The role of inflammation in CNS injury and disease (original) (raw)
Abstract
For many years, the central nervous system (CNS) was considered to be ‘immune privileged', neither susceptible to nor contributing to inflammation. It is now appreciated that the CNS does exhibit features of inflammation, and in response to injury, infection or disease, resident CNS cells generate inflammatory mediators, including proinflammatory cytokines, prostaglandins, free radicals and complement, which in turn induce chemokines and adhesion molecules, recruit immune cells, and activate glial cells. Much of the key evidence demonstrating that inflammation and inflammatory mediators contribute to acute, chronic and psychiatric CNS disorders is summarised in this review. However, inflammatory mediators may have dual roles, with detrimental acute effects but beneficial effects in long-term repair and recovery, leading to complications in their application as novel therapies. These may be avoided in acute diseases in which treatment administration might be relatively short-term. Targeting interleukin (IL)-1 is a promising novel therapy for stroke and traumatic brain injury, the naturally occurring antagonist (IL-1ra) being well tolerated by rheumatoid arthritis patients. Chronic disorders represent a greater therapeutic challenge, a problem highlighted in Alzheimer's disease (AD); significant data suggested that anti-inflammatory agents might reduce the probability of developing AD, or slow its progression, but prospective clinical trials of nonsteroidal anti-inflammatory drugs or cyclooxygenase inhibitors have been disappointing. The complex interplay between inflammatory mediators, ageing, genetic background, and environmental factors may ultimately regulate the outcome of acute CNS injury and progression of chronic neurodegeneration, and be critical for development of effective therapies for CNS diseases.
Keywords: Cytokine, neurodegeneration, COX, NO, interleukin, tumour necrosis factor, complement
Introduction
Inflammation is a cardinal host defence response to injury, tissue ischaemia, autoimmune responses or infectious agents. Locally, within tissues outside the brain, inflammation manifests by the classical features of swelling, redness, heat and often pain. More mechanistic definitions of inflammation have now been established, including invasion of circulating immune cells (lymphocytes and macrophages), and induction or activation of inflammatory mediators such as kinins, cyclooxygenase products and cytokines. Many of these molecules are produced locally and have proven involvement in tissue inflammation, and are thus key targets for therapeutic intervention in a whole range of diseases.
Inflammation often elicits a generalised sequence of events known as the acute phase response. This represents another host defence mechanism which can limit proliferation of invading pathogens (Suffredini et al., 1999). It includes production of acute phase proteins by the liver, activation of the sympathetic nervous system, changes in cardiovascular function, altered neuroendocrine status (most notably activation of the hypothalamic pituitary adrenal axis leading to production of anti-inflammatory steroids), behavioural changes which lead to energy conservation such as increased sleep, lethargy, reduced appetite, and the most common feature of infection, fever, which can limit bacterial proliferation. These local and generalised inflammatory responses have clear benefits in infectious states, and when activated in a regulated manner for a defined period of time. However, sustained, excessive or inappropriate inflammation is the cause of numerous diseases including rheumatoid arthritis, psoriasis and inflammatory bowel disease. Inflammation is a major component of the damage caused by autoimmune diseases, and is also a fundamental contributor to diseases such as cancer, diabetes and cardiovascular disease. Atherosclerosis, once viewed as a rather passive state of vascular deposition of material leading to plaque formation, is established as an inflammatory condition, and acute or chronic inflammation is a risk factor for coronary and vascular disease, and stroke.
The brain as an immune privileged site
Until just over a decade ago, the brain was regarded as an ‘immune privileged' organ, which was not susceptible to inflammation or immune activation, and was thought to be largely unaffected by systemic inflammatory and immune responses. This view has been revised significantly.
There is no doubt that the brain differs significantly from other tissues in its responses to pathogenic challenges. Oedema is limited by the cranium, but can have devastating effects since the swelling which accompanies disorders such as stroke and brain injury, leads to raised intracranial pressure, impaired function and sometimes death. Infection or inflammation elicit rather different responses in the brain to those in other tissues. This is most evident in leukocyte recruitment, which is rapid in many systemic organs, but modest and delayed in the brain. In spite of these notable differences, the brain does exhibit key features of inflammation (Table 1).
Table 1. Key features of CNS inflammation.
| Glial activation |
|---|
| Oedema |
| MHC expression |
| Systemic acute phase response with general inflammation and acute phase protein synthesis |
| Complement activation – e.g. anaphylatoxins, membrane attack complex |
| Synthesis of inflammatory mediators – e.g. cytokines, free radicals, prostaglandins |
| Expression of adhesion molecules |
| Invasion of immune cells |
While leukocyte invasion may be delayed in response to acute insults, activation of brain microglia and release of inflammatory mediators are rapid – occurring within minutes or hours. Most importantly, there is now extensive evidence that inflammation within the CNS contributes to many acute and chronic degenerative disorders and perhaps some psychiatric diseases.
CNS regulation of inflammation
The realisation that the CNS regulates some, or even many, aspects of systemic inflammation and immune responses was unexpected. Yet the first member identified of a family of proteins critical for the regulation of inflammation, the cytokines, was interleukin (IL)-1. IL-1 was discovered as an important endogenous pyrogen, and is now recognised as the ‘prototypic inflammatory cytokine' (reviewed by Rothwell & Luheshi, 2000). IL-1 is a potent endogenous and exogenous inducer of fever, a phenomenon which has long been recognised to result from a change in hypothalamic thermoregulation. Since IL-1 was first shown to act in the brain, numerous CNS-mediated effects of IL-1 on systemic injury, infection and inflammation have been identified, and it has been recognised that the brain regulates many aspects of systemic inflammation and the acute phase response.
The afferent signals from inflamed, injured or infected tissues to the CNS have not been fully elucidated, but appear to include release of circulating inflammatory mediators such as IL-6 (IL-1 is not readily detected in plasma), and local (e.g. C-fibre) and more general, notably vagal, neuronal signals. The brain clearly senses peripheral insults rapidly since it can activate responses such as fever and neuroendocrine changes within 30 min. It is not surprising that the CNS regulates thermoregulatory and neuroendocrine responses to disease and injury, but more unexpectedly, it can also affect peripheral immune function.
It is now accepted that the brain coordinates and regulates many aspects of the host defence response, which may begin to explain the behavioural responses to disease such as fatigue and depression, and how psychological state can influence susceptibility to disease and subsequent recovery. The most intense interest in inflammation in the CNS has arisen from its potential role in diseases including acute brain injury, stroke, epilepsy, multiple sclerosis, motor neurone disease, movement disorders and Alzheimer's disease, and more recently some psychiatric disorders such as depression, anxiety and schizophrenia.
