Insulin induces phosphatidylinositol-3-phosphate formation through TC10 activation (original) (raw)
Abstract
Phosphatidylinositol-3-phosphate (PtdIns-3-P) is considered as a lipid constitutively present on endosomes; it does not seem to have a dynamic role in signalling. In contrast, phosphatidylinositol-3,4,5-trisphosphate (PtdIns-3,4,5-P3) plays a crucial role in different signalling pathways including translocation of the glucose transporter protein GLUT4 to the plasma membrane upon insulin receptor activation. GLUT4 translocation requires activation of two distinct pathways involving phosphatidylinositol 3-kinase (PI 3-K) and the small GTP-binding protein TC10, respectively. The contribution of each pathway remains to be elucidated. Here we show that insulin specifically induces the formation of PtdIns-3-P in insulin- responsive cells. The insulin-mediated formation of PtdIns-3-P occurs through the activation of TC10 at the lipid rafts subdomain of the plasma membrane. Exogenous PtdIns-3-P induces the plasma membrane translocation of both overexpressed and endogenous GLUT4. These data indicate that PtdIns-3-P is specifically produced downstream from insulin-mediated activation of TC10 to promote the plasma membrane translocation of GLUT4. These results give a new insight into the intracellular role of PtdIns-3-P and shed light on some aspects of insulin signalling so far not completely understood.
Keywords: GLUT4/insulin/phosphatidylinositol 3-kinase/phosphatidylinositol-3-phosphate/TC10
Introduction
Phosphatidylinositol 3-kinases (PI 3-Ks) phosphorylate position D-3 of phosphoinositides leading to the formation of 3′-phosphorylated phosphoinositides. Different PI 3-Ks have been identified and grouped in three classes on the basis of their sequence homology and in vitro substrate specificity (Vanhaesebroeck et al., 2001). Among the different products of PI 3-Ks, resting mammalian cells contain detectable levels of phosphatidylinositol-3-phosphate (PtdIns-3-P) mainly localized on endosomes (Gillooly et al., 2001) that do not change upon cellular stimulation. On the contrary, phosphatidylinositol-3,4,5-trisphosphate (PtdIns-3,4,5-P3) is barely detected in resting cells but its intracellular concentration rapidly increases upon stimulation by growth factors and cytokines and it plays a pivotal role in many different physiological events, including cell proliferation, motility, receptor internalization and glucose transport (Rameh and Cantley, 1999).
Disposal of glucose into fat and muscle cells requires the insulin-induced translocation of the GLUT4 glucose transporter protein from intracellular storage sites to the plasma membrane (Summers et al., 1999; Watson and Pessin, 2001). The signalling pathway that links the insulin receptor to GLUT4 translocation is still controversial. It has been well established that activation of a PI 3-K-dependent pathway is necessary for this trafficking event (Shepherd et al., 1998) but several lines of evidence suggest that it is not sufficient and a second signalling pathway is also required (Isakoff et al., 1995; Wiese et al., 1995; Jiang et al., 1998; Czech and Corvera, 1999). Apparently, this additional pathway is PI 3-K-independent and involves activation of the small GTP-binding protein TC10 (Baumann et al., 2000; Chiang et al., 2001). Although a direct or indirect role in cytoskeleton rearrangement has been proposed for TC10, its precise mechanism of action leading to GLUT4 translocation is still far from being completely understood. Here we show that insulin specifically increases the levels of PtdIns-3-P in L6 cells and 3T3-L1 adipocytes. The insulin-dependent pool of PtdIns-3-P is produced at the level of the plasma membrane and is relatively resistant to PI 3-K inhibitors. A constitutively active mutant of TC10 mimics the effect of insulin, whereas a dominant-negative mutant of TC10 inhibits the formation of PtdIns-3-P upon insulin stimulation, thus indicating that the insulin-mediated formation of PtdIns-3-P occurs through the activation of TC10. Furthermore, the insulin/TC10-mediated formation of PtdIns-3-P occurs at the lipid rafts subdomain of the plasma membrane where TC10 is localized. We finally show that exogenous PtdIns-3-P induces the plasma membrane translocation of both overexpressed and endogenous GLUT4. Taken together, these data demonstrate that PtdIns-3-P, as with other products of PI 3-Ks, can act as a second messenger in a specific pathway, downstream from insulin-mediated activation of TC10, to promote the plasma membrane translocation of GLUT4.
Results
Insulin induces PtdIns-3-P formation in L6 cells and 3T3-L1 adipocytes
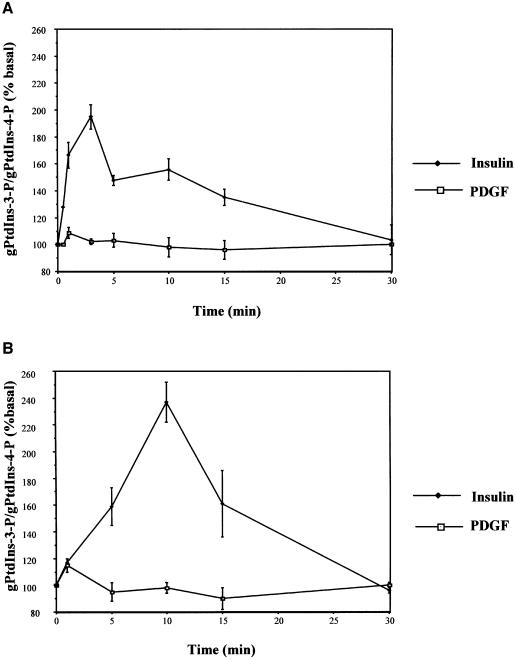
Activation of PI 3-Ks is a common step of several signalling pathways although it leads to different downstream effects. To address whether the peculiar effects of PI 3-Ks activation in insulin signalling could be associated with qualitative or quantitative differences in phosphoinositide metabolism, we labelled L6 cells and 3T3-L1 adipocytes with _myo_-[3H]inositol and performed a time course of insulin effects using HPLC analysis of [3H]glycerophosphoinositides. Surprisingly, we found that insulin increased the levels of PtdIns-3-P both in L6 cells (Figure 1A) and in 3T3-L1 adipocytes (Figure 1B). PtdIns-3-P formation was very rapid in both cell types although the effect was more persistent in 3T3-L1, lasting ∼15 min. No difference in PtdIns-3-P levels was observed in platelet-derived growth factor (PDGF)-stimulated cells (Figure 1A and B). When we analysed the levels of the other 3′-phosphorylated phosphoinositides in insulin- and PDGF-stimulated L6 cells, we observed that insulin induced a persistent formation of PtdIns-3,4,5-P3 and a slower and more persistent formation of phosphatidylinositol-3,4,-bisphosphate (PtdIns-3,4-P2). In contrast, PDGF induced a rapid but less persistent increase in the levels of both phosphoinositides. More interestingly, upon 3–5 min of stimulation, the levels of both PtdIns-3,4,5-P3 and PtdIns-3,4-P2 were higher in PDGF- than in insulin-stimulated cells (Supplementary figure 1, available at The EMBO Journal Online). Increases in the levels of PtdIns-3,4,5-P3 and PtdIns-3,4-P2 upon insulin stimulation have already been reported (Ruderman et al., 1990) although, to our knowledge, our work is the first report of a complete time course of insulin-induced formation of all the 3′-phosphorylated phosphoinositides.
Fig. 1. Insulin induces PtdIns-3-P formation in L6 cells and 3T3-L1 adipocytes. (A) L6 cells and (B) 3T3-L1 adipocytes were labelled with _myo_-[3H]inositol for 24 h and then stimulated with 300 nM insulin or 20 ng/ml PDGF. Phospholipids were extracted at different times of stimulation, deacylated and analysed by HPLC as described in Materials and methods. Data are mean ± SEM (A, n = 6; B, n = 2).
These results argue that PtdIns-3-P can be produced upon cellular stimulation, and that the effect may be insulin-specific.
