TRPV1 activation results in disruption of the blood–brain barrier in the rat (original) (raw)
Abstract
- We have examined the role of TRPV1 activation in disrupting the blood–brain barrier by measuring the permeability of single pial venular capillaries in anaesthetized rats.
- Capsaicin application to the brain surface resulted in increased permeability, maximal 2.1±0.12 × 10−6 cm s−1 (mean±s.e.m.) with log EC50 −4.5±0.10. Substance P methyl ester gave a similar response (maximal 2.0±0.07, _n_=6, log EC50 −4.8±0.07), but the selective NK2 agonist, _β_-Ala8-NKA4–10 peptide, had no effect. Although CGRP decreased the permeability of venules (log EC50 10.3±0.11), its receptor antagonist CGRP8–37 had no effect on the response to capsaicin.
- The TRPV1 antagonist capsazepine (1 mM) reduced the response to capsaicin (100 _μ_M), from 1.78±0.15 to 0.63±0.10 (_n_=4). The NK1 receptor antagonists GR205171 (100 _μ_M) and SDZ NKT 376 (1 mM) also reduced the response to capsaicin (from 1.75±0.14 to 0.46±0.08; _n_=6, and from 1.85±0.13 to 0.48±0.05; _n_=5, respectively), indicating that capsaicin acts via TRPV1 in series with NK1.
- Starch microspheres were used to produce transient focal ischaemia. Permeability was increased on reperfusion to a greater extent and more rapidly in vessels with diameter greater than 40 _μ_m than those less than 15 _μ_m. Capsazepine given intraperitoneally during ischaemia reduced the permeability increase in small venules from 5.9±0.3 to 2.4±0.1, and from 11.4±0.8 to 5.1±0.9 in large venules.
- In conclusion, the TRPV1 receptor is active in the brain microvasculature and has its permeability-increasing effect via substance P. It also plays a role in the immediate blood–brain barrier disruption following ischaemia–reperfusion.
Keywords: Blood–brain barrier, capillary permeability, capsaicin, capsazepine, cerebral ischaemia, NK1, TRPV1
Introduction
Cerebral oedema, which can be fatal, is a consequence of stroke and trauma through raised cerebrovascular permeability and consequent plasma protein leakage into the brain parenchyma. In the last few years, inflammatory processes have been suggested to play a significant role in this increased cerebrovascular permeability. For instance, it has been shown that the kallikrein–kinin system is activated following stroke (Wagner et al., 2002), and that inhibition of the bradykinin B2 receptor can ameliorate the consequences of experimental cerebral ischaemia when an antagonist is administered even as late as 6 h following its induction (Ding-Zhou et al., 2003). The tachykinin system also appears to play an important role: the infarct volume in experimental reversible focal cerebral ischaemia was significantly reduced when a neurokinin 1 (NK1) receptor antagonist was applied (Yu et al., 1997). Subsequent experiments showed that NK1 receptors are transiently induced on cerebral venular endothelial cells close to and within the infarct area (Stumm et al., 2001), and, furthermore, substance P containing nerve endings accumulated in regions close to cerebral venules 2 days following ischaemia.
There are a number of indications that the transient potential receptor vanilloid 1 (TRPV1, formerly known as the vanilloid, VR1, or capsaicin receptor) is also involved in disruption of the blood–brain barrier. Thus, immunoreactive TRPV1 is found widely in the brain on astrocytes and pericytes, which are closely associated with the vasculature, as well as neurones (Toth et al., 2005). Long-term pretreatment with capsaicin, resulting in TRPV1 downregulation, reduces post-traumatic neurological deficit and cerebral oedema (Nimmo et al., 2004). Bradykinin, which is rapidly released during ischaemia (Maier-Hauff et al., 1984), can activate TRPV1 when pH falls, as happens in ischaemia (Vyklicky et al., 1998; Cortright & Szallasi, 2004). The TRPV1 antagonist, capsazepine, would thus be expected to reduce the cerebrovascular permeability increase that follows experimental cerebral ischaemia.
The experiments described here were designed primarily to investigate whether TRPV1 activation acutely increases cerebrovascular permeability, and whether this occurs via substance P release. The possible endogenous activation of TRPV1 receptors following experimental ischaemia was also examined. These studies demonstrate that the functional activation of TRPV1 receptors results in raised cerebrovascular permeability, which may be inhibited by TRPV1 and NK1 antagonists, and that TRPV1 antagonism protects against blood–brain barrier disruption following experimental focal ischaemia.
Methods
Animal preparation
The experiments were performed on Wistar rats of either sex, aged between 23 and 33 days, weighing between 60 and 90 g, and were within the guidelines directed by The Animals (Scientific Procedures) Act 1986. The animals were anaesthetized by an intraperitoneal (i.p.) injection of 60 mg kg−1 body weight sodium pentobarbitone diluted in water (25% (w v−1)), and maintained by supplementary injection of 10% of the original dose when necessary. At the end of the experiment, the animal was killed by administering an overdose of the anaesthetic. Once anaesthetized, a thermal probe was inserted rectally and the animal was kept on a heating blanket connected via the probe to a feedback circuit (CFP 8105 Harvard Instruments) to maintain its body temperature at 37±1°C. The trachea and the right common carotid artery were cannulated routinely, the arterial cannula being placed orthogradely and filled with heparinized saline (100 U ml−1) to prevent clot formation. A section of the frontoparietal bones on the left side, between the coronal and lamboid sutures, was thinned with a dental drill.
