Early anaerobic metabolisms (original) (raw)
Abstract
Before the advent of oxygenic photosynthesis, the biosphere was driven by anaerobic metabolisms. We catalogue and quantify the source strengths of the most probable electron donors and electron acceptors that would have been available to fuel early-Earth ecosystems. The most active ecosystems were probably driven by the cycling of H2 and Fe2+ through primary production conducted by anoxygenic phototrophs. Interesting and dynamic ecosystems would have also been driven by the microbial cycling of sulphur and nitrogen species, but their activity levels were probably not so great. Despite the diversity of potential early ecosystems, rates of primary production in the early-Earth anaerobic biosphere were probably well below those rates observed in the marine environment. We shift our attention to the Earth environment at 3.8 Gyr ago, where the earliest marine sediments are preserved. We calculate, consistent with the carbon isotope record and other considerations of the carbon cycle, that marine rates of primary production at this time were probably an order of magnitude (or more) less than today. We conclude that the flux of reduced species to the Earth surface at this time may have been sufficient to drive anaerobic ecosystems of sufficient activity to be consistent with the carbon isotope record. Conversely, an ecosystem based on oxygenic photosynthesis was also possible with complete removal of the oxygen by reaction with reduced species from the mantle.
Keywords: Archaean, evolution, hydrogen, anoxygenic photosynthesis, iron, metabolism
1. Introduction
Nearly, all primary production on the present Earth is accomplished by photosynthetic organisms producing oxygen. These oxygenic phototrophs include a vast array of different plants, algae and cyanobacteria, and since they accomplish most of the primary production, they also drive the carbon cycle. Thus, oxygenic photosynthesis maintains the levels of biological activity that the Earth presently enjoys.
Oxygen production originated with the evolution of cyanobacteria. However, cyanobacteria were not the earliest photosynthetic organisms (Blankenship 1992; Olson & Blankenship 2004) nor were they members of the earliest ecosystems on Earth (e.g. Cloud 1972; Garrels & Perry 1974; Canfield & Raiswell 1999; Nisbet & Sleep 2001; Knoll 2003; Battistuzzi et al. 2004). Therefore, the earliest Earth ecosystems existed in an anoxic world and their activities were driven by anaerobic metabolisms. Since the activity of the present biosphere is so critically coupled to oxygenic photosynthesis, one can reasonably assume that the activity level of the early biosphere would have been different in the absence of oxygen production. This topic has received very little attention. However, in one report, Des Marais (2000) provided rough calculations suggesting that early anaerobic ecosystems were probably 2–3 orders of magnitude less active than the present biosphere. More recently, Canfield (2005) and Kharecha et al. (2005) have expanded this view, and in different ways, they have found that early anaerobic ecosystems may have enjoyed activity levels higher than previously thought. These ideas will be reviewed and more fully developed in the present contribution, which will focus on the structure and activity level of ancient anaerobic ecosystems.
2. Early energy and possible community structure
There are several entrance points for discussing the structure and activity of early anaerobic microbial communities. We could, for example, begin by considering the history of early metabolic evolution as evidenced through the comparisons of RNA and gene sequences (e.g. Woese 1987). However, modern microbial genomes have been heavily impacted by the lateral transfer of genetic material (Hilario & Gogarten 1993; Brown & Doolittle 1997; Jain et al. 1999). Thus, phylogenies based on genomic data may not necessarily represent the evolutionary history of a particular metabolism (e.g. Hilario & Gogarten 1993; Doolittle 1999). Therefore, we take a different approach, and base our analysis on the availability of electron acceptors and electron donors. We will assume that during the course of early-Earth evolution, organisms had evolved to take advantage of the available energy to fuel them. This is reasonable, but we know it is not strictly valid. For example, water has been available as an electron donor for photosynthesis (producing oxygen) over at least 95% of Earth history (Wilde et al. 2001; Cavosie et al. 2005), and was a necessary prerequisite for the origin of life. Yet, as mentioned earlier, oxygenic photosynthesis was not among the earliest-evolved metabolisms, as it required the earlier evolution of at least two types of anoxygenic photosynthetic reaction centres and pigment synthetic pathways (Blankenship 1992, 2001; Xiong et al. 2000). However, the tree of life demonstrates a wide range of early innovations in anaerobic metabolism (Woese 1987; Stetter 1996; Canfield & Raiswell 1999), and it is reasonable to assume that most, if not all, of the primary metabolisms outlined in tables 1 and 2 indeed predated oxygenic photosynthesis.
Table 1.
Primary electron donors to early-Earth ecosystems.
| electron donor | source | metabolism |
|---|---|---|
| H2 | subaerial and subaqueous volcanoes | methanogenesis |
| anoxygenic photosynthesis | ||
| sulphate reduction | ||
| sulphur reduction | ||
| iron reduction | ||
| acetogenesis | ||
| denitrification | ||
| H2S | subaerial and subaqueous volcanoes | anoxygenic photosynthesis |
| nitrate reduction | ||
| S0 | subaerial volcanoes (atmospheric reactions) | anoxygenic photosynthesis |
| sulphur disproportionation | ||
| nitrate reduction | ||
| Fe2+ | subaqueous volcanoes, weathering | anoxygenic photosynthesis |
| nitrate reduction | ||
| CH4 | subaqueous volcanoes | anaerobic methane oxidation |
| NH4+ | subaqueous volcanoes | anammox |
| CH2O | subaqueous volcanoes | heterotrophic metabolisms |
Table 2.
Primary electron acceptors.
| electron acceptor | source | metabolism |
|---|---|---|
| CO2 | subaerial and subaqueous volcanoes | methanogenesis |
| anoxygenic photosynthesis | ||
| acetogenesis | ||
| CO | atmospheric reactions | acetogenesis |
| SO42− | subaerial volcanoes (atmospheric reactions) | sulphate reduction |
| S0 | subaerial volcanoes (atmospheric reactions) | sulphur reduction |
| NO (NO3− and NO2−) | lightning | denitrification |
| anammox | ||
| nitrate reduction |
With these considerations in mind, we explore the most probable primary electron donors and electron acceptors fuelling early anaerobic metabolisms. We begin by looking at electron donors. Most of these have volcanic sources, emanating from either subaqueous hydrothermal volcanics or subaerial volcanics (table 1). Hydrogen gas is delivered from both subaqueous and subaerial volcanics as H2S (e.g. Kadko et al. 1994; Symonds et al. 1994; Halmer et al. 2002). Elemental sulphur does not have a direct volcanic source. However, it can form through the photolysis of volcanic SO2 gas (Farquhar et al. 2001; Pavlov & Kasting 2002; Ono et al. 2003) or from the SO2 released in hydrothermal settings (Canfield & Raiswell 1999). Ferrous iron has a direct hydrothermal source as observed, for example, at mid-ocean ridge hydrothermal settings (e.g. Kadko et al. 1994), but it can also enter the oceans through the weathering of continental crust. This has probably occurred through all of Earth history where subaerial crust was present. Finally, there may have been an important source of ammonium through the reduction of N2 during mid-ocean ridge hydrothermal circulation (Brandes et al. 1998_a_,b).
A number of primary electron acceptors can also be identified (table 2). Of these, CO2 has a direct volcanic source, while elemental sulphur (as just discussed) and sulphate can form through the photolysis of SO2 in the atmosphere. An early primary source of nitrate was also probably through the oxidation of N2 by lightning (Yung & McElroy 1979; Navarro-González et al. 2001). The electron donors and electron acceptors which become available as products of secondary anaerobic metabolisms are listed in table 3. Most of these are the same as those found from primary sources. A notable exception is iron oxides, which form as a product of anoxygenic photosynthesis with Fe2+ as the electron donor (Widdel et al. 1993; Heising et al. 1999; Jiao et al. 2005).
Table 3.
Secondary electron acceptors and electron donors. (Reactions in italics are believed to have been minor processes on the early Earth.)
| source | |
|---|---|
| electron acceptor | |
| CO2 | organic respiration |
| SO42− | anoxygenic photosynthesis |
| S0 | anoxygenic photosynthesis |
| (nitrate reduction with H 2 S) | |
| (reaction with Fe oxides) | |
| Fe oxides | anoxygenic photosynthesis |
| electron donor | |
| H2 | fermentation |
| N2-fixation | |
| H2S | sulphate reduction |
| S0 reduction | |
| S0 disproportionation | |
| S0 | anoxygenic photosynthesis |
| (nitrate reduction with H 2 S) | |
| (reaction with Fe oxides) | |
| Fe2+ | heterotrophic Fe-oxide reduction |
| (reaction of Fe oxides with H 2 S) | |
| CH4 | methanogenesis |
| NH4+ | ammonification |
| nitrate reduction with H2S, S0 and CH2O | |
| CH2O | anoxygenic photosynthesis, secondary production |
From the lists of electron donors and electron acceptors, and the metabolisms they support, we can construct possible ancient ecosystems. By considering the probable limits on the source strength of the most limiting chemical compound, be it electron donor or electron acceptor, we can place limits on the activity level of the ancient ecosystem. Following Canfield (2005) and Kharecha et al. (2005), we will consider various ecosystems in relative isolation. This means that for each ecosystem, we concentrate on the cycling of a limited number of related elements which are involved in active recycling. There would have indeed been cross-coupling between element cycles, but including this complexity would probably not increase our estimates of the activity level of the ancient biosphere, but it could give us a more realistic picture of the dynamics of elemental cycling. For this reason, we will also discuss in some cases how different element cycles might have been linked.
3. Hydrogen-based ecosystems
Kharecha et al. (2005) considered the dynamics of two different hydrogen-based ecosystems. These included an ecosystem where hydrogen is used primarily as an electron donor in anoxygenic photosynthesis, and another, where hydrogen is used to fuel methanogenesis. The importance of hydrogen as an early fuel for anoxygenic photosynthesis has also been emphasized by Olson (2006). The treatment presented here follows Kharecha et al. (2005) with some modifications. In one sense, the treatment here is simpler, as Kharecha et al. (2005) introduced CO-consuming acetogens into their model. This arises because CO can accumulate in an anoxic atmosphere from the photochemical oxidation of CH4 (see below). We do not consider this in our model. Neglecting this, however, does not influence primary production rates, as in our simplified model we conserve the same amount of electron equivalents in primary produced biomass as do Kharecha et al. (2005).
We will consider first an ecosystem fuelled by hydrogen where anoxygenic phototrophs are the primary producers (figure 1). Anoxygenic photosynthesis with hydrogen can be written quite simply as
| 2H2+CO2+hv→CH2O+H2O. | (3.1) |
|---|
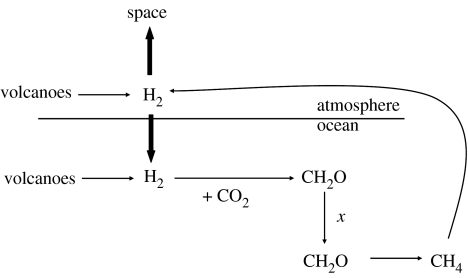
Figure 1.
Early-Earth microbial ecosystem driven by hydrogen-based anoxygenic photosynthesis. The primary sources of hydrogen are subaqueous and subaerial volcanoes. The organic matter produced by photosynthesis is decomposed by methanogenesis, a fraction of which, x, is buried in sediments. Methane is reconverted to hydrogen by photolysis reactions in the atmosphere. See text for details.
The organic matter produced by anoxygenic phototrophs will decompose, and in this simple hydrogen-based ecosystem, methanogenesis is assumed to be the most important mineralization pathway:
Most of the organic matter produced by anoxygenic phototrophs will decompose, but some fraction, x, will be preserved, and this represents a removal vector for hydrogen, where 2H2 are removed for every CH2O preserved. If we define fluxmar as the flux of H2 used to fuel primary production, then the burial flux of H2 as organic matter, fluxbur, is
The methane produced during the decomposition of organic matter will escape to the atmosphere, where through photolysis, it will decompose back to hydrogen gas (Catling et al. 2001; Pavlov et al. 2001), and overall, two molecules of H2 and one molecule of CO2 are produced for every molecule of CH4 photolysed.
