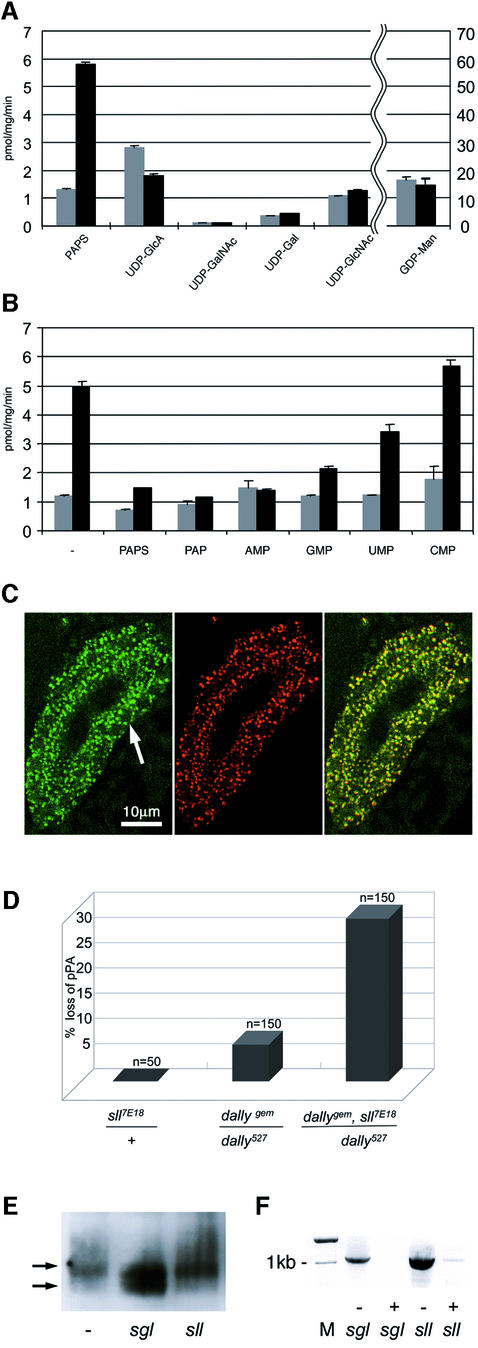
In order to demonstrate directly that Sll has transporter activity, and to determine its substrate specificity, an in vitro vesicle transport assay was used to examine the ability of Sll to facilitate the transport of defined nucleotide substrates into Saccharomyces cerevisae micro somes. Heterologous expression of sll in yeast was favored over other systems since we have successfully used procedures for transfection of nucleotide–sugar genes, high-level preparation of sealed microsomal vesicles and nucleotide–sugar assays in this organism (Segawa et al., 2002). In vesicles derived from cells transfected with a _sll_-expression construct, a 5-fold increase in the uptake of PAPS was detected. Import of several other nucleotide–sugars did not increase after sll expression (Figure 4A). We attribute the background in the control cells to glycosylation reactions, such as mannosylation of dolichol using GDP-Man on the outer surface of the microsomal vesicles (Hirschberg and Snider, 1987). Importantly, the _sll_-dependent PAPS transport activity could be efficiently competed by structurally similar substrate analogs but not by more distantly related nucleotide monophosphates, confirming that the transport was specific (Figure 4B).
Fig. 4. sll encodes a PAPS transporter. (A) Transport activity of Sll for various nucleotide–sugar substrates and PAPS. The microsomes from _sll_-transfected S.cerevisiae were prepared and assayed for transport of the designated nucleotides at a concentration of 5 mM, as described in Materials and methods. Transport activity is shown in picomoles of substrate transported per milligram of microsomes per minute. Transport of GDP-Man is shown on a 10-fold higher scale compared with all other substrates. (B) Effect of nucleotide addition on PAPS transport into microsomal vesicles. Microsomal vesicles were prepared from _sll_-transfected S.cerevisiae and assayed for PAPS 35S-transport activity in the presence of various nucleotide monophosphates at 100 µM concentration. In (A) and (B), the gray bars indicate transport by microsomes isolated from S.cerevisiae and transfected with empty vector. Black bars indicate transport by microsomes isolated from S.cerevisiae and transfected with a _sll_-expression vector. (C) The left panel shows the expression of Sll in salivary glands. Note the perinuclear localization of the staining. The arrow points to the nucleus of one cell visible as a dark area. The bar is 10 µm long. The center panel shows the expression of a Drosophila Golgi specific protein in salivary glands. The right panel was obtained by merging the left and center panels. Note the extensive co-localization of staining. (D) Genetic interaction between dally and sll in loss of the posterior postalar bristle (pPA). (E) Western blot analysis of HA–Dally from S2 cells incubated with buffer (–) or dsRNA specific for sugarless (sgl), or slalom (sll). Upper arrows indicate the size of HA–Dally in untreated cells. The lower arrow indicates the size of HA–Dally after RNAi treatment against sgl (center lane) or sll (right lane). (F) Amplification of sgl or sll RNA by RT–PCR from S2 cells incubated with buffer (–) or dsRNA (+) specific for sugarless (sgl) or slalom (sll). M, DNA size marker.
