Transgenic expression of the coxsackie/adenovirus receptor enables adenoviral-mediated gene delivery in naïve T cells (original) (raw)
Abstract
The inability to easily and efficiently introduce genes into primary T cells has hampered the investigation of the pathways controlling T cell fate. To enable adenoviral-mediated gene transfer into normal naïve T cells, transgenic (Tg) mice expressing the coxsackie/adenovirus receptor (CAR) in their T cell compartment were constructed. Whereas naïve T cells are resistant to adenoviral infection, Tg expression of CAR on T cells greatly facilitates adenoviral-mediated gene expression ex vivo, in vivo, and in differentiated T helper cells. Thus we have developed a technology for efficient gene delivery to naïve T cells. By using adenoviral vectors encoding specific inhibitors, we show that G1 cyclin-dependent kinase, NF-κB, and caspase activities are required for the proliferation of primary T cells. In addition, by expressing Bcl-xL protein at a level that closely approximates mitogen-induced levels, we demonstrate that Bcl-xL expression is sufficient to account for mitogen-mediated survival of primary T cells. Thus, adenoviral-mediated gene delivery to CAR Tg T cells should be useful for the analysis of many genes controlling T cell fate.
The manipulation of gene expression in primary lymphocytes has been successfully approached by the generation of transgenic (Tg) and gene-disrupted mice as well as by using retroviral-mediated gene delivery. These technologies have been invaluable for our understanding of the genes controlling lymphocyte physiology. However, experiments involving retroviral transduction of lymphocytes have been limited by the difficulty in transducing the majority of the cell population, the requirement that the cells be proliferating for viral integration, and the time required for the transduction and expression of the introduced gene. The generation of Tg and gene-disrupted mice is expensive and time consuming, and the genetic manipulation may alter lymphocyte development. As an alternative approach to gene transfer in lymphocytes, we developed methods to allow the introduction of genes by using adenovirus vectors. Adenoviral vectors are attractive in that proliferating or quiescent cells can be efficiently transduced, the expression of the introduced gene is evident by 5 h, the vectors can accommodate large insert sizes, and high-titer stocks are easily generated (1).
Cell entry by adenovirus involves high-affinity binding of the viral fiber capsid protein to a cellular receptor, the coxsackie/adenovirus receptor (CAR) (2, 3). Viral penton base binding to certain cellular integrins then mediates cell entry by receptor-mediated endocytosis via clathrin-coated pits (4). The CAR protein contains two extracellular Ig domains, and the first domain appears sufficient for interaction with the adenoviral fiber protein (5, 6). Unfortunately, naïve lymphocytes are inefficiently infected with adenoviruses, in part because of undetectable expression of CAR (7). We have shown that the expression of CAR in various lymphocyte tumor cell lines facilitates gene transfer by adenovirus recombinants in vitro (8). The cytoplasmic domain of CAR is dispensable for facilitating adenovirus entry (8, 9), suggesting that CAR functions primarily as a docking protein for the adenoviral fiber knob. In particular, lymphoma cells expressing CARΔ1, which encodes a protein with only four amino acids C terminal to the predicted transmembrane domain, and wild-type (wt) CAR were similarly transduced by adenoviruses (8). Importantly, CARΔ1 may be attenuated in its normal function in the cell, and may therefore have less of an effect on cell physiology when exogenously expressed.
Here we describe the generation and analysis of Tg mice that express either wt CAR or CARΔ1 in T cells. T cells from these mice were efficiently transduced with recombinant adenoviruses. Adenovirally manipulated T cells could be maintained in vivo for at least 4 days before immune rejection. T helper (Th) 1 and Th2 clones established from CARΔ1 Tg mice were also efficiently transduced with recombinant adenovectors, which should facilitate the analysis of the genes controlling T cell differentiation and effector functions. By using this technology, we have demonstrated that the activities of cyclin-dependent kinases, NF-κB, and caspases are required for T cell proliferation, and that Bcl-xL expression in the absence of T cell activation can inhibit apoptosis. Lymphocytes that express CAR will be valuable for the analysis of biochemical pathways that control various aspects of cellular physiology, by means of adenoviral-meditated gene transfer, either ex vivo or_in vivo_.
Materials and Methods
Tg Construct and Injection.
The CAR and CARΔ1 cDNAs (from LXSN vectors cut with_Eco_RI/_Xho_I and then ligated to _Asc_I linkers; ref. 8) were cloned into the _Asc_I-digested lckB proximal promoter/human CD2 enhancer Tg construct (a kind gift of Thor Hettmann, University of Chicago). Tg mice were generated by injection of the construct DNAs into the pronuclei of one-cell embryos from FvB/N mice (The Jackson Laboratory) following standard protocols. CAR-positive mice were maintained by backcrossing with FvB, BALB/c (more than five generations), or C57BL/6 mice. Experiments involved mice in the FvB background (congenic) unless otherwise stated.
Antibodies and Flow Cytometry.