The insulin-dependent pool of PtdIns-3-P is generated at the plasma membrane
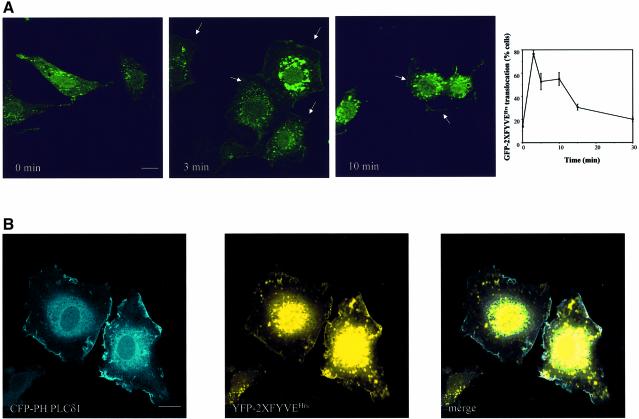
It is well established that constitutive PtdIns-3-P is localized on endosomes (Gillooly et al., 2000). To study the intracellular localization of the insulin-dependent pool of PtdIns-3-P, L6 cells were transfected with a cDNA encoding the double FYVE domain from the hepatocyte-growth-factor-regulated tyrosine kinase substrate (Hrs) fused to green fluorescent protein (GFP–2XFYVEHrs) that is a specific probe for PtdIns-3-P (Gillooly et al., 2000). Confocal microscopy revealed that in L6-transfected cells, GFP–2XFYVEHrs was localized only in endosomal structures in the absence of insulin (Figure 2A, 0 min) but it clearly translocated to the plasma membrane upon 3 min of insulin stimulation. Translocation was still evident at 10 min (Figure 2A) and it almost completely disappeared at 15 min (data not shown). Quantitative analyses revealed that the time course of GFP–2XFYVEHrs translocation to the plasma membrane was similar to the HPLC pattern of PtdIns-3-P formation upon insulin stimulation (plot in Figure 2A). GFP–2XFYVEHrs translocation upon insulin stimulation was also observed in living L6 cells (Supplementary figure 2). Comparison of intracellular and plasma membrane localization of GFP–2XFYVEHrs is further discussed in Supplementary data (Supplementary figure 3). To definitely rule out the possibility that the peripheral accumulation of PtdIns-3-P is associated with sub-membranous endosomes, we decided to stain the plasma membrane without prior permeabilization by using the isolated pleckstrin homology domain from phospholipase Cδ1 (PH PLCδ1) that is known to strongly bind phosphatidylinositol-4,5,-bisphosphate and is constitutively present at the plasma membrane (Maffucci and Falasca, 2001). L6 cells were then co-transfected with cDNAs encoding the 2XFYVEHrs fused to the yellow fluorescent protein (YFP–2XFYVEHrs) and the PH PLCδ1 fused to the cyan fluorescent protein (CFP–PH PLC δ1), respectively. Confocal microscopy analysis of insulin-stimulated cells revealed a clear colocalization of the two proteins at the plasma membrane, confirming that insulin induces translocation of the PtdIns-3-P probe to the plasma membrane (Figure 2B). Translocation of GFP–2XFYVEHrs was specifically induced by insulin since neither epidermal growth factor (EGF) nor PDGF affected the endosomal localization of the probe, as discussed in Supplementary data (Supplementary figure 4).
Fig. 2. Insulin induces the translocation of the PtdIns-3-P probe 2XFYVEHrs to the plasma membrane in L6 cells. (A) GFP–2XFYVEHrs-transfected L6 cells were serum deprived for 6 h and then left untreated (0 min) or stimulated with 300 nM insulin. At the indicated times of stimulation, cells were fixed and analysed by confocal microscopy. Arrows indicate the plasma membrane localization. Bar, 10 µm. Data on plot are mean ± SEM (n = 3–5). (B) L6 cells were co-transfected with cDNAs encoding YFP–2XFYVEHrs and the plasma membrane marker CFP–PH PLCδ1, respectively. After 24 h, cells were serum deprived for 6 h and stimulated with 300 nM insulin for 3 min before fixing for confocal microscopy analysis. Bar, 10 µm.
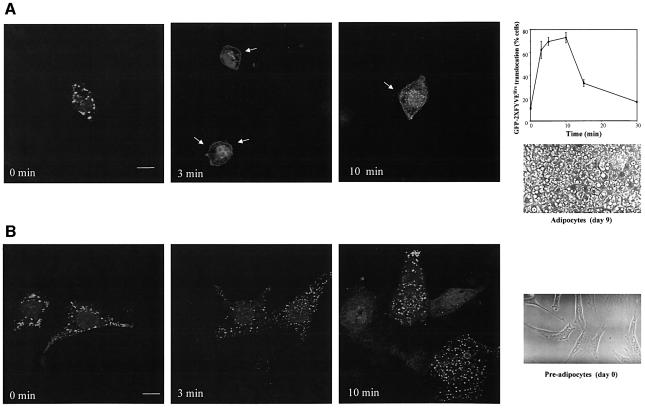
The insulin-mediated translocation of GFP–2XFYVEHrs was also observed in fully differentiated 3T3-L1 adipocytes electroporated with the corresponding cDNA (Figure 3A). As for L6 cells, translocation paralleled the HPLC pattern of PtdIns-3-P (plot in Figure 3A). No plasma membrane translocation of the PtdIns-3-P probe was observed in 3T3-L1 pre-adipocytes upon insulin stimulation (Figure 3B), confirming that the effect was specific to insulin-responsive cells. Full differentiation of adipocytes was confirmed both by accumulation of lipid droplets, shown in the phase-contrast images in Figure 3A and B and assessed by Oil Red O staining (data not shown), and by western blot analyses of GLUT4 expression (data not shown).
Fig. 3. Insulin recruits the GFP–2XFYVEHrs fusion protein to the plasma membrane of 3T3-L1 adipocytes. (A) Fully differentiated 3T3-L1 adipocytes or (B) 3T3-L1 pre-adipocytes were electroporated with the cDNA encoding GFP–2XFYVEHrs. After 24 h, cells were serum deprived for 3 h and then left untreated (0 min) or stimulated with 300 nM insulin for different times before fixing for confocal microscopy analysis. Arrows mark the plasma membrane localization. Bar, 10 µm. Data on plot are mean ± SEM (n = 2–3). Phase-contrast images show 3T3-L1 pre-adipocytes (day 0) and adipocytes at 9 days after initiation of differentiation.
The lipid specificity of these observations was confirmed by using different fluorescent probes for PtdIns-3-P, as described in Supplementary data (Supplementary figure 5). Taken together, these results indicate that, in insulin-responsive cells, insulin can specifically modulate the intracellular levels of PtdIns-3-P by inducing the formation of a pool of this phosphoinositide at the plasma membrane.