Measurement of permeability
The method used in this study, and its theoretical basis, has been described fully (Fraser & Dallas, 1993; Easton & Fraser, 1994). Briefly, the microcirculation of the surface of the brain was exposed by removing the dura; the arachnoid comes away freely in these weanling animals who do have a fully developed blood–brain barrier (Butt et al., 1990). A metal ring (internal diameter 7 mm and outside diameter 13 mm) was glued with cyanoacrylate adhesive onto the cranium surrounding the thinned bone. The thinned cranial surface within the ring was constantly superfused with artificial cerebrospinal fluid (CSF) at the rate of 0.5–1 ml min−1. The fluid was warmed to 37±0.3°C, and delivered through a fine plastic tube to ensure that a layer of fluid was present at all times. The thinned bone within the ring was removed carefully avoiding damage to the meninges and cerebral surface. Pial microvessels were exposed by cutting away the overlying meninges. The rat was then placed on the modified stage of a microscope (ACM, Zeiss Oberkocken) and the exposed cerebral surface was illuminated with a 100 W xenon discharge lamp through a × 20 water-immersion objective lens (Cooke, N.A. 0.5). Sulphorhodamine B dye (580 Da) was introduced into single venular capillaries via a bolus injection into the carotid artery and viewed through a Zeiss ACM fluorescence microscope under 525–535 nm illumination. The fluorescent signal, which has been shown to be linear with the dye concentration, was captured via a microscope and an image-intensifier camera, and analysed through a video densitometer. Permeability was assessed by measuring the rate of loss of dye trapped in a single pial venular capillary by a glass-occluding probe. The fluorescence measurements were made from a small segment 200–300 _μ_m from the open end of the occluded vessel, but not so close to the occluding probe that the vessel diameter was distorted. Permeability was calculated from the rate of decrease in fluorescence under the measured portion of the occluded segment. The diameter of the vessels did not change during the occlusion (Easton & Fraser, 1994). As rate of fall of intravascular concentration of a small polar molecule is independent of the hydrostatic pressure (Fraser & Dallas, 1993), the concentration of the dye early in the occlusion, before axial volume flux distorts the uniform axial concentration of dye in the region of measurement, will be given by C_t_=C_0 × e_−kt, where _k=4_P/d, and P is the permeability and d the diameter of the vessel. We have previously shown that the repeated occlusions have no effect on the permeability measurements for over 2 h (Sarker et al., 2000).
Artificial CSF and drug application
The cerebral surface was superfused with an artificial CSF at the rate of 0.5–1 ml min−1, warmed to 37±1°C and buffered to pH 7.40, which formed a stable meniscus between the ring and the objective. The CSF contained (mM) NaCl, 110.5; KCl, 4.7; CaCl2, 2.5; KH2PO4, 1.1; MgSO4·7H2O, 1.25; NaHCO3, 25 and HEPES (_N_-[2-hydroxyethyl]piperazine-_N_′-[2-ethanesulphonic acid], 15. All chemicals were obtained from Sigma, Poole, Dorset, U.K., except MgSO4·7H2O, which was obtained from Fisons, Loughborough, Leicestershire, U.K. The superfusion was temporarily halted and the drugs were applied to the cerebral surface while measurements were carried out. The stable, specific NK1 agonist substance P methyl ester (SP-o-Me) was obtained from Cambridge Research Biochemicals, the neurokinin 2 (NK2) agonist [_β_-Ala8]-neurokinin A4–10 (_β_-Ala8-NKA4–10) and the TRPV1 agonist capsaicin from Sigma, and the human _α_-calcitonin gene-related peptide (CGRP) and the antagonist CGRP8–37 from Phoenix Pharmaceuticals, U.S.A. The NK1 antagonists SDZ NKT 343 (2nitrophenylcarbamoyl-(S)-prolyl-(S)-3-(2-naphthyl) alanyl-_N_-benzyl-_N_-methylamide), its enantiomer (R,R)-SDZ NKT 343 and GR205171 were supplied by Novartis Institute for Medical Sciences, London, U.K., as was capsazepine. SDZ NKT 343, capsaicin and capsazepine were dissolved in Tween-80 and 95% ethanol and diluted with saline; GR205171 was dissolved in saline. The doses are expressed as the final concentration in the pool, unless otherwise specified.
Permeability measurements were carried out on venular capillaries between 8–15 and 40–60 _μ_m diameter as follows. The selected dye-containing vessel was occluded, and once a stable level of fluorescence was obtained (after about 60 s), the drug, or mixture of drugs, was applied to the brain surface while the occlusion was maintained. The drug mixtures were washed off the brain surface within 60 s. The order of application of the drug mixtures was randomized in any series. All the experiments, unless otherwise stated, were completed within 2 h of the first measurement. Dose–response curves were constructed by applying all the different doses of the drug in pseudorandom order to a single vessel, and at least four vessels (each vessel was from a different rat) were used for each dose–response curve.
Transient ischaemia
The cerebral arterioles and downstream microcirculation were blocked by the injection of degradable starch microspheres (30 mg ml−1, 40 _μ_m diameter; Spherex, Pharmacia, Sweden). The vessels on the surface of the brain were viewed through the microscope during the injection, which was terminated when microspheres were seen in third and fourth branches of the middle cerebral artery. About 200,000 microspheres were needed for this level of blockage, which was designed to last about 30 min before plasma amylase digested the starch. Further microspheres were carefully administered if it appeared that the blockage would not last sufficiently long. Blood pressure was continuously monitored and no significant changes were observed throughout the experiments, not even when the microspheres were infused, with mean pressure ranging from 89 to 114 mmHg. The possibility that degradation products of the starch microspheres themselves contributed to the permeability increase was investigated by microinjecting plasma containing dissolved starch microspheres into the lumen of a venular capillary. No permeability change was observed.
Statistics
Unless otherwise stated, the results are expressed as the mean±s.e.m., and the significance of any changes was assessed by using either the ‘Student' _t_-test or analysis of variance and the appropriate post-test. Sigmoidal dose–response curves were fitted using GraphPad Prism version 4.03 for Windows, GraphPad Software, San Diego CA, U.S.A.