PAPS is synthesized in the cytoplasm and serves as a substrate for sulfotransferases during the post-translational modification of macromolecules in the Golgi. Therefore, the result that sll encodes a PAPS transporter suggested that Sll should be localized to the Golgi membranes. To address this question, we raised peptide antibodies against Sll. Localization of Sll in salivary glands of wild-type embryos, using Sll antiserum, showed a punctate staining pattern in the perinuclear region of cells (Figure 4C, left panel). Co-staining of salivary glands with antibodies against an endogenous Golgi protein (Stanley et al., 1997) (Figure 4C, center panel) revealed extensive co-localization of the two proteins (Figure 4C, right panel), indicating that Sll is a resident Golgi protein. Together, these data suggest that Sll encodes a nucleotide transporter required to translocate the high-energy sulfate donor PAPS into the lumen of the Golgi.
Requirement of sll for proteoglycan function
Several lines of evidence suggest that sll interferes with Wg and Hh signaling due to its requirement for GAG modification. In order to confirm these findings we examined genetic interactions between sll and the heparan sulfate proteoglycan dally, which has previously been implicated in Wg signaling (Lin and Perrimon, 1999; Tsuda et al., 1999). Among other functions, Dally is required for the development of a subset of sensory bristles on the notum which form in, or immediately adjacent to, _wg_-expressing cells (Fujise et al., 2001). Of these macrochaetae, pPA is the one most sensitive to wg activity. In adults, transheterozygous for the hypomorphic dally alleles _dally_527 and _dally_gem, pPA is absent from 5% of all individuals. When one copy of _sll_7E18 is introduced into these flies, the fraction of individuals that lack pPA climbs to 30% (Figure 4D), showing that dally and sll act synergistically in the loss of the pPA macrochaeta. dally_527/dally_gem adults also display defects in wing patterning. In addition to notches in the margin (Figure 2H, arrow), the wings of these mutants show a truncation of the longitudinal vein L5 (Figure 2H, arrowhead). Very similar defects are also seen in wings of _sll_l(3)142214/_sll_7E18 transheterozygotes (Figure 2I). Together, these data suggest that sll function is required for the post-translational modification of heparan sulfate proteoglycans such as dally, which are in turn essential for the activity of several extracellular ligand-dependent signaling pathways including the Wg and Hh pathways.
The influence of sll on post-translational modification of Dally was examined in a Drosophila Schneider (S2) cell assay. S2 cells were transfected with a hemagglutinin-tagged dally (HA-dally) transgene and the expression of several genes involved in GAG metabolism was subjected to RNA interference (RNAi) mediated inhibition (reviewed in Hannon, 2002). Changes in the mobility of HA–Dally due to altered GAG modification were visualized by SDS–PAGE and western blotting. The synthesis of GAG chains on Dally creates a higher molecular weight population of Dally molecules, which are visible as a smear above the main Dally band (Figure 4E, left lane). Following incubation of cells with double-stranded RNA (dsRNA) specific for sgl, the amount of higher molecular weight molecules of Dally is significantly reduced and a lower molecular weight band of HA-dally appears (Figure 4E, center lane). These data suggest that a significant fraction of Dally molecules is not properly GAG modified when sgl activity is compromised. When S2 cells were incubated with dsRNA specific for sll (Figure 4E, right lane), no change in the mobility of HA–Dally was seen, although sll mRNA levels were strongly reduced, as confirmed by RT–PCR (Figure 4F). These observations are consistent with the view that sll (Figure 4E, lane) does not interfere with the synthesis of GAG chains on the Dally core protein but is required only for their subsequent sulfation.
sll expression in the germline and in the embryo
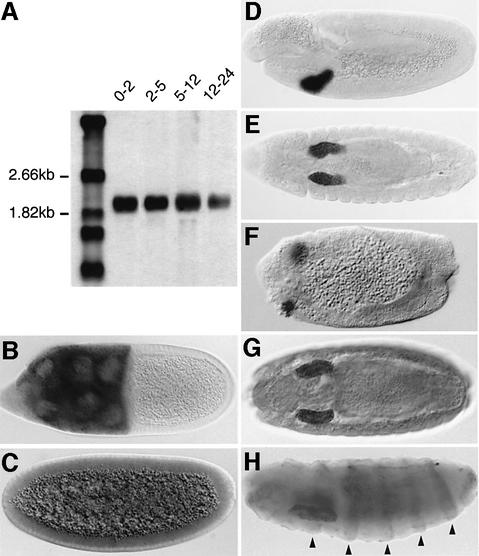
The expression profile of sll transcripts was first analyzed by northern blotting, which showed that sll is expressed throughout embryogenesis (Figure 5A). During oogenesis, sll is expressed strongly in the nurse cells of the germline, confirming that sll transcripts are maternally contributed to the egg (Figure 5B). In situ hybridization to whole-mount embryos detected a low level of ubiquitously expressed maternal sll mRNA at the syncytial blastoderm stage (Figure 5C). Zygotic sll expression was first detectable after germ-band elongation in the invaginating salivary gland placodes (Figure 5D and E). sll remains expressed predominantly in this tissue throughout embryogenesis, but low-level expression below the detection threshold may be present throughout the embryo. sll mRNA is also detectable in salivary gland placodes in _sll_7E18 null mutant embryos (Figure 5F), consistent with the molecular defect found in this mutant. Immunohistochemical stainings of wild-type embryos with Sll antibodies confirmed that Sll protein distribution coincides with the expression of sll transcripts (Figure 5G). In prd-Gal4/UAS-sll embryos, Sll is also detected in epidermal stripes corresponding to the prd expression domains, demonstrating that the antibody specifically recognizes Sll (Figure 5H).