Antibodies to CD3ɛ (145–2C11) and human CAR (RmcB) were purified from hybridoma cell culture supernatants. Antibodies to B220 (allophycocyanin-linked; no. 553092), CD4 (phycoerythrin-linked; no. 09005B), and CD8 (Cy-Chrome-linked; no. 553034) were purchased from PharMingen. Isolated cells from the thymus, lymph nodes, spleen, or heparinized peripheral blood were treated with hemolytic buffer (0.83% NH4Cl/0.1% NaHCO3/0.004% Na2EDTA) to remove red blood cells and washed in 1% goat serum in PBS. Cells were then incubated in 1% goat serum in PBS with a 1:100 dilution of the indicated antibody (except for 1:2 dilution of crude RmcB supernatant) for 45 min on ice. For detection of CAR expression, the cells were then incubated in a 1:100 dilution of biotin-labeled goat anti-mouse IgG1 (no. 02232D; PharMingen) for 45 min, washed, incubated in a 1:100 dilution of phycoerythrin-labeled streptavidin (no. 13025; PharMingen) for 30 min and washed twice. For the detection of intracellular Bcl-xL expression, cells were stained with anti-B220 as above, fixed, permeabilized, and stained with anti-Bcl-xL (Transduction Laboratories, Lexington, KY) as described (10). Stained cells were analyzed (by using a lymphocyte size gate) on a Coulter Epics XL or a Becton Dickinson FACSCalibur flow cytometer. For the analysis of DNA content in T cells, lymphocytes were stained with an allophycocyanin-linked anti-B220, washed, fixed in 1% paraformaldehyde (in PBS) for 15 min on ice, washed with PBS, and permeabilized in 0.1% saponin in PBS for 5 min on ice. The cells were then stained with propidium iodide (20 μg/ml) in PBS for 2 h and DNA content was determined by flow cytometry.
Adenoviruses.
Adenoviruses with a cytomegalovirus promoter (AdCMV) expressing enhanced green fluorescent protein (GFP) (8), p27 (11), IκB (12), Mdm2 (13), and Bcl-xL (14) have been described. All adenoviruses were purified by CsCl gradient centrifugation (1).
Generation of AdUbC-GFP.
The 1.2-kb human ubiquitin (UbC) promoter followed by a polylinker and a 0.8-kb region containing the simian virus 40 late and bovine growth hormone polyadenylation sequences (15) was obtained from the laboratories of J. Kappler and P. Marrack (National Jewish Medical and Research Center, Denver). A _Bam_HI/_Not_I fragment containing the GFP cDNA (from pEGFP-N3; CLONTECH) was cloned into the_Bam_HI/_Not_I sites of the polylinker after the UbC promoter. A _Bgl_II fragment containing the UbC promoter, GFP, and the polyadenylation sequences was then ligated into the_Bgl_II site of the pShuttle vector (16) to obtain pSh-UbC-GFP. Recombinant adenovirus pAdUbC-GFP was generated by the method described (16).
Generation of AdCMV-CrmA.
The CrmA coding region was liberated from pcDNA3-CrmA (kind gift of R. Duke, University of Colorado Health Sciences Center, Denver, CO) by digestion with _Xho_I plus _Hin_dIII and ligated to_Sal_I plus _Hin_dIII-digested adenovirus shuttle vector pACCMV#95 (a kind gift of D. Reed, Ludwig Institute for Cancer Research, Lausanne, Switzerland). Cotransfection of 293 cells with Ad5_dl_327Bstβ-gal–TP complex, plaque purification, and screening to generate AdCMV-CrmA were as described (17).
Virus titers were determined by transduction of 293 cells for 16 h followed by an indirect immunofluorescence assay using an antibody against E2A (1). For GFP-expressing adenoviruses, titers were also determined by transduction of cloned human CAR-expressing EL-4 cells (8) at low multiplicities of infection (MOIs; less than 1) followed by determination of the percentage of cells transduced by using detection of GFP expression by flow cytometry. The latter titering method results in titer values that are about 10-fold lower than that obtained by using 293 cells, but more accurately reflects the actual number of viruses that transduce T cells for a given MOI. All MOIs are expressed by using the EL-4 titering method (for viruses not expressing GFP, titers were converted to the EL-4 method).
Transduction of T Cells.
Harvested peripheral T cells from the spleen or lymph nodes (combined submandibular, axillary, para-aortic, mesenteric, and inguinal lymph nodes) were incubated in hemolytic buffer for 2 min and washed in PBS. Cells (4 × 106) were transduced with recombinant viruses in 100 μl of DMEM (GIBCO/BRL) containing 10 mM Hepes (pH 7.2). Recombinant viruses were stored in 50% glycerol in PBS and dialyzed into DMEM/Hepes before each experiment. Transduction cocktails were incubated at 37°C under a 5% CO2/95% air atmosphere for 30 min with gentle mixing every 10 min. Cells were washed twice in PBS to deplete unbound adenoviruses. For the analysis of ex vivo gene expression from adenovectors, lymphocytes were then cultured in RP10 [RPMI medium 1640 (GIBCO/BRL) supplemented with 10% FBS (HyClone)/0.1 mM 2-mercaptoethanol/1% penicillin-streptomycin] at 37°C under a 5% CO2 atmosphere for the indicated times. For adoptive transfer experiments, hemolytic treatment was not used and the cells were not cultured after the two PBS washes. Cells (4 × 107) were instead resuspended in 200 μl of PBS and transferred by subocular injection into the peripheral blood of anesthetized (250 mg/kg Avertin) recipients.
Northern Analysis of CAR Expression.
Total RNA was isolated from mouse tissues by using Trizol (GIBCO/BRL). RNA was subjected to gel electrophoresis, Northern transfer, and hybridization as has been described (18), except that NorthernMax hybridization buffer (Ambion, Austin, TX) at 45°C and higher stringency washes (to 65°C in 0.1× SSC/0.1% SDS) were used. After washing, the filter was exposed to Kodak Bio-Max film.
Lymphocyte Proliferation.
Lymphocytes (1 × 106) were plated in duplicate in 96-well plates in 200 μl of RP10 medium supplemented with 4 μg/ml Con A (Sigma), 2 ng/ml phorbol 12-myristate 13-acetate (PMA; Sigma), and 50 μCi/ml [3H]thymidine. Cells were incubated at 37°C under a 5% CO2 atmosphere for 48 h and processed, and the amount of incorporated tritium was determined by using a Wallac (Gaithersburg, MD) 96-well harvester and scintillation counter.
