Leflunomide: mode of action in the treatment of rheumatoid arthritis (original) (raw)
Abstract
Leflunomide is a selective inhibitor of de novo pyrimidine synthesis. In phase II and III clinical trials of active rheumatoid arthritis, leflunomide was shown to improve primary and secondary outcome measures with a satisfactory safety profile. The active metabolite of leflunomide, A77 1726, at low, therapeutically applicable doses, reversibly inhibits dihydroorotate dehydrogenase (DHODH), the rate limiting step in the de novo synthesis of pyrimidines. Unlike other cells, activated lymphocytes expand their pyrimidine pool by approximately eightfold during proliferation; purine pools are increased only twofold. To meet this demand, lymphocytes must use both salvage and de novo synthesis pathways. Thus the inhibition of DHODH by A77 1726 prevents lymphocytes from accumulating sufficient pyrimidines to support DNA synthesis. At higher doses, A77 1726 inhibits tyrosine kinases responsible for early T cell and B cell signalling in the G0/G1 phase of the cell cycle. Because the immunoregulatory effects of A77 1726 occur at doses that inhibit DHODH but not tyrosine kinases, the interruption of de novo pyrimidine synthesis may be the primary mode of action. Recent evidence suggests that the observed anti-inflammatory effects of A77 1726 may relate to its ability to suppress interleukin 1 and tumour necrosis factor α selectively over their inhibitors in T lymphocyte/monocyte contact activation. A77 1726 has also been shown to suppress the activation of nuclear factor κB, a potent mediator of inflammation when stimulated by inflammatory agents. Continuing research indicates that A77 1726 may downregulate the glycosylation of adhesion molecules, effectively reducing cell-cell contact activation during inflammation.
Full Text
The Full Text of this article is available as a PDF (174.9 KB).
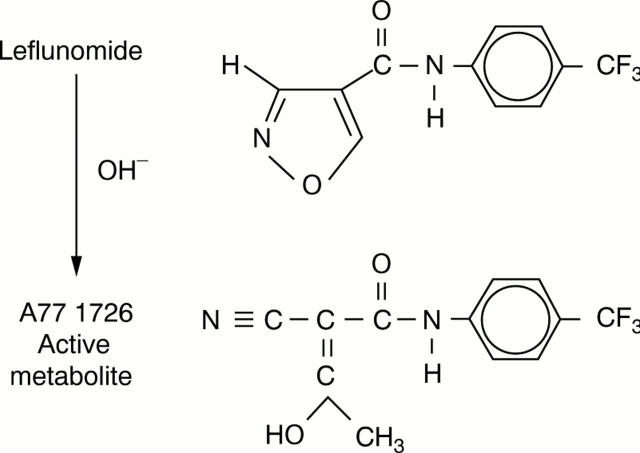
Figure 1 .
Chemical structure of leflunomide and its active metabolite A77 1726.
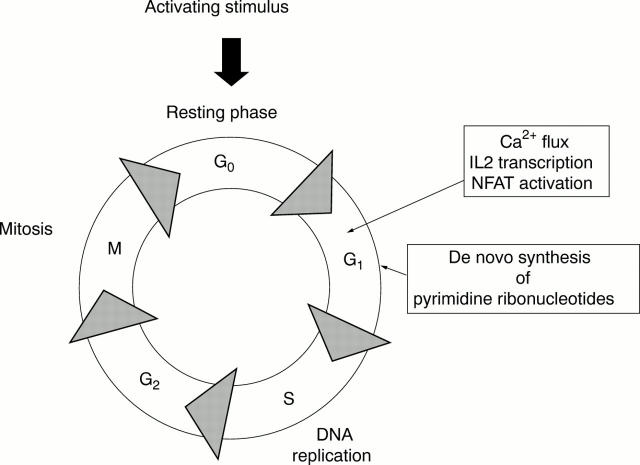
Figure 2 .
Cell cycle modulation. Some agents block signal transduction events in the resting G0 phase. Other agents interfere with ribonucleotide biosynthesis in the G1 phase. In either case, transition into the DNA replication phase, or S phase, of the cell cycle is blocked. NFAT = nuclear factor of activated T cells.