Results
Capsaicin increases permeability via TRPV1 and NK1 receptor activation
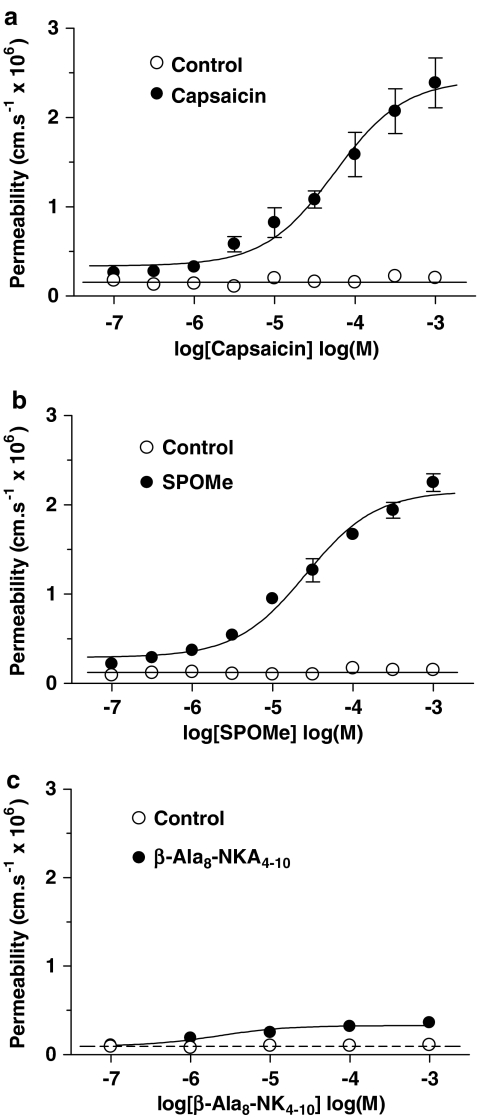
The resting permeability to sulphorhodamine dye of the majority of single venular capillaries used (see below for the exception) in the absence of agonist was 0.1±0.04 × 10−6 cm s−1 (mean±s.d.; 49 vessels: equivalent to a membrane specific resistance of 2400 Ω cm2) and this did not change significantly over the course of the experiments, which lasted about 2 h. Capsaicin application to the brain surface for 1 min resulted in a reversible permeability increase (Figure 1), which was dose dependent, with a maximal estimated permeability increase of 2.1±0.12 × 10−6 cm s−1 (mean±s.e.m.; _n_=13) and log EC50 4.5±0.10 (Figure 2a). The NK1 receptor agonist SP-o-Me (Figure 2b) gave a similar dose–effect relationship (maximal 2.0±0.07, _n_=6, log EC50 4.8±0.07), but the selective NK2 agonist _β_-Ala8-NKA4–10 peptide produced only a very small permeability increase (maximal 0.2±0.03, _n_=7).
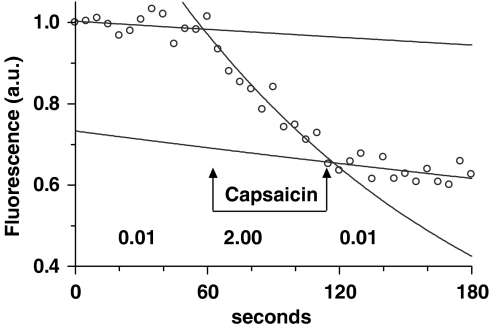
Figure 1.
An occlusion experiment. A selected pial venule was occluded during the passage of a sulphorhodamine dye bolus through the microvasculature, and the fluorescence measured at 5 s intervals. The rate constant of the decrease in fluorescence, obtained from the fitted exponential, was used to calculate the permeability (× 10−6 cm s−1; shown below the relevant sections of the record) before, during and after the application of agonist or agonist/antagonist mixtures.
Figure 2.
Effects of capsaicin, NK1 and NK2 agonists on pial venular permeability. The permeability effect of (a) the TRPV1 agonist capsaicin, (b) the NK1 agonist SP-o-Me and (c) the NK2 agonist _β_-Ala(8)-NK4–10 was plotted against concentration. The data were obtained from six to 13 venular capillaries, diameters between 8 and 15 _μ_m, each vessel from a separate animal, and displayed as mean±s.e.m.
CGRP is often released from the same nerves as substance P and has marked vasodilator effects via adenylate cyclase activation and cAMP formation (see Brain & Grant, 2004). Raised endothelial cAMP reduces permeability, which is not normally detectible in brain venules due to their very low permeability, but we have previously shown that raising cAMP will reduce permeability of vessels that had spontaneously become permeable (Easton et al., 1997). The dose–effect curve in the present experiments (Figure 3a) was obtained by applying CGRP to such vessels: the maximum effect was to reduce permeability from 2.3±0.20 to 0.1±0.1, (log EC50 10.3±0.11, _n_=8). The CGRP receptor antagonist, CGRP8–37, however, had no effect on the capsaicin dose–effect relationship (Figure 3b), which indicates that CGRP is not released when TRPV1 is activated by capsaicin on the brain surface.
Figure 3.
Effects of CGRP on pial venular permeability. (a) The permeability effect of CGRP on the permeability of venules that had spontaneously become slightly leaky was plotted against concentration. Four vessels from four rats. (b) The effect of the CGRP receptor antagonist CGRP8–37 on the capsaicin dose–effect curve; four to 13 vessels for each of the curves, each vessel from a different animal. All the vessel diameters were between 8 and 15 _μ_m.
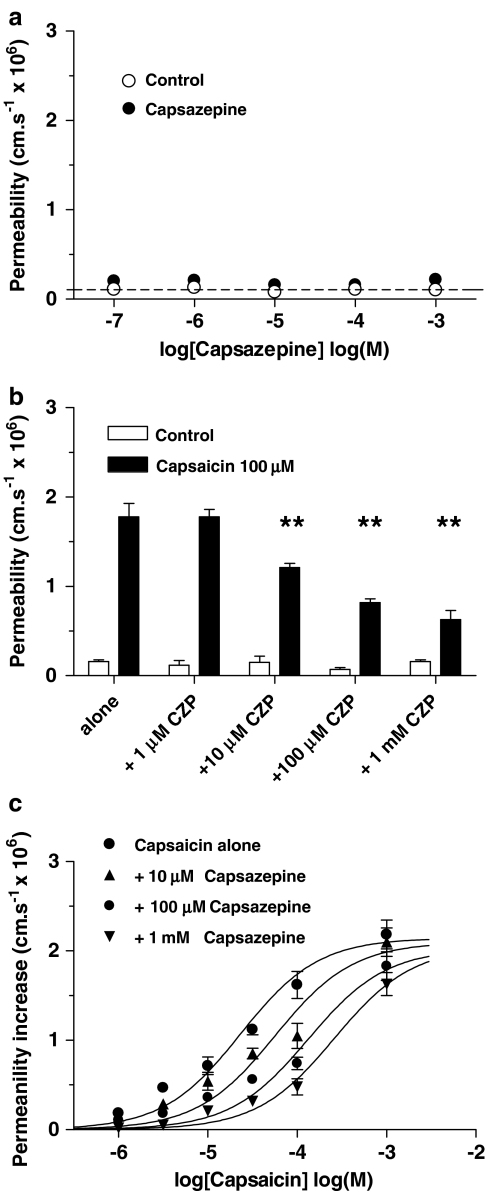
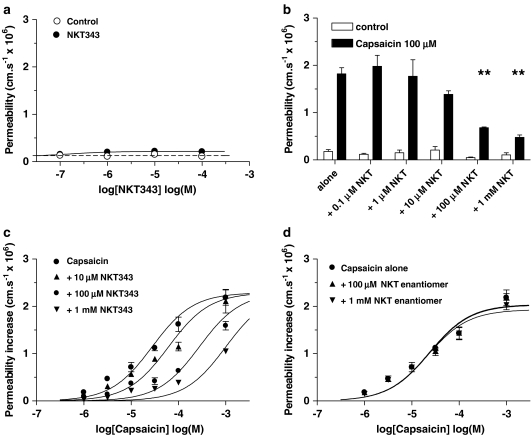
The concept that the capsaicin permeability-increasing effect required the TRPV1 receptor to be activated was confirmed by the experiments with capsazepine. Capsazepine itself had no permeability-increasing effect (Figure 4a). Coapplication of capsazepine (1 _μ_M–1 mM) and 100 _μ_M capsaicin produced a dose-dependent reduction in permeability with 1 mM capsazepine reducing the permeability from 1.78±0.15 to 0.63±0.10 (_n_=4; Figure 4b). Increasing capsazepine concentration also moved the capsaicin dose–response curve progressively to the right: log EC50 −4.6±0.05 for capsaicin alone to −4.3±0.07 with 10 _μ_M capsazepine, to −3.9±0.05 with 100 _μ_M and −3.6±0.05 with 1 mM, but with no significant change to the maximal permeability (Figure 4c; each curve based on four vessels; ANOVA on log EC50 showed a significant dose-dependency P<0.005, _F_=8.0, df 3,16).