Generation of CARΔ1 Tg Th1 and Th2 Clones.
CARΔ1 Tg mice (C57BL/6) were immunized in the hind footpads with the chicken ovalbumin peptide 323–339 (OVA; 1 mg/ml) emulsified in complete Freund's adjuvant and CD4+ T cell clones were derived under conditions favoring Th1 or Th2 selection as described (19). Selected clones were analyzed by flow cytometry for expression of CAR and were transduced with AdCMV-GFP to ascertain transduction efficiency. Transduced cells (1 × 105) were also stimulated with anti-CD3 mAb (1 μg/ml) with or without anti-CD28 mAb (PV1; 2 μg/ml) immobilized to plastic culture wells in 200 μl of RP10, and cytokine content was tested by ELISA on supernatants collected at 24 h.
Results and Discussion
Tg Expression of CAR on T Cells Facilitates Adenoviral-Mediated Gene Delivery.
We have previously shown that the expression of CAR facilitates the transduction of mouse T cell lymphoma cell lines with recombinant adenoviruses in vitro (8). We sought to determine if the expression of CAR on primary T cells would similarly facilitate adenoviral transduction. We generated Tg mice that express either wt human CAR or CARΔ1 from the proximal Lck promoter/CD2 enhancer, which directs expression primarily in lymphocytes. The normal cellular role of the CAR protein is not well understood and Tg expression of wt CAR may have the undesired effect of perturbing cellular physiology. Tg expression of CARΔ1, which lacks the cytoplasmic domain and therefore probably the capacity to signal, may have less of an effect on T cell development and/or physiology.
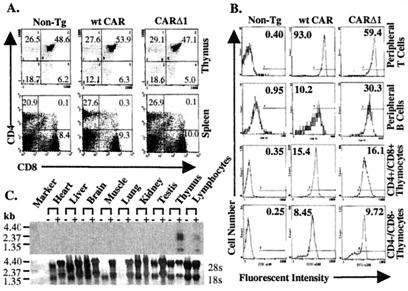
Peripheral blood lymphocytes, splenocytes, and thymocytes were isolated from wt CAR, CARΔ1, or non-Tg (FvB) mice analyzed by flow cytometry for the expression of CAR and markers of B and T cell lineages. The expression of CARΔ1 or wt CAR did not obviously affect lymphocyte development because normal numbers of cells expressing CD4 and CD8 were observed in the thymus and spleen (Fig.1A). CAR was expressed at high and uniform levels on peripheral CD4+ and CD8+ T cells from both Tg mouse strains (Fig.1B). Non-Tg thymocytes, B cells, and T cells did not express detectable CAR. The expression of CAR on B cells and immature CD4+CD8+ thymocytes, whereas clearly increased relative to non-Tg mice, was substantially less than that observed on peripheral T cells (Fig. 1B). More immature CD4−CD8− thymocytes expressed even lower levels of CAR than did CD4+CD8+ thymocytes. We did not observe significant expression of CAR on platelets, macrophages, or red blood cells from wt CAR or CARΔ1 Tg mice (data not shown). To determine whether Tg expression of CAR was limited to lymphoid tissues, we analyzed CAR expression in various tissues from CARΔ1 Tg or non-Tg mice by high-stringency Northern analysis using a human CAR cDNA probe (Fig. 1C). CARΔ1 RNA expression was detected only in lymph node/spleen and thymuses of CARΔ1 Tg mice.
Figure 1.
Expression of CARΔ1 and wt CAR on Tg lymphocytes. (A) CAR expression does not perturb T cell development. Lymphocytes were isolated from the spleen or thymus of either non-Tg, wt CAR Tg, or CARΔ1 Tg mice, stained with fluorescent-conjugated antibodies to CAR, CD4, CD8, and/or B220, and analyzed by flow cytometry (CD4 and CD8 expression in the spleen and thymus is shown). The percentages shown for cells in each quadrant represent the average for four sets of age- and sex-matched mice. Thymic and splenic cellularity did not differ significantly between mice of the different genotypes. Average cell number ± SD (×107) for the thymus: non-Tg, 7.7 ± 3.6; CARΔ1, 8.6 ± 4.7; and wt CAR, 7.5 ± 3.4. For the spleen: non-Tg, 9.9 ± 2.3; CARΔ1, 12.0 ± 5.6; and wt CAR, 14.1 ± 1.6. (B) The expression of CAR on lymphocyte subsets. The expression of CAR on peripheral blood T cells (B220-negative lymphocytes), B cells (B220 positive), CD4+CD8+ thymocytes, or CD4−CD8− thymocytes was determined by flow cytometry as in A. The mean value of fluorescence for the entire cell population is indicated inside each histogram. (C) The CAR Tg mRNA is specifically expressed in the thymus and peripheral lymphocytes. Total RNA was isolated from the indicated tissues from either non-TG (−) or CARΔ1 Tg mice and analyzed for the expression of the human CARΔ1 transgene by Northern blotting. Lymphocyte RNA was isolated from the spleen and lymph nodes combined. The positions of RNA size markers are indicated. The expression of 28S and 18S rRNA (methylene blue stain) is shown as a loading control.
