Vagal afferent nerves with nociceptive properties in guinea-pig oesophagus (original) (raw)
Abstract
Some vagal afferent nerves are thought to mediate autonomic responses evoked by noxious oesophageal stimuli and participate in the perception of pain originating in the oesophagus. However, the vagal nociceptive nerve phenotypes implicated in this function have yet to be identified. In this study, nociceptive fibres were defined by the capacity to discriminate noxious mechanical stimuli (wide range of oesophageal distension with pressure up to 100 mmHg) and detect noxious chemical stimuli (the activators of capsaicin receptor TRPV1). Using immunohistochemical techniques with retrogradely labelled oesophagus-specific neurones and performing extracellular recordings from the isolated vagally innervated oesophagus, we show that in the guinea-pig, the vagus nerves supply the oesophagus with a large population of nociceptive-like afferent nerve fibres. Vagal nociceptive-like fibres in the guinea-pig oesophagus are derived from two embryonically distinct sources: neurones situated in the nodose vagal ganglia and neurones situated in the jugular vagal ganglia. Nodose (placode-derived) nociceptive-like fibres are exclusively C-fibres sensitive to a P2X receptors agonist and rarely express the neuropeptide substance P. In contrast, jugular (neural crest-derived) nociceptive-like fibres include both A-fibres and C-fibres, are insensitive to P2X receptors agonist and mostly express substance P. The non-nociceptive vagal tension mechanoreceptors are distinguished from nociceptors by their saturable response to oesophageal distension and by the lack of TRPV1. These tension mechanoreceptors are exclusively A-fibres arising from the nodose ganglion. We conclude that the vagus nerves supply the guinea-pig oesophagus with nociceptors in addition to tension mechanoreceptors. The vagal nociceptive-like fibres in the oesophagus comprise two distinct subtypes dictated by the ganglionic location of their cell bodies.
Sensory nerves detect stimuli resulting from the physiological activity of the tissue as well as stimuli associated with impending and/or actual tissue damage (noxious stimuli). The sensory nerves capable of discriminating noxious stimuli are termed nociceptors (Sherrington, 1906; Belmonte & Cervero, 1996). The activation of nociceptive nerves may initiate defensive reflexes and/or perceptions of discomfort and pain.
The mammalian oesophagus receives sensory innervation from vagal and spinal pathways (Sengupta, 2000). It is generally accepted that the spinal sensory nerves detect noxious oesophageal stimuli leading to conscious perceptions such as heartburn and pain (e.g. nociceptive nerves; Gebhart, 2000; Berthoud et al. 2004). In contrast, vagal sensory nerves are traditionally thought to participate mainly in the regulation of physiological functions of the oesophagus (Gebhart, 2000; Berthoud et al. 2004). It is increasingly recognized, however, that vagal sensory nerves may also mediate autonomic responses to noxious stimuli and modulate the perception of pain and discomfort originating in the oesophagus (Berthoud & Neuhuber, 2000; Grundy, 2002). Studies on vagal afferent nerves responsive to oesophageal distension have thus far focused on the non-nociceptive mechanosensors, termed ‘tension mechanoreceptors’ or ‘tension receptors’ (Falempin et al. 1978; Clerc & Mei, 1983; Satchell, 1984; Sengupta et al. 1989; Page & Blackshaw, 1998; Zagorodnyuk & Brookes, 2000; Page et al. 2002). The vagal afferent phenotypes responsive to noxious stimuli in the oesophagus have yet to be identified.
Similarly to other cranial nerves, the vagus nerves contain sensory nerve fibres originating from two different embryonic tissues (Baker & Bronner-Fraser, 2001). A proportion of vagal sensory neurones is derived from the embryonic placodes. These neurones are situated in the nodose (inferior) vagal ganglia. The other population of vagal sensory neurones is located in the jugular (superior) vagal ganglia. The jugular neurones are derived from the embryonic neural crest, as are the spinal sensory neurones. Based on the common neural crest origin of nociceptive spinal and vagal jugular sensory neurones, we hypothesized that jugular ganglia supply the oesophagus with nociceptive sensory nerves, a notion also supported by anatomical observations (Wank & Neuhuber, 2001). We have adopted a definition of a nociceptive phenotype based on the discriminative responsiveness to noxious mechanical and chemical stimuli (Sherrington, 1906; Belmonte & Cervero, 1996).
We show that in the guinea-pig, the vagus nerve supplies the oesophagus with a large population of nociceptive afferent nerves. A proportion of oesophageal distension-sensitive nociceptive-like fibres originate from the neural crest-derived jugular ganglion. The placode-derived nodose neurones also contribute to the nociceptive innervation of the oesophagus. Importantly, the phenotype of nodose nociceptors differs from that of the jugular nociceptors, suggesting that the two subtypes of nociceptive nerves may serve different functions. Finally, we show that the oesophageal non-nociceptive tension mechanoreceptors are exclusively A-fibres originating from the placode-derived nodose ganglia.
Methods
All experiments were approved by the Johns Hopkins Animal Care and Use Committee. Male Hartley guinea-pigs (Hilltop Laboratory Animals, Inc., Scottsdale, PA, USA) weighing 100–300 g were used.
Histology
Retrograde tracing and tissue preparation
Guinea-pigs were aneesthetized by intramuscular injection of ketamine (50 mg kg−1) and xylazine (2.5 mg kg−1). The cervical oesophagus was surgically exposed and 1 μl of the retrograde tracer DiI solution (1% diluted in 50% dimethyl sulphoxide in saline) was injected into one or two sites in the left dorsolateral aspect of the oesophageal wall using a 5 μl Hamilton syringe. It has been shown that DiI can be used for immunohistological processing when the use of detergents is limited (Berthoud & Neuhuber, 2000). Ten to 14 days after injection the animals were killed by CO2 asphyxiation. Intrathoracic organs and sensory vagal ganglia (jugular and nodose ganglia) were isolated and fixed in 4% paraformaldehyde for 2 h at 4°C and cryoprotected in sucrose (18%) for 18 h (4°C). Continuous serial cryostat sections (12 μm thick) of the vagal sensory ganglia (nodose or jugular) were thaw-mounted on four different slides, such as the first slide had sections 1, 5, 9…, the second 2, 6, 10… and so on; alternate slides were used for the analysis.
Immunohistochemistry
Slides were rinsed with water and PBS and incubated with goat serum (10%) diluted in PBS containing bovine serum albumin (BSA, 1%) at room temperature for 1 h. Sections were then incubated with primary antibodies diluted in PBS containing BSA (1%) (BSA/PBS) and incubated for 24 h at 4°C. After rinsing with BSA/PBS the sections were covered with secondary (antiprimary) antibodies diluted in BSA/PBS for 2 h at room temperature. The sections were then rinsed with PBS and with saline buffered with phosphate to pH 8.6, coverslipped and viewed immediately.
Double staining for neurofilament 160 kDa (NF) and substance P (SP) immunoreactivity was performed using mouse anti-NF (2.5 μg ml−1, Chemicon, Temecula, CA, USA) and rat anti-SP (5 μg ml−1, Chemicon) as primary antibodies, and goat antirat Alexa Fluor®488-labelled antibody (20 μg ml−1, Molecular Probes, Eugene, OR, USA) and goat antimouse Alexa Fluor®350-labelled antibody (10 μg ml−1, Molecular Probes) used as secondary antibodies. Sections were examined under epifluorescence (Olympus DX60 microscope) using appropriate filter combinations for DiI (excitation filter 510–550 nm, barrier filter 570–590 nm), for Alexa Fluor®488 and FITC (excitation filter 450–480 nm, barrier filter 500–515 nm) and for Alexa Fluor®350 (excitation filter 330–385 nm, barrier filter 400–420 nm). Cervical oesophagus and trachea as well as the adjacent connective tissues were cut in 1–2 mm sections and evaluated for the presence and localization of the neuronal tracer.