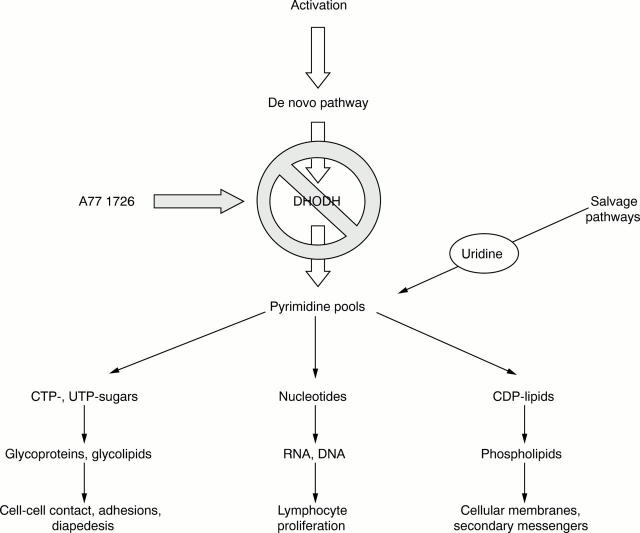
Figure 3 .
Effect of inhibition of de novo pyrimidine synthesis on various mechanisms of activated lymphocytes. (Adapted from Herrmann et al.49a)
Figure 4 .
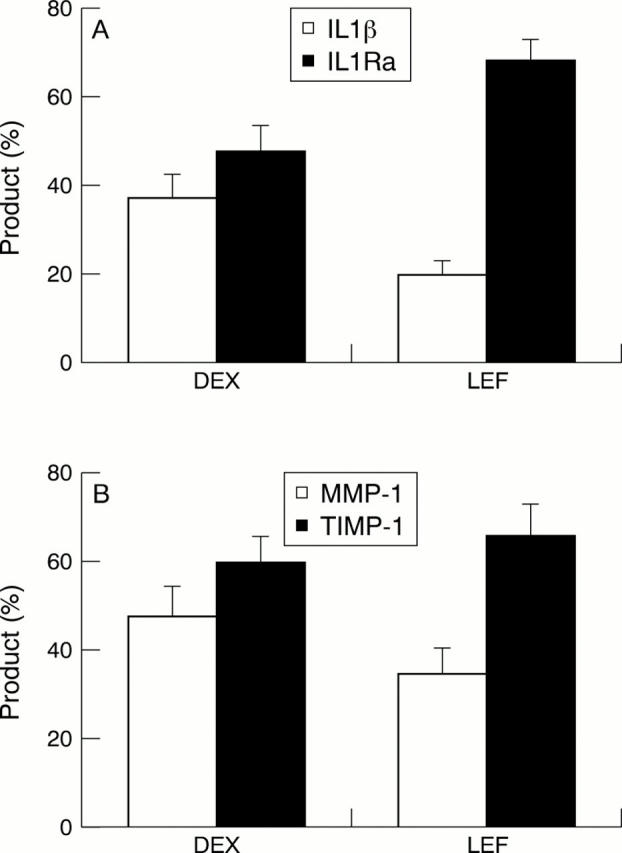
(A) Leflunomide (LEF) differentially inhibits the ability of stimulated T lymphocytes to activate THP-1 cells by direct cellular contact, favouring interleukin 1 receptor antagonist (IL1Ra) production, compared with dexamethasone (DEX). (B) Similarly, LEF differentially inhibits the ability of stimulated T lymphocytes to activate THP-1 cells by direct cellular contact, favouring the production of tissue inhibitor metalloproteinase-1. Concentrations of both LEF and DEX for figs 4A and B are 10−5 mol/l.60
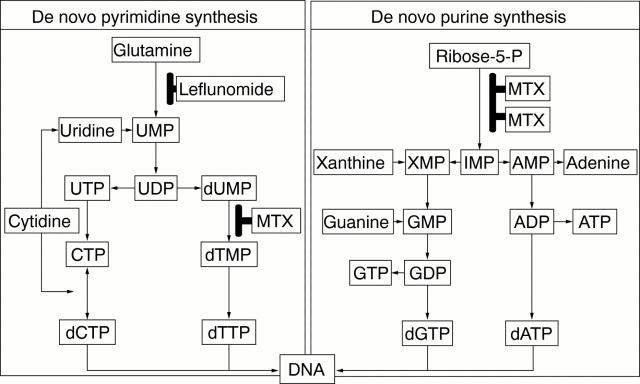
Figure 5 .