Figure 4.
Effect of capsazepine on the capsaicin-induced permeability increase. (a) The effect of the TRPV1 antagonist capsazepine on venular permeability was plotted against concentration; four vessels. (b) The effects of 1–1000 _μ_M capsazepine (CZP) on the permeability-increasing effect of 100 _μ_M capsaicin; ANOVA with Dunnett's multiple comparison test; four vessels, **P<0.01. (c) Dose–effect curves produced by coapplication of capsazepine (1 _μ_M–1 mM) with capsaicin; each curve mean of four vessels from four rats: vessel diameters were between 8 and 15 _μ_m.
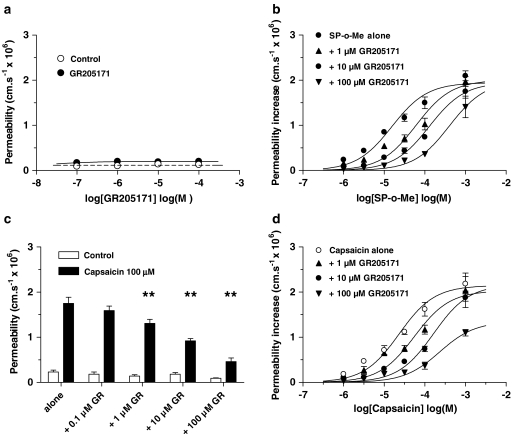
The NK1 antagonist, GR205171, had no effect on permeability itself (Figure 5a), but increasing concentrations of GR205171 displaced the dose–effect curve for SP-o-Me to the right: the log EC50 increased from −4.8±0.06 for SP-o-Me alone to −4.3±0.06 with 1 _μ_M GR205171, to −3.9±0.05 with 10 _μ_M and to −3.4±0.09 with 100 _μ_M, but with no significant change to the maximal permeability (Figure 5b; each curve based on four vessels; ANOVA on log EC50 showed a significant dose-dependency P<0.001, _F_=13.5, df 3,16). GR205171 also dose dependently reduced the permeability response to capsaicin (100 _μ_M) such that with GR205171 (100 _μ_M) permeability was reduced from 1.75±0.14 to 0.46±0.08; _n_=6 (Figure 5c), and progressively increased log EC50 from −4.6±0.05 for capsaicin alone to −4.1±0.08 with 1 _μ_M GR205171, to −3.7±0.04 with 10 _μ_M and to −3.0±0.05 with 100 _μ_M, but with no significant change to the maximal permeability (Figure 5d; each curve based on four vessels; ANOVA on log EC50 showed a significant dose dependency P<0.001, _F_=9.1, df 3,16). A similar pattern of capsaicin response inhibition was found with the structurally different NK1 receptor antagonist SDZ NKT 343. This also had no permeability-increasing effects (Figure 6a), dose dependently reduced the response to 100 _μ_M capsaicin, such that with SDZ NKT 376 (1 mM) permeability was reduced from 1.85±0.13 to 0.48±0.05; _n_=5 (Figure 6b), and progressively increased the log EC50 from −4.6±0.05 for capsaicin alone to −4.2±0.07 with 10 _μ_M SDZ NKT 343, to −3.6±0.06 with 100 _μ_M and to −3.0±0.05 with 1 mM, but with no significant change to the maximal permeability (Figure 6c; each curve based on four vessels; ANOVA on log EC50 showed a significant dose dependency P<0.05, _F_=4.5, df 3,16). The enantiomer, (R,R)-SDZ NKT 343, had no effect on the capsaicin permeability response (Figure 6d; each curve based on four vessels). Schildt plots (not shown) of the antagonist effects gave _K_B of −5.1, −6.0, −5.5 and −6.8 for capsaicin–NKT 343, GR205171, capsazepine and SP-o-Me–GR205171 interaction, respectively, but the gradients were all well below unity (from 0.44 to 0.79).
Figure 5.
The permeability response to capsaicin is inhibited by GR205171. (a) The effect of the NK1 antagonist GR205171 on venular permeability was plotted against concentration. (b) Dose–effect curves produced by coapplication of GR205171 (1–100 _μ_M) with SP-o-Me; each curve mean of four vessels from four rats. (c) The effects of GR205171 (0.1–100 _μ_M) on the permeability response to capsaicin (100 _μ_M); ANOVA with Dunnett's multiple comparison test four vessels, **P<0.01, six vessels from six rats. (d) Dose–effect curves produced by coapplication of GR205171 (1–100 _μ_M) with capsaicin; each curve mean of four vessels from four rats. All vessel diameters were between 8 and 15 _μ_m.
Figure 6.
The permeability response to capsaicin is inhibited by SDZ NKT 343. (a) The effect of the NK1 antagonist SDZ NKT 343 on venular permeability was plotted against concentration. (b) The effects of SDZ NKT 343 (0.1–100 _μ_M) on the permeability response to capsaicin (100 _μ_M); ANOVA with Dunnett's multiple comparison test; four vessels, **P<0.01, four vessels from four rats. (c) Dose–effect curves produced by coapplication of SDZ NKT 343 (10–1000 _μ_M) with capsaicin; four vessels from four rats. (d) Dose–effect curves produced by coapplication of the enantiomer (R,R)-SDZ NKT 343 (0.1 and 1 mM) with capsaicin; each curve mean of four vessels from four rats. All vessel diameters were between 8 and 15 _μ_m.