Expression of inflammatory mediators in the CNS
Most inflammatory mediators have relatively few actions in healthy CNS tissue and are expressed at very low, or undetectable, levels. However, they are induced rapidly in response to tissue injury or infection, and exert diverse actions. Proinflammatory cytokines and other mediators play an essential role in CNS inflammation through the induction of chemokines and adhesion molecules, recruitment of immune cells into the parenchyma and activation of immune cells and endogenous glial cells (Rothwell & Luheshi, 2000).
Neurones, astrocytes, microglia and oligodendrocytes can produce inflammatory mediators, and cytokine receptors are expressed constitutively throughout the CNS, albeit at low levels. The constitutive expression of genes encoding cytokines and their receptors in the brain suggests that cytokines may contribute to normal physiological functions of the CNS. There is increasing evidence for the involvement of IL-1 in sleep, feeding and memory (Pollmacher et al., 2002). In pathophysiological conditions, microglia and blood-derived macrophages are activated by CNS damage or infection, and are recruited rapidly to the site of insult. The presence of damaged cells and debris causes ramified resting microglia to transform into rounded migratory macrophages. In this active state, microglia produce cytokines and trophic factors that can exert damaging or protective effects on neighbouring cells. Cytokines can also cross the blood–brain barrier (BBB), probably either by active transport or through leaky regions of endothelia when the BBB is compromised by a pathological condition. Thus the CNS can be affected not only by inflammatory mediators produced within the brain, but also through the actions of mediators originating from the periphery.
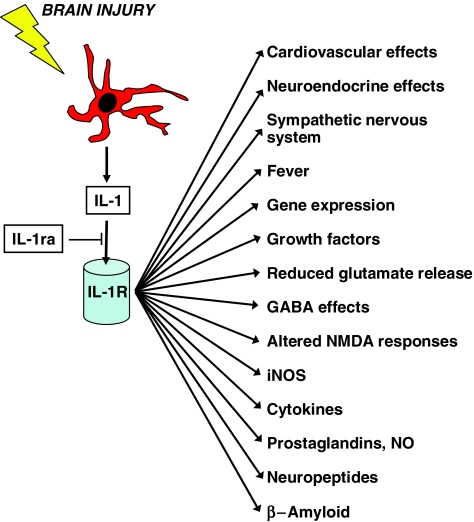
IL-1 is the most widely studied proinflammatory cytokine. It has been implicated in a number of neurodegenerative conditions and is generally believed to have neurotoxic actions, although the mechanisms of these effects are unclear. The most extensively characterised forms of IL-1 are IL-1_α_ and IL-1_β_ (see Rothwell & Luheshi, 2000). IL-1 initiates signal transduction predominantly through the IL-1 type I receptor, and a specific, endogenous receptor antagonist (IL-1ra) prevents activation of the IL-1 receptor complex by IL-1. Following CNS damage, IL-1 is rapidly released from activated microglia. The actions of IL-1 in the CNS are diverse and include induction of growth factors, reduction of glutamate release, enhanced GABA effects, modulation of neuronal responses to NMDA and glycine, and increased activation of inducible nitric oxide synthase (iNOS) (Figure 1).
Figure 1.
Downstream actions of IL-1. Brain injury and inflammation activate cells (e.g. microglia) leading to generation of IL-1, which acts on the IL-1 receptor (type 1) to activate diverse downstream effects. IL-1ra can inhibit the actions of IL-1.
Tumour necrosis factor-α (TNF_α_) is one of the central mediators of tissue inflammation and has been implicated in the pathogenesis of many neurological conditions. This cytokine is reported to have both deleterious and protective actions in neurones, and these opposing effects may be explained by the existence of two distinct TNF-signalling pathways mediated by two receptors, p55 and p75. Both receptor isoforms have been detected in the CNS, and although the functions of p75 in the brain are still unclear, activation of p55 initiates signals leading to neuronal apoptosis. In the CNS, resident macrophages, astrocytes and microglia are able to synthesise TNF_α_, which seems to be proinflammatory during the acute phase of CNS inflammatory responses, but immunosuppressive during the chronic phase.
IL-6 is an endogenous pyrogen which exerts multiple effects that are both beneficial and destructive to CNS cells. IL-6 signals through a specific receptor (IL-6R) to stimulate various signalling pathways which lead to gene activation (Van Wagoner & Benveniste, 1999). Under normal physiological conditions, IL-6 expression in the brain is low but levels are elevated in a large number of human CNS disorders, leading to increased central production of inflammatory cytokines. IL-6 can be induced by a variety of molecules including IL-1, TNF_α_, transforming growth factor-β and prostaglandins (PGs), and many other mediators such as β_-amyloid, interferon-γ (IFN_γ) and IL-4 can potentiate these primary inducers, highlighting the complex nature of IL-6 modulation. For example, IL-2 induces expression of IL-6, but also increases levels of known IL-6 inducers such as IL-1_β_ and TNF_α_.
The mechanisms that control expression of different cytokines are often related; TNF_α_ stimulates expression of IL-1 and IL-6, and IL-1 can induce both IL-6 and TNF_α_. Thus, following an insult to the brain, initial upregulation of cytokines leads to infiltration of other inflammatory mediators to the site of injury, and secondary cytokine signalling is initiated. Although microglia produce the initial increase in cytokine levels, the actual stimuli for cytokine production in the brain are unknown; the rapidity of the response suggests however that the stimuli must be direct and fast, such as excessive neuronal activity.
PGs are inflammatory mediators that are produced via the metabolism of arachidonic acid by cyclooxygenase (COX) and PG synthase enzymes. The effects of PGs are mediated by nine prostanoid-binding G-protein-coupled receptors, and although PGs are classically considered to be potent proinflammatory molecules, they also have anti-inflammatory actions in some settings. COX exists as two major isozymes: COX-1 is expressed constitutively in many tissues, whereas COX-2 is induced in response to inflammatory mediators. PGs produced via COX-2 are therefore generally considered to be responsible for inflammatory effects, whereas those produced by COX-1 are thought to have a role in normal homeostasis.