Hydrogen is also lost through escape from the atmosphere to space. There is currently a great deal of discussion and uncertainty on the probable pathways and magnitude of H2 escape on the early Earth (Catling & Claire 2005; Tian et al. 2005). Most early models assumed that hydrogen escape would have been diffusion limited (Hunten 1973; Walker 1977), where the flux can be easily calculated with the expression
| fluxesc=2.5×1013ftot, | (3.4) |
|---|
where fluxesc represents the escape flux of hydrogen from the atmosphere (molecules cm−2 s−1) and _f_tot represents the mixing ratio (v/v) of all hydrogen-bearing species above the tropopause, where H2O is generally absent
| ftot=f(H2)+2f(CH4)+f(H2O)+⋯. | (3.5) |
|---|
By contrast, Tian et al. (2005) argue that on the early Earth, in the absence of atmospheric oxygen, a lower exobase temperature would have resulted. This, in turn, would have resulted in lower hydrogen escape rates (at the same hydrogen concentration) than predicted by diffusion-limited escape. Catling & Claire (2005) counter that the cold exobase temperatures calculated by Tian et al. (2005) rest on a simplified view of upper atmospheric chemistry and may not be correct. In what follows, we will assume that H2 escape to space from the upper atmosphere is diffusion limited. We then ignore water and combine equations (3.4) and (3.5) to yield
| fluxesc=A[f(H2)+2f(CH4)], | (3.6) |
|---|
where A represents the constant 2.5×1013.
The source of hydrogen is volcanic outgassing, whose flux we is designate as fluxvolc. Overall, a hydrogen balance can be written as
| fluxvolc=fluxesc+fluxbur=fluxesc+xfluxmar. | (3.7) |
|---|
Our ultimate goal will be to calculate fluxmar, which, as discussed earlier, represents the flux of hydrogen used in primary production. Since 2 mol of hydrogen are used to form 1 mol of organic carbon, the primary production rate of organic carbon, fluxphoto is one-half of fluxmar. We assume, as did Kharecha et al. (2005), that primary production is limited by the flux of hydrogen across the sea–water interface. This flux depends on the concentration gradient of hydrogen across this interface, the diffusion coefficient for hydrogen in water and the thickness of the diffusive boundary layer in the upper ocean layer over which diffusion applies. A piston velocity term, _V_p (cm s−1) (e.g. Broecker & Peng 1982), is often used to represent gas transport across the air–sea interface and is calculated by combining the diffusion coefficient for H2 in water (taken here as 5×10−5 cm2 s−1; Kharecha et al. 2005) with the length-scale (taken here as 0.004 cm; Broecker & Peng 1982) over which diffusion applies. Thus, _V_p=1.3×10−2 cm s−1. Overall, fluxmar (molecules cm−2 s−1) is given by
| fluxmar=VpD(αPH2−[H2]), | (3.8) |
|---|
where, in addition to the terms already defined, D is a constant of proportionality (6.02×1020 molecules cm−3 mol−1 l) for converting to the flux units used here, α is the Henry's law constant for H2 (7.8×10−4 M atm−1; see summary in Canfield et al. 2005), PH2 is the partial pressure (atm) of H2 at the ocean surface and [H2] is the concentration (M) of hydrogen in the surface ocean. We assume that in the air just above the ocean surface f(H2)=PH2.
With this assumption, equation (3.8) is combined with equation (3.6) to yield
| fluxesc=A(fluxmar+Vp[H2]D)VpαD+2Af(CH4). | (3.9) |
|---|
This expression is further combined with equation (3.7) to yield
| fluxvol=A(fluxmar+Vp[H2]D)VpαD+2Af(CH4)+xfluxmar. | (3.10) |
|---|
To solve this equation, we must find an alternative expression for f(CH4). Pavlov et al. (2001) have solved an atmospheric model relating f(CH4) with flux of methane to the atmosphere. The model is more or less linear at f(CH4) below 10−4, and the following equation fits the model results:
| f(CH4)=fluxmethane1014.43, | (3.11) |
|---|
where fluxmethane is the flux of methane to the atmosphere in units of molecules cm−2 s−1.
We assume, following Catling & Claire (2005) and Kharecha et al. (2005), that anoxygenic phototrophs will reduce the concentration of hydrogen in the surface ocean to negligible amounts, and therefore [H2] is essentially zero (this will not be the case with the methane-based ecosystem). We are left, then, with the flux of methane to the atmosphere. We assume that the abiotic sources are relatively small, and therefore the decomposition of organic matter produced by anoxygenic phototrophs represents the main methane source to the atmosphere. From equations (3.1) and (3.2), 4 mol of H2 are used to produce 1 mol of methane, and therefore, fluxmethane is represented by
| fluxmethane=0.25(1−x)fluxmar, | (3.12) |
|---|
and from equations (3.11) and (3.12),
| f(CH4)=0.25(1−x)fluxmethane1014.43=B(1−x)fluxmar, | (3.13) |
|---|
where B is 9×10−16. Finally, equations (3.10) and (3.13) are combined to yield
| fluxmar=VpαDfluxvol[A+2ABVpαD(1−x)+xVpαD]. | (3.14) |
|---|
The values for constants used in this equation are summarized in table 4.
Table 4.
Constants used in modelling hydrogen-based ecosystems.
| constant | value | units |
|---|---|---|
| _V_p | 1.3×10−2 | cm yr−1 |
| D | 6.02×1020 | molecules cm−3 mol−1 l |
| α | 7.8×10−4 | M atm−1 |
| A | 2.5×1013 | molecules cm−2 s−1 |
| B | 9×10−16 | molecules−1 cm2 s |
| [H2] | 0 (anoxygenic photosynthesis) | M |
| [H2] | 5×10−9 (methanogenesis) | M |
From equation (3.14), rates of hydrogen used in primary production, fluxmar, are controlled by two variables: the preservation efficiency of hydrogen in organic matter, x, and the volcanic outgassing flux of hydrogen, fluxvol. Reasonable values for x, i.e. the preservation proportion of organic carbon in an anoxic setting, probably range between 0.01 and 0.1 (Canfield 1994; Arthur & Dean 1998). Values for the volcanic flux of hydrogen require some detailed consideration.
The hydrogen flux from hydrothermal systems, such as those found in mid-ocean ridge spreading centres, is determined by combining H2 concentration measurements in the high-temperature vent fluids with the volume flux of water through the high-temperature vents (Kadko et al. 1994). These calculations yield hydrogen fluxes in the range of 7–27×109 mol yr−1 (see table 7), with most of the uncertainty owing to variability in the concentrations of hydrogen in the end-member hydrothermal vent fluids.
Table 7.
Volcanic H2 flux to the surface environment.
| H2 flux (×1011 mol yr−1) | |
|---|---|
| subaerial volcanoes | 0.9–3.4 |
| ocean crust serpentinization | 0.8–1.3 |
| mid-ocean ridge volcanoes | 0.07–0.27 |
| total flux | 1.8–5.0 |
| from Holland (2002) | 47 |
Hydrogen is also produced through the serpentinization of ocean crust peridotite (e.g. Alt & Shanks 2003; Bach et al. 2006), where the electrons for hydrogen formation come from the oxidation of ferrous iron. The formation of hydrogen through this process can be written as follows, although the reaction sequence and reaction products are more complex (Bach et al. 2006):
| 3Fe2SiO2+2H2O→3SiO2+2Fe3O4+2H2. | (3.15) |
|---|
We determine the hydrogen flux by calculating the total amount of serpentinization which is likely to occur in a year. We begin with Alt & Shanks (2003) who estimate that from 3 to 5% of the ocean crust is serpentinized peridotite. Overall, about 3 km2 of new ocean floor are produced every year at ocean spreading centres, and with the crustal depth of about 1 km and a density for crustal peridotite of about 3.5 g cm−3, 3.2–5.3×1014 g of serpentinized peridotite is produced every year. Bach et al. (2006) calculate that 1 mol of H2 is produced for every 4200 g of peridotite serpentinized. Altogether, an H2 production rate of 0.8–1.3×1011 mol yr−1 is calculated (see table 7). This flux is important and exceeds the flux from mid-ocean ridges by several times (see above).
Finally, there is the flux from subaerial volcanoes. This flux is difficult to determine directly, but it can be approximated from the flux of SO2, which is reasonably well constrained by a combination of satellite and ground-based observations (e.g. Halmer et al. 2002). Thus, the hydrogen flux may be determined by combining the SO2 flux with the ratio of SO2 : H2 concentrations in volcanic subaerial emanations. A compilation of volcanic gas data for a variety of different volcanoes is presented in table 5. These volcanoes include those from convergent plate margins (S), as well as those associated with rifting (the formation of new continental plates, R), and hotspot volcanics (H). As can be seen, the concentrations of SO2 and H2 vary widely, as do the SO2 : H2 ratios. As convergent margins and rift-associated volcanics represent different tectonic regimes, we analyse the data both within each regime and taking all volcanoes together. If we calculate the SO2 : H2 ratio from average SO2 and H2 concentrations within each tectonic setting, we see that each setting gives an SO2 : H2 ratio of around 3 (table 6). If we consider all volcanoes together, the same average concentration is obtained. We recognize that a small number of volcanoes have very high concentrations of SO2 and H2, which can greatly skew the calculated average concentrations. Therefore, we also calculate SO2 : H2 from the median concentrations within each tectonic setting. Here, some differences are observed. Subduction-related volcanoes give a ratio of 0.7, whereas those from rift settings give a much higher ratio of 8.4, and a ratio of around 1 is calculated from all volcanoes taken together.
Table 5.
Compilation of volcanic gas data. (S, subduction-related volcanics; R, rift-zone-related volcanics; H, hotspot volcanics; —, not measured. Data from compilations in Halmer et al. (2002) and Symonds et al. (1994).)
| volcano | type | SO2 (mol%) | H2 (mol%) | H2S (mol%) |
|---|---|---|---|---|
| Etna, Italy | S, hawaiite | 25.2 | 0.5 | 3.4 |
| Iwo Shimo, Japan | S, andesite | 0.4 | — | — |
| Usu, Japan | S, dacite–andesite | 0.23 | 0.26 | 0.26 |
| Showa-Shinzan, Japan | S, andesite | 0.21 | 0.31 | 0.53 |
| Mt St Helens, USA | S, dacite | 0.7 | 2.4 | 1.4 |
| Tolbachik, Kamchatka | S, basalt | 0.08 | 1.89 | 0.14 |
| Klyuchevskoy, Kamchatka | S, calcalkaline basalt | 0.1 | 1.15 | — |
| White Island, NZ | S, andesite | 1.1 | — | 0.5 |
| Ngauruhoe, NZ | S, andesite | 10.2 | 2.6 | 6.8 |
| Momotombo, Nicaragua | S, tholeiitic basalt | 0.41 | 0.45 | 0.23 |
| Paos, Costa Rica | S, tholeiitic basalt | 1.63 | 0.58 | 0.06 |
| Gunung, Merapi | S, andesite | 0.64 | 1.07 | 0.059 |
| Mt St Augustine, Alaska | S, andesite | 0.08 | 0.54 | 0.22 |
| Mt St Augustine (spires) | S, andesite | 5.95 | 0.59 | 0.85 |
| Mt St Augustine (domes) | S, andesite | 0.26 | 0.55 | 0.33 |
| Nyiragongo, Africa | R, melilite–nephelinite | 18.3 | 14.9 | 1.6 |
| Erta'Ale, Africa | R, tholeiitic basalt | 14.2 | 1.7 | 0.2 |
| Erta'Ale, Africa | R, tholeiitic basalt | 8.3 | 1.5 | — |
| Aroukoba, Africa | R, tholeiitic basalt | 14.2 | 1.7 | 1.7 |
| Surtsey, Iceland | R, alkali-basalt | 3.3 | 1.7 | 0.12 |
| Kilauea, Hawaii | H, tholeiitic basalt | 14.6 | 0.7 | 0.11 |
Table 6.
Gas ratios (molar) from volcanic emanations.
| SO2 : H2 | SO2 : H2S | |
|---|---|---|
| average concentrations S volcanoes | 3.2 | 2.8 |
| average concentrations R+H volcanoes | 3.3 | 16.2 |
| average concentrations all volcanoes | 3.2 | 5.5 |
| median concentrations S volcanoes | 0.7 | 1.2 |
| median concentrations R+H volcanoes | 8.4 | 5.5 |
| median concentrations all volcanoes | 1.0 | 4.8 |
From this analysis, we conclude that hydrogen enters the atmosphere from subaerial volcanoes with an SO2 : H2 ratio of between 1 and 3. If we combine this with an estimated SO2 volcanic flux of 2.3–3.1×1011 mol yr−1 (Halmer et al. 2002), we obtain an H2 flux ranging from about 0.8 to 3.1×1011 mol yr−1. The hydrogen fluxes from all sources are summarized in table 7. The total hydrogen flux to the ocean–atmosphere system is estimated at 1.8–5.0×1011 mol yr−1. This flux is 10–26 times lower than the estimate of Holland (2002), which is calculated by combining the subaerial volcanic CO2 flux with the assumption that these gases are in equilibrium with the FMQ (quartz, magnetite, fayalite) buffer (table 7). We believe that our flux estimate is well grounded in current observations.
