Mapping the genetic variation of executive attention onto brain activity (original) (raw)
Abstract
Brain imaging data have repeatedly shown that the anterior cingulate cortex is an important node in the brain network mediating conflict. We previously reported that polymorphisms in dopamine receptor (DRD4) and monoamine oxidase A (MAOA) genes showed significant associations with efficiency of handling conflict as measured by reaction time differences in the Attention Network Test (ANT). To examine whether this genetic variation might contribute to differences in brain activation within the anterior cingulate cortex, we genotyped 16 subjects for the DRD4 and MAOA genes who had been scanned during the ANT. In each of the two genes previously associated with more efficient handling of conflict in reaction time experiments, we found a polymorphism in which persons with the allele associated with better behavioral performance showed significantly more activation in the anterior cingulate while performing the ANT than those with the allele associated with worse performance. The results demonstrate how genetic differences among individuals can be linked to individual differences in neuromodulators and in the efficiency of the operation of an appropriate attentional network.
Apopular theory of cognitive control suggests that the dorsal anterior cingulate is part of a network involved in handling conflict between neural areas (1, 2). In support of this general idea, a number of neuroimaging studies have shown activation of the dorsal anterior cingulate in tasks requiring people to respond to one dimension of a stimulus rather than a strong conflicting dimension (1–3). One task in which this has been found (1, 4) involves the person responding to the direction of a central arrow when flanking arrows could point either in the same (congruent) or the opposite (incongruent) direction.
The Attention Network Test (ANT) uses the flanker task to measure conflict and shows strong activation in the dorsal anterior cingulate (4, 5). Because the cingulate is modulated by the ventral tegmental dopamine system (6–8), we previously tested 200 normal persons with the ANT and genotyped them for a number of genes related to the dopamine system (9). We found polymorphisms in two genes were significantly related to the efficiency of conflict. These were the dopamine D4 receptor gene (DRD4) and monoamine oxidase a (MAOA) genes.
In the current study, we ran 16 unselected normal subjects on the ANT with event-related functional MRI (fMRI). We collected cheek cells to search for polymorphisms in the two genes which we previously found to be related to performance on the conflict network of the ANT (9). We considered only alleles possessed by at least six of our subjects, and which we thought might influence dopamine modulation within the conflict network. There were sufficient data to test one such polymorphism in each of the two previously identified genes. One of these is a 30-bp repeat polymorphism in the promoter of the MAOA gene. The other is a single-nucleotide insertion/deletion polymorphism in the 5′ region of the DRD4 gene.
One of the advantages of the molecular genetic method used here is that it can noninvasively probe genes that have been shown to result in variation in protein levels or biochemical activity. For example, transfection experiments show that the three-repeat allele of the 30-bp repeat in the MAOA promoter results in a 5-fold lower transcriptional induction than the four-repeat allele (10). This finding suggests that those subjects with the three-repeat allele may have relatively lower levels of active enzyme and thus relatively higher levels of dopamine. Some polymorphisms in the DRD4 gene have been shown to confer differences in biochemical activity (11) and have been related to behavior (12). The -1217G insertion/deletion polymorphism resides in the upstream region of the DRD4 gene and may affect transcriptional efficiency; however, biochemical evidence in this case has not been reported.
Both of these polymorphisms did show some tendency toward association with behavioral performance when we examined our larger population of 200 subjects. We ask whether they will be associated with different levels of activation in the dorsal anterior cingulate during performance of the ANT, as would be expected if the candidate genes are truly related to monitoring and processing conflict.
Methods
Participants. Participants in the behavioral–genetic study were recruited from advertisements in the New York area and Beijing (for more details, see ref. 9). Participants in the fMRI study were recruited from the New York area only. Participants with a history of psychopathology and/or who were taking medication were excluded. Participants in the fMRI study consisted of 16 right-handed normal adults (mean age, 27.2 years; SD = 5.7; range, 18–36 years; eight female, eight male). They performed the ANT while being scanned in an event-related fMRI experiment.
