Cell therapy for autoimmune diseases: does it have a future? (original) (raw)
Abstract
Almost all current therapeutic concepts in autoimmune diseases are based on the systemic suppression of immune functions and are not curative. The recent introduction of biologicals such as tumour necrosis factor blocking antibodies or receptors has added greater specificity to efficient management of disease by targeted suppression of rheumatic inflammation. It is evident, however, that only the elimination of the cells secreting inflammatory mediators, rather than the blockade of secreted molecules, will offer real specific therapeutic advantages in the future. Merely the elimination of such cells and also cells controlling the secreting effector cells could be curative and induce true long term remissions. We review here the state of the art and future therapeutic concepts that are based on the specific modulation of pathogenic cells that induce and sustain autoimmune inflammation. This sounds visionary, however, a variety of basic tools are at hand now. Thus, direct and specific cell therapy of rheumatic diseases will become a true alternative to conventional therapies.
Full Text
The Full Text of this article is available as a PDF (259.5 KB).
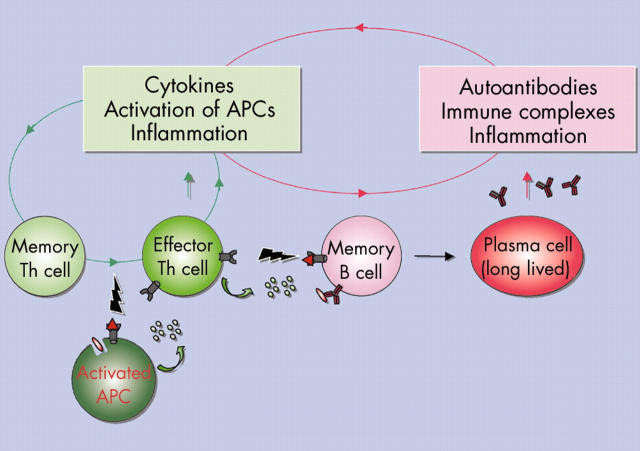
Figure 1.
The chronic autoimmune reaction. Effector immunocytes as cytokine secreting effector/memory T helper (Th) cells and autoantibody secreting plasma cells are major "players" in chronic autoimmune reactions. In addition, activated antigen presenting cells (APCs) also play an important role in both initiating and sustaining autoimmunity. During chronic inflammation non-professional APCs as B cells also might present autoantigens.
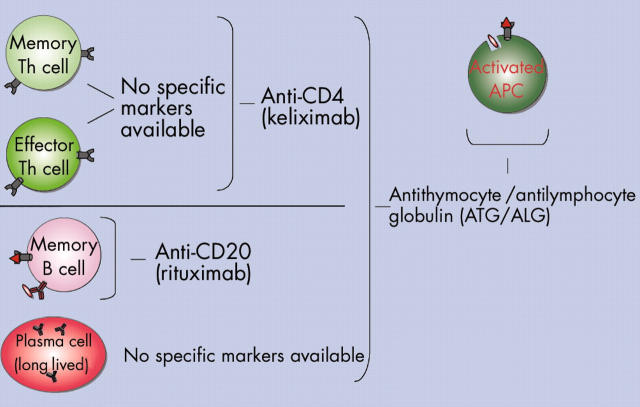
Figure 2.
In vivo targeting of cells of the autoimmune reaction with antibodies. CD4+ T helper (Th) cells can be efficiently targeted by keliximab (anti-CD4). However, both protective memory and effector Th cells are affected. In addition, the targeting of naive (antigen inexperienced) Th cells (not shown) potentially decreases the overall immune competence for new pathogens, particularly since the naive T cell pools are restored slowly in older people. Rituximab (anti-CD20) is a highly efficient B cell depleting drug. However, it is not able to target long lived plasma cells since these have lost CD20 expression. No feasible antibodies for in vivo targeting of plasma cells have yet been introduced. Polyclonal antibody preparations such as ATG or ALG have been used since a long time for in vivo leucocyte depletion in haematological malignancies. Both reagents usually lead to severe and pronounced in vivo depletion of leucocytes including plasma cells and antigen presenting cells (APCs) (not shown).