We are now ready to use equation (3.14) from which rates of primary production can be calculated. We begin with a burial efficiency, x, of 0.01, and with a volcanic H2 flux of 3.4×1011 mol yr−1, which is the average of our estimated modern-day range. With these input parameters, we produce a primary production rate of 2.9×1012 mol C yr−1, a result roughly coincident with that of Kharecha et al. (2005) at the same hydrogen outgassing rate. This rate of primary production is considerably larger than could be supported by the hydrogen flux from volcanoes without any recycling. Thus, as noted by Kharecha et al. (2005), the recycling of hydrogen through methanogenesis and the decomposition of methane in the atmosphere considerably enhances rates of primary production. However, the rates are still very low, over 1000 times less than modern marine rates of primary production in the oceans (Field et al. 1998; table 8).
Table 8.
Productivity of hydrogen-based anoxygenic photosynthetic ecosystem.
| _x_=0.01 | mol yr−1 | ||
|---|---|---|---|
| fluxvolc | fluxphoto | fluxesc | primary production |
| 3.4×1011 | 5.8×1012 | 2.82×1011 | 2.9×1012 |
| 3.4×1012 | 5.8×1013 | 2.82×1012 | 2.9×1013 |
| 4.7×1012 | 8.0×1013 | 3.90×1012 | 4.0×1013 |
| 3.4×1013 | 5.8×1014 | 2.82×1013 | 2.9×1014 |
| present day | 4.0×1015 |
| fluxvolc=3.4×1011 | |||
|---|---|---|---|
| x | |||
| 1 | 3.2×1011 | 1.60×1010 | 1.6×1011 |
| 0.1 | 2.3×1012 | 1.12×1011 | 1.1×1012 |
| 0.01 | 5.8×1012 | 2.81×1011 | 2.9×1012 |
| 0.001 | 6.8×1012 | 3.33×1011 | 3.4×1012 |
| 0.0001 | 6.9×1012 | 3.39×1011 | 3.5×1012 |
It is quite probable that fluxes of hydrogen were higher in the past owing to higher heat flow and perhaps also owing to hydrothermal circulation at mid-ocean ridges under reduced hydrostatic pressure (Kump & Seyfried 2005), which, from thermodynamic grounds, should increase the H2 concentration and the flux from these systems. However, even increasing the hydrogen flux by a factor of 100 produces primary production rates of only 7% of the present-day marine rates. We can also vary x, the burial efficiency of organic carbon (table 8). At higher burial efficiencies than used here (less organic carbon decomposed and more buried), lower rates of primary production are produced at the same volcanic hydrogen outgassing rate. However, as x decreases, a plateau in primary production is reached, and with the value of x we use, 0.01, primary production is about 80% of its maximum at infinitely small values of x. Overall, it appears that an early-Earth hydrogen-based ecosystem supporting anoxygenic photosynthesis may have been dynamic, but not nearly as active as the present marine biosphere.
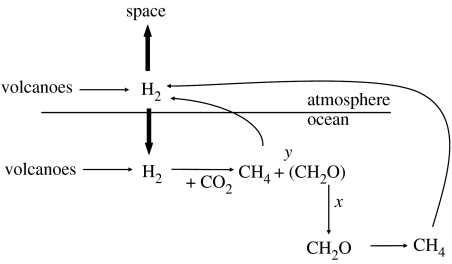
We also explored here, following Kharecha et al. (2005), the case where hydrogen fuels a methanogenic population. This case is shown in figure 2 and is somewhat different from the case with anoxygenic phototrophs, as organic carbon production depends on the growth yield, y, of the organisms. Growth yield can be defined in different ways, but for our purposes here, growth yield will represent the efficiency with which hydrogen is transferred into cell biomass (organic matter) versus methane. A growth efficiency of 0.1 was used by Kharecha et al. (2005), and this value will be maintained here. To proceed, we note that the burial flux of H2 into organic carbon is related to the flux of hydrogen across the sea–water interface by the following expression:
| fluxbur=xyfluxmar. | (3.16) |
|---|
Figure 2.
Early-Earth microbial ecosystem driven by hydrogen-based methanogenesis. Similar to the case outlined in figure 1, the primary sources of hydrogen are subaqueous and subaerial volcanoes. The organic matter is produced as methanogen cell biomass with the growth yield, y. Organic matter is decomposed by methanogenesis, a fraction of which, x, is buried in sediments. Methane is reconverted to hydrogen by photolysis reactions in the atmosphere. See text for details.
This expression is substituted into equation (3.7) yielding
| fluxvolc=fluxesc+xyfluxmar. | (3.17) |
|---|
With this, equation (3.10) is amended to
| fluxvol=A(fluxmar+Vp[H2]D)VpαD+2Af(CH4)+xyfluxmar, | (3.18) |
|---|
which is then solved as above, yielding
| fluxmar=VpαDfluxvol+AVp[H2]D[A+2ABVpαD(1−x)+xyVpαD]. | (3.19) |
|---|
Finally, because only a portion of the hydrogen used by methanogens is shunted into organic carbon production, rates of primary production (in carbon equivalents) are given by
| prim.prod.=0.5yfluxmar. | (3.20) |
|---|
While analysing equations (3.19) and (3.20), we take [H2] to be 5×10−9 M, which is a typical value for marine systems undergoing active methanogenesis, and a value which yields Δ_G_ values of around −20 kJ mol−1 of H2 (see review in Canfield et al. 2005) with typical seawater chemistry. This energy yield is sufficient for ATP production by prokaryotes (Schink 1997).
As for the case with anoxygenic photosynthesis, we explore how the flux of hydrogen into the ocean, fluxmar, and rates of primary production scale with variable parameters including volcanic outgassing rate, fluxvol, organic carbon burial efficiency, x, and growth efficiency, y (table 9). We begin the analysis with values of 0.01 and 0.1 for x and y, respectively. With these values, the flux of hydrogen into the oceans at the same fluxvol is very similar to the case where primary production is dominated by anoxygenic photosynthesis (table 8). However, because only a portion of the hydrogen is channelled into organic matter production, rates of primary production are about an order of magnitude lower than with anoxygenic photosynthesis. This point was also highlighted by Kharecha et al. (2005). With high values of x, meaning high organic carbon burial efficiencies and limited recycling, primary production rates drop (table 9). However, there is very little increase in rates of primary production if we decrease x below 0.01. As would be expected, rates of primary production are tightly coupled to the growth yield, y. However, the growth yield would need to be 1 before rates of primary production in this methane-based ecosystem were comparable to rates in the anoxygenic photosynthetic system. Such a high growth yield would be impossible during methanogenesis. Overall, we agree with Kharecha et al. (2005) that a methane-based ecosystem would be much less productive than an anoxygenic photosynthetic ecosystem at comparable rates of volcanic outgassing.
Table 9.
Productivity of hydrogen-based methanogenic ecosystem.
| _x_=0.01, _y_=0.1 | mol yr−1 | ||
|---|---|---|---|
| fluxvolc | fluxphoto | fluxesc | primary production |
| 3.4×1011 | 6.0×1012 | 3.37×1011 | 3.0×1011 |
| 3.4×1012 | 6.8×1013 | 3.37×1012 | 3.4×1012 |
| 4.7×1012 | 9.4×1013 | 4.65×1012 | 4.7×1012 |
| 3.4×1013 | 6.8×1014 | 3.37×1013 | 3.4×1013 |
| present day | 4.0×1015 |
| fluxvolc=3.4×1011, _y_=0.1 | |||
|---|---|---|---|
| x | |||
| 1.0 | 2.9×1012 | 1.97×1011 | 1.4×1011 |
| 0.1 | 5.4×1012 | 3.13×1011 | 2.7×1011 |
| 0.01 | 6.0×1012 | 3.37×1011 | 3.0×1012 |
| 0.001 | 6.0×1012 | 3.40×1011 | 3.0×1012 |
| fluxvolc=3.4×1011, _x_=0.01 | |||
|---|---|---|---|
| y | |||
| 1.0 | 5.0×1012 | 3.15×1011 | 2.5×1012 |
| 0.1 | 6.0×1012 | 3.37×1011 | 3.0×1011 |
| 0.01 | 6.1×1012 | 3.40×1011 | 3.0×1010 |
4. Sulphur-based ecosystem
Canfield & Raiswell (1999), Des Marais (2000), Canfield (2005) and Kharecha et al. (2005) have all considered the potential productivity of a sulphur-based ecosystem. In different ways, each of these studies has concluded that such an ecosystem would be 2–3 orders of magnitude less productive than the modern marine environment. However, each of these studies used different approaches with different assumptions and our understanding of the early-Earth sulphur system has advanced enormously in the last few years. Therefore, it would seem pertinent to review the structure and activity levels of ancient-Earth ecosystems driven by sulphur.
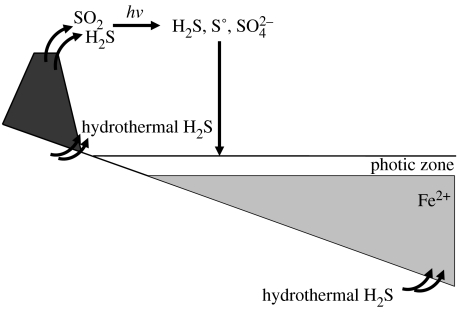
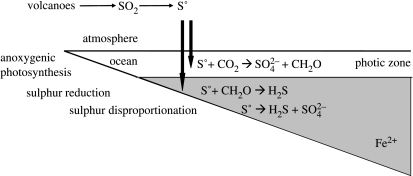
Sulphur would have entered the early-Earth surface environment from a number of avenues (figure 3). These would have included a hydrothermal flux of H2S at divergent plate margins within the ocean (mid-ocean ridge volcanics), sources of SO2 and H2S from subaerial volcanics, and terrestrial hydrothermal H2S. Modern analogues for the latter include hot springs at Yellowstone National Park, USA, and hydrothermal areas in Iceland as well as the North Island of New Zealand. We believe that early-Earth oceans contained dissolved Fe2+ based on the occurrence of banded iron formations (BIFs) in 3.8-Gyr-old rocks at Isua, Greenland (e.g. Dymek & Klein 1988; Rosing et al. 1996). Thus, the deep-ocean hydrothermal flux of H2S would have probably been scavenged by reaction with dissolved iron in the oceans, and would not have entered into the surface environment (see Canfield 2004; Kharecha et al. 2005). Thus, unlike modern mid-ocean ridge systems where sulphide fuels a diverse and dynamic ecosystem based, ultimately, on the oxidation of sulphide, on the early Earth, the sulphide would have been unavailable to fuel microbial metabolisms. (However, some methanogenesis, based on the reaction between hydrothermal CO2 and H2, would have fuelled a small microbial community.) Then, the main sources of sulphur to the surface environment would have been terrestrial hydrothermal sources and subaerial volcanics.
Figure 3.
The early-Earth sulphur cycle. Sulphide enters the cycle from a number of sources including mid-ocean ridge hydrothermal systems, terrestrial hydrothermal systems and volcanic emanations. The sulphide coming from mid-ocean ridge systems would have likely precipitated as iron sulphide minerals in an iron-containing ocean. The sulphur species coming from subaerial volcanics would have been converted to a mix of reaction products through photolysis reactions in the atmosphere.
Modern terrestrial hydrothermal systems support highly interesting sulphur-based ecosystems. This is particularly true at high temperatures (around 70°C) where, among the phototrophs, cyanobacteria are sometimes excluded and primary production is often driven by the anoxygenic phototrophic oxidation of sulphide (see Brock 1985, 1994; Pierson 2001). In such environments, a ‘sulphuretum’ (e.g. Baas Becking 1925) forms with dynamic, coupled, carbon and sulphur cycles, where the organic matter produced by sulphide-oxidizing anoxygenic phototrophs is oxidized by sulphate reducers (obtaining their sulphate from the sulphide-oxidizers), producing sulphide for further oxidation by the phototrophs. In a situation where all the organic matter decomposition occurs by sulphate reduction, the primary production in the system is equal to (Canfield 2005)
| prim.prod.=2fluxsulphidex, | (4.1) |
|---|
where, in addition to the terms already defined, fluxsulphide represents the primary hydrothermal flux of sulphide to the system. The factor 2 represents the stoichiometric relationship between sulphide oxidation (to sulphate) and CO2 fixation by anoxygenic phototrophs. As discussed by Canfield (2005), a typical value for x in a microbial mat is 0.1, so that in such a system, prim. prod.=20fluxsulphide.
