Identification of Arabidopsis rat Mutants (original) (raw)
Abstract
Limited knowledge currently exists regarding the roles of plant genes and proteins in the _Agrobacterium tumefaciens_-mediated transformation process. To understand the host contribution to transformation, we carried out root-based transformation assays to identify Arabidopsis mutants that are resistant to Agrobacterium transformation (rat mutants). To date, we have identified 126 rat mutants by screening libraries of T-DNA insertion mutants and by using various “reverse genetic” approaches. These mutants disrupt expression of genes of numerous categories, including chromatin structural and remodeling genes, and genes encoding proteins implicated in nuclear targeting, cell wall structure and metabolism, cytoskeleton structure and function, and signal transduction. Here, we present an update on the identification and characterization of these rat mutants.
_Agrobacterium tumefaciens_-mediated genetic transformation is widely used to generate transgenic plants of many economically important plant species, but there remain many challenges for applying this technique to numerous recalcitrant species and elite varieties of agronomic and horticultural importance. These include major cereal crops (maize [_Zea mays_], rice [_Oryza sativa_], wheat [_Triticum aestivum_], barley (Hordeum vulgare), oat (Avena sativa), etc.), legumes (soybean [_Glycine max_], common bean [_Phaseolus vulgaris_], and pea [_Pisum sativum_]), cotton (Gossypium hirsutum), fruit, nut, and ornamental trees, and trees used for timber and pulp production (van Wordragen and Dons, 1992; Hansen and Wright, 1999; Pena and Seguin, 2001). The molecular and genetic events within A. tumefaciens leading to plant transformation are reasonably well understood. However, we currently have very limited knowledge of the roles that plant genes and proteins play during this process (for reviews, see Gelvin, 2000, 2003a; Zupan et al., 2000; Tzfira and Citovsky, 2002; Wu and Hohn, 2003). Further investigation of the functions of host genes and manipulation of their expression may lay a foundation for the improvement of transformation of recalcitrant plants (Gelvin, 2003b).
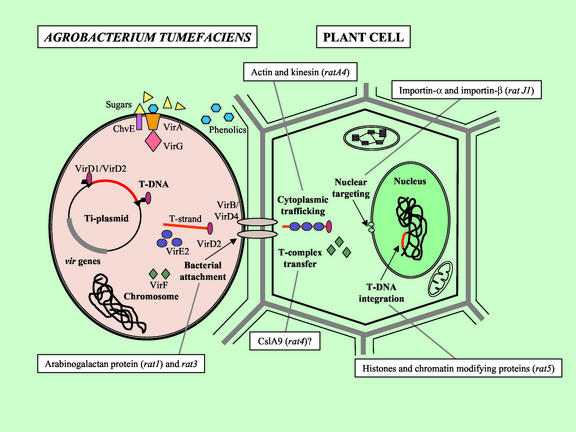
The _A. tumefaciens_-mediated transformation process results from a complex interaction between the host and the bacterium. The events that occur within the bacterium include the perception of phenolic and sugar signals, induction and expression of the vir (virulence) genes, processing of the T-(transferred) DNA from the tumor-inducing plasmid, and export of the T strand (the single-stranded processed form of T-DNA) and virulence proteins from the bacterium using a type IV secretion system encoded by the virB and virD4 genes (see e.g. Christie and Vogel, 2000). Events involving the plant include attachment of the bacterium to the plant surface, transfer of the T-DNA and virulence proteins through the plant cell wall and plasma membrane to the cytoplasm, cytoplasmic trafficking and nuclear targeting of T-strand/protein complexes, T-DNA integration into the host genome, and the resulting expression of T-DNA-encoded genes (Fig. 1). The ultimate outcome of this complex process is the horizontal transfer of genetic information from A. tumefaciens to the plant genome.
Figure 1.
Schematic representation of the process of _A. tumefaciens_-mediated transformation. Phenolic and sugar molecules from wounded plant cells trigger in the bacterium a series of events resulting in the processing of the T-DNA by the VirD1/VirD2 endonuclease and the subsequent transfer of the VirD2/T-strand complex, along with VirE2 and VirF proteins, from the bacterium through the VirB/VirD4 type IV secretion system. Key events in the plant cell include bacterial attachment, T-complex and Vir protein transfer, cytoplasmic trafficking of the T-complex, nuclear targeting, and T-DNA integration. Some of the plant genes necessary for these processes are depicted by representative rat mutants.
One way to dissect the contribution of host factors to the _A. tumefaciens_-mediated plant transformation process is to isolate plant mutants with altered transformation properties and to identify the host genes responsible for the corresponding phenotypes. Therefore, we developed root-based transformation assays (Nam et al., 1997, 1999) because roots are a major natural transformation target for this soil bacterium. Although our initial studies entailed assessing differences in transformation susceptibility among Arabidopsis ecotypes (Nam et al., 1997), we soon focused upon screening Arabidopsis T-DNA insertion lines for plants that are resistant to Agrobacterium transformation (rat mutants; Nam et al., 1999). One advantage of screening libraries of T-DNA insertion lines is the relative ease of recovering plant DNA junction sequences at the T-DNA insertion site using plasmid rescue or thermal asymmetric interlaced PCR techniques (Liu et al., 1995). As part of a National Science Foundation-funded plant genome project, we screened, and continue to screen, several T-DNA disruption libraries for rat mutants. To date, we have identified more than 100 such mutants from approximately 16,500 independent T-DNA insertion lines. In addition, we have utilized several “reverse genetic” approaches to identify specific genes that are involved in the _A. tumefaciens_-mediated transformation process. These include PCR- and computerbased approaches to identify T-DNA insertions in “target” genes suspected to be involved in transformation and the use of antisense and RNAi technologies to decrease expression of “target” genes. In addition, we are currently screening Arabidopsis T-DNA activation-tagged libraries for lines that are hyper-susceptible to Agrobacterium transformation (hat mutants).
To date, we have identified 126 rat mutants and have recovered, from those generated by T-DNA insertion, numerous T-DNA/plant DNA junctions. Based on the putative functions of the encoded proteins and the various steps in the transformation process described above, we have tentatively organized these genes into several functional groups. These include cell wall metabolism and structural genes, cytoskeleton genes, genes whose products may play a role in nuclear targeting, chromatin structural and remodeling genes, and genes whose products are involved in signal transduction and housekeeping processes. The functions of the various mutated genes collectively could be involved in all steps of _A. tumefaciens_-mediated transformation, including bacterial attachment, T-DNA and virulence protein transfer, cytoplasmic trafficking and nuclear targeting of the T-complex, and T-DNA integration and expression. We were able to complement all but one of 14 selected mutants by introduction of the corresponding wild-type gene into the homozygous mutant line. Here, we present an update on the rat mutants that we have identified.
