Roles of Saccharomyces cerevisiae DNA polymerases Polη and Polζ in response to irradiation by simulated sunlight (original) (raw)
Abstract
Sunlight causes lesions in DNA that if unrepaired and inaccurately replicated by DNA polymerases yield mutations that result in skin cancer in humans. Two enzymes involved in translesion synthesis (TLS) of UV-induced photolesions are DNA polymerase η (Polη) and polymerase ζ (Polζ), encoded by the RAD30A and REV3 genes, respectively. Previous studies have investigated the TLS roles of these polymerases in human and yeast cells irradiated with monochromatic, short wavelength UVC radiation (254 nm). However, less is known about cellular responses to solar radiation, which is of higher and mixed wavelengths (310–1100 nm) and produces a different spectrum of DNA lesions, including Dewar photoproducts and oxidative lesions. Here we report on the comparative cytotoxic and mutagenic effects of simulated sunlight (SSL) and UVC radiation on yeast wild-type, _rad30_Δ, _rev3_Δ and _rev3_Δ _rad30_Δ strains. The results with SSL support several previous interpretations on the roles of these two polymerases in TLS of photodimers and (6–4) photoproducts derived from studies with UVC. They further suggest that Polη participates in the non-mutagenic bypass of SSL-dependent cytosine-containing Dewar photoproducts and 8-oxoguanine, while Polζ is mainly responsible for the mutagenic bypass of all types of Dewar photoproducts. They also suggest that in the absence of Polζ, Polη contributes to UVC- and SSL-induced mutagenesis, possibly by the bypass of photodimers containing deaminated cytosine.
INTRODUCTION
The two major types of DNA damage induced by UV radiation are the bipyrimidine photolesions cis_–_syn cyclobutane pyrimidine dimers (photodimers) and pyrimidine (6–4) pyrimidone photoproducts [(6–4) photoproducts]. They are most efficiently produced by wavelengths close to the maximum absorption of bases (250–270 nm), and are formed at a lower yield by longer wavelengths in the UVB range (280–320 nm) (1). The highly mutagenic short-wavelength UVC and far-UVB (λ < 295 nm) radiation do not reach the earth’s surface so that the terrestrial sunlight UV spectrum consists of UVB (λ = 295–320 nm) and UVA (λ = 320– 400 nm). The UVB component of sunlight is mostly responsible for the production of photodimers, (6–4) photoproducts and their photoisomers, the Dewar photoproducts, via direct absorption. UVA radiation is weakly absorbed by DNA. However, it excites cellular chromophores and generates reactive oxygen species that may react with DNA to produce mutagenic oxidative DNA damage (2). For example, 8-oxoguanine is infrequently produced by UVB or UVC, but it is a main lesion induced by UVA radiation (2,3). Thus, the biological effects of sunlight might be more diverse than those resulting from monochromatic UV.
The UV component of sunlight is a major factor in the development of skin cancer (4). Patients with xeroderma pigmentosum (XP) are extremely sensitive to sunlight and have a dramatically increased risk of skin cancer. Cell lines derived from XP patients exhibit increased UV irradiation-induced killing and mutagenesis (1,5). Seven classical XP complementation groups plus the variant type (XPV) have been identified (1). Patients with classic XP suffer from defective nucleotide excision repair (NER) whereas XPV patients carry out normal NER, but are defective in replicating UV-damaged DNA (1,5). The XPV phenotype is caused by mutations in the human RAD30A gene, encoding polymerase η (Polη) (6). This polymerase was originally identified in Saccharomyces cerevisiae (7). In yeast devoid of Polη, UVC-induced mutagenesis and cell sensitivity are moderately enhanced (8–10). Deficiency in Polη strongly reduces the bypass of the TT photodimer, but has little effect on the TT(6–4) photoproduct bypass (11). In vitro data suggest that yeast and human Polη replicate through the TT photodimer with high efficiency, and with accuracy as high as for synthesis on undamaged templates (12). In contrast, Polη is unable to bypass the TT(6–4) photoproduct (12).
Polymerase ζ (Polζ), also discovered in S.cerevisiae, is a DNA polymerase critically important for UV mutagenesis (13). It is a heterodimer formed by the catalytic subunit encoded by the REV3 gene and the accessory factor encoded by REV7, that is sufficient for replicating across a lesion in vitro. In vivo, a third protein, Rev1p, is also required for the bypass activity of Polζ, but its role is still unknown (11,14). Inactivation of Polζ in yeast leads to a decrease in cell survival and a suppression of mutagenesis after UVC exposure (1,11). Deficiency in Polζ or Rev1p strongly reduces the bypass of the TT(6–4) photoproduct (although this bypass is highly mutagenic), but has little effect on the TT photodimer bypass frequency (15). In vitro, yeast Polζ has a limited capacity to insert nucleotides opposite the 3′T of the TT photodimer (16) and the TT(6–4) photoproduct (17). In contrast, Polζ is much more efficient at extending from a nucleotide inserted opposite the 3′ base of the (6–4) photoproduct by another polymerase (12).
Mammalian homologs of Rev3p, Rev7p and Rev1p have been identified. Defects in the REV3 gene in mice result in embryonic lethality (14). Depletion of Polζ in cultured human cells by antisense RNA expression strongly reduces UV-induced mutagenesis. This observation implies a key role for Polζ in the mutagenic bypass of photolesions in humans and a putative involvement of this polymerase in skin carcinogenesis.
Most of our knowledge about the UV sensitivity of Polη- or Polζ-deficient human cell lines and yeast strains derives from using UVC radiation. As noted above, UVC exposure does not accurately simulate exposure to sunlight. Thus, we determined cell sensitivity and mutagenesis induced by simulated sunlight (SSL, 310–1100 nm) in _S.cerevisiae rad30_Δ and _rev3_Δ strains deficient in Polη and Polζ, respectively, and compared the results with effects induced by UVC. We also characterized mutations induced by SSL and UVC at the chromosomal CAN1 locus in the above cells. The comparison of the induced mutation spectra brings new insight into the respective role of Polη and Polζ in sunlight- and UVC-induced mutagenesis.
MATERIALS AND METHODS
Strains and media
Yeast strains used in this study were derived from strain 7B-YUNI300 (_MAT_a _his7-2 leu2-3,112 ura3_-Δ trp1-289 ade2-1 lys2-B12) (18) by complete substitution of target genes with a kanMX cassette (19). The following set of isogenic strains was used: wild-type strain, dl-7B-YUNI300 (_leu2_Δ::kanMX) (20); Polη-deficient strain, eta-7B-YUNI300 (_rad30_Δ::kanMX) (18); Polζ-deficient strain, zeta-7B-YUNI300 (_rev3_Δ:: kanMX) derived in the present study with the primers described earlier (21). A strain lacking both Polη and Polζ, eta-zeta-7B-YUNI300 (_rad30_Δ::kanMX rev3::LEU2), was created from the strain eta-7B-YUNI300 by one-step gene disruption using the XbaI fragment of pAM56 plasmid (constructed by A. Morrison), as described by Holbeck and Strathern (22). Complete YEPD medium and synthetic complete medium SC containing no arginine and supplemented with 60 mg/l l-canavanine (SC + CAN) were used (23).
UV irradiation
Stationary phase yeast cells were harvested and resuspended in cold (+4°C) water at 106 cells/ml. Eight milliliters of this suspension were placed into a 60 × 15 mm plastic Petri dish and used for irradiation. UVC irradiation was performed using a Mazda germicidal 15TG lamp at a dose rate of 0.5 J/m2/s (maximal treatment time 140 s). Simulated solar light (SSL) was produced by a 2500 W xenon compact arc lamp XBO (OSRAM, Molsheim, France) in conjunction with a Schott WG320 cut-off filter (3 mm). The fluence rate was 1500 J/m2/s as determined by a YSI Kettering 65A thermopile (Yellow Springs Instrument, Yellow Springs, OH). The incident emission spectrum was composed of <10–5% UVC, 0.8% UVB, 6% UVA, 44.5% of visible and 48.7% of infrared light (24). Cell suspensions irradiated with SSL were swirled and cooled continuously in an ice bath (maximal treatment time 41 min).
