Gastrulation Defective is a serine protease involved in activating the receptor Toll to polarize the Drosophila embryo (original) (raw)
Abstract
The dorsoventral axis of the Drosophila embryo is induced by a ventrally restricted ligand for the receptor Toll. The Toll ligand is generated by a proteolytic processing reaction, which occurs at the end of a proteolytic cascade and requires the gastrulation defective (gd), nudel, pipe, and windbeutel genes. Here we demonstrate that the GD protein is a serine protease and that the three other genes act to restrict GD activity to the ventral side of the embryo. Our data support a model in which the GD protease catalyzes the ventral activation of the proteolytic cascade that produces the Toll ligand.
A recurring theme in embryonic development is the use of spatially localized signals to inform cells of their relative positions within the embryo. What are these signals, and how are they localized in space and in time? An important experimental system for addressing these questions at the molecular level has emerged from genetic studies of how the embryonic dorsoventral axis is established in Drosophila (for a review, see ref. 1). Here a key signal is conveyed by a ventrally restricted ligand for the membrane receptor Toll, which is uniformly distributed on the surface of the one-cell embryo. Ventral activation of Toll induces dorsoventral asymmetry in the embryo essential for ventral and lateral development. Failure to activate Toll results in all embryonic cells following a dorsal path of development and hence in the production of a dorsalized embryo lacking ventral and lateral structures.
The Toll ligand is generated by a proteolytic cascade that involves the products of maternal genes expressed during oogenesis. One of these genes, spätzle, encodes a secreted protein that appears to be proteolytically processed to the Toll ligand shortly after fertilization within the extracellular compartment between embryo and surrounding eggshell known as the perivitelline space (2, 3). Processing of Spätzle to the Toll ligand requires the snake and easter genes, which both encode serine proteases (2, 4, 5). Like the regulated serine proteases involved in mammalian blood clotting, the Snake and Easter proteases are synthesized as inactive zymogens that become activated by proteolytic cleavage at a defined site between N-terminal “pro” and C-terminal catalytic domains. Snake and Easter appear to be activated shortly after fertilization within the perivitelline space (6–8). Genetic experiments suggest that Snake and Easter act sequentially in a proteolytic cascade with Snake required to activate Easter, which may then process Spätzle to the Toll ligand (6, 9, 10).
The products of four other maternal genes gastrulation defective (gd), nudel (ndl), pipe (pip), and windbeutel (wind) also are required for processing of Spätzle to the Toll ligand (2). In the genetically ordered pathway for production of the Toll ligand, these genes act upstream of the snake gene and therefore are presumed to encode components required to activate the Snake protease (9). According to recent studies, the Pipe and Windbeutel proteins function during oogenesis to generate a spatially localized factor that defines the ventral side of the embryo (11–13). In current models, this factor is hypothesized to direct the ventral production of the Toll ligand by spatially restricting the activation of Snake and Easter, perhaps via a mechanism involving the Nudel and GD proteins (14–16). Nudel, a large modular protein with a central serine protease domain, is functionally complex and apparently involved in several processes important for embryogenesis (17–19). Our laboratory recently showed that the Nudel protease is required for cross-linking of the eggshell, in addition to its role in establishing the embryonic dorsoventral axis (20). This observation has raised the intriguing possibility that the Nudel protease is involved in creating an extracellular matrix structure necessary for activating the proteolytic cascade that produces the Toll ligand.
The role of the GD protein in activating this proteolytic cascade has been particularly enigmatic. GD is suspected to have an important role in this process, in part because it has the structure of a regulated serine protease like Snake and Easter, with a C-terminal domain homologous to serine proteases (21). However, it has been unclear whether GD functions as a serine protease because of peculiarities of its primary structure, such as the absence of a catalytic serine in the conserved position (Fig. 1), or a recognizable proteolytic cleavage site for zymogen activation. It also has been unclear whether GD functions during early embryogenesis, rather than oogenesis, and is therefore directly involved in activating the proteolytic cascade that produces the Toll ligand (21, 22). In addition, the position of GD with respect to Nudel, Pipe, and Windbeutel in the pathway producing the Toll ligand has not been known.
