Novel Alpha Interferon (IFN-α) Variant with Improved Inhibitory Activity against Hepatitis C Virus Genotype 1 Replication Compared to IFN-α2b Therapy in a Subgenomic Replicon System (original) (raw)
Abstract
Hepatitis C virus (HCV) treatment is based on the association of pegylated alpha interferon (IFN-α) and ribavirin. To improve the level of sustained virological response to treatment, especially in patients infected with HCV genotype 1, new IFNs with improved efficacy and toxicity profiles may be developed. In this report, we show that, in the BM4-5 cell line harboring an HCV subgenomic replicon, a novel and naturally occurring human IFN-α17 variant, GEA007.1, which was discovered by using an original population genetics-based drug discovery approach, inhibits HCV genotype 1 RNA replication more efficiently than does IFN-α2b. Moreover, we show that complete viral clearance is obtained in BM4-5 cells after long-term treatment with GEA007.1, while HCV subgenomic RNA is still detected in cells treated with other IFN-α variants or with standard IFN-α2b. Eventually, we demonstrate that the better inhibitory activity of GEA007.1 compared to that of standard IFN-α is likely to be due to stronger and faster activation of the JAK-STAT signaling pathway and to broader expression of IFN-α-responsive genes in cells. Our results demonstrate a superior inhibitory activity of GEA007.1 over that of IFN-α2b in the HCV replicon system. Clinical trials are required to determine whether GEA007.1 could be a potent “next generation” IFN for the treatment of HCV infection, especially in nonresponders or relapsing patients infected with HCV genotype 1 who currently represent a clinical unmet need.
Hepatitis C virus (HCV) infects 170 million people worldwide and leads, in approximately 70% of cases, to chronic infection (3, 24, 26). Current treatments are based on the association of pegylated alpha interferon (IFN-α) 2a or 2b and ribavirin (5, 8, 20). This treatment induces a sustained virological response in approximately 60% of cases. The rate of sustained virological response ranges from 30 to 40% in patients infected with genotype 1 to 80 to 90% in those infected with genotype 2 or 3. Despite rather good efficacy, this treatment is often poorly tolerated because of the side effects (5). It is therefore important to improve current molecules and continue searching for novel antiviral agents with an enhanced anti-HCV activity to overcome the failure of current treatments, especially in patients infected with HCV genotype 1 (4).
Until the very recent development of a complete and robust HCV replication system (19, 27, 28), hepatoma cell lines harboring the HCV subgenomic replicon were a relevant model for studying some aspects of HCV biology as well as the effects and mechanisms of action of antiviral agents against HCV replication (1). Various interferons, including IFN-α, -β, and -γ, were studied using this model, which thus provided some insight on their antiviral effects (6, 7, 10, 18) and mechanisms of action (6, 11, 12, 15). Overall it was shown that the transduction pathway following the interaction of exogenous IFNs with their cellular receptors was functional in most cases, although slight differences could exist between different Huh7 cell lines harboring HCV subgenomic replicons. These differences may account for the variability observed in the amount of interferon used to obtain a given inhibition. Altogether, HCV replicon systems were validated as suitable tools for studying the efficacies and mechanisms of action of interferon molecules. Alpha interferon is known to exhibit several independent biological activities, including immunomodulatory, antiproliferative, and antiviral activities (14, 22, 23). In the context of an antiviral strategy, the pleiotropic effect of IFN-α may account for the adverse effects observed in vivo that are major causes of either noncompliance or premature interruption of the treatment (5). One possible approach to improving the currently used IFN-α is to identify novel IFN-α entities with increased antiviral activities and potentially decreased other activities.
In this study, we evaluated the inhibitory effects of original and functionally relevant variants of different IFN-α subtypes (IFNv) against the replication of the HCV using a cell line harboring a HCV genotype 1b subgenomic replicon (BM4-5 cells) and compared them to the inhibitory effect of standard IFN-α2b (i.e., IFN-α2 wild type [IFN-α2WT]). One of the studied variants, GEA007.1, was found to be seven times more efficient than was the IFN-α2WT molecule for inhibiting HCV RNA synthesis in BM4-5 cells. Interestingly, long-term treatment with GEA007.1 was able to cure BM4-5 cells from the HCV replicon, which was in contrast to the results obtained with the standard IFN-α2WT molecule tested under the same conditions. Moreover, based on mechanistic studies, we propose that the increased efficacy of the novel IFN-α17 variant GEA007.1 against HCV replication is likely to be due to a more potent activation of the JAK-STAT transduction pathway in BM4-5 cells, following the interaction of GEA007.1 with type I IFN receptor.
MATERIALS AND METHODS
Identification and production of IFN-α variants.