RESULTS AND DISCUSSION
rat Mutant Assays
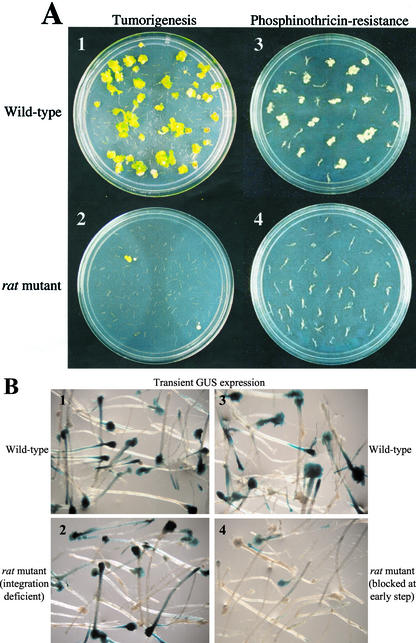
We developed three root-based transformation assays to determine whether a particular T-DNA insertion, antisense, or RNAi Arabidopsis line is a rat mutant. The first assay measures crown gall tumorigenesis at the cut ends of root segments. We classified the morphologies of the tumors into four categories: large green leafy teratomas, small green amorphous, large yellow, and small yellow-white tumors. Generally, wild-type Arabidopsis plants fully develop tumors 4 to 5 weeks after inoculation with A. tumefaciens A208, although tumors can be seen with the aid of a microscope as early as 2 weeks after inoculation. The majority of tumors developing on ecotype Wassilewskija (Ws)-2 (Arabidopsis Biological Resource Center [ABRC] no. CS2360) are generally green (Fig. 2A). Tumors developing on ecotypes Columbia-0 (ABRC no. CS60000) and Columbia-7 (ABRC no. CS3731) are generally amorphous and yellow, although upon extended periods of incubation (6–8 weeks), green teratomas can occasionally develop on these ecotypes. When we score transformation, we count as positive a root segment containing any morphology of tumor. However, plants that are more susceptible to transformation respond with larger and greener tumors than do plants with decreased susceptibility. Therefore, when determining whether a particular mutant is a rat mutant, one must consider not only the percentage of root segments that develop tumors but also the size and morphology of the tumors. Because crown gall tumors represent a long-term response of plants to the overproduction of phytohormones directed by T-DNA-encoded genes (Weiler and Schroder, 1987), this assay measures stable transformation of the root segments. However, it is possible that plants can be stably transformed but not develop crown gall disease if the plant were a hormone response mutant (e.g. Lincoln et al., 1992). Therefore, we utilized a second screen for stable transformation: development of antibiotic resistance or herbicide tolerance encoded by a resistance gene on the T-DNA (Fig. 2A). Plant mutants that show altered susceptibility to stable transformation can be blocked at any step of the transformation process.
Figure 2.
Wild-type and rat mutant phenotypes. A, Stable transformation phenotypes of crown gall tumorigenesis (1 and 2) and ppt resistance (3 and 4) on cut root segments 4 weeks after inoculation. Wild-type ecotype Ws (1 and 3) and typical rat mutants (2 and 4) are shown. B, Transient transformation phenotype of GUS expression 4 d after inoculation of cut root segments. Wild-type ecotype Ws (1 and 3), a rat mutant deficient in the step of T-DNA integration (2), and a rat mutant deficient in an early transformation step (4) are shown after staining with 5-bromo-4-chloro-3-indolyl glucuronide.
Plants can be transiently but not stably transformed by A. tumefaciens if the T-DNA reaches the nucleus and is converted to a double-stranded transcription-competent form, but the T-DNA does not integrate into the plant genome (Nam et al., 1997; Mysore et al., 1998). Thus, we developed an assay that would suggest whether a rat mutant were specifically T-DNA integration deficient (Fig. 2B). If roots of a particular rat mutant were able to express a high level of β-glucuronidase (GUS) activity 2 to 6 d after inoculation, this would indicate efficient transient transformation independent of the process of T-DNA integration. Using these sets of assays, we have confirmed biochemically that Arabidopsis ecotype UE-1 and rat5 are integration deficient (Nam et al., 1997; Mysore et al., 2000b).
During the course of our studies, we have attempted transformation of numerous rat mutants using either a flower vacuum infiltration or a flower dip method (Clough and Bent, 1998). With the exception of the mutant rad5, the transformation of all of these mutants was as efficient as was the transformation of their respective wild-type parental ecotypes (Mysore et al., 2000a; data not shown). rat mutants that can be efficiently transformed by a flower dip protocol include mutants with disruptions in genes putatively involved in cell wall/membrane synthesis or function (rat1, rat3, rat4, and uta1), chromatin proteins (rat5, ratT17, atrx1, ratJ7, HAT4, HAT6, and HDA1), proteins involved in nuclear targeting (ratJ1 and importin α-7), cytoskeletal proteins (act2-1, act7-1, and act7-4), proteins involved in transcription and signal transduction (rat17 [_cpc_], ratA2 [_rcn1_], ratT5, and ratT8), and unidentified or unknown proteins (rat9, rat14, rat15, rat18, rat20, rat21, ratT16, ratH1, and ratT16). These results are consistent with our earlier observations (Mysore et al., 2000a) and further suggest that the efficiency of transformation may depend upon the target tissue (Yi et al., 2002). Table I lists our current collection of rat mutants.
Table I.
rat mutants
*, Mutant complemented with wild-type gene; **, attempted complementation failed; ***, kanamycin (kan) resistance does not cosegregate with rat phenotype; +, mutant scores less than 25% of wild-type; ++, mutant scores less than 33% of wild-type; +++, mutant scores less than 50% of wild-type; ++++, mutant scores more than 50% of wild-type but still a rat mutant; +++++, mutant scores at the level of wild-type for transient GUS activity; N/A, not applicable.