The complement system, a protein cascade involved in immune responses, consists of around 30 fluid-phase and cell-membrane-associated proteins. The activation products of the cascade contribute to the production of other inflammatory mediators, and can therefore promote tissue injury at sites of inflammation. The most potent proinflammatory molecules produced in response to complement activation are the anaphylatoxins C3a and C5a, which exert their actions through specific receptors. Activation of the complement system also leads to formation of the membrane attack complex (MAC), which lyses target cells by forming a pore in the phospholipid bilayer. Neurones, microglia, astrocytes and oligodendrocytes express complement proteins, and neurones are particularly susceptible to complement-mediated damage.
Free radicals damage lipids, DNA and proteins, and can promote CNS injury. In particular, nitric oxide (NO) is emerging as an effector of neurodegeneration (Moro et al., 2004). NO is generated by three NOS enzymes (see also Moncada & Higgs, this issue), all of which have been characterised in CNS cells. Glial cells synthesise NO mainly via iNOS, which is expressed after exposure to IL-1_β_, TNF_α_ and IFN_γ_.
Evidence for the contribution of inflammation to CNS disease
Acute CNS injury
Stroke and cerebral ischaemia
Stroke is the third leading cause of death in the Western world and the leading cause of permanent disability. Recombinant tissue plasminogen activator is currently the only acute therapy being used for stroke, but its narrow therapeutic window and risk of haemorrhage limit its use. It is now clear that proinflammatory cytokines, and in particular IL-1, have a direct involvement in experimentally induced ischaemic injury. Peripheral or central administration of recombinant IL-1ra in rats or mice reduces ischaemic damage after 48 h by around 50%, and mice which lack the genes for both IL-1_α_ and IL-1_β_ exhibit a 70% reduction in infarct volume compared to wild-type animals. Administration of a neutralising anti-IL-1_β_ antibody also inhibits experimental stroke damage in rats. Thus, blocking the actions of endogenous IL-1 reduces CNS cell loss following experimental ischaemic brain injury. In addition, selective inhibition of caspase-1 (or interleukin-converting enzyme (ICE), the enzyme required to produce active IL-1_β_) reduces ischaemic brain damage, and mice which lack the gene encoding caspase-1 also display reduced damage.
Administration of TNF_α_ directly to the brain increases, while neutralising antibodies which block the actions of TNF_α_ reduces, injury induced by experimental ischaemia. However, a significant reduction in infarct size has been observed in mice treated with TNF_α_ prior to the insult, suggesting dual neurotoxic and neuroprotective roles for this cytokine (Hallenbeck, 2002). Given the key role of TNF_α_ in the initiation of inflammatory cascades, inhibitors of this cytokine may have therapeutic potential. TNF_α_-converting enzyme (TACE) is essential for the maturation of TNF_α_ and is an attractive target for development of small-molecule inhibitors of the TNF_α_ system. TACE inhibitors reduce ischaemic damage in preclinical stroke models, and the success of anti-TNF agents in the treatment of rheumatoid arthritis provides encouraging evidence that inhibition of TNF_α_ can reduce inflammation in a clinical setting.
IL-6 mRNA increases markedly in the rodent brain in response to experimental stroke injury, but although IL-6 appears to be involved in the inflammatory response associated with ischaemia, deletion of the IL-6 gene in rodents has no effect on infarct volume or neurological function after middle cerebral artery occlusion. Indeed administration of recombinant IL-6 to the brain reduces experimental ischaemic damage, suggesting that IL-6 may be an inhibitor of neuronal death during cerebral ischaemia.
Increases in COX-2 activity and PG production have been associated with ischaemic damage in experimental stroke. PGD2 contributes to cerebral tissue damage in rats, and mice which constitutively overexpress COX-2 in the striatum, cerebral cortex and hippocampus display increased infarct volumes after experimental ischaemia. NO can exert both protective and deleterious actions in ischaemic events: initially NO produced by endothelial NOS is protective through its vasodilatory action, but subsequently, NO produced via neuronal NOS and iNOS contributes to ischaemic damage. The complement cascade may also have deleterious effects in stroke; inhibition of the complement system using a C1 antagonist reduces neurological deficits and infarct volume following transient focal ischaemia (De Simoni et al., 2003).
Brain trauma
Traumatic brain injury (TBI) is a major cause of mortality in young people, and triggers an inflammatory response that is initiated by the release of proinflammatory cytokines. Delayed CNS damage frequently occurs after TBI, and inflammatory mediators have been implicated in this process. Central injection of recombinant IL-1ra at varying times between 15 min and 48 h after experimental TBI reduces the extent of CNS damage, indicating that endogenous IL-1 is involved in both the acute and delayed damage. Furthermore, in a closed head injury model of TBI, mice which overexpress IL-1ra show no significant acute increase in IL-1, TNF_α_ or IL-6 levels, and exhibit improved neurological recovery compared with wild-type animals (Tehranian et al., 2002). Agents that inhibit TNF_α_ improve both short- and long-term neurological outcome in rats, and IL-6 may be neuroprotective since transgenic IL-6 production increases CNS wound healing after traumatic injury by cryolesion. Administration of a COX-2 inhibitor after experimental TBI improves both neurological and motor outcome in rats and the complement cascade may also have a role in brain damage following TBI. Secondary damage caused by experimental brain injury is reduced in mice which lack complement C3 or complement C5, and C5a receptor antagonists reduce CNS cell loss (Sewell et al., 2004).
Epilepsy
Epilepsy is a common neurological disorder which affects around 50 million people worldwide. Seizures are caused by abnormal, high-frequency discharge of groups of neurones. The underlying neurochemical mechanisms are unknown, although increasing evidence implicates proinflammatory cytokines. Seizures and epilepsy can develop following events which induce a CNS inflammatory response, and expression of IL-1, TNF_α_, IL-1ra and IL-6 are increased by seizure activity. Cytokines affect neuronal excitability directly by acting on ionic currents, and indirectly by inducing gene transcription in glia and neurones.
Exogenous IL-1_β_ enhances chemically induced seizures in rats and IL-1ra inhibits motor seizures and delays their onset (see Vezzani et al., 2002). Transgenic mice which overexpress IL-1ra selectively in astrocytes are less susceptible to seizure induction than wild-type animals, and seizures in these mice have a reduced duration and delayed onset compared to their wild-type counterparts. Furthermore, mice that lack IL-1RI are less susceptible to heat-induced febrile seizures than wild-type mice. Evidence for a proconvulsive role of IL-1_β_ is compelling, but it has also been shown to have anticonvulsive properties. In a rodent amygdala kindling model of epilepsy, fully kindled seizures are inhibited by IL-1_β_, and daily injection of IL-1_β_ during kindling slows the rate of kindling (Sayyah et al., 2005).
