Irritable bowel syndrome (original) (raw)
. Author manuscript; available in PMC: 2007 Jan 3.
Abstract
Conceptually, the irritable bowel syndrome (IBS) has been considered a brain-gut functional disorder, but this paradigm is under serious challenge. There is increasing evidence that organic disease of the gastrointestinal tract can be identified in subsets of patients who fulfil the Rome criteria for IBS. Evidence for subtle inflammatory bowel disease, serotonin dysregulation, bacterial overgrowth and central dysregulation continue to accumulate. The underlying causes of IBS remain to be adequately identified, but postinfectious IBS is a clear-cut entity. Furthermore, a genetic contribution to IBS also seems likely. Diagnosis continues to be based on the symptom profile and the absence of alarm features. A heightened awareness of coeliac disease masquerading as IBS is becoming accepted. Management remains largely based on symptomatic rather than on disease-modifying therapy, but this is likely to change in the near future. Here, recent advances in the pathophysiology and management of IBS are considered.
Keywords: irritable bowel syndrome, infection, inflammation, genetics, therapy
Introduction
The irritable bowel syndrome (IBS) is characterized by unexplained abdominal discomfort or pain associated with disturbed defecation.1 Traditionally, IBS has been conceptualized as a condition arising from brain-gut dysregulation. Hence, IBS is classified as one of the functional gastrointestinal disorders, where functional refers to a variable combination of chronic or recurring gastrointestinal symptoms not explained by structural or biochemical abnormalities.2 Although IBS has been dismissed by many as a non-organic problem, this view has been seriously challenged by a body of recent studies that suggest that IBS, at least in part, has an organic component that can be readily and easily recognized.3 This paradigm shift implies that the term functional as applied to IBS is no longer defensible, although such views remain controversial.3 In this review, some of the newest information about IBS will be presented, to help guide clinicians diagnosing and treating their patients.
Definition and epidemiology
Over the past 15 years, the definition of IBS has evolved, driven largely by expert opinion and based on studies that have identified symptoms that discriminate those labelled as IBS from organic disease, as well as factor analyses that have identified clear symptom clusters (Table 1).2 Classically, IBS presents with abdominal pain or discomfort that is relieved by defecation or is associated at its onset with a change in stool frequency (either an increase or decrease) or a change in the appearance of the stool (to either loose or hard). The absence of red flag (alarm) symptoms such as gastrointestinal bleeding, weight loss, fever, anaemia or an abdominal mass support such a symptom complex as IBS rather than as structural disease.4 A number of other comorbid conditions may occur more often than expected by chance in those with IBS, including gastro-oesophageal reflux, genito-urinary symptoms, fibromyalgia, headache, backache and psychological symptoms.2 Hence, IBS can present to a number of different subspecialists and is often initially misdiagnosed.2
Table 1.
Rome III criteria for irritable bowel syndrome
- 3 months or more
- Abdominal discomfort or pain at least 3 days per month
Relieved by defecation
Associated with a change in stool appearance (form)
Associated with a change in stool frequency
IBS can be subdivided into those who tend to have predominant diarrhoea or predominant constipation.2,3,5 There is also a group of IBS patients who have mixed constipation and diarrhoea. To complicate matters, those with one predominant bowel pattern can alternate with the other. Highly variable bowel symptoms support a diagnosis of IBS, but the coexistence of abdominal pain and disturbed defecation remains a sine qua non for diagnosis.
IBS is a remarkably common condition according to population-based studies (Fig. 1).2,3,6 In Western countries, including the USA and Australia, approximately 10% of the general population fulfil the Rome criteria for IBS, although many do not ever consult for the problem. IBS overlaps with a number of other unexplained gastrointestinal symptom complexes, including chronic constipation and dyspepsia, suggesting that these conditions may not be discrete entities, but represent disorders with a common aetiopathogenesis.7 In the West, there tends to be a female predominance but this is not seen in the East.8 It has been postulated that IBS is underdiagnosed in Asia and the condition will increase in prevalence because of changes in diet and infectious risk factors.8
Figure 1.
