MUC4 expression is a novel prognostic factor in patients with invasive ductal carcinoma of the pancreas (original) (raw)
Abstract
Background: Many patients with invasive ductal carcinoma of the pancreas (IDC) have a poor outcome. MUC4 expression has been implicated as a marker for diagnosis and progression of IDC, but there are no studies of the relation between MUC4 expression and patient prognosis in IDC.
Aims: To investigate the prognostic significance of MUC4 expression in IDC.
Methods: The expression profiles of MUC4, ErbB2, p27, and MUC1 were investigated in IDC tissues from 135 patients by means of immunohistochemistry.
Results: MUC4 was expressed in 43 of the 135 patients with IDC (31.9%). The survival of 21 patients with high MUC4 expression (>20% of neoplastic cells stained) was significantly worse than that of the 114 patients with low MUC4 expression (<20% of neoplastic cells stained) (p = 0.0043). Univariate analysis showed that high MUC4 expression (p = 0.0061), large primary tumour status (>T2) (p = 0.0436), distant metastasis (p = 0.0383), lymphatic invasion (p = 0.0243), and surgical margins (p = 0.0333) were significant risk factors affecting the outcome of patients with IDC. Backward stepwise multivariate analysis showed that MUC4 expression (p = 0.0121), lymph node metastasis (p = 0.0245), and lymphatic invasion (p = 0.0239) were significant independent risk factors. ErbB2, p27, and MUC1 were not independent risk factors.
Conclusions: This study shows that MUC4 expression in IDC is a new independent factor for poor prognosis and predicts the outcome of patients with IDC.
Keywords: pancreatic cancer, mucin, immunohistochemistry, cumulative survival rate, multivariate analysis
Invasive ductal carcinoma of the pancreas (IDC) still has a relatively poor prognosis. In Japan, IDC is the fifth cause of carcinoma related death in men and the sixth cause of carcinoma related death in women.1 The overall five year survival rate for all patients with or without pancreatectomy after diagnosis is 9.7% in Japan, whereas those patients with a successful resection of IDC at the early stage (stage Ia) have a 39.9% five year survival rate.2 Most patients are diagnosed in the advanced stages because of the anatomical location of the pancreas, lack of specific symptoms, infiltration to the surrounding organs, or distant metastasis even from a small primary tumour less than 2 cm in diameter.3
“MUC4 expression is a novel predictor of poor prognosis in patients with intrahepatic cholangiocarcinoma, mass forming type”
Mucins are high molecular weight glycoproteins with oligosaccharides attached to serine or threonine residues of the mucin core protein backbone by _O_-glycosidic linkages, which are produced by various epithelial cells. Core proteins for human mucins (MUC1–9, MUC11–13, and MUC15–17) have been identified.4,5,6,7,8,9,10,11 Mucins are categorised into: membrane associated mucins (MUC1, MUC3, MUC4, MUC12, and MUC17), gel forming mucins (MUC2, MUC5AC, MUC5B, and MUC6), and soluble mucin (MUC7).12 Our previous studies have shown that the expression of MUC1 is related to the invasive proliferation of tumours and poor patient outcome, whereas the expression of MUC2 is related to non-invasive proliferation of tumours and favourable outcome.4,13–18 In pancreatic tumours, IDC showed high expression of various glycoforms of MUC1.4,19 In contrast, the “dark cell type” and “clear cell type” of intraductal papillary mucinous neoplasms showed different expression patterns of glycosylated MUC1 and MUC2.19
MUC4 was first reported as tracheobronchial mucin,20 and has been shown to be expressed in various normal human tissues,21–24 but not in the normal pancreas.25,26 In contrast, MUC4 expression has been detected in IDC.25–28 Recently, Swartz et al reported that MUC4 expression increases progressively in pancreatic intraepithelial neoplasia (PanIN).29,30
In addition, MUC4 is not expressed in the normal biliary epithelium but it is expressed in cholangiocarcinoma.31 We reported for the first time that MUC4 expression is a novel predictor of poor prognosis in patients with intrahepatic cholangiocarcinoma, mass forming type (ICC-MF).32
To date, no studies are available on the relation between the expression of MUC4 and the outcome of patients with IDC. The aims of our study were to investigate the expression profile of MUC4 in IDC and to evaluate the relation between MUC4 expression and patient outcome.
The transmembrane subunit of MUC4 acts as an intramembrane ligand for the receptor tyrosine kinase ErbB2.33–35 MUC4 plays an important role in cell proliferation and differentiation of epithelial cells34 by inducing specific phosphorylation of ErbB2 and enhancing expression of the cyclin dependent kinase inhibitor p27, which inhibits cell cycle progression through the control of the G1 to S phase.36–38 Thus, we also examined the expression of ErbB2 and p27 in IDC and compared it with MUC4 expression. For comparison, we also examined MUC1 expression, which has previously been reported as a useful prognostic factor in various carcinomas.18,32,39,40
MATERIALS AND METHODS
Tissue samples
One hundred and thirty five patients (89 men, 46 women) with surgically resected IDC were retrieved from the files in Kagoshima Medical Association Hospital and Kagoshima University Graduate School of Medical and Dental Sciences (Surgical Oncology and Digestive Surgery), Japan. The mean age of the patients was 65.8 years (range, 30–82). Surgical procedures were as follows: 88 patients were treated by conventional pancreatoduodenectomy, 31 were treated by pylorus preserving pancreatoduodenectomy, 10 were treated by distal pancreatectomy, and six were treated by total pancreatectomy. The 135 cases of IDC were histologically classified according to the TNM postsurgical histopathological classification.41 The study was approved by the Kagoshima University Graduate School of Medical and Dental Sciences human investigation committee.