UV-sensitivity and mutagenesis tests
UV-sensitivity tests were performed for all strains as follows. For each irradiated and unirradiated cell suspension, six independent appropriate dilutions were prepared and cells were plated on six YEPD plates. The percent survival was calculated as a ratio of the median number of colonies present on the six plates from the irradiated culture to the median number of colonies present on the six plates from the unirradiated culture. To determine the frequency of can1 mutants in strains dl-7B-YUNI300 and eta-7B-YUNI300, irradiated and unirradiated cell suspensions were concentrated 10-fold by centrifugation and six 100 µl aliquots were plated on six SC + CAN plates, and six independent appropriately diluted cell suspensions were plated on six YEPD plates. Mutant frequency was calculated as the ratio of total number of colonies present on six SC + CAN plates to the total number of colonies counted on the six YEPD plates. A different strategy was used for strains displaying low induced mutagenesis (zeta-7B-YUNI300 and eta-zeta-7-B-YUNI300). Twenty independent cultures were started from independent colonies of a strain. All 20 cultures were used for determination of spontaneous and UVC-induced mutant frequencies and 15 cultures of the strain were treated with SSL. Cells from irradiated and unirradiated suspensions were collected by centrifugation and resuspended in 1 ml of YEPD broth. The resulting cultures were incubated for 24 h with shaking at 30°C. Three 100 µl aliquots of each culture were plated on three SC + CAN plates and three independent appropriate dilutions were plated on three YEPD plates. The mutant frequency in each culture was calculated as the ratio of total number of colonies on the three SC + CAN plates to the total number of colonies on the three YEPD plates. Then, we used the median of the obtained values as an estimate for the mutant frequency for the given strain. This procedure allows us to measure and compare low UV-induced mutant frequencies in the _REV3_-deficient strains.
To avoid photoreactivation, all manipulations with irradiated cells were performed immediately after treatment, in a shaded place, and the plates were incubated in the dark at 30°C.
DNA sequence analysis
For can1 mutants sequencing, we used the same method for all strains. To minimize the fraction of spontaneous mutants in the samples of irradiated _REV3_-deficient strains, only cultures containing the lowest number of mutants (≤3 × 10–7) were used for irradiation. Yeast cell suspensions were irradiated with UVC at 50 J/m2 or SSL at 27 × 105 J/m2. Irradiated cells were harvested by centrifugation and plated on an appropriate number of SC + CAN plates. Independent canavanine-resistant clones were colony-purified on SC + CAN plates. Yeast genomic DNA was isolated by a miniprep method (23). PCR amplification of the CAN1 gene was performed either using primers CANF (5′-TCT GTC GTC AAT CGA AAG-3′) and CANR (5′-TTC GGT GTA TGA CTT ATG AGG GTG-3′), or P1PCR (5′-CAG ACT TCT TAA CTC CTG-3′) and P3 (5′-GGA ATG TGA TTA AAG GTA ATA AAA CG-3′). Approximately half of the mutants had been sequenced using three primers: P1 (5′-GGA ACT TTG TAC GTC CAA AAT TG-3′), P2 (5′-GGA ACT TAG TGT AGT TGG-3′) and P3 at the Genome Express Company (Meylan, France). The remainder of the samples were sequenced using six primers: 5′-AAA AAA GGC ATA GCA ATG-3′, 5′-ATT CTG TCA CGC AGT CCT-3′, 5′-GAA CTA GTT GGT ATC ACT-3′, 5′-GAA ATG GCG TGG AAA TGT G-3′, 5′-TGT CTC CAT GTA AGC CAA-3′ and 5′-ATA TTA TAC CTG GAC CCC-3′ on an ABI377 Prism or 3100 automatic sequencer using a manufacturer-supplied protocol.
To compare mutation spectra generated by the two types of radiation in the different strains, we used a two-tailed Fisher’s exact test (FET).
RESULTS
Cell survival and mutagenesis after simulated sunlight exposure
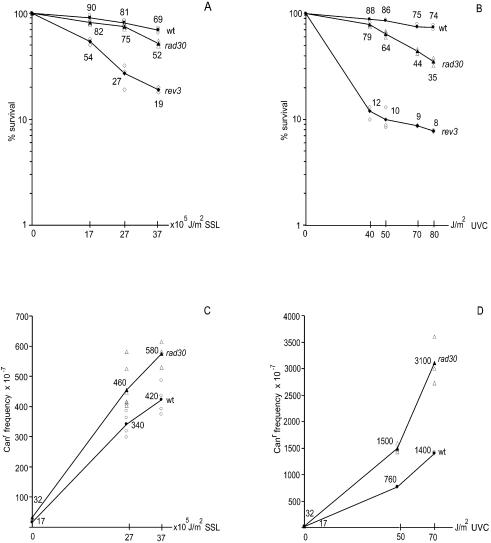
We first examined the SSL sensitivity of yeast deficient in Polη and Polζ. We found that rad30 and rev3 deletion mutants were more sensitive than the wild type to cell killing by SSL or by UVC (Fig. 1A and B). The rev3 strain was more sensitive than the rad30. Notably, in other published works, rad30 strains exhibited similar or enhanced UVC sensitivity, compared with rev3 strains, depending on the UV dose (8,10).
Figure 1.
Sensitivity of wild-type (circles), rad30 (triangles) and rev3 (diamonds) strains to SSL (A) and UVC (B), and induction of can1 mutants in the wild-type (circles) and rad30 (triangles) strains by SSL (C) and UVC (D). The data from each of at least three independent experiments are represented as open symbols and the means are represented as closed symbols. Values for these means are shown near each closed symbol. The differences observed between wild-type and other strains in all cases were statistically significant (linear multivariate regression analysis, P < 0.01).
We then determined the mutation frequencies at the CAN1 locus. In the rad30 mutant, mutagenesis induced by SSL was slightly, but significantly, above that in the wild type (Fig. 1C). It was increased 2-fold after UVC irradiation (Fig. 1D), in accord with what was previously observed at the CAN1 and other loci (8–10). In the rev3 and rev3 rad30 strains, mutagenesis by SSL, although detectable, was strongly diminished (Table 1) (significant by Wilcoxon test, P < 0.05). In the rev3 strain, mutagenesis induced by UVC was also strongly reduced. However, there was no induction of can1 mutants by UVC in the rev3 rad30 double mutant (Table 1).
Table 1. Induction of can1 mutants by SSL and UVC in the rev3 and rev3 rad30 strainsa.
| Strain | Spontaneous | SSL (27 × 105 J/m2) | UVC (50 J/m2) | ||
|---|---|---|---|---|---|
| _f_spont × 10–7 | f × 10–7 | _f_ind × 10–7 | f × 10–7 | _f_ind × 10–7 | |
| rev3 | 7 (6–9) | 44 (40–61) | 38 | 29 (18–43) | 22 |
| rev3 rad30 | 10 (8–15) | 41 (29–52) | 31 | 16 (9–21) | NSb |
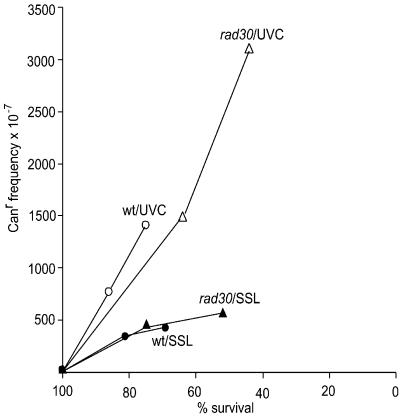
We compared the effects of SSL and UVC on mutagenesis in the wild-type and rad30 strains at equal cell survival (Fig. 2). The difference in the slopes of the curves indicates that SSL produced lesions that were more cytotoxic and less mutagenic than those produced by UVC. This suggests that cytotoxicity and mutagenicity of SSL might be partially due to lesions other than bipyrimidine photoproducts. Indeed, the UVA component of sunlight generates reactive oxygen species which may damage proteins and lipids and induce the formation of DNA single-strand breaks, DNA–protein crosslinks and oxidative base damage (25–29).
Figure 2.
Mutation frequencies as a function of survival level. Frequencies of can1 mutants in the wild-type (circles) and rad30 (triangles) strains upon irradiation with SSL (closed symbols) or UVC (open symbols) were plotted against corresponding surviving fractions.