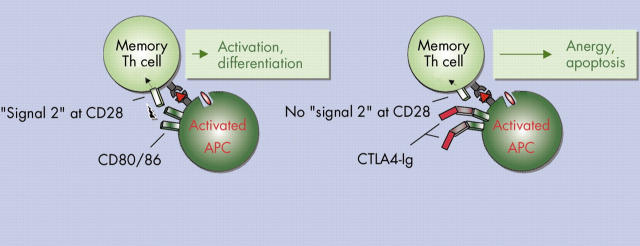
Figure 3.
Targeting antigen presenting cells (APCs)—T helper (Th) cell interaction with CTLA4-Ig. During activation of T cells APCs upregulate CD80 and CD86 that can bind to CD28 on T cells and thereby provide strong costimulatory "signal 2". CTLA4-Ig binds with high affinity to both CD80 and CD86 molecules on the APCs and thereby blocks T cell costimulation leading to anergy or even apoptosis. However, chronically stimulated effector T cells might be less dependent on CD28 mediated costimulation. Thus CTLA4-Ig predominantly modulates primary T cell activation.
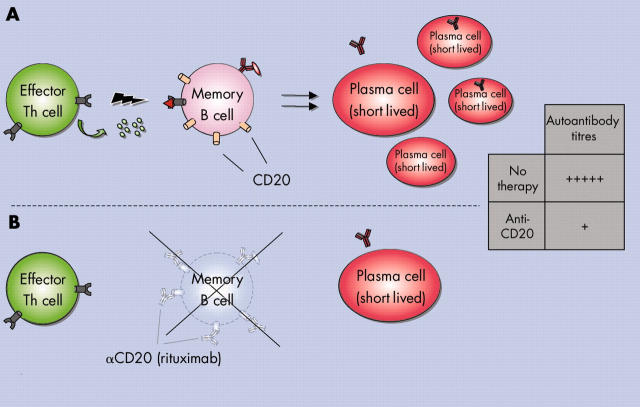
Figure 4.
B cell targeting in autoimmune diseases with anti-CD20. In particular, memory B cells are eliminated during rituximab (anti-CD20) treatment. Depletion of naive B cells is compensated by swift restoration with new B cells from the bone marrow even in older people. While long lived plasma cells lacking CD20 expression cannot be targeted, the supply of pathogenic short lived plasma cells that are generated during activation of autoimmune memory B cells is reduced efficiently. Thereby autoantibody titres originating from short lived plasma cells are decreased drastically (see table) while protective antibodies remain unaltered.
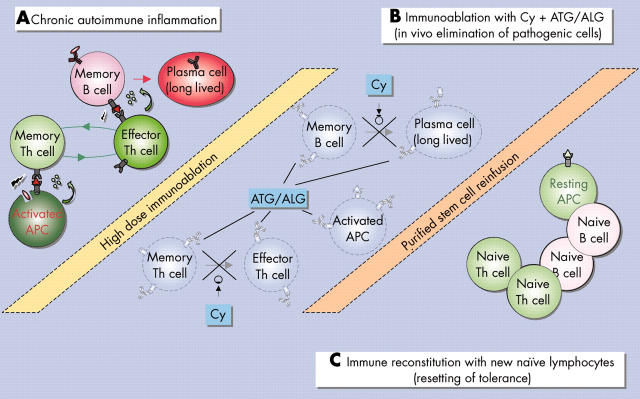
Figure 5.
