Anti-inflammatory effects of leflunomide on cultured synovial macrophages from patients with rheumatoid arthritis (original) (raw)
Abstract
Background: Leflunomide and its active metabolite A77 1726 reversibly inhibits the enzyme dihydro-orotate dehydrogenase, the rate limiting step in de novo synthesis of pyrimidines and progression of the cell cycle in different cell lines, mainly activated T lymphocytes.
Objective: To analyse in vitro the possible anti-inflammatory effects exerted by A77 1726, on cultured macrophages, obtained from the synovial tissues of patients with rheumatoid arthritis (RA).
Methods: The effects of different doses of A77 1726 on intracytoplasmic expression and extracellular concentration of inflammatory cytokines (tumour necrosis factor α (TNFα), interleukin (IL) 1ß, IL6), as well as the influence on production and expression of intercellular adhesion molecule-1 (ICAM-1) and cyclo-oxygenase 2 (COX-2) by primary cultures of synovial macrophages from patients with RA, were evaluated by immunocytochemistry and western blot analysis. The observations were made at four and 24 hours.
Results: A progressive and significant time and dose dependent decrease of the number of positive macrophages for intracellular TNFα and IL1ß, treated with different doses of A77 1726, was found in comparison with untreated cells. The extracellular concentration of TNFα was found to be significantly decreased in media containing cultured macrophages at 24 hours for all tested doses of A77 1726. At 24 hours, a significant time and dose dependent decrease of ICAM-1 and COX-2 expression by cultured macrophages after A77 1726 treatment was found.
Conclusions: In conclusion, the mechanism of antiproliferative activity exerted by leflunomide on activated T lymphocytes seems to be the same mechanism (alteration of the cell cycle progression) which interferes with the functions of other activated cells—namely, the monocytes/macrophages, which are strongly involved in the inflammatory reaction in RA synovial tissue. The positive clinical results seem to confirm that leflunomide exerts an anti-inflammatory action on phagocytic cells in short and long term treatment of RA.
Full Text
The Full Text of this article is available as a PDF (188.2 KB).
Figure 1 .
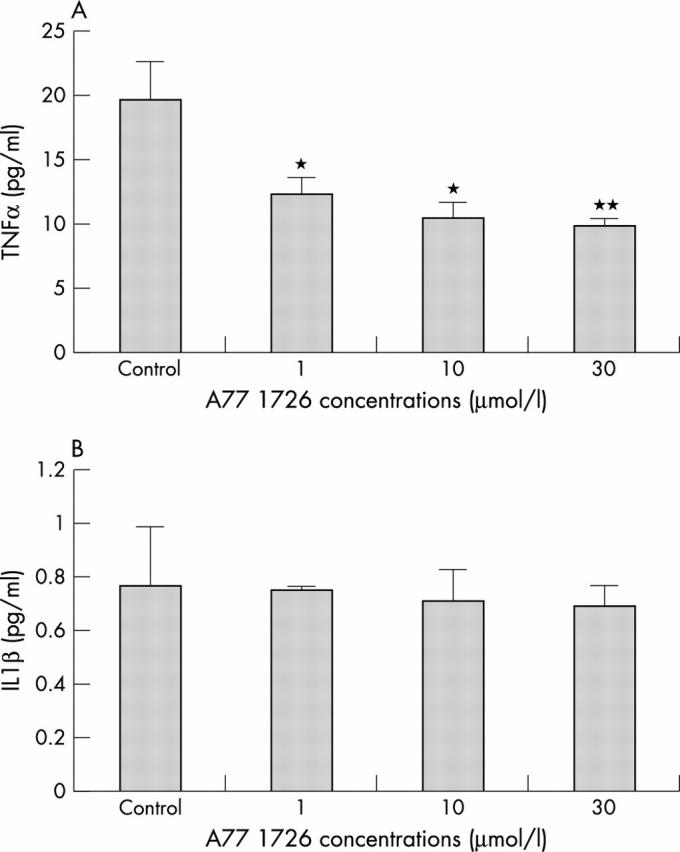
(A) TNFα concentrations at 24 hours in culture media after incubation of RA synovial macrophages with A77 1726 at concentrations of 1, 10, and 30 µmol/l. *p<0.05; **p<0.01 versus controls. (B) IL1ß concentrations at 24 hours in culture media after incubation of RA synovial macrophages with A77 1726 at concentrations of 1, 10, and 30 µmol/l. No significant changes were found.