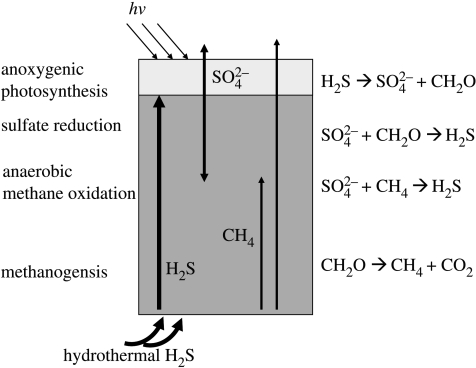
In practice, some of the sulphate produced by the anoxygenic phototrophs might be washed out from the system, leaving an excess of organic matter over the sulphate. In this situation, some of the organic matter would be decomposed by methanogens producing methane. This would lead to further ecological complexity, as a methane–sulphate interface would develop where anaerobic methane oxidation would occur (e.g. Reeburgh 1980; Alperin & Reeburgh 1988; figure 4). The productivity of the system would probably be somewhat lower than that predicted from equation (3.20), as some of the reduced equivalent as methane would probably escape from the system. Unfortunately, it is difficult to place limits on the global productivity of such a sulphuretum. There are no good estimates for the terrestrial hydrothermal flux of sulphide to the surface environment, but such environments probably do not represent a globally significant source of sulphur. Thus, though such an environment would have been extremely interesting ecologically, it may not have been a globally important source of primary production.
Figure 4.
The sulphur cycle associated with a sulphuretum. Hydrothermal sulphide is oxidized by anoxygenic phototrophs producing sulphate. As the microbial mat accretes, organic matter buried below the photic zone will be oxidized by sulphate reduction, recycling the sulphate produced by photosynthesis. Some methanogenesis is also likely to occur, and anaerobic methane oxidation would have occurred at the sulphate–methane transition zone.
A mix of SO2 and H2S is delivered from subaereal volcanics, with an excess of SO2 by about 5 : 1 (tables 5, 6 and 10). Atmospheric photolysis of these compounds with UV radiation creates a mix of reaction products, including SO2, H2S, S0 and H2SO4 (Farquhar et al. 2001; Ono et al. 2003). Modelling suggests that the relative importance of these different reaction products varies depending on the SO2 outgassing rate and the CH4 mixing ratio (Ono et al. 2003). Higher CH4 mixing ratios, giving a more reducing atmosphere, generate more sulphide, while higher SO2 fluxes favour S0 over sulphate. For modern SO2 volcanic fluxes and greater, as would be probable on the early Earth, S0 dominates over all other sulphur forms, and is greatly reduced in significance only at CH4 mixing ratios of 10−5 or less, where H2SO4 and SO2 dominate. In most modelling scenarios (Ono et al. 2003), the proportion of S0 to total sulphur species varies from about 20 to 70%.
Table 10.
Sulphur sources to the surface environment.
| S phase | source | flux (×1011 mol yr−1) |
|---|---|---|
| SO2 | subaerial volcanoes | 2–3 |
| H2S | subaerial volcanoes | 0.4–0.6 |
| H2S | subaqueous volcanoes | 0.9–9.6 |
In the present discussion, we will be mostly interested in the electron donors, which include H2S and S0. As discussed earlier, sulphate could be an electron acceptor in organic matter mineralization or even sulphate reduction with hydrogen (table 1). However, due to its relatively low growth yield (as in methanogenesis), sulphate reduction will not considerably add to the global productivity estimates already forwarded. Therefore, we will focus on the electron donors, and since S0 dominates over sulphide in the photochemical models pertinent here, we will concentrate on S0. To do this, we look more closely at the cycle of elemental sulphur (figure 5). As mentioned, elemental sulphur forms in the atmosphere through photochemistry and settles to the Earth surface as particulate S8 (Ono et al. 2003). Elemental sulphur is an excellent electron donor for anoxygenic photosynthesis (e.g. Pfennig 1975), and in the photic zone of the ocean, this process will produce sulphate and organic matter (figure 5). The S0 settling from the photic zone could fuel heterotrophic S0 reduction, producing sulphide (figure 5), and it could also disproportionate into sulphide and sulphate (Thamdrup et al. 1993).
Figure 5.
The early-Earth cycle of elemental sulphur. Elemental sulphur would have been produced by the photolysis of SO2 gas. The sulphur would have settled into the ocean, some of which would have been oxidized by anoxygenic phototrophs. Some sulphur might also have settled below the euphotic zone into iron-containing waters, where sulphur reduction and sulphur disproportionation would have occurred.
The sulphide produced from both sulphur reduction and sulphur disproportionation would probably precipitate as iron sulphide minerals in the iron-containing water column (figure 5). Therefore, the prospects for active sulphur cycling in such an ocean system are minimal. In other words, significant amounts of elemental sulphur are unlikely to be regenerated through secondary microbial metabolisms or inorganic chemical reactions. This means that maximum rates of primary production will be driven by single-stage S0 oxidation through anoxygenic photosynthesis. We assume that one-half of the volcanic SO2 flux is converted to S0 through photolysis in the atmosphere, and that 2 mol of S0 are used to produce 3 mol of CH2O through anoxygenic photosynthesis by the reaction
| 2S0+3CO2+5H2O+hv→3CH2O+4H++2SO42−. | (4.2) |
|---|
Rates of primary production by this process will depend directly on the SO2 outgassing rate, and the results are presented in table 11. Even with rates of outgassing 10 times the present rate, rates of primary production are still 1000 times lower than those of the present day. Although the discussions differ somewhat, and different processes are highlighted, these results are roughly compatible with those presented by Des Marais (2000), Canfield et al. (2005) and Kharecha et al. (2005).
Table 11.
Primary production fuelled by volcanic SO2 flux.
| subaerial SO2 flux (mol yr−1) | primary production (mol yr−1) |
|---|---|
| 3×1011 | 2.3×1011 |
| 3×1012 | 2.3×1012 |
| 3×1013 | 2.3×1013 |
| present day | 4×1015 |
5. Iron-based ecosystem
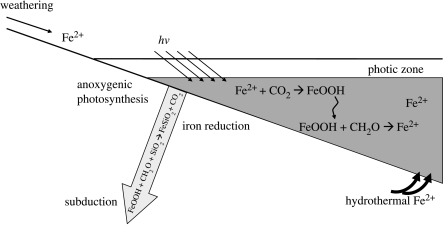
The iron cycle on the early Earth derived inputs from mid-ocean ridge hydrothermal circulation and the weathering of continental rocks. Presently, about 20 times more ‘reactive iron’ (i.e. iron involved in active oxidation–reduction reactions) is brought to the oceans by weathering than by hydrothermal sources (Canfield 1998). Kump & Seyfried (2005) have argued that because they were hotter, mid-ocean ridge systems on the early Earth would have risen higher into the oceans, venting at shallower depths than at present. Thermodynamic calculations suggest that under reduced hydrostatic pressure, more iron would have been delivered from these systems to the oceans. Thus, the flux of iron to the oceans might have been considerably greater than at present. In any event, a dynamic and interesting ecosystem could have resulted, driven by iron-oxidizing phototrophs oxidizing Fe2+ dissolved in the oceans (figure 6). As far as we know, this was first envisioned by Garrels & Perry (1974), and this idea has been considerably strengthened by the discovery of bacteria capable of oxidizing Fe2+ phototrophically (Widdel et al. 1993; Heising et al. 1999; Jiao et al. 2005). Indeed, the link has been made between phototrophic iron oxidation and the deposition of Archaean and Early Proterozoic BIFs (Hartman 1984; Eherenreich & Widdel 1994_a_,b; Kappler et al. 2005).
Figure 6.
The early-Earth iron cycle. Iron would have entered the oceans from continental weathering (although this may not have been a major source, see text) and mid-ocean ridge hydrothermal vents. The iron within the ocean would have been oxidized by anoxygenic phototrophs producing iron oxides and organic matter. The organic matter and iron oxides would have settled into the deep ocean, with iron reduction reducing the iron oxides and oxidizing the organic matter. Subduction and metamorphism would have regenerated reduced iron.
Recycling occurs as the iron oxides formed by phototrophic iron oxidation are re-reduced by iron-reducing bacteria (figure 6). To maintain high activity levels, the geological recycling of the electron donor, Fe2+, is also required. This is especially true if, as at present, weathering represents the most important flux of Fe2+ to the oceans (Holland 1984; Canfield 1998). Garrels & Perry (1974) also recognized this, and they argued that recycling occurs through subduction and metamorphism where Fe2+ is regenerated by the following reaction:
| 2Fe2O3+CH2O+4SiO2→4FeSiO3+CO2+H2O. | (5.1) |
|---|
Following uplift and denudation, the iron silicate minerals formed would be weathered again, delivering Fe2+ in solution back to the oceans.
Both Canfield (2005) and Kharecha et al. (2005) have attempted to evaluate the activity level of such an ecosystem. Each uses similar starting points, but they end up with very different conclusions. We begin with Canfield (2005) who assumes, for the sake of argument, that ocean circulation on the early Earth was the same as today. The next step is to recognize that the present deep-ocean concentration of phosphate, 2.3 μM (Broecker & Peng 1982), supports a marine primary production rate of 4×1015 mol C yr−1 (Field et al. 1998). If primary production was conducted by iron-oxidizing phototrophs, we can calculate how much Fe2+ oxidation would be associated with the cycling of 2.3 μM of phosphate. We begin by assuming a C : P ratio of phototrophic biomass of 106 : 1 (the ‘Redfield’ ratio) and we recognize that 4 mol of Fe2+ are used to fix 1 mol of CO2 into organic carbon:
| 7H2O+4Fe2++CO2→4FeOOH+CH2O+8H+. | (5.2) |
|---|
With these values, we calculate the amount of iron oxidized as 2.3 μM P×106 C/P×4 Fe/C=975 μM Fe2+. Canfield (2005) follows Holland (2004) and places possible limits on Archaean seawater Fe2+ concentrations to between 40 and 120 μM. These concentrations are 8–24 times lower than what would produce present-day levels of primary production, implying that iron-based primary production was similarly 8–24 times lower than today. There are numerous uncertainties in this calculation, most importantly the concentrations of iron on the early Earth as well as the assumption that oceans circulated then as they do now. Nevertheless, this calculation suggests that, in principle, an iron-based marine ecosystem could have been within an order of magnitude as active as at present. Higher iron concentrations could have brought it even closer (assuming that phosphate did not become limiting; see Bjerrum & Canfield 2002).
Kharecha et al. (2005) assume, as did Canfield (2005), that early-Earth oceans circulated as those today. With this assumption, they assigned a global average upwelling rate of 4 m yr−1. Using an Fe2+ concentration of 54 μM, they calculated a primary production rate of 1.9×1013 mol yr−1, about 10 times lower than the estimate provided by Canfield (2005). However, as also recognized by Kharecha et al. (2005), upwelling rates may be much higher in coastal zones and, in particular, in coastal upwelling areas, where upwelled water is sourced not from the deep, but from rather shallow depths around the thermocline (e.g. Leth & Middleton 2004). The significance of this becomes apparent if we use a global average upwelling rate of 4 m yr−1 as suggested by Kharecha et al. (2005) and calculate a global primary production rate using the deep-ocean phosphate concentration of 2.3 μM. Combining these values with the ocean area of 3.6×1014 m2 and the C : P ratio of 106 : 1 for photosynthetically produced organic matter, we calculate a primary production rate of 3.5×1014 mol C yr−1, which is one order of magnitude lower than today's value (see table 12).
Table 12.