ANT. The details of ANT used in the behavioral genetic study are illustrated in ref. 13. In the fMRI study, stimuli and timing were adjusted to measure the brain activity optimally. Stimuli consist of a row of five visually presented horizontal black lines, with arrowheads pointing left or right against a gray background. A single arrow subtended 0.58° of visual angle, and the contours of adjacent arrows or lines were separated by 0.06° of visual angle. The stimuli (one central arrow plus four flankers) subtended a total 3.27° of visual angle. The target is a left or right arrowhead at the center. To introduce a conflict-resolution component, the central arrow is “flanked” by congruent or incongruent stimuli. The target was flanked on either side by two arrows in the same direction (congruent condition) or the opposite direction (incongruent condition). The participants' task was to identify the direction of the centrally presented arrow by pressing one key for the left and a second key for the right direction. To introduce an attentional orienting component to the task, the row of five arrows was presented in one of two locations outside the point at which the participant was fixating either 1.06° above or below the fixation point. To measure the alerting or/and orienting benefits, there were three warning conditions: no cue (baseline), center cue (alerting), and orienting cue (alerting plus orienting). In this paper, we report only the conflict effect that is target related. The conflict effect was calculated on the basis of two measurements: reaction time (RT) and error rate. For the RT, the ratio score of conflict effect was calculated as RT of incongruent condition minus RT of congruent condition divided by mean RT. Only RTs of correct responses were included for the calculation. For the error rate, conflict was calculated as error rate of incongruent condition minus error rate of congruent condition.
fMRI. We used event-related fMRI to study the changes of brain activity of these attentional networks corresponding to the task conditions. In each trial, a fixation cross first appeared in the center of the screen. At the same time, depending on the condition, a cue (asterisk) was (cued condition) or was not (no cue condition) presented for 200 ms. After a variable duration (one of a set of discrete times from 300 to 11,800 ms, approximating an exponential distribution with a mean interval of 2,800 ms), the target and flankers were presented until the participant responded with a button press, but for no longer than 1,700 ms. After the participant made a response, the target and flankers disappeared immediately, and a posttarget fixation cross appeared for a variable duration. The duration between the onset of the target and the start time of the next trial was also a variable duration (a set of discrete times from 3,000 to 15,000 ms with a mean of 6,000 ms, exponentially distributed). We isolated brain activity associated with the subtraction of the congruent condition from incongruent condition for the measurement of the conflict effect.
MRI imaging was carried out by using a General Electric Signa 3 tesla scanner. Blood oxygenation level-dependent functional images were collected by using a T2* weighted gradient echo planar imaging (EPI) sequence (TE, 35 ms; TR, 2,000 ms; flip angle, 80°) with an in-plane resolution of 3.44 × 3.44 mm (64 × 64 matrix; 220 × 220-mm field of view). Twenty-four 5-mm slices (skip 1 mm between slices) were acquired along the anterior commisure to posterior commisure plane, as determined by the midsagittal section. In-plane structural scans were collected by using a T1-weighted sequence in the same orientation as the functional sequences to provide detailed anatomic images aligned to the functional scans. A high-resolution structural MRI sequence was also acquired for the purpose of normalization. Distortion in EPI images was corrected on the basis of estimated parameters of the phase map (14).