The survival period after surgical resection was used as survival time for statistical analysis. The median length of the survival time at the last follow up of the patients was 11.0 months (range, 0–155). Seven patients died in the perioperative period. Clinical outcome data were available in all 135 patients with IDC, and overall survival was analysed. All the specimens were fixed in formalin, embedded in paraffin wax, and cut into 4 μm thick sections for immunohistochemistry, in addition to the usual haematoxylin and eosin staining.
Immunohistochemistry
Antibodies
Immunohistochemistry was carried out using the following antibodies. MUC4 was detected by a mouse monoclonal antibody, clone 8G7.30 ErbB2 was detected by rabbit polyclonal antibody (Dako Cytomation, Glostrup, Denmark). p27 was detected by mouse monoclonal antibody NCL-p27 (Novocastra, Newcastle, UK). MUC1 was detected by mouse monoclonal antibody DF3 (mouse IgG; Toray-Fuji Bionics, Tokyo, Japan),
Biotinylated affinity purified horse antimouse IgG, goat antirabbit IgG, and avidin–biotinylated horseradish peroxidase (ABC) complex were purchased from Vector Laboratories (Burlingame, California, USA) as the Vectastain Elite ABC kit.
Staining procedure
Immunohistochemical staining was performed with an immunoperoxidase method using the ABC complex as described previously.14,15,17,32 Each section was dewaxed with xylene. Endogenous peroxidase was blocked by incubating the sections in 0.3% hydrogen peroxidase in absolute methanol at room temperature for 30 minutes. After hydration in decreasing concentrations of ethanol in water, the sections were washed in 0.01M phosphate buffered saline (PBS), pH 7.4. Antigen retrieval was achieved by waterbath pretreatment at 80°C for 20 minutes in 0.01M citrate buffer (pH 6.0) for MUC4, waterbath pretreatment at 98°C for 40 minutes for ErbB2, and autoclave pretreatment at 120°C for five minutes for p27. The sections were washed twice with PBS and 2% horse or goat serum in PBS was applied for 30 minutes at room temperature to prevent non-specific staining. The sections were then incubated with dilutions of the primary antibodies (anti-MUC4, 1/3000; anti-ErbB2, 1/100; anti-p27, 1/ 40; and anti-MUC1, 1/10) in PBS with 1% bovine serum albumin for 16 hours at 4°C. The sections were washed three times with PBS, incubated with the biotinylated secondary antibodies, and then washed three times with PBS. All sections then received ABC complex for 30 minutes. After washing with PBS three times, the sections were finally reacted with diaminobenzidine substrate for 10 minutes for visualisation, rinsed with tap water, counterstained with haematoxylin, and mounted. Reaction products were not present when non-immune serum or PBS was used instead of the primary antibodies.
Evaluation of the results by scoring
The results of the immunohistochemical staining were evaluated as the percentage of positively stained neoplastic cells. For MUC4, ErbB2, and MUC1, membranous and cytoplasmic immunoreactivity was evaluated. For p27, nuclear immunoreactivity was evaluated. According to our previous studies,14,32 the percentages of positively stained neoplastic cells were graded as follows: –, < 5% of neoplastic cells stained; +, > 5% but < 20% of neoplastic cells stained; ++, > 20% but < 50% of neoplastic cells stained; and +++, > 50% of neoplastic cells stained.
In addition, for statistical analysis, the 135 cases were divided into two groups: the low expression group, composed of the – and + cases (under 20% of neoplastic cells stained), and the high expression group, composed of the ++ and +++ cases (more than 20% of neoplastic cells stained).14
Statistical analysis
Statistical analysis was performed using the Student’s t test, the χ2 test, and the Mann-Whitney U test where appropriate. Survival of the patients was compared between the group with high MUC4, ErbB2, p27, or MUC1 expression, and the group with low expression. Univariate and multivariate survival analyses were performed using the Cox proportional hazards regression model. For the multivariate model, we used 0.20 as the cutoff p value to select the analysed factors from the univariate analysis data. Furthermore, backward stepwise multivariate analysis was used to find independent prognostic factors. A value of p < 0.05 was considered significant.
RESULTS
MUC4, ErbB2, p27, or MUC1 expression in normal pancreatic tissue
MUC4 and ErbB2 were not expressed in normal pancreatic tissue (fig 1A). p27 was expressed in the nuclei of most normal epithelia. MUC1 was expressed in the cell apices of the centroacinar cells, intercalated ducts, and intralobular ducts and focally in the interlobular ducts, but was not expressed in the main pancreatic ducts, acini, or islets.
Figure 1.