Original genetic variants of 14 different human IFN-α protein subtypes were provided by GenOdyssee. The discovery of natural cytokine mutants with improved therapeutic utilities was performed using the following process: a genomic DNA library was constituted with genomic DNA from 239 different individuals, representing altogether 85% of the human ethnic diversity of the worldwide population, and screened for nonsynonymous single-nucleotide polymorphisms in the coding sequences of the 14 different human IFN-α gene subtypes. The identification and selection of IFN-α protein mutants with functionally relevant amino acid mutations was then carried out using bioinformatics tools, including sequence/structure analysis and molecular modeling. IFN-α variants, hereafter named GEA007.1, GEA009.2, GEA011.1, and GEA013.1, derived from such procedures, are natural mutants of human IFN-α17, IFN-α21, IFN-α5, and IFN-α7, respectively (Swiss-Prot primary accession numbers P01571, P01568, P01569, and P01567, respectively). All carry a unique amino acid mutation (G45R for GEA007.1, D95N for GEA013.1, C122S for GEA011.1, and K179E for GEA009.2), with changes in three-dimensional structure and electrostatic isopotential at the receptor binding surface, based on molecular modeling. The recombinant human IFN-α variants used in this study were produced in the methylotrophic yeast Pichia pastoris, with a yield of approximately 10 to 20 mg/liter, following the protocols and recommendations provided by Research Corporation Technologies, Inc. IFNv proteins were secreted into the culture medium and purified using Cibacron and ion exchange chromatography to obtain pure preparations of recombinant human IFNv in a phosphate-buffered saline (PBS) solution. The purity of each preparation was greater than 95%, and endotoxin content, determined by Limulus amoebocyte lysate, was less than 5 IU/μg. IFN-α2b, hereafter named IFN-α2WT, was synthesized and purified using the same process in order to be an relevant control. In our experiments, 1 mg of IFN-α produced in yeast was equivalent to 3 × 108 IU (i.e., 1 IU is equivalent to 3.33 pg). Stock solutions were stored at −80°C. Dilutions of these stock solutions were carried out in PBS extratemporally. Prior to activity testing of the different IFN preparations against HCV in the replicon model, the preparations were tested for their specific activies and standardized in vitro using a gene reporter assay as described previously elsewhere (17). It was shown that the inhibitory activities of IFN-α2WT produced in Pichia pastoris and manufactured IFN-α2b (intron A; Schering Plough Corporation) produced in Escherichia coli are similar, indicating that the yeast Pichia pastoris production system does not affect the activity of IFN-α.
Cell culture.
BM4-5 cells (from a Huh7 cell line harboring an HCV genotype 1b subgenomic replicon), kindly provided by C. Seeger (Fox Chase Cancer Center, Philadelphia, Pa.), were cultured in Dulbecco's modified Eagle's medium medium (Invitrogen) complemented with 10% fetal calf serum (Perbio), antibiotics, and 500 μg/ml of Geneticin (G418 sulfate; Invitrogen) as previously described (10).
Protocols of administration of IFN.
Sixteen hours before starting treatments, BM4-5 cells were seeded in six-well plates at a density of 2.5 × 105 cells/well. IFNv (GEA007.1, GEA009.2, GEA011.1, and GEA013.1) and IFN-α2WT were administered to cells in complete medium in the absence of Geneticin. For short-term treatments, the administration of each drug (at concentrations ranging from 0 to 3,333 pg/ml) was renewed every day for three consecutive days. For long-term treatments, IFN-α2WT or GEA007.1 was administered every day at 333 pg/ml for 21 days. In order to maintain cells in the proliferation/division stage, cells were trypsinized and diluted four times every 4 days. A total of five passages were performed during treatment. At the end of this period of treatment, the administration of IFN-α2WT and GEA007.1 was interrupted and cells were kept for five further passages in medium either containing or not containing Geneticin to monitor a potential rebound of HCV subgenomic RNA replication.
Analysis of IFN toxicity.
Cells were seeded in 96-well plates at a density of 12,500 cells/well. They were treated by IFNs with the same concentrations and conditions as those used for short-term inhibitory assays. Cell viability was measured by both neutral red and MTS (CellTiter 96 Aqueous One Solution cell proliferation assay; Promega) tests. The 50% cytotoxic concentration (CC50) was defined as the concentration of interferon leading to a 50% reduction in the absorbance value compared to that of nontreated cells.
Analysis of HCV subgenomic RNA synthesis.
Total RNA was extracted with the Extract All reagent according to manufacturer's instructions (Eurobio). Northern blot analysis was then performed using the NorthernMax-Gly kit according to manufacturer's instructions (Ambion). Briefly, 5 μg of total RNA were denaturated in glyoxal buffer at 50°C for 30 min, separated on 1.1% agarose gel, and transferred overnight by capillarity onto a charged nylon membrane (HybondN+; Amersham). Hybridization was carried out with three different [32P]CTP-labeled riboprobes obtained by in vitro transcription (riboprobe in vitro transcription system; Promega). Two probes complementary to the NS5A region were used to detect either the negative or the positive HCV subgenomic RNA strands. A third probe was complementary to the β-actin mRNA and obtained by in vitro transcription using the plasmid pTRI-beta-actin-human (Ambion). First, the blot was hybridized for 16 h at 68°C with the two riboprobes directed against the negative strand of HCV subgenomic RNA and β-actin mRNA. After hybridization, the membrane was washed twice in stringent conditions and then exposed sequentially to X-ray film and a phosphor screen for quantification. The β-actin mRNA was used as an internal loading control to standardize the amount of HCV subgenomic RNA detected. The same membrane was subsequently hybridized with the negative-sense riboprobe to visualize and quantify the level of positive HCV subgenomic RNA strand, using the same procedure.
Analysis of the signal transduction pathway induced by IFN-α treatment in BM4-5 cells.
To evaluate the activation of intracellular signaling pathways by IFN-α2WT and IFNv, BM4-5 cells (75 × 103 cells/well in 24 wells plate) were transfected with an interferon-stimulating response element (ISRE)-luciferase plasmid (Stratagene) by using the TransIT-TKO reagent (Mirus) following the manufacturer's recommendations. Twenty-four hours after transfection, duplicate cultures in 24-well plates were either left untreated or treated for 24 h with a range of IFN-α2WT and IFNv of 0.3, 3, 33, 333, and 3,333 pg/ml. Cells were then washed with cold PBS and lysed by passive lysis buffer (Promega), and the luciferase activity was measured as recommended by the manufacturer (Promega). The luciferase activity induced by IFN-α2WT for each concentration was taken as 100%, and other luciferase activities were normalized accordingly.
Western blot analysis.