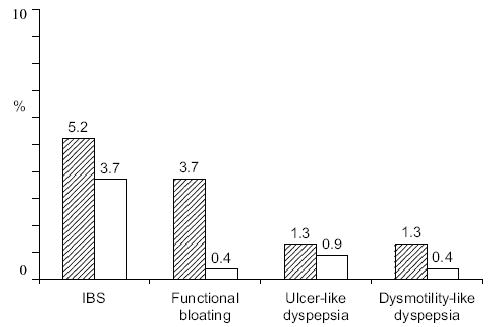
Prevalence of irritable bowel syndrome (IBS) and other major functional gastrointestinal disorders in an Australian population sample based on Rome II criteria.6 n = 762; ▨ females; □ males.
Pathophysiology
Traditionally, IBS has been conceptualized as a condition of visceral hypersensitivity (leading to abdominal discomfort or pain) and gastrointestinal motor disturbances (leading to diarrhoea or constipation).2,3 The gastrointestinal motor disturbances identified, including changes in intestinal transit, do not easily explain mixed or alternating IBS.3 Some have suggested that these abnormalities are secondary to psychological disturbances rather than being of primary relevance.2,3 However, not all patients with IBS have significant psychological overlay and referral bias may partly account for the psychological associations.2,3 Hints as to why visceral hypersensitivity and gastrointestinal motor disturbances may arise are emerging.
Postinfectious and inflammatory IBS
There is now convincing evidence that IBS can arise after bacterial gastroenteritis in up to one-quarter of cases.3 For example, a Spanish study reported that the relative risk of developing IBS after Salmonella gastroenteritis was increased eightfold in the subsequent year.9 How often subclinical cases of gastroenteritis account for those who develop IBS without a history of preceding gastroenteritis is unknown.
There is also accumulating evidence that a subgroup of patients labelled as IBS have subtle inflammatory bowel disease, although the exact prevalence of these findings in IBS overall remains unclear.3 For example, lamina propria T lymphocytes are increased in those with documented postinfectious IBS and in those with non-postinfectious IBS compared with controls.10 Mast cells have also been observed to be increased in those with non-postinfectious IBS based on quantification methods.10 Using conventional histological criteria, these biopsies are normal. Hence, we have probably been overlooking subtle inflammatory bowel disease in IBS in the past.
Not only are there subtle inflammatory changes in colonic biopsies in IBS, but there is also evidence that there is a change in peripheral cytokine profiles in IBS reminiscent of those seen in inflammatory bowel disease. For example, an abnormal interleukin (IL)-10/IL-12 ratio has been observed in IBS, suggesting a pro-inflammatory (Th-1) state.11 Based on evidence such as this, application of the label functional to IBS now seems rather inappropriate.
Serotonin dysregulation
Whether inflammation can alter mucosal control of motility is an area of active research interest.3 Serotonin, present largely in the enterochromaffin cells in the gut, is a major regulator of the peristaltic reflex and sensory relays in the gut.12 Two lines of evidence support the view that serotonin regulation is abnormal in IBS. The release of serotonin in plasma appears to be reduced in those with constipation-predominant IBS and increased in diarrhoea.13 In a seminal paper, rectal biopsy specimens were assessed in patients with IBS, ulcerative colitis and controls.14 A defect in serotonin signalling was noted in both IBS and ulcerative colitis, with a reduction in normal mucosal serotonin and serotonin transporter immunoreactivity in both diseases.14 This implies that there may be a real molecular defect in IBS, which conceivably is acquired possibly after infection.