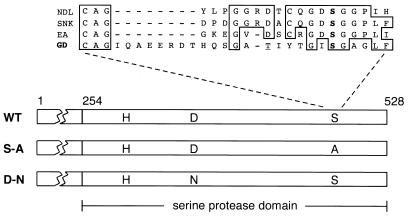
Figure 1.
Structure of GD protein. Within the C-terminal domain of GD having sequence homology to serine proteases, the positions of the putative catalytic triad residues (H, D, and S) are indicated. Two of these residues were altered by site-directed mutagenesis to generate the S-A and D-N mutants. At the top is a comparison of the region around the catalytic serine (boldface type) in the Nudel (NDL), Snake (SNK), and Easter (EA) proteases with a similar region in GD. The candidate catalytic serine in GD is unusual, as it is positioned 8 aa further C terminal in the sequence and lacks a conserved aspartic acid as a neighbor. Our DNA sequence analysis reveals a threonine (underlined) instead of a serine in the GD sequence as previously reported (21). WT, wild type.
Here we provide evidence from a combination of in vivo and in vitro experiments that GD is a serine protease required during early embryogenesis to establish the embryonic dorsoventral axis. Surprisingly, we observed a correspondence between GD activity and the number of embryonic cells that adopt ventral and lateral fates. As such a correspondence had previously been seen for Spätzle, our observation suggests that the amount of Spätzle processed to the Toll ligand is determined by the level of active GD. We also show that Nudel, Pipe, and Windbeutel are required to restrict GD activity to the ventral side of the embryo. Our studies support a model in which the GD protease catalyzes the ventral activation of the proteolytic cascade producing the Toll ligand.
Materials and Methods
Molecular Procedures.
The plasmid containing a full-length gd cDNA was obtained from J. L. Marsh, University of California, Irvine (21). Using a two-step PCR procedure (23), we separately mutated two residues of GD's putative catalytic triad, changing serine 468 to alanine and aspartic acid 347 to asparagine, to generate the S-A and D-N mutants shown in Fig. 1. RNAs encoding wild-type and mutant GD were transcribed from plasmids by using the mMessage mMachine kit (Ambion, Austin, TX) and were dissolved in water at a concentration of 1.5 mg/ml as estimated by UV spectrophotometry.
Fly Stocks and Embryo Injection.
The wild-type strain was Oregon R. The mutations and allelic combinations used here were previously described: gd7/gd7(22), ndll111/l(3)3844 (17), pip386/pip664 and snk073/snk073(24), and _wind_M46/_wind_AR51 (11). Embryos, 0.5–1.5 h postfertilization, were injected after removal of the outer eggshell layer, according to a standard procedure (25). To prevent collapse of the fragile embryos from the nudel mutant, the outer eggshell layer was not removed before injection in this case. Injected embryos were visually examined during gastrulation, and their cuticles were prepared for examination as described (24, 26).
Trypsin Digestion and Affinity Labeling of Recombinant GD.