The insulin-mediated formation of PtdIns-3-P is relatively resistant to PI 3-K inhibitors
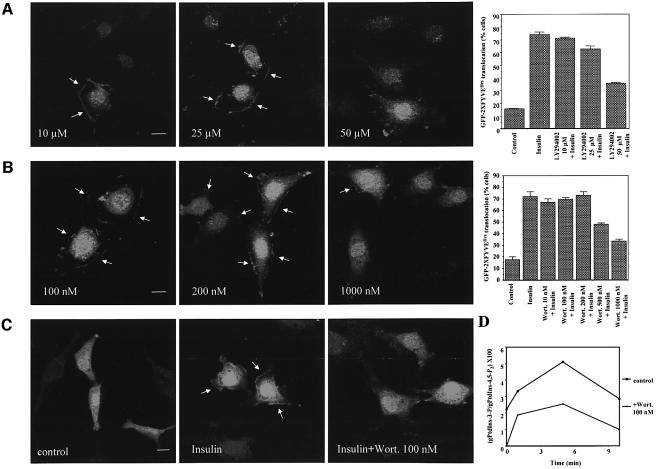
To determine whether the insulin-mediated formation of PtdIns-3-P was sensitive to PI 3-K inhibitors, GFP–2XFYVEHrs-transfected L6 cells were pre-treated with different concentrations of the reversible PI 3-K specific inhibitor LY294002 before stimulation with insulin for 3 min. Confocal microscopy studies and quantitative analyses showed that a pre-treatment with up to 25 µM LY294002 did not inhibit the insulin-induced plasma membrane translocation of GFP–2XFYVEHrs (Figure 4A), whereas it completely removed the endosomal localization of the probe. A concentration of 50 µM LY294002 partially abrogated the translocation (Figure 4A). Similarly, the insulin-induced plasma membrane translocation of GFP–2XFYVEHrs was not affected by a pre-treatment with the irreversible PI 3-K specific inhibitor wortmannin up to 200 nM, although a pre-treatment with 100 nM wortmannin was sufficient to remove the endosomal localization (Figure 4B). Plasma membrane staining was still present, although reduced, after a pre-treatment with 1000 nM wortmannin (Figure 4B). In contrast, a pre-treatment with 100 nM wortmannin completely inhibited the insulin-dependent translocation of the PH domain from protein kinase B fused to the GFP (GFP–PH PKB/Akt) that binds to both PtdIns-3,4-P2 and PtdIns-3,4,5-P3 (Figure 4C). The plasma membrane translocation of GFP–2XFYVEHrs after a pre-treatment with 200 nM wortmannin was also observed in L6-transfected living cells (Supplementary figure 6) and in GFP–PX SNX3- and GFP–PX p40phox-transfected L6 cells (data not shown). To further confirm these results, we compared the levels of PtdIns-3-P at different times of insulin stimulation in untreated L6 cells and in cells pre-treated with 100 nM wortmannin. HPLC analyses revealed that pre-treatment with wortmannin did not inhibit the insulin-dependent formation of PtdIns-3-P (Figure 4D).
Fig. 4. PtdIns-3-P formation at the plasma membrane is relatively resistant to PI 3-K inhibitors. (A and B) GFP–2XFYVEHrs-transfected L6 cells were pre-treated with the indicated concentrations of LY294002 (A) or wortmannin (B) for 15 min at 37°C and then stimulated with 300 nM insulin for 3 min. GFP–2XFYVEHrs intracellular localization was then assessed by confocal microscopy. Bar, 10 µm. Data on plot are mean ± SEM (n = 2). (C) L6 cells were transfected with GFP–PH PKB/Akt, serum deprived for 6 h and then stimulated with 300 nM insulin for 3 min. When indicated, cells were pre-treated with 100 nM wortmannin for 15 min before insulin stimulation. (D) _myo_-[3H]inositol-labelled L6 cells were left untreated or pre-treated with 100 nM wortmannin before stimulation with 300 nM insulin for different times. Deacylated phospholipids were analysed by HPLC as described in Materials and methods. Data are from one experiment representative of two independent experiments.
These data indicate that the formation of the insulin-dependent pool of PtdIns-3-P is relatively resistant to wortmannin and LY294002. In addition, these data definitely rule out the possibility that the insulin-dependent pool of PtdIns-3-P might simply be a degradation product of PtdIns-3,4,5-P3 since wortmannin completely inhibited the formation of PtdIns-3,4,5-P3 upon insulin stimulation (data not shown). Furthermore, the observation that wortmannin completely abolished the endosomal localization of GFP–2XFYVEHrs without inhibiting the insulin-induced formation of PtdIns-3-P rules out the possibility that insulin increases the levels of the endosomal pool of PtdIns-3-P and that the peripheral accumulation of PtdIns-3-P is associated with sub-membranous endosomal structures.
The insulin-mediated formation of PtdIns-3-P occurs through the activation of TC10
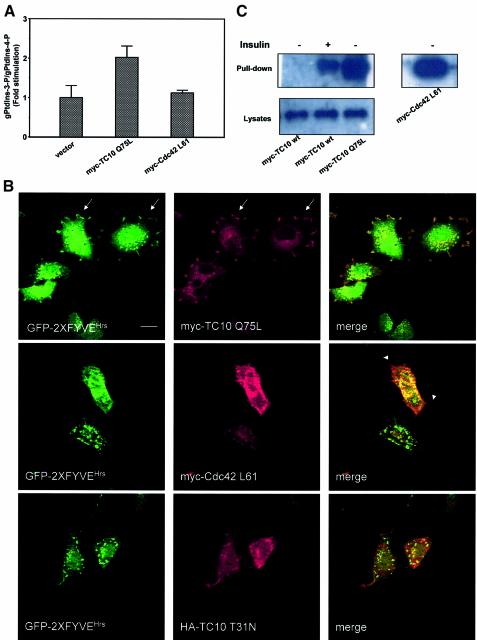
The wortmannin and LY294002 resistance led us to the conclusion that the insulin effect observed here might be related to the TC10-dependent pathway. To determine whether PtdIns-3-P formation occurs downstream of TC10 activation, L6 cells were transfected with a constitutively active mutant of TC10 (myc-TC10 Q75L) or the empty vector or a myc-tagged constitutively active mutant of Cdc42 (myc-Cdc42 L61). After labelling with _myo_-[3H]inositol, phospholipids were extracted, deacylated and the levels of PtdIns-3-P were analysed by HPLC. Overexpression of myc-TC10 Q75L induced a 2-fold increase in the levels of PtdIns-3-P, whereas overexpression of myc-Cdc42 L61 had no effect (Figure 5A). No increase in the levels of PtdIns-3,4,5-P3 was observed in myc-TC10 Q75L-transfected cells, further confirming that, in this context, PtdIns-3-P is not a breakdown product of PtdIns-3,4,5-P3. We next analysed the levels of PtdIns-3-P in L6 cells transfected with a dominant-negative mutant of TC10, HA-TC10 T31N, labelled and stimulated with insulin for 3 min. HPLC analyses revealed that overexpression of the dominant-negative TC10 completely inhibited the insulin-induced formation of PtdIns-3-P (Supplementary figure 7A). The transfection efficiency of both TC10 mutants is shown in Supplementary figure 7B.
Fig. 5. The insulin-mediated formation of PtdIns-3-P occurs through the activation of TC10. (A) L6 cells were transfected with myc-TC10 Q75L, myc-Cdc42 L61 or the empty vector. After 24 h, cells were labelled with _myo_-[3H]inositol and HPLC analysis of PtdIns-3-P levels was performed as described in Materials and methods. Data are mean ± SEM of two independent experiments performed in duplicate. (B) L6 cells were co-transfected with GFP–2XFYVEHrs and either myc-TC10 Q75L, myc-Cdc42 L61 or HA-TC10 T31N. After 24 h, cells were serum starved for 6 h and analysed by confocal microscopy. HA-TC10 T31N-co-transfected cells were stimulated with 300 nM insulin for 3 min before confocal analysis. Arrows indicate the plasma membrane localization. Arrowheads mark the filopodia induced by overexpression of myc-Cdc42 L61. Bar, 10 µm. (C) L6 cells were transfected with myc-TC10 wt, myc-TC10 Q75L or myc-Cdc42 L61. Serum-deprived cells were left untreated or stimulated with insulin for 3 min. Lysates were incubated with GST-Pak1 PBD and pull-down assay was performed as described in Materials and methods. Association of the overexpressed proteins with the GST-Pak1 PBD was assessed in western blot analyses by using an anti-myc antibody (upper panels). Equal amount of the overexpressed proteins in lysates was confirmed by loading an aliquot of each lysate (bottom panel). Blot is representative of three independent experiments.