Comparison of the effect of leflunomide with that of methotrexate on nucleotide synthesis. MTX = methotrexate, dUMP = deoxyuridine monophosphate.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alcón S., Pozo M. J., Salido G. M., Pariente J. A. Histaminergic modulation of hormonal control in the exocrine guinea-pig pancreas. Inflamm Res. 1995 May;44(5):207–211. doi: 10.1007/BF01782260. [DOI] [PubMed] [Google Scholar]
- Arend W. P., Dayer J. M. Inhibition of the production and effects of interleukin-1 and tumor necrosis factor alpha in rheumatoid arthritis. Arthritis Rheum. 1995 Feb;38(2):151–160. doi: 10.1002/art.1780380202. [DOI] [PubMed] [Google Scholar]
- Bartlett R. R., Anagnostopulos H., Zielinski T., Mattar T., Schleyerbach R. Effects of leflunomide on immune responses and models of inflammation. Springer Semin Immunopathol. 1993;14(4):381–394. doi: 10.1007/BF00192310. [DOI] [PubMed] [Google Scholar]
- Bartlett R. R. Immunopharmacological profile of HWA 486, a novel isoxazol derivative--II. In vivo immunomodulating effects differ from those of cyclophosphamide, prednisolone, or cyclosporin A. Int J Immunopharmacol. 1986;8(2):199–204. doi: 10.1016/0192-0561(86)90059-7. [DOI] [PubMed] [Google Scholar]
- Bartlett R. R., Schleyerbach R. Immunopharmacological profile of a novel isoxazol derivative, HWA 486, with potential antirheumatic activity--I. Disease modifying action on adjuvant arthritis of the rat. Int J Immunopharmacol. 1985;7(1):7–18. doi: 10.1016/0192-0561(85)90003-7. [DOI] [PubMed] [Google Scholar]
- Brazelton T. R., Morris R. E. Molecular mechanisms of action of new xenobiotic immunosuppressive drugs: tacrolimus (FK506), sirolimus (rapamycin), mycophenolate mofetil and leflunomide. Curr Opin Immunol. 1996 Oct;8(5):710–720. doi: 10.1016/s0952-7915(96)80090-2. [DOI] [PubMed] [Google Scholar]
- Breedveld F. C. New insights in the pathogenesis of rheumatoid arthritis. J Rheumatol Suppl. 1998 Jul;53:3–7. [PubMed] [Google Scholar]
- Cao W. W., Kao P. N., Aoki Y., Xu J. C., Shorthouse R. A., Morris R. E. A novel mechanism of action of the immunomodulatory drug, leflunomide: augmentation of the immunosuppressive cytokine, TGF-beta 1, and suppression of the immunostimulatory cytokine, IL-2. Transplant Proc. 1996 Dec;28(6):3079–3080. [PubMed] [Google Scholar]
- Cao W. W., Kao P. N., Chao A. C., Gardner P., Ng J., Morris R. E. Mechanism of the antiproliferative action of leflunomide. A77 1726, the active metabolite of leflunomide, does not block T-cell receptor-mediated signal transduction but its antiproliferative effects are antagonized by pyrimidine nucleosides. J Heart Lung Transplant. 1995 Nov-Dec;14(6 Pt 1):1016–1030. [PubMed] [Google Scholar]
- Chen S. F., Perrella F. W., Behrens D. L., Papp L. M. Inhibition of dihydroorotate dehydrogenase activity by brequinar sodium. Cancer Res. 1992 Jul 1;52(13):3521–3527. [PubMed] [Google Scholar]
- Cherwinski H. M., Byars N., Ballaron S. J., Nakano G. M., Young J. M., Ransom J. T. Leflunomide interferes with pyrimidine nucleotide biosynthesis. Inflamm Res. 1995 Aug;44(8):317–322. doi: 10.1007/BF01796261. [DOI] [PubMed] [Google Scholar]