Expression of recombinant GD by transient transfection of Drosophila S2 cells using the metallothionein promoter was accomplished essentially as described (27). Culture medium from transfected cells was mixed with a suspension of trypsin-Sepharose (Worthington) for 5 min at room temperature. After trypsin-Sepharose was removed by centrifugation, one-half of the supernatant was analyzed by Western blot with affinity-purified antibodies to a GD bacterial fusion protein (E.K.L., unpublished work). The other half of the supernatant was incubated with 50 μM of biotinylated peptidyl chloromethylketones BCFPRCK and BCEGRCK (Hematologic Technologies, Essex Junction, VT) for 15 min–2 h at room temperature. This sample then was analyzed by avidin blot, involving SDS/PAGE and subsequent transfer to a nitrocellulose filter for detection of biotinylated proteins with a complex of avidin and biotinylated horseradish peroxidase (Vector Laboratories). Western and avidin blots were visualized by ECL (Amersham Pharmacia). The biotinylated peptidyl chloromethylketones appeared to react variably with GD, perhaps because of a number of factors, including low affinity of GD for reagents originally designed to react specifically with mammalian proteases of blood clotting and fibrinolysis (28). The 29-kDa proteolytic fragment of the wild-type GD protein (see Fig. 3) was strongly labeled by these reagents in 25–100% of parallel reactions (21 samples in four experiments); however, the 29-kDa proteolytic fragment of the GD S-A mutant protein was never labeled (nine samples in three experiments). We did not perform the affinity labeling experiment with the GD D-N mutant protein, as earlier studies had shown that the D-N mutant and wild-type forms of trypsin are equally reactive with and inhibited by a chloromethylketone reagent (29).
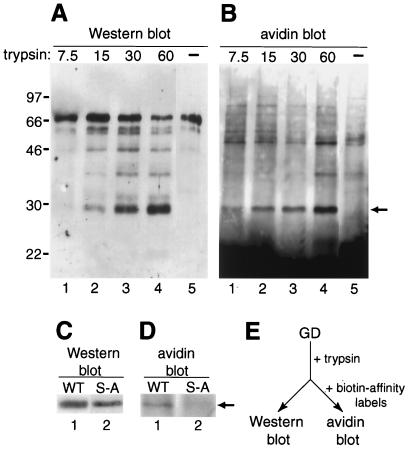
Figure 3.
Identification of active GD protease in vitro. Recombinant GD protein in S2 cell culture medium was treated with immobilized trypsin and then analyzed by Western blot (A and C) or by avidin blot after further incubation with biotinylated affinity reagents specific for active serine proteases (B and D). (A) Increasing amounts of trypsin (nM) converts full-length GD (lane 5) into smaller polypeptides, including a prominent 29-kDa form (lanes 1–4). (B) The 29-kDa polypeptide (arrowhead, lanes 1–4), but not full-length GD (lane 5), is biotinylated. Free reagents are likely the cause of an intense signal toward the bottom of the avidin blot. Molecular masses of markers in kDa are indicated on the left. (C) Trypsin treatment generates a 29-kDa polypeptide from both wild-type GD (WT, lane 1) and mutant GD lacking putative catalytic serine (S-A, lane 2). (D) The 29-kDa polypeptide from wild type (lane 1), but not mutant (lane 2), is biotinylated (arrow). (E) Basic scheme of experiment.
Results
Injection of gd RNA Rescues gd Mutant Embryos.
Embryos lacking the maternal activity of gd (hereafter referred to as gd mutant embryos) fail to undergo ventral and lateral development, therefore developing the dorsalized phenotype evident during gastrulation and in the cuticle pattern (Fig. 2 A and B). To test whether ventral and lateral development in gd mutant embryos can be rescued, we injected these embryos with synthetic RNA encoding the GD protein. Injection of a low concentration of gd RNA resulted in a high percentage of complete rescue, with embryos showing the wild-type pattern of gastrulation movements and cuticular structures, including some that hatched out of the eggshell (Fig. 2 C and D, and Table 1). Complete rescue with normal dorsoventral polarity was observed whether the RNA was injected into the prospective ventral or dorsal side of the embryo as defined by the egg shape. The level of complete rescue was comparable to what has been observed in experiments involving the injection of snake RNA into snake mutant embryos and easter RNA into easter mutant embryos (5, 30). Rescue of ventral and lateral development in gd mutant embryos by injection of gd RNA suggests that the GD protein is required after fertilization to establish the embryonic dorsoventral axis.
Figure 2.