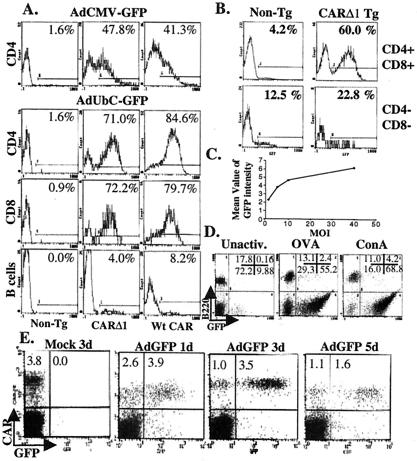
We determined the ability of CAR Tg lymphocytes to be transduced by recombinant adenovirus. Peripheral lymphocytes (from the spleen and lymph nodes) and thymocytes were isolated from CAR wt, Δ1, or non-Tg mice and transduced with the indicated recombinant adenovirus for 30 min and cultured overnight. The cells were then stained with fluorescent-tagged antibodies to CD4 and CD8, and GFP fluorescence was determined by using flow cytometry. As shown in Fig.2A, the expression of either wt CAR or CARΔ1 on peripheral T cells allows for the efficient transduction of these cells with adenovirus expressing GFP from the cytomegalovirus promoter (AdCMV-GFP). However, because the expression from the cytomegalovirus promoter is weak in T cells, we constructed a recombinant adenovirus (AdUbC-GFP) that expresses GFP from the human UbC promoter. Transduction with AdUbC-GFP resulted in high levels of GFP expression in either CD4+ or CD8+ Tg T cells (5- to 10-fold higher than with AdCMV-GFP; Fig. 2A). Almost all peripheral CAR Tg T cells expressed GFP after transduction with AdUbC-GFP (Fig.2A), and the expression of GFP was maintained_in vitro_ with no significant decrease for at least 4 days (data not shown). Importantly, the cytoplasmic domain of CAR was dispensable for facilitating adenoviral-mediated gene transfer in primary T cells. Transduction of T cells with AdUbC-GFP at different MOIs resulted in a dose-dependent increase in the expression of GFP (Fig. 2C), although the relationship was not linear. As expected, non-Tg peripheral lymphocytes and thymocytes showed almost no expression of GFP after transduction. CAR Tg CD4+CD8+ thymocytes were also transduced with adenoviruses (Fig. 2B), although not to the same extent as in peripheral T cells, correlating with the lower expression of CAR on thymocytes. Only a modest increase in transduction was observed in CAR Tg CD4−CD8− thymocytes. Only a small percentage of B cells from CAR Tg mice expressed detectable GFP after transduction with AdUbC-GFP or AdCMV-GFP (Fig.2A). It is not clear why the expression of Tg CAR on B cells did not improve adenoviral transduction more substantially. Whereas higher CAR expression generally correlated with increased adenoviral transduction, other factors (such as integrin expression) probably contribute to the differential transduction efficiencies of T cells, B cells, and thymocytes.
Figure 2.
CAR Δ1 Tg mature T cells and thymocytes are efficiently transduced by recombinant adenovirus. (A) Transduction of mature lymphocytes. Lymphocytes from CAR Tg or non-Tg mice were harvested from the spleen and lymph nodes (pooled), transduced with either AdCMV-GFP or AdUbC-GFP at a MOI of 10, cultured overnight, and stained with fluorescent-tagged antibodies to CD4 and CD8. GFP expression (displayed on the x axis; the y axis represents cell number) was monitored by flow cytometry in lymphocytes gated for the expression of either CD4, CD8, or neither (B cells). In_A_ and B, percentages of GFP-positive cells are indicated inside each histogram. Gating for GFP-positive cells was determined based on the fluorescent intensity of mock-transduced cells. (B) Transduction of CAR Tg thymocytes. Thymocytes from CARΔ1 Tg and non-Tg mice were transduced with AdUbC-GFP at a MOI of 5 as described in A. GFP expression was determined in thymocytes gated for the expression of both CD4 and CD8 or neither (CD4−CD8− thymocytes). (C) Dose-dependent gene expression. Lymphocytes from CARΔ1 Tg mice were transduced with AdUbC-GFP at the indicated MOIs as described in A. The mean value of GFP intensity for transduced T cells is plotted relative to the MOI. (D) T cell activation potentiates CAR-mediated adenoviral delivery. Peripheral lymphocytes (DO11.10 TCR and CARΔ1 double Tg) were harvested and either immediately transduced with AdUbC-GFP at a MOI of 1 (Unactiv.), or cultured in RP10 together with 5 μg/ml OVA (ISQAVHAAHAEINEAGR) or 4 μg/ml Con A for 2 days before transduction with AdUbC-GFP at a MOI of one. Twenty-four hours after transduction, the cells were harvested, stained with anti-B220, and analyzed for GFP expression in lymphocytes by flow cytometry. The upper and lower quadrants represent B cells and T cells, respectively. The percentages in each quadrant are indicated. (E) Manipulation of gene expression in T cells in vivo. Lymphocytes from CARΔ1 Tg mice were harvested and transduced with AdUbC-GFP at a MOI of 10 ex vivo as described in_A_. Transduced cells (or mock-transduced cells) were washed twice with PBS and immediately transferred into recipient FvB mice by subocular injection; 1, 3 and 5 days after transfer, splenocytes from recipient mice were harvested and stained with antibodies to CAR and B220. The expression of CAR and GFP was determined in T cells (B220 negative) by flow cytometry.