Electrophysiology
Tissue preparation
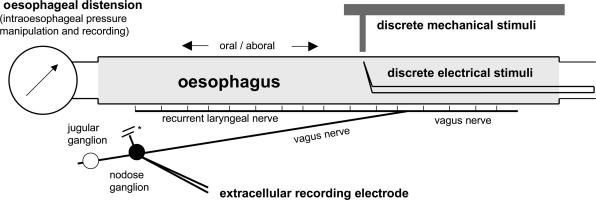
The method used to record extracellularly from vagal sensory neurones projecting to the guinea-pig oesophagus was modified from a method extensively used to record from guinea-pig vagal bronchopulmonary afferent fibres (Riccio et al. 1996; Undem et al. 2004). Guinea-pigs were killed by CO2 inhalation and exsanguination, and the oesophagus and trachea with intact bilateral extrinsic vagal innervation (including jugular and nodose ganglia) were dissected. As schematically shown in Fig. 1, the tissue was pinned in a small Sylgard-lined Perspex chamber filled with Krebs bicarbonate buffer (KBS, composed of (mm): NaCl, 118; KCl, 5.4; NaH2PO4, 1.0; MgSO4, 1.2; CaCl2, 1.9; NaHCO3, 25.0; and dextrose, 11.1, and gassed with 95% O2–5% CO2, pH 7.4, 35°C) containing indomethacin (3 μm). The chamber had two compartments: the oesophagus with attached trachea (to support the recurrent laryngeal nerves) and the vagus were pinned in the tissue compartment, and the rostral aspect of the vagus nerves including the nodose and jugular ganglia were pinned in the recording compartment. The two compartments were separately superfused with KBS (pH 7.4, 35°C, 4–6 ml min−1). Polyethylene tubing was inserted 3–5 mm into the cranial and caudal oesophagus and secured for perfusion with KBS. The superior laryngeal nerves that enter the oesophagus at its cranial end were damaged by securing the PT tubing and were therefore severed. In some experiments, the oesophageal mucosal layer was removed whilst preserving the tube shape of the oesophagus or, in other experiments, the oesophagus was cut longitudinally on the ventral side and pinned, mucosal side up, in the recording chamber. The results of these experiments are described separately.
Figure 1. Ex vivo selective recording from the oesophageal vagal afferent nerve fibres originating in nodose and jugular vagal ganglia.
The extracellular recording electrode was positioned in the nodose (shown in figure) or jugular ganglion. Oesophageal distension was induced by manipulation of intraoesophageal pressure. The agonists were delivered in the intraluminal and/or serosal perfusate. * Note that the superior laryngeal nerve was severed.
Oesophageal distension and recording of intraoesophageal pressure
The pressure in the fluid (KBS)-filled oesophagus was measured with a differential pressure transducer connected in series to the oesophagus and recorded simultaneously with neural activity by the chart recorder (TA240S, Gould, Valley View, OH, USA; Fig. 1). In the preparation used, the lumen of the oesophagus spontaneously collapsed and the lowest pressure required to restore the perfusion (resting pressure) was 0–4 mmHg. Isobaric (constant pressure) distension of the oesophagus was achieved by increasing intraluminal oesophageal pressure to 4–100 mmHg (Fig. 4, pressure traces). The pressure was generated by a calibrated device utilizing fluid (KBS) columns.
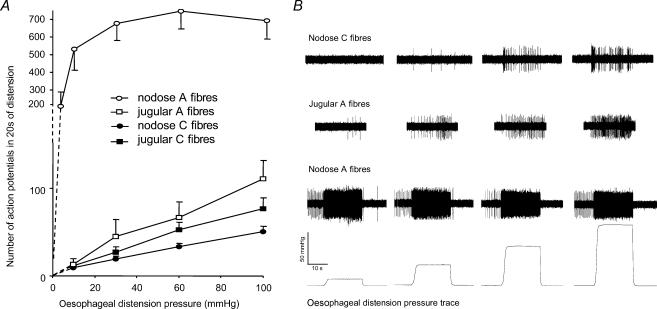
Figure 4. Response of vagal afferent nerve fibres to oesophageal distension.
Average data (A) and representative traces (B) are shown separately for afferent nerve types defined by the combination of anatomical (nodose, jugular) and neurophysiological properties (A-fibres, C-fibres). The response of nodose A-fibres saturated (< 60 mmHg), while the response of the remaining fibres did not (up to 100 mmHg). The response of nodose A-fibres also differed quantitatively (P < 0.05). Note the change in the y axis scale required for visualization of the nodose A-fibre response.
Recording of nerve activity
Extracellular recordings were performed using an aluminosilicate glass microelectrode (pulled with a Flaming-Brown micropipette puller, Sutter Instrument Company, Novato, CA, USA) and filled with 3 m sodium chloride (electrode resistance 2 MΩ. The electrode was placed into an electrode holder connected directly to the headstage (A-M Systems, Everett, WA, USA). A return electrode of silver–silver chloride wire and earthed silver–silver chloride pellet were placed in the perfusion fluid of the recording compartment. The recorded signal was amplified (Microelectrode AC amplifier 1800, A-M Systems) and filtered (low cut-off, 0.3 kHz; high cut-off, 1 kHz) and the resultant activity was displayed on an oscilloscope (TDS 340, Tektronix, Beaverton, OR, USA) and a model TA240 chart recorder (Gould). The data were stored and analysed on a Macintosh computer using the software TheNerveOfIt (sampling frequency 33 kHz; PHOCIS, Baltimore, MD, USA) and further processed using spreadsheet software (Microsoft Excel 98).
Identification of a vagal afferent nerve fibre projecting to the oesophagus
The recording electrode was micromanipulated into the nodose or jugular ganglion (left or right). A distension-sensitive unit was identified when oesophageal distension (in most experiments with a rapid increase in intraluminal pressure to 100 mmHg for 5 s) evoked action potential discharge. The serosal surface of the oesophagus was then searched with a punctate mechanical probe (Von Frey hair, 1 mN, filament diameter < 0.5 mm) applied to the tissue, with a firm probe (outside diameter ∼1 mm) having already been inserted into the oesophageal lumen. A mechanosensitive receptive field was located when the punctate stimulus evoked discharge of action potentials with waveforms identical to the action potentials evoked by distension. The receptive field was then stimulated electrically (pulse duration 1 ms, frequency 1 Hz) with a concentric electrode inserted into the oesophagus with the tip positioned at the site of the mechanosensitive receptive field. The initial voltage (100 V) was gradually reduced to the lowest voltage (threshold voltage) at which each stimulation pulse was followed by a single action potential (30–90 V for most afferent nerve fibres recorded). The waveforms of the electrically evoked action potentials were identical to those induced by distension and the punctate mechanical stimulus. Conduction time was measured as the time between the stimulation pulse and the action potential (visualized by oscilloscope). Variability of conduction time (during the train stimulation, 10 Hz, 10 s) less than 3 ms indicated direct electrical stimulation (indirect activation of a unit by electrically evoked muscle contractions was readily discernable by high variability > 20 ms of conduction time). Conduction velocity was calculated by dividing the length of the approximated nerve pathway by conduction time. In the instances when the action potential of the recorded unit could be identified in the compound action potential evoked by electrical stimulation of the vagus nerve trunk, the conduction velocity was also calculated from the nerve pathway length and conduction time obtained from the vagus nerve trunk stimulation. Although the oesophagus was not extensively searched for multiple mechanically sensitive receptive fields, when identified, the conduction velocity was calculated separately for each mechanosensitive receptive field. In some experiments the oesophagus was searched with intraluminal pressure of 10–60 mmHg or with intraluminal electrical stimulation. Thus the afferent unit was identified before distending the oesophagus with larger pressures. In these experiments no significant desensitization of distension-evoked response was noted to repeated distension up to 100 mmHg. In the preparations with the longitudinally cut oesophagus pinned mucosal side up, the receptive fields were searched with mucosal electrical (100 V, 1 ms, 1 Hz) and punctate mechanical (1 mN) stimuli.