Rescue of ventral and lateral development in embryos by injection of gd RNA. After injection, live embryos at the gastrulation stage (A, C, E, and G) or cuticles produced by the embryos (B, D, F, and H) were examined. Embryos are oriented anterior end to the left and dorsal side up. (A and B) Embryos lacking maternal gd develop the dorsalized phenotype, symmetric folds both dorsally and ventrally at gastrulation, and a cuticle lacking all ventral and lateral structures. (C and D) After injection with 0.03 mg/ml gd RNA, the majority of gd mutant embryos are completely rescued, as evident by the lateral head fold (*) and anterior-ward displacement of pole cells (arrowhead) from original posterior position. These embryos produce a normal dorsoventral cuticle pattern showing ventral denticles (inverted v) and the dorsolateral Filzkörper (arrow). (E and F) Injection of ≥0.75 mg/ml gd RNA causes gd mutant embryos to develop the ventralized phenotype, recognizable by head fold on dorsal side (*) and retention of pole cells at posterior. These embryos produce a cuticle showing mainly ventral denticles in a disorganized pattern. (G and H) After injection with ≥0.75 mg/ml gd RNA, embryos from a nudel or pipe mutant develop the lateralized phenotype, as evident by prominence of head fold both dorsally and ventrally (*). These embryos develop a cuticle pattern similar to the ventralized phenotype. Embryos from the nudel mutant were injected without prior removal of the outer eggshell layer. The bright structure surrounding the cuticles in B, F, and H is the inner eggshell layer.
Table 1.
The cuticle patterns produced by gd mutant embryos after injection of gd RNA
| gd RNA, mg/ml | Number of cuticles | % Partially rescued | % Rescued to wild type | % Ventralized |
|---|---|---|---|---|
| 0.03 | 91 | 31 | 66 | 3 |
| 0.15 | 40 | 38 | 20 | 42 |
| 0.75 | 52 | 6 | 2 | 92 |
| 1.50 | 64 | 0 | 0 | 100 |
High Levels of Injected gd RNA Produce a Ventralized Phenotype.
Interestingly, injection of high concentrations of gd RNA caused gd mutant embryos to develop a ventralized phenotype in which more cells than normal behave like ventral and lateral cells (Fig. 2 E and F). A concentration only five times the level required to obtain hatching embryos produced both hatching embryos as well as embryos with the ventralized phenotype, whereas higher concentrations produced mostly to exclusively the ventralized phenotype (Table 1). Wild-type embryos injected with a high concentration of gd RNA also developed the ventralized phenotype (Table 2). Our results suggest that the amount of GD determines the number of cells in the embryo that adopt ventral and lateral fates.
Table 2.
The gastrulation patterns of embryos injected with wild-type or mutant gd RNA
| Recipient embryos | gd RNA | Number of embryos | Dorsalized, % | Ventralized, % | Lateralized, % |
|---|---|---|---|---|---|
| Wild type | Wild type | 63 | 0 | 100 | 0 |
| nudel | Wild type | 112 | 77 | 3 | 20 |
| pipe | Wild type | 116 | 16 | 0 | 84 |
| windbeutel | Wild type | 79 | 4 | 77 | 19 |
| snake | Wild type | 75 | 100 | 0 | 0 |
| gd | S-A | 324 | 100 | 0 | 0 |
| gd | D-N | 267 | 100 | 0 | 0 |
gd Can Bypass the Requirement for Other Genes in the Toll Signaling Pathway.