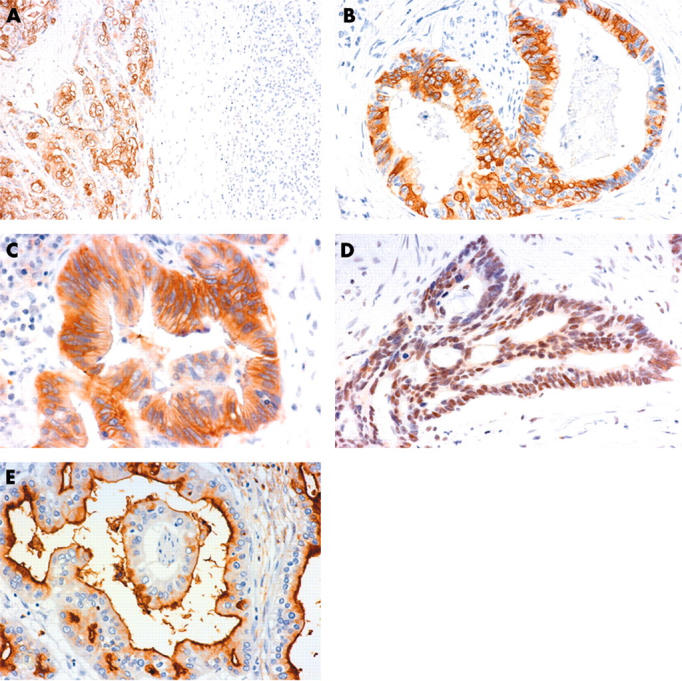
Immunohistochemical staining of (A, B) MUC4, (C) ErbB2, (D) p27, and (E) MUC1 in invasive ductal carcinoma of the pancreas. MUC4 was expressed in the carcinoma tissue (A, left), but not in the normal pancreas tissue (A, right) (original magnification, ×40). MUC4 expression was seen in the cytoplasm and/or membrane of the carcinoma cells (B, original magnification, ×160). ErbB2 was expressed in the membrane and/or cytoplasm of the carcinoma cells (C, original magnification, ×160). p27 expression was seen in the nuclei of the carcinoma cells (D, original magnification, ×160). MUC1 was expressed in the membrane and/or cytoplasm of the carcinoma cells (E, original magnification, ×160).
MUC4, ErbB2, p27, or MUC1 expression profile in IDC
Figure 1 shows representative expression patterns of MUC4, ErbB2, p27, and MUC1 in IDC. Positive staining for MUC4 was seen in the cytoplasm and/or membrane of the carcinoma cells in 43 (31.9%) of 135 IDCs (fig 1A, B).
ErbB2 was expressed at the membrane and/or cytoplasm of the carcinoma cells in 46 (34.1%) of the 135 IDCs (fig 1C), p27 was seen in the nuclei of the carcinoma cells in 62 (45.9%) (fig 1D), and MUC1 was expressed in the membrane and/or cytoplasm of the carcinoma cells in 116 (85.9%) (fig 1E).
Association between MUC4, ErbB2, p27, and MUC1 expression in IDC
There was no significant association between the expression of MUC4 and the expression of ErbB2, p27, or MUC1 (p = 0.7595, 0.0604, and 0.5999, respectively; table 1).
Table 1.
Correlation between MUC4, ErbB2, p27, and MUC1 expression in 135 patients with invasive ductal carcinoma of the pancreas
| MUC4 expression | |||||
|---|---|---|---|---|---|
| − | + | ++ | +++ | p Value | |
| ErbB2 expression | |||||
| − | 59 | 14 | 7 | 9 | 0.7595 |
| + | 12 | 5 | 1 | 0 | |
| ++ | 16 | 2 | 2 | 1 | |
| +++ | 5 | 1 | 1 | 0 | |
| p27 expression | |||||
| − | 52 | 15 | 1 | 5 | 0.0604 |
| + | 9 | 1 | 4 | 1 | |
| ++ | 15 | 3 | 4 | 1 | |
| +++ | 16 | 3 | 2 | 3 | |
| MUC1 expression | |||||
| − | 16 | 3 | 0 | 0 | 0.5999 |
| + | 14 | 2 | 2 | 2 | |
| ++ | 27 | 9 | 3 | 2 | |
| +++ | 35 | 8 | 6 | 6 |
Association between expression of MUC4, ErbB2, p27, or MUC1 and clinicopathological features
Table 2 shows the association between the expression of MUC4, ErbB2, p27, or MUC1 and the clinicopathological features. In the 135 patients examined, 21 (15.6%) had high MUC4 expression and 114 (84.4%) had low MUC4 expression. MUC4 expression in IDC was not related to the clinicopathological features listed in table 2. Twenty eight (20.7%) patients had high ErbB2 expression and 107 (79.3%) had low ErbB2 expression. The expression of ErbB2 was related to histological grade (p = 0.0234). Neither expression of p27 nor MUC1 was related to the clinicopathological features listed in table 2.
Table 2.
