Expression of matrix metalloproteinases in patients with Wegener's granulomatosis (original) (raw)
Abstract
Background: Enhanced activity of matrix metalloproteinases (MMPs) has been reported to have a pathogenic role in several diseases such as cancer and cardiovascular disorders, and seems also to play a part in certain autoimmune diseases.
Objective: To examine whether enhanced MMP activity may also have a role in the pathogenesis of Wegener's granulomatosis (WG).
Methods: In a study group of 15 patients with WG and 15 controls, plasma levels and gene expression were measured in freshly isolated peripheral blood mononuclear cells (PBMCs) of several MMPs and their endogenous inhibitors (that is, tissue inhibitors of metalloproteinases (TIMPs)) by enzyme immunoassays and RNase protection assay, respectively.
Results: Whereas patients with WG in remission had enhanced gene expression of several MMPs and TIMPs in PBMCs, those with active disease had a selective up regulation of MMP-2 and MMP-8 compared with healthy controls, and a down regulation of TIMP-1 and TIMP-3 compared with other patients with WG. Moreover, plasma levels of TIMP-1 and MMP-8 correlated significantly with C reactive protein levels, further supporting an association between activation of the MMP/TIMP system and disease activity in WG. Finally, these changes in MMP/TIMP expression in WG were accompanied by increased total MMP activity in PBMC supernatants, particularly in those with active disease, suggesting a matrix degrading net effect.
Conclusion: These findings suggest that disturbed MMP and TIMP activity has a role in the pathogenesis of WG.
Full Text
The Full Text of this article is available as a PDF (90.4 KB).
Figure 1.
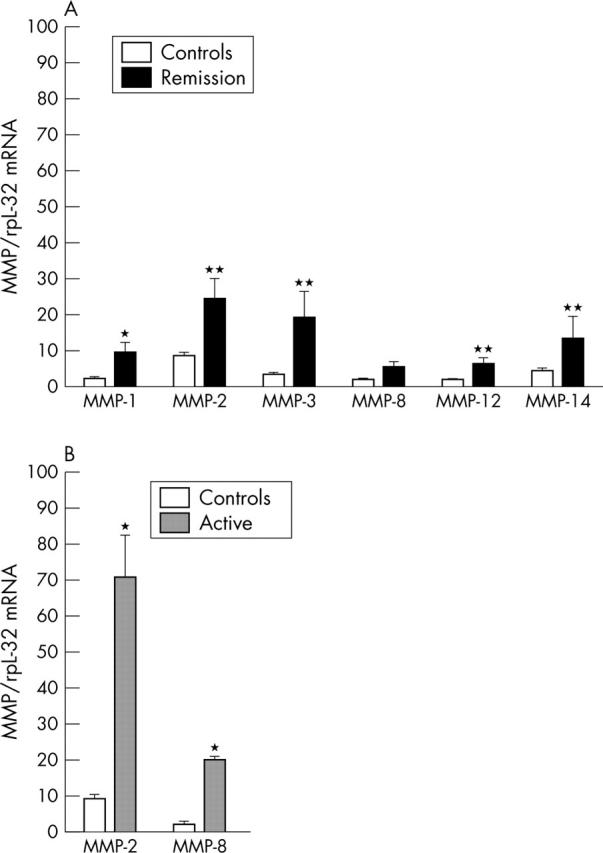
Gene expression (RPA) of MMPs in relation to the control gene rpL32 in PBMCs from patients with WG classified as being in remission (n = 8) (A) or as having active disease (n = 7) (B) according to the BVAS, serum levels of CRP, and ESR (see table 1) and 15 healthy controls. Data are given as mean (SEM). *p<0.01 and **p<0.001 versus healthy controls.