| Mutant | Identificationa | Collection | Tumorigenesis | Phosphinothricin (ppt) Resistance | Transient GUS | Zygosity | Resistance Marker | Gene Affected | Insertion Site |
|---|---|---|---|---|---|---|---|---|---|
| rat1* | F | Feldmann | + | + | + | homo | kan | Arabinogalactan protein | 5′-Untranslated region (UTR) |
| rat3* | F | Feldmann | + | + | +++ | homo | kan | Likely cell wall protein | Intergenic |
| rat4* | F | Feldmann | + | + | + | homo | kan | Cellulose synthase-like protein (CslA-09) | 3′-UTR |
| rat5* | F | Feldmann | + | + | +++++ | homo | kan | Histone H2A-1 | 3′-UTR |
| rat6 | F | Feldmann | + | + | + | kan | |||
| rat7 | F | Feldmann | + | + | + | kan | Unknown protein | ||
| rat8 | F | Feldmann | +++ | +++ | ++ | kan | |||
| rat9 | F | Feldmann | + | + | + | kan | Unknown protein | ||
| rat10 | F | Feldmann | + | + | ++ | kan | |||
| rat11 | F | Feldmann | + | + | ++ | kan | |||
| rat12 | F | Feldmann | + | + | + | kan | |||
| rat13 | F | Feldmann | + | + | + | kan | |||
| rat14 | F | Feldmann | + | + | ++ | kan | Unknown protein | 3′-UTR | |
| rat15 | F | Feldmann | + | + | + | kan | |||
| rat16 | F | Feldmann | + | +++ | ++ | kan | |||
| rat17*** | F | Feldmann | + | + | +++++ | kan | Myb transcription factor (cpc) | 3′-UTR | |
| rat18 | F | Feldmann | + | ++ | +++++ | kan | |||
| rat19 | F | Feldmann | + | + | + | kan | Intergenic | ||
| rat20 | F | Feldmann | + | +++ | +++++ | kan | |||
| rat21 | F | Feldmann | + | + | +++ | kan | |||
| rat22 | F | Feldmann | + | ++ | ++++ | kan | Unknown protein | Intergenic | |
| rat A1 | F | Feldmann | + | + | homo | kan | |||
| rat A2* | F, R | Feldmann | + | + | + | homo | kan | phosphatase 2A (rcn1) | Sixth exon |
| rat A3 | F | Feldmann | + | + | homo | kan | |||
| rat A4 | F | Feldmann | + | ++++ | homo | kan | Kinesin protein | First intron | |
| rat A5 | F | Feldmann | + | ++++ | homo | kan | Unknown protein | ||
| rat A6 | F | Feldmann | + | + | homo | kan | |||
| ratJ1* | F | Feldmann | + | ++ | + | homo | kan | Importin β-3 | 18th intron |
| ratJ2 | F | Feldmann | + | + | kan | MADS box protein | Fifth intron | ||
| ratJ3 | F | Feldmann | + | kan | |||||
| ratJ4 | F | Feldmann | + | ++ | kan | ||||
| ratJ5 | F | Feldmann | + | + | kan | ||||
| ratJ6 | F | Feldmann | + | + | kan | 3- Isopropylmalate dehydrogenase | Sixth exon | ||
| ratJ7 | F | Feldmann | + | + | homo | kan | DEAD box RNA helicase | Third intron | |
| ratJ8 | F | Feldmann | + | + | kan | ||||
| ratJ9 | F | Feldmann | + | + | kan | Mitochondrial chaperonin hsp60 | Fourth exon | ||
| ratJ10 | F | Feldmann | ++ | + | kan | ||||
| ratJ11 | F | Feldmann | + | + | kan | ||||
| ratJ12 | F | Feldmann | + | + | kan | ||||
| ratJ13 | F | Feldmann | + | + | kan | ||||
| ratJ14 | F | Feldmann | + | + | kan | ||||
| ratJR1 | F | Feldmann | + | + | kan | ||||
| ratJR2 | F | Feldmann | ++ | + | kan | ||||
| ratJR3 | F | Feldmann | + | + | kan | ||||
| ratJR4 | F | Feldmann | ++ | ++++ | kan | ||||
| ratJR5 | F | Feldmann | ++ | +++ | kan | ||||
| ratJR6 | F | Feldmann | ++++ | ++ | kan | ||||
| ratJR7 | F | Feldmann | + | + | + | kan | |||
| ratJR8 | F | Feldmann | + | ++ | ++ | kan | |||
| ratJR9 | F | Feldmann | +++ | + | ++++ | kan | |||
| ratJR10 | F | Feldmann | ++++ | + | ++++ | kan | |||
| ratJR11 | F | Feldmann | ++++ | + | + | kan | |||
| ratL1 | F | Bressan | +++ | ppt | Cyclin/cinnamoyl transferase | Third intron/3′-UTR | |||
| ratL2 | F | Bressan | +++ | ppt | |||||
| ratL3 | F | Bressan | +++ | ppt | ARID protein/METHF dehydrogenase | 5′-UTR/Fourth exon | |||
| ratL4 | F | Bressan | +++ | ppt | ATP citrate lyase/glucosidase | 5′-UTR | |||
| ratL5 | F | Bressan | + | ppt | A-T-rich repeat region | Intergenic | |||
| ratL6 | F | Bressan | ++ | ppt | Hypothetical protein | Exon | |||
| ratL7 | F | Bressan | + | ppt | CAAT repeat region | 3′-UTR | |||
| ratT2 | F | Feldmann | ++ | ++ | kan | ||||
| ratT3 | F | Feldmann | +++ | + | kan | rac GTPase-activating protein | 5′-UTR | ||
| ratT4 | F | Feldmann | + | + | kan | Ethylene-responsive element binding factor | 3′-UTR | ||
| ratT5* | F | Feldmann | + | + | ++ | homo | kan | DREB2A | Second exon |
| ratT6 | F | Feldmann | + | + | kan | ||||
| ratT7 | F | Feldmann | + | + | kan | ||||
| ratT8* | F | Feldmann | + | + | + | homo | kan | Receptor-like kinase | 3′-UTR |
| ratT9 | F | Feldmann | + | + | kan | Receptor-like kinase | 3′-UTR | ||
| ratT10 | F | Feldmann | + | + | kan | Unknown protein | 3′-UTR | ||
| ratT11 | F | Feldmann | + | + | kan | ||||
| ratT12 | F | Feldmann | + | ++ | kan | ||||
| ratT13 | F | Feldmann | + | + | kan | Unknown protein | 5′-UTR | ||
| ratT14 | F | Feldmann | ++ | +++ | kan | ||||
| ratT15 | F | Feldmann | + | + | kan | Unknown protein | 5′-UTR | ||
| ratT16* | F | Feldmann | + | + | + | homo | kan | Unknown protein | 6th intron |
| ratT17* | F | Feldmann | + | ++ | ++++ | homo | kan | Histone H3 | Intergenic |
| ratT18 | F | Feldmann | + | + | kan | β-Expansin | Intron | ||
| ratT19 | F | Feldmann | + | ||||||
| uta1* | F | Bressan | + | homo | ppt | Voltage-dependent anion channel | First exon | ||
| uta2 | F | Bressan | + | homo | ppt | F-box protein | Exon | ||
| act2-1 | R | Feldmann | + | + | ++ | homo | kan | Actin-2 (root actin) | First intron |
| act7-4* | R | Feldmann | + | + | +++ | homo | kan | Actin-7 (root actin) | First intron |
| act7-1* | R | Feldmann | + | +++ | +++ | homo | kan | Actin-7 (root actin) | Fourth intron |
| Importin α-7* | R | Feldmann | + | ++ | +++ | homo | kan | Importin α-7 | Seventh intron |
| ratH1 | R | Feldmann | + | + | +++ | kan | Unknown protein | Intergenic | |
| cep1 | R | Feldmann | + | + | homo | kan | Constitutive expression of PR1,2,5 genes | ||