Effect of capsazepine on experimental cerebral ischaemia
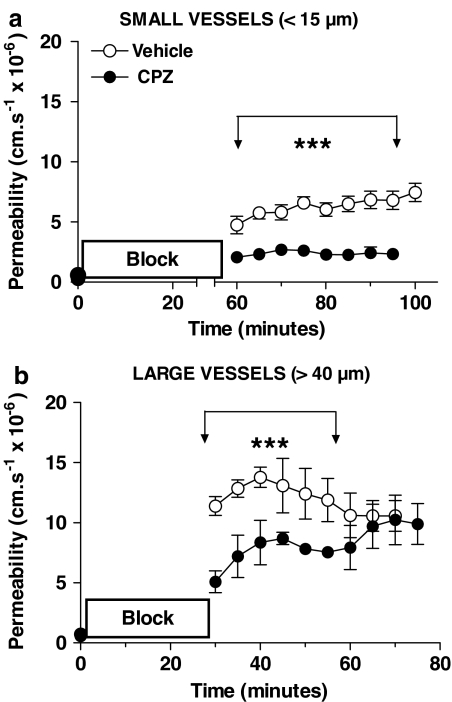
Capsazepine was administered i.p. (at 1 _μ_mol kg−1) 20 min after the microspheres were administered. The starch microsphere embolism method of producing cerebral ischaemia and reperfusion was chosen as it produced a total cessation of flow in the pial vessels, and the start of reperfusion was easily observed in the pial vessels. Permeabilities were measured immediately before and at 5 min intervals for at least 30 min after reperfusion started in similar-sized (diameters between 8 and 15 _μ_m) venules as used for the acute study. Preliminary experiments showed that a blockage that lasted 30 min produced a small permeability increase only (ca. 2 × 10−6 cm s−1), and accordingly the blockage time was increased to 60 min. The mean permeability over the 35 min reperfusion following that was 5.9±0.3 × 10−6 cm s−1, _n_=8, which capsazepine treatment reduced to 2.4±0.1, _n_=4, (Figure 7a). The effect of the treatment did not diminish during the 35 min that it was followed.
Figure 7.
The effect of capsazepine on blood–brain barrier disruption following experimental cerebral ischaemia–reperfusion studied in single pial venules. The permeability to sulforhodamine dye was measured immediately before, and at 5 min intervals following starch microsphere embolization. Vehicle or capsazepine (1 _μ_mol kg−1) was injected i.v. 20 min after the embolization started. (a) In the small venular capillaries capsazepine resulted in a significantly smaller permeability increase after microsphere embolism that lasted 60 min, which showed no sign of diminishing with increased time of reperfusion (***P<0.0001 unpaired _t_-test with Welch's correction for unequal variances, eight vessels for vehicle, four vessels capsazepine, one vessel from each animal). (b) The larger venules gave a much larger permeability response, even for a shorter duration of embolism, and this too was significantly attenuated by capsazepine treatment, but the differences in the permeabilities of the two groups diminished as reperfusion progressed, until 30 min after reperfusion when there was no difference between the treated and untreated vessels (***P<0.0001 unpaired _t_-test, eight vessels for vehicle, five vessels capsazepine, one vessel from each animal).
Parallel experiments in the laboratory indicated that larger venules are more reactive to ischaemia than the smaller ones. We examined the permeability responses of these vessels (diameters between 40 and 60 _μ_m), which although having the same very low permeability before the microsphere embolism had a much greater permeability on reperfusion after 30 min blockage (11.39±0.78, _n_=8). This rose and then fell slightly in the following 30 min, the mean being 12.6±0.4 (_n_=8; see Figure 7). With capsazepine treatment, the initial postblockage permeability was less than half that of the untreated vessels (5.10±0.91, _n_=5), but this reduction lessened with time, so that after 30 min reperfusion, there was no difference between the treated and untreated vessels.
Discussion
The experiments described here demonstrate that capsaicin applied to the brain surface increases the permeability of the pial microvasculature, and that an NK1 agonist, which is likely to be substance P, is a key mediator of the response. Furthermore, we have shown that activation of TRPV1 is likely to be responsible for a component of blood–brain barrier disruption in the early phase of the response to cerebral ischaemia.
The possibility that TRPV1 has a role in cerebral oedema formation consequent to ischaemia was investigated by using starch microspheres to produce transient focal occlusion of blood flow in cerebral microvessels. They provided a relatively easy and effective means of generating severe cerebral ischaemia that resulted in increased permeability in the microvessels being measured. The pial vessels were exposed to atmospheric oxygen throughout, so the permeability increase was not due to direct effects of hypoxia, nor could it be due to direct contact with the microspheres because they lodge on the arterial side of the circulation only. The permeability increase is therefore most probably due to release of a substance, or substances, from ischaemic tissue, which diffused to the pial vessels that were measured. Degradable starch microspheres have been used in chemotherapeutic drug administration for hepatic cancer (see Hakansson et al., 1997), but have not been widely used as emboli to produce reversible experimental ischaemia. Where they have been used previously to occlude the rat cerebral vasculature, it was found that the irreversible damage was related to the number of microspheres injected (Laccourreye et al., 1993). An electron microscopy study of starch microsphere interaction with rat liver and kidney microvessels showed that they changed shape and occupied almost the whole of the lumen before being digested by plasma amylase (Makita et al., 1989). We found that blocking TRPV1 with capsazepine during experimental focal cerebral ischaemia–reperfusion reduced the blood–brain barrier disruption considerably.
Other observations in our laboratory had led us to consider the possibility that larger venules were more reactive than smaller ones. This was borne out in the event as their permeability response was larger, and more rapid, which is also in accord with the cardiac arrest of model transient ischaemia (Pluta et al., 1994). Permeability of both the large and the small venules was greatly reduced by the capsazepine treatment, but surprisingly these protective effects were only transient in the larger venules, lasting for less than 30 min of reperfusion.
Capsazepine was apparently much more effective in reducing permeability postischaemia than against a direct capsaicin challenge. One possible reason for this is the longer period for equilibration in the postischaemia experiments than with acute application to the pia (see below), but it is also possible that the responsiveness of the ischaemic preparation is likely to be considerably enhanced over that in the normal situation. We have previously found that interleukin-1_β_, which is released in ischaemia, will rapidly enhance the permeability response to bradykinin (Hu & Fraser, 1997) and preliminary evidence in the laboratory indicates that this also occurs with substance P.