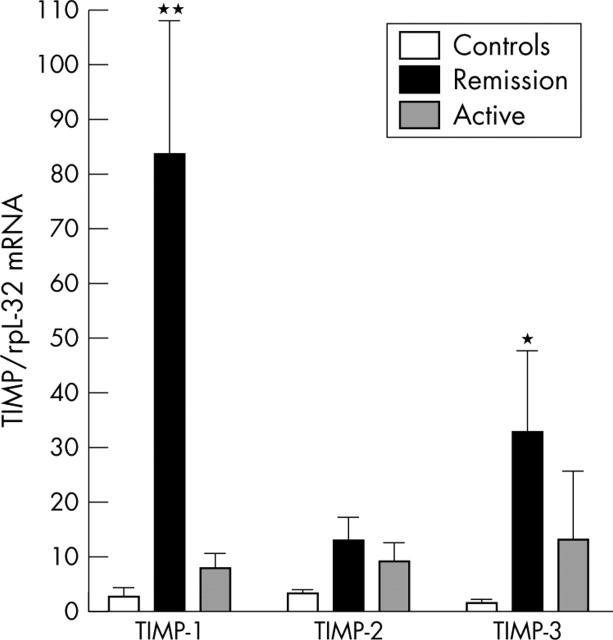
Figure 2.
Gene expression (RPA) of TIMPs in relation to the control gene rpL32 in PBMCs from 15 patients with WG classified as having active disease (n = 7) or being in remission (n = 8) according to the BVAS, serum levels of CRP, and ESR (see table 1) and 15 healthy controls. Data are given as mean (SEM). *p<0.01 and **p<0.001 versus active disease and healthy controls.
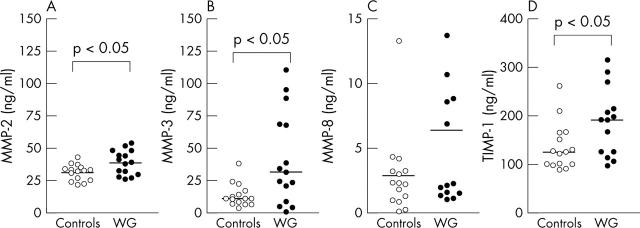
Figure 3.
Plasma levels of MMP-2 (A), MMP-3 (B), MMP-8 (C), and TIMP-1 (D) in 15 patients with WG and 15 healthy controls. Horizontal lines represent median values.
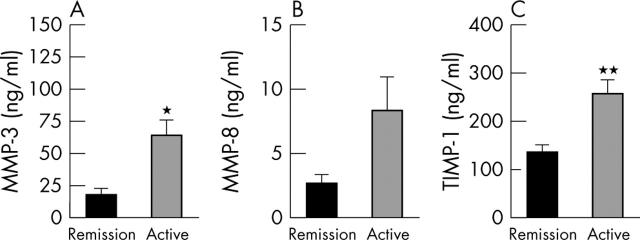
Figure 4.
Plasma levels of MMP-3 (A), MMP-8 (B), and TIMP-1 (C) in 15 patients with WG classified as having active disease (n = 7) or being in remission (n = 8) according to the BVAS, serum levels of CRP, and ESR (see table 1). Data are given as mean (SEM). *p<0.01 and **p<0.005 versus patients in remission.
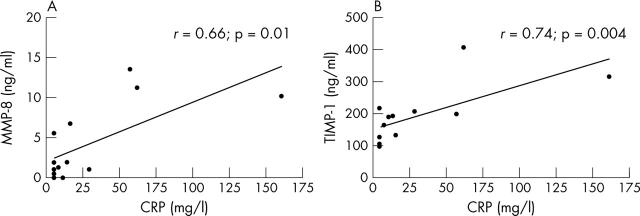
Figure 5.
Correlation between plasma levels of CRP and MMP-8 (A) and TIMP-1 (B) in 15 patients with WG.
Figure 6.