| atrx1* | R | Feldmann | + | homo | kan | Atrx1 | |||
| rad5 | R | + | ++++ | ++ | Point mutation | ||||
| HTA2 | R | Feldmann | ++ | homo | kan | Histone H2A-2 | 5′-UTR | ||
| HTA3 | R | Feldmann | + | homo | kan | Histone H2A-3 | 3′-UTR | ||
| HTA10 | R | Feldmann | ++++ | +++++ | homo | kan | Histone H2A-10 | 3′-UTR | |
| HTA11 | R | Feldmann | + | homo | kan | Histone H2A-11 | 3′-UTR | ||
| HTA13 | R | Feldmann | ++++ | +++++ | homo | kan | Histone H2A-13 | 5′-UTR | |
| HTB5 | R | Feldmann | ++ | homo | kan | Histone H2B-5 | 5′-UTR | ||
| HTB6 | R | Feldmann | ++ | +++++ | homo | kan | Histone H2B-6 | 3′-UTR | |
| HTR4/5 | F, R | Feldmann | +++ | homo | kan | Histone H3-4/5 | Intergenic | ||
| HFO3 | R | Feldmann | + | homo | kan | Histone H4-3 | 3′-UTR | ||
| HFO4 | R | Feldmann | + | +++++ | homo | kan | Histone H4-4 | 3′-UTR | |
| HAT6 | R | Feldmann | + | + | +++ | homo | kan | Histone acetyl transferase-6 | 5′-UTR |
| HAC11 | R | Feldmann | ++ | +++ | homo | kan | Histone acetyl transferase-11 | 3′-UTR | |
| HDA1 | R | Feldmann | + | +++++ | homo | kan | Histone deacetylase-1 | Exon | |
| HDA2 | R | Feldmann | ++++ | +++++ | homo | kan | Histone deacetylase-2 | Exon | |
| HDA6 | R | Feldmann | +++ | homo | kan | Histone deacetylase-6 | 3′-UTR | ||
| HDA9 | R | Feldmann | ++++ | homo | kan | Histone deacetylase-9 | 3′-UTR | ||
| HXA1 | R | Feldmann | +++ | + | hetero | kan | Histone acetylase complex HXA1 | 5′-UTR | |
| HXA2 | R | Feldmann | + | ++++ | kan | Component ADA2 homolog HXA2 | 5′-UTR | ||
| CS2491 | R | Feldmann | + | homo | kan | Disease resistance gene | |||
| Unknown (HAT4) | R | Feldmann | + | homo | kan | Homologous to phytoene hydroxylase | |||
| RNAi CHA 6A | R | ++++ | hyg | Chromatin-remodeling complex subunit 6 | N/A | ||||
| RNAi CHA 6C | R | ++++ | hyg | Chromatin- remodeling complex subunit 6 | N/A | ||||
| RNAi HAC8 | R | Line 156 A | + | homo | hyg | Histone acetyl transferase-8 | N/A | ||
| R | Line 156 B | + | homo | hyg | Histone acetyl transferase-8 | N/A | |||
| RNAi NFA2 | R | Line 300 A | +++ | homo | hyg | Nucleosome assembly factor A | N/A | ||
| R | Line 422 A | ++++ | homo | hyg | Nucleosome assembly factor A | N/A | |||
| RNAi SGA1 | R | Line 524 A | ++++ | homo | hyg | Chromatin-silencing group 1 | N/A | ||
| R | Line 524 B | + | homo | hyg | Chromatin-silencing group 1 | N/A | |||
| RNAi BTI1 | R | Line 23 | + | + | ppt | Unknown protein | N/A | ||
| RNAi BTI2 | R | Line 45 | + | + | ppt | Unknown protein | N/A | ||
| RNAi BTI3 | R | Line 16 | + | + | ppt | Unknown protein | N/A | ||
| RNAi AtRAB8 | R | Line 22 | + | + | ppt | AtRAB8 | N/A | ||
| rat4 Antisense lines | R | Line L | +++ | hyg | CsIA-09 | N/A | |||
| R | Line M | ++++ | hyg | CsIA-09 | N/A | ||||
| R | Line N | ++++ | hyg | CsIA-09 | N/A | ||||
| Importin α-1 antisense lines | R | Line 1 | +++ | +++ | ppt | Importin α-1 | N/A | ||
| R | Line 2 | ++++ | ++++ | ppt | Importin α-1 | N/A | |||
| R | Line 3 | ++ | ++ | ppt | Importin α-1 | N/A | |||
| R | Line 4 | +++ | ++ | ppt | Importin α-1 | N/A | |||
| BTI1 antisense | R | Line 132 | ++ | + | + | kan | Unknown protein | N/A | |
| BTI2 antisense | R | Line 107 | + | +++ | + | kan | Unknown protein | N/A | |
| RAB8 antisense | R | Line 19 | + | +++ | +++ | kan | AtRAB8 | N/A | |
| VIP1 antisense | R | (In tobacco [_Nicotiana tabacum_]) | + | kan | VirE2-interacting protein | N/A |
rat Mutants from T-DNA Insertion Libraries
We have screened and continue to screen mutagenized Arabidopsis plants from three T-DNA insertion libraries for the rat phenotype. These include the Feldmann collection of 6,500 mutants (ABRC no. CS6502), the Institut National de la Recherche Agronomique (Versailles, France) collection of 3,900 mutants (ABRC nos. CS5455 and CS5600), and an 80,000-member collection of T-DNA insertion mutants generated in the laboratory of Dr. Ray Bressan (Purdue University, West Lafayette, IN). In addition, we have searched the SIGnAL TDNA-Express site (http://signal.salk.edu/cgi-bin/tdnaexpress) to identify T-DNA insertions in specific genes of interest. Consistent with an earlier report (Nam et al., 1999), approximately 0.7% of the 16,500 independent lines screened displayed a rat phenotype, suggesting that there may be as many as 200 Arabidopsis genes involved in the _A. tumefaciens_-mediated plant transformation process.
We have conducted genetic analyses of several rat mutants (Nam et al., 1999; Mysore et al., 2000b; data not shown). We successfully complemented all but one (ratT8) of 14 selected mutants. In addition, kanamycin resistance encoded by the T-DNA insertion did not cosegregate with the rat phenotype in the mutant rat17 (C.T.R. Kumar, unpublished data). The T-DNA insertion site in rat17 is in the 3′-UTR of the cpc (caprice) gene. Another mutant containing a T-DNA insertion in the coding region of this gene (Wada et al., 1997) is not a rat mutant (C.T.R. Kumar, unpublished data). We have recovered plant DNA/T-DNA junction sequences from more than one-half of the rat mutants. In most cases, the T-DNA inserted outside of the predicted coding region of the gene. However, a few rat mutants contain T-DNA insertions within a predicted intron or a predicted exon. The paucity of T-DNA insertions in predicted exons and introns of rat mutants is striking and suggests that insertions in the open reading frames of these genes may be deleterious to plant survival. A propensity to recover T-DNA insertions outside protein coding regions of the gene has been noted by others (Rios et al., 2002; Szabados et al., 2002) and may reflect the tendency of the T-DNA to target A-T-rich regions of the genome for integration (Brunaud et al., 2002).