There have been a number of other indications that TRPV1 activation will result in brain injury and cerebral oedema, and the endocannabinoid, anandamide, which increases following cerebral ischaemia (Berger et al., 2004), could be the endogenous ligand for this effect. It is interesting that anandamide administered into the cerebral ventricles results in cerebral oedema, which was greatly reduced by coadministration with capsazepine (Cernak et al., 2004). The recent finding that capsaicin treatment administered shortly before transient global cerebral ischaemia, protected neurones, apparently conflicts with the idea that TRPV1 is a key player in stroke (Pegorini et al., 2005), but it is possible that in that study capsaicin was acting by desensitizing TRPV1.
The processes that lead from ischaemia to blood–brain barrier disruption appear to involve a number of different inflammatory mediators. We have found that both blocking the bradykinin B2 receptor with topically applied HOE 140, and free radical scavengers, reduce postischaemic pial venular permeability dramatically (Kurokawa & Fraser, 1995), but this is the first time that a systemic treatment has had such an effect in this model of severe transient ischaemia. It is probable that substance P, and other inflammatory mediators, such as VEGF and interleukin-1_β_, act synergistically, and blocking any one of them results in a large decrease in the resultant effect.
Previous experiments with capsaicin did not show any effects on the blood–brain barrier permeability. Thus, systemically administered capsaicin (intravenously (i.v.) at 5 _μ_mol kg−1 over 20 min) did not increase the uptake of _α_-aminoisobutyric acid (Reid & McCulloch, 1987). A much higher concentration of capsaicin was used in the present experiments, and topically applied capsaicin gave a barely discernible response at 5 _μ_M (EC50≈50 _μ_M). Capsaicin behaves as a competitive uptake inhibitor of substrates into a cancer cell line (Nabekura et al., 2005), so it is also possible that it is a substrate for p-glycoprotein, which is expressed at the blood–brain barrier (Bauer et al., 2005), thus preventing the uptake of i.v. capsaicin and its access to TRPV1.
The brain TRPV1 receptor has a higher affinity for capsaicin than that of the periphery (Szabo et al., 2002), so it is a little surprising that we needed such high doses. On the other hand, the antiretching effect of capsaicin applied to the fourth ventricle required a dose of 330 μ_M to have a significant effect (Shiroshita et al., 1997). It is possible that capsaicin is rapidly metabolized to inactive compounds in the brain, as is its derivative DA-5018 (Kang & Kim, 1999), but it is also possible that the TRPV1 receptor is not located directly on the pial surface. The permeability measurements in the present experiments were made within 1 min of drug application to avoid complications that can arise from the rapid release of other substances, such as occurs with prolonged bradykinin application and interleukin-1_β release (Hu & Fraser, 1997), and this did not allow sufficient time for equilibration between drug and receptor to give a true estimate of pD2. TRPV1 has been found on cultured brain endothelium (Golech et al., 2004), and its activation leads to a rise in endothelial [Ca2+], a sufficient condition for a rapid permeability response in this preparation (Sarker et al., 1998). The present experiments, however, show that a tachykinin NK1 agonist, but not an NK2 agonist (Nicolau et al., 1993), is a necessary intermediate between capsaicin and the pial venular permeability response; hence, it appears that TRPV1 cannot be active on pial venular endothelium.
Substance P methyl ester was used to stimulate the NK1 receptor, rather than substance P itself, as it is less prone to rapid degradation, but it is nevertheless a substrate for neutral endopeptidase (Skidgel et al., 1984), which is expressed on the brain surface (Norman et al., 2003). This could also contribute to the high EC50 (≈25 _μ_M) for the SP-o-Me permeability-increasing effect: we have previously shown that the EC50 for bradykinin was reduced by three orders of magnitude when ACE and endopeptidase inhibitors were coapplied (Sarker et al., 2000). On the other hand, the pial venules were exquisitely sensitive to the permeability reducing effect of CGRP, with an EC50≈50 pM, similar to vasodilatation of cerebral arterioles at 28 pM (Mori et al., 1997). Interestingly, the principal enzyme responsible for CGRP degradation, endopeptidase 24.11 (Mentlein & Roos, 1996), has little activity in the rat meninges (Zajac et al., 1987). The failure of the CGRP receptor antagonist CGRP8–37 to alter the capsaicin dose–response curve, and the failure of the neurokinin A agonist to increase permeability, indicates that TRPV1 activation results in substance P release. This is supported by the experiments in which NK1 antagonists were coapplied with capsaicin. Higher doses of SDZ NKT 343 than of GR205171 were required for a similar permeability reduction, but this accords with its relative lack of activity on the rat NK1 receptor (Campbell et al., 2000). It appears that TRPV1 activation results in substance P release alone, which is a little different from the recent studies in the trigeminal sensory nucleus where it was found that in only 2% of neurones did TRPV1 colocalize with substance P alone, but it was 44% with CGRP (Bae et al., 2004). Presumably the situation is different on the pial surface as it appears that capsaicin has its permeability-increasing effects via activating neuronal TRPV1 that results in substance P release.
In conclusion, we have shown that the TRPV1 receptor is active in the brain microvasculature and has its permeability-increasing effect via an NK1 agonist, which is likely to be substance P. It also appears that this receptor plays a role in the immediate, if transient, blood–brain barrier disruption following ischaemia–reperfusion.
Acknowledgments
This work was supported by Novartis Institute for Medical Science. A.S.E. was an MRC–Pfizer collaborative student.