Haematopoietic stem cell transplantation for autoimmunity. (A) Chronic autoimmune reaction disturbs the homoeostasis of peripheral immunocyte populations. (B) During immunoablation antithymocyte globulin (ATG)/antilymphocyte globulin (ALG) efficiently eliminate resting long lived plasma cells, antigen presenting cells (APCs), or resting memory lymphocytes, and cyclophosphamide (Cy) targets the differentiation of memory B and T cells into effector immunocytes. (C) Purification of autologous stem cells prevents reinfusion with autoreactive immunocytes. Immune reconstitution with new naive B and T cells help to reset and maintain tolerance and normal immunocyte homoeostasis.
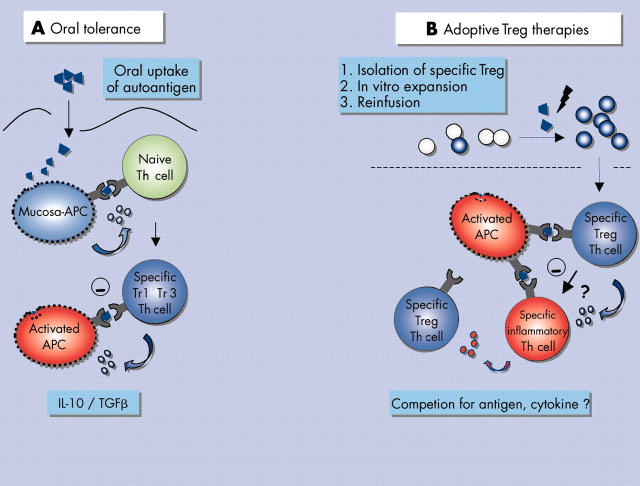
Figure 6.
Control of pathogenic autoimmune reactions with regulatory T cells. (A) Uptake of target autoantigen via mucosal routes can lead to in vivo induction of regulatory T cells either producing transforming growth factor ß (TGFß) (Th 3 cells) or interleukin (IL)-10 (Tr 1 cells). Both Th 3 and Tr 1 cells target activated antigen presenting cells (APCs) presenting the autoantigen or in the end compete with pathogenic inflammatory T cells (not shown). TGFß and IL-10 also modulate T cell functions. However, active chronic immune reactions might be refractory to modulation by oral tolerance. (B) An alternative strategy involves the isolation of specific regulatory T cells or Tregs from the peripheral blood of patients with autoimmunity. After isolation, autoantigen specific regulatory T cells can be further expanded and reinfused in high numbers back into the patient. At the site of inflammation they exert their suppressive anti-inflammatory effects after specific activation from activated APCs. Here regulatory T cells potentially compete with inflammatory T cells for antigen and growth factors but they also most likely suppress local inflammation through secretion of suppressive cytokines (for example IL-10).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akamizu Takashi. Monoclonal antibodies to thyroid specific autoantigens. Autoimmunity. 2003 Sep-Nov;36(6-7):361–366. doi: 10.1080/08916930310001603055. [DOI] [PubMed] [Google Scholar]
- Breedveld F. C. Monoclonal antibodies to CD4. Rheum Dis Clin North Am. 1998 Aug;24(3):567–578. doi: 10.1016/s0889-857x(05)70026-0. [DOI] [PubMed] [Google Scholar]
- Burt R. K., Traynor A. E., Pope R., Schroeder J., Cohen B., Karlin K. H., Lobeck L., Goolsby C., Rowlings P., Davis F. A. Treatment of autoimmune disease by intense immunosuppressive conditioning and autologous hematopoietic stem cell transplantation. Blood. 1998 Nov 15;92(10):3505–3514. [PubMed] [Google Scholar]
- Burt Richard K., Slavin Shimon, Burns William H., Marmont Alberto M. Induction of tolerance in autoimmune diseases by hematopoietic stem cell transplantation: getting closer to a cure? Int J Hematol. 2002 Aug;76 (Suppl 1):226–247. doi: 10.1007/BF03165251. [DOI] [PubMed] [Google Scholar]