Classification of Genes Involved in the Rat Phenotype
Considering T-DNA insertion, antisense, and RNAi mutants, we have identified a wide range of genes that contribute to _A. tumefaciens_-mediated transformation. These can be classified into several general groups (Table II).
Table II.
Steps of the transformation process putatively disrupted in selected Arabidopsis rat mutants +, Mutant scores less than 25% of the wild-type; ++, mutant scores less than 33% of the wild-type; +++, mutant scores less than 50% of the wild-type; ++++, mutant scores more than 50% of the wild-type but still a rat mutant; +++++, mutant scores at the level of wild-type for transient GUS activity; *, mutant has been complemented with the wild-type gene; ND, not determined.
| Mutant | Tumorigenesis | Transient GUS | Gene Affected |
|---|---|---|---|
| Bacterial attachment/T-DNA transfer | |||
| rat1* | + | + | Arabinogalactan protein |
| rat3* | + | +++ | Likely cell wall protein |
| rat4* | + | + | AtCslA-09 |
| ratT18 | + | ND | β-Expansin |
| Antisense rat4 | +++ | ND | AtCslA-09 |
| Antisense F9 | ++ | + | Unknown protein |
| Antisense F8 | + | + | Unknown protein |
| Antisense RAB8 | + | +++ | AtRAB8 |
| RNAi BTI1 | + | + | Unknown protein |
| RNAi BTI2 | + | + | Unknown protein |
| RNAi BTI3 | + | + | Unknown protein |
| RNAi AtRAB8 | + | + | AtRAB8 |
| Cytoplasmic trafficking/cytoskeleton | |||
| act2-1 | + | ++ | Actin-2 |
| act7-4* | + | +++ | Actin-7 |
| act7-1* | + | +++ | Actin-7 |
| rat A4 | + | ND | Kinesin protein |
| Nuclear targeting | |||
| ratj1* | + | + | Importin β-3 |
| Importin α-7* | + | +++ | Importin α-7 |
| Antisense importin α-1 | ++/+++ | ++/+++ | Importin α-1 |
| T-DNA integration/chromatin structure and remodeling | |||
| rat5* | + | ++++ | Histone H2A-1 |
| HTA2 | ++ | ND | Histone H2A-2 |
| HTA3 | + | ND | Histone H2A-3 |
| HTA10 | ++++ | +++++ | Histone H2A-10 |
| HTA11 | + | ND | Histone H2A-11 |
| HTA13 | ++++ | +++++ | Histone H2A-13 |
| HTB5 | ++ | ND | Histone H2B-5 |
| HTB6 | ++ | +++++ | Histone H2B-6 |
| HTR4/5 | +++ | ND | Histone H3-4/5 |
| HFO3 | + | ND | Histone H4-3 |
| HFO4 | + | +++++ | Histone H4-4 |
| HDA1 | + | +++++ | Histone deacetylase-1 |
| HDA2 | ++++ | +++++ | Histone deacetylase-2 |
| HDA6 | +++ | ND | Histone deacetylase-6 |
| HDA9 | ++++ | ND | Histone deacetylase-9 |
| HAT6 | + | +++ | Histone acetyl transferase-6 |
| HAC11 | ++ | + | Histone acetyl transferase-11 |
| HXA1 | +++ | + | Histone acetylase complex HXA1 |
| HXA2 | + | ++++ | Histone acetylase complex HXA2 |
| RNAi CHA6 | ++++ | ND | Chromatin-remodeling complex subunit 6 |
| RNAi HAC 8-1 | + | ND | Histone acetyl transferase-8 |
| RNAi NFA2-1 | +++ | ND | Nucleosome assembly factor A |
| RNAi SGA1 | + | ND | Chromatin-silencing group 1 |
Chromatin Structure and Remodeling Genes
Based on our initial discovery that rat5 contains a disruption of the histone H2A gene HTA1 and that this gene is involved in T-DNA integration (Mysore et al., 2000b), we have conducted an extensive search for T-DNA insertions in all Arabidopsis core histone, histone acetyltransferase, and histone deacetylase genes. We have coupled this search with an analysis of Arabidopsis lines containing RNAi constructions directed against numerous chromatin genes (see ChromDB at http://www.chromdb.org/). rat mutants within this group include disruptions of five additional histone H2A genes, two histone H2B genes, two histone H3 genes, and two histone H4 genes. The RAT5 histone H2A gene HTA1 may encode a “replacement” histone because it is expressed in cells that are not carrying out mitotic division, although these cells may be undergoing endoreduplication (Yi et al., 2002). A T-DNA disruption between two closely spaced histone H3 genes (HTR5 and HTR4) also results in a rat phenotype. HTR4 and HTR5 encode “replacement” histones (Chaubet et al., 1992; Chaubet-Gigot et al., 2001). These results suggest that “replacement” histones may be involved in _A. tumefaciens_-mediated transformation. T-DNA or RNAi disruptions of other chromatin modifying genes, including those that encode four histone deacetylases, five histone acetyl transferases, and three other chromatin-modifying proteins, also result in a rat phenotype. Many of these rat mutants remain susceptible to transient transformation, suggesting the importance of chromatin structure in T-DNA integration.
Nuclear-Targeting Genes
T-complex protein components VirD2 and VirE2 interact in yeast (Saccharomyces cerevisiae) with a number of Arabidopsis proteins that are involved in nuclear targeting of karyophilic proteins, including importin-α and VIP1 (Ballas and Citovsky, 1997; Tzfira and Citovsky, 2001; Tzfira et al., 2001; S. Bhattacharjee and S.B. Gelvin, unpublished data). Disruption of importin-α7 and importin-β3 (ratJ1) by T-DNA insertion and importin-α1 and VIP1 by antisense inhibition result in a rat phenotype. These results emphasize the importance of nuclear transport of the T-complex as a key step of the _A. tumefaciens_-mediated transformation process.
Cytoskeleton Genes
Mutations in two root-expressed actins (actin-2 and actin-7; McKinney et al., 1995; Kandasamy et al., 2001), but not the pollen-expressed actin-12, result in a rat phenotype. A mutant with a T-DNA insertion in a kinesin gene is also a rat mutant. However, the bot1 mutant that has altered cortical microtubule organization (Bichet et al., 2001) does not show a rat phenotype. VirD2 and VirE2 proteins interact with polymerized actin in vitro, and pharmacological inhibitors of actin cytoskeleton structure or the myosin motor reversibly inhibit transformation of tobacco BY-2 cells (P. Rao, M. Duckely, B. Hohn, and S.B. Gelvin, unpublished data). These results suggest a role for the plant cytoskeleton in the transformation process, possibly by mediating cytoplasmic trafficking of the T-complex.