Clinicopathological features and the expression of MUC4, ErbB2, p27, and MUC1 in 135 patients with invasive ductal carcinoma of the pancreas
| Category | N (%) | MUC4 | ErbB2 | p27 | MUC1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low expression* | High expression* | p Value | Low expression | High expression | p Value | Low expression | High expression | p Value | Low expression | High expression | p Value | ||
| Age (years) | |||||||||||||
| ⩽65 | 51 (37.8) | 43 (84.3) | 8 (15.7) | 0.9740 | 41 (80.4) | 10 (19.6) | 0.8003 | 31 (60.8) | 20 (39.2) | 0.4888 | 16 (31.4) | 35 (68.6) | 0.6198 |
| >65 | 84 (62.2) | 71 (84.5) | 13 (15.5) | 66 (78.6) | 18 (21.4) | 56 (66.7) | 28 (33.3) | 23 (27.4) | 61 (72.6) | ||||
| Sex | |||||||||||||
| Male | 89 (65.9) | 75 (84.3) | 14 (15.7) | 0.9379 | 72 (80.9) | 17 (19.1) | 0.5134 | 60 (67.4) | 29 (32.6) | 0.3158 | 28 (31.5) | 61 (68.5) | 0.3591 |
| Female | 46 (34.1) | 39 (84.8) | 7 (15.2) | 35 (76.1) | 11 (23.9) | 27 (58.7) | 19 (41.3) | 11 (23.9) | 35 (76.1) | ||||
| Primary tumour | |||||||||||||
| ⩽T2 | 20 (14.8) | 19 (95.0) | 1 (5.0) | 0.1582 | 17 (85.0) | 3 (15.0) | 0.4927 | 16 (80.0) | 4 (20.0) | 0.1153 | 6 (30.0) | 14 (70.0) | 0.9054 |
| >T2 | 115 (85.2) | 95 (82.6) | 20 (17.4) | 90 (78.3) | 25 (21.7) | 71 (61.7) | 44 (38.3) | 33 (28.7) | 82 (71.3) | ||||
| Lymph node metastasis | |||||||||||||
| No | 59 (43.7) | 52 (88.1) | 7 (11.9) | 0.2971 | 43 (72.9) | 16 (27.1) | 0.1073 | 39 (66.1) | 20 (33.9) | 0.7230 | 19 (32.2) | 40 (67.8) | 0.4541 |
| Yes | 76 (56.3) | 62 (81.6) | 14 (18.4) | 64 (84.2) | 12 (15.8) | 48 (63.2) | 28 (36.8) | 20 (26.3) | 56 (73.7) | ||||
| Distant metastasis | |||||||||||||
| No | 128 (94.8) | 108 (84.4) | 20 (15.6) | 0.9242 | 101 (78.9) | 27 (21.1) | 0.6653 | 83 (64.8) | 45 (35.2) | 0.6785 | 39 (30.5) | 89 (69.5) | 0.0833 |
| Yes | 7 (5.2) | 6 (85.7) | 1 (14.3) | 6 (85.7) | 1 (14.3) | 4 (57.1) | 3 (42.9) | 0 (0.0) | 7 (100.0) | ||||
| Histological grade | |||||||||||||
| G1 | 37 (27.4) | 32 (86.5) | 5 (13.5) | 0.7762 | 24 (64.9) | 13 (35.1) | 0.0234 | 27 (73.0) | 10 (27.0) | 0.2388 | 15 (40.5) | 22 (59.5) | 0.1171 |
| G2 | 73 (54.1) | 62 (84.9) | 11 (15.1) | 60 (82.2) | 13 (17.8) | 47 (64.4) | 26 (35.6) | 16 (21.9) | 57 (78.1) | ||||
| G3 | 25 (18.5) | 20 (80.0) | 5 (20.0) | 23 (92.0) | 2 (8.0) | 13 (52.0) | 12 (48.0) | 8 (32.0) | 17 (68.0) | ||||
| Lymphatic invasion | |||||||||||||
| No | 9 (6.7) | 9 (100.0) | 0 (0.0) | 0.1826 | 6 (66.7) | 3 (33.3) | 0.3348 | 8 (88.9) | 1 (11.1) | 0.1128 | 4 (44.4) | 5 (55.6) | 0.2865 |
| Yes | 126 (93.3) | 105 (83.3) | 21 (16.7) | 101 (80.2) | 25 (19.8) | 79 (62.7) | 47 (37.3) | 35 (27.8) | 91 (72.2) | ||||
| Venous invasion | |||||||||||||
| No | 35 (25.9) | 31 (88.6) | 4 (11.4) | 0.4338 | 28 (80.0) | 7 (20.0) | 0.9001 | 23 (65.7) | 12 (34.3) | 0.8553 | 8 (22.9) | 27 (77.1) | 0.3603 |
| Yes | 100 (74.1) | 83 (83.0) | 17 (17.0) | 79 (79.0) | 21 (21.0) | 64 (64.0) | 36 (36.0) | 31 (31.0) | 69 (69.0) | ||||
| Perineural invasion | |||||||||||||
| No | 13 (9.6) | 12 (92.3) | 1 (7.7) | 0.4106 | 13 (100.0) | 0 (0.0) | 0.0524 | 10 (76.9) | 3 (23.1) | 0.3228 | 5 (38.5) | 8 (61.5) | 0.4231 |
| Yes | 122 (90.4) | 102 (83.6) | 20 (16.4) | 94 (77.0) | 28 (23.0) | 77 (63.1) | 45 (36.9) | 34 (27.9) | 88 (72.1) | ||||
| Surgical margins | |||||||||||||
| Negative | 78 (57.8) | 66 (84.6) | 12 (15.4) | 0.9489 | 62 (79.5) | 16 (20.5) | 0.9391 | 52 (66.7) | 26 (33.3) | 0.5281 | 22 (28.2) | 56 (71.8) | 0.8375 |
| Positive | 57 (42.2) | 48 (84.2) | 9 (15.8) | 45 (78.9) | 12 (21.1) | 35 (61.4) | 22 (38.6) | 17 (29.8) | 40 (70.2) |
Association between expression of MUC4, ErbB2, p27, MUC1, or histological parameters and cumulative survival rate
Among the 135 patients examined, 87 died during the follow up period. Forty eight patients were alive at the last follow up and the longest survivor was alive at 155 months after surgery. The overall one, three, and five year survival rates of patients with surgical resection were 58.7%, 19.0%, and 11.4%, respectively.