We tested whether a high level of gd RNA could elicit ventral and lateral development in the absence of other genes that, like gd, act at the beginning of the Toll signaling pathway. Injection of a high concentration of gd RNA resulted in ventral and lateral development in embryos from strongly dorsalizing mutants of nudel and pipe; however, these embryos developed a lateralized phenotype lacking the dorsoventral asymmetry of ventralized embryos (Fig. 2 G and H). The response to injection was stronger in pipe mutant embryos than in nudel mutant embryos, as most of the latter remained dorsalized (Table 2). With a moderately dorsalizing windbeutel mutant, injection of gd RNA resulted in most embryos becoming ventralized and a smaller number of embryos becoming lateralized (Table 2). Because gd can induce ventral and lateral development in the absence of normal nudel, pipe, or windbeutel activity, gd must act downstream of these genes. Injection of a high level of gd RNA did not elicit ventral and lateral development in snake mutant embryos, consistent with the previous finding that gd acts upstream of snake (ref. 9; Table 2).
Mutagenesis of Putative Catalytic Triad Residues Destroys Biological Activity of gd RNA.
Within its C-terminal domain homologous to serine proteases, GD has aspartic acid and histidine residues at the appropriate positions to be part of the catalytic triad necessary for enzymatic activity; however, the best candidate to be the catalytic serine is unusually positioned (see Fig. 1). We used site-directed mutagenesis to change this serine to an alanine (Fig. 1). Embryos injected with RNA encoding this mutant protein were not rescued and developed the dorsalized phenotype, like uninjected gd mutant embryos, indicating that the mutation destroyed the rescuing activity of gd RNA (Table 2). We also separately mutated the putative catalytic aspartic acid, changing this residue to asparagine (Fig. 1). Injection of RNA encoding this altered protein failed to rescue ventral and lateral development in gd mutant embryos (Table 2). The destruction of gd RNA's biological activity by alteration of putative catalytic triad residues is consistent with GD being an active serine protease.
Identification of an Active GD Protease in Vitro.
To prove that GD is an active serine protease, it is necessary to demonstrate its enzymatic activity in vitro. For this purpose, we made a recombinant form of GD by using cultured Drosophila S2 cells. The culture medium of these cells after transfection with a plasmid encoding GD contained a major polypeptide of 66 kDa, which is detectable with polyclonal antibodies raised against a GD bacterial fusion protein (Fig. 3A, lane 5). This polypeptide, which was not detected in culture medium of mock-transfected cells, is similar in size to the polypeptide made by in vitro translation and the baculovirus system from a gd cDNA (data not shown). These data indicate that the 66-kDa polypeptide secreted by transfected S2 cells is the GD protein.
To test whether GD is proteolytically activated, we digested the recombinant GD protein with trypsin, which cleaves after (that is, C terminal to) basic residues. We chose trypsin because of the possibility that GD is activated in vivo by the Nudel protease, which is predicted to be specific for basic residues (17) and is the only known protease in the Toll signaling pathway that could function before GD (see above). We incubated the culture medium containing recombinant GD with increasing amounts of trypsin bound to Sepharose beads. After the immobilized trypsin was removed by centrifugation, the supernatant was analyzed by Western blot. This treatment generated 4–5 smaller polypeptides, including a prominent one of about 29 kDa (Fig. 3A, lane 4).
We wanted to determine whether full-length GD or any of the smaller polypeptides generated by trypsin digestion is an active serine protease. As the amount of protein was apparently not sufficient for enzyme assays using synthetic substrates, we decided to use a more sensitive affinity labeling method for detecting serine protease activity. Specifically, we used biotinylated peptidyl chloromethylketones, synthetic inhibitors of trypsin-like serine proteases that react covalently with active proteases but not inactive zymogens (28, 31). After the supernatants from trypsin digestion were incubated with these reagents, biotinylated proteins in the supernatants were visualized by avidin blot. As shown in Fig. 3B (lanes 1–4), the predominant biotinylated polypeptide appears to be the 29-kDa fragment of GD, suggesting that this polypeptide represents an active serine protease. The polypeptide corresponding to full-length GD was not affinity labeled (Fig. 3B, lane 5). The 29-kDa polypeptide was not detected in control reactions in which water or culture medium from mock-transfected cells were treated with immobilized trypsin and incubated with the affinity labels (data not shown). We also performed the same experiment with a recombinant form of the GD S-A protein, in which the catalytic serine was mutated to alanine (Fig. 1). As expected, the 29-kDa polypeptide generated by trypsin digestion of the mutant protein did not react with the affinity reagents (Fig. 3 C and D, lane 2). These results strongly suggest that the 29-kDa polypeptide generated by trypsin digestion of the wild-type GD protein is an active serine protease.