Bacterial overgrowth
In the presence of dysregulated gastrointestinal motor function, it is conceivable that stasis promotes small intestinal bacterial overgrowth to occur, inducing fermentation and leading to production of excess gas. The gas in turn may be trapped and induce some of the symptoms of IBS, including discomfort and bloating.15 There is some direct and indirect evidence in support of this hypothesis, which has management implications, although further work is required to confirm the observations.15
Central dysregulation
Psychosocial factors do appear to be important in IBS, although whether these factors directly alter gastrointestinal function remains uncertain.2,3 It is also possible that gastrointestinal dysfunction modulates central processes too. For example, there is good evidence now that abuse in childhood or adulthood is associated with IBS, although whether it is of aetiological importance remains in dispute.16 Anxiety and depression are also common in IBS.2,3 Some have conceptualized IBS as a somatization disorder, but the clear evidence for an organic pathophysiology makes this unlikely.3,16
There are differences in brain responses in patients with IBS that have been documented. For example, measures of regional cerebral blood flow during rectal distention have shown that IBS patients have greater activation of the anterior cingulate cortex, amygdala and dorsomedial frontal cortex, in contrast to patients with ulcerative colitis and controls.17 It has been postulated that the brains of people without IBS are better able to activate endogenous pain inhibition areas. This could represent a genetic predisposition to IBS. The antidepressant amitriptyline has been shown to reduce rectal pain and this has been correlated to activation of the right prefrontal cortex, right insula and perigenual anterior cingulate cortex.18 Such central changes might explain the potential benefit of antidepressants in IBS.
Genetics
Twin studies and familial studies suggest that there is a genetic contribution to IBS, although the importance of this remains in dispute.19 A search for candidate genes continues, with the working hypothesis that environmental factors likely play an important role in the pathogenesis in the genetically primed individual.
Diagnosis
Diagnosis of IBS is based on positive history of abdominal discomfort or pain associated with disturbed defecation (the Rome criteria), in the absence of obvious alarm features.2,3,20 It is no longer a diagnosis of exclusion; there is good evidence that a positive diagnosis is robust.20 Furthermore, current evidence does not suggest that blood tests, stool studies, abdominal imaging or endoscopy are required to make a diagnosis in the setting of positive symptoms and no alarm features. A negative colonoscopy does not appear to improve reassurance or quality of life in IBS.21
One area of controversy surrounds coeliac disease that can present with typical symptoms of IBS in the absence of weight loss or other obvious features. It has been estimated that testing for coeliac disease serologically (typically using tissue transglutaminase) is cost-effective, assuming the prevalence of coeliac disease exceeds 8% in patients with IBS in local clinical practice.22 Further work is needed to confirm that coeliac disease is an important problem in terms of misdiagnosis in those with Rome criteria for IBS, but at this stage testing seems reasonable.
Management
A positive diagnosis complemented by reassurance, explanation and investigation of psychosocial issues is all important in the management of IBS. Medical treatment options, however, remain limited; most current therapies do not target the likely underlying aetiopathogenesis.
The placebo response in IBS is approximately 40% in clinical trials.23 Making use of the placebo response seems sensible. Providing self-help information seems to be beneficial based on a randomized study in primary care, where the introduction of a guidebook resulted in a 60% reduction in primary care consultations.24 There are good patient-based materials available, which might be useful.25 Patient support groups exist and all those with persistent or troublesome symptoms certainly should be encouraged to join these organizations.
Dietary therapy for IBS is of limited value.26,27 There is now reasonable evidence that constipation will improve with an increased fibre intake, but pain and diarrhoea probably do not do any better on fibre than with placebo.26,27 However, anecdotally some with diarrhoea will have a firming up of their stools with the introduction of fibre. Fibre supplements may be better tolerated but need to be started at a low dose and built up slowly because of the increase in bloating that often occurs with their use. Antispasmodic agents have been shown in clinical trials to probably be superior to placebo in terms of improvement of abdominal pain; however, the quality of the trials has not been high.3 Mebeverine seems to be a useful drug and is worth a trial.27
If diarrhoea is a predominant problem, loperamide is useful and will reduce diarrhoea, but will not alter abdominal pain or other IBS symptoms over placebo based on the current published reports.26,27 Use of the drug to prevent episodes of diarrhoea in those with symptoms after meals or stress can be particularly useful.
Antidepressants are often prescribed for patients with resistant symptoms.26,27 The low-dose tricyclic antidepressants are thought to potentially work locally and centrally. They may be particularly useful in those with more diarrhoea than constipation because of anticholinergic effects, although the evidence supporting their use in IBS subgroups is still limited. Secondary amines such as desipramine or imipramine tend to have less anticholinergic side-effects. Unfortunately, even with low dose, side-effects limit their use. It is reasonable if benefit accrues to continue treatment for approximately 6 months and then taper off the medication based on clinical experience (but not clinical trials).