Mitogen-mediated up-regulation of αv integrin expression on human peripheral T cells has been shown to allow some adenoviral entry (up to 15%) that is independent of the interaction of the viral fiber protein with CAR (7). Consistent with these results, activation of non-Tg mouse T cells with Con A before transduction resulted in a modest enhancement of transduction (from 0.36% to 2.54% at a MOI of 10; data not shown). Lymphocytes were harvested from mice Tg for both CARΔ1 and the DO11.10 T cell antigen receptor (TCR) (BALB/c), which recognizes OVA presented by I-Ad MHC (20), and transduced with AdUbC-GFP at a MOI of 1 to accentuate differences in transduction efficiencies. Whereas only 12% (9.88/82.08) of unactivated double-Tg T cells expressed GFP (comparable to the transduction of CARΔ1 Tg mice in the FvB background at this low MOI), most OVA- or Con-A-activated T cells expressed GFP after transduction (65% and 81% of T cells, respectively; Fig. 2D). Even B cell transduction was improved by Con A activation, from less than 1% without activation to 28% with Con A activation. Thus, lymphocyte activation cooperates with the expression of CAR to further facilitate adenoviral entry, presumably by the up-regulation of integrin expression. Thus, both naïve and preactivated CAR Tg T cells are amenable to adenoviral-mediated gene delivery.
CAR (wt or Δ1) Tg T cells, either nontransduced or adenovirally transduced at the MOIs used here, did not show significant differences from nontransduced normal T cells in terms of cell proliferation and IL-2 production after TCR/CD28 activation (data not shown). Transduction of CAR Tg T cells and thymocytes at these MOIs also did not appreciably affect cell viability or the expression of activation markers (CD69, CD62L, and CD25) after antigenic stimulation. Finally, we did not observe significant differences in integrin expression (β1, αIIb, and αM, αv, and lymphocyte function-associated antigen integrins) on wt CAR or CARΔ1 Tg relative to non-Tg T cells, either naïve or activated (data not shown). Thus, neither the expression of CAR nor adenoviral transduction at reasonable MOIs (≤10) substantially affected T cell physiology.
The Manipulation of Gene Expression in T Cells in Vivo.
To facilitate the study of the pathways that contribute to antigen-induced T cell responses under physiological conditions, we evaluated the utility of adoptive transfer of adenovirally manipulated CAR Tg T cells into recipient mice. Peripheral lymphocytes (pooled spleen and lymph nodes) were isolated from CARΔ1 Tg mice and were transduced ex vivo with AdUbC-GFP. The cells were washed twice to deplete noninternalized viruses and were immediately transferred by subocular injection into multiple congenic (FvB) recipient mice. A representative experiment out of four is shown. Recipients were killed 1, 3, and 5 days after transfer and splenic lymphocytes were harvested and analyzed for the expression of B220, CAR, and GFP (Fig. 2E). At both 1 and 3 days after adoptive transfer, 3–4% of splenic T cells (B220-negative) expressed GFP. Of the recovered CARΔ1-expressing T cells at 3 days, 78% (3.5/4.5) were GFP+, which is comparable to the transduction efficiency in this and other experiments (Fig.2A). However, by 5 days after transfer, a reduction (on average, 50%) of GFP-expressing T cells was observed (Fig.2E). Similar results were obtained after adoptive transfer of transduced wt CAR Tg lymphocytes (data not shown). These results indicate that adenoviral transduction of CAR Tg T cells can allow the manipulation of gene expression and the determination of cell fate in T cells in vivo for several days after adoptive transfer.
Peripheral blood was also isolated from the tail veins of recipient mice at 1, 4, and 8 days after adoptive transfer of transduced lymphocytes in several different experiments. No significant loss of the CAR- or GFP-expressing transferred T cells occurred within 4 days (about 90% of day 1 percentages remained), but by 8 days, substantially reduced (about 10%) GFP+ T cells were recovered (data not shown). In contrast, mock-transduced lymphocytes were maintained in recipients for at least 8 days without substantial cell loss. The eventual loss of adenoviral-transduced T cells in vivo appears to be immune mediated. Recipient mice that 7 days before had received AdUbC-GFP-transduced CARΔ1 lymphocytes rapidly eliminated (within 16 h) GFP-expressing T cells after a second adoptive transfer of similarly transduced lymphocytes (data not shown). Nonetheless, the adoptive transfer of adenovirally manipulated CAR Tg T cells into recipient mice provides a window (4 days) to study various aspects of T cell function, and is in marked contrast to the direct intrasplenic injection of adenovirus into CAR Tg mice, which results in very inefficient transduction and immune rejection within 1 day (data not shown). Either the use of “gutted” adenovectors that encode for fewer or no viral genes or transfer of transduced T cells into immunocompromised hosts may allow for more prolonged analysis of transduced T cells in vivo.
Th1 and Th2 Cells Established from CAR Tg Mice Are Efficiently Transduced with Recombinant Adenoviruses.
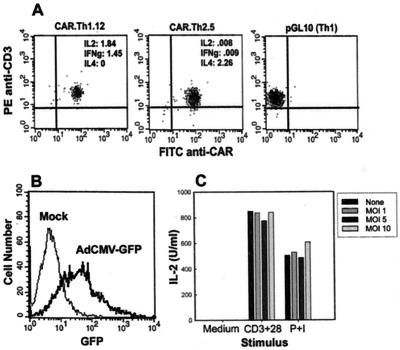
To facilitate future studies of the molecular control of T cell effector function, CARΔ1 Tg CD4+ Th1 and Th2 clones were generated. CARΔ1 Tg mice were immunized with OVA, and clones were derived under limiting dilution conditions in the presence of IFN-γ or IL-4, to preferentially select for Th1 or Th2 cells, respectively. Indeed, both Th1 and Th2 clones were obtained at the expected frequencies (21), and differentiated clones retained high expression of CAR (Fig. 3A), indicating that expression of CAR on T cells does not disrupt peripheral T cell differentiation. It was important to confirm that these cells remained transducible with adenovirus, and that transduction with adenovirus did not disrupt T cell function. As shown in Fig. 3 B and C for the clone CAR.Th1.12, efficient expression of AdCMV-GFP was obtained under conditions that did not significantly interfere with IL-2 production induced by CD3/CD28 ligation. Comparable results were obtained with a Th2 clone (data not shown). These results indicate that CAR Tg T cell clones will be a valuable tool for the genetic manipulation of differentiated effector cells.