To confirm these results, L6 cells were co-transfected with GFP–2XFYVEHrs and myc-TC10 Q75L or myc-Cdc42 L61, and the intracellular localization of the fluorescent probe was analysed by confocal microscopy. In serum-starved cells, overexpression of myc-TC10 Q75L mimicked the insulin effect, inducing the plasma membrane translocation of the probe (Figure 5B). Furthermore, the merged image reveals the co-localization of GFP–2XFYVEHrs and myc-TC10 Q75L at the plasma membrane. Neither myc-Cdc42 L61 (Figure 5B) nor a myc-tagged constitutively active mutant of Rac1 (data not shown) induced the GFP–2XFYVEHrs plasma membrane translocation. We then analysed the intracellular localization of the PtdIns-3-P probe in L6 cells co-transfected with GFP–2XFYVEHrs and the dominant-negative HA-TC10 T31N and stimulated with insulin for 3 min. Overexpression of the dominant-negative mutant of TC10 inhibited the insulin-induced translocation of the probe (Figure 5B). In contrast, no inhibition was observed when the dominant-negative mutants of Cdc42 or Rac1 were co-expressed (data not shown). These data indicate that activation of TC10 induces the formation of the plasma membrane-associated pool of PtdIns-3-P.
To test whether insulin can activate TC10 in L6 cells, we performed the same assay used to demonstrate TC10 activation in 3T3-L1 adipocytes. The insulin-induced activation of TC10 has been demonstrated by using a GST-p21-activated kinase 1 (Pak1) pull-down assay in 3T3-L1 adipocytes overexpressing a tagged wild-type TC10 (Chiang et al., 2001). L6 cells were then transfected with a myc-tagged wild-type TC10 and lysates from untreated and insulin-stimulated cells were incubated with a GST–Pak1 PBD fusion protein that specifically binds to and precipitates Rac/Cdc42 and TC10 in their GTP-bound form. Precipitation of myc-TC10 wt was revealed only in extracts from insulin-stimulated cells (Figure 5C), indicating that insulin induced activation of TC10. Pull-down assays from L6 cells transfected with the constitutively active myc-TC10 Q75L and myc-Cdc42 L61 revealed that both proteins were efficiently precipitated (Figure 5C), confirming that these mutants were in their GTP-bound form. In the same assay, PDGF was not able to activate myc-TC10 wt (Supplementary figure 7C), in agreement with our observation that PDGF did not alter the levels (Figure 1A) and the intracellular localization (Supplementary figure 4) of PtdIns-3-P. These data demonstrate that insulin specifically can activate TC10 in L6 cells. Taken together, these data indicate that insulin induces PtdIns-3-P formation through TC10 activation.
The insulin/TC10-dependent pool of PtdIns-3-P is generated at the lipid rafts subdomain of the plasma membrane
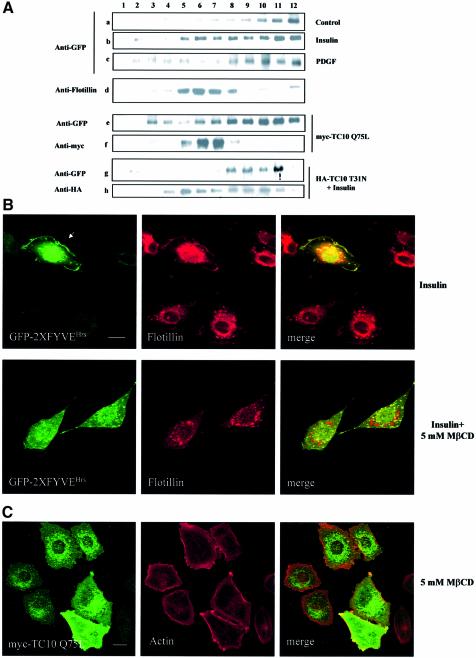
It has been reported that TC10 is localized in the lipid rafts subdomain of the plasma membrane (Watson et al., 2001). To check whether the TC10-mediated formation of PtdIns-3-P occurs at the level of this subdomain, we performed sucrose gradient fractionations of GFP–2XFYVEHrs-transfected L6 cells untreated or stimulated with insulin for 3 min. The localization of the fusion protein in the different fractions was then assessed in western blot analyses by using an anti-GFP antibody. Flotillin was used as a marker of the rafts fractions. A clear translocation of GFP–2XFYVEHrs in the flotillin-enriched fractions (fractions 5–8) was observed in insulin- but not in PDGF-stimulated cells (Figure 6A).
Fig. 6. The insulin/TC10-dependent pool of PtdIns-3-P is generated at the lipid rafts subdomain of the plasma membrane. (A) GFP–2XFYVEHrs-transfected L6 cells (a–d) were serum deprived for 6 h and then left untreated (a and d) or stimulated with 300 nM insulin for 3 min (b) or with 20 ng/ml PDGF (c). Cells were then homogenized and fractionated as described in Materials and methods. GFP–2XFYVEHrs localization in the different fractions was then assessed in western blot analyses by using an anti-GFP antibody (a–c). Flotillin was used as a marker of rafts fractions (d). Alternatively, L6 cells were transfected with both GFP–2XFYVEHrs and myc-TC10 Q75L (e and f). After 24 h, cells were serum deprived for 6 h and sucrose gradient fractions were prepared. The fusion proteins were revealed by using an anti-GFP (e) and an anti-myc (f) antibody, respectively. Alternatively, L6 cells were transfected with both GFP–2XFYVEHrs and HA-TC10 T31N (g and h). After 24 h, cells were serum deprived for 6 h, stimulated with 300 nM insulin for 3 min and then fractionated as above. The fusion proteins were revealed by using an anti-GFP (g) and an anti-HA (h) antibody, respectively. Blot is representative of at least three independent experiments. (B) GFP–2XFYVEHrs-transfected L6 cells were serum deprived for 6 h and then left untreated or incubated with 5 mM MβCD for 50 min at 37°C before stimulation with 300 nM insulin for 3 min. After fixation, cells were permeabilized, incubated with an anti-flotillin antibody and analysed by confocal microscopy. Bar, 10 µm. (C) myc-TC10 Q75L-transfected L6 cells were incubated with 5 mM MβCD for 50 min at 37°C. After fixation, cells were permeabilized, incubated with an anti-myc antibody and Alexa 594–phalloidin and analysed by confocal microscopy. Bar, 10 µm.
To confirm that the insulin-dependent translocation of GFP–2XFYVEHrs to the lipid rafts occurs upon TC10 activation, sucrose gradient fractionations were performed in serum-deprived L6 cells co-transfected with the GFP–2XFYVEHrs and the constitutively active mutant myc-TC10 Q75L. Localization of both proteins was then assessed in western blot analysis by using an anti-GFP and an anti-myc antibody, respectively. Overexpression of myc-TC10 Q75L mimicked the insulin effect inducing the translocation of GFP–2XFYVEHrs to the rafts fractions (Figure 6A). Moreover, co-localization of flotillin and TC10 was observed in the rafts fractions (Figure 6A). These data confirm that overexpression of constitutively active TC10 can reproduce the insulin-induced formation of PtdIns-3-P leading to an intracellular re-localization of the GFP–2XFYVEHrs fusion protein. To confirm these results, similar experiments were performed in L6 cells transfected with the GFP–2XFYVEHrs and the dominant-negative mutant HA-TC10 T31N and stimulated with insulin. Localization of both proteins was then assessed in western blot analysis by using an anti-GFP and an anti-HA antibody, respectively. As shown in Figure 6A, overexpression of the dominant-negative mutant of TC10 completely abrogated the insulin-induced translocation of GFP–2XFYVEHrs to the rafts fractions. The different distribution of myc-TC10 Q75L and HA-TC10 T31N is in agreement with the different intracellular localization of the two mutants (Chang et al., 2002). Taken together, these data indicate that the insulin-dependent pool of PtdIns-3-P is generated at the lipid rafts subdomain of the plasma membrane through TC10 activation.
Confocal microscopy analyses of GFP–2XFYVEHrs-transfected L6 cells stimulated with insulin confirmed the co-localization of the fusion protein with the endogenous rafts marker flotillin (Figure 6B).