Tegaserod is a serotonin type 4 agonist that has been shown to have modest efficacy in constipation-predominant IBS in women.26,27 There is no convincing evidence of efficacy in men, although the clinical trials were underpowered to determine efficacy here. The drug appears to be reasonably safe, although diarrhoea and headache are well-recognized side-effects. There have been reports of ischaemic colitis in patients on tegaserod, but there appears to be an increased incidence of ischaemic colitis in IBS and these reports may not reflect a cause-and-effect correlation.25 The major problem with tegaserod use in Australia is its cost. Alosetron is a serotonin type 3 antagonist that has been shown to be efficacious in women and men with diarrhoea-predominant IBS.27,28 The efficacy may be less in men based on recent clinical trial data.28 The drug is available in the USA, but ischaemic colitis and severe constipation have limited its use.
Several antibiotics to treat suspected bacterial overgrowth in IBS has gained momentum following recent trials. There is evidence that rifaximin, a non-absorbable antibiotic, may be superior to placebo in terms of reducing some IBS symptoms, although large, high-quality clinical trials are yet to be conducted and the length of response remains unknown.29 Based on the current evidence, it is premature to recommend treatment with antibiotics. Probiotics (e.g. bifidobacterium) may be an alternative approach and seem promising.11
Alternative therapies
Several alternative approaches have been used to try and manage IBS.25 There is evidence that sleep is disturbed in some patients with IBS and this has led to work with melatonin as a sleep-promoting agent. In one randomized trial from Singapore, 3 mg of melatonin at bedtime for 2 weeks did reduce abdominal pain as well as rectal pain sensitivity.30 Further work is needed to assess the value of melatonin in IBS.
Psychological treatments have been tested in IBS and current evidence does support the view that these approaches can reduce symptoms and improve well-being.2,3 Some have suggested that this is because psychological therapies activate endogenous pain regulation pathways in the brain. Hypnosis is one method where there is increasing empirical evidence of benefit, although large randomized controlled trials are lacking.31
Conclusion
Currently, IBS is relatively straightforward to diagnose based on clinical grounds. However, management remains frustrating, as current treatments largely do not target the underlying pathogenesis, but rather take a symptom-based approach. Cure of IBS will rely on further understanding the underlying causes and pathophysiology and developing treatments that can prevent and/or reverse these abnormalities. There is now hope that in the future this will indeed be possible. Hence, research into IBS and related, unexplained gastrointestinal syndromes is currently in a particularly exciting phase. Expect to see current research findings translate into clinical practice in the relatively near future.
Footnotes
Funding: This work was supported in part by NIH grant U01DK 65713-1 to Dr Talley.
Potential conflicts of interest: Consultant or research support: Novartis, Solvay, Axcan, Bohringer-Ingelheim, Giaconda and GlaxoSmithKline.