- Cherwinski H. M., Cohn R. G., Cheung P., Webster D. J., Xu Y. Z., Caulfield J. P., Young J. M., Nakano G., Ransom J. T. The immunosuppressant leflunomide inhibits lymphocyte proliferation by inhibiting pyrimidine biosynthesis. J Pharmacol Exp Ther. 1995 Nov;275(2):1043–1049. [PubMed] [Google Scholar]
- Cherwinski H. M., McCarley D., Schatzman R., Devens B., Ransom J. T. The immunosuppressant leflunomide inhibits lymphocyte progression through cell cycle by a novel mechanism. J Pharmacol Exp Ther. 1995 Jan;272(1):460–468. [PubMed] [Google Scholar]
- Chizzolini C., Chicheportiche R., Burger D., Dayer J. M. Human Th1 cells preferentially induce interleukin (IL)-1beta while Th2 cells induce IL-1 receptor antagonist production upon cell/cell contact with monocytes. Eur J Immunol. 1997 Jan;27(1):171–177. doi: 10.1002/eji.1830270125. [DOI] [PubMed] [Google Scholar]
- Chong A. S., Finnegan A., Jiang X., Gebel H., Sankary H. N., Foster P., Williams J. W. Leflunomide, a novel immunosuppressive agent. The mechanism of inhibition of T cell proliferation. Transplantation. 1993 Jun;55(6):1361–1366. doi: 10.1097/00007890-199306000-00028. [DOI] [PubMed] [Google Scholar]
- Chong A. S., Huang W., Liu W., Luo J., Shen J., Xu W., Ma L., Blinder L., Xiao F., Xu X. In vivo activity of leflunomide: pharmacokinetic analyses and mechanism of immunosuppression. Transplantation. 1999 Jul 15;68(1):100–109. doi: 10.1097/00007890-199907150-00020. [DOI] [PubMed] [Google Scholar]
- Chong A. S., Rezai K., Gebel H. M., Finnegan A., Foster P., Xu X., Williams J. W. Effects of leflunomide and other immunosuppressive agents on T cell proliferation in vitro. Transplantation. 1996 Jan 15;61(1):140–145. doi: 10.1097/00007890-199601150-00026. [DOI] [PubMed] [Google Scholar]
- Davis J. P., Cain G. A., Pitts W. J., Magolda R. L., Copeland R. A. The immunosuppressive metabolite of leflunomide is a potent inhibitor of human dihydroorotate dehydrogenase. Biochemistry. 1996 Jan 30;35(4):1270–1273. doi: 10.1021/bi952168g. [DOI] [PubMed] [Google Scholar]
- Déage V., Burger D., Dayer J. M. Exposure of T lymphocytes to leflunomide but not to dexamethasone favors the production by monocytic cells of interleukin-1 receptor antagonist and the tissue-inhibitor of metalloproteinases-1 over that of interleukin-1beta and metalloproteinases. Eur Cytokine Netw. 1998 Dec;9(4):663–668. [PubMed] [Google Scholar]
- Elder R. T., Xu X., Williams J. W., Gong H., Finnegan A., Chong A. S. The immunosuppressive metabolite of leflunomide, A77 1726, affects murine T cells through two biochemical mechanisms. J Immunol. 1997 Jul 1;159(1):22–27. [PubMed] [Google Scholar]
- Emery P., Breedveld F. C., Lemmel E. M., Kaltwasser J. P., Dawes P. T., Gömör B., Van Den Bosch F., Nordström D., Bjorneboe O., Dahl R. A comparison of the efficacy and safety of leflunomide and methotrexate for the treatment of rheumatoid arthritis. Rheumatology (Oxford) 2000 Jun;39(6):655–665. doi: 10.1093/rheumatology/39.6.655. [DOI] [PubMed] [Google Scholar]