Characterization of the types of mutations induced in Polη- and Polζ-deficient cells
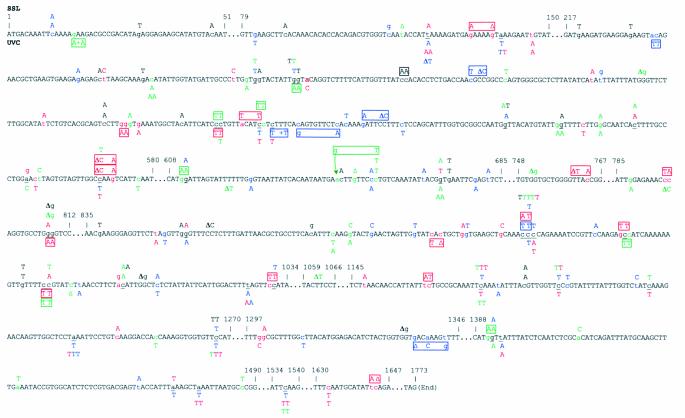
In order to get insight into the role of Polη and Polζ in sunlight mutagenesis, mutation spectra were determined at the CAN1 locus in the wild-type, rad30 and rev3 strains for SSL and UVC, and in the rev3 rad30 double mutant for SSL. The CAN1 gene is 1.8 kb long and has many detectable sites. Figure 3 shows the distribution of our collection of 232 induced mutants characterized by a single nucleotide change or a tandem or non-tandem mutation. Mutational events are dispersed along the gene.
Figure 3.
Distribution of UVC- and SSL-induced mutations along the CAN1 gene. Mutations that occurred in the wild-type (blue), rad30 (red), rev3 (green) and rev3 rad30 (black) strains are shown. The sequence is that of the non-transcribed strand. Only 1 nt alterations are shown. SSL- and UVC-induced changes are represented above and below the sequence, respectively. Tandem and non-tandem closely spaced double mutations are boxed. The lower case letters in the gene sequence indicate the altered base. Altered bases in the gene sequence where mutations occurred in more than one strain are underlined.
SSL produced predominantly single base pair substitutions in all the strains (Table 2 and Fig. 3). More complex mutations, including tandem and non-tandem mutations, were also observed, in particular in the rad30 strain. All tandem double mutations in the wild-type and rev3 strains were CC→TT, whereas in the rad30 strain, we obtained other changes as well: CC→TT, CC→TA, CC→AT, GC→TT, TC→AT and TC→AΔ (Fig. 3). In the rev3 rad30 strain, the single tandem mutation found was a CC→AA change. Non-tandem double mutations were also recovered in all strains except the rev3 rad30 strain. All involved near-adjacent bases separated by 2–8 bp (Fig. 3). The proportion of tandem and non-tandem double mutations was significantly higher in the rad30 strain than in the wild-type strain (FET, P = 0.03).
Table 2. DNA sequence changes in can1 mutants induced by SSL and UVC.
| Mutation type | SSL (27 × 105 J/m2) | UVC (50 J/m2) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wild type | rad30 | rev3 | rev3 rad30 | Wild type | rad30 | rev3 | ||||||||
| No. (%) | f × 10–7 | No. (%) | f × 10–7 | No. (%) | f × 10–7 | No. (%) | f × 10–7 | No. (%) | f × 10–7 | No. (%) | f × 10–7 | No. (%) | f × 10–7 | |
| Single | ||||||||||||||
| Base substitutions (BS) | 28 (88) | 300 | 22 (67) | 310 | 25 (68) | 30 | 29 (83) | 34 | 27 (84) | 640 | 30 (86) | 1300 | 24 (73) | 21 |
| Deletions 1 nt | – | – | – | – | 6 (16) | 7 | 4 (11) | 5 | 1 (3) | 24 | – | – | 3 (9) | 3 |
| Deletions > 1 nt | – | – | – | – | – | – | 1 (3)a | 1 | – | – | – | – | 1 (3)b | 1 |
| Duplications > 1 nt | – | – | – | – | 1 (3)c | 1 | – | – | – | – | – | – | 1 (3)d | 1 |
| Tandem double | ||||||||||||||
| CC→TT | 1 (3) | 11 | 1 (3) | 14 | 4 (11) | 5 | – | – | – | – | 4 (11) | 170 | 3 (9) | 3 |
| Other | – | – | 5 (15) | 70 | – | – | 1 (3) | 1 | 1 (3) | 24 | – | – | – | – |
| Non-tandem double | ||||||||||||||
| BS + BS | – | – | 1 (3) | 14 | 1 (3) | 1 | – | – | 1 (3) | 24 | – | – | – | – |
| BS + Δ1nt | 2 (6) | 21 | 4 (12) | 56 | – | – | – | – | – | – | 1 (3) | 43 | – | – |
| BS + insertion 1 nt | – | – | – | – | – | – | – | – | 1 (3) | 24 | – | – | 1 (3) | 1 |
| Multiple | – | – | – | – | – | – | – | – | 1 (3)e | 24 | – | – | – | – |
| Complex | 1 (3)f | 11 | – | – | – | – | – | – | – | – | – | – | – | – |
| Total mutants | 32 | 340 | 33 | 460 | 37 | 44 | 35 | 41 | 32 | 760 | 35 | 1500 | 33 | 29 |
The mutation spectra obtained for UVC resemble that produced by SSL, and are also characterized by a predominance of single base substitutions (Table 2), as usually observed (3). However, in contrast to SSL, all tandem double mutations found in the rad30 strain were CC→TT (Table 2 and Fig. 3). In addition, there was no significant increase in the tandem and non-tandem double mutations in the rad30 strain in comparison with wild type. It appears that, at least in the rad30 strain, mutations produced by SSL are more complex than that observed for UVC, implying differences in the premutagenic lesions.
Characterization of the base substitutions induced in Polη- and Polζ-deficient cells
The comparison of the types of base changes induced by SSL and UVC in the wild-type strain shows major differences as described below. Most of the base changes generated by SSL occurred at G·C pairs (84%) (Table 3). The mutation spectrum induced by SSL was characterized by approximately equal proportions of transitions and transversions and was dominated by G·C→A·T transitions (50%) and G·C→T·A transversions (28%). UVC induced changes at A·T and at G·C pairs with equal frequency (47 and 53%, respectively) (Table 3). Transversions predominated (62%), mainly consisting of A·T→T·A (41%), while G·C→A·T transitions represented 38% of the mutant collection. The increased proportion of base changes at G·C pairs after SSL irradiation in comparison with UVC was significant (FET, P = 0.008). A similar observation was previously made for wild-type yeast irradiated with UVC or natural sunlight (30,31). This may correlate with some biochemical data indicating that SSL produces relatively more cytosine-containing photodimers than UVC (24,32,33).
Table 3. Types of base substitutions induced by SSL and UVCa.
| | SSL (27 × 105 J/m2) | UVC (50 J/m2) | | | | | | | | | | | | | | | ---------------------- | ------------- | ------- | ------------ | --------- | ---------- | ------- | ---------- | ------- | ---------- | ------- | ---------- | ------- | ---------- | -- | | | Wild type | rad30 | rev3 | rev3 rad30 | Wild type | rad30 | rev3 | | | | | | | | | | No. (%) | f × 10–7 | No. (%) | f × 10–7 | No. (%) | f × 10–7 | No. (%) | f × 10–7 | No. (%) | f × 10–7 | No. (%) | f × 10–7 | No. (%) | f × 10–7 | | | Changes at | | | | | | | | | | | | | | | | A·T | 5 (16) | 54 | 12 (31) | 150 | 4 (11) | 4 | – | – | 16 (47) | 340 | 12 (31) | 510 | 1 (3) | 1 | | G·C | 27 (84) | 270 | 27 (69) | 280 | 31 (89) | 32 | 31 | 35 | 18 (53) | 390 | 27 (69) | 970 | 30 (97) | 21 | | Transitions | | | | | | | | | | | | | | | | A·T→G·C | 1 (3) | 11 | 4 (10) | 56 | 2 (6) | 2 | – | – | – | – | – | – | – | – | | G·C→A·T | 16 (50) | 150 | 18 (46) | 180 | 25 (71) | 25 | 6 (19) | 7 | 13 (38) | 290 | 25 (64) | 880 | 26 (84) | 18 | | Total | 17 (53) | 160 | 22 (56) | 240 | 27 (77) | 27 | 6 (19) | 7 | 13 (38) | 290 | 25 (64) | 880 | 26 (84) | 18 | | Transversions | | | | | | | | | | | | | | | | G·C→T·A | 9 (28) | 96 | 8 (21) | 84 | 5 (14) | 6 | 24 (77) | 27 | 4 (12) | 83 | 1 (3) | 43 | 4 (13) | 3 | | A·T→T·A | 3 (9) | 32 | 8 (21) | 91 | 1 (3) | 1 | – | – | 14 (41) | 320 | 11 (28) | 470 | – | – | | C·G→G·C | 2 (6) | 21 | 1 (3) | 14 | 1 (3) | 1 | 1 (3) | 1 | 1 (3) | 12 | 1 (3) | 43 | – | – | | A·T→C·G | 1 (3) | 11 | – | – | 1 (3) | 1 | – | – | 2 (6) | 16 | 1 (3) | 43 | 1 (3) | 1 | | Total | 15 (47) | 160 | 17 (44) | 190 | 8 (23) | 9 | 25 (81) | 28 | 21 (62) | 430 | 14 (36) | 600 | 5 (16) | 4 |
The comparison of the types of base substitutions induced by SSL in the four strains shows the following features (Table 3). The distribution of the types of base substitutions did not significantly vary between wild-type and rad30 strains, implying a role for Polη in the non-mutagenic bypass of most of the potentially mutagenic lesions induced by SSL. In the rev3 strain, transitions, mainly G·C→A·T (71%), were the major type of base substitutions, while in the rev3 rad30 strain, transversions, essentially G·C→T·A (77%), predominated. Such a distribution of mutations contrasts with that observed in wild-type and rad30 strains. Further insight is gained in the comparison of the types of base substitutions induced by UVC in the four strains. In the rev3 strain, 97% of base substitutions occur at G·C pairs. This is more than in the wild-type and rad30 strains (53 and 69%, respectively, with no significant difference, FET, P = 0.2). In comparison with the wild-type strain, the proportion of transitions was significantly higher in the rad30 (64%) and rev3 (84%) strains (FET, P = 0.04 and 0.0003, respectively) (Table 3). All transitions found in all three strains were G·C→A·T. As in the wild-type strain, the major type of transversion observed in the rad30 strains was A·T→T·A. Remarkably, no A·T→T·A transversions were recovered in the rev3 strain. The last observation suggests a role for Polζ in generating this type of mutation.