Mechanical stimulation
Isobaric oesophageal distension for 20 s with an intraluminal pressure of 10–100 mmHg separated by at least 60 s was used to determine the distension pressure–nerve activity relationship of an oesophageal afferent fibre (Fig. 4, pressure traces). To assess the reproducibility of distension-evoked activation, this distension protocol was repeated after at least 5 min. Distension with a pressure of 60 mmHg (20 s) was routinely used to assess the viability and mechanical responsiveness of an afferent fibre during experiments. This was reproducible over 2 h of experimentation. The distension-evoked response was quantified as the total number of action potentials discharged during the 20 s of distension from which the spontaneous activity (if present) was subtracted. Data are presented as means ± s.e.m. The adaptation of nerve fibre activation evoked by sustained distension was analysed by comparing the action potentials discharged in the initial 10 s and last 10 s of the 20 s distension period and quantified by the adaptation index. The adaptation index (expressed as a percentage) was defined as the difference between expected and actual number of action potentials discharged during the last 10 s normalized to the expected discharge (adaptation index = (initial 10 s – last 10 s)/initial 10 s × 100%). An adaptation index of 0% reflects no adaptation; an adaptation index of 100% reflects complete adaptation; and a negative value of adaptation index reflects an increase in action potential discharge during the course of sustained distension. Adaptation data are presented as means ± s.e.m. Sharp and blunt probes were used to deliver intraluminal mechanical stimuli. In some experiments, after the response to oesophageal distension was characterized, the mucosal layer was removed and the receptive field was tested again for the response to punctate mechanical stimulus (1 mN).
Pharmacological stimulation
The drugs diluted in KBS were delivered to both the mucosal surface in the luminal perfusate and the serosal surface of the oesophagus in the external perfusate for 10 min. In preliminary experiments, we found that extending the drug exposure to 20 min did not uncover the response of afferents that were not activated during the initial 10 min. The nerve activity (action potential discharge) was monitored continuously and analysed in 1 s bins (yielding the number of action potentials in each second, Hz). The peak frequency (Hz) was defined as the maximal frequency of action potential discharge. The response to the particular agonist was considered positive when the drug evoked action potential discharge with a peak frequency of at least 3 Hz (in the fibres with no baseline activity) or a peak frequency at least three times the frequency of baseline activity. The time to onset of the response was defined as the time elapsed between adding the drug to the tissue to the onset of the action potential discharge. The time to onset of activation and the peak frequency of action potential discharge are presented as means ± s.e.m. In some experiments, when no response was obtained to any pharmacological stimulus, the mucosal layer was gently removed and the denuded tissue exposed to pharmacological stimuli. The agonists capsaicin and α,β-methylene-ATP were used in their maximally effective concentrations of 1 and 30 μm, respectively. In preliminary experiments, we found that the threshold for capsaicin and α,β-methylene-ATP-induced activation was < 1 μm(n = 3) and ≤ 3 μm(n = 4), respectively. The stock solutions of capsaicin (10 mm) and α,β-methylene-ATP (10 mm) in ethanol and water, respectively, were stored at −20°C and diluted in KBS to final concentration on the day of use. Both drugs were purchased from Sigma-Aldrich.
Results
Location of cell bodies
Injection of the retrograde tracer DiI into the wall of the cervical oesophagus resulted in bilateral labelling of vagal sensory neurones in both nodose and jugular ganglia (Fig. 2). In four animals, a total of 1124 and 2485 neurones retrogradely labelled from the oesophagus were studied in nodose (161 ± 24, n = 7) and jugular ganglia (311 ± 20, n = 8), respectively. The labelled neurones were distributed almost equally between the right (55%) and left ganglia (45%). Qualitatively, the same results were obtained when the left and right ganglia were analysed separately, so the pooled data are presented.
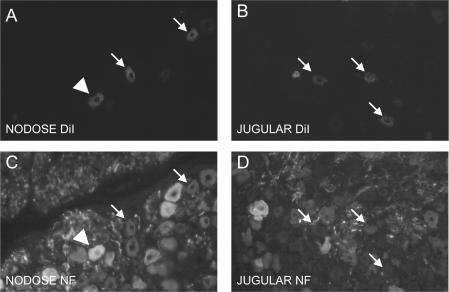
Figure 2. Both nodose (A and C) and jugular vagal sensory neurones (B and D) innervate the oesophagus in guinea-pigs.
Injection of the retrograde tracer DiI into the cervical oesophagus resulted in bilateral labelling of nodose (A) and jugular sensory neurones (B). C and D, staining for neurofilament 160 kDa (NF) was used to discriminate between A-fibre (NF-positive, arrowheads) and C-fibre neurones (NF-negative, arrows). Labelled nodose neurones were nearly evenly divided between NF-positive (putative A-fibre neurones, 56 ± 6%) and NF-negative neurones (putative C-fibre neurones, 44 ± 6%), while labelled jugular neurones were mostly (92 ± 1%) NF-negative putative C-fibre neurones.
Staining for neurofilament (NF 160 kDa) was used to discriminate histologically between the C-fibre and A-fibre neurons (Fig. 2). In primary sensory neurones, high levels of NF are immunohistochemically detected only in those neurones that project myelinated nerve fibres conducting in the A range (Lawson & Waddell, 1991; Riccio et al. 1996). The jugular ganglia were found to project mainly NF-negative fibres (putative C-fibres) to the cervical oesophagus (92 ± 1% of the jugular oesophagus-specific neurones were NF negative, n = 8). Nodose neurones labelled from the cervical oesophagus were almost equally divided between NF-positive (putative A-fibre neurones, 56 ± 6%, n = 7) and NF-negative neurones (putative C-fibre neurones, 44 ± 6%, n = 7).
In agreement with the retrograde tracing experiments, electrophysiological recordings showed that both the nodose and jugular ganglia project afferent nerve fibres to the oesophagus. The nerve activity was recorded from 69 nodose and 43 jugular distension-sensitive oesophageal afferent nerve fibres. Since the ganglion was arbitrarily chosen at the beginning of the experiment, these numbers do not reflect the relative contribution of nodose and jugular ganglia to oesophageal afferent innervation. We noted no difference in the probability of finding distension-sensitive afferent nerve fibres in the left and right ganglia. Similarly, we noted no difference in the studied properties between the afferent nerves originating from the left and right side and so the pooled data are presented.