- Fairbanks L. D., Bofill M., Ruckemann K., Simmonds H. A. Importance of ribonucleotide availability to proliferating T-lymphocytes from healthy humans. Disproportionate expansion of pyrimidine pools and contrasting effects of de novo synthesis inhibitors. J Biol Chem. 1995 Dec 15;270(50):29682–29689. [PubMed] [Google Scholar]
- Fox R. I., Herrmann M. L., Frangou C. G., Wahl G. M., Morris R. E., Kirschbaum B. J. How does leflunomide modulate the immune response in rheumatoid arthritis? BioDrugs. 1999 Oct;12(4):301–315. doi: 10.2165/00063030-199912040-00007. [DOI] [PubMed] [Google Scholar]
- Furst D. E. Clinical pharmacology of combination DMARD therapy in rheumatoid arthritis. J Rheumatol Suppl. 1996 Mar;44:86–90. [PubMed] [Google Scholar]
- Greene S., Watanabe K., Braatz-Trulson J., Lou L. Inhibition of dihydroorotate dehydrogenase by the immunosuppressive agent leflunomide. Biochem Pharmacol. 1995 Sep 7;50(6):861–867. doi: 10.1016/0006-2952(95)00255-x. [DOI] [PubMed] [Google Scholar]
- Hambleton P., McMahon S. Drug actions on delayed-type hypersensitivity in rats with developing and established adjuvant arthritis. Agents Actions. 1990 Mar;29(3-4):328–332. doi: 10.1007/BF01966465. [DOI] [PubMed] [Google Scholar]
- Herrmann M. L., Schleyerbach R., Kirschbaum B. J. Leflunomide: an immunomodulatory drug for the treatment of rheumatoid arthritis and other autoimmune diseases. Immunopharmacology. 2000 May;47(2-3):273–289. doi: 10.1016/s0162-3109(00)00191-0. [DOI] [PubMed] [Google Scholar]
- Isler P., Vey E., Zhang J. H., Dayer J. M. Cell surface glycoproteins expressed on activated human T cells induce production of interleukin-1 beta by monocytic cells: a possible role of CD69. Eur Cytokine Netw. 1993 Jan-Feb;4(1):15–23. [PubMed] [Google Scholar]
- Jones M. E. Pyrimidine nucleotide biosynthesis in animals: genes, enzymes, and regulation of UMP biosynthesis. Annu Rev Biochem. 1980;49:253–279. doi: 10.1146/annurev.bi.49.070180.001345. [DOI] [PubMed] [Google Scholar]
- Kremer J. M. Methotrexate and leflunomide: biochemical basis for combination therapy in the treatment of rheumatoid arthritis. Semin Arthritis Rheum. 1999 Aug;29(1):14–26. doi: 10.1016/s0049-0172(99)80034-1. [DOI] [PubMed] [Google Scholar]
- Kuo E. A., Hambleton P. T., Kay D. P., Evans P. L., Matharu S. S., Little E., McDowall N., Jones C. B., Hedgecock C. J., Yea C. M. Synthesis, structure-activity relationships, and pharmacokinetic properties of dihydroorotate dehydrogenase inhibitors: 2-cyano-3-cyclopropyl-3-hydroxy-N-[3'-methyl-4'-(trifluoromethyl)phenyl ] propenamide and related compounds. J Med Chem. 1996 Nov 8;39(23):4608–4621. doi: 10.1021/jm9604437. [DOI] [PubMed] [Google Scholar]
- Küchle C. C., Thoenes G. H., Langer K. H., Schorlemmer H. U., Bartlett R. R., Schleyerbach R. Prevention of kidney and skin graft rejection in rats by leflunomide, a new immunomodulating agent. Transplant Proc. 1991 Feb;23(1 Pt 2):1083–1086. [PubMed] [Google Scholar]
- Lacraz S., Isler P., Vey E., Welgus H. G., Dayer J. M. Direct contact between T lymphocytes and monocytes is a major pathway for induction of metalloproteinase expression. J Biol Chem. 1994 Sep 2;269(35):22027–22033. [PubMed] [Google Scholar]