Cell Wall Structural and Metabolism Genes
Attachment of A. tumefaciens to plant cells is required for efficient transformation (Matthysse, 1987). Several rat mutants contain T-DNA insertions in or near genes implicated in cell wall synthesis or modification. rat1 contains a T-DNA insertion in the promoter region of a gene encoding an arabinogalactan protein, and A. tumefaciens do not attach well to roots of this mutant (Nam et al., 1999; Y.M. Gaspar, J. Nam, C.J. Schultz, L.-Y. Lee, P. Gilson, S.B. Gelvin, and A. Bacic, unpublished data). Arabinogalactan proteins are important for transformation. Incubation of Arabidopsis roots with β-glucosyl Yariv reagent, a chemical that binds arabinogalactan proteins, inhibits transformation (J. Nam and S.B. Gelvin, unpublished data). rat4 contains a T-DNA insertion that disrupts transcription of a cellulose synthase-like gene (cslA-09). This mutant shows a decreased number of lateral roots (Y. Zhu and S.B. Gelvin, unpublished data). A β-expansin mutant was also isolated that shows a strong rat phenotype. Finally, we have identified, using a yeast two-hybrid approach, a number of “unknown” Arabidopsis proteins that may serve as putative receptors for the A. tumefaciens T-pilus. These proteins localize to the periphery of the plant cell. Antisense and RNAi inhibition of expression of the genes that encode these proteins results in a rat phenotype (H.-H. Hwang and S.B. Gelvin, unpublished data).
Other RAT Genes
Numerous other mutants were obtained containing disruptions in genes likely involved in signal transduction processes, including a receptor-like protein kinase (ratT8 and ratT9) and a type 2A phosphoprotein phosphatase (ratA2). Other rat mutants contain T-DNA insertions in genes whose products may be involved in the process of gene expression or protein function. These include a DEAD box RNA helicase (ratJ7), a MADS box protein (ratJ2), a stress-related DREB2A transcription factor (ratT5), and an F-box protein (uta2). DNA sequence analysis of plant DNA/T-DNA junctions from a large number of rat mutants identified proteins of unknown function, or “hypothetical” proteins.
CONCLUSIONS
Using a combination of forward and several reverse genetic strategies, we have identified 126 Arabidopsis rat mutants. Many of these mutants can be transiently transformed, suggesting that in these mutants the step of T-DNA integration is specifically disrupted. Other mutants show defects in bacterial attachment to roots. Although we could genetically complement 13 of 14 selected mutants to transformation proficiency with the corresponding wild-type gene, we have not attempted complementation of the majority of mutants. Thus, the reader should be aware that we have not proven that disruption of many genes by T-DNA insertion is responsible for the rat phenotype. We continue to characterize these rat mutants and search for additional rat mutants. We shall periodically update the results of these activities (http://www.bio.purdue.edu/courses/gelvinweb/gelvin.html). All rat mutants are available for further investigation (please contact Stanton B. Gelvin at gelvin@bilbo.bio.purdue.edu).
MATERIALS AND METHODS
Agrobacterium tumefaciens Strains and Culture Conditions
All A. tumefaciens strains were cultured in liquid Yeast Extract-Peptone medium (Lichtenstein and Draper, 1986) containing the appropriate antibiotics (10 μg mL–1 rifampicin and 25 μg mL–1 kanamycin). Crown gall tumorigenesis assays were conducted using A. tumefaciens A208 (Sciaky et al., 1978), which incites large, green teratomas on the roots of Arabidopsis ecotype Ws. A. tumefaciens At872 (Nam et al., 1999), containing a plant-active bar gene on the binary vector pCAS1, was used to incite ppt-resistant calli. A. tumefaciens At849 (Nam et al., 1999) contains the binary vector pBISN1 (Narasimhulu et al., 1996). pBISN1 contains a plant-active nptII gene and a _gusA_-intron gene under the control of a “super-promoter” (Ni et al., 1995). The intron in the gusA gene prevents expression of GUS activity in bacteria (Liu et al., 1992). A. tumefaciens At849 was used to incite kanamycin-resistant calli and to monitor transient GUS expression in inoculated root segments.
Root Transformation Assays
We have previously described seed sterilization and germination, plant growth, preparation of A. tumefaciens, and in vitro root inoculation procedures (Nam et al., 1997, 1998, 1999; Mysore et al., 2000b; Yi et al., 2002). In brief, surface-sterilized Arabidopsis seeds were placed on Gamborg's B5 medium (Gibco-BRL, Gaithersburg, MD) solidified with 0.75% (w/v) Bactoagar (Difco, Detroit) and containing the appropriate selective agent (50 μg mL–1 kan or 10 μg mL–1 ppt). After incubation at 4°C for 2 d, the plates were incubated under a 16-h-light/8-h-dark photoperiod at 25°C for 7 to 10 d. Individual seedlings were transferred into baby food jars containing solidified B5 medium lacking a selective agent and grown for 7 to 10 d. Roots were cut into 3- to 5-mm segments, and bundles of roots from an individual plant were inoculated with the appropriate A. tumefaciens strain. After 2 d, the root bundles were separated into individual segments and transferred to solidified medium containing 100 μg mL–1 timentin to kill the bacteria and the appropriate agent to select for transformation. We used Murashige and Skoog medium (Gibco-BRL) lacking phytohormones to select for crown gall tumors and callus-inducing medium (Nam et al., 1997) containing either kan (50 μg mL–1) or ppt (10 μg mL–1) to select for antibiotic- or herbicide-resistant calli, respectively. For GUS activity assays, root segments were placed on solidified callus-inducing medium for 4 to 6 d, after which they were stained with 5-bromo-4-chloro-3-indolyl glucuronide (Jefferson et al., 1987).