Summary of early-Earth primary production rate estimates.
| process | primary production rate (mol yr−1) |
|---|---|
| H2-based anoxygenic photosynthesis | 2.9×1013 |
| H2-based methanogenesis | 3.4×1012 |
| S0-based anoxygenic photosynthesis | 2.3×1012 |
| N-based anammox | 1.4×109 |
| Fe-based anoxygenic photosynthesis | 1.7–5.0×1014 |
| present day | 4×1015 |
| early Earth (3.8 Gyr ago) | 2.8×1014 |
6. Nitrogen-based ecosystem
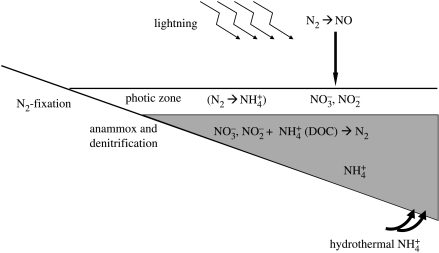
An interesting nitrogen-based ecosystem might have also been active on the early Earth before oxygenic photosynthesis (figure 7). We can identify a primary source of oxidized nitrogen (NO) from lightning (Yung & McElroy 1979; Navarro-González et al. 2001) and of ammonia from the reduction of N2 at high temperatures in mid-ocean ridge hydrothermal circulation systems (Brandes et al. 1998_a_,b). In addition, there is the possibility of biological nitrogen fixation, fixing atmospheric N2 to ammonia for use in biomolecules. In an anoxic early-Earth atmosphere, the NO produced by lightning will be reduced with H to form a nitroxyl molecule (HNO; Kasting & Walker 1981), which, according to Mancinelli & McKay (1988), would decompose in the oceans to NO2− and NO3−. Nitrate (NO3−) and nitrite (NO2−) would be used by heterotrophic denitrifiers to oxidize organic matter, producing N2 and possibly some NH4+ as reaction products. In addition, nitrate (through a nitrite) and nitrite might be used to oxidize ammonia to N2 gas through the anammox process (e.g. Dalsgaard et al. 2005), which occurs widely in anoxic, sulphide-free zones of the global ocean (e.g. Dalsgaard et al. 2003; Kuypers et al. 2003, 2005). This nitrogen cycle lacks the recycling aspects of the other element cycles as there is no known pathway from ammonia to nitrate in the absence of oxygen (a phototrophic pathway is in principle possible, but has not yet been described).
Figure 7.
The early-Earth nitrogen cycle. NO would have been produced by lightning, and settled into the oceans where nitrate and nitrite would have formed. Nitrate and nitrite would have been used in both the denitrification and the anammox reaction. Anammox is a source of primary production, where the ammonia would have come from hydrothermal vents.
The anammox reaction represents primary production, the rates of which can be estimated. Navarro-González et al. (2001) estimate an NO production rate of about 2×1010 mol yr−1 for early anoxic atmospheres. If we assume that this NO is quantitatively converted to nitrate and nitrite, then it will oxidize an equimolar amount of ammonia through anammox. In fluidized bioreactors, growth of anammox bacteria produces about 0.07 mol of CH2O for each mole of NO2− reduced (van Dongen et al. 2001). Maintaining this growth yield, the early-Earth production rate of NO would support primary production rates of about 1.4×109 mol yr−1 of organic carbon through the anammox reaction. In making this calculation, we assume that ammonia is available in sufficient amounts to fuel the anammox reaction, either through primary hydrothermal sources or from the organic nitrogen liberated during organic matter mineralization. Overall, anammox would have probably been a relatively minor player in global primary production on the early Earth.
7. Discussion
Our primary production rate estimates are summarized in table 12. For the hydrogen- and sulphur-based ecosystems, we assume that rates of H2 and SO2 outgassing were 10 times greater than the present rate. Higher estimates of outgassing will produce higher primary production rate estimates as shown in tables 8, 9 and 11. Our iron-based ecosystem production rates do not depend on outgassing rates, but depend on the dissolved iron concentrations in the deep ocean, which we have attempted to bracket, but for which considerable uncertainty exists. In addition, these different ecosystems would not operate in complete isolation as our modelling might suggest. For example, H2 is an excellent substrate for sulphate reducers, sulphur reducers, iron reducers and denitrifiers. Thus, some hydrogen would have been used to drive these processes, leaving less for hydrogen-based photosynthesis and methanogenesis. It is difficult to quantify just how hydrogen might have been distributed among these different electron acceptors. However, our overall productivity estimates change very little if hydrogen is channelled in the other respiratory processes other than methanogenesis. This is because we might expect similar growth yields from H2 use by these other processes.
A great influence on our productivity estimates is the relative channelling of H2 into photosynthetic versus non-photosynthetic metabolisms. As mentioned earlier, non-photosynthetic pathways using H2 should have similar associated carbon production rates, but these are all probably one order of magnitude less than hydrogen-based photosynthesis. Thus, the productivity of our hydrogen-based photosynthetic ecosystem should be viewed as a maximum, with reductions occurring as proportionally more H2 is used by non-photosynthetic pathways.
We can conclude that for all the ecosystems explored, primary production rates were probably considerably less than those of today. The iron-based ecosystem comes closest to matching present rates, but, if our assumptions about bottom water iron concentrations are correct, such an ecosystem is still considerably less active than what we find at present. This analysis reinforces earlier discussions (Knoll & Bauld 1989; Des Marais 2000; Canfield 2005) highlighting how the evolution of oxygenic photosynthesis led to a considerable acceleration of the activity level of the biosphere. With oxygen photosynthesis, the electron donors (H2O) and the electron acceptors (CO2) are not limiting, and production rate is thus limited by the availability of nutrients such as N, P and Fe and other trace metals.
What does the geologic record tell us about the activity level of the ancient biosphere? Indeed, as highlighted by many authors (Schidlowski 1988; Des Marais et al. 1992; Holland 2002; Bjerrum & Canfield 2004; Hayes & Waldbauer 2006), the isotope record of inorganic and organic carbon provides an indication of organic carbon burial rates on the ancient Earth, which is related to rates of primary production. The isotope record reveals that presently about 20% of all carbon (both organic and inorganic) removed from the oceans is organic carbon (e.g. Garrels & Lerman 1981; Hayes et al. 1999). Traditional readings of the isotope record would suggest that this burial proportion has not varied greatly over the last 3.8 Gyr, with a burial proportion of 0.14 indicated 3.5–3.8 Gyr ago (Des Marais et al. 1992; Bjerrum & Canfield 2004; Hayes & Waldbauer 2006). Recently, Bjerrum & Canfield (2004) have suggested that if a significant amount of inorganic carbon was removed by reaction with ocean crust (see Sleep & Zahnle 2001), and if a significant isotope difference existed between carbonate buried on the continents and in ocean crust, then the isotope record cannot be taken at face value. Indeed, burial proportions of organic carbon as low as 0.05 might have occurred. Thus far, evidence from the isotopic composition of inorganic carbon reacted from seawater and associated with ancient basalts does not support his view (Nakamura & Kato 2004), and the higher burial proportions of 0.14 are indicated. However, it remains to be demonstrated that these ancient inorganic carbonates were precipitated in deep-ocean water.
What do these burial proportions mean for primary production rates in the early ocean? To convert the organic carbon burial proportions into primary production rates, we need to know the input rates of inorganic carbon to the Earth surface, and the burial efficiency, x, of the organic carbon. We will assume a burial efficiency of 0.01 as we have done with the ecosystem modelling discussed earlier. Presently, this value is closer to 0.001 (Holland 1978; Berner 2004), but much higher values would be expected for anoxic organic carbon deposition (Canfield 1994). The input rates of inorganic carbon to the Earth surface depend on the fluxes of CO2 from the mantle as well as the recycling of carbon owing to subduction, metamorphism and continental weathering (e.g. Berner 2004). The early-Earth carbon cycle has been recently treated in some detail by Godderis & Veizer (2000), Sleep & Zahnle (2001) and Hayes & Waldbauer (2006). Of particular importance here are the calculations of Hayes & Walker (1977), who show that the combined continental reservoir of organic and inorganic carbon probably grew very slowly through the Earth history and was negligible before 3.5 Gyr ago (see also Godderis & Veizer 2000). This means that continental weathering would have been an insignificant source of inorganic carbon to the early-Earth biosphere, and that most of the carbon released from the mantle to the surface environment was subducted again (see also Sleep & Zahnle 2001).
In making their calculations, Hayes & Waldbauer (2006) have assumed that the mantle to Earth surface flux of CO2 at 4 Gyr ago was 10 times the modern flux. With falling heat flow, this CO2 flux decreased rapidly, and Hayes & Waldbauer (2006) give values of 24×1012 mol yr−1 at 4 Gyr ago, dropping to 20×1012 mol yr−1 at 3.8 Gyr ago and 13.5×1012 mol yr−1 at 3.5 Gyr ago. We use the mantle flux of CO2 at 3.8 Gyr ago, and assume that this was the most significant source of CO2 to the biosphere. With an organic carbon burial proportion of 0.14 and an x of 0.01, primary production rates at 4 Gyr ago are calculated as 2.8×1014 mol yr−1. This value is 14 times lower than present rates and shows, rather paradoxically, that relatively low rates of primary production might have accompanied much higher rates of carbon input from the mantle. This is owing to reduced, or even insignificant, carbon inputs from the weathering of the continental crust.
We find that our estimated Early Archaean rates of primary production are comparable to the rates we have calculated for our iron-based anoxygenic photosynthetic ecosystem (table 12), and about a factor of 10 greater than those predicted from our hydrogen-based anoxygenic photosynthetic ecosystem. All the other ecosystems fall short by at least two orders of magnitude. In order to better appreciate the similarities and differences in these comparisons, we must revisit some of the assumptions and the implications of our modelling.
We begin with the iron-based ecosystem. Our calculations are based on possible constraints on early-Earth bottom water ocean chemistry, and the assumption that the oceans at this time circulated as they do today. We can look at the implications of this ecosystem from another perspective. We can calculate that with an organic carbon burial proportion of 0.14, and our assumed values for the input rate of inorganic carbon to the biosphere (see above), organic carbon was buried at a rate of 2.8×1012 mol yr−1. With productivity based on Fe2+ oxidation by anoxygenic photosynthesis, this amount of organic carbon burial would need to be associated with the burial of 1.1×1013 mol yr−1 of ferric iron. Analysis of Archaean BIFs shows Fe3+ : Fe2+ ratios of 0.4–0.6 (see summary in Bjerrum & Canfield 2002). If we assume that these ratios apply to early-Earth sedimentary iron in general, then we need an input of around 2.2×1013 mol yr−1 of reactive iron, i.e. iron which is involved in active cycling and not just bound in unreactive phases. The present input of reactive iron to the oceans is mainly from riverine particulates (4×1012 mol yr−1; Canfield 1998), with a much smaller input from mid-ocean hydrothermal vents (1.7×1011 mol yr−1; see Canfield 1998). With little continental area on the early Earth, the riverine flux of reactive iron would have been insignificant, and the bulk of the reactive iron would have originated from mid-ocean ridge hydrothermal sources. To account for the reactive iron necessary to drive our calculated rates of early-Earth carbon burial, hydrothermal iron sources would need to be elevated by a factor of 100 over today. As mentioned earlier, Kump & Seyfried (2005) have argued for much higher hydrothermal iron fluxes on the early Earth. Whether such fluxes could be elevated by a factor of 100 over those of today requires more detailed consideration.
We now take a look at our hydrogen-based ecosystems. In our calculations of these ecosystems, much of the H2 coming from volcanoes is lost to space (tables 8 and 9). If hydrogen escape was less efficient, more hydrogen would be cycled through the ecosystem and more would be associated with organic carbon burial. As discussed earlier, Tian et al. (2005) have argued that hydrogen escape on the early Earth might have been more sluggish than the diffusion-limited escape we have assumed in our modelling. However, this view has been strongly opposed by Catling & Claire (2005). We can, however, match our calculated early-Earth rates of primary production if we reduce hydrogen escape by one order of magnitude (by reducing A in equation (3.6) by a factor of 10) and if we increase the volcanic hydrogen flux to about 30 times the present flux (table 13).
Table 13.
Primary productivity in hydrogen-based anoxygenic photosynthetic ecosystem with reduced H2 escape.
| _x_=0.01 | mol yr−1 | ||
|---|---|---|---|
| fluxvolc | fluxphoto | fluxesc | primary production |
| 3.4×1011 | 2.3×1013 | 1.12×1011 | 1.1×1013 |
| 3.4×1012 | 2.3×1014 | 1.12×1012 | 1.1×1014 |
| 3.4×1013 | 2.3×1015 | 1.12×1013 | 1.1×1015 |
| 1.1×1014 | 7.4×1015 | 3.62×1013 | 3.8×1015 |
| present day | 4.0×1015 | ||
| 3.8 Gyr ago | 2.8×1014 |
As discussed earlier, higher heat flow would have led to greater tectonic activity on the early Earth. This would have probably accelerated hydrogen flux to the surface environment from all identified sources including subduction-related subaerial volcanics, the serpentinization of ocean-crust peridotites and the flux from mid-ocean ridge hydrothermal systems. In an earlier study, Kump et al. (2001) argued for substantially higher volcanic hydrogen fluxes owing to a more reducing mantle on the early Earth. However, the partitioning of trace metals into the volcanic rocks of all ages suggests that the oxidation level of the mantle has changed very little through recorded Earth history (Delano 2001; Canil 2002). More recently, and as discussed earlier, Kump & Seyfried (2005) have argued for higher H2 fluxes in early-Earth mid-ocean ridge settings owing to, mostly, the absence of sulphate in circulating vent fluids. Further consideration is required to constrain how much of this flux might have reasonably been accelerated over today. It is also critical to better understand the controls of hydrogen escape on the early Earth.