- Lang R., Wagner H., Heeg K. Differential effects of the immunosuppressive agents cyclosporine and leflunomide in vivo. Leflunomide blocks clonal T cell expansion yet allows production of lymphokines and manifestation of T cell-mediated shock. Transplantation. 1995 Feb 15;59(3):382–389. [PubMed] [Google Scholar]
- Li J. M., Isler P., Dayer J. M., Burger D. Contact-dependent stimulation of monocytic cells and neutrophils by stimulated human T-cell clones. Immunology. 1995 Apr;84(4):571–576. [PMC free article] [PubMed] [Google Scholar]
- Linke S. P., Clarkin K. C., Di Leonardo A., Tsou A., Wahl G. M. A reversible, p53-dependent G0/G1 cell cycle arrest induced by ribonucleotide depletion in the absence of detectable DNA damage. Genes Dev. 1996 Apr 15;10(8):934–947. doi: 10.1101/gad.10.8.934. [DOI] [PubMed] [Google Scholar]
- Linke S. P., Clarkin K. C., Wahl G. M. p53 mediates permanent arrest over multiple cell cycles in response to gamma-irradiation. Cancer Res. 1997 Mar 15;57(6):1171–1179. [PubMed] [Google Scholar]
- Manna S. K., Aggarwal B. B. Immunosuppressive leflunomide metabolite (A77 1726) blocks TNF-dependent nuclear factor-kappa B activation and gene expression. J Immunol. 1999 Feb 15;162(4):2095–2102. [PubMed] [Google Scholar]
- McChesney L. P., Xiao F., Sankary H. N., Foster P. F., Sharma S., Haklin M., Williams J. W. An evaluation of leflunomide in the canine renal transplantation model. Transplantation. 1994 Jun 27;57(12):1717–1722. [PubMed] [Google Scholar]
- Mladenovic V., Domljan Z., Rozman B., Jajic I., Mihajlovic D., Dordevic J., Popovic M., Dimitrijevic M., Zivkovic M., Campion G. Safety and effectiveness of leflunomide in the treatment of patients with active rheumatoid arthritis. Results of a randomized, placebo-controlled, phase II study. Arthritis Rheum. 1995 Nov;38(11):1595–1603. doi: 10.1002/art.1780381111. [DOI] [PubMed] [Google Scholar]
- Morris R. E., Huang X., Cao W., Zheng B., Shorthouse R. A. Leflunomide (HWA 486) and its analog suppress T- and B-cell proliferation in vitro, acute rejection, ongoing rejection, and antidonor antibody synthesis in mouse, rat, and cynomolgus monkey transplant recipients as well as arterial intimal thickening after balloon catheter injury. Transplant Proc. 1995 Feb;27(1):445–447. [PubMed] [Google Scholar]
- Pasternak R. D., Wadopian N. S., Wright R. N., Siminoff P., Gylys J. A., Buyniski J. P. Disease modifying activity of HWA 486 in rat adjuvant-induced arthritis. Agents Actions. 1987 Aug;21(3-4):241–243. doi: 10.1007/BF01966478. [DOI] [PubMed] [Google Scholar]
- Paulus H. E. The use of combinations of disease-modifying antirheumatic agents in rheumatoid arthritis. Arthritis Rheum. 1990 Jan;33(1):113–120. doi: 10.1002/art.1780330116. [DOI] [PubMed] [Google Scholar]
- Peters G. J., Veerkamp J. H. Purine and pyrimidine metabolism in peripheral blood lymphocytes. Int J Biochem. 1983;15(2):115–123. doi: 10.1016/0020-711x(83)90051-4. [DOI] [PubMed] [Google Scholar]
- Popovic S., Bartlett R. R. Disease modifying activity of HWA 486 on the development of SLE in MRL/1-mice. Agents Actions. 1986 Dec;19(5-6):313–314. doi: 10.1007/BF01971235. [DOI] [PubMed] [Google Scholar]