IFN-α2WT or GEA007.1 was administered to BM4-5 cells at a concentration of 3,333 pg/ml or 33 pg/ml for 30 min and 1, 2, 4, 8, 12, 24, 48, and 72 h. Administration was renewed every 24 h. At the indicated time, cells were washed twice with cold PBS and then lysed using a buffer (0.2% NP-40, 150 mM NaCl, 20 mM Tris, 2 mM EDTA, 0.1% glycerol, 10 mM dithiothreitol) containing serine/threonine phosphatase inhibitors (50 mM NaF, 200 μM orthovanadate) and protease inhibitor (Complete protease inhibitor; Roche). Proteins of cell lysates were denaturated at 100°C in Laemmli buffer (50 mM Tris, pH 6.8, 2% sodium dodecyl sulfate, 10% glycerol, 0.1% bromophenol blue, 1.25% 2-mercaptoethanol), separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and transferred onto polyvinylidene difluoride membranes (Amersham Pharmacia Biotech). Nonspecific binding sites were blocked for 2 h at room temperature with 5% milk, 0.1% Tween 20 in PBS. The membrane was then incubated overnight at 4°C with monoclonal antibodies raised against PKR (Santa Cruz Biotechnology), p48 (Becton Dickinson), or β-actin (Sigma). Membranes were then washed three times and incubated at room temperature for 1 h with horseradish peroxidase-conjugated goat anti-mouse or anti-rabbit immunoglobulin (Immunotech) at 1/10,000. Bound antibodies were detected by using ECL technology (ECL+ for p48 and ECL for PKR and β-actin) as specified by the manufacturer (Amersham Biosciences).
Analysis of IFN-α-specific gene expression using GEArray Q series array.
BM4-5 cells were incubated in six-well plates at 80% confluence. The cells either were left untreated or were incubated with IFN-α2WT or GEA007.1 at the same concentration of 33 pg/ml. After 8 h of incubation, total RNA was extracted with the Extract All reagent (Eurobio) and stored at −80°C. For one set of experiments, six arrays (GEArray Q series Human Interferon α,β Response gene array; SuperArray Bioscience Corporation) were used following the manufacturer's recommendation. For each array, 96 gene-specific cDNA fragments are printed on a nylon membrane of 3.8 by 4.8 cm. The list of the genes is accessible from the SuperArray web site (document HS-054). A total of 3 μg of each RNA sample was used as a template to generate cDNA by reverse transcription. cDNA was then amplified and labeled with biotin-16-dUTP (Roche) by a linear polymerase reaction (AmpoLabeling-LPR kit). After a step of prehybridization, the membranes were hybridized overnight, with the different probes corresponding to the different treatments. The signal was revealed by chemiluminescent reaction using alkaline phosphatase-conjugated streptavidin and detected by a scanner camera (Bio-Rad). All results were obtained from the analysis of nonsaturated images after 2 min of exposure. Spot quantification was performed with Quantify-One software (Bio-Rad). The mean intensity of blank spots served as negative controls and was subtracted from other values. The mean intensity of β-actin gene served as a positive control and was used to normalize the intensities of the spots to allow relative comparisons between the different arrays. To determine which genes were up-regulated, the intensities of spots resulting from hybridization with cDNA obtained from total RNA extracted from untreated cells were used to establish the basal expression level. The up-regulation of genes under interferon treatment was reported as a positive severalfold increase compared to those of untreated control samples.
RESULTS
Identification of novel IFN-α variants with improved inhibitory properties.
Based on the hypothesis that, in human populations, the natural evolution of the human genome has likely created a series of unpredictable and functionally relevant genetic variants with various structural and biological properties, nonsynonymous single-nucleotide polymorphisms were searched in the genomes of 239 individuals representing altogether 85% of the current ethnic diversity of the human population to identify interesting novel alleles of IFN-α genes coding for IFN-α proteins with superior or novel therapeutic properties compared to those of standard IFN-α2a or IFN-α2b. Following this approach, several IFNv, 17, 21, 5, and 7, referred to as GEA007.1, GEA009.2, GEA011.1, and GEA013.1, respectively, were selected for their significantly improved (GEA007.1 and GEA013.1) or decreased (GEA009.2 and GEA011.1) inhibitory properties compared to those of standard IFN-α2WT, based on preliminary data obtained with both an in vitro model of vesicular stomatitis virus-infected WISH cells and an in vivo model of encephalomyocarditis virus-infected mice (data not shown).
Anti-HCV effect of novel IFN-α variants.
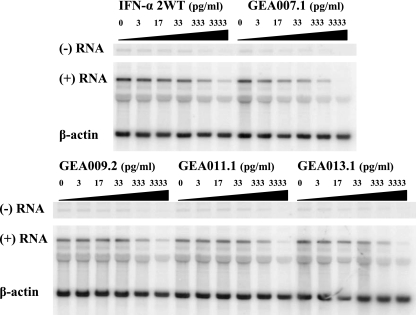
To evaluate and quantify the inhibitory effect of these novel IFNv, BM4-5 cells were treated for 3 days with increasing concentrations of GEA007.1, GEA009.2, GEA011.1, and GEA013.1 as well as with IFN-α2WT used as a reference. Total RNA was extracted and subjected to Northern blot analysis with three different riboprobes designed to detect both negative and positive HCV subgenomic RNA strands and β-actin mRNA (Fig. 1). A dose-dependent reduction in the amount of HCV subgenomic RNA was observed with all IFNv tested, and the greatest effect was obtained with GEA007.1. Three independent experiments were performed to obtain statistically relevant data. The quantification of the relative intensity of HCV subgenomic RNA and control β-actin signals after exposure to phosphor screens allowed the calculation of the 50% effective concentration (EC50), which was defined as the concentration required to induce a reduction of 50% of the amount of HCV subgenomic RNA detected by Northern blotting after normalization for each IFN tested (Table 1). Two IFNv, GEA007.1 and to a lesser extent GEA013.1, had better EC50s than did IFN-α2WT, suggesting higher efficacies of these molecules compared to that of the control. The better inhibitory activity of GEA007.1 (×7) and GEA013.1 (×1.5) over that of IFN-α2WT remained significant when the EC90s of the drugs were concerned (Table 1). This inhibitory activity was not due to toxicity, as no IFNs tested showed any cytotoxic effect on the BM4-5 cells, with the CC50 (i.e., 50% cytotoxic concentration) always superior to 3,333 pg/ml, the highest dose used in our experiments (Table 1).