Fig. 5. Expression of sll mRNA during embryonic development. (A) Northern blot showing expression of sll transcripts at different time intervals during embryogenesis. (B) Maternal expression of sll RNA in the nurse cells of the germline during oogenesis. (C–F) In situ hybridization of sll RNA to wild-type (C–E) and _sll_7E18 mutant (F) embryos. (C) Maternal expression of sll RNA in the syncytial blastoderm embryo (stage 4). (D) Zygotic expression of sll RNA is first detected in the salivary gland placodes at stage 11. (E) Salivary gland specific expression of sll RNA at stage 14. (F) Expression of sll RNA in the salivary gland placodes of a _sll_7E18 null mutant at stage 11. (G) Expression of Sll protein in the salivary glands of a wild-type embryo. (H) Expression of Sll protein in a prd-Gal4/UAS-sll embryo showing that the α-Sll antibody recognizes Sll specifically. Arrowheads indicate expression in epidermal stripes in the abdominal region. (C, D, F and H) Lateral view. (E and G) Ventral view. Anterior is to the left.
sll is required for D/V axis determination
Two other Drosophila genes, windbeutel (wbl) (Nilson and Schüpbach, 1998) and pip (Sen et al., 1998), are predominantly expressed in the salivary glands during embryogenesis. While the function of these genes in the salivary glands is unclear, both are also required for the establishment of the D/V axis of the egg. During oogenesis, pip is expressed specifically in the ventral follicle cell epithelium, where it is involved in the generation of a localized signal triggering the D/V signaling cascade. It encodes a protein with similarity to vertebrate heparan sulfate 2-_O_-sulfotransferases. However, the enzymatic activity of Pip has not been demonstrated directly.
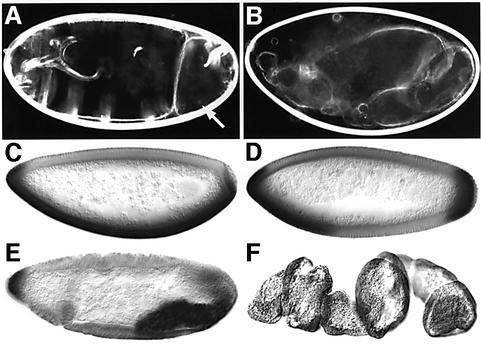
The similarities in the expression patterns of pip and sll, as well as the molecular functions of both genes, raised the possibility that sll function might also be required for D/V axis determination. To test this hypothesis, females carrying sll mutant follicle cell clones were generated and phenotypes associated with these clones were analyzed. Cuticles developed from eggs laid by these females frequently exhibited dorsalization of varying severity, ranging from partial and spatially restricted (Figure 6A, arrow) to complete loss of ventral structural elements (Figure 6B). Consistent with the cuticle phenotypes, the ventral mesodermal marker twist (Figure 6C) often showed discontinuities, or total loss of expression, in embryos produced by females carrying mosaic ovaries (Figure 6D–F). These data strongly suggest that sll activity is required for activation of the D/V signaling cascade initiated by pip. To investigate whether the requirement of sll in D/V polarity is mediated by GAGs, we generated follicle cell clones homozygous mutant for _sgl_08310 or _frc_00073. Despite the fact that the ability of _sgl_08310 or _frc_00073 mutant cells to synthesize GAGs is severely reduced, D/V polarity defects similar to those in sll mutant clones could not be detected (data not shown). This suggests that GAGs, such as heparan sulfate, chondroitin sulfate or dermatan sulfate, which require GlcA for their formation, may not play a role in D/V axis determination but that sll function may be required for sulfation of a different substrate of pip in this process.
Fig. 6. Role of sll in the follicle cell epithelium. (A and B) Examples of cuticle phenotypes of embryos derived from females carrying _sll_7E18 mutant clones in the follicle cell epithelium. Note the absence of ventral denticle belts in posterior abdominal segments (arrow in A) and the complete dorsalization of the cuticle in (B). (C) Twi expression in wild type at the cellular blastoderm stage (stage 5). (D–F) Examples of alterations in expression of the ventral marker Twi in embryos derived from females carrying _sll_7E18 mutant clones in the follicle epithelium. (D) Dorsalization in the abdominal region. (E) Dorsalization in the gnathal and thoracic region. (F) Complete dorsalization of the entire embryo. Anterior is to the left, dorsal side up.