The difference in the types of base substitutions induced by SSL and UVC seems to hold in the distribution of transversions, i.e. a majority of G·C→T·A changes for SSL and A·T→T·A for UVC. The A·T→T·A events were rare after SSL exposure, except in the rad30 strain. The increased proportion of G·C→T·A transversions in the rad30 strain after SSL irradiation was significant (FET, P = 0.03), although that in the wild-type strain was not (FET, P = 0.1). However, the proportion of SSL-induced G·C→T·A transversions in the rad30 strain was similar to that observed in the wild-type strain (28 and 21%, respectively). Interestingly, there was no significant difference in the distribution of base substitutions in the rev3 strain after SSL and UVC irradiation. On the other hand, the fraction of transitions in the rev3 strain after SSL and UVC irradiation (77 and 84%, respectively) was higher than expected spontaneously in the rev3 strains in the CAN1 (45%) and _SUP4_-o (44%) loci (34,35). Taking into account the experimental conditions used to minimize the fraction of spontaneous mutants (see Materials and Methods), most mutations were indeed induced by irradiation in this strain.
The many differences observed in the base substitutions induced by SSL and UVC demonstrate that the premutagenic lesions greatly differ for these two types of radiation. The remarkable similarity and low frequency of base changes induced by SSL and UVC in the rev3 strain reveal that most of the premutagenic lesions are processed by the polymerase encoded by the REV3 gene. It also indicates that the mutations observed in the absence of this polymerase in both cases are generated by the same mechanism. For both SSL and UVC, the distribution of base substitutions in wild-type and rad30 strains does not drastically differ, indicating an essentially non-mutagenic bypass of lesions processed by the polymerase encoded by the RAD30 gene.
Site specificity of base substitutions
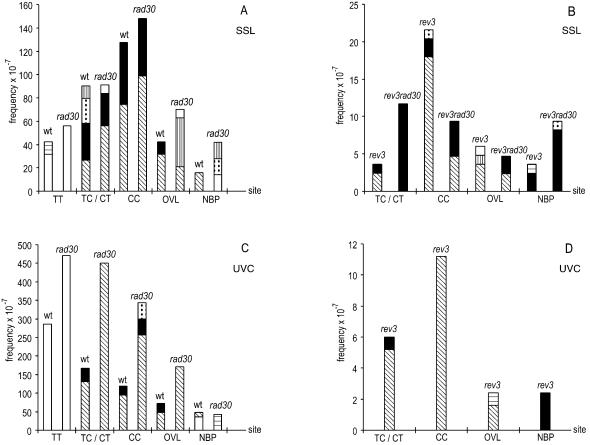
Assuming that a majority of SSL and UVC lesions giving rise to mutations involved bipyrimidine photoproducts, at least in the wild-type and rad30 strains (3,36,37), we presented the data as changes at pyrimidines for all tested strains (Table 4 and Fig. 4). The comparison of sites and types of base changes observed following SSL (Fig. 4A and B) and UVC (Fig. 4C and D) exhibits major differences as follows.
Table 4. Site specifity of base substitutionsa.
| Site | SSL (27 × 105 J/m2) | UVC (50 J/m2) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wild type | rad30 | rev3 | rev3 rad30 | Wild type | rad30 | rev3 | ||||||||
| No. (%) | f × 10–7 | No. (%) | f × 10–7 | No. (%) | f × 10–7 | No. (%) | f × 10–7 | No. (%) | f × 10–7 | No. (%) | f × 10–7 | No. (%) | f × 10–7 | |
| TT | 4 (13) | 43 | 4 (12) | 56 | – | – | – | – | 12 (39) | 290 | 11 (31) | 470 | – | – |
| 5′TC3′ | 5 (16) | 53 | 4 (12) | 56 | 2 (7) | 2 | 5 (17) | 6 | 4 (13) | 95 | 8 (23) | 340 | 7 (25) | 6 |
| 5′CT3′ | 1 (3) | 11 | 3 (9) | 42 | 1 (3) | 1 | 2 (7) | 2 | 1 (3) | 24 | 1 (3) | 43 | – | – |
| TCT | 3 (10) | 32 | 1 (3) | 14 | – | – | 3 (10) | 4 | 3 (10) | 71 | 2 (6) | 86 | 1 (4) | 1 |
| CCb | 12 (39) | 130 | 11 (33) | 150 | 18 (60) | 22 | 8 (27) | 9 | 5 (16) | 120 | 8 (23) | 340 | 14 (50) | 11 |
| OVLc | 4 (13) | 43 | 6 (18) | 84 | 5 (17) | 6 | 4 (13) | 5 | 3 (10) | 71 | 4 (11) | 170 | 3 (11) | 2 |
| NId | – | – | 1 (3) | 14 | 1 (3) | 1 | – | – | 1 (3) | 24 | – | – | – | – |
| NBPe | 2 (7) | 17 | 3 (9) | 42 | 3 (10) | 4 | 8 (27) | 9 | 2 (7) | 48 | 1 (3) | 43 | 3 (11) | 2 |
| Total | 31 | 330 | 33 | 460 | 30 | 36 | 30 | 35 | 31 | 740 | 35 | 1500 | 28 | 22 |
Figure 4.
The frequencies and types of base changes associated with defined sites induced by SSL (A and B) and UVC (C and D). Changes indicated: T→A (open bars), T→G (horizontal lined bars), T→C (vertical lined bars), C→T (oblique bars), C→A (black bars), C→G (dotted bars). Overlapped sites (CCT, TTC, TCC and CTT) are indicated as OVL and non-bipyrimidine sites as NBP.
After SSL irradiation, ≥90% of base changes in the wild-type, rad30 and rev3 strains occurred at bipyrimidine sites (Table 4). However, we observed a significant increase of mutations at non-bipyrimidine sites (NBP) in the rev3 rad30 strain (27%) relative to the wild-type strain (7%) (FET, P = 0.04). The CAN1 gene sequence contains 1362 (77%) bipyrimidine sites and 409 (23%) NBP. The proportion of mutations at NBP in the rev3 rad30 strain was close to the proportion of those sites in the CAN1 gene. Thus, we propose that SSL-induced mutations in the rev3 rad30 strain (a majority of G·C→T·A transversions) arose mostly independently from bipyrimidine photoproducts.