Since lipid rafts are cholesterol-sphingolipids-rich membrane subdomains, cholesterol-extracting drugs have been widely used as tools for disrupting rafts-associated signalling (Watson et al., 2001). To further confirm the lipid rafts compartmentalization of PtdIns-3-P formation, L6 cells were pre-treated with the cholesterol-extracting drug methyl-β-cyclodextrin (MβCD) before insulin stimulation. A pre-treatment with 5 mM MβCD completely removed the plasma membrane localization of flotillin and prevented the insulin-induced translocation of GFP–2XFYVEHrs (Figure 6B). Similarly, this pre-treatment completely removed the plasma membrane localization of myc-TC10 Q75L (Figure 6C) without perturbing the cytoskeleton (as shown by the actin staining in Figure 6C). A pre-treatment with 0.5 mM MβCD had no effect on the plasma membrane localization of both flotillin and myc-TC10 Q75L, and it did not inhibit the GFP–2XFYVEHrs plasma membrane translocation upon insulin stimulation (data not shown). Similar results were obtained in GFP–2XFYVEHrs-expressing 3T3-L1 adipocytes (Supplementary figure 8).
These data indicate that preservation of intact rafts subdomain at the plasma membrane is required for the insulin-mediated formation of PtdIns-3P.
Taken together, these results indicate that the insulin/TC10-dependent pool of PtdIns-3-P is specifically produced at the level of the lipid rafts subdomain of the plasma membrane.
PtdIns-3-P is involved in GLUT4 translocation to the plasma membrane
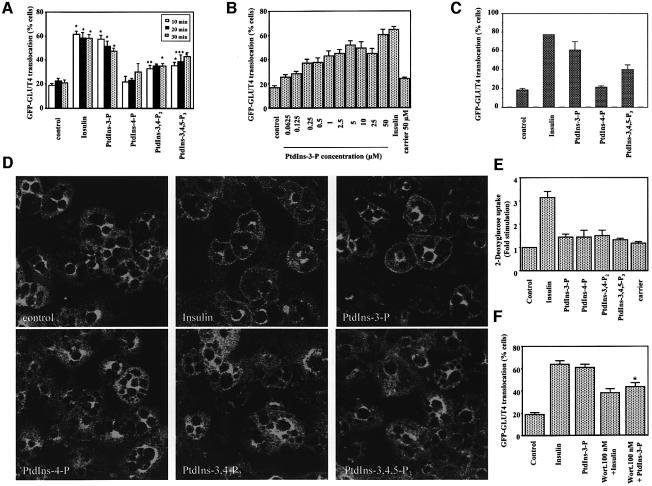
It is well established that endosomal PtdIns-3-P plays a crucial role in trafficking events. The major trafficking event occurring downstream of insulin receptor activation is translocation of GLUT4 from intracellular storage sites to the plasma membrane. To check whether the insulin-dependent pool of PtdIns-3-P is involved in this process, we tested the effect of exogenous PtdIns-3-P on both exogenous and endogenous GLUT4 intracellular localization. Efficient delivery of exogenous phosphoinositides by using polyamine carriers (Ozaki et al., 2000) is demonstrated in thr Supplementary data (Supplementary figure 9). L6 cells were transfected with a cDNA encoding the GFP–GLUT4 fusion protein. Serum-deprived cells were then incubated with insulin or with exogenous phosphoinositides for different times (Figure 7A). Quantitative analyses revealed that insulin induced the translocation of the fusion protein to the plasma membrane, as expected. More interestingly, we observed that PtdIns-3-P induced the GFP–GLUT4 plasma membrane translocation to the same extent as insulin (Figure 7A). No GFP–GLUT4 translocation was observed with PtdIns-4-P (Figure 7A) and PtdIns-5-P (data not shown), whereas PtdIns-3,4-P2 and PtdIns-3,4,5-P3 induced a partial translocation of GLUT4 at longer periods of stimulation (Figure 7A). When we tested the effect of increasing concentrations of PtdIns-3-P on GLUT4 translocation, we observed that a concentration of 5 µM was almost completely able to mimic the insulin effect, with a maximum reached at a concentration of 50 µM (Figure 7B). By contrast, carrier alone did not induce the GFP–GLUT4 plasma membrane translocation (Figure 7B). To confirm these results, GFP–GLUT4-expressing 3T3-L1 adipocytes were stimulated with insulin or incubated with different phosphoinositides. As for L6 cells, PtdIns-3-P induced the plasma membrane translocation of exogenous GLUT4 at a similar extent to insulin (Figure 7C).
Fig. 7. (A–C) Exogenous PtdIns-3-P induces plasma membrane translocation of exogenous GLUT4. (A) L6 cells were transfected with GFP–GLUT4. After 24 h, cells were serum deprived for 6 h and then stimulated with 300 nM insulin or with the indicated phosphoinositides at a final concentration of 50 µM. After incubation at 37°C for 10, 20 or 30 min, cells were fixed for quantitative analyses. (B) GFP–GLUT4-transfected L6 cells were incubated for 10 min with 300 nM insulin, or the complex carrier/PtdIns-3-P at the indicated lipid final concentrations or carrier alone at a final concentration of 50 µM and then fixed for quantitative analyses. Data are mean ± SEM (n = 3–10). Significantly different from control: *P < 0.001; **P < 0.01; ***P < 0.05. (C) 3T3-L1 adipocytes were electroporated with 500 µg of a cDNA encoding GFP–GLUT4. After 24 h, cells were serum deprived for 3 h and stimulated with 300 nM insulin or incubated with the complex carrier/phosphoinositide at a lipid final concentration of 50 µM for 30 min at 37°C. Data are mean ± SEM (n = 2). (D) Exogenous PtdIns-3-P induces plasma membrane translocation of endogenous GLUT4. 3T3-L1 adipocytes were serum deprived overnight and then stimulated with insulin or with each complex carrier/phosphoinositide for 10 min. After fixing and permeabilization, the localization of endogenous GLUT4 was assessed by using an anti-GLUT4 antibody followed by a FITC-conjugated secondary antibody and analysed by confocal microscopy. (E) 3T3-L1 adipocytes were serum deprived overnight and then stimulated with insulin or incubated with either a complex carrier/phosphoinositide or carrier alone. Glucose uptake was performed as described in Materials and methods. Data are mean ± SEM (n = 3–5). (F) GFP–GLUT4-transfected L6 cells were serum deprived for 6 h and then left untreated or incubated with 100 nM wortmannin at 37°C. After 15 min, cells were stimulated with 300 nM insulin or incubated with the complex carrier/PtdIns-3-P for an additional 10 min. Data are mean ± SEM (n = 3–5). Significantly different from PtdIns-3-P: *P < 0.05.
To confirm these results in a more physiological context, we checked the effect of exogenous phosphoinositides on endogenous GLUT4 intracellular localization. Serum-deprived 3T3-L1 adipocytes were incubated with insulin or with different exogenous phosphoinositide and localization of the endogenous GLUT4 was assessed by confocal microscopy (Figure 7D). Insulin induced GLUT4 translocation to the plasma membrane, as expected. Among the different phosphoinositides tested, PtdIns-3-P alone was able to reproduce the insulin-mediated GLUT4 translocation to the plasma membrane, whereas PtdIns-4-P, PtdIns-3,4-P2 and PtdIns-3,4,5-P3 did not affect the intracellular localization of the protein. These observations were very convincing since almost all cells displayed a plasma membrane localization upon both insulin and PtdIns-3-P stimulation, whereas no cell showed a plasma membrane rim in unstimulated coverslips or in coverslips incubated with the other phosphoinositides. The observation that PtdIns-3,4,5-P3 induced a partial translocation of GFP–GLUT4 (Figure 7C) but had no visually detectable effect on endogenous GLUT4 (Figure 7D) suggests that PtdIns-3,4,5-P3 has a small effect on GLUT4 translocation that can be easily detected only when GLUT4 is overexpressed.
Taken together, these data indicate that exogenous PtdIns-3-P is able to induce the plasma membrane translocation of both overexpressed and endogenous GLUT4, thus indicating that this phosphoinositide plays a role in GLUT4 translocation.