Identification of mechanosensitive nerve terminals
Transient (∼5 s) distension of the oesophagus induced by an increase in intraluminal pressure from the resting pressure to 100 mmHg was used to search for afferent nerve fibres. For nearly all distension-sensitive vagal oesophageal afferents, a receptive field sensitive to punctate mechanical stimulus (von Frey filament, 1 mN), delivered perpendicularly to the serosal surface (Fig. 1), could be identified. In rare instances the mechanosensitive receptive field could not be localized and the fibre was not studied further. In six fibres two mechanosensitive receptive fields separated by a mechanically insensitive area (> 1 mm) were noted. In these cases, the identical action potential waveforms and conduction velocities obtained from stimulation of the two separate receptive fields confirmed that they were supplied by a single afferent nerve fibre. Although finding more than one receptive field for a single nerve was a relatively rare occurrence, it should be noted that an extensive effort to identify multiple receptive fields was not made.
Conduction velocities
The distribution of the conduction velocities of both nodose and jugular distension-sensitive afferent nerve fibres was bimodal with two peaks at ∼0.8 and 4–5 m s−1. Based on the anatomical location of the afferent neurone (nodose versus jugular) and its electrophysiological characteristics (conduction velocity), the vagal oesophageal distension-sensitive afferent nerve fibres were divided into four groups (mean ± s.e.m. of conduction velocities in parentheses): nodose A-fibres (4.8 ± 0.6 m s−1, n = 15); nodose C-fibres (0.8 ± 0.04 m s−1, n = 54); jugular A-fibres (3.9 ± 0.5 m s−1, n = 10); and jugular C-fibres (0.9 ± 0.07 m s−1, n = 33; Table 1).
Table 1.
Mechanosensitive and pharmacological characteristics of vagal distension-sensitive afferents in the guinea-pig oesophagus
| Nodose A-fibre | Nodose C-fibre | Jugular A-fibre | Jugular C-fibre | |
|---|---|---|---|---|
| Conduction velocitya (m s−1) | 4.8 ± 0.6 (n = 15) | 0.83 ± 0.03 (n = 53) | 3.7 ± 0.5 (n = 9) | 0.91 ± 0.07 (n = 33) |
| Peak frequency (Hz) of distention-evoked action potential dischargea, b | 73 ± 10* (n = 11) | 9 ± 1 (n = 37) | 8 ± 3 (n = 8) | 7 ± 1 (n = 24) |
| Saturation of response to oesophageal distension (10–100 mmHg) | Yes (∼30 mmHg) | No | No | No |
| TRPV1 receptor | No | Yes | Yes | Yes |
| Peak frequency of capsaicin (1 μm)-induced action potential dischargea (Hz) | — | 11 ± 2 | 9 ± 1 | 13 ± 2 |
| Proportion responsivec | 0/6 | 15/16 | 5/5 | 16/16 |
| P2X receptors | Yes | Yes | No | No |
| Peak frequency of α,β-methylene-ATP (30 μm)-induced action potential dischargea (Hz) | 16 ± 4 | 9 ± 2 | — | — |
| Proportion responsiveb | 9/9 | 13/16 | 0/5 | 0/8 |
These groups were analysed separately for their mechanosensitive and pharmacological properties. In jugular A-fibres, but not nodose A-fibres, the conduction velocity was in some instances noticeably higher when determined from the nerve trunk than from the receptive field (the apparent length of the nerve pathways was 30 and 50–80 mm, respectively). This observation suggests that some jugular A-fibres, but not nodose A-fibres, follow a less direct path to their termination and/or lose their myelin sheath after entering the oesophagus. The afferent fibres with higher conduction velocity in the proximal part of the axon have been described in the vagus nerves in cats (Duclaux et al. 1976).
Location of mechanosensitive nerve terminals
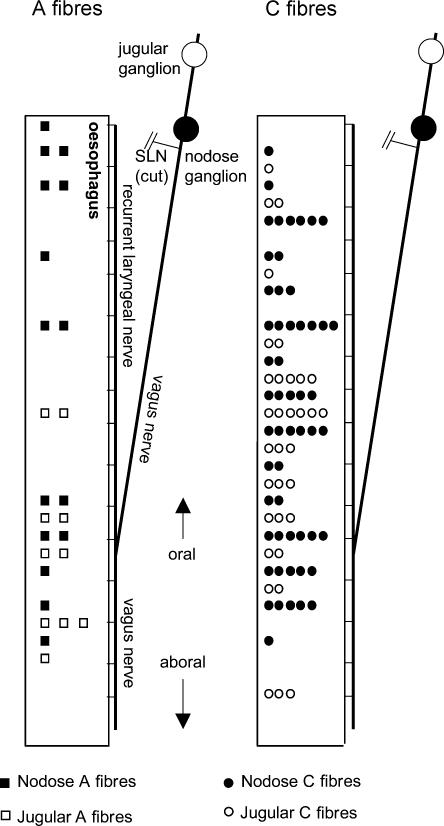
As described in the Methods, the superior laryngeal nerves were severed during preparation for the electrophysiological studies (Fig. 1). As seen in Fig. 3, nodose fibres innervating the oesophagus via oesophageal branches of the vagus and the recurrent laryngeal nerve (filled symbols) were located at every level of the oesophagus. In contrast, jugular fibres innervating the oesophagus via oesophageal branches of the vagus and the recurrent laryngeal nerve (open symbols) were found mainly (∼75%) in the distal half of the oesophagus. In a separate series of experiments, in a preparation with intact superior laryngeal nerves, we found that the jugular neurones project mechanosensitive afferent nerve fibres to the proximal oesophagus also via the superior laryngeal nerve (n = 3). These data show that the oesophageal branches of the vagus and the recurrent laryngeal nerves convey nodose afferents to both proximal and distal oesophagus. In contrast, the jugular afferent nerves reach the proximal oesophagus mostly through the superior laryngeal nerve whist projecting to the distal oesophagus through the vagus and the recurrent laryngeal nerves. These electrophysiological data are consistent with previous anatomical studies in rat oesophagus (Wank & Neuhuber, 2001).
Figure 3. Distribution of the distension-sensitive nerve terminals of the A-fibres and C-fibres originating in nodose (closed symbols) and jugular vagal ganglia (open symbols).
The mechanosensitive nerve terminal was identified by oesophageal distension and located by punctate mechanical stimulus. The conduction velocity was determined from electrical stimulation of the mechanosensitive nerve ending. Note that the superior laryngeal nerve (SLN) was severed in these experiments. This probably accounts for the relative paucity of recorded jugular afferent nerve terminals in the proximal half of the oesophagus.
Experiments were not designed to address specifically the location of nerve terminals within the tissue. Nevertheless, we noted that gently probing the mucosal surface with a mechanical probe failed to activate any distension-sensitive fibres. In the guinea-pig oesophagus, the mucosal layer that contains the epithelium and mucosal smooth muscle can easily be removed (peeled off) from the striated muscle layer (Zagorodnyuk & Brookes, 2000). We observed in at least two of each type of fibre (nodose A and C-fibres, and jugular A and C-fibres) that careful removal of the mucosal layer did not result in the loss of mechanosensitivity of nerve fibres first identified in the intact preparations. Considered together, these data are consistent with the hypothesis that the terminals of the distension-sensitive oesophageal afferent nerves are situated beneath the mucosal layer.