- De Vita Salvatore, Zaja Francesco, Sacco Stefania, De Candia Alessandro, Fanin Renato, Ferraccioli Gianfranco. Efficacy of selective B cell blockade in the treatment of rheumatoid arthritis: evidence for a pathogenetic role of B cells. Arthritis Rheum. 2002 Aug;46(8):2029–2033. doi: 10.1002/art.10467. [DOI] [PubMed] [Google Scholar]
- Elliott Peter J., Zollner Thomas Matthias, Boehncke Wolf-Henning. Proteasome inhibition: a new anti-inflammatory strategy. J Mol Med (Berl) 2003 Mar 26;81(4):235–245. doi: 10.1007/s00109-003-0422-2. [DOI] [PubMed] [Google Scholar]
- Euler H. H., Marmont A. M., Bacigalupo A., Fastenrath S., Dreger P., Hoffknecht M., Zander A. R., Schalke B., Hahn U., Haas R. Early recurrence or persistence of autoimmune diseases after unmanipulated autologous stem cell transplantation. Blood. 1996 Nov 1;88(9):3621–3625. [PubMed] [Google Scholar]
- Groux H., O'Garra A., Bigler M., Rouleau M., Antonenko S., de Vries J. E., Roncarolo M. G. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997 Oct 16;389(6652):737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- Hammaker D., Sweeney S., Firestein G. S. Signal transduction networks in rheumatoid arthritis. Ann Rheum Dis. 2003 Nov;62 (Suppl 2):ii86–ii89. doi: 10.1136/ard.62.suppl_2.ii86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser Anja E., Debes Gudrun F., Arce Sergio, Cassese Giuliana, Hamann Alf, Radbruch Andreas, Manz Rudolf A. Chemotactic responsiveness toward ligands for CXCR3 and CXCR4 is regulated on plasma blasts during the time course of a memory immune response. J Immunol. 2002 Aug 1;169(3):1277–1282. doi: 10.4049/jimmunol.169.3.1277. [DOI] [PubMed] [Google Scholar]
- Horneff G., Burmester G. R., Emmrich F., Kalden J. R. Treatment of rheumatoid arthritis with an anti-CD4 monoclonal antibody. Arthritis Rheum. 1991 Feb;34(2):129–140. doi: 10.1002/art.1780340202. [DOI] [PubMed] [Google Scholar]
- Hoyer Bimba F., Moser Katrin, Hauser Anja E., Peddinghaus Anette, Voigt Caroline, Eilat Dan, Radbruch Andreas, Hiepe Falk, Manz Rudolf A. Short-lived plasmablasts and long-lived plasma cells contribute to chronic humoral autoimmunity in NZB/W mice. J Exp Med. 2004 Jun 1;199(11):1577–1584. doi: 10.1084/jem.20040168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keysser G., Alpermann U., Burmester G. R. Anti-CD4-Therapie in der Behandlung der rheumatoiden Arthritis--sind die Würfel schon gefallen? Z Rheumatol. 1998 Oct;57(5):320–325. doi: 10.1007/s003930050119. [DOI] [PubMed] [Google Scholar]
- Kimmig Sonja, Przybylski Grzegorz K., Schmidt Christian A., Laurisch Katja, Möwes Beate, Radbruch Andreas, Thiel Andreas. Two subsets of naive T helper cells with distinct T cell receptor excision circle content in human adult peripheral blood. J Exp Med. 2002 Mar 18;195(6):789–794. doi: 10.1084/jem.20011756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer Joel M., Westhovens Rene, Leon Marc, Di Giorgio Eduardo, Alten Rieke, Steinfeld Serge, Russell Anthony, Dougados Maxime, Emery Paul, Nuamah Isaac F. Treatment of rheumatoid arthritis by selective inhibition of T-cell activation with fusion protein CTLA4Ig. N Engl J Med. 2003 Nov 13;349(20):1907–1915. doi: 10.1056/NEJMoa035075. [DOI] [PubMed] [Google Scholar]