Discussion
Molecular identification of PAPS translocators
The sulfation of macromolecules is carried out by sulfotransferases in the Golgi and constitutes an important step in the post-translational modification of proteins and the maturation of GAGs in higher organisms. All sulfotransferase reactions require the high-energy donor PAPS. PAPS is synthesized via the sequential actions of ATP sulfurylase and APS kinase in the cytoplasm, and subsequently must be translocated into the Golgi for utilization by luminal sulfotransferases (Capasso and Hirschberg, 1984; Lyle et al., 1994). We report the first molecular identification of a PAPS transporter in eukary otes and demonstrate the importance of sulfate transport into the lumen of the Golgi for pattern-formation processes during development.
The sll sequence is unique in the Drosophila genome, but homologs are found throughout the animal kingdom, including mice and humans. Proteins with PAPS transport activity have previously been purified from mammalian cells, but their molecular identity has remained elusive. The size of these mammalian PAPS translocators has been reported as 75 or 230 kDa, respectively (Mandon et al., 1994; Ozeran et al., 1996), neither of which corresponds to the predicted 47 kDa of mammalian Sll homologs. One possible explanation for this could be that these proteins may be post-translationally modified in a manner that accounts for the size discrepancy. The high degree of conservation between sll and its mammalian homologs suggests that they are true orthologs; however, the final evaluation of this will have to await functional characterization of the mammalian proteins.
sll and growth factor signaling
The phenotypical analysis of sll mutants shows that sll function is required for Wg and Hh signaling, and suggests that sll may also play a role in the activation of other signaling pathways, such as the TGF-β pathway and possibly others. The broad requirement for sll raises the question of how sulfation influences the function of multiple secreted signaling factors. Proteins entering the secretory pathway are ubiquitously sulfated on specific tyrosine residues. However, according to sulfation consensus prediction algorithms, neither Wg nor Hh is predicted to be a substrate for tyrosine sulfation (see ‘Sulfinator’ at http://www.expasy.org/tools/sulfinator/) (Monigatti et al., 2002). In addition, our overexpression experiments show that wg and hh have the ability to at least partially activate their respective signaling cascades in sll mutants, which would be unlikely if the signaling factors themselves were non-functional due to lack of sulfate modification. Therefore, it is unlikely that lack of tyrosine sulfation of either Wg or Hh proteins is responsible for the defects in Wg or Hh signaling pathways in sll mutants.
The Wg and Hh signaling pathways are activated through interaction of secreted ligands with their respective cell surface receptors. This activation relies on the action of GAGs attached to proteoglycan core proteins of the glypican family (Lin and Perrimon, 1999). In the case of Wg, the proteoglycans are thought to act as coreceptors, retaining the ligand at the cell surface in the vicinity of its receptor. Hh has been proposed to depend on GAGs for efficient transport to its target cells. In the absence of functional GAGs, the signaling molecules are not present in sufficiently high concentrations at the receptor to activate the signal transduction pathway. This defect can be compensated by overexpression of the signaling molecule. Therefore, a characteristic feature of mutations in genes involved in GAG metabolism is that their segment polarity defects can be rescued by overexpression of the ligand. Our data show that Wg protein levels and Hh signaling activity are reduced in sll mutant clones. The segment polarity defects of sll mutants can be rescued by overexpression of wg or hh. In addition sll and dally mutants affect Wg signaling synergistically. Our data also suggest that the ability of cells to sulfate GAGs is dramatically reduced in sll mutants because the sulfate donor necessary for sfl to modify the polysaccharide chains cannot be transported into the Golgi in sufficient amounts. Taken together, these observations strongly suggest that lack of functional GAGs is responsible for the segment polarity defects in sll mutants.
Interestingly, the overall size of sugar chains attached to the glypican Dally does not appear to be affected in sll mutants. Consistent with this result, it has been observed that the overall level of HS, which is reduced to trace amounts in the sgl mutant that affects GAG biosynthesis, is not markedly changed in sfl mutants, which should affect sulfate modification but not synthesis of GAG chains (Toyoda et al., 2000). However, it cannot be excluded that residual sulfation of GAGs occurs in the cell culture assay owing to incomplete inhibition of the PAPS transport activity of sll by dsRNA interference. However, the phenotypic analysis of sll suggests that sll function is essential for at least two sulfotransferases, sfl and pip, and that the GAG chains present in sll mutants are not able to fulfill their normal function owing to altered sulfation patterns.
sll and D/V patterning
The signal determining the D/V axis of the Drosophila embryo is produced by a proteinase cascade active in the perivitelline space of the egg chamber during oogenesis. Activation of this cascade requires localized expression of pip in the ventral follicle cells of the ovarian egg chamber in which the egg is constructed, but the mechanism leading to activation remains enigmatic. pip has significant similarity to a family of mammalian heparan sulfate 2-_O_-sulfotransferases; however, the enzymatic activity of pip has not been demonstrated directly. Our data show that transport of the sulfate donor PAPS into the Golgi is essential for establishment of the D/V axis. The requirement of PAPS for D/V patterning strongly supports the notion that sulfotransferase activity is an essential feature of pip function.