The role of TNF_α_ in seizures is not clear, although systemic infusion of TNF_α_ exacerbates experimentally induced seizures in rats and subcutaneous injection of a TACE inhibitor significantly reduces seizure activity in a rat model of pneumococcal meningitis. However, recent evidence suggests that TNF_α_ is protective against experimental seizures in mice. Transgenic mice that overexpress TNF_α_ are less susceptible to seizure induction than wild-type animals, and mice which lack TNF_α_ p75 receptors suffer prolonged seizures (Balosso et al., 2005). In contrast, p55 knockout mice exhibit reduced seizure activity compared with wild-type mice, providing further evidence for opposing effects of the two TNF_α_ receptors.
Although IL-6 levels increase after seizures, IL-6 knockout mice are more susceptible to experimental audiogenic seizures than wild-type mice, suggesting that IL-6 may be protective. In fact, mice lacking IL-6 are more susceptible to seizure induction in many different experimental paradigms. In contrast, mice which overexpress IL-6 are much more sensitive to glutamatergic seizures than wild-type animals (Samland et al., 2003).
COX-2 and PG levels increase markedly following rapid seizure kindling in mice, and mice which are deficient in COX-2 display reduced hippocampal seizure activity (Takemiya et al., 2003). However, selective COX-2 inhibitors aggravate kainate seizures and the associated neuronal cell death in mice. Deposition of the complement MAC may also contribute to epileptic seizures; in rats, seizures are induced by the sequential infusion of individual proteins of the MAC into the hippocampus.
Chronic CNS disease
Chronic CNS diseases have a more complex aetiology than acute injuries, and are generally multifactorial, with environmental factors and genetic background contributing to the development and progression of the disease (reviewed by Campbell, 2004). All these factors also contribute to CNS inflammation, which is further exacerbated by ageing. Many of the same inflammatory mediators increase in chronic neurodegenerative diseases: activation of microglia leads to production of cytokines, superoxide radicals, NO and components of the complement system.
Multiple sclerosis
Multiple sclerosis (MS) is a chronic disorder in which inflammation plays a clear role. Invasion of the CNS by T cells and macrophages leads to damage to the myelin sheaths surrounding axons, loss of neuronal function and death. Since the injured areas of the CNS vary widely, the clinical symptoms are heterogeneous, and can include fatigue, muscle weakness, areas of numbness and paralysis. Characteristically, the disease progresses in cycles of relapse, often associated with systemic infection and inflammation, and remission. No single gene has been linked to MS, although there may be an association with alleles of the major histocompatability complex (MHC) class II. Microarray analysis has revealed that many genes related to inflammatory processes are upregulated in the marginal zones of active demyelinating lesions (Mycko et al., 2003). Environmental factors may be as important as genetic predisposition to the development of MS, and it has been proposed that the disease may be triggered in genetically susceptible individuals by viral infection, although no virus has been isolated from post-mortem brain tissue from MS patients (Gilden, 2005).
Many inflammatory mediators are upregulated in MS and associated with the demyelinating lesions (reviewed by Raivich & Banati, 2004). In animal models of the disease (usually generated by injection with myelin basic protein or viral infection), iNOS, complement, and the cytokines IL-1, IL-12 and TNF_α_ are increased, changes which are also observed in MS patients and correlate with the stage of the disease. COX-2 is also induced, often in association with iNOS, in areas with evidence of recent demyelination. Chronic administration of IL-1 can lead to extensive demyelination in the rat, mimicking MS, and blocking IL-1 and TNF_α_ alone or together can reduce the disease in animals. However, the relationship between IL-1 gene polymorphisms and MS is controversial, with some reports describing a link, but many finding no association. Current clinical treatments for MS include anti-inflammatory agents and interferon-β (IFN_β_), which interestingly can lead to elevations of IL-1ra. Lovastatin blocks progression of experimental allergic encephalitis (a model of MS), reducing cytokine and iNOS expression, but also transmigration of monocytes, perhaps by reducing expression of _α_1_β_1 integrin (Stanislaus et al., 2001).
Alzheimer's disease
Alzheimer's disease (AD) is characterised by the progressive inability to form new memories and access existing ones, due to neuronal cell death in the hippocampus and frontal cortex. Activated microglia surround the amyloid plaques that are one of the hallmarks of the disease (Sheng et al., 1998). These cells initiate a cycle of events – once activated by the amyloid peptide, A_β_, they synthesise and release cytokines (IL-1, IL-6 and TNF_α_) and chemokines, leading to monocyte migration across the BBB. Indeed increased levels of these cytokines have been detected in brain tissue and the cerebrospinal fluid (CSF) of patients with AD. Both TNF_α_ and IL-1 can then increase expression of amyloid precursor protein and A_β_ peptide. These results suggest that CNS inflammation at least participates in amplification of the disease state. Furthermore, proteins of the complement system are associated with AD lesions, and one risk factor for AD is acute brain injury, leading to long-term inflammation, with increased expression of MHC class II, IL-1 and TNF_α_. Perhaps the most convincing evidence however for a causal role of inflammation in AD comes from many studies demonstrating associations between polymorphisms in the genes encoding members of the IL-1 family and AD (e.g. Rainero et al., 2004), particularly IL-1_α_. Statins may have protective effects in AD and other types of dementia. Cross-sectional analysis of three hospital databases suggest that prevalence of AD in patients taking statins is 60% lower than in patients taking other medications used in the treatment of cerebrovascular diseases (Liao & Laufs, 2005).
Based on this significant body of evidence, a plausible hypothesis was that anti-inflammatory agents might reduce the probability of developing AD, or slow its progression. In particular, COX-2 inhibitors protect neuronal cells from amyloid toxicity in vitro, and promote neuronal survival in animal models of ischaemic and excitotoxic neurodegeneration. Retrospective epidemiological studies showed that patients taking anti-inflammatory drugs for long periods of time have a reduced incidence of AD. Although other epidemiological studies also suggest a beneficial role for anti-inflammatory agents in AD (McGeer et al., 1996), the general view from prospective trials of nonsteroidal inflammatory drugs (NSAIDs) is that they are disappointingly variable. In one trial, patients with mild to moderate AD were treated with a selective COX-2 inhibitor, a traditional NSAID or placebo for a period of 1 year. Patients receiving the COX-2 inhibitor or NSAID showed no slowing of cognitive decline compared with patients receiving placebo (Aisen et al., 2003). A larger trial involving the COX-2 inhibitor Vioxx was then terminated following a dramatic increase in the incidence of cardiovascular side effects (Couzin, 2004), which is worrying for ageing AD patients who are already at high risk of heart disease. The mechanism of action of these drugs is not totally clear, since although NSAIDs generally are COX inhibitors, many (e.g. ibuprofen) also reduce generation of A_β_. Dosage may also be part of the problem in the clinical trials, since the degree of inflammation in specific brain areas during AD may be significantly greater than in arthritic joints. Agents that combine anti-inflammatory and antioxidant activity may prove to be more efficacious in the future.