Discussion
GD Is a Serine Protease Directly Involved in Activating Toll.
The fact that we could rescue gd mutant embryos by injection of gd RNA indicates that the GD protein can function during early embryogenesis and thus could have a direct role in producing the Toll ligand. The downstream proteases Snake and Easter likely function in the perivitelline space of the embryo (6, 7). We presume that GD is also a component of this extracellular compartment, as it has a signal sequence and is efficiently secreted by transfected S2 cells. However, we were unable to obtain rescue by injecting the perivitelline space of gd mutant embryos with recombinant GD in S2 cell culture medium, perhaps because of insufficient amount of protein that also precluded extensive biochemical analysis (see above).
We have provided genetic as well as biochemical evidence that GD functions as a serine protease. First, the biological activity of gd RNA is destroyed by site-directed mutagenesis of putative catalytic serine and aspartic acid residues in GD. Second, a 29-kDa proteolytic fragment of recombinant GD protein reacts in vitro with affinity reagents specific for active serine proteases. The affinity labeling experiments also suggest that GD is synthesized as a zymogen that becomes activated by proteolytic cleavage, because a proteolytic fragment of GD, but not the full-length protein, reacted with the affinity reagents. GD lacks a recognizable zymogen activation site, so where cleavage normally occurs for GD to become an active serine protease is unclear. An active protease was generated by digestion of GD by trypsin, which is specific for basic residues, implying that GD could be normally activated by cleavage after a basic residue.
The identification of GD as a serine protease raises the question, what is GD's substrate? A possible candidate is Snake, which is immediately downstream of GD in the Toll signaling pathway (9). The Snake zymogen may be activated by cleavage after a leucine residue and therefore by a chymotrypsin-like enzyme that prefers large hydrophobic residues (30). Whether GD has this specificity is unclear, as it diverges in sequence from other serine proteases in the region containing certain key residues that determine amino acid specificity (21). The fact that the 29-kDa form of GD reacts with affinity reagents designed for trypsin-like enzymes might suggest that GD is specific for basic residues. However, chymotrypsin also can cleave after basic residues, albeit inefficiently, and therefore could react with these affinity reagents under favorable conditions (32). Clearly, further biochemical experiments will be required to discern the amino acid specificity of the GD protease and to determine whether GD directly activates Snake.
Regulated GD Activity Is Important for Establishment of the Embryonic Dorsoventral Axis.
Our RNA injection experiments revealed an important relationship between GD activity and Toll signaling. We observed that injection of embryos with high concentrations of gd RNA caused more embryonic cells than normal to adopt ventral and lateral fates. A similar result has been observed in analogous experiments with spätzle RNA and with RNAs encoding “preactivated” Snake and Easter but not the zymogen forms of these proteases (2, 5, 6, 9, 30). Thus, the level of GD activity may determine the amount of Spätzle processed to the Toll ligand, presumably by controlling the level of active Snake and Easter. In this case, an important function of Snake and Easter could be to amplify the signal generated by GD into the appropriate amount of Toll ligand necessary to establish the embryonic dorsoventral axis.
Our experiments also revealed that gd acts downstream of nudel, pipe, and windbeutel, because the normal activity of each of these three genes is not required for injected gd RNA to induce ventral and lateral structures. After injection with a high level of gd RNA, embryos lacking maternal activity of nudel, pipe, or windbeutel developed a lateralized phenotype, which lacks the dorsoventral asymmetry of the ventralized phenotype seen when wild-type and gd mutant embryos are similarly injected. Thus, GD activity appears to be spatially uniform in the absence of normal Pipe, Windbeutel or Nudel function, implying that these three proteins are required to restrict GD activity to the ventral side of the embryo.