This view is also supported by the specific expression patterns of pip and sll during development. In the embryo, both genes are highly expressed in the developing salivary glands. Another gene involved in PAPS biosynthesis, PAPS synthetase, is also highly expressed in salivary glands (Jullien et al., 1997), suggesting that these genes act together in a common pathway providing the sulfate donor for the modification of macromolecules produced in the salivary glands in late embryos and larvae.
Interestingly, the defects in D/V polarity associated with sll mutants are not observed in mutants of the UDP-glucose dehydrogenase sgl or the UDP-glucuronic acid transporter frc. The activity of both genes is required for the formation of GAGs, such as heparan sulfate, chondroitin sulfate or dermatan sulfate, and levels of these GAGs are dramatically reduced in sgl mutants (Toyoda et al., 2000). These observations support the possibility that GAGs may not play a role in D/V axis determination and that, despite its homology to heparan sulfate 2-_O_-sulfotransferases, pip may be required for sulfation of a non-GAG substrate. This view is also consistent with the highly restricted expression pattern of pip, which suggests that pip is not generally involved in GAG modification.
The central position of sll in sulfate metabolism along the secretory pathway makes it an interesting tool for the identification of developmental pathways sensitive to sulfate modification. Our results demonstrate that several cell–cell communication pathways are critically dependent on the sulfation of macromolecules, and highlight the importance of sulfation during pattern formation and development.
Materials and methods
Molecular biology
Genomic DNA flanking sll P-element insertions was recovered by plasmid rescue (Huang et al., 2000). Insertion sites were determined by nucleotide sequencing of plasmid rescue DNA fragments. Insertion points were determined to be nucleotide 140633 in AE003718.2 for _sll_l(3)24533 and nucleotide 140365 in the same genomic segment for _sll_l(3)142214. The sll cDNA SD04670 (DDBJ/EMBL/GenBank accession No. AI533003), which is 1.9 kb long and contains an ORF for a protein of 465 amino acids, was obtained from the Drosophila gene collection (BDGP). The point mutation in _sll_7E18 is a C to T transition at nucleotide 127 of the ORF and was identified by PCR amplification of the sll locus from _sll_7E18 mutants and subsequent nucleotide sequencing. The UAS-sll transgene was produced by cloning the _Eco_RI–_Spe_I fragment of SD04670 into pUASP (Rorth, 1998).
Genetics
The _sll_7E18 mutation is located at 90B3-4 on the third chromosome. The P-element insertion allele _sll_l(3)24533 was identified by complementation analysis. To demonstrate that the lethality of _sll_l(3)24533 is associated with the P-element insertion, the transposase source Δ2–3 was used to mobilize the transposon. This led to excision of the P-element and reversion of the lethality, demonstrating that _sll_l(3)24533 is responsible for the mutation. Embryos homozygous for the _sll_7E18 and _sll_l(3)24533 mutations and trans-heterozygotes of these alleles are embryonic lethal. _sll_l(3)142214 was identified in a screen for P-element mutations which, when made homozygous in the ovarian follicle cell layer (Duffy et al., 1998), would give rise to dorsalized embryonic progeny. Subsequently, complementation analysis demonstrated that _sll_l(3)142214 is an allele of sll. Homozygotes of _sll_l(3)142214 and trans-heterozygotes of this allele with _sll_7E18 or _sll_l(3)24533 are viable and show adult visible phenotypes in the wings.
Mosaics
Germ-line clones were induced in w*, P{hsFLP}22/+; P{ry+t7.2= neoFRT82b, sll}/P{neo FRT82b, ovo_D}_ females. Heat shocks were performed during the third larval instar for 2 h at 37°C on two consecutive days. Homozygous mutant clones in imaginal discs were generated by crossing y_1, w_*; P{w+mW.hs=en2.4-GAL4}e22c, P{w+mC=UAS-FLP1.D}JD1/CyO; P{ry+t7.2=neoFRT82b, sll}/TM3, Sb flies to P{ry+t7.2=neoFRT}82B P{w+mC=Ubi-GFP}83. Larvae were dissected at the third larval instar. Follicle cell clones were generated using the ‘directed mosaic’ system (Duffy et al., 1998). Virgins of the genotype w; e22c-GAL4, UAS-FLP/+; _FRT_82B _/FRT_82B _sll_7E18 were collected and crossed to Oregon R wild-type males. The e22c-Gal4 enhancer trap line is known to be expressed in the presumptive follicular stem cells.
Misexpression, immunohistochemistry and northern analysis
UAS-wg, UAS-hh, prd-Gal4 and act-Gal4 lines have been described previously (Selva et al., 2001). Primary antibodies used were mouse α-Wg (4D4; Dev. Studies Hybridoma Bank), mouse α-En (4D9; Dev. Studies Hybridoma Bank), rat α-Ci (Motzny and Holmgren, 1995), α-Drosophila Golgi (Calbiochem; 1:400), mouse α-Myc (Invitrogen) and rabbit α-Twi (Roth et al., 1989). Polyclonal rabbit α-Sll antibodies were raised against the peptide acyl-DQLDAGTSTADKDRC and used at a dilution of 1:2000. Secondary antibodies used in clonal analyses of imaginal discs were α-mouse Cy3 (Amersham) and α-rat Cy5 IgG, H+L (Jackson ImmunoResearch).