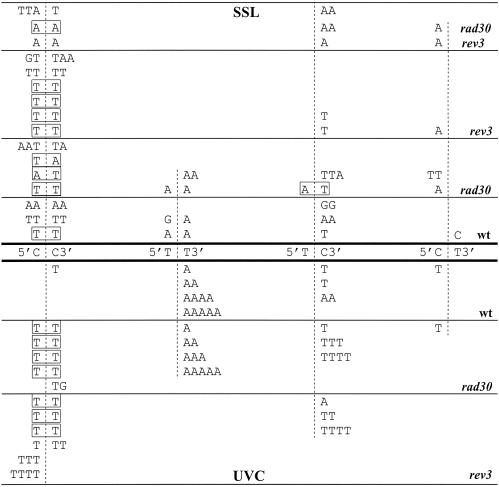
The site distribution of mutations after SSL irradiation was in the following order: CC > (5′TC3′/5′CT3′) > TT in the wild-type and rad30 strains, CC > (5′TC3′/5′CT3′) in the rev3 strain and (5′TC3′/5′CT3′) > CC in the rev3 rad30 strain. Most of the mutations at CC and 5′TC3′/5′CT3′ sites observed in the wild-type and rad30 strains were C→T transitions and C→A transversions (Fig. 4A); they were C→T transitions in the rev3 strain, but C→A transversions in the rev3 rad30 strain (Fig. 4B). Most of the mutations in all tested strains at 5′TC3′ and 5′CT3′ sites occurred at the 3′C of TC sites or at the 5′C of CT sites, and at both the 5′C and 3′C positions of CC sites (some of them were tandem double) (Fig. 5). Most, if not all, changes at TT sites in the wild-type and rad30 strains were T→A transversions at both the 3′T or 5′T position. Interestingly, SSL did not induce mutations at TT sites in the rev3 and rev3 rad30 strains. Mutations at NBPs observed in the rev3 rad30 strain consisted mostly (88%) of G·C→T·A transversions.
Figure 5.
Position specificity (3′ or 5′) of base substitutions at bipyrimidine sites 5′CC3′, 5′TT3′, 5′TC3′ and 5′CT3′ induced by SSL (above the sites) and UVC (below the sites). Tandem double mutations are boxed.
After UVC irradiation, 89–97% of base changes in all strains tested were found at bipyrimidine sites (Table 4). The distribution of changes at bipyrimidine sites was TT ≥ (5′TC3′/5′CT3′) > CC in the wild-type and rad30 strains. Most of the changes recovered at CC and 5′TC3′/5′CT3′ sites in all strains tested were C→T transitions occurring at the 3′C of TC sites or at the 5′C of CT sites, and in both 5′C and 3′C positions of CC sites (compare Fig. 4C and D with A and B, and see Fig. 5). Relatively more mutations at TT sites were observed for UVC than for SSL (Table 4). All changes recovered at TT sites in the wild-type and rad30 strains were T→A transversions, but only at the 3′T position of the bipyrimidine sequence (Figs 4C and 5). However, UVC, like SSL, did not produce mutations at TT sites in the rev3 strain (Figs 4D and 5).
No significant strand specificity of mutations induced by SSL
Preferential repair of photolesions in the transcribed strand (TS), namely transcription-coupled repair, has been demonstrated in prokaryotes and eukaryotes, including yeast (38). After SSL irradiation we observed a slightly increased fraction of mutated bipyrimidine sites in the non-transcribed strand (NTS) in the wild-type, rad30 and rev3 strains (19 NTS:10 TS in the wild-type and rad30 strains, and 16 NTS:10 TS in the rev3 strain), but not in the rev3 rad30 strain (9 NTS:13 TS). After UVC exposure, mutated bipyrimidine sites distributed almost equally between TS and NTS in the wild-type and rev3 strains (16 NTS:12 TS and 12 NTS:13 TS, respectively), while their distribution is in favor of the NTS in the rad30 strain (26 NTS:8 TS). However, these data are insufficient to assess any role for transcription-coupled repair in SSL- and UVC-induced mutagenesis.
Main features of SSL- and UVC-induced mutagenesis in yeast proficient or deficient in bypass DNA polymerases
We demonstrated that SSL produced cytotoxic lesions that were less mutagenic than those produced by UVC. SSL, like UVC, induced mostly single base changes at bipyrimidine sites, but also tandem and non-tandem double mutations. Compared with UVC, SSL induced less mutations at TT sites, which were almost all T→A transversions occurring exclusively at the 3′T of the TT sites after UVC exposure, but at both the 3′T and 5′T after SSL irradiation. Moreover, the main type of transversion recovered after SSL irradiation was G·C→T·A, whereas after UVC exposure it was A·T→T·A, at least in the wild-type strain.
Inactivation of the RAD30 gene (i) increased SSL- and UVC-induced mutagenesis, (ii) enhanced the proportion of UVC-induced G·C→A·T transitions at bipyrimidine sites and (iii) increased the diversity in the types of SSL-induced tandem double mutations at CC and 5′TC3′ sites.
Inactivation of the REV3 gene (i) strongly decreased SSL- and UVC-induced mutagenesis and (ii) led to the absence of mutations at TT sites after both SSL- and UVC-irradiation, while mutations at other bipyrimidine sites still occurred and were, in both cases, mostly G·C→A·T transitions.
Inactivation of both RAD30 and REV3 genes (i) completely suppressed UVC-induced mutagenesis, whereas SSL-induced mutagenesis was still observed and (ii) drastically increased the proportion of SSL-induced G·C→T·A transversions which did not occur preferentially at bipyrimidine sites.
DISCUSSION
Polη was initially defined as a lesion bypass polymerase capable of non-mutagenic replication past adducts induced by UVC radiation. Polζ was known to be indispensable for UVC-induced mutagenesis (11,12). Part of our knowledge comes from studies investigating the ability of such purified enzymes to bypass (insert opposite and elongate after insertion) a unique lesion [a TT photodimer or TT(6–4) photoproduct] on a template (12). It is still difficult to corroborate such in vitro data with in vivo experiments. In particular, the genetic studies explored only UVC-induced mutagenesis and simply at fixed sites by a locus-specific reversion assay, or examined the bypass of a unique lesion in a plasmid, in yeast strains proficient or deficient in the _RAD30_-encoded Polη (11,12,39,40). In humans, the lack of Polη leads to the XPV phenotype characterized by enhanced UV sensitivity, UVC-induced hypermutagenesis and predisposition to skin cancer in sunlight exposed areas (1,5). On the other hand, the damage distribution induced by sunlight and UVC differs (36). In particular, in cells exposed to natural sunlight, (6–4) photoproducts were mostly converted into their Dewar valence isomers (41). To fill the gap in our knowledge we analyzed and compared SSL- and UVC-induced mutation spectra generated in isogenic yeast strains proficient or deficient in defined bypass polymerases. The results, in conjunction with published biochemical and other genetic data, bring new insights into the molecular mechanism of solar mutagenesis, as emphasized below.
Polζ is required for mutagenic bypass of TT photolesions
SSL and UVC did not produce mutations at TT sites in Polζ-deficient strains (Table 4 and Fig. 4). This indicates that Polζ is required for mutation generation at TT photolesions. At such sites, almost all events were T→A transversions in both the wild-type and rad30 strains, all occurring at the 3′T of TT sites after UVC exposure, but at both the 3′T and 5′T in the case of SSL irradiation (Figs 4 and 5). What TT photolesion(s) could be responsible for the Polζ-dependent mutagenesis?
It was reported that no more than 0.4% of cis_–_syn TT photodimer bypass events in wild-type yeast were mutagenic (42). In the rad30 strain, the TT photodimer bypass was reduced to 15% of the wild type, and it was completely absent in the rev3 rad30 or rev1 rad30 double mutants (11). However, in the rad30 strain, TT photodimers were much more mutagenic than in the wild-type strain (12% of bypass events resulted in 3′T→A or 3′T→C mutations) (11). These data indicate that in yeast, Polη plays a major role in efficient and non-mutagenic bypass of TT photodimers, whereas mutagenic bypass of this lesion requires Polζ and Rev1p, but is infrequent. In contrast to the TT photodimer, the bypass of the TT(6–4) photoproduct in yeast was found to be far more mutagenic (40% of bypass events were mutagenic) and the major types of mutations were 3′T→C transitions, 3′T→A or 5′T→A transversions (43). The in vivo bypass of the TT(6–4) photoproduct required Polζ and Rev1p (15), while the absence of Polη had little effect (11). Therefore, it is likely that TT(6–4) photoproducts rather than TT photodimers are responsible for most of the Polζ-dependent mutations occurring at TT sites upon UVC irradiation.