FIG. 1.
GEA007.1 has improved inhibitory properties compared to IFN-α2WT and other IFN-α variants in the replicon system. BM4-5 cells were treated during 3 days by IFN-α2WT, GEA007.1., GEA009.2, GEA011.1, or GEA013.1 at 0, 3, 17, 33, 333, and 3,333 pg/ml. Total RNA was isolated from the cells and analyzed by Northern blotting for negative and positive HCV subgenomic RNA. β-Actin serves as an internal control for loading of cellular RNA.
TABLE 1.
Inhibitory features and toxicity of various interferonsa
| Interferon | EC50 (pg/ml) | Pb | EC90 (pg/ml) | Pb | CC50 (pg/ml) |
|---|---|---|---|---|---|
| IFN-α2WT | 154.2 ± 8.8 | 3,080 ± 406 | >3,333 | ||
| GEA007.1 | 22.7 ± 9.4 | <0.05 | 471 ± 276 | <0.05 | >3,333 |
| GEA009.2 | 483.3 ± 86.6 | <0.05 | >3,333 | >0.05 | >3,333 |
| GEA011.1 | 187.9 ± 57.5 | >0.05 | >3,333 | >0.05 | >3,333 |
| GEA013.1 | 95.6 ± 86.6 | <0.05 | 2,156 ± 300 | >0.05 | >3,333 |
Long-term administration of GEA007.1 cures cells from the HCV genotype 1b replicon subgenome.
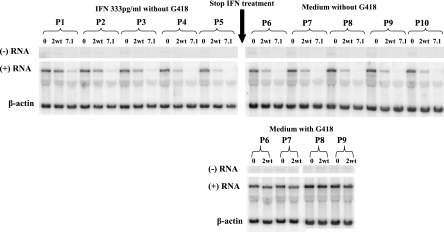
Having established that GEA007.1 had the best inhibitory activity compared to that of IFN-α2WT, with a very good EC90, we asked whether a prolonged administration of this IFNv could induce a better-sustained inhibitory effect than that of IFN-α2WT and lead to the rapid eradication of HCV replication in cells. BM4-5 cells were treated during 20 days through five passages with either IFN-α2WT or GEA007.1 at a single concentration of 333 pg/ml in medium without Geneticin as described in Materials and Methods. The results of a Northern blot analysis presented in Fig. 2 show that HCV subgenomic RNA synthesis was inhibited more intensively by GEA007.1 than by IFN-α2WT, thus confirming the better inhibitory activity of GEA007.1. Moreover, after 20 days of treatment and five passages, HCV subgenomic RNAs (both strands) were no longer detectable in cells treated with 333 pg/ml of GEA007.1, whereas the positive-strand HCV subgenomic RNA was still detectable in standard IFN-α2WT-treated cells. To monitor the potential rebound of HCV replication after the cessation of treatment, cells were kept untreated for at least four additional passages in either the absence or the presence or Geneticin. The presence of Geneticin was meant to select back more efficiently HCV replication from the very low level of residual replication after 20 days of treatment with IFN. In the absence of Geneticin, HCV subgenomic RNA remained undetectable after five passages posttreatment in GEA007.1-treated cells, whereas a small amount of positive-strand HCV subgenomic RNA was maintained in IFN-α2WT-treated cells (Fig. 2). When cells were cultured in a medium with Geneticin after treatment cessation, the cells that had been treated with IFN-α2WT could survive, but it took five more days for these cells to reach confluence relative to untreated cells. Interestingly, cells that had been treated with GEA007.1 were no longer able to survive in the presence of Geneticin, indicating that GEA007.1 was able to cure the cells from HCV subgenomic RNA (Fig. 2). In our experimental conditions, GEA007.1, but not IFN-α2WT, was able to eliminate HCV replication from BM4-5 cells, thus once again indicating the better efficacy of this variant molecule over that of the wild type.
FIG. 2.
GEA007.1 is able to clear HCV from BM4-5 cells after prolonged administration. BM4-5 cells were left untreated or treated every day with either IFN-α2WT or GEA007.1 at 333 pg/ml in the absence of Geneticin (upper panel left). At confluence, cells were passaged by a 1:4 dilution. After five passages, the treatment was stopped and cells were further passaged by a 1:4 dilution in medium free of Geneticin (upper panel right) or containing Geneticin (lower panel right). In the latter case, no result was presented for GEA007.1, as cells died in the presence of Geneticin (see the text for details). At each passage, total RNA was isolated from cells, separated on an agarose gel, and analyzed by Northern blotting for the detection of negative and positive RNAs. β-Actin served as an internal loading control. P, cell passage; 0, no IFN treatment; 2wt, treatment with IFN-α2WT; 7.1, treatment with GEA007.1.
Stronger and faster expression of proteins of type I IFN signal transduction pathway in BM4-5 cells is obtained with GEA007.1 compared to that with IFN-α2WT.