Figure 2 .
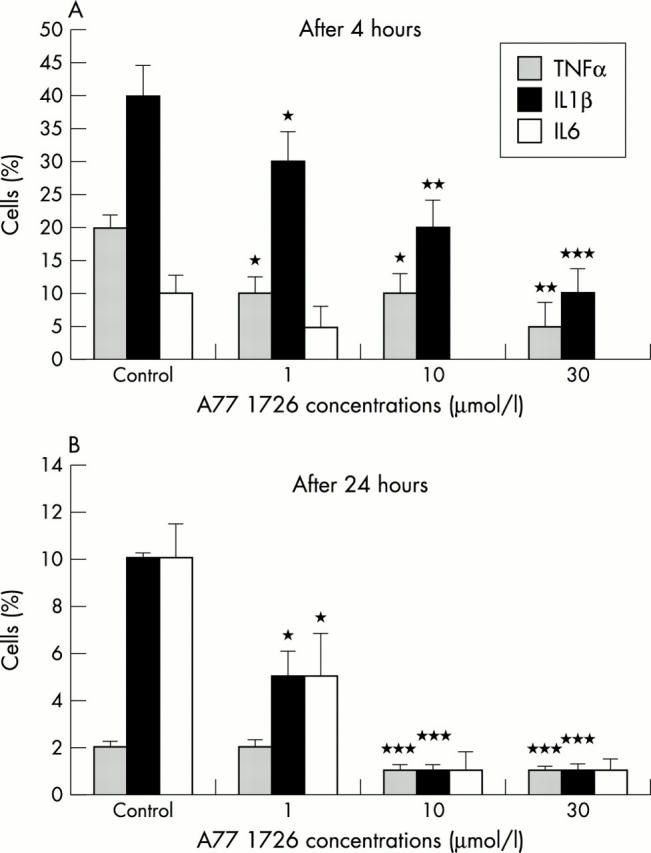
(A) Intracytoplasmic expression of TNFα, IL1ß and IL6 in RA synovial macrophages treated with different concentrations of A77 1726 after four hours. The percentage of TNFα and IL1ß positive cells decreased significantly in a dose dependent manner. *p<0.05; **p<0.01; ***p<0.001 versus controls. IL6 was poorly expressed and the effects of A77 1726 were not significant. (B) Intracytoplasmic expression of TNFα, IL1ß, and IL6 in RA synovial macrophage cells with different concentrations of A77 1726 after 24 hours. The percentage of IL1ß and IL6 positive cells decreased significantly in a dose dependent manner. *p<0.05; ***p<0.01 versus controls. TNFα was poorly expressed at 24 hours and the effect of A77 1726 was not significant.
Figure 5 .
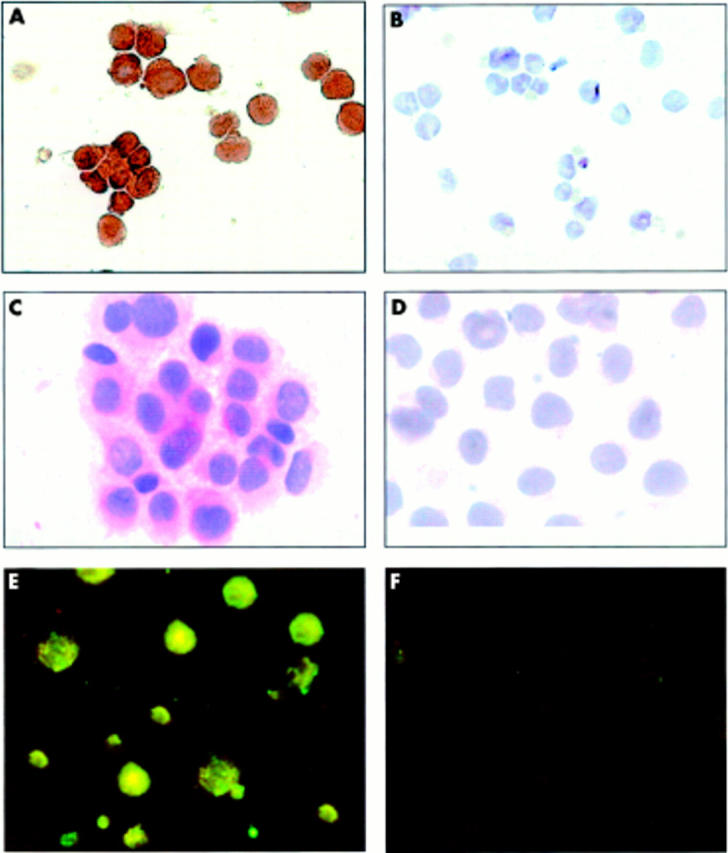
COX-2 expression by cultured RA synovial macrophages with (A) and without (B) treatment with A77 1726 (30 µmol/l). ICAM-1 expression by cultured RA synovial macrophages with (C) and without (D) treatment with A77 1726 (30 µmol/l). Intracytoplasmic IL1 expression by cultured RA synovial macrophages with (E) and without (F) treatment with A77 1726 (30 µmol/l).