It was reported that the in vitro translesion synthesis opposite a TT(6–4) photoproduct by yeast Polζ was limited, with a predominant incorporation of an A or T opposite the 3′T and an A opposite the 5′T (17). An alternative mutagenic pathway would involve the consecutive action of Polη and Polζ. It was shown that in vitro, Polη inserts a G opposite the 3′T, and that Polζ efficiently elongates the reaction product by inserting an A opposite the 5′T (12). Thus, according to these in vitro data, a direct bypass of TT(6–4) photoproducts by Polζ would produce 3′T→A transversions, while a bypass performed consecutively by Polη and Polζ would generate 3′T→C transitions. Hence, we propose that 3′T→A transversions observed in our study result from a direct bypass of TT(6–4) photoproducts by Polζ. Alternatively, another polymerase (not Polη, since its absence has little effect on mutations at TT sites) inserts a T opposite the 3′T and Polζ elongates from the inserted T, fixing the T→A mutation at the 3′ position.
A substantial fraction of the (6–4) photoproducts induced by the UVB component of SSL is converted by the UVA component into their Dewar valence isomeric forms (36,41). These last photoproducts were not produced by UVC in biologically relevant amounts (36). On the other hand, biochemical data suggest that upon SSL irradiation most TT(6–4) photoproducts (up to 90%) are isomerized into their Dewar form, both in isolated DNA and in mammalian cells (44). In the case of SSL, Dewar TT photoproducts may be responsible for most of the Polζ-dependent mutations occurring at TT sites. We propose that Polζ is required for bypass of Dewar TT photoproducts, as well as TT(6–4) photoproducts. In Escherichia coli, the Dewar TT isomer is less mutagenic, but produces a broader spectrum of changes, in comparison with the TT(6–4) photoproduct (45). We observe that, in contrast to UVC, SSL produces T→A transversions in both 3′ and 5′ positions of TT sites (Fig. 5). Thus, we presume that the Polζ-mediated bypass of TT Dewar photoproducts in yeast occurs with different specificity as for the TT(6–4) photoproduct.
Polζ is essential for mutagenic bypass of cytosine-containing photolesions, while Polη is involved in their non-mutagenic bypass
Our data showed that, in the absence of Polζ, SSL- and UVC-induced mutagenesis at CC, 5′TC3′ and 5′CT3′ sites were strongly diminished but not completely suppressed (see mutation frequencies in Fig. 4). This observation is consistent with a major role for Polζ in the mutagenic bypass of cytosine-containing photolesions. Polζ was responsible for most of the C→T transitions (compare the mutation frequencies at cytosine-containing sites in the wild-type, rad30 and rev3 strains in Fig. 4). This might result from a direct bypass of cytosine-containing (6–4) photoproducts by Polζ or from sequential action of some other polymerase and Polζ.
We found that UVC induced an increased proportion of C→T transitions at bipyrimidine sites in the rad30 strain relative to the wild-type strain (Table 3 and Fig. 4C). This shows a loss of non-mutagenic bypass of cytosine-containing photolesions in the absence of Polη. This is in accordance with a recent report showing an increase in the frequency of UVC-induced C→T transitions at two unique CC and 5′TC3′ sites in yeast strains lacking Polη (10). Meanwhile, our data further demonstrate that the non-mutagenic bypass of C-containing photolesions by Polη is a general phenomenon. Our data are consistent with the fact that, in vivo, Polη performs a non-mutagenic bypass of C-containing photodimers and may participate in the non-mutagenic bypass of (6–4) photoproducts by inserting a ‘correct’ G opposite the 3′C of the lesions with subsequent elongation by Polζ, as proposed originally by Yu et al. (10).
In the rad30 strain, SSL irradiation induced a variety of tandem double mutations at CC and 5′TC3′ sites, while in the Polη-proficient strains only CC→TT tandem double changes were recovered (Table 2, Figs 3 and 5). Only CC→TT tandem double changes occurred after UVC exposure in the rad30 strain. These data may be related to the following observation made in E.coli. The Dewar 5′TC3′ photoproduct led to enhanced mutagenicity and relaxed mutagenic specificity, comparatively to the 5′TC3′ (6–4) photoproduct, with a strong increase in various double substitutions (46). We propose that the various tandem double mutations induced by SSL in the rad30 strain are due to the mutagenic bypass of Dewar photoproducts. Thus, Polη may be involved in the non-mutagenic bypass of cytosine-containing Dewar photoproducts. In the absence of Polη, such lesions are bypassed by Polζ (or Polζ and an unidentified polymerase) with less specificity compared with the corresponding (6–4) photoproducts.
In the absence of Polζ, Polη contributes to mutagenesis at C-containing bipyrimidine photoproducts
The participation of Polη (presumably in co-operation with Polζ as shown in vitro) in the mutagenic bypass of TT(6–4) photoproducts in yeast was described recently (39,40). Here, we provide the first evidence that, in the absence of Polζ, Polη may contribute to UV-induced mutagenesis at cytosine-containing bipyrimidine sites. We found that mutagenesis by UVC was weak in the Polζ-deficient strain, while it was completely abolished when both Polη and Polζ were absent (Table 1). This observation is in favor of an involvement of Polη in UVC-induced mutagenesis when Polζ is inactivated. All of the SSL- and UVC-induced mutations at bipyrimidine sites in the Polζ-deficient strain occurred at CC and 5′TC3′/5′CT3′ sites, and most of them were C→T transitions (Table 4, Figs 4 and 5). These data indicate that Polη-dependent mutagenic bypass of C-containing photolesions occurred with an insertion of A opposite C in the lesion. It was shown that, in vitro, cytosines in photodimers are quite unstable and deaminate to uracil (47). However, the contribution of cytosine deamination to UV-induced mutagenesis in wild-type yeast is presumably low (10). We propose that in the absence of the major mutagenic Polζ-dependent pathway, Polη may contribute to SSL- and UVC-induced mutagenesis by replicating through deaminated photodimers with preferable insertion of an ‘A’ opposite U, which creates a mutation. Thus, the frequency of C→T transitions at CC and 5′TC3′/5′CT3′ sites in the rev3 strain may correlate with the rate of cytosine deamination in photodimers. Another possibility is that Polη-dependent mutagenesis is due to errors made by Polη during the bypass of cytosine-containing photodimers.
The contribution of bypass polymerases to ‘non-targeted mutagenesis’
The analysis of non-tandem double mutations leads to an interesting observation. These mutations were observed after SSL and UVC exposure in all strains except rev3 rad30 (Table 2). All non-tandem double mutations can be described as a one base change plus an additional base change or 1 nt deletion or insertion, separated by 2–8 bp (Fig. 3). We propose that such mutations are the result of a mutagenic bypass of a photolesion followed by low-fidelity DNA synthesis up to 8 nt downstream by Polη or Polζ. The absence of non-tandem double mutations in the rev3 rad30 strain is consistent with this proposal. Indeed, it was reported that, in vitro, Polη copies a DNA template with low fidelity, with a misinsertion rate of 10–2–10–3 and a single-base deletion or addition frequency of ∼10–3 (48–50). In contrast to Polη, Polζ is supposed to copy undamaged DNA more accurately, since its misinsertion frequency in vitro is 10–4–10–5, which is comparable with the fidelity of replicative polymerases lacking proofreading capability (12). On the other hand, Polζ is a powerful extender of the mismatches (12). Moreover, it was reported that Polζ was responsible for the occurrence of closely spaced (<10 nt) spontaneous multiple mutations, including +1 bp insertions, in yeast strains deficient in NER (51).
8-Oxoguanine possibly contributes to SSL-induced mutagenesis
We observed that SSL, in contrast to UVC, was mutagenic for the strain lacking both Polζ and Polη, and that 77% of the base changes in this strain were G·C→T·A transversions which did not occur preferentially at bipyrimidine sites. It is well known that G·C→T·A transversions are a ‘mutagenic signature’ of 8-oxoguanine (52), a major oxidative damage produced by UVA and SSL in DNA (28,29). It is also known that 8-oxoguanine is produced by SSL at a yield only 50 times lower than photodimers in mammalian cells and that it is not formed by UVC at biologically relevant doses (29). In a preliminary study, we observed that in an ogg1 yeast strain, defective in 8-oxoguanine-DNA-glycosylase, UVA-induced mutagenesis was highly enhanced, relative to that in the wild-type strain (data not shown). Thus, SSL-induced G·C→T·A transversions are presumably due to the presence of 8-oxoguanine.