PCR-Based Reverse Genetic Approach to Identify T-DNA Insertions in Genes
We used a PCR-based approach similar to that described by Krysan et al. (1996) to identify Arabidopsis (ecotype Ws) mutants containing a T-DNA insertion in or near a gene of interest. Pooled samples of DNA from 1,000, 100, and 20 plants from the Feldmann T-DNA insertion library (Feldmann and Marks, 1987; Forsthoefel et al., 1992) were successively assayed for insertions, followed by assay of individual plants from the pool of 20 mutant plants. The zygosity of a particular allele was determined using forward and reverse primers specific to the particular gene and one primer specific to a particular gene in combination with either a T-DNA left or right border primer. T-DNA primer sequences were: left border, 5′-GATGCACTCGAAATCAGCCAATTTTAGAC-3′; and right border, 5′-TCCTTCAATCGTTGCGGTTCTGTCAGTTC-3′. To identify T-DNA insertions in or near large genes, primer sets were designed approximately every 2 kb along the gene, including primers reading “out” from the 5′ and 3′ ends of the open reading frame. PCR was carried out using 0.5 units of ExTaq (Takara, Shiga, Japan) DNA polymerase with robocyclers (Stratagene, La Jolla, CA) using the following amplification conditions: 95°C for 5 min, 30 to 36 cycles at 94°C for 40 to 60 s, 56°C to 60°C (depending upon the primer melting temperature) for 1 min, 72°C for 3 min, 72°C for 10 min, and 4°C hold. We used 0.24 μm of each primer, 0.2 mm of each dNTP, and either 100 ng of DNA (for screening super-pools of 1,000 plants) or 20 ng of DNA (for screening pools of 100, 20, or individual plants) in a 50-μL final reaction volume.
Antisense and RNAi Reverse Genetic Approaches
To construct plasmids to express antisense versions of a given gene, large portions of the cDNA of that gene were cloned into the T-DNA binary vector pE1546 under the control of an enhanced cauliflower mosaic virus 35S promoter in an antisense orientation. pE1546 contains a plant-active hpt gene for selection of hygromycin-resistant plants. The resulting construction was introduced into A. tumefaciens GV3101 (Koncz and Schell, 1986) by electroporation, and this strain was used to transform the appropriate Arabidopsis mutant line using a “flower dip” technique (Clough and Bent, 1998). Although rat mutants are highly recalcitrant to root transformation, they are easily transformed by “flower vacuum infiltration” or “flower dip” techniques (Mysore et al., 2000a). Plants containing the antisense gene were identified by selection on 20 μg mL–1 hygromycin. To construct plasmids for RNAi experiments, large portions of the cDNA encoding the open reading frame of the gene of interest were cloned in both sense and antisense orientation into the T-DNA binary vectors pFGC1008 (hygromycin selection in plants) or pFGC5941 (ppt selection in plants; http://www.chromdb.org/). The resulting plasmids were introduced into GV3101, and the A. tumefaciens strain was used to transform the relevant mutant plant as described above.
Acknowledgments
The authors thank Dr. Ray Bressan for generating the T-DNA disruption-tagged library, Dr. Alison DeLong for providing complemented lines of the PP2A mutant, and Ms. Sara Oakeley for help designing Figure 1.
1
The majority of this work was funded by the National Science Foundation (Plant Genome grant no. 99–75715 to S.B.G.). We acknowledge additional support for this work from the sources: the National Science Foundation (Plant Genome grant no. 99–75930 to S.B.G.), the U.S. Department of Agriculture (grant nos. 9801261 and 0191113 to S.B.G.), the Corporation for Plant Biotechnology Research (to S.B.G.), the Biotechnology Research and Development Corporation (to S.B.G.), The Novartis Research Foundation (to B.H.), the National Science Foundation (2010 Program grant no. 0210992 to V.C.), the U.S. Department of Agriculture (grant no. 00–35304–9333 to V.C.), the NIH (grant no. GM50224 to V.C.), the Basic Research Program of the Korea Science & Engineering Foundation (grant no. R05–2000–00170 to J.N.), and the Jagiellonian University (grant no. N–25/CRBW–VII–1/2002 to A.Z.).
References
- Ballas N, Citovsky V (1997) Nuclear localization signal binding protein from Arabidopsis mediates nuclear import of Agrobacterium VirD2 protein. Proc Natl Acad Sci USA 94**:** 10723–10728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bichet A, Desnos T, Turner S, Grandjean O, Hofte H (2001) BOTERO1 is required for normal orientation of cortical microtubules and anisotropic cell expansion in Arabidopsis. Plant J 25**:** 137–148 [DOI] [PubMed] [Google Scholar]
- Brunaud V, Balzergue S, Dubreucq B, Aubourg S, Samson F, Chauvin S, Bechtold N, Cruaud C, DeRose R, Pelletier G et al. (2002) T-DNA integration into the Arabidopsis genome depends on sequences of preinsertion sites. EMBO Rep 3**:** 1152–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaubet N, Clement B, Gigot C (1992) Genes encoding a histone H3.3-like variant in Arabidopsis contain intervening sequences. J Mol Biol 225**:** 569–574 [DOI] [PubMed] [Google Scholar]
- Chaubet-Gigot N, Kapros T, Flenet M, Kahn K, Gigot C, Waterborg JH (2001) Tissue-dependent enhancement of transgene expression by introns of replacement histone H3 genes of Arabidopsis. Plant Mol Biol 45**:** 17–30 [DOI] [PubMed] [Google Scholar]
- Christie PJ, Vogel JP (2000) Bacterial type IV secretion: conjugation systems adapted to deliver effector molecules to host cells. Trends Microbiol 8**:** 354–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: A simplified method for _Agrobacterium_-mediated transformation of Arabidopsis thaliana. Plant J 16**:** 735–743 [DOI] [PubMed] [Google Scholar]
- Feldmann KA, Marks MD (1987) _Agrobacterium_-mediated transformation of germinating seeds of Arabidopsis thaliana: a non-tissue culture approach. Mol Gen Genet 208**:** 1–9 [Google Scholar]
- Forsthoefel NR, Wu Y, Schulz B, Bennett MJ, Feldmann KA (1992) T-DNA insertion mutagenesis in Arabidopsis: prospects and perspectives. Aust J Plant Physiol 19**:** 353–366 [Google Scholar]
- Gelvin SB (2000) Agrobacterium and plant genes involved in T-DNA transfer and integration. Annu Rev Plant Physiol Plant Mol Biol 51**:** 223–256 [DOI] [PubMed] [Google Scholar]
- Gelvin SB (2003a) Agrobacterium and plant transformation: the biology behind the “gene-jockeying” tool. Microbiol Mol Biol Rev 67**:** 16–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelvin SB (2003b) Improving plant genetic engineering by manipulating the host. Trends Biotechnol 21**:** 95–98 [DOI] [PubMed] [Google Scholar]