Statistical parametric mapping (SPM) was conducted by using SPM99 (developed by Wellcome Department of Cognitive Neurology, London). A timing correction procedure was used to correct differences in image acquisition time between slices by using sinc interpolation. The spatial alignment was performed to realign the time series of images acquired from the same subject by using a least-squares approach and a six-parameter (rigid body) spatial transformation. All volumes from each participant were realigned to the first volume. EPI images were registered to each participant's T1 in-plane images and then to the high-resolution images. The high-resolution images were normalized to a standard template supplied with SPM, which approximates that of the space described in the atlas of ref. 15. Then the transformation parameters were applied to the EPI images. Voxels were resampled with 2 × 2 × 2-mm3 voxel size. An 8 × 8 × 12-mm full-width half-maximum Gaussian kernel was used to smooth the EPI images. For statistical analysis, high-pass filtering was applied to the time series of EPI images to remove the low-frequency drift in the EPI signal. The global changes in signal intensity were removed by proportional scaling. Statistical analysis was then conducted with general linear modeling. Regressors were created by convolving a train of δ functions that represents the individual trials with the base functions, which were a synthetic hemodynamic response function composed of two γ functions and its derivative (16). Six realignment parameters were used as covariates. A random-effects analysis was carried out to make inferences to the population with the resultant parameter estimates for each contrast from each subject as the input. To understand the source of the interaction between conditions (congruent and incongruent) and genotypic groups, simple comparisons were conducted. The threshold for the random effect model is P ≤ 0.05 for the height and k ≥ 50 voxel for the extent. The brain activity related to conflict was defined as the contrast of incongruent condition minus congruent condition. To examine whether genetic variations might contribute to differences in brain activation, two sample t tests were conducted.
A region-of-interest analysis was conducted to obtain activation values (the resultant parameter estimates for the contrast) from the 16 subjects for the activated anterior cingulate cortex (ACC) cluster based on the results of t test. Analysis of covariance was then conducted for the control of potential confounding variables of gender, age, the conflict effect calculated by using the RT ratio scores, and the conflict effect based on error rate.
Genotyping Analysis. Buccal swabs were obtained via buccal cell brush from consenting subjects and prepared as directed by the manufacturer. We used the MasterAMP buccal swab DNA extraction kit (Epicentre Technologies, Madison, WI). Yields range from 0.5 to 3 μg of DNA from each buccal sample and were determined spectrophotometrically by absorbance at 260 nm. Taq polymerase, PCR buffer, and dNTPs were obtained from Qiagen (Chatsworth, CA) and used at recommended concentrations for a 20-μl PCR reaction. PCR reactions and restriction digests (PCR-restriction fragment length polymorphism) are optimized for each marker and performed on the PTC-100 Programmable Thermal Controller (MJ Research, Cambridge, MA) outfitted with a heated lid for oil-free amplifications. A “touchdown” PCR cycling regimen and the addition of DMSO (10% final vol/vol) was used to automatically optimize the hybridization stringency. Gel electrophoresis in LE agarose followed by staining in ethidium bromide was used to resolve and visualize DNA fragments.
For genotyping of the MAOA LPR as described (10), forward: 5′-ACAGCCTGACCGTGGAGAAG-3′ and reverse, 5′-GAACGGACGCTCCATTCGGA-3′ primers were used. The insertion/deletion of a guanosine or “G” residue at the upstream position -1,217 was genotyped according to ref. 11 by using forward, 5′-TGCACAAGAGGGACTGAGCCTGGCT-3′ and reverse, 5′-GCGGCGCACATCCTGATGCTCTAGT-3′ followed by digestion with _Bst_EII.
Results
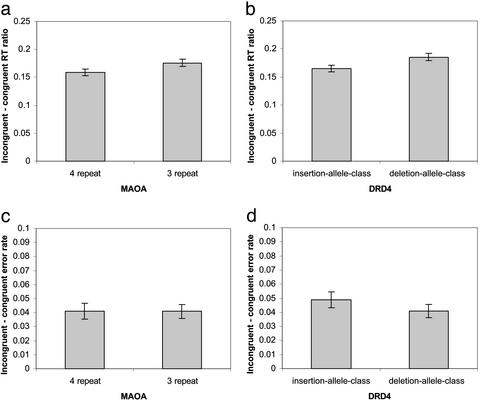
Behavioral Results. Fig. 1 a and b shows the conflict effects for the different groups defined by two genotypes and constructed from our overall sample of 200 subjects.
Fig. 1.