Construction of expression plasmids
To add myc-His tags, the sll cDNA was amplified by PCR using sll N-_Bam_HI (5′-CGAAAGGATCCACATGTACGCCT) and sll C-_Apa_I (5′-CCGGGCCCGACAGCCATTTTC) as primers. The resulting cDNA fragment, which has a proline codon instead of the stop codon, was inserted into the mammalian expression vector pcDNA3.1/myc-His (Invitrogen) at its _Bam_HI–_Apa_I sites. For sll expression in yeast, a modified pYEX-BX vector (Clontech Laboratories, Palo Alto, CA) was used (Segawa et al., 2002). pYEX-sll/_myc_-His was constructed by inserting a 1.4 kb fragment, excised _Bam_HI and _Pme_I from pcDNA3.1-sll/Myc-His, into the _Bam_HI and _Sma_I site of pYEX. Saccharomyces cerevisiae YPH500 cells were transformed with pYEX and pYEX-sll/myc-His.
Standard nucleotide–sugar transport and PAPS assay
sll (epitope tagged at the C-terminus with Myc and His6 sequences) was subcloned into the copper-inducible expression vector pYEX-BX and transformed into S.cerevisiae strain YPH500 as described (Segawa et al., 2002). Expression of sll was confirmed by western blotting with anti-Myc antibodies (data not shown). Subcellular fractionation and intact microsomal vesicles were prepared as described previously (Sun-Wada et al., 1998) with minor modification (Goto et al., 2001). The membrane fractions obtained by centrifugation at 10 000 and 100 000 g were combined and used in the transport assay. Yeast microsomal preparations exhibited linearity with protein, time and temperature. In the standard transport assay, microsomes (50 µg of protein) were incubated in 0.1 ml of TSE buffer (10 mM Tris–HCl pH 7.0, 0.8 M glucitol, 2 mM EDTA, 50 mM dimercaptopropanol) containing 1 µM radioactive substrate (6400 Ci/mol unless otherwise specified) at 30°C for 1 min. The PAPS transport, UDP-GlcA transport and GDP-Man transport were carried out using [35S]PAPS (2990 Ci/mol), [1-14C(U)]UDP-GlcA (300 Ci/mol) and GDP-[14C]Man (150 Ci/mol). The reaction was terminated by 10-fold dilution with ice-cold TSE. The radioactive material incorporated into microsomes was trapped on a nitrocellulose filter (Millipore, Bedford, MA) and the radioactivity retained on the filter was measured.
Acknowledgments
Acknowledgements
We express our thanks to Antonio Giraldez and Stephen Cohen for supplying materials and for their help with the cell culture assays. We thank Sverker Nystedt for the α-Ci antibody and Wilma Martinez Arias for help with northern blotting. D.S. would like to thank Poornima Parameswaran and Jason Goltz for technical assistance. This work is supported by the Swedish Foundation for Strategic Research (SSF) Developmental Biology Programme, providing a junior research group to U.H., the Swedish Research Council VR (U.H.), the Swedish Cancer Foundation (U.H.) and NIH grant AI20941 (S.J.T.). Work in the laboratory of D.S. was supported by NIH grant GM52761.
References
- Amiri A. and Stein,D. (2002) Dorsoventral patterning: a direct route from ovary to embryo. Curr. Biol., 12, R532–R354. [DOI] [PubMed] [Google Scholar]
- Baeg G.H., Lin,X., Khare,N., Baumgartner,S. and Perrimon,N. (2001) Heparan sulfate proteoglycans are critical for the organization of the extracellular distribution of Wingless. Development, 128, 87–94. [DOI] [PubMed] [Google Scholar]
- Bellaiche Y., The,I. and Perrimon,N. (1998) Tout-velu is a Drosophila homologue of the putative tumour suppressor EXT-1 and is needed for Hh diffusion. Nature, 394, 85–88. [DOI] [PubMed] [Google Scholar]
- Binari R.C., Staveley,B.E., Johnson,W.A., Godavarti,R., Sasisekharan,R. and Manoukian,A.S. (1997) Genetic evidence that heparin-like glycosaminoglycans are involved in wingless signaling. Development, 124, 2623–2632. [DOI] [PubMed] [Google Scholar]
- Capasso J.M. and Hirschberg,C.B. (1984) Mechanisms of glycosylation and sulfation in the Golgi apparatus: evidence for nucleotide sugar/nucleoside monophosphate and nucleotide sulfate/nucleoside monophosphate antiports in the Golgi apparatus membrane. Proc. Natl Acad. Sci. USA, 81, 7051–7055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy J.B., Harrison,D.A. and Perrimon,N. (1998) Identifying loci required for follicular patterning using directed mosaics. Development, 125, 2263–2271. [DOI] [PubMed] [Google Scholar]