How is GD activated? Although Nudel is the only known protease in the Toll signaling pathway that acts genetically upstream of GD, our data suggest that Nudel is not essential for GD protease activity. GD could be activated by an unidentified protease; alternatively, GD could self-activate. Proteases that initiate the protease cascades of blood clotting and apoptosis have a low level of enzymatic activity as zymogens, but become fully active upon a conformational change or upon oligomerization induced by a nonenzymatic cofactor (33, 34). If GD is activated by a similar mechanism, then it should have a low level of protease activity as a zymogen (presumably below the sensitivity of detection in our affinity labeling experiments). In this case, the high concentration of GD protein produced by RNA injection into the embryo may have led to spontaneous GD activation that mimicked and bypassed the normal activation mechanism. A model consistent with available data is that the Nudel protease activates a cofactor, perhaps the ventral determinant provided by Pipe and Windbeutel, which in turn is necessary for activating the GD protease.
Acknowledgments
We thank Larry Marsh for providing the gd cDNA, Rob DeLotto for discussions, and Lynn Cooley and Michael Koelle for helpful comments on the manuscript. E.K.L. was supported by postdoctoral fellowships from the American Heart Association, Heritage Affiliate, and the National Institutes of Health (HD-08041). This work was supported by National Institutes of Health Grant GM-49370.
Abbreviation
GD
Gastrulation Defective
References
- 1.Morisato D, Anderson K V. Annu Rev Genet. 1995;29:371–399. doi: 10.1146/annurev.ge.29.120195.002103. [DOI] [PubMed] [Google Scholar]
- 2.Morisato D, Anderson K V. Cell. 1994;76:677–688. doi: 10.1016/0092-8674(94)90507-x. [DOI] [PubMed] [Google Scholar]
- 3.Schneider D S, Jin Y, Morisato D, Anderson K V. Development (Cambridge, UK) 1994;120:1243–1250. doi: 10.1242/dev.120.5.1243. [DOI] [PubMed] [Google Scholar]
- 4.DeLotto R, Spierer P. Nature (London) 1986;323:688–692. doi: 10.1038/323688a0. [DOI] [PubMed] [Google Scholar]
- 5.Chasan R, Anderson K V. Cell. 1989;56:391–400. doi: 10.1016/0092-8674(89)90242-0. [DOI] [PubMed] [Google Scholar]
- 6.Chasan R, Jin Y, Anderson K V. Development (Cambridge, UK) 1992;115:607–616. doi: 10.1242/dev.115.2.607. [DOI] [PubMed] [Google Scholar]
- 7.Smith C L, Giordano H, Schwartz M, DeLotto R. Development (Cambridge, UK) 1995;121:4127–4135. doi: 10.1242/dev.121.12.4127. [DOI] [PubMed] [Google Scholar]
- 8.Misra S, Hecht P, Maeda R, Anderson K V. Development (Cambridge, UK) 1998;125:1261–1267. doi: 10.1242/dev.125.7.1261. [DOI] [PubMed] [Google Scholar]
- 9.Smith C L, DeLotto R. Nature (London) 1994;368:548–551. doi: 10.1038/368548a0. [DOI] [PubMed] [Google Scholar]
- 10.DeLotto Y, DeLotto R. Mech Dev. 1998;72:141–148. doi: 10.1016/s0925-4773(98)00024-0. [DOI] [PubMed] [Google Scholar]