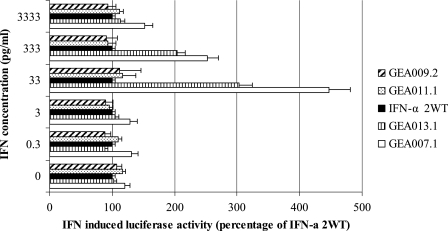
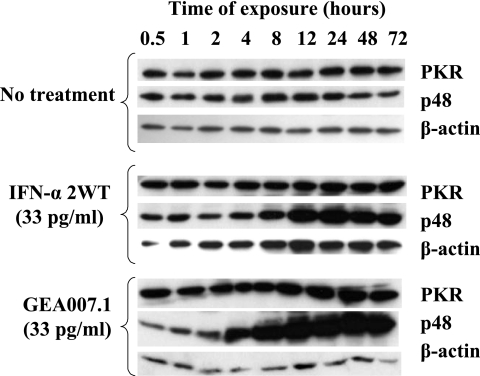
Having established that GEA007.1 had better anti-HCV properties than did IFN-α2WT in the replicon system, we sought to determine whether the higher activity of GEA007.1 was associated with a better activation of key cell signaling proteins involved in type I IFN transduction pathways. To this end, two sets of experiments were performed. First, BM4-5 cells were transfected with a reporter plasmid containing an interferon-stimulating response element upstream driving the expression of the firefly luciferase. Twenty-four hours after transfection, culture either was left untreated or was treated with a range of IFN-α2WT and IFNv of 0.3, 3, 33, 333, and 3,333 pg/ml. Luciferase activity was measured 24 h later, and results obtained with IFNv for each concentration were normalized to IFN-α2WT-induced activity (Fig. 3). The results showed an increased activation of the IFN-stimulating pathway with GEA007.1 compared to that of the others IFNs with the following order GEA007.1 > GEA013.1 > IFN-α2WT = GEA011.1 > GEA009.2. By comparison with that of IFN-α2WT, increased activations of three- and twofold of the IFN signaling pathways was observed with GEA007.1 at the concentrations of 33 and 333 pg/ml, respectively. At a higher concentration, i.e., 3,333 pg/ml, the difference of activation was not significant, most likely because of the saturation of the IFN receptor. Further experiments were therefore performed with a low IFN concentration of 33 pg/ml (a value equivalent to 10 IU) to clearly see differences between IFN-α2WT and GEA007.1. Western blot analyses were performed to further characterize the expression of proteins involved in the IFN signaling pathway. BM4-5 cells were treated with either GEA007.1 or IFN-α2WT at 33 pg/ml for 30 min and 1, 2, 4, 8, 12, 24, 48, and 72 h, and proteins were extracted and subjected to Western blot analysis using antibodies directed against p48 (interferon regulatory factor 9) and PKR, two important proteins of the type I IFN transduction pathways (25). With these conditions, GEA007.1 triggered faster and stronger expression of PKR and recruitment of p48 proteins in BM4-5 cells than did IFN-α2WT. The difference in activation of IFN-inducible proteins was more marked for p48. In our experimental conditions, the low dose of IFN-α2WT did not allow us to visualize a significant increase of the expression of p48 and PKR proteins (Fig. 4).
FIG. 3.
GEA007.1 is a better inducer of the IFN signaling pathways than is IFN-α2WT. BM4.5 cells were transfected with an ISRE luciferase construct. Twenty-four hours after transfection, the cells were incubated without or with IFN-α2WT and GEA007.1 for 24 h. The cells were then harvested and assayed for luciferase activity. The luciferase activity induced by IFN-α2WT for each concentration was taken as 100%, and other luciferase activities were normalized accordingly. Mean values ± standard deviations (error bars) of three independent experiments are shown. The absolute luciferase values obtained with increasing concentration of IFN-α2WT of 0, 0.3, 3, 33, 333, and 3,333 pg/ml were 205, 234, 226, 217, 798, and 2,004, respectively.
FIG. 4.
Effect of GEA007.1 on PKR and p48 (interferon regulatory factor 9) protein expression. BM4-5 cells were cultured in the absence or presence of 33 pg/ml of IFN-α2WT or GEA007.1. At different time points following the administration of IFNs, total cellular proteins were extracted and subjected to Western blot analysis using anti-PKR or anti-p48 antibodies. As a loading control, Western blot analysis was performed with anti-actin antibodies.
IFN-responsive gene expression is stronger and broader after treatment with GEA007.1 than after treatment with IFN-α2WT.
To broaden results obtained by Western blotting analysis and gain a global view of the differences in transduction pathway activation activities elicited by GEA007.1 and IFN-α2WT in BM4-5 cells, the expression of IFN-α-stimulated genes (ISGs) was analyzed using cDNA expression arrays. BM4-5 cells were left untreated or treated with either 33 pg/ml of GEA007.1 or IFN-α2WT for 8 h. RNA was then extracted and used for RT-PCR. Labeled cDNA was used for the hybridization of arrays as described in Materials and Methods. Relative intensities for the different genes and conditions of treatment were calculated, and results from significant up-regulation in BM4-5 cells induced by the administration of either IFN are reported in Table 2. An increase in gene expression of more than twofold was considered significant. Two IFN-responsive genes, MX1 and ISG56 (25), were found significantly up-regulated following IFN-α2WT treatment, while a stronger and a broader up-regulation of genes (MX1, ISG56, ISG15, ISG60, ADAR, KIAA1268, and STAT1) was found associated with GEA007.1 inhibitory activity in BM4-5 cells. In this experiment, the dose of IFN-α2WT chosen to point out differences with GEA007.1 was not high enough to trigger a strong activation of gene expression, in particular for PKR and p48, at least in our experimental conditions. This result is in good correlation with the lack of increase of the protein amount observed by Western blotting (Fig. 4). Collectively, these results confirm better activation of type I IFN signal transduction in these cells with GEA007.1 than with IFN-α2WT at the same concentration and time of exposure. This better ability to induce a stronger, faster, and broader cell signaling response was therefore associated with a superior inhibitory property of GEA007.1 over that of IFN-α2WT observed in cells harboring the HCV genotype 1b replicon.
TABLE 2.