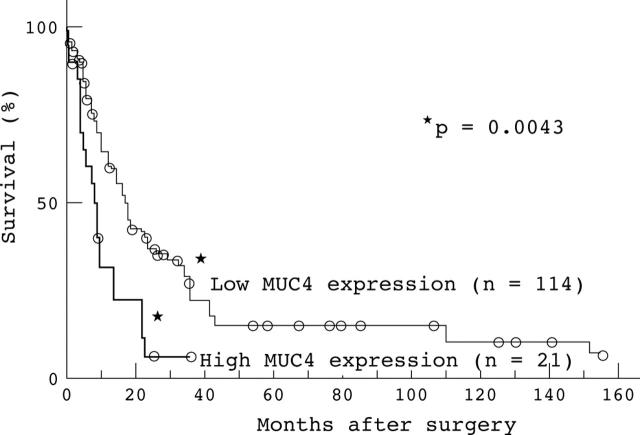
The one, three, and five year survival rates of the high MUC4 expression group were 31.5%, 5.6%, and 0.0%, respectively, whereas those of the low MUC4 expression group were 64.2%, 22.0%, and 13.9%, respectively. The survival of patients with high MUC4 expression was significantly worse than those with low MUC4 expression (p = 0.0043; log rank test; fig 2). There were no significant associations between survival and the expression of erbB2, p27, or MUC1 (p = 0.5400, 0.0726, and 0.9730, respectively; log rank test).
Figure 2.
Correlation between MUC4 expression and cumulative survival rate in 135 patients with invasive ductal carcinoma, as determined by the Kaplan–Meier method. The survival of patients with high MUC4 expression was worse than those with low MUC4 expression (p = 0.0043). The open circles represent those patients who were alive at the last follow up.
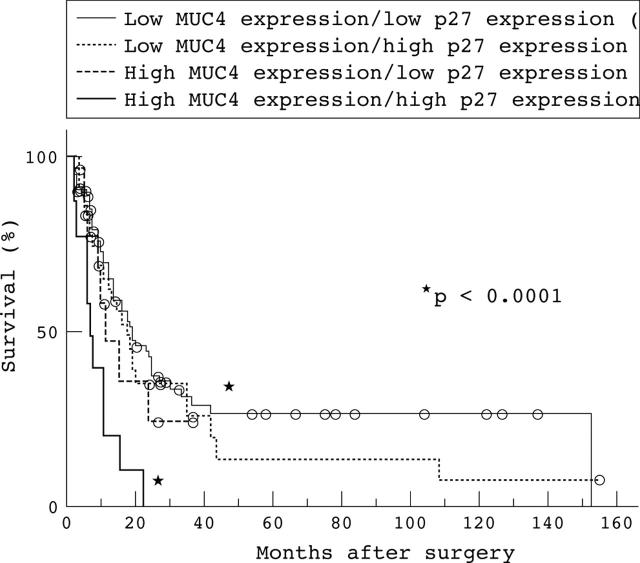
In the combined evaluation of MUC4 and p27 expression, patients with both high MUC4 and p27 expression had a worse outcome than those with both low MUC4 and p27 expression. A significant difference for survival was found between the two groups (p < 0.0001; fig 3). There was no significant difference in the combined evaluation of MUC4 and ErbB2 expression, or in that of MUC4 and MUC1 expression (p = 0.5369 and 0.0582, respectively).
Figure 3.
Combined evaluation of MUC4 and p27 expression. Patients with both high MUC4 and p27 expression had the worst outcome. In contrast, patients with both low MUC4 and p27 expression had the best outcome. A significant difference for survival was found between the two groups (p < 0.0001). The open circles represent those patients who were alive at the last follow up.
The survival of patients with large primary tumour status (> T2), positive distant metastasis, positive lymphatic invasion, or positive surgical margins was significantly worse than those with small primary tumour status (⩽ T2) (p = 0.0089), no distant metastasis (p = 0.0292), no lymphatic invasion (p = 0.0156), or negative surgical margins (p = 0.0287) (log rank test).
Univariate analysis of prognostic factors
Table 3 shows the results of univariate analysis of prognostic factors for IDC. MUC4 expression (p = 0.0061; hazard ratio (HR), 2.127; 95% confidence interval (CI), 1.240 to 3.649), large primary tumour status (> T2) (p = 0.0436; HR, 1.846; 95% CI, 1.018 to 3.350), distant metastasis (p = 0.0383; HR, 2.438; 95% CI, 1.049 to 5.665), lymphatic invasion (p = 0.0243; HR, 3.208; 95% CI, 1.163 to 8.846), and surgical margins (p = 0.0333; HR, 1.608; 95% CI, 1.038 to 2.490) were significant poor risk factors affecting the outcome of the patients.
Table 3.
Univariate analysis of prognostic factors
| Variable | HR | 95% CI | p Value |
|---|---|---|---|
| Age (years) | |||
| ⩽65 (n = 51) | 1 | ||
| >65 (n = 84) | 1.130 | 0.737 to 1.735 | 0.5749 |
| Sex | |||
| Male (n = 89) | 1 | ||
| Female (n = 46) | 1.080 | 0.675 to 1.729 | 0.7470 |
| Primary tumour | |||
| ⩽T2 (n = 20) | 1 | ||
| >T2 (n = 11) | 1.846 | 1.018 to 3.350 | 0.0436 |
| Lymph node metastasis | |||
| No (n = 59) | 1 | ||
| Yes (n = 76) | 1.542 | 0.983 to 2.420 | 0.0595 |
| Distant metastasis | |||
| No (n = 128) | 1 | ||
| Yes (n = 7) | 2.438 | 1.049 to 5.665 | 0.0383 |
| Histological grade | |||
| G1 (n = 37) | 1 | ||
| G2 (n = 73) | 1.123 | 0.565 to 2.231 | 0.7401 |
| G3 (n = 25) | 1.280 | 0.676 to 2.425 | 0.4483 |
| Lymphatic invasion | |||
| No (n = 9) | 1 | ||
| Yes (n = 126) | 3.208 | 1.163 to 8.846 | 0.0243 |
| Venous invasion | |||
| No (n = 35) | 1 | ||
| Yes (n = 100) | 1.007 | 0.641 to 1.584 | 0.9745 |
| Perineural invasion | |||
| No (n = 13) | 1 | ||
| Yes (n = 122) | 1.520 | 0.725 to 3.186 | 0.2673 |
| Surgical margins | |||
| Negative (n = 78) | 1 | ||
| Positive (n = 57) | 1.608 | 1.038 to 2.490 | 0.0333 |
| MUC4 expression | |||
| Low (<20%) (n = 114) | 1 | ||
| High (⩾20%) (n = 21) | 2.127 | 1.240 to 3.649 | 0.0061 |
| ErbB2 expression | |||
| Low (<20%) (n = 107) | 1 | ||
| High (⩾20%) (n = 28) | 1.182 | 0.685 to 2.039 | 0.5482 |
| p27 expression | |||
| Low (<20%) (n = 87) | 1 | ||
| High (⩾20%) (n = 48) | 1.469 | 0.955 to 2.258 | 0.0798 |
| MUC1 expression | |||
| Low (<20%) (n = 39) | 1 | ||
| High (⩾20%) (n = 96) | 1.001 | 0.635 to 1.599 | 0.9735 |
Multivariate analysis of prognostic factors
Table 4 shows the results of multivariate analysis of IDC prognostic factors. Among the seven factors selected from the univariate analysis data, based on 0.20 as the cutoff p value, MUC4 expression (p = 0.0165, HR, 1.956; 95% CI, 1.130 to 3.384) was the only significant poor risk factor affecting the outcome of the patients.