Figure 3 .
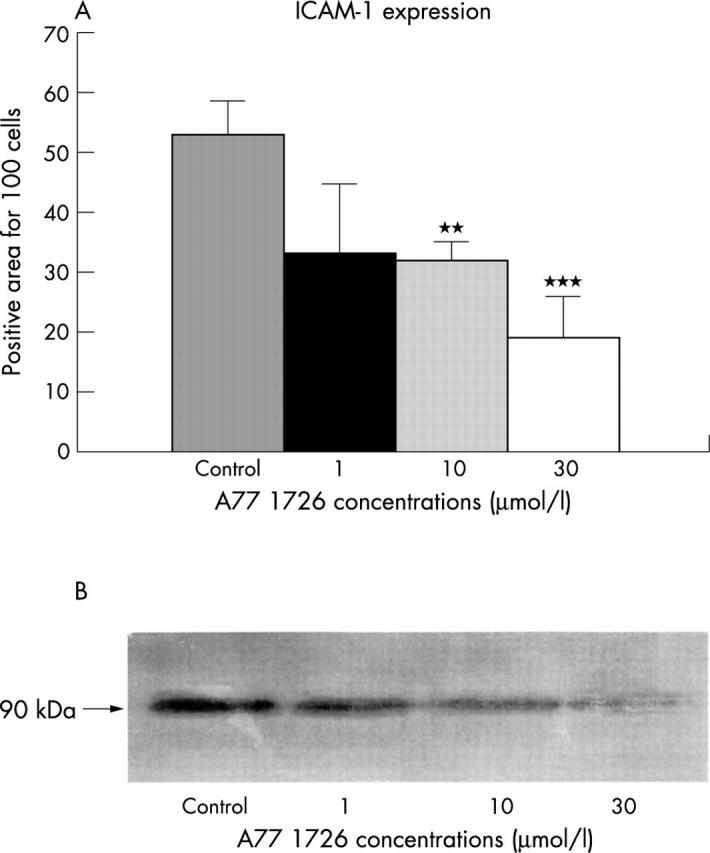
(A) ICAM-1 expression in RA synovial macrophages treated with different concentrations of A77 1726 after 24 hours as evaluated by immunocytochemistry and image analysis. The staining positivity was expressed as a percentage for cell number. **p<0.01; ***p<0.001. (B) ICAM-1 expression in RA synovial macrophages treated with different concentrations of A77 1726 after 24 hours as evaluated by western blot analysis.
Figure 4 .