Relative gene induction in BM4-5 cells after 8 h treatment with IFN-α2WT or GEA007.1a
| Gene name | Increase (_n_-fold) with indicated treatmentb | |
|---|---|---|
| IFN-α2WT | GEA007.1 | |
| MX1 | 17.83 | 25.92 |
| ISG-56 | 2.055 | 4.455 |
| ISG-15 | 1.7 | 4.125 |
| ISG-60 | 1.27 | 3.985 |
| ADAR | 1.38 | 2.91 |
| KIAA1268 | 1.19 | 2.35 |
| IFI6-16 | 0.795 | 2.13 |
| STAT1 | 1.38 | 2.08 |
| MAPKK1/MEK1 | 0.65 | 1.72 |
| PKR | 0.89 | 1.535 |
| P48/IRF-9 | 0.82 | 1.365 |
DISCUSSION
While we wait for the development and clinical use of new anti-HCV molecules, alpha interferon remains a major component of the anti-HCV therapy. Its use may also remain mandatory in combination with novel HCV inhibitors to prevent resistance to the latter drugs (8). However, the currently used IFN-α2a and IFN-α2b are not efficient in all cases. Nonresponding and relapsing patients are observed with current pegylated IFN-α/ribavirin combination therapies. Many factors may explain this resistance of the virus to short- or long-term viral clearance activity of IFN-α2, including host genetic and virological factors. For instance, it is well known that patients infected with HCV genotype 1 have significantly lower response rates (5, 6) and that genetic host factors may influence the response rate to interferon-based therapies (2, 16).
The main objective of this work was to determine whether better anti-HCV genotype 1 IFN molecules than the clinically used alpha interferon 2a and 2b could be identified among the novel IFN-α variants GEA007.1 and GEA013.1 with improved inhibitory properties against vesicular stomatitis virus and encephalomyocarditis virus (data not shown). For this study, IFN-α2b (IFN-α2WT) served as the standard IFN-α and additional IFN-α variants with low-inhibitory properties, GEA009.2 and GEA011.1, were included as negative controls. GEA007.1, GEA013.1, GEA009.2, and GEA011.1 were all identified with an original approach using the natural genetic variation of cytokine genes. Basically, the genomes of 239 individuals, representing altogether 85% of the human ethnic diversity, were searched for new and natural functionally relevant genetic mutants of the different human IFN-α subtypes, and variants with potential superior activities compared to that of IFN-α2 were selected. BM4-5 cells from a Huh7 cell line harboring an HCV genotype 1b subgenomic replicon were used to test the inhibitory properties of IFN-α variants. Interestingly, among the four interferon variants tested in this study, GEA007.1 and to a lesser extent GEA013.1 were found to be significantly more potent than IFN-α2WT for inhibiting the replication of an HCV genotype 1b subgenomic replicon in BM4-5 cells. GEA007.1 was the best molecule tested with an EC50 of 22.7 pg/ml, which is seven times lower than the EC50 obtained from standard IFN-α2WT (i.e., 154.2 pg/ml) in the same conditions (Table 1). Moreover, we found that GEA007.1, administered at 333 pg/ml every day for 20 days, was able to clear the HCV subgenomic replicon from BM4-5 cells, while HCV subgenomic RNA was still detected in cells treated with standard IFN-α2WT under the same conditions (Fig. 2). This result demonstrates the superiority of GEA007.1 over IFN-α2b in an HCV genotype 1b subgenomic replicon system. However, the results obtained from the HCV subgenomic replicon system may not translate into antiviral activity in vivo. Therefore, our results warrant further evaluation of GEA007.1 in vitro, using systems allowing a complete multiplication of HCV (19, 27, 28), and in vivo, in the frame of clinical trials.
The biological activity of IFN-α is related to its capacity to induce type I IFN signal transduction pathways after interaction with its cellular receptor. Different mechanisms have been proposed to explain the inhibitory effects of IFN-α against HCV replication, including noncytopathic and cytopathic processes (12). It was shown that the in vitro noncytopathic inhibition of HCV RNA replication by IFN-α in Huh7 cells is dependent on a functional JAK-STAT pathway and proteasome. However, double-stranded RNA-dependent antiviral pathways, such as the PKR and RNase L pathways, were shown not to be involved in IFN-induced inhibition of HCV replication in Huh7 cells (12). The IFN-α-induced antiviral program (i) could affect HCV internal ribosome entry site-directed translation and therefore cause a reduction in viral protein and (ii) could in turn inhibit viral RNA amplification as a result of a tight linkage between translation, assembly of replication complexes, and viral RNA synthesis (11). Interestingly, using an ISRE reporter assay, we showed an increased activation of the IFN-stimulating pathway by GEA007.1 relative to IFN-α2WT, which was associated with a better inhibitory activity on HCV subgenomic RNA synthesis. Then we analyzed key cell signaling proteins involved in the type I IFN transduction pathway after BM4-5 cells were exposed to GEA007.1 or IFN-α2WT. Western blot analyses were performed to monitor the level of expression of some key proteins like p48 or PKR, and cDNA expression array experiments were performed to monitor the expression of IFN-α-responsive genes. GEA007.1 induced a stronger expression of p48 than did IFN-α2WT. This result suggests that p48 may be recruited more efficiently by the STAT1P and STAT2P heterodimer complex to form the ISGF3 complex (25) after treatment with GEA007.1 than after treatment with IFN-α2WT. Our results are in