Several lines of evidence suggest that GLUT4 translocation and glucose uptake are distinct processes, and that a full stimulation of glucose transport requires both translocation and activation of the transporter (Hausdorff et al., 1999; Sweeney et al., 1999; Somwar et al., 2001). To gain further insight into the precise role of PtdIns-3-P in insulin signalling, we tested the effect of exogenous phosphoinositides on glucose uptake in 3T3-L1 adipocytes. None of the phosphoinositides tested alone was able to induce glucose uptake (Figure 7E). These data are in agreement with the observation that PtdIns-3,4,5-P3 itself is not able to induce efficient uptake (Jiang et al., 1998) and further support the hypothesis that the sole GLUT4 translocation to the plasma membrane is not sufficient for efficient activation of glucose uptake. Furthermore, these results clearly indicate that PtdIns-3-P plays a crucial role specifically in GLUT4 translocation but other factors are required for full stimulation of glucose transport.
We have shown that a pre-treatment with 100 nM wortmannin does not inhibit the insulin-induced formation of PtdIns-3-P (Figure 4B and D). On the contrary, it has been reported that this pre-treatment almost completely blocks GLUT4 translocation (Hausdorff et al., 1999; Somwar et al., 2001). The observation that the insulin-mediated formation of PtdIns-3-P and the GLUT4 translocation show a different sensitivity to wortmannin seems to be in conflict with the hypothesis of a role in GLUT4 translocation for PtdIns-3-P. To better understand this aspect, we compared the effect of PI 3-K inhibition on insulin- or PtdIns-3-P-mediated GLUT4 translocation. GFP–GLUT4-transfected L6 cells were pre-treated with wortmannin before stimulation with insulin or incubation with PtdIns-3-P. A pre-treatment with 100 nM wortmannin clearly reduced the insulin- and the PtdIns-3-P-induced GLUT4 translocation to the same extent (Figure 7F). Similar results were obtained using 25 µM LY294002 (data not shown), thus suggesting that a wortmannin/LY294002-sensitive step downstream from PtdIns-3-P formation is also required for efficient GLUT4 translocation.
Discussion
PtdIns-3-P, as for other products of PI 3-Ks, acts as a second messenger upon cellular stimulation
It has been well established that, among the different products of PI 3-Ks, PtdIns-3,4,5-P3 plays a crucial role in many signalling events (Rameh and Cantley, 1999). By contrast, PtdIns-3-P has been essentially considered as a lipid constitutively present and restricted to endosomes and intracellular vesicles. In contrast to this general idea, it has been demonstrated that an integrin-dependent signalling in human platelets activates the transient formation of PtdIns-3-P, followed by the generation of PtdIns-3,4-P2 but not PtdIns-3,4,5-P3 (Zhang et al., 1998) Furthermore, we have previously shown that PtdIns-3-P increases by ∼50% after stimulation of COS7 and HeLa cells with lysophosphatidic acid (Razzini et al., 2000). More recently, two reports have shown a rapid upregulation of PtdIns-3-P on phagosomal membranes following closure of the phagosome (Ellson et al., 2001; Vieira et al., 2001), further supporting the hypothesis of the existence of a stimulatory control of PtdIns-3-P formation. Our data here provide the first clear demonstration that, apart from a constitutive pool of PtdIns-3-P in endosomal structures, another pool of this phosphoinositide can be specifically produced upon cellular stimulation at the level of the lipid rafts subdomain of the plasma membrane. These results give a new insight into the intracellular role of PtdIns-3-P and clearly state that PtdIns-3-P, as for other products of PI 3-Ks, can act as a lipid second messenger. Furthermore, the observation that different growth factors, such as EGF or PDGF, do not induce the formation of the plasma membrane-associated pool of PtdIns-3-P indicates that this phosphoinositide plays a role specifically upon insulin receptor activation.
The insulin-dependent pool of PtdIns-3-P is generated downstream from TC10 activation and is involved in GLUT4 translocation
Insulin stimulates glucose uptake into fat and muscle cells through two pathways involving PI 3-K and the small GTP-binding protein TC10, respectively. The second pathway has been described recently and the precise mechanism of action and downstream effectors of TC10 remain to be completely identified. It has been proposed that TC10 may produce a cytoskeletal rearrangement to facilitate the exocytosis of GLUT4. In particular, it has been suggested that the actin-regulatory neural Wiskott–Aldrich syndrome protein (N-WASP) may function downstream from TC10 to mobilize cortical F-actin (Jiang et al., 2002). In addition, a novel TC10-interacting protein, CIP4/2, has been recently identified in a two-hybrid screen (Chang et al., 2002) and it has been suggested that CIP4/2 may act as an adaptor protein by recruiting additional molecules to the plasma membrane.
Our data clearly identify PtdIns-3-P as a new downstream product of TC10 activation since the insulin-induced formation of PtdIns-3-P can be specifically reproduced by a constitutively active mutant (TC10 Q75L) and specifically inhibited by a dominant-negative mutant (TC10 T31N) of the small GTP-binding protein. Our results suggest that, once activated, TC10 activates a PI 3-K more resistant than type IA PI 3-K to inhibitors such as wortmannin and LY294002. The major candidate is the class II enzyme PI 3-KC2α, which is activated by insulin (Brown et al., 1999) and is wortmannin resistant (Domin et al., 1997).
Furthermore, our data indicate that the TC10-dependent pool of PtdIns-3-P is involved in the translocation of GLUT4 to the plasma membrane, and suggest that this may account, at least in part, for the contribution of the TC10 pathway to this event following insulin receptor activation.
Our observation that overexpression of TC10 Q75L induces the formation of PtdIns-3-P, which in turn may induce GLUT4 translocation, seems to be in contrast to the first report indicating that overexpression of TC10 Q75L inhibits GLUT4 translocation (Chiang et al., 2001). However, it has recently been reported that the plasma membrane translocation of the adaptor protein CIP4/2 is essential for insulin-stimulated GLUT4 translocation. Interestingly, overexpression of TC10 Q75L induces the plasma membrane translocation of CIP4/2 suggesting that, in this context, TC10 Q75L is a positive regulator of GLUT4 translocation (Chang et al., 2002). In agreement with these results, our data on the TC10-mediated formation of PtdIns-3-P and the role of PtdIns-3-P in GLUT4 translocation suggest that the activated state of TC10 is required for at least some events leading to a full GLUT4 translocation.
In our assays, PtdIns-3-P was able to induce GLUT4 translocation to the same extent as insulin. Although these results seem to indicate that PtdIns-3-P is sufficient for this trafficking event, a concerted action of PtdIns-3-P and other factors, for instance PtdIns-3,4,5-P3, is more likely to be responsible for an efficient GLUT4 translocation in vivo. This possibility is supported by our observation that the PtdIns-3-P-mediated GLUT4 translocation is reduced by pre-treatment with PI 3-K-inhibitors, suggesting that some wortmannin/LY294002-sensitive steps, downstream from PtdIns-3-P formation, are also required for an efficient GLUT4 translocation. Furthermore, the hypothesis of a concerted action of both phosphoinositides is in agreement with the well established role of PtdIns-3,4,5-P3 in this process.
We finally observed that the effect of PtdIns-3-P is restricted to GLUT4 translocation and neither this phosphoinositide nor other phosphoinositides tested, including PtdIns-3,4,5-P3, alone is sufficient to induce glucose uptake. These data are in agreement with the current idea that full stimulation of glucose uptake by insulin requires a combination of increased GLUT4 translocation and increased intrinsic activity of GLUT4 (Sweeney et al., 1999). Furthermore, it has been reported that GLUT4 translocation and glucose uptake show a different sensitivity to wortmannin and LY294002 (Hausdorff et al., 1999; Somwar et al., 2001), thus suggesting that they are distinct processes, probably involving different PI 3-Ks. Our results are in agreement with the hypothesis of a concerted activation of different pathways for a full stimulation of glucose uptake and strongly support a specific role in trafficking of the transporter for PtdIns-3-P.