We must also consider the possibility that the Early Archaean carbon isotope record reflects an ecosystem driven by oxygenic photosynthesis. This view has been advocated by Rosing (1999) and Rosing & Frei (2004). They note the appearance of finely laminated organic carbon in distal marine turbidites and pelagic shales from 3.8-Gyr-old deposits from Isua, Greenland. These have an isotopic composition consistent with carbon fixation by the Calvin cycle (using Rubisco) as conducted by oxygenic phototrophs. The lack of oxidized iron (or iron of any kind) in these deposits would seem to rule out carbon fixation by iron-oxidizing phototrophs, at least at this location. However, it is still possible that hydrogen-based phototrophy could have produced the organic carbon. For example, members of the purple sulphur bacteria, which are anoxygenic phototrophs capable of using H2, also contain Rubisco (see summary in Canfield et al. 2005). However, Rosing & Frei (2004) also note that the same deposits have experienced uranium mobilization, which is consistent with locally oxic conditions at the time of sediment deposition. This is further evidence for the presence of oxygenic photosynthesis.
If this is true, and oxygenic photosynthesis was responsible for primary production then, as today, why did the surface environment remain predominantly anoxic, which is consistent with the available geological and geochemical evidence (e.g. Holland 1984; Farquhar et al. 2000; Canfield 2005). One explanation would be that there was a sufficient flux of reduced species like H2, H2S and Fe2+ to titrate the oxygen produced by oxygenic photosynthesis (e.g. Kump et al. 2001; Holland 2002). Overall, with the burial of 2.8×1012 mol yr−1 of organic carbon, as we calculate for the Earth 3.8 Gyr ago, we would produce an equal amount of oxygen, and need a comparable flux of reduced equivalents to keep oxygen from accumulating. As discussed earlier, the necessary iron flux would need to be substantially elevated over today's flux, and whether this is reasonable requires further consideration. The flux of sulphide from mid-ocean ridge hydrothermal systems was probably negligible, and if the volcanic flux of H2S was 10 times today (table 10), this would be 5×1011 mol yr−1, which could remove 1×1012 mol yr−1 of O2, about 35% of the necessary flux. If we elevate the present-day H2 flux by a factor of 10, we would deliver between about 2 and 5×1012 mol yr−1 of H2, which could remove between 1 and 2.5×1012 mol yr−1 of O2. This, either alone or in combination with the other mantle-derived reduced compounds, would appear to be sufficient to remove the required amounts of oxygen from the atmosphere.
The caveat with the hydrogen flux calculation is whether or not any of this flux would have been lost to space. We could imagine that if most of the hydrogen was delivered to the surface environment through ocean crust serpentinization and mid-ocean ridge hydrothermal circulation, then much of this hydrogen would be scavenged by oxygen higher in the surface layers of the water column where oxygenic phototrophs were active. Thus, in the presence of oxygenic phototrophs, much of the hydrogen would be scavenged before it could escape to the atmosphere and further to space.
Taken together, our understanding of the carbon cycle as revealed from the carbon isotope record would allow for the carbon cycle 3.8 Gyr ago based on anaerobic metabolisms if the fluxes of Fe2+ and H2 were elevated over the present fluxes by factors of 1–2 orders of magnitude. Further constraints need to be imposed to decide whether or not the necessary magnitudes of increase are reasonable. Equally allowable is a carbon cycle driven by oxygenic photosynthesis where the oxygen produced is completely removed by reaction with reduced species from the mantle.
8. Summary
There was a sufficient diversity of electron donors and electron acceptors delivered to the early Earth to have allowed for a variety of dynamic, and even complex, anaerobic microbial ecosystems. We can envision a number of different ecosystems based on the cycling of individual elements. Some of these could have been more active, particularly those based on the anoxygenic photosynthetic oxidation of H2 and Fe2+. Of these two, an ecosystem based on Fe2+ oxidation would apparently have been the most active. Element cycles involving sulphur and nitrogen would have also led to diverse and interesting ecosystems, but these would have been considerably less active than those based on H2 and Fe. Overall, none of these ecosystems matches the primary productivity of the present marine environment, although an ecosystem based on anoxygenic photosynthesis with iron comes within a factor of 10.
The natural question arises as to what sort of ecosystem might have been responsible for carbon production in the Early Archaean, where the carbon isotope record suggests that organic carbon accounted for 14% of the total carbon. Among the anaerobic ecosystems explored here, the most probable candidates are those based on anoxygenic photosynthesis, both H2 and Fe2+. In each case, the fluxes of reduced compounds to the surface environment need to be elevated over present-day fluxes by factors of 30–100. Whether such high fluxes are reasonable deserves further attention. Alternatively, the Early Archaean ecosystem might have been based on oxygenic photosynthesis, and there is some evidence to support this. The flux of H2 and Fe2+ from the interior of the Earth would need to have been elevated 1 to 2 orders of magnitude times the present fluxes to have maintained an anoxic atmosphere.
Acknowledgments
We wish to acknowledge fruitful discussions with Dave Pyle, Jeff Alt, Wolfgang Bach, John Hayes, Bo Thamdrup and Jim Kasting. We are particularly indebted to Dave Pyle, Wolfgang Bach and Jeff Alt for sharing calculations and ideas. We acknowledge the expert technical assistance of Mette Andersen. Finally, we are grateful to the Danish National Research Foundation (Dansk Grundforskningsfond) for their generous support.
Footnotes
One contribution of 19 to a Discussion Meeting Issue ‘Conditions for the emergence of life on the early Earth’.
Discussion
D. W. Deamer (Department of Chemistry and Biochemistry, University of California, Santa Cruz, USA). What was the basis of the calculated 40–120 μM Fe2+ in the early deep ocean?
D. E. Canfield. This estimate is based on the reasoning of Dick Holland who noted that calcite (CaCO3) and siderite (FeCO3) are sometimes found together in Archaean sedimentary successions. This means that the water from which these minerals formed was saturated with both calcite and siderite, and if so, the molar ratio of dissolved Fe2+ : Ca2+ is equal to the ratio of the solubility products of siderite and calcite. Therefore,
mFe2+mCa2+=KsidKcal=10−10.810−8.4=4×10−3.
We don't know the concentration of calcium in nearly oceans, but if we assume that it was bracketed between the Phanerozoic extremes of 10 and 30 mM, then we obtain our estimate for dissolved Fe2+ of 40–120 μM.
J. F. Kasting (Department of Geosciences, Penn State University, PA, USA): (Comment on the above point.) H. D. Holland's estimate of deep oceanal dissolved Fe2+ concentrations in the Archaean is supported by evidence from the Hamerschy BIFs. If one assumes that the mm-scale microbonding is annual, and if one simply adopts modern upwelling rates, then one can account for the iron in the BIFs. This is in Holland's 1984 book (Evolution of the Atmosphere and Oceans).
J. I. Lunine (Lunar and Planetary Sciences Department, University of Arizona, USA). You have mentioned a variety of different possible metabolisms that may have been important prior to the start of photosynthesis. But evidence for these metabolisms must be sought in the fossil record. Are there distinct isotopic signatures in carbon or other elements that would allow one to distinguish the existence of these metabolisms on the ancient earth?
D. E. Canfield. In some instances, stable isotope signatures in early-Earth rocks can be traced to specific metabolisms, and I will give you two examples. In the first example, sulphate-reducing bacteria (SRB) fractionate sulphur isotopes during their metabolism when sufficient sulphate, greater than about 200 μM, is available to them. Indeed, we found stable isotope evidence for the activities of SRB in 3.5-Gyr-old sediments from the Dresser Formation at North Pole, Australia (Shen et al. 2001). We were attracted to this place because an important component of the sedimentary succession is barite, which was originally precipitated as gypsum, implying high concentrations of sulphate. Otherwise, most Archaean sulphides show only small fractionations consistent with low sulphate concentrations, but not by themselves diagnostic of sulphate reduction. The second example is a recent report (Ueno et al. 2006) of biogenic methane within silica dikes also found in the Dresser Formation, North Pole, Australia. The authors make a good case that this is biogenic gas because the isotopic composition of the methane is very 13C-depleted, and not easily explained by abiological processes. Other than these examples, sedimentary carbon isotopes from the Archaean provide evidence for biological activity, but the isotopic compositions themselves are not diagnostic of specific microbial metabolisms.
D. W. Schwartzman (Department of Biology, Howard University, Washington, DC, USA). The interpretation of the sedimentary C-isotopic record in the Archaean may be more uncertain than suggested, since the 13C : 12C input ratio could have been different from today as could the fraction of organic C that was buried.
D. E. Canfield. I agree that our interpretation of the early-Earth carbon isotope record is quite uncertain, and indeed, our understanding of the early-Earth carbon cycle is very poor. To answer your question directly, the input carbon isotope ratio could change if there was a long-term evolution of this ratio with time from the mantle (of which we have no evidence), or with isotopically variable inputs due to crustal carbon cycling. Unfortunately, we have no way at present to isolate these various possible influences. I am encouraged, however, by recent carbon cycle modelling of the type recently presented by Hayes & Waldbauer (2006) which attempt to hindcast the evolution of the carbon cycle, highlighting interactions between the crust and the mantle. I believe this is necessary before we can begin to understand quantitatively what the carbon isotope record is telling us about the dynamics of carbon cycling through time.
J. F. Kasting. I will rephrase this question in writing it down because you reminded us that you and Bjerrum (right?-Yes) have re-analysed the long-term carbon isotope record.
The ‘standard’ interpretation (Schidlowsli, Holland, others) of the S13C record in carbonates is at present that ca 20% of the CO2 outgassed from volcanoes is reduced to organic carbon and buried in sediments. If the atmosphere–ocean–sediment system is in steady state, this requires that an equivalent amount of H2 (or other reductants) be outgassed from volcanoes, even in the modern system. This requirement is part of what leads Holland to propose relatively large volcanic H2 fluxes in the modern Earth system. In the standard C-isotope interpretation, the constancy of the S13C values of carbonate carbon at ca 0% throughout geologic time implies that ca 20% of the outgassed CO2 has been buried as organic C all the way back to ca 3.5 Gyr ago. My question would be: Is the model that you presented, in which volcanic H2 fluxes are ca 50 times smaller than Holland's values, consistent with this carbon isotope record?
D. E. Canfield. Christian Bjerrum and I (Bjerrum & Canfield 2004) argued that the carbon cycle is more complicated than the standard carbon isotope models considered. In particular, the removal of inorganic carbon by the reaction with ocean crust provides a carbon exit pathway, which has not before been considered. If this carbon is isotopically distinct from the surface carbon reservoir, then this pathway will influence our interpretation of carbon isotope record. The jury is still out on the significance of this pathway in our interpretation of the carbon isotope record.
However, even in the absence of this extra carbon removal pathway, the Early Archaean isotope record is most consistent with burial proportions of organic carbon ranging between about 12 and 15% of the total carbon burial. I believe that the proposals presented here on the activity levels of the early Earth are in line with this. Thus, as outlined in the manuscript, carbon burial rates in the Early Archaean may have been of the order of 2.8×1012 mol yr−1. This calculation assumes that the carbon cycle is driven mainly by CO2 degassing from the mantle about 10 times greater than today, and with a burial proportion of organic carbon of 0.14. Present-day H2 fluxes are of the order of 3×1011 mol yr−1, the volcanic (subaerial) H2S flux is around 5×1010 mol yr−1 and the hydrothermal flux of iron is around 1.7×1011 mol yr−1. Altogether, these could contribute to the burial of about 2.9×1011 mol yr−1 of organic carbon, about 10 times less than we assume for the Early Archaean. If, however, the flux of these reduced species from the mantle was higher by a factor of 10 in the Early Archaean, as we have assumed for CO2, then this flux of reduced species could account for the burial of organic carbon as we would calculate directly from the carbon isotope record.