- Rückemann K., Fairbanks L. D., Carrey E. A., Hawrylowicz C. M., Richards D. F., Kirschbaum B., Simmonds H. A. Leflunomide inhibits pyrimidine de novo synthesis in mitogen-stimulated T-lymphocytes from healthy humans. J Biol Chem. 1998 Aug 21;273(34):21682–21691. doi: 10.1074/jbc.273.34.21682. [DOI] [PubMed] [Google Scholar]
- Schorlemmer H. U., Seiler F. R., Bartlett R. R. Prolongation of allogeneic transplanted skin grafts and induction of tolerance by leflunomide, a new immunosuppressive isoxazol derivative. Transplant Proc. 1993 Feb;25(1 Pt 1):763–767. [PubMed] [Google Scholar]
- Siemasko K. F., Chong A. S., Williams J. W., Bremer E. G., Finnegan A. Regulation of B cell function by the immunosuppressive agent leflunomide. Transplantation. 1996 Feb 27;61(4):635–642. doi: 10.1097/00007890-199602270-00020. [DOI] [PubMed] [Google Scholar]
- Siemasko K., Chong A. S., Jäck H. M., Gong H., Williams J. W., Finnegan A. Inhibition of JAK3 and STAT6 tyrosine phosphorylation by the immunosuppressive drug leflunomide leads to a block in IgG1 production. J Immunol. 1998 Feb 15;160(4):1581–1588. [PubMed] [Google Scholar]
- Silva H. T., Cao W., Shorthouse R., Morris R. E. Mechanism of action of leflunomide: in vivo uridine administration reverses its inhibition of lymphocyte proliferation. Transplant Proc. 1996 Dec;28(6):3082–3084. [PubMed] [Google Scholar]
- Silva H. T., Jr, Cao W., Shorthouse R. A., Löffler M., Morris R. E. In vitro and in vivo effects of leflunomide, brequinar, and cyclosporine on pyrimidine biosynthesis. Transplant Proc. 1997 Feb-Mar;29(1-2):1292–1293. doi: 10.1016/s0041-1345(96)00523-4. [DOI] [PubMed] [Google Scholar]
- Silva H. T., Jr, Morris R. E. Leflunomide and malononitriloamides. Expert Opin Investig Drugs. 1997 Jan;6(1):51–64. doi: 10.1517/13543784.6.1.51. [DOI] [PubMed] [Google Scholar]
- Smolen J. S., Kalden J. R., Scott D. L., Rozman B., Kvien T. K., Larsen A., Loew-Friedrich I., Oed C., Rosenburg R. Efficacy and safety of leflunomide compared with placebo and sulphasalazine in active rheumatoid arthritis: a double-blind, randomised, multicentre trial. European Leflunomide Study Group. Lancet. 1999 Jan 23;353(9149):259–266. doi: 10.1016/s0140-6736(98)09403-3. [DOI] [PubMed] [Google Scholar]
- Strand V., Cohen S., Schiff M., Weaver A., Fleischmann R., Cannon G., Fox R., Moreland L., Olsen N., Furst D. Treatment of active rheumatoid arthritis with leflunomide compared with placebo and methotrexate. Leflunomide Rheumatoid Arthritis Investigators Group. Arch Intern Med. 1999 Nov 22;159(21):2542–2550. doi: 10.1001/archinte.159.21.2542. [DOI] [PubMed] [Google Scholar]
- Thoss K., Henzgen S., Petrow P. K., Katenkamp D., Brauer R. Immunomodulation of rat antigen-induced arthritis by leflunomide alone and in combination with cyclosporin A. Inflamm Res. 1996 Feb;45(2):103–107. doi: 10.1007/BF02265123. [DOI] [PubMed] [Google Scholar]
- Vey E., Burger D., Dayer J. M. Expression and cleavage of tumor necrosis factor-alpha and tumor necrosis factor receptors by human monocytic cell lines upon direct contact with stimulated T cells. Eur J Immunol. 1996 Oct;26(10):2404–2409. doi: 10.1002/eji.1830261021. [DOI] [PubMed] [Google Scholar]