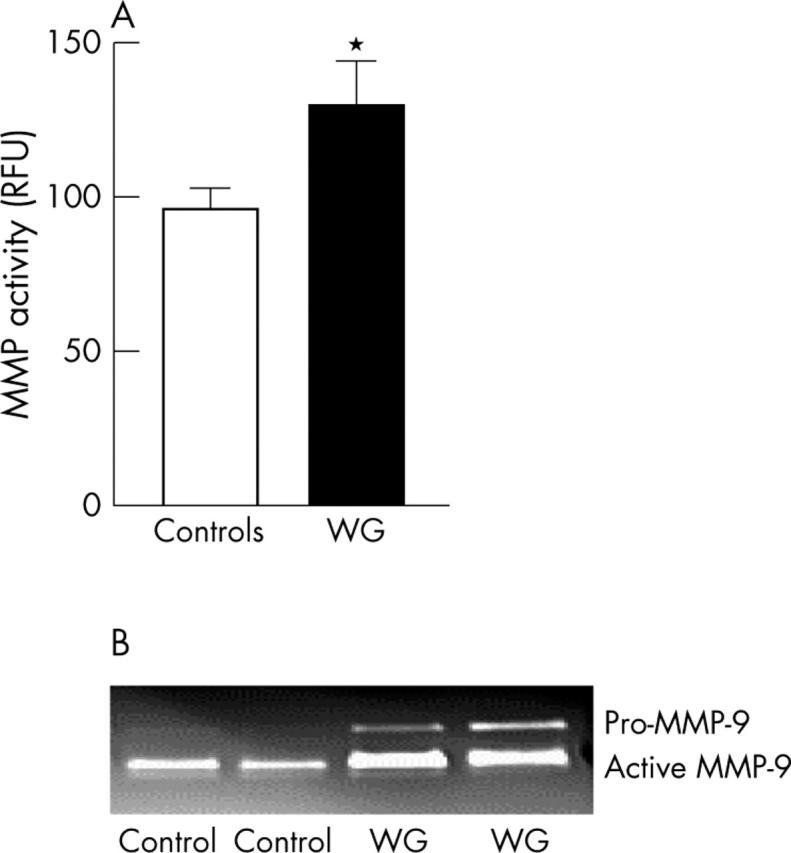
MMP activity in PBMC supernatants from patients with WG and healthy controls. (A) Total MMP activity in nine patients with WG and eight healthy controls using a fluorogenic peptide substrate (see "Patients and methods"). (B) Gelatinolytic (MMP-9) activity in PBMC supernatants after incubation for 18 hours in two representative patients with WG and two healthy controls. Gelatinolytic activity was seen as lytic bands and the majority of these were identified as pro-MMP-9 and active MMP-9. Note: pro-MMP-9 was only detected in the WG group. RFU, relative fluorescence units.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson Jeffrey J., Senior Robert M. Matrix metalloproteinase-9 in lung remodeling. Am J Respir Cell Mol Biol. 2003 Jan;28(1):12–24. doi: 10.1165/rcmb.2002-0166TR. [DOI] [PubMed] [Google Scholar]
- Billinghurst R. C., Wu W., Ionescu M., Reiner A., Dahlberg L., Chen J., van Wart H., Poole A. R. Comparison of the degradation of type II collagen and proteoglycan in nasal and articular cartilages induced by interleukin-1 and the selective inhibition of type II collagen cleavage by collagenase. Arthritis Rheum. 2000 Mar;43(3):664–672. doi: 10.1002/1529-0131(200003)43:3<664::AID-ANR24>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Bottomley K. M., Borkakoti N., Bradshaw D., Brown P. A., Broadhurst M. J., Budd J. M., Elliott L., Eyers P., Hallam T. J., Handa B. K. Inhibition of bovine nasal cartilage degradation by selective matrix metalloproteinase inhibitors. Biochem J. 1997 Apr 15;323(Pt 2):483–488. doi: 10.1042/bj3230483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damås J. K., Eiken H. G., Oie E., Bjerkeli V., Yndestad A., Ueland T., Tonnessen T., Geiran O. R., Aass H., Simonsen S. Myocardial expression of CC- and CXC-chemokines and their receptors in human end-stage heart failure. Cardiovasc Res. 2000 Sep;47(4):778–787. doi: 10.1016/s0008-6363(00)00142-5. [DOI] [PubMed] [Google Scholar]
- Faber-Elmann A., Sthoeger Z., Tcherniack A., Dayan M., Mozes E. Activity of matrix metalloproteinase-9 is elevated in sera of patients with systemic lupus erythematosus. Clin Exp Immunol. 2002 Feb;127(2):393–398. doi: 10.1046/j.1365-2249.2002.01758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanemaaijer R., Sorsa T., Konttinen Y. T., Ding Y., Sutinen M., Visser H., van Hinsbergh V. W., Helaakoski T., Kainulainen T., Rönkä H. Matrix metalloproteinase-8 is expressed in rheumatoid synovial fibroblasts and endothelial cells. Regulation by tumor necrosis factor-alpha and doxycycline. J Biol Chem. 1997 Dec 12;272(50):31504–31509. doi: 10.1074/jbc.272.50.31504. [DOI] [PubMed] [Google Scholar]