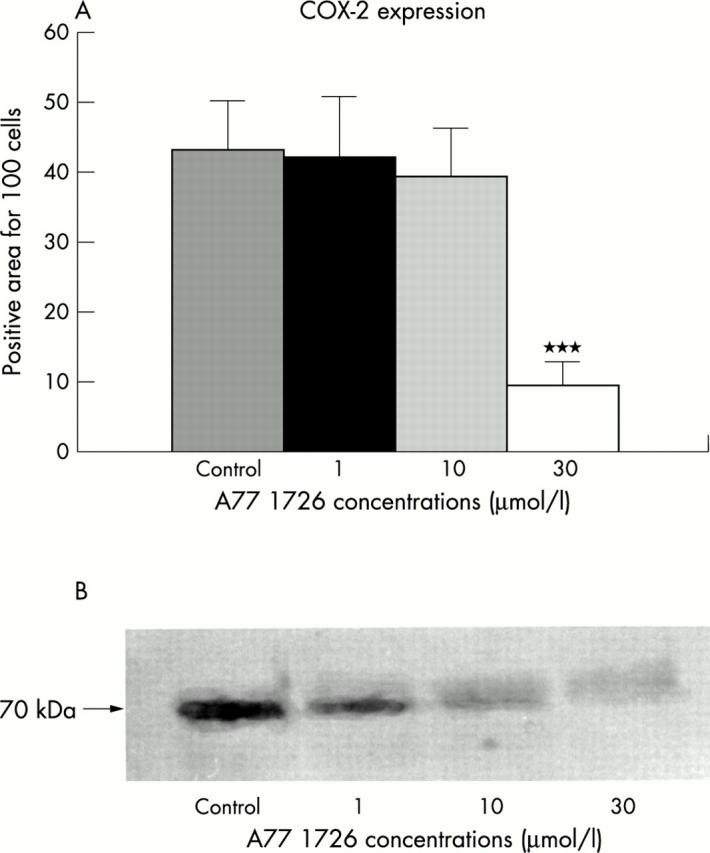
(A) COX-2 expression in RA synovial macrophages treated with different concentrations of A77 1726 after 24 hours as evaluated by immunocytochemistry and image analysis. The staining positivity was expressed as a percentage for cell number. ***p<0.001. (B) COX-2 expression in RA synovial macrophages treated with different concentrations of A77 1726 after 24 hours as evaluated by western blot analysis.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson U., Andersson J., Lindfors A., Wagner K., Möller G., Heusser C. H. Simultaneous production of interleukin 2, interleukin 4 and interferon-gamma by activated human blood lymphocytes. Eur J Immunol. 1990 Jul;20(7):1591–1596. doi: 10.1002/eji.1830200727. [DOI] [PubMed] [Google Scholar]
- Arend W. P., Dayer J. M. Inhibition of the production and effects of interleukin-1 and tumor necrosis factor alpha in rheumatoid arthritis. Arthritis Rheum. 1995 Feb;38(2):151–160. doi: 10.1002/art.1780380202. [DOI] [PubMed] [Google Scholar]
- Brazelton T. R., Morris R. E. Molecular mechanisms of action of new xenobiotic immunosuppressive drugs: tacrolimus (FK506), sirolimus (rapamycin), mycophenolate mofetil and leflunomide. Curr Opin Immunol. 1996 Oct;8(5):710–720. doi: 10.1016/s0952-7915(96)80090-2. [DOI] [PubMed] [Google Scholar]
- Breedveld F. C., Dayer J. M. Leflunomide: mode of action in the treatment of rheumatoid arthritis. Ann Rheum Dis. 2000 Nov;59(11):841–849. doi: 10.1136/ard.59.11.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks P., Emery P., Evans J. F., Fenner H., Hawkey C. J., Patrono C., Smolen J., Breedveld F., Day R., Dougados M. Interpreting the clinical significance of the differential inhibition of cyclooxygenase-1 and cyclooxygenase-2. Rheumatology (Oxford) 1999 Aug;38(8):779–788. doi: 10.1093/rheumatology/38.8.779. [DOI] [PubMed] [Google Scholar]
- Cao W. W., Kao P. N., Chao A. C., Gardner P., Ng J., Morris R. E. Mechanism of the antiproliferative action of leflunomide. A77 1726, the active metabolite of leflunomide, does not block T-cell receptor-mediated signal transduction but its antiproliferative effects are antagonized by pyrimidine nucleosides. J Heart Lung Transplant. 1995 Nov-Dec;14(6 Pt 1):1016–1030. [PubMed] [Google Scholar]
- Cherwinski H. M., Byars N., Ballaron S. J., Nakano G. M., Young J. M., Ransom J. T. Leflunomide interferes with pyrimidine nucleotide biosynthesis. Inflamm Res. 1995 Aug;44(8):317–322. doi: 10.1007/BF01796261. [DOI] [PubMed] [Google Scholar]
- Chong A. S., Rezai K., Gebel H. M., Finnegan A., Foster P., Xu X., Williams J. W. Effects of leflunomide and other immunosuppressive agents on T cell proliferation in vitro. Transplantation. 1996 Jan 15;61(1):140–145. doi: 10.1097/00007890-199601150-00026. [DOI] [PubMed] [Google Scholar]
- Cutolo M., Bisso A., Sulli A., Felli L., Briata M., Pizzorni C., Villaggio B. Antiproliferative and antiinflammatory effects of methotrexate on cultured differentiating myeloid monocytic cells (THP-1) but not on synovial macrophages from patients with rheumatoid arthritis. J Rheumatol. 2000 Nov;27(11):2551–2557. [PubMed] [Google Scholar]