Parkinson's disease
Parkinson's disease (PD) is characterised by loss of dopaminergic neurones in the substantia nigra, and motor symptoms of tremor, muscle rigidity and bradykinesia. Although there are associations with mutations in the genes encoding α_-synuclein and parkin, PD is otherwise sporadic, and various environmental agents including pesticides and infections, may contribute to the disease. A role of inflammation has been strongly implicated (reviewed by Gao et al., 2003), and activated microglia are found close to degenerating substantia nigral neurones of patients with PD. Inhibiting microglial activation with agents such as naloxone, an opioid receptor antagonist, or the tetracycline minocycline is neuroprotective in animal models for PD. The complement cascade is activated, and mRNAs for complement proteins are upregulated in PD (and AD) to a greater extent than in peripheral inflammatory diseases such as rheumatoid arthritis (reviewed by McGeer & McGeer, 2004). Although PD has been linked with polymorphisms in IL-1 genes, the role of cytokines remains controversial, with some reports showing that TNF_α and IL-1 contribute to neuronal loss, whereas others conclude that IL-1 and IL-6, which are both elevated in the CSF of PD patients, are neuroprotective. Certainly, COX-2 is linked with the progression of PD, and COX-2 inhibitors reduce neuronal damage in the experimental model of PD generated by administration of the neurotoxin MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine).
Other chronic conditions
Inflammation undoubtedly contributes to other chronic CNS disorders, such as amyotropic lateral sclerosis (ALS) and Creutzfeldt–Jakob disease (CJD). ALS, a rapidly progressing motor neurone disease, is associated with mutation of superoxide dismutase (SOD1) gene, and mice that overexpress mutant SOD1 show upregulation of TNFα (Yoshihara et al., 2002), suggesting activation of microglia. Similarly, IL-1 levels are elevated in CSF of CJD patients, and activated microglia are detected in mice infected with CJD (Van et al., 2002).
For more than a decade, inflammation has been implicated in chronic psychiatric disorders. Acute phase proteins and cytokines such as IL-1_β_ and IL-6 are elevated in the serum of depressed patients, and IL-1ra and IFN_γ_ are increased in bipolar disorder. The aetiology of schizophrenia remains unexplained, but recently a vascular-inflammatory-genetic theory has been proposed (Hanson & Gottesman, 2005), bringing together environmental and genetic factors that influence the inflammatory response and potentially contribute to the disease. Serum levels of many cytokines are increased in schizophrenia, including IL-1_β_ and IL-6. Interestingly, antipsychotic drugs have long been known to be immunomodulatory, targeting cytokine networks, and raising the possibility of an alternative mechanistic explanation for the actions of these agents. Drugs that modify immune mediators are now being explored for psychiatric disease; preliminary clinical data suggest that COX-2 inhibitors may be beneficial in schizophrenia and depression.
Inflammation, repair and recovery
Although the information presented so far points to a deleterious role for inflammation in CNS disease, it may also be beneficial. In response to a brain insult, astrocytes become activated, increasing expression of glial fibrillary acidic protein, and producing cytokines; they also contribute to the formation of the glial scar, which isolates the damaged area, but also acts as a barrier to reinnervation. Prevention of this reactive astrogliosis might be predicted to be beneficial for repair and recovery, but in fact this increases neuronal loss from the injury site and inhibits repair of the BBB and axonal remyelination (Faulkner et al., 2004), partly because astrocytes produce neurotrophic factors such as nerve growth factor and brain-derived growth factor that are upregulated by injury. Similarly microglia produce proinflammatory mediators, but also have beneficial effects, removing debris and harmful compounds from the brain parenchyma, although their phagocytic capacity is limited.
Cytokines themselves may also have dual roles, with detrimental acute effects, but beneficial effects in the longer term. Both TNF_α_ and IL-1 are strongly implicated in neuronal loss during acute and chronic neurodegenerative disease, but also participate in repair and recovery. Although TNF_α_ is found associated with active MS lesions, induces death of oligodendrocytes, and increases disease severity in animal models of MS, blocking this cytokine in human clinical trials was not beneficial, and in fact, worsened the disease (The Lenercept Multiple Sclerosis Study Group and The University of British Columbia MS/MRI Analysis Group, 1999). The duality of the role of TNF_α_ has been elegantly demonstrated in mice lacking this cytokine: acutely after cortical impact injury, the TNF_α_ knockout mice show less behavioural impairment than the wild-type controls, but while the wild-type mice recovered in the weeks following injury, the knockout mice did not, remaining significantly impaired both in terms of histological damage and behaviour (Scherbel et al., 1999). These divergent activities may arise from the existence of two TNF_α_ receptors.
Furthermore, cytokine ‘priming' or ‘preconditioning' prior to disease onset can be beneficial. For example, pretreatment with IL-1 is neuroprotective against ischaemic injury, and reduces neurological deficits in an animal model of MS (Huitinga et al., 2000). IL-1 is also reported to influence remyelination, since IL-1 knockout mice fail to remyelinate MS-like lesions. These effects may be at least partly explained by induction of neuroprotective growth factors including insulin-like growth factor by IL-1.
Key points for pharmacological intervention
Although considerable data suggest that inflammation contributes to many CNS diseases, and therefore represents a plausible therapeutic target for intervention, the potential for beneficial as well as detrimental effects leads to complications in the development of therapies. These may be avoided in acute diseases such as stroke, in which the treatment can be administered over a fairly short term, when experimental and clinical data suggest that inflammation is clearly deleterious. Chronic disorders represent a potentially greater therapeutic challenge, but an ideal target might be to block the transition from acute to chronic inflammation. Ultimately combination therapies may be the most beneficial; for example, combining L-DOPA therapy with anti-inflammatory agents for PD.