Figure 3.
Generation of CAR Tg Th1 and Th2 cell clones. (A) CAR expression on Th1 and Th2 cell clones. T cell clones CAR.Th1.12 and CAR.Th2.5 were stained with antibodies to CAR and CD3 and analyzed by flow cytometry. The previously established wt Th1 clone pGL10 is shown for comparison. Cytokine ELISA OD after stimulation with anti-CD3 for 24 h are displayed in the Inset. (B) Transduction of CAR.Th1.12. The Th1 clone was transduced with AdCMV-GFP at a MOI of 10, and flow cytometric analysis of GFP expression was performed 16 h later. (C) IL-2 production. CAR.Th1.12 cells were transduced with increasing concentrations of AdCMV-GFP, cells were stimulated with either anti-CD3 plus anti-CD28 mAbs or 50 ng/ml PMA/0.5 μM ionomycin, and IL-2 production was measured at 24 h.
Inhibition of Activation-Induced T Cell Proliferation by Using Adenoviral-Mediated Delivery of IκB, p27KIP1, or CrmA.
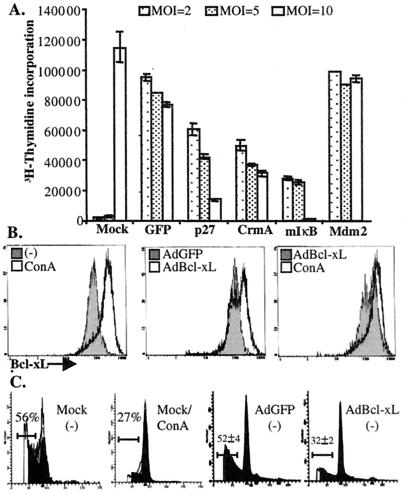
NF-κB is activated by TCR and B cell antigen receptor signaling, as well as by tumor necrosis factor receptor stimulation (22). Receptor signaling results in the phosphorylation and degradation of IκBα and the release of transcriptionally active NF-κB. NF-κB activity is required for activation-induced T cell proliferation as demonstrated by using IκB Tg T cells (23, 24). Cyclin D- and cyclin E-dependent kinase (G1 cdk) activities are also increased in activated T cells during G1 and S phase progression. IL-2 stimulation results in decreased expression of p27KIP1 in peripheral human T cells, contributing to the induction of G1 Cdk activity (25). As shown in Fig. 4A, adenoviral-mediated expression of a mutant IκB superrepressor lacking phosphorylation sites required for signaling-induced degradation (12) or of the p27 Cdk inhibitor in CAR Tg mature T cells reduced proliferation in response to PMA/Con A stimulation in a dose-dependent manner. Interestingly, the expression of CrmA, an inhibitor of Fas-mediated apoptosis, also blocked proliferation (Fig. 4A). In contrast, the expression of Mdm2, a p53 inhibitor, did not significantly affect T cell proliferation. Transduction with AdUbC-GFP caused a modest inhibition of DNA synthesis, although it is not clear whether this is because of the expression of GFP or adenoviral products. Although the inhibition of proliferation by CrmA was unexpected, recent reports have suggested a role for caspase activation in T cell proliferation (26, 27). Our results support such a role specifically for caspases 8 and/or 1, which are inhibited by CrmA. A similar ability of CrmA or IκB to block anti-CD3-induced T cell proliferation was also observed by carboxyfluorescein diacetate succinimidyl ester labeling, as a direct measure of the extent of division of individual T cells (data not shown). Notably, Tg expression of a dominant-negative mutant of the Fas-associated death domain (FADD), but not CrmA, in T cells blocked anti-CD3-induced T cell proliferation, whereas the expression of either dominant-negative FADD or CrmA inhibited Fas-induced apoptosis (28, 29). These apparently discrepant results suggest that either the expression of the CrmA transgene is not as high as that obtained by using adenoviral delivery, or the expression of CrmA during T cell development results in mature T cells that are resistant to CrmA-mediated inhibition of proliferation.
Figure 4.
Adenoviral-mediated manipulation of T cell proliferation and survival pathways. (A) The expression of p27KIP, CrmA, and IκB can block T cell proliferation. Lymphocytes from CAR Tg mice (BALB/c) were harvested and either mock transduced or transduced with adenovectors expressing either p27, CrmA, IκB, Mdm2, or GFP at the indicated MOI. One day after transduction, T cells were cultured with PMA and Con A in RP10 with [3H]thymidine for 2 days, and the amount of incorporated [3H]thymidine (±SE) was determined by scintillation counting. The first two bars represent mock-transduced cells that did not receive PMA or Con A. (B) Adenoviral-mediated expression of Bcl-xL in T cells. Lymphocytes from CARΔ1 Tg mice (BALB/c) were harvested and transduced with AdUbC-GFP or AdCMV-Bcl-xL at a MOI of 10, and then cultured in RP10 for 2 days. (Left) Cells were mock transduced and cultured without (−) or with 4 μg/ml Con A. Cells were stained with anti-B220, fixed, permeabilized, and then stained with an antibody to Bcl-xL. The cells were analyzed by flow cytometry for the expression of Bcl-xL in T cells (B220 negative). (C) Bcl-xL expression increases T cell survival in the absence of mitogenic stimulation. Apoptosis was determined in the cells from the same experiments described in B. The cells were harvested, stained with allophycocyanin-linked anti-B220, stained with propidium iodide, and DNA content (x axis) was determined in T cells (B220 negative) by flow cytometry. The percentage of T cells with a sub-G1 DNA content (apoptotic) representing the average of two experiments (±SE) is indicated.