- 11.Konsolaki M, Schüpbach T. Genes Dev. 1998;12:120–131. doi: 10.1101/gad.12.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nilson L A, Schüpbach T. Cell. 1998;93:253–262. doi: 10.1016/s0092-8674(00)81576-7. [DOI] [PubMed] [Google Scholar]
- 13.Sen J, Goltz J S, Stevens L, Stein D. Cell. 1998;95:471–481. doi: 10.1016/s0092-8674(00)81615-3. [DOI] [PubMed] [Google Scholar]
- 14.Anderson K V. Cell. 1998;95:439–442. doi: 10.1016/s0092-8674(00)81610-4. [DOI] [PubMed] [Google Scholar]
- 15.Roth S. Curr Biol. 1998;8:906–910. [Google Scholar]
- 16.LeMosy E K, Hong C C, Hashimoto C. Trends Cell Biol. 1999;9:102–107. doi: 10.1016/s0962-8924(98)01494-9. [DOI] [PubMed] [Google Scholar]
- 17.Hong C C, Hashimoto C. Cell. 1995;82:785–794. doi: 10.1016/0092-8674(95)90475-1. [DOI] [PubMed] [Google Scholar]
- 18.Hong C C, Hashimoto C. Genetics. 1996;143:1653–1661. doi: 10.1093/genetics/143.4.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.LeMosy E K, Leclerc C L, Hashimoto C. Genetics. 2000;154:247–257. doi: 10.1093/genetics/154.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.LeMosy E K, Hashimoto C. Dev Biol. 2000;217:352–361. doi: 10.1006/dbio.1999.9562. [DOI] [PubMed] [Google Scholar]
- 21.Konrad K D, Goralski T J, Mahowald A P, Marsh J L. Proc Natl Acad Sci USA. 1998;95:6819–6824. doi: 10.1073/pnas.95.12.6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Konrad K D, Goralski T J, Mahowald A P. Dev Biol. 1988;127:133–142. doi: 10.1016/0012-1606(88)90195-9. [DOI] [PubMed] [Google Scholar]
- 23.Cormack B. In: Current Protocols in Molecular Biology. Ausubel F M, Kingston R, Moore D, Seidman J, Smith J, Struhl K, editors. New York: Wiley; 1997. pp. 8.5.1–8.5.10. [Google Scholar]
- 24.Anderson K V, Jürgens G, Nüsslein-Volhard C. Cell. 1985;42:779–789. doi: 10.1016/0092-8674(85)90274-0. [DOI] [PubMed] [Google Scholar]
- 25.Anderson K V, Nüsslein-Volhard C. Nature (London) 1984;311:223–227. doi: 10.1038/311223a0. [DOI] [PubMed] [Google Scholar]
- 26.Wieschaus E, Nüsslein-Volhard C. In: Drosophila: A Practical Approach. Roberts D B, editor. Oxford: IRL; 1986. pp. 199–227. [Google Scholar]
- 27.Bunch T A, Grinblat Y, Goldstein L S. Nucleic Acids Res. 1988;16:1043–1061. doi: 10.1093/nar/16.3.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williams E B, Mann K G. Methods Enzymol. 1993;222:503–513. doi: 10.1016/0076-6879(93)22031-a. [DOI] [PubMed] [Google Scholar]
- 29.Craik C S, Roczniak S, Largman C, Rutter W J. Science. 1987;237:909–913. doi: 10.1126/science.3303334. [DOI] [PubMed] [Google Scholar]
- 30.Smith C, Giordano H, DeLotto R. Genetics. 1994;136:1355–1365. doi: 10.1093/genetics/136.4.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams E B, Krishnaswamy S, Mann K G. J Biol Chem. 1989;264:7536–7544. [PubMed] [Google Scholar]
- 32.Hedstrom L, Szilagyi L, Rutter W J. Science. 1992;255:1249–1253. doi: 10.1126/science.1546324. [DOI] [PubMed] [Google Scholar]
- 33.Furie B C, Furie B. N Engl J Med. 1992;326:800–806. doi: 10.1056/NEJM199203193261205. [DOI] [PubMed] [Google Scholar]
- 34.Thornberry N A, Lazebnik Y. Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]