A wealth of potential inflammatory targets for intervention have been proposed, including microglial activation, leukocyte extravasation via adhesion molecules or matrix metalloproteinases, NO and iNOS, COX-2, and cytokines such as IL-1 and TNF_α_ (Table 2). Some agents have multiple targets, such as minocycline, a tetracycline antibiotic, that has anti-inflammatory and neuroprotective activities and might be beneficial in many CNS disorders. Minocycline inhibits microglial activation, reducing cytokine expression, but it also inhibits matrix metalloproteinase and iNOS activities, and breakdown of the BBB. Complications of its use however include inhibition of remyelination in MS models, and observations that microglial activation may be associated with anti-inflammatory cytokine expression rather than proinflammatory (Perry, 2004).
Table 2. Summary of potential inflammatory targets in CNS disease.
| CNS disease | Inflammatory mediators | Potential therapeutic interventions |
|---|---|---|
| Stroke | IL-1 | IL-1ra, caspase-1 inhibitors, soluble receptor |
| TNF_α_ | TACE inhibitors, soluble receptor, antibodies | |
| IL-6 | ||
| Prostaglandins | COX inhibitors | |
| Nitric oxide | iNOS inhibitors | |
| Complement | Complement antagonists | |
| General inflammation | Statins | |
| Traumatic brain injury | IL-1 | IL-1ra |
| TNF_α_ | TACE inhibitors, soluble receptor, antibodies | |
| IL-6 | ||
| Prostaglandins | COX inhibitors | |
| Complement | Complement antagonists | |
| Epilepsy | IL-1 | IL-1ra |
| TNF_α_ | TACE inhibitors, soluble receptor, antibodies | |
| IL-6 | ||
| Prostaglandins | COX inhibitors | |
| Complement | Complement antagonists | |
| Multiple sclerosis | IL-1 | IL-1ra |
| TNF_α_ | TACE inhibitors, soluble receptor, antibodies | |
| IL-12 | ||
| Prostaglandins | COX inhibitors | |
| Nitric oxide | iNOS inhibitors | |
| Complement | Complement antagonists | |
| IFN_β_ | ||
| Alzheimer's disease | IL-1 | IL-1ra |
| TNF_α_ | TACE inhibitors, soluble receptor, antibodies | |
| IL-6 | ||
| Prostaglandins | COX inhibitors | |
| General inflammation | Statins, NSAIDs | |
| Parkinson's disease | IL-1 | IL-1ra |
| TNF_α_ | TACE inhibitors, soluble receptor, antibodies | |
| IL-6 | ||
| Prostaglandins | COX inhibitors | |
| Complement | Complement antagonists | |
| Microglial activation | Minocycline, naloxane | |
| Depression | Prostaglandins | COX inhibitors |
| IL-1 | IL-1ra | |
| IL-6 | ||
| Acute phase proteins | ||
| Schizophrenia | Prostaglandins | COX inhibitors |
| IL-6 | ||
| IL-8 | ||
| Soluble IL-2R | ||
| IFN_γ_ |
Increased NO and iNOS expression contribute to many CNS diseases, and have been explored as potential targets for intervention, via scavengers for NO or inhibitors of iNOS; examples of potential agents include the naturally occurring 1,25-dihydrovitamin D3, and L-NMMA (N(omega)-monomethyl-L-arginine).
Blockade of molecules that mediate adhesion of circulating leukocytes to the endothelium of the BBB also has the potential to reduce CNS inflammation, and be of therapeutic benefit. Indeed, experimental stroke in rats is reduced either by antibodies to integrin _α_4 (Becker et al., 2001), or peptides derived from the extracellular matrix protein fibronectin. A small molecule inhibitor of integrin _α_4 also significantly reduces inflammation and disease pathology in an animal model of MS (Piraino et al., 2002).
Statins are potent inhibitors of cholesterol biosynthesis that are used for the prevention of coronary heart disease. However, they have also been shown to have anti-inflammatory effects, and in large clinical trials of statins, there is a consistent reduction in the incidence of ischaemic stroke. For example, the Heart Protection Study reported a 28% reduction in ischaemic strokes in 20,000 people with cerebrovascular disease (Liao & Laufs, 2005).
Targeting IL-1 represents one of the most promising potential therapies for CNS disorders, largely because the naturally occurring antagonist (IL-1ra) is well tolerated, being used in the clinic for treatment of rheumatoid arthritis. Furthermore, the beneficial effects of IL-1ra have been extensively demonstrated in acute disorders such as stroke, and its levels are elevated by IFN_β_ treatment of MS patients.
Conclusions
Increasingly over the past decade, it has been realised that inflammation plays a key role in CNS disease, and that bidirectional communication between the CNS and the periphery contributes to many disorders, and the sickness behaviour (fever, lethargy, and appetite suppression) that arises from peripheral inflammation. The hallmark of CNS inflammation, microglial activation, initiates generation and release of a diverse array of downstream mediators, including cytokines, NO, PGs, free radicals and complement. Since many of these agents are proinflammatory and lead to neuronal loss, they represent candidates for potential therapeutic targets for CNS disease. However, there is much still to be understood about the nature of CNS inflammation before it can be successfully exploited for the development of clinical treatments. Morphological microglial activation in some situations may be associated with production of anti-inflammatory agents rather than proinflammatory ones (Perry, 2004). Indeed many of the mediators produced as a result of microglial activation have dual characteristics: they can be neuroprotective as well as neurotoxic, and may participate in long-term repair and recovery. The complex interplay and balance between these diverse mediators, ageing, genetic background, and environmental factors may ultimately determine the outcome of acute CNS injury and regulate initiation and progression of chronic neurodegeneration – understanding these interactions in greater detail will hopefully lead to the development of effective therapies for intractable CNS diseases.