- Hayashi K., Horikoshi S., Osada S., Shofuda K., Shirato I., Tomino Y. Macrophage-derived MT1-MMP and increased MMP-2 activity are associated with glomerular damage in crescentic glomerulonephritis. J Pathol. 2000 Jul;191(3):299–305. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH637>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Hui W., Rowan A. D., Cawston T. Insulin-like growth factor 1 blocks collagen release and down regulates matrix metalloproteinase-1, -3, -8, and -13 mRNA expression in bovine nasal cartilage stimulated with oncostatin M in combination with interleukin 1alpha. Ann Rheum Dis. 2001 Mar;60(3):254–261. doi: 10.1136/ard.60.3.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennette J. C., Falk R. J., Andrassy K., Bacon P. A., Churg J., Gross W. L., Hagen E. C., Hoffman G. S., Hunder G. G., Kallenberg C. G. Nomenclature of systemic vasculitides. Proposal of an international consensus conference. Arthritis Rheum. 1994 Feb;37(2):187–192. doi: 10.1002/art.1780370206. [DOI] [PubMed] [Google Scholar]
- Komocsi Andras, Lamprecht Peter, Csernok Elena, Mueller Antje, Holl-Ulrich Konstanze, Seitzer Ulrike, Moosig Frank, Schnabel Armin, Gross Wolfgang Ludwig. Peripheral blood and granuloma CD4(+)CD28(-) T cells are a major source of interferon-gamma and tumor necrosis factor-alpha in Wegener's granulomatosis. Am J Pathol. 2002 May;160(5):1717–1724. doi: 10.1016/s0002-9440(10)61118-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamprecht P., Kumanovics G., Mueller A., Csernok E., Komocsi A., Trabandt A., Gross W. L., Schnabel A. Elevated monocytic IL-12 and TNF-alpha production in Wegener's granulomatosis is normalized by cyclophosphamide and corticosteroid therapy. Clin Exp Immunol. 2002 Apr;128(1):181–186. doi: 10.1046/j.1365-2249.2002.01801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leavitt R. Y., Fauci A. S., Bloch D. A., Michel B. A., Hunder G. G., Arend W. P., Calabrese L. H., Fries J. F., Lie J. T., Lightfoot R. W., Jr The American College of Rheumatology 1990 criteria for the classification of Wegener's granulomatosis. Arthritis Rheum. 1990 Aug;33(8):1101–1107. doi: 10.1002/art.1780330807. [DOI] [PubMed] [Google Scholar]
- Lechapt-Zalcman E., Coste A., d'Ortho M. P., Frisdal E., Harf A., Lafuma C., Escudier E. Increased expression of matrix metalloproteinase-9 in nasal polyps. J Pathol. 2001 Feb;193(2):233–241. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH771>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Luqmani R. A., Bacon P. A., Moots R. J., Janssen B. A., Pall A., Emery P., Savage C., Adu D. Birmingham Vasculitis Activity Score (BVAS) in systemic necrotizing vasculitis. QJM. 1994 Nov;87(11):671–678. [PubMed] [Google Scholar]
- Matrisian L. M. Metalloproteinases and their inhibitors in matrix remodeling. Trends Genet. 1990 Apr;6(4):121–125. doi: 10.1016/0168-9525(90)90126-q. [DOI] [PubMed] [Google Scholar]
- McCawley L. J., Matrisian L. M. Matrix metalloproteinases: they're not just for matrix anymore! Curr Opin Cell Biol. 2001 Oct;13(5):534–540. doi: 10.1016/s0955-0674(00)00248-9. [DOI] [PubMed] [Google Scholar]