Table 4.
Multivariate analysis of prognostic factors
| Variable | HR | 95% CI | p Value |
|---|---|---|---|
| Primary tumour | |||
| ⩽T2 (n = 20) | 1 | ||
| >T2 (n = 115) | 1.057 | 0.546 to 2.048 | 0.8691 |
| Lymph node metastasis | |||
| No (n = 59) | 1 | ||
| Yes (n = 76) | 1.547 | 0.955 to 2.507 | 0.0764 |
| Distant metastasis | |||
| No (n = 128) | 1 | ||
| Yes (n = 7) | 1.532 | 0.641 to 3.664 | 0.3372 |
| Lymphatic invasion | |||
| No (n = 9) | 1 | ||
| Yes (n = 126) | 2.672 | 0.889 to 8.034 | 0.0801 |
| Surgical margins | |||
| Negative (n = 78) | 1 | ||
| Positive (n = 57) | 1.372 | 0.880 to 2.140 | 0.1631 |
| MUC4 expression | |||
| Low (<20%) (n = 114) | 1 | ||
| High (⩾20%) (n = 21) | 1.956 | 1.130 to 3.384 | 0.0165 |
| p27 expression | |||
| Low (<20%) (n = 87) | 1 | ||
| High (⩾20%) (n = 48) | 1.280 | 0.820 to 1.997 | 0.2775 |
Backward stepwise multivariate analysis of prognostic factors
Table 5 shows the results of the backward stepwise multivariate analysis of the seven prognostic factors listed in table 4. MUC4 expression (p = 0.0121; HR, 2.005; 95% CI, 1.164 to 3.452), lymph node metastasis (p = 0.0245; HR, 1.687; 95% CI, 1.069 to 2.660), and lymphatic invasion (p = 0.0239; HR, 3.282; 95% CI, 1.170 to 9.207) were significant independent risk factors.
Table 5.
Backward stepwise multivariate analysis of prognostic factors
| Variable | HR | 95% CI | p Value |
|---|---|---|---|
| Lymph node metastasis | |||
| No (n = 59) | 1 | ||
| Yes (n = 76) | 1.687 | 1.069 to 2.660 | 0.0245 |
| Lymphatic invasion | |||
| No (n = 9) | 1 | ||
| Yes (n = 126) | 3.282 | 1.170 to 9.207 | 0.0239 |
| MUC4 expression | |||
| Low (<20%)(n = 114) | 1 | ||
| High (⩾20%)(n = 21) | 2.005 | 1.164 to 3.452 | 0.0121 |
DISCUSSION
The expression of MUC4 has been studied in IDC tissue samples and cell lines, and its relation to malignant progression has been elucidated.25–28 The expression of MUC4 is thought to be a useful indicator of malignancy potential in IDCs,28,30 but there have been no studies of the direct association between the expression of MUC4 and the outcome of patients with IDC. We have reported that MUC4 expression is a very useful predictor of poor prognosis in patients with ICC-MF: the survival rate of MUC4 positive patients was significantly worse than MUC4 negative patients.32
In our present study, we demonstrated for the first time that patients with IDC who have high MUC4 expression had a worse survival rate than those with low MUC4 expression, similar to patients with ICC-MF. Univariate analysis of the prognostic factors in our present study showed that MUC4 expression, primary tumour size, distant metastasis, lymphatic invasion, and surgical margins were significant risk factors affecting the outcome of patients with IDC. Furthermore, MUC4 expression was the only significant prognostic factor for patients with IDC in the multivariate analysis. In the evaluation of independent risk factors using backward stepwise multivariate analysis, MUC4, lymph node metastasis, and lymphatic invasion were significant independent risk factors in patients with IDC. These findings are in line with the progression model for pancreatic adenocarcinoma reported by Swartz et al, showing that MUC4 expression increases with increasing grade of PanIN.30
MUC4/sialomucin complex (SMC) is a rat homologue of the human mucin gene MUC4, and its transmembrane subunit acts as an intramembrane ligand for the receptor tyrosine kinase ErbB2 to induce the phosphorylation of the Tyr-1248 of ErbB2.34,36,42 MUC4/SMC leads to the expression of the cell cycle inhibitor p27.34 In addition, MUC4/SMC and neuregulin act synergistically to enhance phosphorylation of both ErbB2 and ErbB3, resulting in the downregulation of p27 and activation of protein kinase B/Akt.35,36 It is proposed that complex formation between MUC4/SMC and ErbB2 has an effect on epithelial cell behaviour, as a switch in epithelial differentiation and proliferation.35,36 Furthermore, Singh et al proposed that MUC4 participates in tumour growth and metastasis by directly altering tumour cell properties and/or via modulating ErbB2 expression.43 However, in the IDCs examined in our present study, ErbB2 expression was not a significant prognostic factor, and neither was combined MUC4 and ErbB2 expression. Nevertheless, high ErbB2 expression was more frequently seen in well differentiated than in poorly differentiated IDCs. This finding is compatible with a report that that well or moderately differentiated colorectal cancers more frequently express ErbB2 proteins than poorly differentiated tumours.44