Genetic variation in MAOA and DRD4 and executive attention. The y axis of a and b shows the ratio conflict scores: (incongruent RT - congruent RT) divided by mean RT. The x axis in a and c indicates that subjects were grouped according to whether they were homozygous/hemizygous for the four-repeat allele of the MAOA LPR (four-repeat class, n = 55) or, alternatively, whether they were homozygous/hemizygous or heterozygous for the MAOA LPR (three-repeat class, n = 115). The x axis in b and d indicates that subjects were grouped according to whether they were homozygous for the insertion of a guanosine residue at position -1217 (insertion class, n = 112) or whether they were heterozygous for the “G” insertion/deletion polymorphism at this site (delection class, n = 71). The y axis of c and d is the conflict score based on the error/incongruent error rate - congruent error rate. (Error bar = ± 1 SE.)
In Fig. 1_a_, subjects were grouped according to whether they were homozygous/hemizygous for the four-repeat allele of the MAOA LPR (four-repeat class, n = 55) or alternatively whether they were homozygous/hemizygous or heterozygous for the three-repeat allele of the MAOA LPR (three-repeat class, n = 115). Between these two groups, the difference on ratio score of conflict effect was marginally significant [four-repeat class: conflict RT ratio, 0.159, SE, 0.006; three-repeat class, conflict RT ratio, 0.175, SE, 0.006; t (148), 1.80; P = 0.075, two-tailed, equal variances not assumed]. The differences in error rate between the two groups (see Fig. 1_c_) were not significant [four-repeat class: conflict error, 0.040, SE, 0.006; three-repeat class, conflict error, 0.040; SE, 0.005; t (168) = -0.033, P = 0.973].
In Fig. 1_b_, subjects were grouped according to whether they were homozygous for the insertion of a guanosine residue at position -1217 (insertion class, n = 112) of the DRD4 gene or whether they were heterozygous for the “G” insertion/deletion polymorphism at this site (deletion class, n = 71). Between these two groups, the difference on ratio score of conflict effect was significant [insertion class: conflict RT ratio, 0.165, SE, 0.006; deletion class, conflict RT ratio, 0.185, SE, 0.009; t (181) = 1.97, P = 0.051, two-tailed]. The differences in conflict effect of error rate (see Fig. 1_d_) were not significant [insertion class: conflict error rate, 0.048, SE, 0.008; deletion class: conflict error rate, 0.040, SE, 0.006; t (181) = 0.713, P = 0.433]. For all above statistics, analyses of covariance with age and gender as the covariates did not show significant changes.
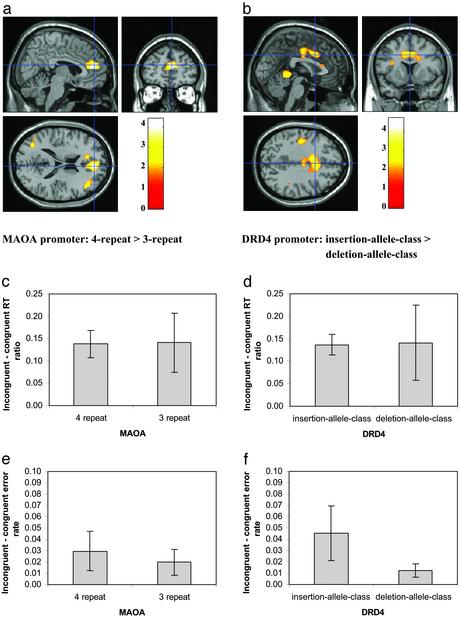
Fig. 2 c–f shows the behavioral results of the fMRI study. Although the subjects were grouped in the same fashion and showed trends (differences between genotypic groups) similar to the larger sample, no trend was significant in the sample of fMRI study.
Fig. 2.