References
- 1.Drossman DA, Corrazziari E, Delvaux M, Spiller R, Talley NJ, Thompson WG, et al. Rome III: The Functional Gastrointestinal Disorders. McLean, VA: Degnon Associates; 2006. [Google Scholar]
- 2.Drossman DA, Corrazziari E, Talley NJ, Thompson WG, Whitehead WE. Rome II: The Functional Gastrointestinal Disorders. Diagnosis, Pathophysiology, and Treatment: A Multinational Consensus. McLean, VA: Degnon Associates; 2000. [Google Scholar]
- 3.Talley N, Spiller RC. Irritable bowel syndrome: a little understood organic bowel disease? Lancet. 2002;360:555–64. doi: 10.1016/S0140-6736(02)09712-X. [DOI] [PubMed] [Google Scholar]
- 4.Hammer J, Eslick G, Howell S, Altiparmak E, Talley NJ. Diagnostic yield of alarm features in irritable bowel syndrome and functional dyspepsia. Gut. 2004;53:666–72. doi: 10.1136/gut.2003.021857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guilera M, Balboa A, Mearin F. Bowel habit subtypes and temporal patterns in irritable bowel syndrome: systematic review. Am J Gastroenterol. 2005;100:1174–84. doi: 10.1111/j.1572-0241.2005.40674.x. [DOI] [PubMed] [Google Scholar]
- 6.Boyce PM, Talley NJ, Burke C, Koloski NA. Epidemiology of the functional gastrointestinal disorders diagnosed according to Rome II criteria: an Australian population-based study. Intern Med J. 2006;36:28–36. doi: 10.1111/j.1445-5994.2006.01006.x. [DOI] [PubMed] [Google Scholar]
- 7.Locke GR, 3rd, Zinsmeister AR, Fett SL, Melton LJ, 3rd, Talley NJ. Overlap of gastrointestinal symptom complexes in a US community. Neurogastroenterol Motil. 2005;17:29–34. doi: 10.1111/j.1365-2982.2004.00581.x. [DOI] [PubMed] [Google Scholar]
- 8.Gwee KA. Irritable bowel syndrome in developing countries – a disorder of civilization or colonization? Neurogastroenterol Motil. 2005;17:317–24. doi: 10.1111/j.1365-2982.2005.00627.x. [DOI] [PubMed] [Google Scholar]
- 9.Mearin F, Perez-Oliveras M, Perello A, Vinyet J, Ibanez A, Coderch J, et al. Dyspepsia and irritable bowel syndrome after a Salmonella gastroenteritis outbreak: one-year follow-up cohort study. Gastroenterology. 2005;129:98–104. doi: 10.1053/j.gastro.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 10.Dunlop SP, Jenkins D, Spiller RC. Distinctive clinical, psychological, and histological features of postinfective irritable bowel syndrome. Am J Gastroenterol. 2003;98:1578–83. doi: 10.1111/j.1572-0241.2003.07542.x. [DOI] [PubMed] [Google Scholar]
- 11.O’Mahony L, McCarthy J, Kelly P, Hurley G, Luo F, Chen K, et al. Lactobacillus and bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology. 2005;128:541–51. doi: 10.1053/j.gastro.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 12.Talley NJ. Serotoninergic neuroenteric modulators. Lancet. 2001;358:2061–8. doi: 10.1016/S0140-6736(01)07103-3. [DOI] [PubMed] [Google Scholar]
- 13.Dunlop SP, Coleman NS, Blackshaw E, Perkins AC, Singh G, Marsden CA, et al. Abnormalities of 5-hydroxytryptamine metabolism in irritable bowel syndrome. Clin Gastroenterol Hepatol. 2005;3:349–57. doi: 10.1016/s1542-3565(04)00726-8. [DOI] [PubMed] [Google Scholar]
- 14.Coates MD, Mahoney CR, Linden DR, Sampson JE, Chen J, Blaszyk H, et al. Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology. 2004;126:1657–64. doi: 10.1053/j.gastro.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 15.Lin HC. Small intestinal bacterial overgrowth: a framework for understanding irritable bowel syndrome. JAMA. 2004;292:852–8. doi: 10.1001/jama.292.7.852. [DOI] [PubMed] [Google Scholar]
- 16.Koloski NA, Talley NJ, Boyce PM. A history of abuse in community subjects with irritable bowel syndrome and functional dyspepsia: the role of other psychosocial variables. Digestion. 2005;72:86–96. doi: 10.1159/000087722. [DOI] [PubMed] [Google Scholar]