- Leandro M. J., Edwards J. C. W., Cambridge G. Clinical outcome in 22 patients with rheumatoid arthritis treated with B lymphocyte depletion. Ann Rheum Dis. 2002 Oct;61(10):883–888. doi: 10.1136/ard.61.10.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looney R. John, Anolik Jennifer, Sanz Inãki. B cells as therapeutic targets for rheumatic diseases. Curr Opin Rheumatol. 2004 May;16(3):180–185. doi: 10.1097/00002281-200405000-00003. [DOI] [PubMed] [Google Scholar]
- MacDonald T. T. T cell immunity to oral allergens. Curr Opin Immunol. 1998 Dec;10(6):620–627. doi: 10.1016/s0952-7915(98)80079-4. [DOI] [PubMed] [Google Scholar]
- Manz R. A., Thiel A., Radbruch A. Lifetime of plasma cells in the bone marrow. Nature. 1997 Jul 10;388(6638):133–134. doi: 10.1038/40540. [DOI] [PubMed] [Google Scholar]
- Martin Flavius, Chan Andrew C. Pathogenic roles of B cells in human autoimmunity; insights from the clinic. Immunity. 2004 May;20(5):517–527. doi: 10.1016/s1074-7613(04)00112-8. [DOI] [PubMed] [Google Scholar]
- Mason Ursula, Aldrich Jose, Breedveld Ferdinand, Davis Charles B., Elliott Michael, Jackson Mildred, Jorgensen Christian, Keystone Edward, Levy Robert, Tesser John. CD4 coating, but not CD4 depletion, is a predictor of efficacy with primatized monoclonal anti-CD4 treatment of active rheumatoid arthritis. J Rheumatol. 2002 Feb;29(2):220–229. [PubMed] [Google Scholar]
- McIntyre Kim W., Shuster David J., Gillooly Kathleen M., Dambach Donna M., Pattoli Mark A., Lu Pin, Zhou Xia-Di, Qiu Yuping, Zusi F. Christopher, Burke James R. A highly selective inhibitor of I kappa B kinase, BMS-345541, blocks both joint inflammation and destruction in collagen-induced arthritis in mice. Arthritis Rheum. 2003 Sep;48(9):2652–2659. doi: 10.1002/art.11131. [DOI] [PubMed] [Google Scholar]
- Moreland Larry W., Alten Rieke, Van den Bosch Filip, Appelboom Thierry, Leon Marc, Emery Paul, Cohen Stanley, Luggen Michael, Shergy William, Nuamah Isaac. Costimulatory blockade in patients with rheumatoid arthritis: a pilot, dose-finding, double-blind, placebo-controlled clinical trial evaluating CTLA-4Ig and LEA29Y eighty-five days after the first infusion. Arthritis Rheum. 2002 Jun;46(6):1470–1479. doi: 10.1002/art.10294. [DOI] [PubMed] [Google Scholar]
- Mottet Christian, Uhlig Holm H., Powrie Fiona. Cutting edge: cure of colitis by CD4+CD25+ regulatory T cells. J Immunol. 2003 Apr 15;170(8):3939–3943. doi: 10.4049/jimmunol.170.8.3939. [DOI] [PubMed] [Google Scholar]
- Nonomura Yoshinori, Kohsaka Hitoshi, Nagasaka Kenji, Miyasaka Nobuyuki. Gene transfer of a cell cycle modulator exerts anti-inflammatory effects in the treatment of arthritis. J Immunol. 2003 Nov 1;171(9):4913–4919. doi: 10.4049/jimmunol.171.9.4913. [DOI] [PubMed] [Google Scholar]
- Quattrocchi E., Dallman M. J., Feldmann M. Adenovirus-mediated gene transfer of CTLA-4Ig fusion protein in the suppression of experimental autoimmune arthritis. Arthritis Rheum. 2000 Aug;43(8):1688–1697. doi: 10.1002/1529-0131(200008)43:8<1688::AID-ANR4>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Rastetter William, Molina Arturo, White Christine A. Rituximab: expanding role in therapy for lymphomas and autoimmune diseases. Annu Rev Med. 2004;55:477–503. doi: 10.1146/annurev.med.55.091902.104249. [DOI] [PubMed] [Google Scholar]