- Cutolo M., Sulli A., Pizzorni C., Seriolo B., Straub R. H. Anti-inflammatory mechanisms of methotrexate in rheumatoid arthritis. Ann Rheum Dis. 2001 Aug;60(8):729–735. doi: 10.1136/ard.60.8.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutolo Maurizio, Sulli Alberto, Craviotto Chiara, Felli Lamberto, Pizzorni Carmen, Seriolo Bruno, Villaggio Barbara. Antiproliferative-antiinflammatory effects of methotrexate and sex hormones on cultured differentiating myeloid monocytic cells (THP-1). Ann N Y Acad Sci. 2002 Jun;966:232–237. doi: 10.1111/j.1749-6632.2002.tb04220.x. [DOI] [PubMed] [Google Scholar]
- Davis J. P., Cain G. A., Pitts W. J., Magolda R. L., Copeland R. A. The immunosuppressive metabolite of leflunomide is a potent inhibitor of human dihydroorotate dehydrogenase. Biochemistry. 1996 Jan 30;35(4):1270–1273. doi: 10.1021/bi952168g. [DOI] [PubMed] [Google Scholar]
- Déage V., Burger D., Dayer J. M. Exposure of T lymphocytes to leflunomide but not to dexamethasone favors the production by monocytic cells of interleukin-1 receptor antagonist and the tissue-inhibitor of metalloproteinases-1 over that of interleukin-1beta and metalloproteinases. Eur Cytokine Netw. 1998 Dec;9(4):663–668. [PubMed] [Google Scholar]
- Elder R. T., Xu X., Williams J. W., Gong H., Finnegan A., Chong A. S. The immunosuppressive metabolite of leflunomide, A77 1726, affects murine T cells through two biochemical mechanisms. J Immunol. 1997 Jul 1;159(1):22–27. [PubMed] [Google Scholar]
- Fairbanks L. D., Bofill M., Ruckemann K., Simmonds H. A. Importance of ribonucleotide availability to proliferating T-lymphocytes from healthy humans. Disproportionate expansion of pyrimidine pools and contrasting effects of de novo synthesis inhibitors. J Biol Chem. 1995 Dec 15;270(50):29682–29689. [PubMed] [Google Scholar]
- Ford D. K. Understanding rheumatoid arthritis. J Rheumatol. 1997 Aug;24(8):1464–1466. [PubMed] [Google Scholar]
- Guidelines for monitoring drug therapy in rheumatoid arthritis. American College of Rheumatology Ad Hoc Committee on Clinical Guidelines. Arthritis Rheum. 1996 May;39(5):723–731. [PubMed] [Google Scholar]
- Hamilton L. C., Vojnovic I., Warner T. D. A771726, the active metabolite of leflunomide, directly inhibits the activity of cyclo-oxygenase-2 in vitro and in vivo in a substrate-sensitive manner. Br J Pharmacol. 1999 Aug;127(7):1589–1596. doi: 10.1038/sj.bjp.0702708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann M. L., Schleyerbach R., Kirschbaum B. J. Leflunomide: an immunomodulatory drug for the treatment of rheumatoid arthritis and other autoimmune diseases. Immunopharmacology. 2000 May;47(2-3):273–289. doi: 10.1016/s0162-3109(00)00191-0. [DOI] [PubMed] [Google Scholar]
- Jones M. E. Pyrimidine nucleotide biosynthesis in animals: genes, enzymes, and regulation of UMP biosynthesis. Annu Rev Biochem. 1980;49:253–279. doi: 10.1146/annurev.bi.49.070180.001345. [DOI] [PubMed] [Google Scholar]