- Vey E., Zhang J. H., Dayer J. M. IFN-gamma and 1,25(OH)2D3 induce on THP-1 cells distinct patterns of cell surface antigen expression, cytokine production, and responsiveness to contact with activated T cells. J Immunol. 1992 Sep 15;149(6):2040–2046. [PubMed] [Google Scholar]
- Vidic-Dankovic B., Kosec D., Damjanovic M., Apostolski S., Isakovic K., Bartlett R. R. Leflunomide prevents the development of experimentally induced myasthenia gravis. Int J Immunopharmacol. 1995 Apr;17(4):273–281. doi: 10.1016/0192-0561(95)00009-q. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Linke S. P., Paulson T. G., Huang L. C. Maintaining genetic stability through TP53 mediated checkpoint control. Cancer Surv. 1997;29:183–219. [PubMed] [Google Scholar]
- Wilke W. S., Clough J. D. Therapy for rheumatoid arthritis: combinations of disease-modifying drugs and new paradigms of treatment. Semin Arthritis Rheum. 1991 Oct;21(2 Suppl 1):21–34. doi: 10.1016/0049-0172(91)90048-5. [DOI] [PubMed] [Google Scholar]
- Williams J. W., Xiao F., Foster P. F., Chong A., Sharma S., Bartlett R., Sankary H. N. Immunosuppressive effects of leflunomide in a cardiac allograft model. Transplant Proc. 1993 Feb;25(1 Pt 1):745–746. [PubMed] [Google Scholar]
- Williamson R. A., Yea C. M., Robson P. A., Curnock A. P., Gadher S., Hambleton A. B., Woodward K., Bruneau J. M., Hambleton P., Moss D. Dihydroorotate dehydrogenase is a high affinity binding protein for A77 1726 and mediator of a range of biological effects of the immunomodulatory compound. J Biol Chem. 1995 Sep 22;270(38):22467–22472. doi: 10.1074/jbc.270.38.22467. [DOI] [PubMed] [Google Scholar]
- Williamson R. A., Yea C. M., Robson P. A., Curnock A. P., Gadher S., Hambleton A. B., Woodward K., Bruneau J. M., Hambleton P., Spinella-Jaegle S. Dihydroorotate dehydrogenase is a target for the biological effects of leflunomide. Transplant Proc. 1996 Dec;28(6):3088–3091. [PubMed] [Google Scholar]
- Wilske K. R. Approaches to the management of rheumatoid arthritis: rationale for early combination therapy. Br J Rheumatol. 1993 Mar;32 (Suppl 1):24–27. [PubMed] [Google Scholar]
- Xu X., Blinder L., Shen J., Gong H., Finnegan A., Williams J. W., Chong A. S. In vivo mechanism by which leflunomide controls lymphoproliferative and autoimmune disease in MRL/MpJ-lpr/lpr mice. J Immunol. 1997 Jul 1;159(1):167–174. [PubMed] [Google Scholar]
- Xu X., Williams J. W., Bremer E. G., Finnegan A., Chong A. S. Inhibition of protein tyrosine phosphorylation in T cells by a novel immunosuppressive agent, leflunomide. J Biol Chem. 1995 May 26;270(21):12398–12403. doi: 10.1074/jbc.270.21.12398. [DOI] [PubMed] [Google Scholar]
- Xu X., Williams J. W., Gong H., Finnegan A., Chong A. S. Two activities of the immunosuppressive metabolite of leflunomide, A77 1726. Inhibition of pyrimidine nucleotide synthesis and protein tyrosine phosphorylation. Biochem Pharmacol. 1996 Aug 23;52(4):527–534. doi: 10.1016/0006-2952(96)00303-6. [DOI] [PubMed] [Google Scholar]