- Fujise M., Izumi,S., Selleck,S.B. and Nakato,H. (2001) Regulation of dally, an integral membrane proteoglycan and its function during adult sensory organ formation of Drosophila. Dev. Biol., 235, 433–448. [DOI] [PubMed] [Google Scholar]
- Gerardy-Schahn R., Oelmann,S. and Bakker,H. (2001) Nucleotide sugar transporters: biological and functional aspects. Biochimie, 83, 775–782. [DOI] [PubMed] [Google Scholar]
- Goto S., Taniguchi,M., Muraoka,M., Toyoda,H., Sado,Y., Kawakita,M. and Hayashi,S. (2001) UDP-sugar transporter implicated in glycosylation and processing of Notch. Nat. Cell Biol., 3, 816–822. [DOI] [PubMed] [Google Scholar]
- Häcker U., Lin,X. and Perrimon,N. (1997) The Drosophila sugarless gene modulates Wingless signaling and encodes an enzyme involved in polysaccharide biosynthesis. Development, 124, 3565–3573. [DOI] [PubMed] [Google Scholar]
- Haerry T.E., Heslip,T.R., Marsh,J.L. and O’Connor,M.B. (1997) Defects in glucuronate biosynthesis disrupt Wingless signaling in Drosophila. Development, 124, 3055–3064. [DOI] [PubMed] [Google Scholar]
- Hannon G.J. (2002) RNA interference. Nature, 418, 244–251. [DOI] [PubMed] [Google Scholar]
- Hirschberg C.B. and Snider,M.D. (1987) Topography of glycosylation in the rough endoplasmic reticulum and Golgi apparatus. Annu. Rev. Biochem., 56, 63–87. [DOI] [PubMed] [Google Scholar]
- Huang M.A., Rehm,E.J. and Rubin,G.M. (2000) Recovery of DNA sequences flanking P-element insertions: inverse PCR and plasmid rescue. In Sullivan.,W., Ashburner,M. and Hawley,R.S. (eds), Drosophila Protocols. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp. 429–437. [DOI] [PubMed] [Google Scholar]
- Irvine K.D. (1999) Fringe, Notch and making developmental boundaries. Curr. Opin. Genet. Dev., 9, 434–441. [DOI] [PubMed] [Google Scholar]
- Ishida N., Miura,N., Yoshioka,S. and Kawakita,M. (1996) Molecular cloning and characterization of a novel isoform of the human UDP-galactose transporter and of related complementary DNAs belonging to the nucleotide–sugar transporter gene family. J. Biochem. (Tokyo), 120, 1074–1078. [DOI] [PubMed] [Google Scholar]
- Jullien D., Crozatier,M. and Kas,E. (1997) cDNA sequence and expression pattern of the Drosophila melanogaster PAPS synthetase gene: a new salivary gland marker. Mech. Dev., 68, 179–186. [DOI] [PubMed] [Google Scholar]
- Khare N. and Baumgartner,S. (2000) Dally-like protein, a new Drosophila glypican with expression overlapping with wingless. Mech. Dev., 99, 199–202. [DOI] [PubMed] [Google Scholar]
- Lin X. and Perrimon,N. (1999) Dally cooperates with Drosophila Frizzled 2 to transduce Wingless signalling. Nature, 400, 281–284. [DOI] [PubMed] [Google Scholar]
- Lin X., Buff,E.M., Perrimon,N. and Michelson,A.M. (1999) Heparan sulfate proteoglycans are essential for FGF receptor signaling during Drosophila embryonic development. Development, 126, 3715–3723. [DOI] [PubMed] [Google Scholar]
- Lyle S., Stanczak,J., Ng,K. and Schwartz,N.B. (1994) Rat chondrosarcoma ATP sulfurylase and adenosine 5′-phosphosulfate kinase reside on a single bifunctional protein. Biochemistry, 33, 5920–5925. [DOI] [PubMed] [Google Scholar]
- Mandon E.C., Milla,M.E., Kempner,E. and Hirschberg,C.B. (1994) Purification of the Golgi adenosine 3′-phosphate 5′-phosphosulfate transporter, a homodimer within the membrane. Proc. Natl Acad. Sci. USA, 91, 10707–10711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura N., Ishida,N., Hoshino,M., Yamauchi,M., Hara,T., Ayusawa,D. and Kawakita,M. (1996) Human UDP-galactose translocator: molecular cloning of a complementary DNA that complements the genetic defect of a mutant cell line deficient in UDP-galactose translocator. J. Biochem. (Tokyo), 120, 236–241. [DOI] [PubMed] [Google Scholar]
- Monigatti F., Gasteiger,E., Bairoch,A. and Jung,E. (2002) The Sulfinator: predicting tyrosine sulfation sites in protein sequences. Bioinformatics, 18, 769–770. [DOI] [PubMed] [Google Scholar]
- Motzny C.K. and Holmgren,R. (1995) The Drosophila cubitus interruptus protein and its role in the wingless and hedgehog signal transduction pathways. Mech. Dev., 52, 137–150. [DOI] [PubMed] [Google Scholar]