“Patients with both high MUC4 and p27 expression had a significantly worse outcome than those with both low MUC4 and p27 expression, indicating that p27 upregulation is induced by MUC4 expression in invasive ductal carcinoma”
Low expression of p27 is a poor prognostic factor in gastric cancer, colorectal carcinoma, and intrahepatic cholangiocarcinoma.45–47 However, in the IDCs examined in our present study, p27 expression was not an independent prognostic factor, although patients with both high MUC4 and p27 expression had a significantly worse outcome than those with both low MUC4 and p27 expression. This result indicates that p27 upregulation is induced by MUC4 expression in IDC. The relation between p27 upregulation and MUC4 expression may be partly explained by the model proposed by Carraway et al and Jepson et al.35,36
MUC4 extends at least 1.12–2.12 μm above the cell membrane, far above all other membrane associated proteins, such as adhesion molecules.43,48 With its rigid and extended structure, MUC4 is thought to be a modulator of cell–cell and cell–extracellular matrix interactions.49 Komatsu et al showed that SMC disrupts integrin mediated cell adhesion to extracellular matrix proteins.49,50 In addition, overexpression of SMC masks the surface antigens on target tumour cells and effectively suppresses tumour cell killing by cytotoxic lymphocytes.49 These phenomena may be related to the poor outcome of patients with high MUC4 expression, although MUC4 expression was not related to morphological invasive parameters, such as lymphatic invasion, venous invasion, perineural invasion, and distant lymph node metastasis.
MUC1 is also a transmembrane protein, and MUC1 expression is related to the development of tumours.51,52 MUC1 expression enhances tumour detachment from the primary site and accelerates distant metastasis, through binding to E selectin and/or intercellular adhesion molecule 1 of the endothelial cells.53–55 In previous studies, MUC1 expression was related to poor outcome in patients with various carcinomas.18,32,39,40 In our previous study of IDC,55 MUC1 expression was also a poor prognostic factor in advanced cases, although our present study, which includes not only advanced but also early cases, found no relation between the expression of MUC1 and the outcome of patients with IDC.
In ICC-MF, MUC4 expression is a more significant prognostic factor than MUC1 expression in multivariate analysis.32 In ICC-MF, MUC1 was expressed at the luminal surface membrane and/or in the cytoplasm, whereas MUC4 expression was seen in the cytoplasm only. In our present study, we confirmed the cytoplasmic expression pattern of MUC4 in IDC also. MUC4 is a membrane mucin, as is MUC1, but MUC4 acts by a different mechanism from that of MUC1. MUC1 acts as a docking protein for signalling molecules, whereas MUC4 acts as a receptor ligand.34 The different expression patterns of MUC1 and MUC4 suggest the possibility of different mechanisms of expression of MUC1 and MUC4, both of which are membrane mucins with cell signalling functions.
Take home messages.
- The survival of patients with invasive ductal carcinoma (IDC) of the pancreas with high MUC4 expression (>20% of neoplastic cells stained) was significantly worse than that of those with low MUC4 expression (<20% of neoplastic cells stained)
- Thus, MUC4 expression in IDC is a new independent factor for poor prognosis and predicts the outcome of patients with IDC
The combined evaluation of MUC4 and MUC1 expression also showed no significant difference in survival for patients with IDC in our present study, although there was a significant difference between MUC4 and MUC1 positive patients and MUC4 and MUC1 negative patients in ICC-MF.32
In conclusion, our results provide new and important information on the importance of MUC4 expression as a useful indicator to predict the outcome of patients with surgically resected IDC. MUC4 has potential as a prognostic marker for clinical management of patients with IDC.
Acknowledgments
We are grateful to Mr Y Atsuji, Ms Y Arimura, and Ms Y Nishimura for their excellent technical assistance. This work was supported by Grants-in-Aid 13220016 (S Yonezawa) and 12218234 (K Imai) from the Ministry of Education, Science, Sports, Culture and Technology, Japan, and USPHS grant CA 78590 from the National Institutes of Health (SK Batra).