Glossary
A_β_
amyloid β
AD
Alzheimer's disease
ALS
amyotropic lateral sclerosis
BBB
blood--brain barrier
CJD
Creutzfeldt–Jakob disease
CNS
central nervous system
COX
cyclooxygenase
CSF
cerebral spinal fluid
GABA
_γ_-amino butyric acid
IFN
interferon
IL
interleukin
IL-1R
interleukin-1 receptor
IL-1ra
interleukin-1 receptor antagonist
IL-6R
interleukin-6 receptor
iNOS
inducible nitric oxide synthase
L-NMMA
N(omega)-monomethyl-L-arginine
MAC
membrane attack complex
MHC
major histocompatability complex
MPTP
1-methyl-4-phenyl-l,2,3,6-tetrahydropyridine
MS
multiple sclerosis
NMDA
_N_-methyl-D-aspartate
NO
nitric oxide
NSAID
nonsteroidal anti-inflammatory drug
PD
Parkinson's disease
PG
prostaglandin
SOD
superoxide dismutase
TACE
TNF_α_-converting enzyme
TBI
traumatic brain injury
TNF
tumour necrosis factor
References
- AISEN P.S., SCHAFER K.A., GRUNDMAN M., PFEIFFER E., SANO M., DAVIS K.L., FARLOW M.R., JIN S., THOMAS R.G., THAL L.J. Effects of rofecoxib or naproxen vs placebo on Alzheimer disease progression: a randomized controlled trial. JAMA. 2003;289:2819–2826. doi: 10.1001/jama.289.21.2819. [DOI] [PubMed] [Google Scholar]
- BALOSSO S., RAVIZZA T., PEREGO C., PESCHON J., CAMPBELL I.L., DE SIMONI M.G., VEZZANI A. Tumor necrosis factor-alpha inhibits seizures in mice via p75 receptors. Ann. Neurol. 2005;57:804–812. doi: 10.1002/ana.20480. [DOI] [PubMed] [Google Scholar]
- BECKER K., KINDRICK D., RELTON J.K., HARLAN J.M., WINN R. Antibody to the alpha4 integrin decreases infarct size in transient focal cerebral ischemia in rats. Stroke. 2001;32:206–211. doi: 10.1161/01.str.32.1.206. [DOI] [PubMed] [Google Scholar]
- CAMPBELL A. Inflammation, neurodegenerative diseases, and environmental exposures. Ann. N.Y. Acad. Sci. 2004;1035:117–132. doi: 10.1196/annals.1332.008. [DOI] [PubMed] [Google Scholar]
- COUZIN J. Clinical trials. Nail-biting time for trials of COX-2 drugs. Science. 2004;306:1673–1675. doi: 10.1126/science.306.5702.1673. [DOI] [PubMed] [Google Scholar]
- DE SIMONI M.G., STORINI C., BARBA M., CATAPANO L., ARABIA A.M., ROSSI E., BERGAMASCHINI L. Neuroprotection by complement (C1) inhibitor in mouse transient brain ischemia. J. Cereb. Blood Flow Metab. 2003;23:232–239. doi: 10.1097/01.WCB.0000046146.31247.A1. [DOI] [PubMed] [Google Scholar]
- FAULKNER J.R., HERRMANN J.E., WOO M.J., TANSEY K.E., DOAN N.B., SOFRONIEW M.V. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J. Neurosci. 2004;24:2143–2155. doi: 10.1523/JNEUROSCI.3547-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAO H.M., LIU B., ZHANG W., HONG J.S. Novel anti-inflammatory therapy for Parkinson's disease. Trends Pharmacol. Sci. 2003;24:395–401. doi: 10.1016/S0165-6147(03)00176-7. [DOI] [PubMed] [Google Scholar]
- GILDEN D.H. Infectious causes of multiple sclerosis. Lancet Neurol. 2005;4:195–202. doi: 10.1016/S1474-4422(05)01017-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALLENBECK J.M. The many faces of tumor necrosis factor in stroke. Nat. Med. 2002;8:1363–1368. doi: 10.1038/nm1202-1363. [DOI] [PubMed] [Google Scholar]
- HANSON D.R., GOTTESMAN I.I. Theories of schizophrenia: a genetic-inflammatory-vascular synthesis. BMC Med. Genet. 2005;6:7. doi: 10.1186/1471-2350-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUITINGA I., SCHMIDT E.D., VAN DER CAMMEN M.J., BINNEKADE R., TILDERS F.J. Priming with interleukin-1beta suppresses experimental allergic encephalomyelitis in the Lewis rat. J. Neuroendocrinol. 2000;12:1186–1193. doi: 10.1046/j.1365-2826.2000.00574.x. [DOI] [PubMed] [Google Scholar]
- LIAO J.K., LAUFS U. Pleiotropic effects of statins. Annu. Rev. Pharmacol. Toxicol. 2005;45:89–118. doi: 10.1146/annurev.pharmtox.45.120403.095748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCGEER P.L., MCGEER E.G. Inflammation and the degenerative diseases of aging. Ann. N.Y. Acad. Sci. 2004;1035:104–116. doi: 10.1196/annals.1332.007. [DOI] [PubMed] [Google Scholar]
- MCGEER P.L., SCHULZER M., MCGEER E.G. Arthritis and anti-inflammatory agents as possible protective factors for Alzheimer's disease: a review of 17 epidemiologic studies. Neurology. 1996;47:425–432. doi: 10.1212/wnl.47.2.425. [DOI] [PubMed] [Google Scholar]
- MORO M.A., CARDENAS A., HURTADO O., LEZA J.C., LIZASOAIN I. Role of nitric oxide after brain ischaemia. Cell Calcium. 2004;36:265–275. doi: 10.1016/j.ceca.2004.02.011. [DOI] [PubMed] [Google Scholar]
- MYCKO M.P., PAPOIAN R., BOSCHERT U., RAINE C.S., SELMAJ K.W. cDNA microarray analysis in multiple sclerosis lesions: detection of genes associated with disease activity. Brain. 2003;126:1048–1057. doi: 10.1093/brain/awg107. [DOI] [PubMed] [Google Scholar]
- PERRY V.H. The influence of systemic inflammation on inflammation in the brain: implications for chronic neurodegenerative disease. Brain Behav. Immun. 2004;18:407–413. doi: 10.1016/j.bbi.2004.01.004. [DOI] [PubMed] [Google Scholar]
- PIRAINO P.S., YEDNOCK T.A., FREEDMAN S.B., MESSERSMITH E.K., PLEISS M.A., VANDEVERT C., THORSETT E.D., KARLIK S.J. Prolonged reversal of chronic experimental allergic encephalomyelitis using a small molecule inhibitor of alpha4 integrin. J. Neuroimmunol. 2002;131:147–159. doi: 10.1016/s0165-5728(02)00273-4. [DOI] [PubMed] [Google Scholar]