- Roncarolo M. G., Bacchetta R., Bordignon C., Narula S., Levings M. K. Type 1 T regulatory cells. Immunol Rev. 2001 Aug;182:68–79. doi: 10.1034/j.1600-065x.2001.1820105.x. [DOI] [PubMed] [Google Scholar]
- Rosen O., Thiel A., Massenkeil G., Hiepe F., Häupl T., Radtke H., Burmester G. R., Gromnica-Ihle E., Radbruch A., Arnold R. Autologous stem-cell transplantation in refractory autoimmune diseases after in vivo immunoablation and ex vivo depletion of mononuclear cells. Arthritis Res. 2000 Jun 8;2(4):327–336. doi: 10.1186/ar107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi Shimon. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- Schulze-Koops H., Lipsky P. E. Anti-CD4 monoclonal antibody therapy in human autoimmune diseases. Curr Dir Autoimmun. 2000;2:24–49. doi: 10.1159/000060506. [DOI] [PubMed] [Google Scholar]
- Shastri K. A., Logue G. L. Autoimmune neutropenia. Blood. 1993 Apr 15;81(8):1984–1995. [PubMed] [Google Scholar]
- Sieper J., Kary S., Sörensen H., Alten R., Eggens U., Hüge W., Hiepe F., Kühne A., Listing J., Ulbrich N. Oral type II collagen treatment in early rheumatoid arthritis. A double-blind, placebo-controlled, randomized trial. Arthritis Rheum. 1996 Jan;39(1):41–51. doi: 10.1002/art.1780390106. [DOI] [PubMed] [Google Scholar]
- Silverman Gregg J., Weisman Stuart. Rituximab therapy and autoimmune disorders: prospects for anti-B cell therapy. Arthritis Rheum. 2003 Jun;48(6):1484–1492. doi: 10.1002/art.10947. [DOI] [PubMed] [Google Scholar]
- Vincent Angela. Unravelling the pathogenesis of myasthenia gravis. Nat Rev Immunol. 2002 Oct;2(10):797–804. doi: 10.1038/nri916. [DOI] [PubMed] [Google Scholar]
- Voll R. E., Mikulowska A., Kalden J. R., Holmdahl R. Amelioration of type II collagen induced arthritis in rats by treatment with sodium diethyldithiocarbamate. J Rheumatol. 1999 Jun;26(6):1352–1358. [PubMed] [Google Scholar]
- Webb L. M., Walmsley M. J., Feldmann M. Prevention and amelioration of collagen-induced arthritis by blockade of the CD28 co-stimulatory pathway: requirement for both B7-1 and B7-2. Eur J Immunol. 1996 Oct;26(10):2320–2328. doi: 10.1002/eji.1830261008. [DOI] [PubMed] [Google Scholar]
- Weiner H. L. Oral tolerance: immune mechanisms and the generation of Th3-type TGF-beta-secreting regulatory cells. Microbes Infect. 2001 Sep;3(11):947–954. doi: 10.1016/s1286-4579(01)01456-3. [DOI] [PubMed] [Google Scholar]
- Wulffraat N., van Royen A., Bierings M., Vossen J., Kuis W. Autologous haemopoietic stem-cell transplantation in four patients with refractory juvenile chronic arthritis. Lancet. 1999 Feb 13;353(9152):550–553. doi: 10.1016/S0140-6736(98)05399-9. [DOI] [PubMed] [Google Scholar]
- van der Lubbe P. A., Dijkmans B. A., Markusse H. M., Nässander U., Breedveld F. C. A randomized, double-blind, placebo-controlled study of CD4 monoclonal antibody therapy in early rheumatoid arthritis. Arthritis Rheum. 1995 Aug;38(8):1097–1106. doi: 10.1002/art.1780380812. [DOI] [PubMed] [Google Scholar]