References
- Alperin M.J, Reeburgh W.S. Carbon and hydrogen isotope fractionation resulting from anaerobic methane oxidation. Global Biogeochem. Cycles. 1988;2:279–288. [Google Scholar]
- Alt J.C, Shanks W.C., III Serpentinization of abyssal peridotites from the MARK area, Mid-Atlantic Ridge: sulfur geochemistry and reaction modeling. Geochim. Cosmochim. Acta. 2003;67:641–653. doi:10.1016/S0016-7037(02)01142-0 [Google Scholar]
- Arthur M.A, Dean W.E. Organic-matter production and preservation and evolution of anoxia in the Holocene Black Sea. Paleoceanography. 1998;13:395–411. doi:10.1029/98PA01161 [Google Scholar]
- Baas Becking L.G.M. Studies on the sulphur bacteria. Ann. Bot. 1925;39:613–650. [Google Scholar]
- Bach W, Paulick H, Garrido C.J, Ildefonse B, Meurer W.P, Humphris S.E. Unraveling the sequence of serpentinization reactions: mineral chemistry, and petrophysics of serpentinites from MAR 15 °N (ODP Leg 209, Site 1274) Geophys. Res. Lett. 2006;33:L13306. doi:10.1029/2006GL025681 [Google Scholar]
- Battistuzzi F.U, Feijao A, Blair Hedges S. A genomic timescale of prokaryote evolution: insights into the origin of methanogenesis, phototrophy, and the colonization of land. BMC Evol. Biol. 2004;4 doi: 10.1186/1471-2148-4-44. doi:10.1186/1471-2148-4-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berner R.A. Oxford University Press; Oxford, UK: 2004. The phanerozoic carbon cycle: CO2 and O2. [Google Scholar]
- Bjerrum C.J, Canfield D.E. Ocean productivity before about 1.9 Gyr ago limited by phosphorus adsorption onto iron oxides. Nature. 2002;417:159–162. doi: 10.1038/417159a. doi:10.1038/417159a [DOI] [PubMed] [Google Scholar]
- Bjerrum C.J, Canfield D.E. New insights into the burial history of organic carbon on the early Earth. Geochem. Geophys. Geosyst. 2004;5:Q08001. doi:10.1029/2004GC000713 [Google Scholar]
- Blankenship R.E. Origin and early evolution of photosynthesis. Photosynth. Res. 1992;33:91–111. doi:10.1007/BF00039173 [PubMed] [Google Scholar]
- Blankenship R.E. Molecular evidence for the evolution of photosynthesis. Trends Plant Sci. 2001;6:4–6. doi: 10.1016/s1360-1385(00)01831-8. doi:10.1016/S1360-1385(00)01831-8 [DOI] [PubMed] [Google Scholar]
- Brandes J.A, Boctor N.Z, Cody G.D, Cooper B.A, Hazen R.M, Yoder H.S., Jr Abiotic nitrogen reduction on the early Earth. Nature. 1998a;395:365–367. doi: 10.1038/26450. doi:10.1038/26450 [DOI] [PubMed] [Google Scholar]
- Brandes J.A, Devol A.H, Yoshinari T, Jayakumar D.A, Naqvi S.W.A. Isotopic composition of nitrate in the central Arabian Sea and eastern tropical North Pacific: a tracer for mixing and nitrogen cycles. Limnol. Oceanogr. 1998;43:1680–1689. [Google Scholar]
- Brock T.D. Life at high temperatures. Science. 1985;230:132–138. doi: 10.1126/science.230.4722.132. [DOI] [PubMed] [Google Scholar]
- Brock T.D. Yellowstone Association for Natural Science, History & Education, Inc; Yellowstone National Park, WY: 1994. Life at high temperatures. [Google Scholar]
- Broecker W.S, Peng T.-H. Eldigio; Palisades, NY: 1982. Tracers in the sea. [Google Scholar]
- Brown J.R, Doolittle W.F. Archaea and the prokaryote-to-eukaryote transition. Microbiol. Mol. Biol. Rev. 1997;61:456–502. doi: 10.1128/mmbr.61.4.456-502.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canfield D.E. Factors influencing organic carbon preservation in marine sediments. Chem. Geol. 1994;114:315–329. doi: 10.1016/0009-2541(94)90061-2. doi:10.1016/0009-2541(94)90061-2 [DOI] [PubMed] [Google Scholar]
- Canfield D.E. A new model for Proterozoic ocean chemistry. Nature. 1998;396:450–453. doi:10.1038/24839 [Google Scholar]
- Canfield D.E. The evolution of the Earth surface sulfur reservoir. Am. J. Sci. 2004;304:839–861. doi:10.2475/ajs.304.10.839 [Google Scholar]
- Canfield D.E. The early history of atmospheric oxygen: homage to Robert M. Garrels. Annu. Rev. Earth Planet. Sci. 2005;33:1–36. doi:10.1146/annurev.earth.33.092203.122711 [Google Scholar]
- Canfield D.E, Raiswell R. The evolution of the sulfur cycle. Am. J. Sci. 1999;299:697–723. doi:10.2475/ajs.299.7-9.697 [Google Scholar]
- Canfield D.E, Kristensen E, Thamdrup B. Academic Press; San Diego, CA: 2005. Aquatic geomicrobiology. [DOI] [PubMed] [Google Scholar]
- Canil D. Vanadium in peridotites, mantle redox and tectonic environments: Archean to present. Earth Planet. Sci. Lett. 2002;195:75–90. doi:10.1016/S0012-821X(01)00582-9 [Google Scholar]
- Catling D.C, Claire M.W. How Earth's atmosphere evolved to an oxic state: a status report. Earth Planet. Sci. Lett. 2005;237:1–20. doi:10.1016/j.epsl.2005.06.013 [Google Scholar]
- Catling D.C, Zahnle K.J, McKay C.P. Biogenic methane, hydrogen escape, and the irreversible oxidation of early life. Science. 2001;293:839–843. doi: 10.1126/science.1061976. doi:10.1126/science.1061976 [DOI] [PubMed] [Google Scholar]
- Cavosie A.J, Valley J.W, Wilde S.A. Magmatic delta O-18 in 4400–3900 Ma detrital zircons: a record of the alteration and recycling of crust in the Early Archean. Earth Planet. Sci. Lett. 2005;235:663–681. doi:10.1016/j.epsl.2005.04.028 [Google Scholar]
- Cloud P.E., Jr A working model of the primitive Earth. Am. J. Sci. 1972;272:537–548. [Google Scholar]
- Dalsgaard T, Canfield D.E, Petersen J, Thamdrup B, Acuña-Gonzalez J. N2 production by the anammox reaction in the anoxic water column of Golfo Dulce, Costa Rica. Nature. 2003;422:606–608. doi: 10.1038/nature01526. doi:10.1038/nature01526 [DOI] [PubMed] [Google Scholar]
- Dalsgaard T, Thamdrup B, Canfield D.E. Anaerobic ammonium oxidation (anammox) in the marine environment. Res. Microbiol. 2005;156:457–464. doi: 10.1016/j.resmic.2005.01.011. doi:10.1016/j.resmic.2005.01.011 [DOI] [PubMed] [Google Scholar]
- Delano J.W. Redox history of the Earh's interior since ∼3900 Ma: implications for prebiotic molecules. Orig. Life Evol. B. 2001;31:311–341. doi: 10.1023/a:1011895600380. doi:10.1023/A:1011895600380 [DOI] [PubMed] [Google Scholar]
- Des Marais D.J. Evolution: when did photosynthesis emerge on Earth? Science. 2000;289:1703–1705. [PubMed] [Google Scholar]
- Des Marais D.J, Strauss H, Summons R.E, Hayes J.M. Carbon isotope evidence for the stepwise oxidation of the Proterozoic environment. Nature. 1992;359:605–609. doi: 10.1038/359605a0. doi:10.1038/359605a0 [DOI] [PubMed] [Google Scholar]
- Doolittle W.F. Phylogenetic classification and the universal tree. Science. 1999;284:2124–2128. doi: 10.1126/science.284.5423.2124. doi:10.1126/science.284.5423.2124 [DOI] [PubMed] [Google Scholar]
- Dymek R.F, Klein C. Chemistry, petrology and origin of banded iron-formation lithologies from the 3800-ma Isua supracrustal Belt, West Greenland. Precambrian Res. 1988;39:247–302. doi:10.1016/0301-9268(88)90022-8 [Google Scholar]
- Ehrenreich A, Widdel F. Anaerobic oxidation of ferrous iron by purple bacteria, a new type of phototrophic metabolism. Appl. Environ. Microbiol. 1994a;60:4517–4526. doi: 10.1128/aem.60.12.4517-4526.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenreich A, Widdel F. Phototrophic oxidation of ferrous minerals: a new aspect in the redox microbiology of iron. In: Stal L.J, Caumette P, editors. Microbial mats. vol. G 35. Springer; Berlin, Germany: 1994b. pp. 393–402. [Google Scholar]
- Farquhar J, Bao H.M, Thiemens M. Atmospheric influence of Earth's earliest sulfur cycle. Science. 2000;289:756–758. doi: 10.1126/science.289.5480.756. doi:10.1126/science.289.5480.756 [DOI] [PubMed] [Google Scholar]
- Farquhar J, Savarino J, Airieau S, Thiemens M.H. Observation of the wavelength-sensitive mass-dependent sulfur isotope effects during SO2 photolysis: Implications for the early atmosphere. J. Geophys. Res. 2001;106:32 829–32 839. doi:10.1029/2000JE001437 [Google Scholar]
- Field C.B, Behrenfeld M.J, Randerson J.T, Falkowski P. Primary production of the biosphere: integrating terrestrial and oceanic components. Science. 1998;281:237–240. doi: 10.1126/science.281.5374.237. doi:10.1126/science.281.5374.237 [DOI] [PubMed] [Google Scholar]
- Garrels R.M, Lerman A. Phanerozoic cycles of sedimentary carbon and sulfur. Proc. Natl Acad. Sci. USA. 1981;78:4652–4656. doi: 10.1073/pnas.78.8.4652. doi:10.1073/pnas.78.8.4652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrels R.M, Perry E.A., Jr . Cycling of carbon, sulfur, and oxygen through geologic time. In: Goldberg E.D, editor. The sea. vol. 5. Wiley; New York, NY: 1974. pp. 303–336. [Google Scholar]
- Godderis Y, Veizer J. Tectonic control of chemical and isotopic composition of ancient oceans: the impact of continental growth. Am. J. Sci. 2000;300:434–461. doi:10.2475/ajs.300.5.434 [Google Scholar]
- Halmer M.M, Schmincke H.-U, Graf H.-F. The annual volcanic gas input into the atmosphere, in particular into the stratosphere: a global data set for the past 100 years. J. Volcanol. Geotherm. Res. 2002;115:511–528. doi:10.1016/S0377-0273(01)00318-3 [Google Scholar]
- Hartman H. The evolution of photosynthesis and microbial mats: a speculation on the banded iron formations. In: Cohen Y, Castenholz R.W, Halvorson H.O, editors. Microbial mats: stromatolites. Alan R. Liss, Inc; New York, NY: 1984. pp. 449–453. [Google Scholar]
- Hayes J.M, Waldbauer J.R. The carbon cycle and associated redox processes through time. Phil. Trans. R. Soc. B. 2006;361:931–950. doi: 10.1098/rstb.2006.1840. doi:10.1098/rstb.2006.1840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes J.M, Strauss H, Kaufman A.J. The abundance of 13C in marine organic matter and isotopic fractionation in the global biogeochemical cycle of carbon during the past 800 Ma. Chem. Geol. 1999;161:103–125. doi:10.1016/S0009-2541(99)00083-2 [Google Scholar]
- Heising S, Richter L, Ludwig W, Schink B. Chlorobium ferrooxidans sp nov., a phototrophic green sulfur bacterium that oxidizes ferrous iron in coculture with a “Geospirillum” sp strain. Arch. Microbiol. 1999;172:116–124. doi: 10.1007/s002030050748. doi:10.1007/s002030050748 [DOI] [PubMed] [Google Scholar]
- Hilario E, Gogarten J.P. Horizontal transfer of ATPase genes—the tree of life becomes a net of life. BioSystems. 1993;31:111–119. doi: 10.1016/0303-2647(93)90038-e. doi:10.1016/0303-2647(93)90038-E [DOI] [PubMed] [Google Scholar]
- Holland H.D. Wiley; New York, NY: 1978. The chemistry of the atmosphere and oceans. [Google Scholar]
- Holland H.D. Princeton University Press; Princeton, NJ: 1984. The chemical evolution of the atmosphere and oceans. Princeton series in geochemistry. [Google Scholar]
- Holland H.D. Volcanic gases, black smokers, and the great oxidation event. Geochim. Cosmochim. Acta. 2002;66:3811–3826. doi:10.1016/S0016-7037(02)00950-X [Google Scholar]
- Holland H.D. The geologic history of seawater. In: Holland H.D, Turekian K.K, editors. Treatise on geochemistry. vol. 6. Elsevier; Amsterdam, The Netherlands: 2004. pp. 583–625. [Google Scholar]
- Hunten D. The escape of light gases from planetary atmospheres. J. Atmos. Sci. 1973;30:1481–1494. doi:10.1175/1520-0469(1973)030<1481:TEOLGF>2.0.CO;2 [Google Scholar]
- Jain R, Rivera M.C, Lake J.A. Horizontal gene transfer among genomes: the complexity hypothesis. Proc. Natl Acad. Sci. USA. 1999;96:3801–3806. doi: 10.1073/pnas.96.7.3801. doi:10.1073/pnas.96.7.3801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y, Kappler A, Croal L.R, Newman D.K. Isolation and characterization of a genetically-tractable photoautotrophic Fe(II)-oxidizing bacterium, Rhodopseudomonas palustris strain TIE-1. Appl. Environ. Microbiol. 2005;71:4487–4496. doi: 10.1128/AEM.71.8.4487-4496.2005. doi:10.1128/AEM.71.8.4487-4496.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadko, D., Baker, E., Alt, J. & Baross, J. A. 1994 Global impact of submarine hydrothermal processes: Ridge/Vents Workshop, NSF RIDGE Initiative and NOAA Vents Program.