accordance with previous works showing that type I IFN signaling pathways are qualitatively functional in Huh7 and BM4-5 cell lines, as persistent HCV replication does not modify gene expression patterns after IFN-α treatment (13, 21). In addition, in our experimental conditions, IFN-α-responsive genes activated in Huh7 cells harboring HCV subgenomic replicon by both GEA007.1 and IFN-α2WT did not differ from those described and reported in other studies (13, 21). Lastly, a stronger and broader expression of IFN-α-responsive genes was seen in BM4-5 cells treated with GEA007.1 than those treated with IFN-α2WT. Among the most activated genes found were Mx1, ISG56, ISG15, ISG60, ADAR, KIAA1268, and STAT1. Mx1 (also named MxA) was the most induced gene in our system. Mx1 was also found previously as an important IFN-α-inducible gene in IFN-α-treated replicon cell lines (13). A stronger expression of Mx1 was observed in cells treated with GEA007.1 which in turn could have contributed to the better inhibitory effect of GEA007.1 compared to IFN-α2WT. In view of the results obtained by others, showing the absence of inhibition of HCV RNA replication by Mx1 (6), it might be hypothesized that the activation of the different genes described can be only a reflection of the activation of the Jak-Stat pathway, without predicting a direct inhibitory role for a specific gene. Moreover, it is not possible at this stage to rule out the possibility that GEA007.1 may also induce unknown IFN-inducible genes responsible for an enhanced inhibitory activity. The ability of GEA007.1 to trigger a strong antiviral response in HCV-infected BM4-5 cells is most likely due to either a better interaction of this IFN to the cellular receptor or a better activity of the complex IFN receptor in transmitting the antiviral signal. The affinity of IFN to its receptor is a major issue in terms of efficacy. For instance, it was shown that different pegylation patterns of IFN could lead to different efficacy of binding to its cellular receptor and activation of the corresponding cell signaling pathway (9). One may hypothesize that GEA007.1 has a better affinity to the type I IFN receptor (IFNAR) and/or that the complex formed between GEA007.1 and IFNAR has a better capacity of signal transduction than the complex formed between IFN-α2 and IFNAR. Work is in progress to confirm this hypothesis. The difference in binding properties of GEA007.1 and standard IFN-α2WT could be due to the differences observed in the amino acid sequence of GEA007.1. In particular, GEA007.1 possesses a unique G45R mutation with significant change in three-dimensional structure and electrostatic isopotential at the receptor binding site, relative to IFN-α17WT protein or IFN-α2WT protein.
In conclusion, this study shows that the IFN-α variant GEA007.1 exhibits a more potent and sustained inhibitory activity on HCV genotype 1b subgenomic replicon replication than does standard IFN-α2b. This enhanced inhibitory activity is associated with a more efficient induction of the JAK STAT cell signaling pathway. These results warrant further evaluation to determine whether this enhanced activity in the HCV subgenomic replicon system could translate into a clinical benefit, especially in naïve HCV genotype 1b-infected patients who do not respond well to the currently available IFN-α2/ribavirin combination therapies as well as in nonresponder patients who represent a growing population of difficult-to-treat patients. Clinical trials are required to determine the potential of GEA007.1 to fulfill this unmet clinical need. It will be also interesting to see the inhibitory effect of GEA007.1 on other HCV genotypes both in vitro and in vivo. Furthermore, an IFN-α variant with an enhanced anti-HCV genotype 1 activity may be of utmost clinical relevance in the perspective of the new generation of treatment regimens, combining IFNs with specific small-molecule HCV inhibitors in current development (4).
Acknowledgments
This work was supported in part by a grant of the European Community and is part of the activities of the VIRGIL European Network of Excellence (contract LSHM-CT-2004-503359).
Footnotes
▿
Published ahead of print on 25 September 2006.
REFERENCES
- 1.Bartenschlager, R., and V. Lohmann. 2001. Novel cell culture systems for the hepatitis C virus. Antivir. Res. 52**:**1-17. [DOI] [PubMed] [Google Scholar]
- 2.Chen, L., I. Borozan, J. Feld, J. Sun, L. L. Tannis, C. Coltescu, J. Heathcote, A. M. Edwards, and I. D. McGilvray. 2005. Hepatic gene expression discriminates responders and nonresponders in treatment of chronic hepatitis C viral infection. Gastroenterology 128**:**1437-1444. [DOI] [PubMed] [Google Scholar]
- 3.Davis, G. L., J. E. Albright, S. F. Cook, and D. M. Rosenberg. 2003. Projecting future complications of chronic hepatitis C in the United States. Liver Transpl. 9**:**331-338. [DOI] [PubMed] [Google Scholar]
- 4.De Francesco, R., and G. Migliaccio. 2005. Challenges and successes in developing new therapies for hepatitis C. Nature 436**:**953-960. [DOI] [PubMed] [Google Scholar]
- 5.Feld, J. J., and J. H. Hoofnagle. 2005. Mechanism of action of interferon and ribavirin in treatment of hepatitis C. Nature 436**:**967-972. [DOI] [PubMed] [Google Scholar]
- 6.Frese, M., T. Pietschmann, D. Moradpour, O. Haller, and R. Bartenschlager. 2001. Interferon-alpha inhibits hepatitis C virus subgenomic RNA replication by an MxA-independent pathway. J. Gen. Virol. 82**:**723-733. [DOI] [PubMed] [Google Scholar]