Bcl-xL Expression in Response to T Cell Activation Is Sufficient to Prevent Apoptosis.
As a further demonstration of the power of adenoviral-mediated gene delivery to CAR Tg T cells, we evaluated the ability of Bcl-xL expression to inhibit T cell apoptosis in response to cell culture in the absence of activation. Tg overexpression of Bcl-xL, similar to Tg expression of Bcl-2, protects thymocytes from apoptosis induced by a variety of stimuli (30). Decreased Bcl-xL expression in CD28−/− T cells at least in part accounts for the reduced survival of these cells and ectopic expression of Bcl-xL in T cell lines prevented death in response to anti-CD3 or IL-2 withdrawal (31, 32). Primary T cell culture in the absence of cytokines or TCR stimulation resulted in progressive apoptosis over several days, coinciding with low expression of Bcl-xL (data not shown and Fig. 4 B and C). In contrast, activation with Con A resulted in enhanced T cell survival and increased Bcl-xL expression. Notably, the expression of Bcl-xL (from AdCMV-Bcl-xL) substantially reduced cell death (Fig. 4C). Increased Bcl-xL expression was observed in the majority of T cells transduced with AdCMV-Bcl-xL. The weak expression of Bcl-xL from the cytomegalovirus promoter in T cells actually represents an advantage in that the adenoviral-mediated expression of Bcl-xL approximates, but does not exceed, the physiological levels observed in activated T cells (Fig. 4B Right). These experiments demonstrate not only that Bcl-xL expression can prevent apoptosis in response to culture in the absence of antigenic stimulation but also that the enhanced expression of Bcl-xL in stimulated cells is sufficient to underlie mitogen-induced survival.
Advantages of Adenoviral-Mediated Gene Transfer to Primary T Cells.
Adenoviral-mediated gene delivery to CAR Tg T cells is a fast, cheap, and efficient method to determine the role of gene products in T cell function. Certainly, retroviral and Tg expressions of genes are still the methods of choice when stable gene expression is required. Adenoviral-mediated gene delivery results in transient expression of the gene product because of the dilution of the episomal adenoviral genome as cells proliferate. Nonetheless, the analysis of the activation, proliferation, apoptosis, and function of either naïve or differentiated T cells and the study of thymocyte negative selection should be amenable to the methods described here. One potential concern is that adenoviral transduction can have nonspecific effects, and we did observe an antiproliferative effect at high MOIs (>40). However, gene expression using low MOIs where nonspecific effects were not observed was quite effective. An advantage of the methods described here is that because the cells of interest are the targets of gene delivery, there is no effect on T cell maturation or repertoire formation. Furthermore, T cell activation and prolonged culture are not required. These features are particularly important where the expressed gene product might inhibit proliferation or induce apoptosis. Even when mature T cells are generated in a Tg mouse despite perturbation of T cell development, these T cells may differ from normal T cells by more than just the expression of the transgene. Also, T cells from the same CAR Tg mouse can be transduced with various gene products, thus eliminating variability because of background heterogeneity. Finally, coexpression of multiple gene products should be straightforward by using adenoviral transduction of CAR Tg T cells, and expression levels can be controlled by the choice of promoter or the MOI used. In conclusion, adenoviral-mediated gene delivery to CAR Tg T cells should complement other gene transfer techniques by facilitating the analysis of gene function in primary naïve T cells when rapid and efficient gene transduction is required.
Acknowledgments
We thank M. Kimbrough, R. Finberg, T. Mitchell, B. Schaefer, P. Marrack, J. Kappler, P. Angel, B. Vogelstein, R. Duke, D. Reed, and F. Miller for reagents, assistance, and advice; K. Helm, P. Schor, and M. Ashton of the Cancer Center Flow Cytometry Core (supported by National Cancer Institute Grant 2 P30 CA 46934-09), and J. Anderson and R. Henderson of the University of Colorado Cancer Center Transgenic Mouse Core; and P. Skavlen and Center for Laboratory Animal Care for excellent veterinary care. J.D. is supported by grants from the V Foundation, National Institutes of Health (RO1 CA77314–01), and a Scholar Award from the Leukemia and Lymphoma Society. T.F.G. is supported by National Institute of Allergy and Infectious Diseases Grant P01 AI35294 and J.S. by National Institutes of Health Grant RO1 VHL58344. All animal experiments were approved by the University of Colorado Health Sciences Center Animal Care and Use Committee.