It has been suggested that, in vitro, yeast Polη is capable of replicating past 8-oxoguanine, by inserting the correct C, and that such a bypass is more efficient and more accurate than that performed by the replicative polymerase Polδ (12,53). In one published study, a yeast ogg1 rad30 strain showed a synergistic increase in spontaneous mutations compared with the single mutant strains, implying that Polη is involved in the non-mutagenic bypass of 8-oxoguanine in vivo (12). We found that, in contrast to that observed in the rev3 rad30 double mutant, SSL was not effective in producing G·C→T·A transversions in the rev3 single mutant strain (Table 3). In agreement with the above observation, our data suggest an involvement of Polη in the non-mutagenic bypass of 8-oxoguanine in vivo. Accordingly, the enhancement of G·C→T·A transversions observed in the rev3 rad30 strain (in which the bypass of bipyrimidinic photoproducts is drastically reduced and Polη-performed replication past 8-oxoguanine is absent) may be totally due to the ambivalent replication of 8-oxoguanine carried out by replicative DNA polymerases.
CONCLUSIONS
Our results suggest the following roles for specialized DNA polymerases Polη and Polζ in sunlight-induced mutagenesis.
(i) Sunlight, like UVC, produces photodimers and (6–4) photoproducts. Polη is mainly responsible for the non-mutagenic bypass of photodimers and participates in the non-mutagenic bypass of cytosine-containing (6–4) photoproducts. Polη performs the mutagenic bypass of photodimers containing deaminated cytosine. Polζ is responsible for the mutagenic bypass of all types of (6–4) photoproducts. Both polymerases may introduce ‘non-targeted’ mutations when bypassing photolesions.
(ii) In contrast to UVC, sunlight converts a fraction of (6–4) photoproducts to Dewar isomers, and induces the formation of 8-oxoguanine. Polη participates in the non-mutagenic bypass of cytosine-containing Dewar photoproducts and 8-oxoguanine. Polζ is mainly responsible for the mutagenic bypass of all types of Dewar photoproducts.
Acknowledgments
ACKNOWLEDGEMENTS
We are grateful to Drs S. Boiteux, L. Gellon and M. de Padula (CEA/CNRS, Fontenay-aux-Roses, France), and D. Nguyen (NIEHS) for help in the can1 gene sequencing. We thank Drs Y. de Rycke and C. Elie (Institut Curie, Section Médicale) for their help in statistical analysis of results. We are thankful to Drs R. M. Schaaper, B. van Houten (NIEHS) and R. Devoret (Institut Curie) for carefully reading the manuscript. This work was supported by Centre National de la Recherche Scientifique (CNRS), Institut Curie and National Institutes of Health. S.K. was a recipient of fellowships from Institut Curie, Association pour la Recherche sur le Cancer and CNRS.
REFERENCES
- 1.Friedberg E.C., Walker,G.C. and Siede,W. (1995) DNA Repair and Mutagenesis. ASM Press, Washington, DC. [Google Scholar]
- 2.Ravanat J.L., Douki,T. and Cadet,J. (2001) Direct and indirect effects of UV radiation on DNA and its components. J. Photochem. Photobiol., 63, 88–102. [DOI] [PubMed] [Google Scholar]
- 3.Sage E. (1999) DNA damage and mutations induced by solar UV radiation. In Baumstark-Khan,C., Kozubek,S. and Horneck,G. (eds), Fundamentals for the Assessment of Risks from Environmental Radiation. Kluwer Academic Publishers, The Netherlands, pp. 115–126. [Google Scholar]
- 4.Brash D.E. (1997) Sunlight and the onset of skin cancer. Trends Genet., 13, 410–414. [DOI] [PubMed] [Google Scholar]
- 5.Stary A. and Sarasin,A. (2002) Molecular mechanisms of UV-induced mutations as revealed by the study of DNA polymerase η in human cells. Res. Microbiol., 153, 441–445. [DOI] [PubMed] [Google Scholar]
- 6.Masutani C., Kusumoto,R., Yamada,A., Dohmae,N., Yokoi,M., Yuasa,M., Araki,M., Iwai,S., Takio,K. and Hanaoka,F. (1999) The XPV (xeroderma pigmentosum variant) gene encodes human DNA polymerase η. Nature, 399, 700–704. [DOI] [PubMed] [Google Scholar]
- 7.Johnson R.E., Prakash,S. and Prakash,L. (1999) Efficient bypass of a thymine-thymine dimer by yeast DNA polymerase, Polη. Science, 283, 1001–1004. [DOI] [PubMed] [Google Scholar]
- 8.McDonald J.P., Levine,A.S. and Woodgate,R. (1997) The Saccharomyces cerevisiae RAD30 gene, a homologue of Escherichia coli dinB and umuC, is DNA damage inducible and functions in a novel error-free postreplication repair mechanism. Genetics, 147, 1557–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson R.E., Prakash,S. and Prakash,L. (1999) Requirement of DNA polymerase activity of yeast Rad30 protein for its biological function. J. Biol. Chem., 274, 15975–15977. [DOI] [PubMed] [Google Scholar]
- 10.Yu S.L., Johnson,R.E., Prakash,S. and Prakash,L. (2001) Requirement of DNA polymerase η for error-free bypass of UV-induced CC and TC photoproducts. Mol. Cell. Biol., 21, 185–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lawrence C.W. (2002) Cellular roles of DNA polymerase ζ and Rev1 protein. DNA Repair, 1, 425–435. [DOI] [PubMed] [Google Scholar]
- 12.Prakash S. and Prakash,L. (2002) Translesion DNA synthesis in eukaryotes: a one- or two-polymerase affair. Genes Dev., 16, 1872–1883. [DOI] [PubMed] [Google Scholar]
- 13.Morrison A., Christensen,R.B., Alley,J., Beck,A.K., Bernstine,E.G., Lemontt,J.F. and Lawrence,C.W. (1989) REV3, a Saccharomyces cerevisiae gene whose function is required for induced mutagenesis, is predicted to encode a nonessential DNA polymerase. J. Bacteriol., 171, 5659–5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lawrence C.W. and Maher,V.M. (2001) Eukaryotic mutagenesis and translesion replication dependent on DNA polymerase ζ and Rev1 protein. Biochem. Soc. Trans, 29, 187–191. [DOI] [PubMed] [Google Scholar]
- 15.Nelson J.R., Gibbs,P.E., Nowicka,A.M., Hinkle,D.C. and Lawrence,C.W. (2000) Evidence for a second function for Saccharomyces cerevisiae Rev1p. Mol. Microbiol., 37, 549–554. [DOI] [PubMed] [Google Scholar]
- 16.Nelson J.R., Lawrence,C.W. and Hinkle,D.C. (1996) Thymine-thymine dimer bypass by yeast DNA polymerase ζ. Science, 272, 1646–1649. [DOI] [PubMed] [Google Scholar]
- 17.Guo D., Wu,X., Rajpal,D.K., Taylor,J.S. and Wang,Z. (2001) Translesion synthesis by yeast DNA polymerase ζ from templates containing lesions of ultraviolet radiation and acetylaminofluorene. Nucleic Acids Res., 29, 2875–2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pavlov Y.I., Nguyen,D. and Kunkel,T.A. (2001) Mutator effects of overproducing DNA polymerase η (Rad30) and its catalytically inactive variant in yeast. Mutat. Res., 478, 129–139. [DOI] [PubMed] [Google Scholar]
- 19.Wach A., Brachat,A., Pohlmann,R. and Philippsen,P. (1994) New heterologous modules for classical or PCR-based gene disruptions in Saccharomyces cerevisiae. Yeast, 10, 1793–1808. [DOI] [PubMed] [Google Scholar]
- 20.Pavlov Y.I., Newlon,C.S. and Kunkel,T.A. (2002) Yeast origins establish a strand bias for replicational mutagenesis. Mol. Cell, 10, 207–213. [DOI] [PubMed] [Google Scholar]