- Mullor J.L., Calleja,M., Capdevila,J. and Guerrero,I. (1997) Hedgehog activity, independent of decapentaplegic, participates in wing disc patterning. Development, 124, 1227–1237. [DOI] [PubMed] [Google Scholar]
- Nakato H., Futch,T.A. and Selleck,S.B. (1995) The division abnormally delayed (dally) gene: a putative integral membrane proteoglycan required for cell division patterning during postembryonic development of the nervous system in Drosophila. Development, 121, 3687–3702. [DOI] [PubMed] [Google Scholar]
- Nilson L.A. and Schüpbach,T. (1998) Localized requirements for windbeutel and pipe reveal a dorsoventral prepattern within the follicular epithelium of the Drosophila ovary. Cell, 93, 253–262. [DOI] [PubMed] [Google Scholar]
- Ozeran J.D., Westley,J. and Schwartz,N.B. (1996) Identification and partial purification of PAPS translocase. Biochemistry, 35, 3695–3703. [DOI] [PubMed] [Google Scholar]
- Park Y., Rangel,C., Reynolds,M.M., Caldwell,M.C., Johns,M., Nayak,M., Welsh,C.J., McDermott,S. and Datt,S. (2003) Drosophila perlecan modulates FGF and hedgehog signals to activate neural stem cell division. Dev. Biol., 253, 247–257. [DOI] [PubMed] [Google Scholar]
- Peifer M., Rauskolb,C., Williams,M., Riggleman,B. and Wieschaus,E. (1991) The segment polarity gene armadillo interacts with the wingless signaling pathway in both embryonic and adult pattern formation. Development, 111, 1029–1043. [DOI] [PubMed] [Google Scholar]
- Perrimon N. (1994) The genetic basis of patterned baldness in Drosophila. Cell, 76, 781–784. [DOI] [PubMed] [Google Scholar]
- Perrimon N. and Bernfield,M. (2000) Specificities of heparan sulfate proteoglycans in developmental processes. Nature, 404, 725–729. [DOI] [PubMed] [Google Scholar]
- Rorth P. (1998) Gal4 in the Drosophila female germline. Mech. Dev., 78, 113–118. [DOI] [PubMed] [Google Scholar]
- Roth S., Stein,D. and Nusslein-Volhard,C. (1989) A gradient of nuclear localization of the dorsal protein determines dorsoventral pattern in the Drosophila embryo. Cell, 59, 1189–1202. [DOI] [PubMed] [Google Scholar]
- Segawa H., Kawakita,M. and Ishida,N. (2002) Human and Drosophila UDP-galactose transporters transport UDP-N-acetylgalactosamine in addition to UDP-galactose. Eur. J. Biochem., 269, 128–138. [DOI] [PubMed] [Google Scholar]
- Selva E.M., Hong,K., Baeg,G.-H., Beverley,S.M., Turco,S.J., Perrimon,N. and Häcker,U. (2001) Dual role of the fringe connection gene in both heparan sulfate and fringe-dependent signalling events. Nat. Cell Biol., 3, 809–815. [DOI] [PubMed] [Google Scholar]
- Sen J., Goltz,J.S., Stevens,L. and Stein,D. (1998) Spatially restricted expression of pipe in the Drosophila egg chamber defines embryonic dorsal–ventral polarity. Cell, 95, 471–481. [DOI] [PubMed] [Google Scholar]
- Spring J., Paine-Saunders,S.E., Hynes,R.O. and Bernfield,M. (1994) Drosophila syndecan: conservation of a cell-surface heparan sulfate proteoglycan. Proc. Natl Acad. Sci. USA, 91, 3334–3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley H., Botas,J. and Malhotra,V. (1997) The mechanism of Golgi segregation during mitosis is cell type-specific. Proc. Natl Acad. Sci. USA, 94, 14467–14470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strigini M. and Cohen,S.M. (1997) A Hedgehog activity gradient contributes to AP axial patterning of the Drosophila wing. Development, 124, 4697–4705. [DOI] [PubMed] [Google Scholar]
- Sun-Wada G.H., Yoshioka,S., Ishida,N. and Kawakita,M. (1998) Functional expression of the human UDP-galactose transporters in the yeast Saccharomyces cerevisiae. J. Biochem. (Tokyo), 123, 912–917. [DOI] [PubMed] [Google Scholar]
- Toyoda H., Kinoshita-Toyoda,A., Fox,B. and Selleck,S.B. (2000) Structural analysis of glycosaminoglycans in animals bearing mutations in sugarless, sulfateless and tout-velu. Drosophila homologues of vertebrate genes encoding glycosaminoglycan biosynthetic enzymes. J. Biol. Chem., 275, 21856–21861. [DOI] [PubMed] [Google Scholar]
- Tsuda M. et al. (1999) The cell-surface proteoglycan Dally regulates Wingless signalling in Drosophila. Nature, 400, 276–280. [DOI] [PubMed] [Google Scholar]