Characteristics of the distension-sensitive nerve terminals
All afferent nerve fibres studied responded to oesophageal distension evoked by step increases in the intraoesophageal pressure in a pressure-dependent manner (Fig. 4). The distension-evoked response of the nodose A-fibres differed markedly (qualitatively and quantitatively) from the responses of the nodose C-fibres, jugular A-fibres and jugular C-fibres and is therefore described separately.
Nodose A-fibres
At the resting pressure, the nodose A-fibres (13/15) displayed regular baseline action potential discharge (3.8 ± 0.7 Hz; Fig. 4_B_, nodose A-fibre trace). The nodose A-fibres were exquisitely sensitive to increases in intraluminal pressure. The lowest attainable increase in oesophageal pressure (to 4–6 mmHg, varying between the preparations) induced a substantial response in the nodose A-fibres and the activation at a pressure of 10 mmHg amounted to ∼70% of their maximal response (Fig. 4_A_). The response intensity (action potential frequency) of the nodose A-fibres reached an asymptotic plateau at pressures ≤ 30 mmHg (Fig. 4_A_). Thus the distension encoding capacity of the nodose A-fibres is saturable.
The adaptation of the nerve response to a constant mechanical stimulus (isobaric step distension, 20 s) was analysed by comparing the number of action potentials which occurred in the initial 10 s and the last 10 s. The adaptation was quantified by an adaptation index expressing the degree of adaptation as a percentage. Nodose A-fibres either failed to adapt, or adapted slowly to the isobaric distension (Fig. 4_B_, nodose A-fibre trace). Detailed analysis revealed that the action potential discharge of nodose A-fibres peaked during the initial dynamic phase of distension and then decayed within a few seconds to an average frequency, and the discharge of action potentials adapted only moderately (17 ± 9%, n = 15) over the rest of the distension period. The distension-evoked action potential discharge of nodose A-fibres was consistently followed by a variable (5–60 s) silent period (cessation of baseline activity, Fig. 4_B_, nodose A-fibre trace), which was proportional to the distension pressure.
Nodose C-fibres, jugular C-fibres and jugular A-fibres
Most of the nodose C-fibres (60%), jugular A-fibres (80%) and jugular C-fibres (50%) had no activity under resting conditions (Fig. 4_B_). The resting activity of the remaining afferents was irregular and averaged 0.3, 0.2 and 0.2 Hz in nodose C-fibres, jugular A-fibres and jugular C-fibres, respectively. The intensity of distension-induced action potential discharge in these types of fibres was much lower than that observed in nodose A-fibres (Fig. 4_A_ and Table 1). Also, in contrast to nodose A-fibres, the activity of nodase C-fibres, jugular C-fibres and jugular A-fibres increased monotonically (linearly) with the oesophageal pressure up to 100 mmHg. (Fig. 4_A_). The activation at a pressure of 10 mmHg amounted to < 20% of the response at 100 mmHg (Fig. 4_A_). Since a pressure of 100 mmHg was arbitrarily chosen as the largest pressure used in this study, this percentage is likely to underestimate the maximal attainable response.
The response to distension was highly reproducible. In nodose C-fibres, repeated distension with pressures of 30, 60 and 100 mmHg caused responses of 102 ± 17, 102 ± 14 and 94 ± 8% of the control responses, respectively (n = 11). In jugular C-fibres, repeated distension with pressures of 30, 60 and 100 mmHg caused responses 101 ± 22, 110 ± 13 and 109 ± 25% of the control responses, respectively (n = 10). The response to isobaric distension adapted in most nodose C-fibres, and this effect was independent of the intensity of distension (Fig. 4_B_, nodose C-fibre trace). The adaptation index of nodose C-fibres at 60 mmHg was 48 ± 6%. Classical rapid adaptation (the cessation of action potential discharge after the first few seconds of activation) was encountered in 5 of 35 nodose C-fibres. The adaptation did not influence the ability of nodose C-fibres to encode the intensity of oesophageal distension (10–100 mmHg, P > 0.1, data not shown). The response of jugular C-fibres to isobaric distension was more variable. In most cases the response did not adapt, but rather increased during the course of 20 s of isobaric distension. This is reflected by a negative value of the adaptation index (−13 ± 15% at 60 mmHg, P < 0.01 compared to nodose C-fibres). The low number and variability of the distension-evoked response of jugular A-fibres precluded thorough analysis, but a non-adapting response resembling jugular C-fibres was uniformly observed at higher pressures (Fig. 4_B_, jugular A-fibre trace).
Pharmacological stimuli
The pharmacological studies of the vagal oesophageal afferent nerves are shown in Fig. 5 and summarized in Table 1. The agonists used were the TRPV1 receptor selective agonist, capsaicin (1 μm), and the P2X receptor selective agonist, α,β-methylene-ATP (30 μm), both of which were applied for 10 min. Both agonists were used in their maximally effective concentrations. None of the vagal afferent nerve fibres found using the oesophageal distension-based protocol was activated by intraluminal (mucosal) administration of any agonist, although responses were induced by subsequent serosal administration of the same agonist in the external perfusate (n = 5–10 for each subtype of vagal afferent). These observations show that the normal oesophageal mucosa is an effective barrier that prevents chemicals from effectively penetrating to the receptive field within the tissue. Some vagal afferents that failed to respond to both mucosal and serosal administration of an agonist were activated when this agonist was administered to the denuded tissue after the removal of the mucosal layer (n = 4). Thus, some afferents are not accessible by diffusion from the serosal surface of oesophagus. These caveats were taken into account when the responsiveness of the vagal afferents to pharmacological stimuli was interpreted. An afferent nerve is reported as unresponsive to the agonist only if access of the agonist to the nerve terminal was confirmed by a response to another agonist. The nerve terminals of the afferents that did not respond to any agonist could be either insensitive (‘silent’) or situated in sites that were inaccessible to drugs. Included in this group of fibres were four nodose A-fibres, eight nodose C-fibres, five jugular C-fibres and two jugular A-fibres. We found no differences with respect to their conduction velocities, mechanical responsiveness, or cranio-caudal location along the oesophagus that would distinguish these fibres from those that responded to application of agonists.
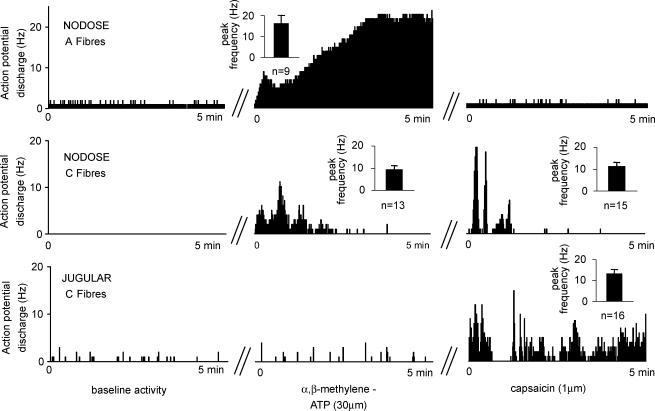
Figure 5. Representative responses of vagal oesophageal afferent nerve fibre types to the P2X receptor agonist, α,β-methylene-ATP (30 μm), and the TRPV1 receptor agonist, capsaicin (1 μm).