Genetic variation in MAOA and DRD4 and brain activity. (a) Greater brain activity among subjects who were grouped according to genotype at the MAOA LPR four-repeat class (n = 8) in comparison with three-repeat class (n = 8). (b) Greater brain activity among subjects who were grouped according to genotype at the DRD4 insertion class (n = 6) in comparison with deletion class (n = 10). The color bar represents the level of t value. (c and d) The y axis shows the conflict effect based on the difference between incongruent and congruent conditions on ratio scores of RT for subjects in the corresponding genetic groups. (e and f) The y axis shows the error rate differences between incongruent and congruent conditions for each genetic group. (Error bar = ± 1 SE.)
fMRI Results. Fig. 2 a and b shows the significant differences of conflict effect in ACC between genotypic groups for the MAOA and DRD4 polymorphisms, respectively. Tables 1 and 2 show other areas in the brain where greater conflict effect was found. In the case of MAOA, the four-repeat genotypic group (n = 8) showed greater conflict effect than the three-repeat group (n = 8) in the ACC. Further analysis of the conflict effect showed that the significant interaction between allele (two groups) and task conditions (congruent and incongruent) occurred because the four-repeat class showed greater conflict effect (greater activity for the incongruent than for the congruent condition), whereas the three-repeat class showed no significant conflict effect. The difference between the two groups was not significant for the congruent condition. The difference between the two groups was significant for the incongruent condition.
Table 1. Regions showing significant differences in conflict between two genotypic groups: MAOA promoter four-repeat class minus three-repeat class.
| Region | BA | x | y | z | Maximum Z | P* | Voxel† |
|---|---|---|---|---|---|---|---|
| L/R anterior cingulate gyrus | 32 | 6 | 48 | 18 | 3.43 | 0.000 | 731 |
| L insula | -30 | -18 | 0 | 2.93 | 0.002 | 703 | |
| L middle occipital gyrus | 19 | -36 | -68 | 30 | 2.88 | 0.002 | 306 |
| R superior temporal gyrus | 22 | 54 | 4 | 2 | 2.53 | 0.006 | 298 |
| R middle frontal gyrus | 46 | 42 | 36 | 22 | 2.51 | 0.006 | 241 |
| L inferior temporal gyrus | 37 | -40 | -52 | -6 | 2.37 | 0.009 | 75 |
| R inferior parietal lobule | 40 | 62 | -42 | 36 | 2.04 | 0.021 | 61 |
Table 2. Regions showing significant differences of conflict between two genotypic groups: DRD4 promoter insertion allele class minus deletion allele class.
| Region | BA | x | y | z | Maximum Z | P* | Voxel† |
|---|---|---|---|---|---|---|---|
| L/R anterior cingulate gyrus | 32/24 | -8 | 4 | 42 | 3.60 | 0.000 | 1982 |
| Vermis | 0 | -44 | -2 | 2.82 | 0.002 | 575 | |
| R postcentral gyrus | 3 | 48 | -26 | 48 | 2.78 | 0.003 | 1207 |
| L precentral gyrus | 3 | -40 | -8 | 44 | 2.63 | 0.004 | 1033 |
| L superior parietal lobule | 7 | -20 | -64 | 54 | 2.58 | 0.005 | 268 |
| L inferior frontal gyrus | 45 | -40 | 32 | 8 | 2.46 | 0.007 | 83 |
| R middle frontal gyrus | 46 | 36 | 42 | 20 | 2.13 | 0.017 | 95 |
| R middle frontal gyrus | 8 | 28 | 4 | 54 | 2.05 | 0.020 | 64 |
| L/R anterior cingulate gyrus | 24 | 0 | 24 | 16 | 2.00 | 0.023 | 60 |
In the case of DRD4, the insertion class (n = 6) showed greater conflict effect than deletion class (n = 10) in the ACC. The difference between two groups in ACC arose because the insertion class showed significantly greater conflict effect, whereas the deletion class did not. ACC activation of the insertion class was less than that of the deletion class for the congruent condition. However, there was no significant ACC activation difference between the two groups for the incongruent condition.
A cluster of ACC activation was extracted. Analysis of covariance with ACC activation as the dependent variable and gender, age, conflict effect calculated using the ratio scores, and conflict effect based on error rate as the covariate variables showed that the difference between the two MAOA groups was still significant, F(1, 10) = 15.78, P < 0.003. The other factors were not significantly correlated to the ACC activation. For DRD4, the difference between the two groups was significant, F(1, 10) = 22.64, P < 0.001. Age was positively correlated with ACC activation, F(1, 10) = 12.33, P < 0.006. Ratio score of conflict effect based on RT was positively correlated to ACC activation for the conflict effect, F(1, 10) = 6.52, P < 0.029.