- Hansen G, Wright MS (1999) Recent advances in the transformation of plants. Trends Plant Sci 4**:** 226–231 [DOI] [PubMed] [Google Scholar]
- Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6**:** 3901–3907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandasamy MK, Gilliland LU, McKinney EC, Meagher RB (2001) One plant actin isovariant, ACT, is induced by auxin and required for normal callus formation. Plant Cell 13**:** 1541–1554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koncz C, Schell J (1986) The promoter of TL-DNA gene 5 controls the tissue-specific expression of chimeric genes carried by a novel type of Agrobacterium binary vector. Mol Gen Genet 204**:** 383–396 [Google Scholar]
- Krysan PJ, Young JC, Tax F, Sussman MR (1996) Identification of transferred DNA insertions within Arabidopsis genes involved in signal transduction and ion transport. Proc Natl Acad Sci USA 93**:** 8145–8150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenstein C, Draper J (1986) Genetic engineering of plants. In DM Glover, ed, DNA Cloning: A Practical Approach, Vol 2. IRL Press, Oxford, pp 67–119 [Google Scholar]
- Lincoln C, Turner J, Estelle M (1992) Hormone-resistant mutants of Arabidopsis have an attenuated response to Agrobacterium strains. Plant Physiol 98**:** 979–983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C-N, Li X-Q, Gelvin SB (1992) Multiple copies of virG enhance the transient transformation of celery, carrot, and rice tissues by Agrobacterium tumefaciens. Plant Mol Biol 20**:** 1071–1087 [DOI] [PubMed] [Google Scholar]
- Liu Y-G, Mitsukawa N, Oosumi T, Whittier RF (1995) Efficient isolation and mapping of Arabidopsis thaliana T-DNA insert junctions by thermal asymmetric interlaced PCR. Plant J 8**:** 457–463 [DOI] [PubMed] [Google Scholar]
- Matthysse AG (1987) Characterization of nonattaching mutants of Agrobacterium tumefaciens. J Bacteriol 169**:** 313–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney EC, Ali N, Traut A, Feldmann KA, Belostotsky DA, McDowell JM, Meagher RB (1995) Sequence-based identification of T-DNA insertion mutations in Arabidopsis: actin mutants act2-1 and act4-1. Plant J 8**:** 613–622 [DOI] [PubMed] [Google Scholar]
- Mysore KS, Bassuner B, Deng X-b, Darbinian NS, Motchoulski A, Ream W, Gelvin SB (1998) Role of the Agrobacterium tumefaciens VirD2 protein in T-DNA transfer and integration. Mol Plant-Microbe Interact 11**:** 668–683 [DOI] [PubMed] [Google Scholar]
- Mysore KS, Kumar CTR, Gelvin SB (2000a) Arabidopsis ecotypes and mutants that are recalcitrant to Agrobacterium root transformation are susceptible to germ-line transformation. Plant J 21**:** 9–16 [DOI] [PubMed] [Google Scholar]
- Mysore KS, Nam J, Gelvin SB (2000b) An Arabidopsis histone H2A mutant is deficient in Agrobacterium T-DNA integration. Proc Natl Acad Sci USA 97**:** 948–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam J, Matthysse AG, Gelvin SB (1997) Differences in susceptibility of Arabidopsis ecotypes to crown gall disease may result from a deficiency in T-DNA integration. Plant Cell 9**:** 317–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam J, Mysore KS, Gelvin SB (1998) Agrobacterium tumefaciens transformation of the radiation hypersensitive Arabidopsis thaliana mutants uvh1 and rad5. Mol Plant-Microbe Interact 11**:** 1136–1141 [DOI] [PubMed] [Google Scholar]
- Nam J, Mysore KS, Zheng C, Knue MK, Matthysse AG, Gelvin SB (1999) Identification of T-DNA tagged Arabidopsis mutants that are resistant to transformation by Agrobacterium. Mol Gen Genet 261**:** 429–438 [DOI] [PubMed] [Google Scholar]
- Narasimhulu SB, Deng X-B, Sarria R, Gelvin SB (1996) Early transcription of Agrobacterium T-DNA genes in tobacco and maize. Plant Cell 8**:** 873–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni M, Cui D, Einstein J, Narasimhulu S, Vergara CE, Gelvin SB (1995) Strength and tissue specificity of chimeric promoters derived from the octopine and mannopine synthase genes. Plant J 7**:** 661–676 [Google Scholar]
- Pena L, Seguin A (2001) Recent advances in the genetic transformation of trees. Trends Biotechnol 19**:** 500–506 [DOI] [PubMed] [Google Scholar]
- Rios G, Lossow A, Hertel B, Breuer F, Schaefer S, Broich M, Kleinow T, Jasik J, Winter J, Ferrando A et al. (2002) Rapid identification of Arabidopsis insertion mutants by non-radioactive detection of T-DNA tagged genes. Plant J 32**:** 243–253 [DOI] [PubMed] [Google Scholar]
- Sciaky DA, Montoya AL, Chilton M-D (1978) Fingerprints of Agrobacterium Ti plasmids. Plasmid 1**:** 238–253 [DOI] [PubMed] [Google Scholar]
- Szabados L, Kovacs I, Oberschall A, Abraham E, Kerekes I, Zsigmond L, Nagy R, Alvarado M, Krasovskaja I, Gal M et al. (2002) Distribution of 1000 sequenced T-DNA tags in the Arabidopsis genome. Plant J 32**:** 233–242 [DOI] [PubMed] [Google Scholar]
- Tzfira T, Citovsky V (2001) Comparison between nuclear localization of nopaline- and octopine-specific Agrobacterium VirE2 proteins in plant, yeast and mammalian cells. Mol Plant Pathol 2**:** 171–176 [DOI] [PubMed] [Google Scholar]
- Tzfira T, Citovsky V (2002) Partners-in-infection: host proteins involved in the transformation of plant cells by Agrobacterium. Trends Cell Biol 12**:** 121–128 [DOI] [PubMed] [Google Scholar]
- Tzfira T, Vaidya M, Citovsky V (2001) VIP1, an Arabidopsis protein that interacts with Agrobacterium VirE2, is involved in ViE2 nuclear import and Agrobacterium infectivity. EMBO J 20**:** 3596–3607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wordragen MF, Dons HJM (1992) _Agrobacterium tumefaciens_-mediated transformation of recalcitrant crops. Plant Mol Biol Rep 10**:** 12–36 [Google Scholar]
- Wada T, Tachibana T, Shimura Y, Okada K (1997) Epidermal cell differentiation in Arabidopsis determined by a myb homolog, CPC. Science 277**:** 1113–1116 [DOI] [PubMed] [Google Scholar]
- Weiler EW, Schroder J (1987) Hormone genes and crown gall disease. Trends Biol Sci 12**:** 271–275 [Google Scholar]
- Wu Y-Q, Hohn B (2003) Transfer to plants and integration into chromosomal DNA of T-DNA of Agrobacterium tumefaciens. In G Stacey, N Keen, eds, Molecular Plant-Microbe Interactions, Vol 6. Kluwer, Dordrecht, The Netherlands (in press)
- Yi HC, Mysore KS, Gelvin SB (2002) Expression of the Arabidopsis histone H2A-1 gene correlates with susceptibility to Agrobacterium transformation. Plant J 32**:** 285–298 [DOI] [PubMed] [Google Scholar]
- Zupan J, Muth TR, Draper O, Zambryski P (2000) The transfer of DNA from Agrobacterium tumefaciens into plants: a feast of fundamental insights. Plant J 23**:** 11–28 [DOI] [PubMed] [Google Scholar]