The response is shown as the number of action potentials in 1 s bins. Baseline activity was recorded for 5 min in the absence of agonists. Note the regular baseline activity (1 Hz) of the nodose A-fibre, which is typical for this type of fibre. The majority of nodose C-fibres, jugular A-fibres and jugular C-fibres had no baseline activity. Irregular baseline activity, shown in the jugular C-fibre, was encountered in a minority of these fibres. In the agonist experiments, the oesophageal tissue containing the receptive field was superfused with the agonist for 10 min. The nerve activity is shown from the onset of action potential discharge, which followed a variable delay (< 3 min, not shown in the figure) in the continuous presence of agonist. Each agonist administration was separated by a 20 min washout period, during which the baseline activity was restored (not shown in figure). Insets show the mean ± s.e.m. of peak frequency of action potential discharge; n is the number of fibres (usually 1 fibre studied per animal). The data are summarized in Table 1.
Nodose A-fibres
Serosal administration of the selective P2X receptors agonist, α,β-methylene-ATP (30 μm), consistently evoked action potential discharge in nodose A-fibres (n = 9; Fig. 5 and Table 1). The response was delayed up to 2 min (on average 55 ± 12 s). Capsaicin (1 μm) failed to activate five of six nodose A-fibres (Fig. 5) and induced only a trivial transient response (average frequency over the baseline 0.8 Hz) in the remaining fibre (probably via indirect mechanical effects).
Nociceptive-like fibres (nodose C-fibres, jugular C-fibres and jugular A-fibres)
Amongst the nodose C-fibres with confirmed drug accessibility, the vast majority were responsive to serosal capsaicin (1 μm; 15/16) and α,β-methylene-ATP (30 μm; 13/16; Fig. 5). The average onset time of the response to capsaicin and α,β-methylene-ATP was 104 ± 17 and 51 ± 8 s, respectively.
Capsaicin (1 μm) evoked action potential discharge in jugular C-fibres (16/16, onset at 131 ± 25 s, Fig. 5) and jugular A-fibres (5/5, onset at 0–300 s). Neither the capsaicin-sensitive jugular C-fibres (8/8) nor the capsaicin-sensitive jugular A-fibres (4/5) responded to α,β-methylene-ATP (30 μm, given before capsaicin treatment). In one jugular A-fibre, α,β-methylene-ATP induced trivial transient activation (average frequency 0.4 Hz).
Substance P content
The data described above indicate that nodose and jugular C-fibres represent two distinct phenotypes of nociceptors. It has been shown that the expression of substance P in oesophagus-specific neurones differs between nodose and jugular ganglia (Wank & Neuhuber, 2001). Here we investigated immunoreactivity for substance P in the C-fibre populations of nodose and jugular ganglia. We found that the majority (74 ± 5%) of jugular-derived NF-negative (putative C-fibre) neurones expressed substance P. In contrast, only 19 ± 5% of NF-negative nodose neurones contained substance P.
Discussion
These data show that vagus nerves supply the guinea-pig oesophagus with two general types of afferent nerve fibres sensitive to oesophageal distension: low-threshold mechanosensors referred to in the literature as ‘tension (mechano)receptors’ (Page & Blackshaw, 1998; Zagorodnyuk & Brookes, 2000) and afferent fibres best described as nociceptors (or, more conservatively, ‘nociceptive-like’ fibres). While vagal oesophageal non-nociceptive low-threshold mechanosensors have been extensively studied, this is, to our knowledge, the first comprehensive study of vagal nociceptive nerve phenotypes in the oesophagus. In addition, we present data showing that the nociceptive-like vagal afferent fibres in the oesophagus comprise two unique subtypes based on pharmacology and ganglionic origin.
Our histological and electrophysiological studies show unequivocally that vagal afferent nerves innervating the guinea-pig oesophagus originate from both the nodose (inferior) and jugular (superior) vagal ganglia. Neurones within these disparate vagal ganglia express distinct neurotrophin receptors and are derived from distinct embryonic tissues (Baker & Bronner-Fraser, 2001). The neurones in the nodose ganglia originate from epibranchial placodes, whereas those in the jugular ganglia are of neural crest origin. It may not be surprising, therefore, that the vagal afferent phenotype is dictated in part by the ganglionic location of the afferent's cell body.
The distension-sensitive vagal afferent nerve fibres in the isolated guinea-pig oesophagus responded to increasing oesophageal distension in two distinct fashions (Fig. 4). In one type of fibre, typified by nodose A-fibres, the response was intense and saturated at relatively low (non-noxious) pressure (∼30 mmHg). The other type, typified by all jugular fibres, as well as nodose C-fibres, responded less intensely to oesophageal distension and in a manner that did not saturate even at noxious distension pressure (up to 100 mmHg). The mechanosensitive properties of the afferent nerves in the first group are identical to the properties of the vagal ‘tension mechanoreceptors’ described in many species, including guinea-pigs (Falempin et al. 1978; Clerc & Mei, 1983; Satchell, 1984; Sengupta et al. 1989; Blackshaw et al. 2000; Zagorodnyuk & Brookes, 2000). The mechanosensitive properties of afferent fibres in the second group are consistent with the nociceptive phenotype, i.e. a phenotype capable of encoding differences between innocuous and noxious distension. It is not possible to define the intraoesophageal pressure that becomes a noxious stimulus in our ex vivo model. Nevertheless, it is likely that distending pressures > 60 mmHg would be a noxious stimulus. This notion is consistent with the studies showing that distension of proximal gut with pressure over 60 mmHg causes responses consistent with pain in rats (Lamb et al. 2003). In keeping with the nociceptive-like character of the fibres in the second group is their responsiveness to capsaicin (see below). Based on these data we conclude that in the guinea-pig oesophagus, vagal tension mechanoreceptors are exclusively nodose A-fibres, whereas vagal oesophageal nodose C-fibres, jugular C-fibres and jugular A-fibres have mechanosensitive properties consistent with nociceptors.
In vivo studies demonstrated that the adapted (10 s) response of vagal tension mechanoreceptors to increasing oesophageal distension saturates in all species studied, including the opossum (Sengupta et al. 1989, 1992), ferret (Blackshaw et al. 2000) and dog (Satchell, 1984). This pattern was reproduced in our ex vivo experiments, showing that this aspect of mechanosensitivity is preserved in the isolated tissue. In agreement with recent findings in the guinea-pig, we also observed that oesophageal tension mechanoreceptors are sensitive to P2X agonist (Zagorodnyuk et al. 2003). We also found that vagal tension mechanoreceptors do not directly respond to the selective TRPV1 agonist, capsaicin. In previous studies, capsaicin has been found to activate a small proportion of oesophageal tension mechanoreceptors (Blackshaw et al. 2000). This is probably explained by mechanical effects caused by capsaicin-induced local release of tachykinins in the tissue (Kerr, 2002). Activation of tension mechanoreceptors following muscle contraction caused by indirect excitatory effects induced by drugs such as bradykinin and methacholine has also been reported in other studies (Sengupta et al. 1992).