Discussion
Many cognitive processes have been studied at the network level by neuroimaging. These include attentional networks (1–5), word reading (17, 18), music (19), faces (20), episodic memory (21), and many others. Each of these networks involves different anatomical areas, and each has its own time course of development. Although the general anatomy of each network is relatively fixed, there are clearly individual anatomical variations (22) and behavioral differences in the efficiency of their operation (13).
We have been working with attentional networks, particularly the executive attention network, which is involved in control of cognition and emotion (1) and is frequently activated by conflict (2). The most commonly activated node of this network is the anterior cingulate gyrus. Because this is a dopamine-rich brain area, we have surveyed a number of dopaminergic genes to see whether they influence performance on a RT test known to tap activity in the anterior cingulate.
We have found two genes that influence the efficiency with which normal people handle conflict (9). Although our imaging study did not have sufficient subjects in each genotypic class to examine these two polymorphisms, we did have enough to examine two other polymorphisms in these genes. These two polymorphisms showed similar but nonsignificant differences in the conflict network of the ANT. However, we did find that the two polymorphisms produced significant differences in the degree of activation in an important node of the executive attention network. This finding closes the loop in showing that genes involved in modulating behavioral performance influence brain activity in a node of the network that mediates that performance. We expect that, in a larger study, we would find similar activation differences for the other alleles of the DRD4 and MAOA genes.
We found that higher efficiency in resolving conflict is associated with more activity in the anterior cingulate. In neuroimaging studies, it is common for practice, priming, or maturation to be associated with decreased activity as higher levels of skill are obtained. We are not sure why the opposite is true here. In the case of maturation, there is some question; for example, Schlaggar et al. (23) found some areas of the brain that showed increases in strength of activation with age and others that showed decreases. Priming and practice usually increase efficiency and reduce neuronal activity, but these are within-subject effects. The effects that we report are between subjects and, in this case, it is less clear whether increases in skill between subjects will be associated with more or less activation.
One study compared adult subjects normal in attention with adults with attentional difficulties in a conflict task similar to the one used in our study (24). The normal subjects, presumably with higher attentional efficiency, showed more cingulate activation than those suffering from Attention Deficit Hyperactivity Disorder. An exact understanding of the mechanism by which variations in the MAOA and DRD4 genes influence neural activity and behavior will require additional human and perhaps animal studies.
We have mainly discussed differences in cingulate activity, both because that was our original hypothesis and because it is the strongest overall area of activation in both Tables 1 and 2. However, Table 1 shows strong activation of the insula, which is sometimes thought to be an additional pathway important for more automatic forms of behavior (25). Table 2 shows a strong activation in the cerebellar vermis. Recent evidence has shown this brain region to be a target of the dopamine neuromodulatory system (26).
The development of attentional networks must involve both genes and specific experience. The executive attention network as indexed by the ANT shows a strong development from 4 to 7 years of age but does not change from age 7 to adulthood (27). During this period, the efficiency of executive control as measured by our test also predicts a number of behavioral and questionnaire measures of attentional regulation among normal persons (28). Our findings should allow the possibility of tracing differences in the development of this network for children with different genotypes. We have developed training exercises designed to influence the rate of development of the network. The use of these exercises with children from different genetic backgrounds gives the opportunity for specific studies of the genetic–environmental interaction (28).