In conclusion, we demonstrate for the first time that, apart from a pool of PtdIns-3-P constitutively associated with endosomes, another pool of this phosphoinositide can be produced at the lipid rafts subdomain of the plasma membrane upon cellular stimulation. This regulated pool of PtdIns-3-P is specifically produced upon insulin stimulation through activation of the small GTP-binding protein TC10 and is involved in the translocation of the glucose transporter protein GLUT4. Taken together, these results clearly assess that PtdIns-3-P, as for other products of PI 3-Ks, is a lipid second messenger and adds an important new component in the recently described TC10-dependent cascade downstream from insulin receptor activation. Our data provide a potential framework for understanding insulin signal specificity, and may have significant implications for approaches to prevent the complications of diabetes mellitus and other related diseases.
Materials and methods
Materials and cell culture
All reagents, cell culture and transfection are described in Supplementary data.
HPLC analyses
Cells were incubated with 10 µCi/well _myo_-[3H]inositol (Perkin-Elmer) in M199 medium for 24 h and then left untreated or stimulated as specified. Phospholipids were extracted, deacylated and separated by anion-exchange HPLC on a Partisil 10 SAX column using a non-linear water (buffer A)/1 M ammonium phosphate pH 3.35 (buffer B) gradient (0–45 min, 0–1.5% buffer B; 45–46 min, 1.5–2.4% buffer B; 46–80 min, 2.4–4.5% buffer B; 80–81 min, 4.5–6.0% buffer B; 81–141 min, 6.0–35% buffer B; 141–142 min, 35–100% buffer B; 142–147 min, 100% buffer B; 147–150 min, 100–0% buffer B; 150–180 min, 0% buffer B wash). The retention times of the different glycerophosphoinositides were as follows: gPtdIns, 23 min; gPtdIns-3-P, 67 min; gPtdIns-4-P, 75 min; gPtdIns-3,4-P2, 114.5 min; gPtdIns-4,5-P2, 118 min; gPtdIns-3,4,5-P3, 146 min. The different glycerophosphoinositides were identified as described (Meijer et al., 2001). The levels of PtdIns-3-P were normalized to the amount of PtdIns-4-P. In Figure 4D, the levels of PtdIns-3-P were normalized to the amount of PtdIns-4,5-P2 since wortmannin may affect the levels of PtdIns-4-P.
Microscopy and quantitative analyses
Cells grown on glass coverslips were fixed with PBS containing 4% paraformaldehyde for 10 min at room temperature. Where necessary, cells were permeabilized with Triton X-100 (0.25% in PBS) and incubated with the indicated primary antibodies followed by TRITC or FITC secondary antibodies. Microscopy was performed using a Zeiss laser confocal microscope system (LSM 510) connected to an Axiovert 100M (Zeiss) and a Zeiss 63× objective. Images were collected at 3.5 µm from the bottom and all conditions were imaged at the same distance from the glass. For experiments with the CFP and YFP constructs (Figure 2B), microscopy was performed using a Bio-Rad RADIANCE 2000 confocal system and a Nikon E1000 microscope equipped with a 60× water immersion objective lens. Quantitative analyses of GFP–2XFYVEHrs and GFP–GLUT4 translocation were performed by blind scoring of at least 100 cells per condition (no score, 0–25% membrane localization; score 1, >25% membrane localization). The number of positive cells was expressed as a percentage of total cells. Statistical analyses were performed by paired Student’s _t_-test.
Pak1 PBD affinity precipitation assay
L6 cells were transfected with myc-TC10 wt, myc TC10 Q75L or myc-Cdc42 L61. After 24 h, cells were serum deprived overnight and then left untreated or stimulated with 300 nM insulin or 20 ng/ml PDGF for 3 min. The affinity precipitation assay was performed by using 7 µg/ml lysate of the Pak1 PBD (Rac/Cdc42 assay reagent), agarose (Upstate), according to the manufacturer. The agarose beads were resuspended in 15 µl of 2× Laemmli sample buffer, collected by a microcentrifuge pulse and the supernatants were separated by SDS–PAGE. Western blot analyses were performed using an anti-myc antibody.
Detergent-free cell fractionations
L6 cells were transfected with GFP–2XFYVEHrs alone or in combination with either myc-TC10 Q75L or HA-TC10 T31N. After 24 h, cells were serum deprived for 6 h and then left untreated or stimulated with 300 nM insulin or 20 ng/ml PDGF. After washing three times in ice-cold PBS, cells were scraped into 2 ml of 500 mM sodium carbonate pH 11.0, and then homogenized on ice with both 10 strokes in a glass Dounce homogenizer and a sonicator (three 20-s bursts; Soniprep 150; Sanyo). The homogenate was then adjusted to 45% sucrose by addition of 2 ml of 90% sucrose in MES-buffered saline, MBS (25 mM MES pH 6.5, 150 mM NaCl) and plated at the bottom of an ultracentrifuge tube. A 5–35% discontinuous gradient was formed above (4 ml of 5% sucrose/4 ml of 35% sucrose both in MBS containing 250 mM sodium carbonate) and centrifuged at 39 000 r.p.m. for 18 h in an SW41 rotor (Beckman Instrument). From the top of each gradient, 1 ml fractions were collected and separated by SDS–PAGE. Western blot analyses were performed by using the indicated primary antibodies followed by HRP-conjugated secondary antibodies. Bands were then revealed by enhanced chemiluminescence (Amersham Biosciences).
Phosphoinositide delivery experiments
Unlabeled carrier 2 or unlabeled carrier 3 (Echelon Research Laboratories) and phosphoinositide (CellSignals, Inc.) were added at a 1:1 molar ratio (200 µM final concentration) in a test tube. After brief vortexing and bath sonication, the complex was incubated at room temperature for 5 min and then added to medium of adherent cells in order to achieve the indicated phosphoinositide final concentration.
Glucose uptake
3T3-L1 adipocytes were serum deprived overnight and then stimulated with 300 nM insulin or incubated with a complex carrier/phosphoinositide (at a lipid final concentration of 50 µM) or carrier alone. After 20 min, cells were washed with KRP buffer (116 mM NaCl, 4.6 mM KCl, 1.2 mM KH2PO4, 25.3 mM NaHCO3, 2.5 mM CaCl2, 1.16 mM MgSO4 pH 7.2 containing 0.1% BSA) and incubated with 10 µM 2-deoxyglucose (0.5 µCi/ml) for 10 min. Uptake was terminated by washing three times with ice-cold PBS and cells were lysed with 1 M NaOH. Liquid scintillation counting assessed radioactivity. Non-specific uptake was determined in the presence of 10 µM cytochalasin B (Sigma) and was subtracted from total uptake.
Supplementary data
Supplementary data are available at The EMBO Journal Online.
Acknowledgments
Acknowledgements
We thank Dr M.J.Quon, Prof. M.A.Horton, Prof. M.A.Lemmon and Dr A.Sala for critical readings of the manuscript, Dr H.Stenmark, Dr P.T.Hawkins, Dr G.W.Zhou, Dr J.M.Tavaré, Dr A.Hall and Dr J.E.Pessin for the gift of constructs and Dr D.Kelly for help with experiments in living cells. This work is supported by Diabetes UK grant BDA: RD02/0002388. M.F. is supported by an endowment from the Dr Mortimer and Mrs Theresa Sackler Trust.