- 7.Frese, M., V. Schwarzle, K. Barth, N. Krieger, V. Lohmann, S. Mihm, O. Haller, and R. Bartenschlager. 2002. Interferon-gamma inhibits replication of subgenomic and genomic hepatitis C virus RNAs. Hepatology 35**:**694-703. [DOI] [PubMed] [Google Scholar]
- 8.Fried, M. W., M. L. Shiffman, K. R. Reddy, C. Smith, G. Marinos, F. L. Goncales, Jr., D. Haussinger, M. Diago, G. Carosi, D. Dhumeaux, A. Craxi, A. Lin, J. Hoffman, and J. Yu. 2002. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N. Engl. J. Med. 347**:**975-982. [DOI] [PubMed] [Google Scholar]
- 9.Grace, M. J., S. Lee, S. Bradshaw, J. Chapman, J. Spond, S. Cox, M. Delorenzo, D. Brassard, D. Wylie, S. Cannon-Carlson, C. Cullen, S. Indelicato, M. Voloch, and R. Bordens. 2005. Site of pegylation and polyethylene glycol molecule size attenuate interferon-alpha antiviral and antiproliferative activities through the JAK/STAT signaling pathway. J. Biol. Chem. 280**:**6327-6336. [DOI] [PubMed] [Google Scholar]
- 10.Guo, J. T., V. V. Bichko, and C. Seeger. 2001. Effect of alpha interferon on the hepatitis C virus replicon. J. Virol. 75**:**8516-8523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo, J. T., J. A. Sohn, Q. Zhu, and C. Seeger. 2004. Mechanism of the interferon alpha response against hepatitis C virus replicons. Virology 325**:**71-81. [DOI] [PubMed] [Google Scholar]
- 12.Guo, J. T., Q. Zhu, and C. Seeger. 2003. Cytopathic and noncytopathic interferon responses in cells expressing hepatitis C virus subgenomic replicons. J. Virol. 77**:**10769-10779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayashi, J., R. Stoyanova, and C. Seeger. 2005. The transcriptome of HCV replicon expressing cell lines in the presence of alpha interferon. Virology 335**:**264-275. [DOI] [PubMed] [Google Scholar]
- 14.Iqbal Ahmed, C. M., and H. M. Johnson. 2003. Interferon gene therapy for the treatment of cancer and viral infections. Drugs Today 39**:**763-766. [DOI] [PubMed] [Google Scholar]
- 15.Ishida, H., K. Ohkawa, A. Hosui, N. Hiramatsu, T. Kanto, K. Ueda, T. Takehara, and N. Hayashi. 2004. Involvement of p38 signaling pathway in interferon-alpha-mediated antiviral activity toward hepatitis C virus. Biochem. Biophys. Res. Commun. 321**:**722-727. [DOI] [PubMed] [Google Scholar]
- 16.Kinzie, J. L., P. H. Naylor, M. G. Nathani, R. R. Peleman, M. N. Ehrinpreis, M. Lybik, J. R. Turner, J. J. Janisse, M. Massanari, and M. G. Mutchnick. 2001. African Americans with genotype 1 treated with interferon for chronic hepatitis C have a lower end of treatment response than Caucasians. J. Viral Hepat. 8**:**264-269. [DOI] [PubMed] [Google Scholar]
- 17.Lallemand, C., P. Lebon, P. Rizza, B. Blanchard, and M. G. Tovey. 1996. Constitutive expression of specific interferon isotypes in peripheral blood leukocytes from normal individuals and in promonocytic U937 cells. J. Leukoc. Biol. 60**:**137-146. [DOI] [PubMed] [Google Scholar]
- 18.Lanford, R. E., B. Guerra, H. Lee, D. R. Averett, B. Pfeiffer, D. Chavez, L. Notvall, and C. Bigger. 2003. Antiviral effect and virus-host interactions in response to alpha interferon, gamma interferon, poly(i)-poly(c), tumor necrosis factor alpha, and ribavirin in hepatitis C virus subgenomic replicons. J. Virol. 77**:**1092-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lindenbach, B. D., M. J. Evans, A. J. Syder, B. Wolk, T. L. Tellinghuisen, C. C. Liu, T. Maruyama, R. O. Hynes, D. R. Burton, J. A. McKeating, and C. M. Rice. 2005. Complete replication of hepatitis C virus in cell culture. Science 309**:**623-626. [DOI] [PubMed] [Google Scholar]
- 20.Manns, M. P., J. G. McHutchison, S. C. Gordon, V. K. Rustgi, M. Shiffman, R. Reindollar, Z. D. Goodman, K. Koury, M. Ling, and J. K. Albrecht. 2001. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet 358**:**958-965. [DOI] [PubMed] [Google Scholar]
- 21.Pai, M., R. Prabhu, A. Panebra, S. Nangle, S. Haque, F. Bastian, R. Garry, K. Agrawal, S. Goodbourn, and S. Dash. 2005. Activation of interferon-stimulated response element in huh-7 cells replicating hepatitis C virus subgenomic RNA. Intervirology 48**:**301-311. [DOI] [PubMed] [Google Scholar]
- 22.Parmar, S., and L. C. Platanias. 2003. Interferons: mechanisms of action and clinical applications. Curr. Opin. Oncol. 15**:**431-439. [DOI] [PubMed] [Google Scholar]
- 23.Platanias, L. C. 2005. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat. Rev. Immunol. 5**:**375-386. [DOI] [PubMed] [Google Scholar]
- 24.Poynard, T., M. F. Yuen, V. Ratziu, and C. L. Lai. 2003. Viral hepatitis C. Lancet 362**:**2095-2100. [DOI] [PubMed] [Google Scholar]
- 25.Samuel, C. E. 2001. Antiviral actions of interferons. Clin. Microbiol. Rev. 14**:**778-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seeff, L. B. 2002. Natural history of chronic hepatitis C. Hepatology 36**:** S35-46. [DOI] [PubMed] [Google Scholar]
- 27.Wakita, T., T. Pietschmann, T. Kato, T. Date, M. Miyamoto, Z. Zhao, K. Murthy, A. Habermann, H. G. Krausslich, M. Mizokami, R. Bartenschlager, and T. J. Liang. 2005. Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nat. Med. 11**:**791-796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhong, J., P. Gastaminza, G. Cheng, S. Kapadia, T. Kato, D. R. Burton, S. F. Wieland, S. L. Uprichard, T. Wakita, and F. V. Chisari. 2005. Robust hepatitis C virus infection in vitro. Proc. Natl. Acad. Sci. USA 102**:**9294-9299. [DOI] [PMC free article] [PubMed] [Google Scholar]