- 17.Mayer EA, Berman S, Suyenobu B, Labus J, Mandelkern MA, Naliboff BD, et al. Differences in brain responses to visceral pain between patients with irritable bowel syndrome and ulcerative colitis. Pain. 2005;115:398–409. doi: 10.1016/j.pain.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 18.Morgan V, Pickens D, Gautam S, Kessler R, Mertz H. Amitriptyline reduces rectal pain related activation of the anterior cingulate cortex in patients with irritable bowel syndrome. Gut. 2005;54:601–7. doi: 10.1136/gut.2004.047423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saito YA, Petersen GM, Locke GRI, Talley NJ. The genetics of irritable bowel syndrome. Clin Gastroenterol Hepatol. 2005;3:1057–65. doi: 10.1016/s1542-3565(05)00184-9. [DOI] [PubMed] [Google Scholar]
- 20.Cash BD, Chey WD. Irritable bowel syndrome – an evidence-based approach to diagnosis. Aliment Pharmacol Ther. 2004;19:1235–45. doi: 10.1111/j.1365-2036.2004.02001.x. [DOI] [PubMed] [Google Scholar]
- 21.Spiegel BM, Gralnek IM, Bolus R, Chang L, Dulai GS, Naliboff B, et al. Is a negative colonoscopy associated with reassurance or improved health-related quality of life in irritable bowel syndrome? Gastrointest Endosc. 2005;62:892–9. doi: 10.1016/j.gie.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 22.Spiegel BM, DeRosa VP, Gralnek IM, Wang V, Dulai GS. Testing for celiac sprue in irritable bowel syndrome with predominant diarrhea: a cost-effectiveness analysis. Gastroenterology. 2004;126:1721–32. doi: 10.1053/j.gastro.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 23.Patel SM, Stason WB, Legedza A, Ock SM, Kaptchuk TJ, Conboy L, et al. The placebo effect in irritable bowel syndrome trials: a meta-analysis. Neurogastroenterol Motil. 2005;17:332–40. doi: 10.1111/j.1365-2982.2005.00650.x. [DOI] [PubMed] [Google Scholar]
- 24.Robinson A, Lee V, Kennedy A, Middleton L, Rogers A, Thompson DG, et al. A randomised controlled trial of self-help interventions in patients with a primary care diagnosis of irritable bowel syndrome. Gut. 2006;55:643–8. doi: 10.1136/gut.2004.062901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Talley NJ. Conquering Irritable Bowel Syndrome. Hamilton, Ontario, Canada: BC Decker; 2005. [Google Scholar]
- 26.Quartero A, Meineche-Schmidt V, Muris JW, Rubin G, de Wit N. Bulking agents, antispasmodic and antidepressant medication for the treatment of irritable bowel syndrome. Cochrane Database Syst Rev. 2005;(2):CD 003460. doi: 10.1002/14651858.CD003460.pub2. [DOI] [PubMed] [Google Scholar]
- 27.Lesbros-Pantoflickova D, Michetti P, Fried M, Beglinger C, Blum AL. Meta-analysis: the treatment of irritable bowel syndrome. Aliment Pharmacol Ther. 2004;20:1253–69. doi: 10.1111/j.1365-2036.2004.02267.x. [DOI] [PubMed] [Google Scholar]
- 28.Chang L, Ameen VZ, Dukes GE, McSorley DJ, Carter EG, Mayer EA. A dose-ranging, phase II study of the efficacy and safety of alosetron in men with diarrhea-predominant IBS. Am J Gastroenterol. 2005;100:115–23. doi: 10.1111/j.1572-0241.2005.40365.x. [DOI] [PubMed] [Google Scholar]
- 29.Sharara AI, Aoun E, Abdul-Baki H, Mounzer R, Sidani S, Elhajj I. A randomized double-blind placebo-controlled trial of rifaximin in patients with abdominal bloating and flatulence. Am J Gastroenterol. 2006;101:326–33. doi: 10.1111/j.1572-0241.2006.00458.x. [DOI] [PubMed] [Google Scholar]
- 30.Song GH, Leng PH, Gwee KA, Moochhala SM, Ho KY. Melatonin improves abdominal pain in irritable bowel syndrome patients who have sleep disturbances: a randomised, double blind, placebo controlled study. Gut. 2005;54:1402–7. doi: 10.1136/gut.2004.062034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whitehead WE. Hypnosis for irritable bowel syndrome: the empirical evidence of therapeutic effects. Int J Clin Exp Hypn. 2006;54:7–20. doi: 10.1080/00207140500328708. [DOI] [PubMed] [Google Scholar]