References
- Baumann C.A., Ribon,V., Kanzaki,M., Thurmond,D.C., Mora,S., Shigematsu,S., Bickel,P.E., Pessin,J.E. and Saltiel,A.R. (2000) CAP defines a second signalling pathway required for insulin-stimulated glucose transport. Nature, 407, 202–207. [DOI] [PubMed] [Google Scholar]
- Brown R.A., Domin,J., Arcaro,A., Waterfield,M.D. and Shepherd,P.R. (1999) Insulin activates the α isoform of class II phosphoinositide 3-kinase. J. Biol. Chem., 274, 14529–14532. [DOI] [PubMed] [Google Scholar]
- Chang L., Adams,R.D. and Saltiel,A.R. (2002) The TC10-interacting protein CIP4/2 is required for insulin-stimulated Glut4 translocation in 3T3L1 adipocytes. Proc. Natl Acad. Sci. USA, 99, 12835–12840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang S.H., Baumann,C.A., Kanzaki,M., Thurmond,D.C., Watson,R.T., Neudauer,C.L., Macara,I.G., Pessin,J.E. and Saltiel,A.R. (2001) Insulin-stimulated GLUT4 translocation requires the CAP-dependent activation of TC10. Nature, 410, 944–948. [DOI] [PubMed] [Google Scholar]
- Czech M.P. and Corvera,S. (1999) Signaling mechanisms that regulate glucose transport. J. Biol. Chem., 274, 1865–1868. [DOI] [PubMed] [Google Scholar]
- Domin J., Pages,F., Volinia,S., Rittenhouse,S.E., Zvelebil,M.J., Stein,R.C. and Waterfield,M.D. (1997) Cloning of a human phosphoinositide 3-kinase with a C2 domain that displays reduced sensitivity to the inhibitor wortmannin. Biochem. J., 326, 139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellson C.D., Anderson,K.E., Morgan,G., Chilvers,E.R., Lipp,P., Stephens,L.R. and Hawkins,P.T. (2001) Phosphatidiylinositol 3-phosphate is generated in phagosomal membranes. Curr. Biol., 11, 1631–1635. [DOI] [PubMed] [Google Scholar]
- Gillooly D.J., Morrow,I.C., Lindsay,M., Gould,R., Bryant,N.J., Gaullier,J.M., Parton,R.G. and Stenmark,H. (2000) Localization of phosphatidylinositol 3-phosphate in yeast and mammalian cells. EMBO J., 19, 4577–4588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillooly D.J., Simonsen,A. and Stenmark,H. (2001) Cellular functions of phosphatidylinositol 3-phosphate and FYVE domain proteins. Biochem. J., 355, 249–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausdorff S.F., Fingar,D.C., Morioka,K., Garza,L.A., Whiteman,E.L., Summers,S.A. and Birnbaum,M.J. (1999) Identification of wortmannin-sensitive targets in 3T3-L1 adipocytes. Dissociation of insulin-stimulated glucose uptake and glut4 translocation. J. Biol. Chem., 274, 24677–24684. [DOI] [PubMed] [Google Scholar]
- Isakoff S.J., Taha,C., Rose,E., Marcusohn,J., Klip,A. and Skolnik,E.Y. (1995) The inability of phosphatidylinositol 3-kinase activation to stimulate GLUT4 translocation indicates additional signalling pathways are required for insulin-stimulated glucose uptake. Proc. Natl Acad. Sci. USA, 92, 10247–10251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang T., Sweeney,G., Rudolf,M.T., Klip,A., Traynor-Kaplan,A. and Tsien,R.Y. (1998) Membrane-permeant esters of phosphatidylinositol-3,4,5-trisphosphate. J. Biol. Chem., 273, 11017–11024. [DOI] [PubMed] [Google Scholar]
- Jiang Z.Y., Chawla,A., Bose,A., Way,M. and Czech,M.P. (2002) A phosphatidylinositol 3-kinase-independent insulin signaling pathway to N-WASP/Arp2/3/F-actin required for GLUT4 glucose transporter. J. Biol. Chem., 277, 509–515. [DOI] [PubMed] [Google Scholar]
- Maffucci T. and Falasca,M. (2001) Specificity in pleckstrin homology (PH) domain membrane targeting: a role for a phosphoinositide-protein co-operative mechanism. FEBS Lett., 506, 173–179. [DOI] [PubMed] [Google Scholar]
- Meijer H.J., Berrie,C.P., Iurisci,C., Divecha,N., Musgrave,A. and Munnik,T. (2001) Identification of a new polyphosphoinositide in plants, phosphatidylinositol 5-monophosphate (PtdIns5P), and its accumulation upon osmotic stress. Biochem. J., 360, 491–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki S., DeWald,D.B., Shope,J.C., Chen,J. and Prestwich,G. (2000) Intracellular delivery of phosphoinositides and inositol phosphates using polyammine carriers. Proc. Natl Acad. Sci. USA, 97, 11286–11291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rameh L.E. and Cantley,L.C. (1999) The role of phosphoinositide 3-kinase lipid products in cell function. J. Biol. Chem., 274, 8347–8350. [DOI] [PubMed] [Google Scholar]
- Razzini G., Brancaccio,A., Lemmon,M.A., Guarnieri,S. and Falasca,M. (2000) The role of pleckstrin homology domain in membrane targeting and activation of phospholipase Cβ1. J. Biol. Chem., 275, 14873–14881. [DOI] [PubMed] [Google Scholar]
- Ruderman N.B., Kapeller,R., White,M.F. and Cantley,L.C. (1990) Activation of phosphatidylinositol 3-kinase by insulin. Proc. Natl Acad. Sci. USA, 87, 1411–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd P.R., Withers,D.J. and Siddle,K. (1998) Phosphoinositide 3-kinase: the key switch mechanism in insulin signalling. Biochem. J., 333, 471–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somwar R., Niu,W., Kim,D.Y., Sweeney,G., Randhawa,V.K., Huang,C., Ramlal,T. and Klip,A. (2001) Differential effects of phosphatidylinositol 3-kinase inhibition on intracellular signals regulating GLUT4 translocation and glucose transport. J. Biol. Chem., 276, 46079–46087. [DOI] [PubMed] [Google Scholar]
- Summers S.A., Yin,V.P., Whiteman,E.L., Garza,L.A., Cho,H., Tuttle,R.L. and Birnbaum,M.J. (1999) Signaling pathways mediating insulin-stimulated glucose transport. Ann. NY Acad. Sci., 892, 169–186. [DOI] [PubMed] [Google Scholar]
- Sweeney G., Somwar,R., Ramlal,T., Volchuk,A., Ueyama,A. and Klip,A. (1999) An inhibitor of p38 mitogen-activated protein kinase prevents insulin-stimulated glucose transport but not glucose transporter translocation in 3T3-L1 adipocytes and L6 myotubes. J. Biol. Chem., 274, 10071–10078. [DOI] [PubMed] [Google Scholar]
- Vanhaesebroeck B., Leevers,S.J., Ahmadi,K., Timms,J., Katso,R., Driscoll,P.C., Woscholski,R., Parker,P.J. and Waterfield,M.D. (2001) Synthesis and function of 3-phosphorylated inositol lipids. Annu. Rev. Biochem., 70, 535–602. [DOI] [PubMed] [Google Scholar]
- Vieira O.V., Botelho,R.J., Rameh,L., Brachmann,S.M., Matsuo,T., Davidson,H.W., Schreiber,A., Backer,J.M., Cantley,L.C. and Grinstein,S. (2001) Distinct roles of class I and class III phosphatidylinositol 3-kinases in phagosome formation and maturation. J. Cell Biol., 155, 19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson R.T. and Pessin,J.E. (2001) Subcellular compartmentalization and trafficking of the insulin-responsive glucose transporter, GLUT4. Exp. Cell Res., 271, 75–83. [DOI] [PubMed] [Google Scholar]
- Watson R.T., Shigematsu,S., Chiang,S.H., Mora,S., Kanzaki,M., Macara,I.G., Saltiel,A.R. and Pessin,J.E. (2001) Lipid raft microdomain compartmentalization of TC10 is required for insulin signaling and GLUT4 translocation. J. Cell Biol., 154, 829–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiese R.J., Mastick,C.C., Lazar,D.F. and Saltiel,A.R. (1995) Activation of mitogen-activated protein kinase and phosphatidylinositol 3′-kinase is not sufficient for the hormonal stimulation of glucose uptake, lipogenesis, or glycogen synthesis in 3T3-L1 adipocytes. J. Biol. Chem., 270, 3442–3446. [DOI] [PubMed] [Google Scholar]
- Zhang J., Banfic,H., Straforini,F., Tosi,L., Volinia,S. and Rittenhouse,S.E. (1998) A type II phosphoinositide 3-kinase is stimulated via activated integrin in platelets. J. Biol. Chem., 273, 14081–14084. [DOI] [PubMed] [Google Scholar]