Abbreviations
CAR
coxsackie/adenovirus receptor
Tg
transgenic
GFP
green fluorescent protein
MOI
multiplicity of infection
Th
T helper
wt
wild-type
UbC
ubiquitin
PMA
phorbol 12-myristate 13-acetate
OVA
chicken ovalbumin peptide 323–339
AdCMV
adenoviral vector with cytomegalovirus promoter
TCR
T cell antigen receptor
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.250356297.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.250356297
References
- 1.Nevins J R, DeGregori J, Jakoi L, Leone G. Methods Enzymol. 1997;283:205–219. doi: 10.1016/s0076-6879(97)83017-0. [DOI] [PubMed] [Google Scholar]
- 2.Bergelson J M, Cunningham J A, Droguett G, Kurt-Jones E A, Krithivas A, Hong J S, Horwitz M S, Crowell R L, Finberg R W. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 3.Tomko R P, Xu R, Philipson L. Proc Natl Acad Sci USA. 1997;94:3352–3356. doi: 10.1073/pnas.94.7.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nemerow G R, Stewart P L. Microbiol Mol Biol Rev. 1999;63:725–734. doi: 10.1128/mmbr.63.3.725-734.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bewley M C, Springer K, Zhang Y B, Freimuth P, Flanagan J M. Science. 1999;286:1579–1583. doi: 10.1126/science.286.5444.1579. [DOI] [PubMed] [Google Scholar]
- 6.Freimuth P, Springer K, Berard C, Hainfeld J, Bewley M, Flanagan J. J Virol. 1999;73:1392–1398. doi: 10.1128/jvi.73.2.1392-1398.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang S, Endo R I, Nemerow G R. J Virol. 1995;69:2257–2263. doi: 10.1128/jvi.69.4.2257-2263.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leon R P, Hedlund T, Meech S J, Li S, Schaack J, Hunger S P, Duke R C, DeGregori J. Proc Natl Acad Sci USA. 1998;95:13159–13164. doi: 10.1073/pnas.95.22.13159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang X, Bergelson J M. J Virol. 1999;73:2559–2562. doi: 10.1128/jvi.73.3.2559-2562.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marrack P, Kappler J, Mitchell T. J Exp Med. 1999;189:521–530. doi: 10.1084/jem.189.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leone G, DeGregori J, Jakoi L, Cook J G, Nevins J R. Proc Natl Acad Sci USA. 1999;96:6626–6631. doi: 10.1073/pnas.96.12.6626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hellerbrand C, Jobin C, Iimuro Y, Licato L, Sartor R B, Brenner D A. Hepatology. 1998;27:1285–1295. doi: 10.1002/hep.510270514. [DOI] [PubMed] [Google Scholar]
- 13.Kowalik T F, DeGregori J, Leone G, Jakoi L, Nevins J R. Cell Growth Differ. 1998;9:113–118. [PubMed] [Google Scholar]
- 14.Aloyz R S, Bamji S X, Pozniak C D, Toma J G, Atwal J, Kaplan D R, Miller F D. J Cell Biol. 1998;143:1691–1703. doi: 10.1083/jcb.143.6.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schorpp M, Jager R, Schellander K, Schenkel J, Wagner E F, Weiher H, Angel P. Nucleic Acids Res. 1996;24:1787–1788. doi: 10.1093/nar/24.9.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He T C, Zhou S, da Costa L T, Yu J, Kinzler K W, Vogelstein B. Proc Natl Acad Sci USA. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schaack J, Langer S, Guo X. J Virol. 1995;69:3920–3923. doi: 10.1128/jvi.69.6.3920-3923.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ingelbrecht I L, Mandelbaum C I, Mirkov T E. BioTechniques. 1998;25:420–423. doi: 10.2144/98253st03. and 425–426. [DOI] [PubMed] [Google Scholar]
- 19.Gajewski T F, Fitch F W. In: Current Protocols in Immunology. Coligan J, Kruisbeek A, Margulies D, Shevach E, Strober W, editors. New York: Greene & Wiley; 1997. pp. 3.13.1–3.13.14. [Google Scholar]
- 20.Murphy K M, Heimberger A B, Loh D Y. Science. 1990;250:1720–1723. doi: 10.1126/science.2125367. [DOI] [PubMed] [Google Scholar]
- 21.Gajewski T F, Joyce J, Fitch F W. J Immunol. 1989;143:15–22. [PubMed] [Google Scholar]
- 22.Baldwin A S., Jr Annu Rev Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 23.Boothby M R, Mora A L, Scherer D C, Brockman J A, Ballard D W. J Exp Med. 1997;185:1897–1907. doi: 10.1084/jem.185.11.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferreira V, Sidenius N, Tarantino N, Hubert P, Chatenoud L, Blasi F, Korner M. J Immunol. 1999;162:6442–6450. [PubMed] [Google Scholar]
- 25.Nourse J, Firpo E, Flanagan W M, Coats S, Polyak K, Lee M-H, Massague J, Crabtree G R, Roberts J M. Nature (London) 1994;372:570–573. doi: 10.1038/372570a0. [DOI] [PubMed] [Google Scholar]
- 26.Alam A, Cohen L Y, Aouad S, Sekaly R P. J Exp Med. 1999;190:1879–1890. doi: 10.1084/jem.190.12.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kennedy N J, Kataoka T, Tschopp J, Budd R C. J Exp Med. 1999;190:1891–1896. doi: 10.1084/jem.190.12.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Newton K, Harris A W, Bath M L, Smith K G C, Strasser A. EMBO J. 1998;17:706–718. doi: 10.1093/emboj/17.3.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walsh C M, Wen B G, Chinnaiyan A M, O'Rourke K, Dixit V M, Hedrick S M. Immunity. 1998;8:439–449. doi: 10.1016/s1074-7613(00)80549-x. [DOI] [PubMed] [Google Scholar]
- 30.Chao D T, Linette G P, Boise L H, White L S, Thompson C B, Korsmeyer S J. J Exp Med. 1995;182:821–828. doi: 10.1084/jem.182.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boise L H, Minn A J, Noel P J, June C H, Accavitti M A, Lindsten T, Thompson C B. Immunity. 1995;3:87–98. doi: 10.1016/1074-7613(95)90161-2. [DOI] [PubMed] [Google Scholar]
- 32.Dahl A M, Klein C, Andres P G, London C A, Lodge M P, Mulligan R C, Abbas A K. J Exp Med. 2000;191:2031–2038. doi: 10.1084/jem.191.12.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]