- Mengshol John A., Mix Kimberlee S., Brinckerhoff Constance E. Matrix metalloproteinases as therapeutic targets in arthritic diseases: bull's-eye or missing the mark? Arthritis Rheum. 2002 Jan;46(1):13–20. doi: 10.1002/1529-0131(200201)46:1<13::aid-art497>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Muller Kobold A. C., Kallenberg C. G., Tervaert J. W. Monocyte activation in patients with Wegener's granulomatosis. Ann Rheum Dis. 1999 Apr;58(4):237–245. doi: 10.1136/ard.58.4.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohbayashi Hiroyuki. Matrix metalloproteinases in lung diseases. Curr Protein Pept Sci. 2002 Aug;3(4):409–421. doi: 10.2174/1389203023380549. [DOI] [PubMed] [Google Scholar]
- Power C., O'Connor C. M., MacFarlane D., O'Mahoney S., Gaffney K., Hayes J., FitzGerald M. X. Neutrophil collagenase in sputum from patients with cystic fibrosis. Am J Respir Crit Care Med. 1994 Sep;150(3):818–822. doi: 10.1164/ajrccm.150.3.8087357. [DOI] [PubMed] [Google Scholar]
- Schnabel A., Csernok E., Braun J., Gross W. L. Activation of neutrophils, eosinophils, and lymphocytes in the lower respiratory tract in Wegener's granulomatosis. Am J Respir Crit Care Med. 2000 Feb;161(2 Pt 1):399–405. doi: 10.1164/ajrccm.161.2.9904076. [DOI] [PubMed] [Google Scholar]
- Shaida A., Kenyon G., Devalia J., Davies R. J., MacDonald T. T., Pender S. L. Matrix metalloproteinases and their inhibitors in the nasal mucosa of patients with perennial allergic rhinitis. J Allergy Clin Immunol. 2001 Nov;108(5):791–796. doi: 10.1067/mai.2001.119024. [DOI] [PubMed] [Google Scholar]
- Siwik D. A., Chang D. L., Colucci W. S. Interleukin-1beta and tumor necrosis factor-alpha decrease collagen synthesis and increase matrix metalloproteinase activity in cardiac fibroblasts in vitro. Circ Res. 2000 Jun 23;86(12):1259–1265. doi: 10.1161/01.res.86.12.1259. [DOI] [PubMed] [Google Scholar]
- Visse Robert, Nagase Hideaki. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003 May 2;92(8):827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- Waehre T., Halvorsen B., Damås J. K., Yndestad A., Brosstad F., Gullestad L., Kjekshus J., Frøland S. S., Aukrust P. Inflammatory imbalance between IL-10 and TNFalpha in unstable angina potential plaque stabilizing effects of IL-10. Eur J Clin Invest. 2002 Nov;32(11):803–810. doi: 10.1046/j.1365-2362.2002.01069.x. [DOI] [PubMed] [Google Scholar]
- Wiercinska-Drapalo Alicja, Jaroszewicz Jerzy, Flisiak Robert, Prokopowicz Danuta. Plasma matrix metalloproteinase-1 and tissue inhibitor of metalloproteinase-1 as biomarkers of ulcerative colitis activity. World J Gastroenterol. 2003 Dec;9(12):2843–2845. doi: 10.3748/wjg.v9.i12.2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi E. S., Colby T. V. Wegener's granulomatosis. Semin Diagn Pathol. 2001 Feb;18(1):34–46. [PubMed] [Google Scholar]
- Yoshihara Y., Nakamura H., Obata K., Yamada H., Hayakawa T., Fujikawa K., Okada Y. Matrix metalloproteinases and tissue inhibitors of metalloproteinases in synovial fluids from patients with rheumatoid arthritis or osteoarthritis. Ann Rheum Dis. 2000 Jun;59(6):455–461. doi: 10.1136/ard.59.6.455. [DOI] [PMC free article] [PubMed] [Google Scholar]