Abbreviations
_β_-Ala8-NKA4–10
[_β_-Ala8]-neurokinin A4–10
CGRP
_α_-calcitonin gene-related peptide
GR20171
(2_S_-cis)-_N_-{[2-methoxy-5-(5-(trifluoromethyl)-1-_H_-tetrazol-1-yl)-phenyl]methyl}-2-phenyl-3-piperidinamine dihydrochloride
NK1
neurokinin 1
NK2
neurokinin 2
SDZ NKT 343
2nitrophenylcarbamoyl-(S)-prolyl-(S)-3-(2-naphthyl) alanyl-_N_-benzyl-_N_-methylamide
SP-o-Me
substance P methyl ester
TRPV1
transient potential receptor vanilloid 1
References
- BAE Y.C., OH J.M., HWANG S.J., SHIGENAGA Y., VALTSCHANOFF J.G. Expression of vanilloid receptor TRPV1 in the rat trigeminal sensory nuclei. J. Comp. Neurol. 2004;478:62–71. doi: 10.1002/cne.20272. [DOI] [PubMed] [Google Scholar]
- BAUER B., HARTZ A.M., FRICKER G., MILLER D.S. Modulation of p-glycoprotein transport function at the blood–brain barrier. Exp. Biol. Med. (Maywood) 2005;230:118–127. doi: 10.1177/153537020523000206. [DOI] [PubMed] [Google Scholar]
- BERGER C., SCHMID P.C., SCHABITZ W.R., WOLF M., SCHWAB S., SCHMID H.H. Massive accumulation of N-acylethanolamines after stroke. Cell signalling in acute cerebral ischemia. J. Neurochem. 2004;88:1159–1167. doi: 10.1046/j.1471-4159.2003.02244.x. [DOI] [PubMed] [Google Scholar]
- BRAIN S.D., GRANT A.D. Vascular actions of calcitonin gene-related peptide and adrenomedullin. Physiol. Rev. 2004;84:903–934. doi: 10.1152/physrev.00037.2003. [DOI] [PubMed] [Google Scholar]
- BUTT A.M., JONES H.C., ABBOTT N.J. Electrical resistance across the blood–brain barrier in anaesthetized rats: a developmental study. J. Physiol. 1990;429:47–62. doi: 10.1113/jphysiol.1990.sp018243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMPBELL E.A., GENTRY C., PATEL S., KIDD B., CRUWYS S., FOX A.J., URBAN L. Oral anti-hyperalgesic and anti-inflammatory activity of NK(1) receptor antagonists in models of inflammatory hyperalgesia of the guinea-pig. Pain. 2000;87:253–263. doi: 10.1016/S0304-3959(00)00288-8. [DOI] [PubMed] [Google Scholar]
- CERNAK I., VINK R., NATALE J., STOICA B., LEA P.M., MOVSESYAN V., AHMED F., KNOBLACH S.M., FRICKE S.T., FADEN A.I. The ‘dark side' of endocannabinoids: a neurotoxic role for anandamide. J. Cereb. Blood Flow Metab. 2004;24:564–578. doi: 10.1097/00004647-200405000-00011. [DOI] [PubMed] [Google Scholar]
- CORTRIGHT D.N., SZALLASI A. Biochemical pharmacology of the vanilloid receptor TRPV1. An update. Eur. J. Biochem. 2004;271:1814–1819. doi: 10.1111/j.1432-1033.2004.04082.x. [DOI] [PubMed] [Google Scholar]
- DING-ZHOU L., MARGAILL I., PALMIER B., PRUNEAU D., PLOTKINE M., MARCHAND-VERRECCHIA C. LF 16-0687 Ms, a bradykinin B2 receptor antagonist, reduces ischemic brain injury in a murine model of transient focal cerebral ischemia. Br. J. Pharmacol. 2003;139:1539–1547. doi: 10.1038/sj.bjp.0705385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EASTON A.S., FRASER P.A. Variable restriction of albumin diffusion across inflamed cerebral microvessels of the anaesthetized rat. J. Physiol. 1994;475:147–157. doi: 10.1113/jphysiol.1994.sp020056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EASTON A.S., SARKER M.H., FRASER P.A. Two components of blood–brain barrier disruption in the rat. J. Physiol. 1997;503:613–623. doi: 10.1111/j.1469-7793.1997.613bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRASER P.A., DALLAS A.D. Permeability of disrupted cerebral microvessels in the frog. J. Physiol. 1993;461:619–632. doi: 10.1113/jphysiol.1993.sp019532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLECH S.A., MCCARRON R.M., CHEN Y., BEMBRY J., LENZ F., MECHOULAM R., SHOHAMI E., SPATZ M. Human brain endothelium: coexpression and function of vanilloid and endocannabinoid receptors. Brain Res. Mol. Brain Res. 2004;132:87–92. doi: 10.1016/j.molbrainres.2004.08.025. [DOI] [PubMed] [Google Scholar]
- HAKANSSON L., HAKANSSON A., MORALES O., THORELIUS L., WARFVING T. Spherex (degradable starch microspheres) chemo-occlusion – enhancement of tumor drug concentration and therapeutic efficacy: an overview. Semin. Oncol. 1997;24:S6. [PubMed] [Google Scholar]
- HU D.-E., FRASER P.A. Evidence for interleukin-1β mediating enhanced permeability responses to bradykinin in single pial venular capillaries of anaesthetized rats. J. Physiol. 1997;505:53P. [Google Scholar]
- KANG Y.S., KIM J.M. Permeability of a capsaicin derivative, [14C]DA-5018 to blood–brain barrier corrected with HPLC method. Arch. Pharm. Res. 1999;22:165–172. doi: 10.1007/BF02976541. [DOI] [PubMed] [Google Scholar]
- KUROKAWA T., FRASER P.A. A bradykinin antagonist prevents cerebral microvascular permeability increase following reperfusion in rats. J. Physiol. 1995;483:140P. [Google Scholar]
- LACCOURREYE O., LAURENT A., POLIVKA M., WASSEF M., DOMAS L., BRASNU D., MERLAND J.J. Biodegradable starch microspheres for cerebral arterial embolization. Invest. Radiol. 1993;28:150–154. doi: 10.1097/00004424-199302000-00014. [DOI] [PubMed] [Google Scholar]
- MAIER-HAUFF K., BAETHMANN A.J., LANGE M., SCHURER L., UNTERBERG A. The kallikrein–kinin system as mediator in vasogenic brain edema. Part 2: studies on kinin formation in focal and perifocal brain tissue. J. Neurosurg. 1984;61:97–106. doi: 10.3171/jns.1984.61.1.0097. [DOI] [PubMed] [Google Scholar]
- MAKITA T., ICHIMAL H., KAGABU S., MANBA K., NAITO I., LINDBLOM R., HATSUOKA M., OKAWA T. Scanning and transmission electron microscopy of biodegradable microspheres (DSM) in blood vessels. Cell Biol. Int. Rep. 1989;13:427–436. doi: 10.1016/0309-1651(89)90137-9. [DOI] [PubMed] [Google Scholar]