We found that the tension mechanoreceptors in the guinea-pig oesophagus (composed of striated muscle) were exclusively A-fibres. This is similar to findings in the striated muscle portion of the oesophagus in sheep (Falempin et al. 1978) and dogs (Satchell, 1984). In an ex vivo ferret oesopahgeal preparation, Page & Blackshaw (1998) found that all tension receptors located above the squamo-columnar epithelial junction were A-fibres. In contrast, some tension mechanoreceptors in the distal, predominately smooth muscle portion of the oesophagus in opossums and cats are C-fibre afferents (Clerc & Mei, 1983; Sengupta et al. 1989). These observations could be reconciled if the tension mechanoreceptors in the striated muscle portion of the oesophagus are exclusively A-fibres, whereas the predominately smooth muscle portion of the oesophagus is innervated by C-fibre as well as A-fibre tension mechanoreceptors. This notion is supported by the findings that the low-threshold mechanosensors in the lower oesophageal sphincter and stomach are slow-conducting C-fibre afferents (Falempin et al. 1978; Blackshaw & Grundy, 1990; Ozaki et al. 1999).
Most of the distension-sensitive vagal afferent fibres we investigated had characteristics that were not consistent with low-threshold non-nociceptive tension mechanosensors. These fibres included all nodose C-fibres, jugular A-fibres and jugular C-fibres. These afferent fibres can collectively be called nociceptors (or, more parsimoniously, nociceptor-like). The nociceptive nature of these afferents is independently supported by the pattern of their response to mechanical and chemical stimulation (Table 1 and Fig. 4). Mechanically, as discussed above, these fibres are capable of distinguishing between physiological and noxious oesophageal distension. Chemically, these fibres are responsive to capsaicin, a selective agonist of the TRPV1 receptor (the prototypical marker of nociceptors in the somatosensory system). This nociceptive-like phenotype of vagal oesophageal afferent nerves has not been, to our knowledge, systematically investigated. However, it is possible that some of the capsaicin-sensitive oesophageal afferent nerves with less intense response to ‘circular tension’ described in the open ex vivo oesophageal preparation in ferrets (Blackshaw et al. 2000) correspond to the nociceptive vagal afferents recorded in this study.
It seems from the ex vivo distension pressure–response curve that the physiological (non-noxious) motility of the oesophagus may activate many of the vagal oesophageal nociceptive-like fibres. However, this is also true for a proportion of spinal oesophageal afferents recognized as nociceptors (Sengupta et al. 1990, 1992). The ability of this type of afferent nerve to signal noxious stimuli is explained by the intensity-based discrimination of innocuous and noxious stimuli (Cervero & Laird, 1999; Gebhart, 2000; Grundy, 2002).
Although the nodose C-fibres, jugular A-fibres and jugular C-fibres all have nociceptive-like phenotypes, we found some distinguishing features among them. Mechanically, the nodose nociceptive-like fibres adapted to prolonged distension more than the jugular fibres. Pharmacologically, nodose but not jugular nociceptive-like fibres were activated by the purinergic P2X receptor agonist. The third differentiating characteristic between nodose and jugular nociceptors was seen in their substance P content. The majority of nodose (placode-derived) C-fibre neurones were substance P negative, whereas > 75% of the jugular (neural crest-derived) C-fibre neurones were substance P positive.
The site within the oesophagus where the subtypes of distension-sensitive nociceptive-like fibres terminate is unknown. The location of their mechanotransduction sites below the mucosal layer is directly shown by the persistence of mechanosensitivity after the physical removal of the mucosal layer. Also supporting the location of the receptive fields below the mucosa is the inaccessibility of the mechanotransduction site to drugs added to the mucosal surface of the oesophagus. In addition, our search protocol selected only distension-sensitive nerves, while mucosal sensory nerves were reported to be poorly responsive to oesophageal distension (Clerc & Mei, 1983; Blackshaw & Grundy, 1990; Page & Blackshaw, 1998; Page et al. 2002). This probably explains why the mucosal afferent fibres were not encountered in this study.
An elegant study in the guinea-pig oesophagus has demonstrated that the mechanosensitive nerve terminals of tension mechanoreceptors colocalize with intraganglionic laminar endings (IGLE; Zagorodnyuk & Brookes, 2000). In addition to IGLE, another morphologically defined vagal nerve terminal type in the oesophageal muscular layer is the intramuscular array (Phillips & Powley, 2000). The terminals of the nociceptive-like fibres are probably not intramuscular arrays, since these structures are restricted to the vicinity of the lower oesophageal sphincter (Wang & Powley, 2000), while we found distension-sensitive nociceptive-like fibres along the whole oesophagus. It is possible that, like tension mechanoreceptors, distension-sensitive nociceptive terminals are IGLE. It seems unlikely, however, that a single structure would underlie two fundamentally different types of response to mechanical perturbation.
The vagal afferent nerves that terminate within the oesophageal mucosa have not been investigated in this study. It is possible that some of them serve a nociceptive function. Such mucosal nerves would be expected to have high thresholds for punctate mechanical stimuli. However, the vagal mucosal afferent fibres in the oesophagus have been reported to have very low thresholds for mechanical stimuli (Clerc & Mei, 1983; Clerc, 1984; Page & Blackshaw, 1998; Page et al. 2002). For example, Page & Blackshaw (1998) found that all mucosal fibres studied had a mechanical threshold < 0.5 mN. These fibres are therefore unlikely candidates for nociceptors, but the presence of mucosal nociceptive afferent fibres cannot be excluded.
The innervation of the oesophagus by nodose and jugular afferent nerves may reflect a general pattern of vagal innervation of thoracic viscera. We have shown that similar duality applies to vagal afferent innervation of the guinea-pig airways and lungs (Riccio et al. 1996; Undem et al. 2004; Canning et al. 2004). It is tempting to speculate that the nodose and jugular nociceptive fibres mediate different aspects of the complex conscious and autonomic response to noxious stimuli. This notion is further supported by the findings that nodose and jugular afferents innervating the oesophagus differ in their central projections (Wank & Neuhuber, 2001).
Acknowledgments
This work was supported by The Johns Hopkins University Blaustein Pain Research Fund, NIH and Astra Zeneca.