We found differences in brain activity in the anterior cingulate with many fewer subjects than would have been necessary to find differences in our behavioral task. This finding suggests that the genetic effect is more closely linked to brain activity than to actual behavioral output. Two recent studies of cognitive networks (21, 29) underlying episodic and working memory provide examples of the strategy used in the current paper. One study examined the influence of the L. catechol-d-methyl transferase (COMT) gene on prefrontal activation (29). The authors found significant differences between alleles on the efficiency of activation during a working memory task. One area of activation was in the anterior cingulate, which fits with the view that working memory involves executive attention as one component. In the other study (21), a polymorphism in the brain-derived neurotrophic factor (BDNF) gene operated in the hippocampus to influence the performance of normal people on a learning task. The authors suggest that it may be possible to apply this method to other cognitive networks, and that relatively fewer subjects may be needed to detect differences in fMRI than would be required to see these effects in behavior. Our results support both of these ideas. The attention networks involve brain areas quite distinct from the hippocampal area. In these networks, we also find that specific polymorphisms influence local activation within the network. In our case as well, much larger samples would have been required to obtain statistical significance than was true for the fMRI result. These results support the use of candidate genes as an approach to understanding the individual development of cognitive networks.
Acknowledgments
We thank Drs. Yihong Yang, Hong Pan, and Hong Gu for help and members of the Sackler Institute for helpful discussions. This work was supported by National Science Foundation Grant BCS 9907831 and James S. McDonnell 21st Century Award. J.F. acknowledges support from the National Institute of Mental Health (National Research Service Award No. 1 F32 MH64360-01A1), a Young Investigator Award from the National Alliance for Research on Schizophrenia and Depression, and a DeWitt Wallace–Reader's Digest Research Fellowship in Psychiatry.
Abbreviations: ANT, Attention Network Test; DRD4, dopamine D4 receptor gene; MAOA, monoamine oxidase a; fMRI, functional MRI; EPI, echo planar imaging; ACC, anterior cingulate cortex; RT, reaction time.
References
- 1.Bush, G., Luu, P. & Posner, M. I. (2000) Trends Cognit. Sci. 4**,** 215-222. [DOI] [PubMed] [Google Scholar]
- 2.Botvinick, M. M., Braver, T. S., Barch, D. M., Carter, C. S. & Cohen, J. D. (2001) Psychol. Rev. 108**,** 624-652. [DOI] [PubMed] [Google Scholar]
- 3.MacDonald, A. W., Cohen, J. D., Stenger, V. A. & Carter, C. S. (2000) Science 288**,** 1835-1838. [DOI] [PubMed] [Google Scholar]
- 4.Fan, J., Flombaum, J. I., McCandliss, B. D., Thomas, K. M. & Posner, M. I. (2003) NeuroImage 18**,** 42-57. [DOI] [PubMed] [Google Scholar]
- 5.Fan, J., McCandliss, B. D., Flombaum, J. I. & Posner, M. I. (2001) in Society for Neuroscience 2001 Annual Meeting (Soc. Neurosci., San Diego), p. 215.
- 6.Berger, B., Trottier, S., Verney, C., Gaspar, P. & Alvarez, C. (1988) J. Comp. Neurol. 273**,** 99-119. [DOI] [PubMed] [Google Scholar]
- 7.Gaspar, P., Bloch, B. & Le Moine, C. (1995) Eur. J. Neurosci. 7**,** 1050-1063. [DOI] [PubMed] [Google Scholar]
- 8.Williams, S. M. & Goldman-Rakic, P. S. (1998) Cereb. Cortex 8**,** 321-345. [DOI] [PubMed] [Google Scholar]
- 9.Fossella, J., Sommer, T., Fan, J., Wu, Y., Swanson, J. M., Pfaff, D. W. & Posner, M. I. (2002) BMC Neurosci. 3**,** 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sabol, S. Z., Hu, S. & Hamer, D. (1998) Hum. Genet. 103**,** 273-279. [DOI] [PubMed] [Google Scholar]
- 11.Okuyama, Y., Ishiguro, H., Nankai, M., Shibuya, H., Watanabe, A. & Arinami, T. (2000) Mol. Psychiatry 5**,** 64-69. [DOI] [PubMed] [Google Scholar]
- 12.Swanson, J., Oosterlaan, J., Murias, M., Moyzis, R., Schuck, S., Mann, M., Feldman, P., Spence, M. A., Sergeant, J., Smith, M., et al. (2000) Proc. Natl. Acad. Sci. USA 97**,** 4754-4749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fan, J., McCandliss, B. D., Sommer, T., Raz, A. & Posner, M. I. (2002) J. Cognit. Neurosci. 14**,** 340-347. [DOI] [PubMed] [Google Scholar]
- 14.Jezzard, P. & Balaban, R. S. (1995) Magn. Reson. Med. 34**,** 65-73. [DOI] [PubMed] [Google Scholar]
- 15.Talairach, J. & Tournoux, P. (1988) Co-Planar Stereotaxic Atlas of the Human Brain (Theime, New York).