- Kraan M. C., Reece R. J., Barg E. C., Smeets T. J., Farnell J., Rosenburg R., Veale D. J., Breedveld F. C., Emery P., Tak P. P. Modulation of inflammation and metalloproteinase expression in synovial tissue by leflunomide and methotrexate in patients with active rheumatoid arthritis. Findings in a prospective, randomized, double-blind, parallel-design clinical trial in thirty-nine patients at two centers. Arthritis Rheum. 2000 Aug;43(8):1820–1830. doi: 10.1002/1529-0131(200008)43:8<1820::AID-ANR18>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Manna S. K., Aggarwal B. B. Immunosuppressive leflunomide metabolite (A77 1726) blocks TNF-dependent nuclear factor-kappa B activation and gene expression. J Immunol. 1999 Feb 15;162(4):2095–2102. [PubMed] [Google Scholar]
- Manna S. K., Mukhopadhyay A., Aggarwal B. B. Leflunomide suppresses TNF-induced cellular responses: effects on NF-kappa B, activator protein-1, c-Jun N-terminal protein kinase, and apoptosis. J Immunol. 2000 Nov 15;165(10):5962–5969. doi: 10.4049/jimmunol.165.10.5962. [DOI] [PubMed] [Google Scholar]
- Reece Richard J., Kraan Maarten C., Radjenovic Aleksandra, Veale Douglas J., O'Connor P. J., Ridgway John P., Gibbon W. W., Breedveld Ferdinand C., Tak Paul P., Emery Paul. Comparative assessment of leflunomide and methotrexate for the treatment of rheumatoid arthritis, by dynamic enhanced magnetic resonance imaging. Arthritis Rheum. 2002 Feb;46(2):366–372. doi: 10.1002/art.10084. [DOI] [PubMed] [Google Scholar]
- Salmi M., Jalkanen S. Human leukocyte subpopulations from inflamed gut bind to joint vasculature using distinct sets of adhesion molecules. J Immunol. 2001 Apr 1;166(7):4650–4657. doi: 10.4049/jimmunol.166.7.4650. [DOI] [PubMed] [Google Scholar]
- Silva H. T., Jr, Cao W., Shorthouse R. A., Löffler M., Morris R. E. In vitro and in vivo effects of leflunomide, brequinar, and cyclosporine on pyrimidine biosynthesis. Transplant Proc. 1997 Feb-Mar;29(1-2):1292–1293. doi: 10.1016/s0041-1345(96)00523-4. [DOI] [PubMed] [Google Scholar]
- Smith M. D., Kraan M. C., Slavotinek J., Au V., Weedon H., Parker A., Coleman M., Roberts-Thomson P. J., Ahern M. J. Treatment-induced remission in rheumatoid arthritis patients is characterized by a reduction in macrophage content of synovial biopsies. Rheumatology (Oxford) 2001 Apr;40(4):367–374. doi: 10.1093/rheumatology/40.4.367. [DOI] [PubMed] [Google Scholar]
- Weithmann K. U., Jeske S., Schlotte V. Effect of leflunomide on constitutive and inducible pathways of cellular eicosanoid generation. Agents Actions. 1994 May;41(3-4):164–170. doi: 10.1007/BF02001911. [DOI] [PubMed] [Google Scholar]
- Xu X., Williams J. W., Bremer E. G., Finnegan A., Chong A. S. Inhibition of protein tyrosine phosphorylation in T cells by a novel immunosuppressive agent, leflunomide. J Biol Chem. 1995 May 26;270(21):12398–12403. doi: 10.1074/jbc.270.21.12398. [DOI] [PubMed] [Google Scholar]
- Xu X., Williams J. W., Gong H., Finnegan A., Chong A. S. Two activities of the immunosuppressive metabolite of leflunomide, A77 1726. Inhibition of pyrimidine nucleotide synthesis and protein tyrosine phosphorylation. Biochem Pharmacol. 1996 Aug 23;52(4):527–534. doi: 10.1016/0006-2952(96)00303-6. [DOI] [PubMed] [Google Scholar]