References
- Baker CV, Bronner-Fraser M. Vertebrate cranial placodes. I. Embryonic induction. Dev Biol. 2001;232:1–61. doi: 10.1006/dbio.2001.0156. [DOI] [PubMed] [Google Scholar]
- Belmonte C, Cervero F. Neurobiology of Nociceptors. Oxford: Oxford University Press; 1996. [Google Scholar]
- Berthoud HR, Blackshaw LA, Brookes SJ, Grundy D. Neuroanatomy of extrinsic afferents supplying the gastrointestinal tract. Neurogastroenterol Motil. 2004;16(Suppl. 1):28–33. doi: 10.1111/j.1743-3150.2004.00471.x. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Neuhuber WL. Functional and chemical anatomy of the afferent vagal system. Auton Neurosci. 2000;85:1–17. doi: 10.1016/S1566-0702(00)00215-0. [DOI] [PubMed] [Google Scholar]
- Blackshaw LA, Grundy D. Effects of cholecystokinin (CCK-8) on two classes of gastroduodenal vagal afferent fibre. J Auton Nerv Syst. 1990;31:191–201. doi: 10.1016/0165-1838(90)90185-l. [DOI] [PubMed] [Google Scholar]
- Blackshaw LA, Page AJ, Partosoedarso ER. Acute effects of capsaicin on gastrointestinal vagal afferents. Neuroscience. 2000;96:407–416. doi: 10.1016/s0306-4522(99)00547-3. [DOI] [PubMed] [Google Scholar]
- Canning BJ, Mazzone SB, Meeker SN, Mori N, Reynolds SM, Undem BJ. Identification of the tracheal and laryngeal afferent neurones mediating cough in anaesthetized guinea-pigs. J Physiol. 2004;557:543–558. doi: 10.1113/jphysiol.2003.057885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervero F, Laird JM. Visceral pain. Lancet. 1999;353:2145–2148. doi: 10.1016/S0140-6736(99)01306-9. [DOI] [PubMed] [Google Scholar]
- Clerc N. Afferent innervation of the lower esophageal sphincter of the cat. Pathways and functional characteristics. J Auton Nerv Syst. 1984;10:213–216. doi: 10.1016/0165-1838(84)90015-8. [DOI] [PubMed] [Google Scholar]
- Clerc N, Mei N. Vagal mechanoreceptors located in the lower oesophageal sphincter of the cat. J Physiol. 1983;336:487–498. doi: 10.1113/jphysiol.1983.sp014593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duclaux R, Mei N, Ranieri F. Conduction velocity along the afferent vagal dendrites: a new type of fibre. J Physiol. 1976;260:487–495. doi: 10.1113/jphysiol.1976.sp011527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falempin M, Mei N, Rousseau JP. Vagal mechanoreceptors of the inferior thoracic oesophagus, the lower oesophageal sphincter and the stomach in the sheep. Pflugers Arch. 1978;373:25–30. doi: 10.1007/BF00581145. 10.1007/BF00581145. [DOI] [PubMed] [Google Scholar]
- Gebhart GF. Visceral pain – peripheral sensitisation. Gut. 2000;47(Suppl. 4):iv54–55. doi: 10.1136/gut.47.suppl_4.iv54. discussion iv58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy D. Neuroanatomy of visceral nociception: vagal and splanchnic afferent. Gut. 2002;51(Suppl. 1):i2–5. doi: 10.1136/gut.51.suppl_1.i2. 10.1136/gut.51.6.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr KP. The guinea-pig oesophagus is a versatile in vitro preparation for pharmacological studies. Clin Exp Pharmacol Physiol. 2002;29:1047–1054. doi: 10.1046/j.1440-1681.2002.03774.x. 10.1046/j.1440-1681.2002.03774.x. [DOI] [PubMed] [Google Scholar]
- Lamb K, Kang YM, Gebhart GF, Bielefeldt K. Gastric inflammation triggers hypersensitivity to acid in awake rats. Gastroenterology. 2003;125:1410–1418. doi: 10.1016/j.gastro.2003.07.010. 10.1016/j.gastro.2003.07.010. [DOI] [PubMed] [Google Scholar]
- Lawson SN, Waddell PJ. Soma neurofilament immunoreactivity is related to cell size and fibre conduction velocity in rat primary sensory neurons. J Physiol. 1991;435:41–63. doi: 10.1113/jphysiol.1991.sp018497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki N, Sengupta JN, Gebhart GF. Mechanosensitive properties of gastric vagal afferent fibers in the rat. J Neurophysiol. 1999;82:2210–2220. doi: 10.1152/jn.1999.82.5.2210. [DOI] [PubMed] [Google Scholar]
- Page AJ, Blackshaw LA. An in vitro study of the properties of vagal afferent fibres innervating the ferret oesophagus and stomach. J Physiol. 1998;512:907–916. doi: 10.1111/j.1469-7793.1998.907bd.x. 10.1111/j.1469-7793.1998.907bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page AJ, Martin CM, Blackshaw LA. Vagal mechanoreceptors and chemoreceptors in mouse stomach and esophagus. J Neurophysiol. 2002;87:2095–2103. doi: 10.1152/jn.00785.2001. [DOI] [PubMed] [Google Scholar]
- Phillips RJ, Powley TL. Tension and stretch receptors in gastrointestinal smooth muscle: re-evaluating vagal mechanoreceptor electrophysiology. Brain Res Brain Res Rev. 2000;34:1–26. doi: 10.1016/s0165-0173(00)00036-9. 10.1016/S0165-0173(00)00036-9. [DOI] [PubMed] [Google Scholar]
- Riccio MM, Kummer W, Biglari B, Myers AC, Undem BJ. Interganglionic segregation of distinct vagal afferent fibre phenotypes in guinea-pig airways. J Physiol. 1996;496:521–530. doi: 10.1113/jphysiol.1996.sp021703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satchell PM. Canine oesophageal mechanoreceptors. J Physiol. 1984;346:287–300. doi: 10.1113/jphysiol.1984.sp015022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta JN. An overview of esophageal sensory receptors. Am J Med. 2000;108(Suppl. 4a):87S–89S. doi: 10.1016/s0002-9343(99)00344-7. 10.1016/S0002-9343(99)00344-7. [DOI] [PubMed] [Google Scholar]
- Sengupta JN, Kauvar D, Goyal RK. Characteristics of vagal esophageal tension-sensitive afferent fibers in the opossum. J Neurophysiol. 1989;61:1001–1010. doi: 10.1152/jn.1989.61.5.1001. [DOI] [PubMed] [Google Scholar]
- Sengupta JN, Saha JK, Goyal RK. Stimulus-response function studies of esophageal mechanosensitive nociceptors in sympathetic afferents of opossum. J Neurophysiol. 1990;64:796–812. doi: 10.1152/jn.1990.64.3.796. [DOI] [PubMed] [Google Scholar]
- Sengupta JN, Saha JK, Goyal RK. Differential sensitivity to bradykinin of esophageal distension-sensitive mechanoreceptors in vagal and sympathetic afferents of the opossum. J Neurophysiol. 1992;68:1053–1067. doi: 10.1152/jn.1992.68.4.1053. [DOI] [PubMed] [Google Scholar]
- Sherrington SC. The Integrative Action of the Nervous System. New Haven: Yale University Press; 1906. [Google Scholar]
- Undem BJ, Chuaychoo B, Lee MG, Weinreich D, Myers AC, Kollarik M. Subtypes of vagal afferent C-fibres in guinea-pig lungs. J Physiol. 2004;556:905–917. doi: 10.1113/jphysiol.2003.060079. 10.1113/jphysiol.2003.060079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang FB, Powley TL. Topographic inventories of vagal afferents in gastrointestinal muscle. J Comp Neurol. 2000;421:302–324. 10.1002/(SICI)1096-9861(20000605)421:3<302::AID-CNE2>3.3.CO;2-E. [PubMed] [Google Scholar]
- Wank M, Neuhuber WL. Local differences in vagal afferent innervation of the rat esophagus are reflected by neurochemical differences at the level of the sensory ganglia and by different brainstem projections. J Comp Neurol. 2001;435:41–59. doi: 10.1002/cne.1192. 10.1002/cne.1192. [DOI] [PubMed] [Google Scholar]
- Zagorodnyuk VP, Brookes SJ. Transduction sites of vagal mechanoreceptors in the guinea pig esophagus. J Neurosci. 2000;20:6249–6255. doi: 10.1523/JNEUROSCI.20-16-06249.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagorodnyuk VP, Chen BN, Costa M, Brookes SJ. Mechanotransduction by intraganglionic laminar endings of vagal tension receptors in the guinea-pig oesophagus. J Physiol. 2003;553:575–587. doi: 10.1113/jphysiol.2003.051862. 10.1113/jphysiol.2003.051862. [DOI] [PMC free article] [PubMed] [Google Scholar]