- Kappler A, Pasquero C, Konhauser K, Newman D.K. Deposition of banded iron formations by phototrophic Fe(II)-oxidizing bacteria. Geology. 2005;33:865–868. doi:10.1130/G21658.1 [Google Scholar]
- Kasting J.F, Walker J.C.G. Limits on oxygen concentration in the prebiotic atmosphere and the rate of abiotic fixation of nitrogen. J. Geophys. Res. Oceans Atmos. 1981;86:1147–1158. [Google Scholar]
- Kharecha P, Kasting J, Siefert J. A coupled atmosphere-ecosystem model of the early Archean Earth. Geobiology. 2005;3:53–76. doi:10.1111/j.1472-4669.2005.00049.x [Google Scholar]
- Knoll A.H. Princeton University Press; Princeton, NJ: 2003. Life on a young planet. The first three billion years of evolution on Earth; p. 287. [Google Scholar]
- Knoll A.H, Bauld J. The evolution of ecological tolerance in Prokaryotes. Trans. R. Soc. Edin. Earth. 1989;80:209–223. doi: 10.1017/s0263593300028650. [DOI] [PubMed] [Google Scholar]
- Kump L.R, Seyfried W.E., Jr Hydrothermal Fe fluxes during the Precambrian: effect of low oceanic sulfate concentration and low hydrostatic pressure on the composition of black smokers. Earth Planet. Sci. Lett. 2005;235:654–662. doi:10.1016/j.epsl.2005.04.040 [Google Scholar]
- Kump L.R, Kasting J.F, Barley M.E. Rise of atmospheric oxygen and the “upside-down” Archean mantle. Geochem. Geophys. Geosyst. 2001;2 doi:10.1029/2000GC000114 [Google Scholar]
- Kuypers M.M.M, Sliekers A.O, Lavik G, Schmid M, Jorgensen B.B, Kuenen J.G, Damste J.S.S, Strous M, Jetten M.S.M. Anaerobic ammonium oxidation by anammox bacteria in the Black Sea. Nature. 2003;422:608–611. doi: 10.1038/nature01472. doi:10.1038/nature01472 [DOI] [PubMed] [Google Scholar]
- Kuypers M.M.M, Lavik G, Woebken D, Schmid M, Fuchs B.M, Amann R, Jørgensen B.B, Jetten M.S.M. Massive nitrogen loss from the Benguela upwelling system through anaerobic ammonium oxidation. Proc. Natl Acad. Sci. USA. 2005;102:6478–6483. doi: 10.1073/pnas.0502088102. doi:10.1073/pnas.0502088102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leth O, Middleton J.F. A mechanism for enhanced upwelling off central Chile: Eddy advection. J. Geophys. Res. 2004;109:C12020. doi:10.1029/2003JC002129 [Google Scholar]
- Mancinelli R.L, McKay C. The evolution of the nitrogen cycle. Orig. Life Evol. B. 1988;18:311–325. doi: 10.1007/BF01808213. doi:10.1007/BF01808213 [DOI] [PubMed] [Google Scholar]
- Nakamura K, Kato Y. Carbonatization of oceanic crust by the seafloor hydrothermal activity and its significance as a CO2 sink in the Early Archean. Geochim. Cosmochim. Acta. 2004;68:4595–4618. doi:10.1016/j.gca.2004.05.023 [Google Scholar]
- Navarro-González R, McKay C.P, Mvondo D.N. A possible nitrogen crisis for Archean life due to reduced nitrogen fixation by lightning. Nature. 2001;412:61–64. doi: 10.1038/35083537. doi:10.1038/35083537 [DOI] [PubMed] [Google Scholar]
- Nisbet E.G, Sleep N.H. The habitat and nature of early life. Nature. 2001;409:1083–1091. doi: 10.1038/35059210. doi:10.1038/35059210 [DOI] [PubMed] [Google Scholar]
- Olson J.M. Photosynthesis in the Archean era. Photosynth. Res. 2006;88:109–117. doi: 10.1007/s11120-006-9040-5. doi:10.1007/s11120-006-9040-5 [DOI] [PubMed] [Google Scholar]
- Olson J.M, Blankenship R.E. Thinking about the evolution of photosynthesis. Photosynth. Res. 2004;80:373–386. doi: 10.1023/B:PRES.0000030457.06495.83. doi:10.1023/B:PRES.0000030457.06495.83 [DOI] [PubMed] [Google Scholar]
- Ono S, Eigenbrode J.L, Pavlov A.A, Kharecha P, Rumble D, III, Kasting J.F, Freeman K.H. New insights into Archean sulfur cycle from mass-independent sulfur isotope records from the Hamersley Basin, Australia. Earth Planet. Sci. Lett. 2003;213:15–30. doi:10.1016/S0012-821X(03)00295-4 [Google Scholar]
- Pavlov A.A, Kasting J.F. Mass-independent fractionation of sulfur isotopes in Archean sediments: strong evidence for an anoxic Archean atmosphere. Astrobiology. 2002;2:27–41. doi: 10.1089/153110702753621321. doi:10.1089/153110702753621321 [DOI] [PubMed] [Google Scholar]
- Pavlov A.A, Brown L.L, Kasting J.F. UV shielding of NH3 and O2 by organic hazes in the Archean atmosphere. J. Geophys. Res. 2001;106:23 267–23 287. doi:10.1029/2000JE001448 [Google Scholar]
- Pfennig N. The phototrophic bacteria and their role in the sulfur cycle. Plant Soil. 1975;43:1–16. doi:10.1007/BF01928472 [Google Scholar]
- Pierson B.K. Phylum BVI. Chloroflexi phy.nov. Family I. “Chloroflexaceae”. In: Boone D.R, Castenholz R.W, Garrity G.M, editors. Bergey's manual of systematic bacteriology. vol. I. Springer; New York, NY: 2001. pp. 427–429. [Google Scholar]
- Reeburgh W.S. Anaerobic methane oxidation: rate depth distributions in Skan Bay sediments. Earth Planet. Sci. Lett. 1980;47:345–352. doi:10.1016/0012-821X(80)90021-7 [Google Scholar]
- Rosing M.T. 13C-depleted carbon microparticles in >3700-Ma sea-floor sedimentary rocks from West Greenland. Science. 1999;283:674–676. doi: 10.1126/science.283.5402.674. doi:10.1126/science.283.5402.674 [DOI] [PubMed] [Google Scholar]
- Rosing M.T, Frei R. U-rich Archaean sea-floor sediments from Greenland—indications of >3700 Ma oxygenic photosynthesis. Earth Planet. Sci. Lett. 2004;217:237–244. doi:10.1016/S0012-821X(03)00609-5 [Google Scholar]
- Rosing M.T, Rose N.M, Bridgwater D, Thomsen H.S. Earliest part of Earth's stratigraphic record: a reappraisal of the >3.7 Ga Isua (Greenland) supracrustal sequence. Geology. 1996;24:43–46. doi:10.1130/0091-7613(1996)024<0043:EPOESS>2.3.CO;2 [Google Scholar]
- Schidlowski M. A 3,800-million-year isotopic record of life from carbon in sedimentary rocks. Nature. 1988;333:313–318. doi:10.1038/333313a0 [Google Scholar]
- Schink B. Energetics of syntrophic cooperation in methanogenic degradation. Microbiol. Mol. Biol. Rev. 1997;61:262–280. doi: 10.1128/mmbr.61.2.262-280.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleep N.H, Zahnle K. Carbon dioxide cycling and implications for climate on ancient Earth. J. Geophys. Res. 2001;106:1373–1399. doi:10.1029/2000JE001247 [Google Scholar]
- Stetter K.O. Hyperthermophiles in the history of life. In: Bock G.R, Goode J.A, editors. Evolution of hydrothermal ecosystems on Earth (and Mars?) Wiley; New York, NY: 1996. pp. 1–10. [Google Scholar]
- Symonds R.B, Rose W.I, Bluth G.J.S, Gerlach T.M. Volcanic-gas studies: methods, results, and applications. In: Carroll M.R, Holloway J.R, editors. Volatiles in magmas. vol. 30. Mineralogical Society of America; Washington, DC: 1994. pp. 1–66. [Google Scholar]
- Thamdrup B, Finster K, Hansen J.W, Bak F. Bacterial disproportionation of elemental sulfur coupled to chemical reduction of iron or manganese. Appl. Environ. Microbiol. 1993;59:101–108. doi: 10.1128/aem.59.1.101-108.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian F, Toon O.B, Pavlov A.A, De Sterck H. A hydrogen-rich early earth atmosphere. Science. 2005;308:1014–1017. doi: 10.1126/science.1106983. doi:10.1126/science.1106983 [DOI] [PubMed] [Google Scholar]
- van Dongen L.G.J.M, Jetten M.S.M, van Loosdrecht M.C.M. IWA Publishing; London, UK: 2001. The combined SHARON/Anammox process. [Google Scholar]
- Walker J.C.G. Macmillan; New York, NY: 1977. Evolution of the atmosphere. [Google Scholar]
- Widdel F, Schnell S, Heising S, Ehrenreich A, Assmus B, Schink B. Ferrous iron oxidation by anoxygenic phototrophic bacteria. Nature. 1993;362:834–835. doi:10.1038/362834a0 [Google Scholar]
- Wilde S.A, Valley J.W, Peck W.H, Grahams C.M. Evidence from detrital zircons for the existence of continental crust and oceans on the earth 4.4 Gyr ago. Nature. 2001;409:175–178. doi: 10.1038/35051550. doi:10.1038/35051550 [DOI] [PubMed] [Google Scholar]
- Woese C.R. Bacterial evolution. Microbiol. Rev. 1987;51:221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong J, Fischer W.M, Inoue K, Nakahara M, Bauer C.E. Molecular evidence for the early evolution of photosynthesis. Science. 2000;289:1724–1730. doi: 10.1126/science.289.5485.1724. doi:10.1126/science.289.5485.1724 [DOI] [PubMed] [Google Scholar]
- Yung Y.L, McElroy M.C. Fixation of nitrogen in the prebiotic atmosphere. Science. 1979;203:1002–1004. doi: 10.1126/science.203.4384.1002. [DOI] [PubMed] [Google Scholar]
Additional references
- Shen Y, Buick R, Canfield D.E. Isotopic evidence for microbial sulphate reduction in the early Archaean era. Nature. 2001;410:77–81. doi: 10.1038/35065071. doi:10.1038/35065071 [DOI] [PubMed] [Google Scholar]
- Ueno Y, Yamada K, Yoshida N, Maruyama S, Isozaki Y. Evidence from fluid inclusions for microbial methanogenesis in the early Archaean era. Nature. 2006;440:516–519. doi: 10.1038/nature04584. doi:10.1038/nature04584 [DOI] [PubMed] [Google Scholar]