- POLLMACHER T., HAACK M., SCHULD A., REICHENBERG A., YIRMIYA R. Low levels of circulating inflammatory cytokines – do they affect human brain functions. Brain Behav. Immun. 2002;16:525–532. doi: 10.1016/s0889-1591(02)00004-1. [DOI] [PubMed] [Google Scholar]
- RAINERO I., BO M., FERRERO M., VALFRE W., VAULA G., PINESSI L. Association between the interleukin-1alpha gene and Alzheimer's disease: a meta-analysis. Neurobiol Aging. 2004;25:1293–1298. doi: 10.1016/j.neurobiolaging.2004.02.011. [DOI] [PubMed] [Google Scholar]
- RAIVICH G., BANATI R. Brain microglia and blood-derived macrophages: molecular profiles and functional roles in multiple sclerosis and animal models of autoimmune demyelinating disease. Brain Res. Brain Res. Rev. 2004;46:261–281. doi: 10.1016/j.brainresrev.2004.06.006. [DOI] [PubMed] [Google Scholar]
- ROTHWELL N.J., LUHESHI G.N. Interleukin 1 in the brain: biology, pathology and therapeutic target. Trends Neurosci. 2000;23:618–625. doi: 10.1016/s0166-2236(00)01661-1. [DOI] [PubMed] [Google Scholar]
- SAMLAND H., HUITRON-RESENDIZ S., MASLIAH E., CRIADO J., HENRIKSEN S.J., CAMPBELL I.L. Profound increase in sensitivity to glutamatergic – but not cholinergic agonist-induced seizures in transgenic mice with astrocyte production of IL-6. J. Neurosci. Res. 2003;73:176–187. doi: 10.1002/jnr.10635. [DOI] [PubMed] [Google Scholar]
- SAYYAH M., BEHESHTI S., SHOKRGOZAR M.A., ESLAMI-FAR A., DELJOO Z., KHABIRI A.R., HAERI R.A. Antiepileptogenic and anticonvulsant activity of interleukin-1 beta in amygdala-kindled rats. Exp. Neurol. 2005;191:145–153. doi: 10.1016/j.expneurol.2004.08.032. [DOI] [PubMed] [Google Scholar]
- SCHERBEL U., RAGHUPATHI R., NAKAMURA M., SAATMAN K.E., TROJANOWSKI J.Q., NEUGEBAUER E., MARINO M.W., MCINTOSH T.K. Differential acute and chronic responses of tumor necrosis factor-deficient mice to experimental brain injury. Proc. Natl. Acad. Sci. 1999;96:8721–8726. doi: 10.1073/pnas.96.15.8721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEWELL D.L., NACEWICZ B., LIU F., MACVILAY S., ERDEI A., LAMBRIS J.D., SANDOR M., FABRY Z. Complement C3 and C5 play critical roles in traumatic brain cryoinjury: blocking effects on neutrophil extravasation by C5a receptor antagonist. J. Neuroimmunol. 2004;155:55–63. doi: 10.1016/j.jneuroim.2004.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHENG J.G., GRIFFIN W.S., ROYSTON M.C., MRAK R.E. Distribution of interleukin-1-immunoreactive microglia in cerebral cortical layers: implications for neuritic plaque formation in Alzheimer's disease. Neuropathol. Appl. Neurobiol. 1998;24:278–283. doi: 10.1046/j.1365-2990.1998.00122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STANISLAUS R., SINGH A.K., SINGH I. Lovastatin treatment decreases mononuclear cell infiltration into the CNS of Lewis rats with experimental allergic encephalomyelitis. J. Neurosci. Res. 2001;66:155–162. doi: 10.1002/jnr.1207. [DOI] [PubMed] [Google Scholar]
- SUFFREDINI A.F., FANTUZZI G., BADOLATO R., OPPENHEIM J.J., O'GRADY N.P. New insights into the biology of the acute phase response. J. Clin. Immunol. 1999;19:203–214. doi: 10.1023/a:1020563913045. [DOI] [PubMed] [Google Scholar]
- TAKEMIYA T., SUZUKI K., SUGIURA H., YASUDA S., YAMAGATA K., KAWAKAMI Y., MARU E. Inducible brain COX-2 facilitates the recurrence of hippocampal seizures in mouse rapid kindling. Prostaglandins Other Lipid Mediat. 2003;71:205–216. doi: 10.1016/s1098-8823(03)00040-6. [DOI] [PubMed] [Google Scholar]
- TEHRANIAN R., NDELL-JONSSON S., BENI S.M., YATSIV I., SHOHAMI E., BARTFAI T., LUNDKVIST J., IVERFELDT K. Improved recovery and delayed cytokine induction after closed head injury in mice with central overexpression of the secreted isoform of the interleukin-1 receptor antagonist. J. Neurotrauma. 2002;19:939–951. doi: 10.1089/089771502320317096. [DOI] [PubMed] [Google Scholar]
- THE LENERCEPT MULTIPLE SCLEROSIS STUDY GROUP AND THE UNIVERSITY OF BRITISH COLUMBIA MS/MRI ANALYSIS GROUP TNF neutralization in MS: results of a randomized, placebo-controlled multicenter study. Neurology. 1999;53:457. [PubMed] [Google Scholar]
- VAN E.B., DEWULF E., PALS P., LUBKE U., MARTIN J.J., CRAS P. The role of cytokines, astrocytes, microglia and apoptosis in Creutzfeldt–Jakob disease. Neurobiol. Aging. 2002;23:59–64. doi: 10.1016/s0197-4580(01)00236-6. [DOI] [PubMed] [Google Scholar]
- VAN WAGONER N.J., BENVENISTE E.N. Interleukin-6 expression and regulation in astrocytes. J. Neuroimmunol. 1999;100:124–139. doi: 10.1016/s0165-5728(99)00187-3. [DOI] [PubMed] [Google Scholar]
- VEZZANI A., MONETA D., RICHICHI C., ALIPRANDI M., BURROWS S.J., RAVIZZA T., PEREGO C., DE SIMONI M.G. Functional role of inflammatory cytokines and antiinflammatory molecules in seizures and epileptogenesis. Epilepsia. 2002;43 Suppl 5:30–35. doi: 10.1046/j.1528-1157.43.s.5.14.x. [DOI] [PubMed] [Google Scholar]
- YOSHIHARA T., ISHIGAKI S., YAMAMOTO M., LIANG Y., NIWA J., TAKEUCHI H., DOYU M., SOBUE G. Differential expression of inflammation- and apoptosis-related genes in spinal cords of a mutant SOD1 transgenic mouse model of familial amyotrophic lateral sclerosis. J. Neurochem. 2002;80:158–167. doi: 10.1046/j.0022-3042.2001.00683.x. [DOI] [PubMed] [Google Scholar]