- 16.Friston, K. J., Fletcher, P., Josephs, O., Holmes, A., Rugg, M. D. & Turner, R. (1998) NeuroImage 7**,** 30-40. [DOI] [PubMed] [Google Scholar]
- 17.Shaywitz, S. E., Shaywitz, B. A., Pugh, K. R., Fulbright, R. K., Constable, R. T., Mencl, W. E., Shankweiler, D. P., Liberman, A. M., Skudlarski, P., Fletcher, J. M., et al. (1998) Proc. Natl. Acad. Sci. USA 95**,** 2636-2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Posner, M. I. & McCandliss, B. D. (1999) in Converging Methods for Understanding Reading and Dyslexia, eds. Klein, R. & McMullen, P. (MIT Press, Cambridge, MA), pp. 305-337.
- 19.Janata, P., Birk, J. L., Van Horn, J. D., Leman, M., Tillmann, B. & Bharucha, J. J. (2002) Science 298**,** 2167-2170. [DOI] [PubMed] [Google Scholar]
- 20.Liu, J., Harris, A. & Kanwisher, N. (2002) Nat. Neurosci. 5**,** 910-916. [DOI] [PubMed] [Google Scholar]
- 21.Egan, M. F., Kojima, M., Callicott, J. H., Goldberg, T. E., Kolachana, B. S., Bertolino, A., Zaitsev, E., Gold, B., Goldman, D., Dean, M., et al. (2003) Cell 112**,** 257-269. [DOI] [PubMed] [Google Scholar]
- 22.Miller, M. B., Van Horn, J. D., Wolford, G. L., Handy, T. C., Valsangkar-Smyth, M., Inati, S., Grafton, S. & Gazzaniga, M. S. (2002) J. Cognit. Neurosci. 14**,** 1200-1214. [DOI] [PubMed] [Google Scholar]
- 23.Schlaggar, B. L., Brown, T. T., Lugar, H. M., Visscher, K. M., Miezin, F. M. & Petersen, S. E. (2002) Science 296**,** 1476-1479. [DOI] [PubMed] [Google Scholar]
- 24.Bush, G., Frazier, J. A., Rauch, S. L., Seidman, L. J., Whalen, P. J., Jenike, M. A., Rosen, B. R. & Biederman, J. (1999) Biol. Psychiatry 45**,** 1542-1552. [DOI] [PubMed] [Google Scholar]
- 25.Raichle, M. E., Fiez, J. A., Videen, T. O., MacLeod, A. M., Pardo, J. V., Fox, P. T. & Petersen, S. E. (1994) Cereb. Cortex 4**,** 8-26. [DOI] [PubMed] [Google Scholar]
- 26.Melchitzky, D. S. & Lewis, D. A. (2000) Neuropsychopharmacology 22**,** 466-472. [DOI] [PubMed] [Google Scholar]
- 27.Rueda, M. R., Posner, M. I. & Rothbart, M. K. (2003) in Handbook of Self Regulation, eds. Baumeister, R. F. & Vohs, K. D. (Guilford, New York), in press.
- 28.Posner, M. I. & Rothbart, M. K. (2000) Dev. Psychopathol. 12**,** 427-441. [DOI] [PubMed] [Google Scholar]
- 29.Egan, M. F., Goldberg, T. E., Koachana, B. S., Callicott, J. H., Mazzanti, C. M., Straub, R. E., Goldman, D. & Weinberger, D. R. (2001) Proc. Natl. Acad. Sci. USA 98**,** 6917-6922. [DOI] [PMC free article] [PubMed] [Google Scholar]