- MENTLEIN R., ROOS T. Proteases involved in the metabolism of angiotensin II, bradykinin, calcitonin gene-related peptide (CGRP), and neuropeptide Y by vascular smooth muscle cells. Peptides. 1996;17:709–720. doi: 10.1016/0196-9781(96)00066-6. [DOI] [PubMed] [Google Scholar]
- MORI Y., TAKAYASU M., SUZUKI Y., SHIBUYA M., YOSHIDA J., HIDAKA H. Effects of adrenomedullin on rat cerebral arterioles. Eur. J. Pharmacol. 1997;330:195–198. doi: 10.1016/s0014-2999(97)01028-5. [DOI] [PubMed] [Google Scholar]
- NABEKURA T., KAMIYAMA S., KITAGAWA S. Effects of dietary chemopreventive phytochemicals on P-glycoprotein function. Biochem. Biophys. Res. Commun. 2005;327:866–870. doi: 10.1016/j.bbrc.2004.12.081. [DOI] [PubMed] [Google Scholar]
- NICOLAU M., SIROIS M.G., BUI M., PLANTE G.E., SIROIS P., REGOLI D. Plasma extravasation induced by neurokinins in conscious rats: receptor characterization with agonists and antagonists. Can. J. Physiol. Pharmacol. 1993;71:217–221. doi: 10.1139/y93-034. [DOI] [PubMed] [Google Scholar]
- NIMMO A.J., CERNAK I., HEATH D.L., HU X., BENNETT C.J., VINK R. Neurogenic inflammation is associated with development of edema and functional deficits following traumatic brain injury in rats. Neuropeptides. 2004;38:40–47. doi: 10.1016/j.npep.2003.12.003. [DOI] [PubMed] [Google Scholar]
- NORMAN M.U., REEVE S.B., DIVE V., SMITH A.I., LEW R.A. Endopeptidases 3.4.24.15 and 24.16 in endothelial cells: potential role in vasoactive peptide metabolism. Am. J. Physiol. Heart Circ. Physiol. 2003;284:H1978–H1984. doi: 10.1152/ajpheart.01116.2002. [DOI] [PubMed] [Google Scholar]
- PEGORINI S., BRAIDA D., VERZONI C., GUERINI-ROCCO C., CONSALEZ G.G., CROCI L., SALA M. Capsaicin exhibits neuroprotective effects in a model of transient global cerebral ischemia in Mongolian gerbils. Br. J. Pharmacol. 2005;144:727–735. doi: 10.1038/sj.bjp.0706115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PLUTA R., LOSSINSKY A.S., WISNIEWSKI H.M., MOSSAKOWSKI M.J. Early blood–brain barrier changes in the rat following transient complete cerebral ischemia induced by cardiac arrest. Brain Res. 1994;633:41–52. doi: 10.1016/0006-8993(94)91520-2. [DOI] [PubMed] [Google Scholar]
- REID J., MCCULLOCH J. Capsaicin and blood–brain barrier permeability. Neurosci. Lett. 1987;81:165–170. doi: 10.1016/0304-3940(87)90359-4. [DOI] [PubMed] [Google Scholar]
- SARKER M.H., EASTON A.S., FRASER P.A. Regulation of cerebral microvascular permeability by histamine in the anaesthetized rat. J. Physiol. 1998;507:909–918. doi: 10.1111/j.1469-7793.1998.909bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SARKER M.H., HU D.E., FRASER P.A. Acute effects of bradykinin on cerebral microvascular permeability in the anaesthetized rat. J. Physiol. 2000;528:177–187. doi: 10.1111/j.1469-7793.2000.00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHIROSHITA Y., KOGA T., FUKUDA H. Capsaicin in the 4th ventricle abolishes retching and transmission of emetic vagal afferents to solitary nucleus neurons. Eur. J. Pharmacol. 1997;339:183–192. doi: 10.1016/s0014-2999(97)01370-8. [DOI] [PubMed] [Google Scholar]
- SKIDGEL R.A., ENGELBRECHT S., JOHNSON A.R., ERDOS E.G. Hydrolysis of substance p and neurotensin by converting enzyme and neutral endopeptidase. Peptides. 1984;5:769–776. doi: 10.1016/0196-9781(84)90020-2. [DOI] [PubMed] [Google Scholar]
- STUMM R., CULMSEE C., SCHAFER M.K., KRIEGLSTEIN J., WEIHE E. Adaptive plasticity in tachykinin and tachykinin receptor expression after focal cerebral ischemia is differentially linked to gabaergic and glutamatergic cerebrocortical circuits and cerebrovenular endothelium. J. Neurosci. 2001;21:798–811. doi: 10.1523/JNEUROSCI.21-03-00798.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SZABO T., BIRO T., GONZALEZ A.F., PALKOVITS M., BLUMBERG P.M. Pharmacological characterization of vanilloid receptor located in the brain. Brain Res. Mol. Brain Res. 2002;98:51–57. doi: 10.1016/s0169-328x(01)00313-8. [DOI] [PubMed] [Google Scholar]
- TOTH A., BOCZAN J., KEDEI N., LIZANECZ E., BAGI Z., PAPP Z., EDES I., CSIBA L., BLUMBERG P.M. Expression and distribution of vanilloid receptor 1 (TRPV1) in the adult rat brain. Brain Res. Mol. Brain Res. 2005;135:162–168. doi: 10.1016/j.molbrainres.2004.12.003. [DOI] [PubMed] [Google Scholar]
- VYKLICKY L., KNOTKOVA-URBANCOVA H., VITASKOVA Z., VLACHOVA V., KRESS M., REEH P.W. Inflammatory mediators at acidic pH activate capsaicin receptors in cultured sensory neurons from newborn rats. J. Neurophysiol. 1998;79:670–676. doi: 10.1152/jn.1998.79.2.670. [DOI] [PubMed] [Google Scholar]
- WAGNER S., KALB P., LUKOSAVA M., HILGENFELDT U., SCHWANINGER M. Activation of the tissue kallikrein–kinin system in stroke. J. Neurol. Sci. 2002;202:75–76. doi: 10.1016/s0022-510x(02)00208-3. [DOI] [PubMed] [Google Scholar]
- YU Z., CHENG G., HUANG X., LI K., CAO X. Neurokinin-1 receptor antagonist SR140333: a novel type of drug to treat cerebral ischemia. Neuroreport. 1997;8:2117–2119. doi: 10.1097/00001756-199707070-00006. [DOI] [PubMed] [Google Scholar]
- ZAJAC J.M., CHARNAY Y., SOLEILHIC J.M., SALES N., ROQUES B.P. Enkephalin-degrading enzymes and angiotensin-converting enzyme in human and rat meninges. FEBS Lett. 1987;216:118–122. doi: 10.1016/0014-5793(87)80768-8. [DOI] [PubMed] [Google Scholar]