- 21.Pavlov Y.I., Shcherbakova,P.V. and Kunkel,T.A. (2001) In vivo consequences of putative active site mutations in yeast DNA polymerases α, ε, δ and ζ. Genetics, 159, 47–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holbeck S.L. and Strathern,J.N. (1997) A role for REV3 in mutagenesis during double-strand break repair in Saccharomyces cerevisiae. Genetics, 147, 1017–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rose M.D., Winston,F. and Hieter,P. (1990) Methods in Yeast Genetics. A Laboratory Course Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 24.Drobetsky E.A., Moustacchi,E., Glickman,B.W. and Sage,E. (1994) The mutational specificity of simulated sunlight at the aprt locus in rodent cells. Carcinogenesis, 15, 1577–1583. [DOI] [PubMed] [Google Scholar]
- 25.Peak M.J., Peak,J.G. and Carnes,B.A. (1987) Induction of direct and indirect single-strand breaks in human cell DNA by far- and near-ultraviolet radiations: action spectrum and mechanisms. Photochem. Photobiol., 45, 381–387. [DOI] [PubMed] [Google Scholar]
- 26.Tyrrell R.M. and Keyse,S.M. (1990) New trends in photobiology. The interaction of UVA radiation with cultured cells. J. Photochem. Photobiol. B., 4, 349–361. [DOI] [PubMed] [Google Scholar]
- 27.Peak J.G. and Peak,M.J. (1991) Comparison of initial yields of DNA-to-protein crosslinks and single-strand breaks induced in cultured human cells by far- and near-ultraviolet light, blue light and X-rays. Mutat. Res., 246, 187–191. [DOI] [PubMed] [Google Scholar]
- 28.Kielbassa C., Roza,L. and Epe,B. (1997) Wavelength dependence of oxidative DNA damage induced by UV and visible light. Carcinogenesis, 18, 811–816. [DOI] [PubMed] [Google Scholar]
- 29.Douki T., Perdiz,D., Grof,P., Kuluncsics,Z., Moustacchi,E., Cadet,J. and Sage,E. (1999) Oxidation of guanine in cellular DNA by solar UV radiation: biological role. Photochem. Photobiol., 70, 184–190. [PubMed] [Google Scholar]
- 30.Armstrong J.D. and Kunz,B.A. (1990) Site and strand specificity of UVB mutagenesis in the _SUP4_-o gene of yeast. Proc. Natl Acad. Sci. USA, 87, 9005–9009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Armstrong J.D. and Kunz,B.A. (1992) Excision repair influences the site and strand specificity of sunlight mutagenesis in yeast. Mutat. Res., 274, 123–133. [DOI] [PubMed] [Google Scholar]
- 32.Mitchell D.L., Jen,J. and Cleaver,J.E. (1992) Sequence specificity of cyclobutane pyrimidine dimers in DNA treated with solar (ultraviolet B) radiation. Nucleic Acids Res., 20, 225–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sage E., Drobetsky,E.A., Brulay,E. and Moustacchi,E. (1995) Molecular analysis of mutations induced by simulated solar light at an endogenous locus in mammalian cells. Detection of lesions and genetic consequences. In Dubertret,L., Santus,R. and Morlière,P. (eds), Ozone, Sun, Cancer. Molecular and Cellular Mechanisms. Prevention. Les Editions INSERM, Paris, pp. 145–151. [Google Scholar]
- 34.Huang M.E., Rio,A.G., Galibert,M.D. and Galibert,F. (2002) Pol32, a subunit of Saccharomyces cerevisiae DNA Polymerase δ, suppresses genomic deletions and is involved in the mutagenic bypass pathway. Genetics, 160, 1409–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roche H., Gietz,R.D. and Kunz,B.A. (1995) Specificities of the Saccharomyces cerevisiae rad6, rad18 and rad52 mutators exhibit different degrees of dependence on the REV3 gene product, a putative nonessential DNA polymerase, Genetics, 140, 443–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perdiz D., Grof,P., Mezzina,M., Nikaido,O., Moustacchi,E. and Sage,E. (2000) Distribution and repair of bipyrimidine photoproducts in solar UV-irradiated mammalian cells. Possible role of Dewar photoproducts in solar mutagenesis. J. Biol. Chem., 275, 26732–26742. [DOI] [PubMed] [Google Scholar]
- 37.Yoon J.H., Lee,C.S., O’Connor,T.R., Yasui,A. and Pfeifer,G.P. (2000) The DNA damage spectrum produced by simulated sunlight. J. Mol. Biol., 299, 681–693. [DOI] [PubMed] [Google Scholar]
- 38.Svejstrup J.Q. (2002) Mechanisms of transcription-coupled DNA repair. Nature Rev. Mol. Cell. Biol., 3, 21–29. [DOI] [PubMed] [Google Scholar]
- 39.Zhang H. and Siede,W. (2002) UV-induced T→C transition at a TT photoproduct site is dependent on Saccharomyces cerevisiae polymerase η in vivo. Nucleic Acids Res., 30, 1262–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bresson A. and Fuchs,R.P. (2002) Lesion bypass in yeast cells: Polη participates in a multi-DNA polymerase process. EMBO J., 21, 3881–3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clingen P.H., Arlett,C.F., Roza,L., Mori,T., Nikaido,O. and Green,M.H. (1995) Induction of cyclobutane pyrimidine dimers, pyrimidine(6–4)pyrimidone photoproducts and Dewar valence isomers by natural sunlight in normal human mononuclear cells. Cancer Res., 55, 2245–2248. [PubMed] [Google Scholar]
- 42.Lawrence C.W., Gibbs,P.E., Borden,A., Horsfall,M.J. and Kilbey,B.J. (1993) Mutagenesis induced by single UV photoproducts in E. coli and yeast. Mutat. Res., 299, 157–163. [DOI] [PubMed] [Google Scholar]
- 43.Gibbs P.E., Borden,A. and Lawrence,C.W. (1995) The T-T pyrimidine (6–4) pyrimidinone UV photoproduct is much less mutagenic in yeast than in Escherichia coli. Nucleic Acids Res., 23, 1919–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Douki T., Reynaud-Angelin,A., Cadet,J. and Sage,E. (2003) Bypyrimidine photoproducts rather than oxidative lesions are the main type of DNA damage involved in the genotoxic effect of solar UVA radiation. Biochemistry, in press. [DOI] [PubMed] [Google Scholar]
- 45.LeClerc J.E., Borden,A. and Lawrence,C.W. (1991) The thymine-thymine pyrimidine-pyrimidone (6–4) ultraviolet light photoproduct is highly mutagenic and specifically induces 3′ thymine-to-cytosine transitions in Escherichia coli. Proc. Natl Acad. Sci. USA, 88, 9685–9689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Horsfall M.J. and Lawrence,C.W. (1994) Accuracy of replication past the T-C (6–4) adduct. J. Mol. Biol., 235, 465–471. [DOI] [PubMed] [Google Scholar]
- 47.Tessman I., Kennedy,M.A. and Liu,S.K. (1994) Unusual kinetics of uracil formation in single and double-stranded DNA by deamination of cytosine in cyclobutane pyrimidine dimers. J. Mol. Biol., 235, 807–812. [DOI] [PubMed] [Google Scholar]
- 48.Washington M.T., Johnson,R.E., Prakash,S. and Prakash,L. (1999) Fidelity and processivity of Saccharomyces cerevisiae DNA polymerase η. J. Biol. Chem., 274, 36835–36838. [DOI] [PubMed] [Google Scholar]
- 49.Matsuda T., Bebenek,K., Masutani,C., Hanaoka,F. and Kunkel,T.A. (2000) Low fidelity DNA synthesis by human DNA polymerase η. Nature, 404, 1011–1013. [DOI] [PubMed] [Google Scholar]
- 50.Matsuda T., Bebenek,K., Masutani,C., Rogozin,I.B., Hanaoka,F. and Kunkel,T.A. (2001) Error rate and specificity of human and murine DNA polymerase η. J. Mol. Biol., 312, 335–346. [DOI] [PubMed] [Google Scholar]
- 51.Harfe B.D. and Jinks-Robertson,S. (2000) DNA polymerase ζ introduces multiple mutations when bypassing spontaneous DNA damage in Saccharomyces cerevisiae. Mol. Cell, 6, 1491–1499. [DOI] [PubMed] [Google Scholar]
- 52.Boiteux S., Gellon,L. and Guibourt,N. (2002) Repair of 8-oxoguanine in Saccharomyces cerevisiae: interplay of DNA repair and replication mechanisms. Free Radic. Biol. Med., 32, 1244–1253. [DOI] [PubMed] [Google Scholar]
- 53.Einolf H.J. and Guengerich,F.P. (2001) Fidelity of nucleotide insertion at 8-oxo-7,8-dihydroguanine by mammalian DNA polymerase δ. Steady-state and pre-steady-state kinetic analysis. J. Biol. Chem., 276, 3764–3771. [DOI] [PubMed] [Google Scholar]