Abbreviations
- ABC, avidin–biotinylated horseradish peroxidase complex
- CI, confidence interval
- HR, hazard ratio
- ICC-MF, intrahepatic cholangiocarcinoma mass forming type
- IDC, invasive ductal carcinoma of the pancreas
- PanIN, pancreatic intraepithelial neoplasia
- PBS, phosphate buffered saline
- SMC, sialomucin complex
REFERENCES
- 1.Matsuno S, Egawa S, Fukuyama S, et al. Pancreatic cancer registry in Japan: 20 years of experience. Pancreas 2004;28:219–30. [DOI] [PubMed] [Google Scholar]
- 2.Isaji S, Kawarada Y, Uemoto S. Classification of pancreatic cancer: comparison of Japanese and UICC classifications. Pancreas 2004;28:231–4. [DOI] [PubMed] [Google Scholar]
- 3.Egawa S, Takeda K, Fukuyama S, et al. Clinicopathological aspects of small pancreatic cancer. Pancreas 2004;28:235–40. [DOI] [PubMed] [Google Scholar]
- 4.Yonezawa S, Sato E. Expression of mucin antigens in human cancers and its relationship with malignancy potential. Pathol Int 1997;47:813–30. [DOI] [PubMed] [Google Scholar]
- 5.D’Cruz OJ, Dunn TS, Pichan P, et al. Antigenic cross-reactivity of human tracheal mucin with human sperm and trophoblasts correlates with the expression of mucin 8 gene messenger ribonucleic acid in reproductive tract tissues. Fertil Steril 1996;66:316–26. [DOI] [PubMed] [Google Scholar]
- 6.Lapensee L, Paquette Y, Bleau G. Allelic polymorphism and chromosomal localization of the human oviductin gene (MUC9). Fertil Steril 1997;68:702–8. [DOI] [PubMed] [Google Scholar]
- 7.Williams SJ, McGuckin MA, Gotley DC, et al. Two novel mucin genes down-regulated in colorectal cancer identified by differential display. Cancer Res 1999;59:4083–9. [PubMed] [Google Scholar]
- 8.Williams SJ, Wreschner DH, Tran M, et al. Muc13, a novel human cell surface mucin expressed by epithelial and hemopoietic cells. J Biol Chem 2001;276:18327–36. [DOI] [PubMed] [Google Scholar]
- 9.Pallesen LT, Berglund L, Rasmussen LK, et al. Isolation and characterization of MUC15, a novel cell membrane-associated mucin. Eur J Biochem 2002;269:2755–63. [DOI] [PubMed] [Google Scholar]
- 10.Yin BW, Dnistrian A, Lloyd KO. Ovarian cancer antigen CA125 is encoded by the MUC16 mucin gene. Int J Cancer 2002;98:737–40. [DOI] [PubMed] [Google Scholar]
- 11.Gum JR Jr, Crawley SC, Hicks JW, et al. MUC17, a novel membrane-tethered mucin. Biochem Biophys Res Commun 2002;291:466–75. [DOI] [PubMed] [Google Scholar]
- 12.Moniaux N, Escande F, Porchet N, et al. Structural organization and classification of the human mucin genes. Front Biosci 2001;6:D1192–206. [DOI] [PubMed] [Google Scholar]
- 13.Yonezawa S, Nakamura A, Horinouchi M, et al. The expression of several types of mucin is related to the biological behavior of pancreatic neoplasms. J Hepatobiliary Pancreat Surg 2002;9:328–41. [DOI] [PubMed] [Google Scholar]
- 14.Tamada S, Goto M, Nomoto M, et al. Expression of MUC1 and MUC2 mucins in extrahepatic bile duct carcinomas: its relationship with tumor progression and prognosis. Pathol Int 2002;52:713–23. [DOI] [PubMed] [Google Scholar]
- 15.Nakamura A, Horinouchi M, Goto M, et al. New classification of pancreatic intraductal papillary-mucinous tumour by mucin expression: its relationship with potential for malignancy. J Pathol 2002;197:201–10. [DOI] [PubMed] [Google Scholar]
- 16.Utsunomiya T, Yonezawa S, Sakamoto H, et al. Expression of MUC1 and MUC2 mucins in gastric carcinomas: its relationship with the prognosis of the patients. Clin Cancer Res 1998;4:2605–14. [PubMed] [Google Scholar]
- 17.Higashi M, Yonezawa S, Ho JJ, et al. Expression of MUC1 and MUC2 mucin antigens in intrahepatic bile duct tumors: its relationship with a new morphological classification of cholangiocarcinoma. Hepatology 1999;30:1347–55. [DOI] [PubMed] [Google Scholar]
- 18.Sagara M, Yonezawa S, Nagata K, et al. Expression of mucin 1 (MUC1) in esophageal squamous-cell carcinoma: its relationship with prognosis. Int J Cancer 1999;84:251–7. [DOI] [PubMed] [Google Scholar]
- 19.Horinouchi M, Nagata K, Nakamura A, et al. Expression of different glycoforms of membrane mucin (MUC1) and secretory mucin (MUC2, MUC5AC and MUC6) in pancreatic neoplasms. Acta Histochem Cytochem 2003;36:443–53. [Google Scholar]
- 20.Porchet N, Nguyen VC, Dufosse J, et al. Molecular cloning and chromosomal localization of a novel human tracheo-bronchial mucin cDNA containing tandemly repeated sequences of 48 base pairs. Biochem Biophys Res Commun 1991;175:414–22. [DOI] [PubMed] [Google Scholar]
- 21.Buisine MP, Devisme L, Copin MC, et al. Developmental mucin gene expression in the human respiratory tract. Am J Respir Cell Mol Biol 1999;20:209–18. [DOI] [PubMed] [Google Scholar]
- 22.Audie JP, Janin A, Porchet N, et al. Expression of human mucin genes in respiratory, digestive, and reproductive tracts ascertained by in situ hybridization. J Histochem Cytochem 1993;41:1479–85. [DOI] [PubMed] [Google Scholar]
- 23.Audie JP, Tetaert D, Pigny P, et al. Mucin gene expression in the human endocervix. Hum Reprod 1995;10:98–102. [DOI] [PubMed] [Google Scholar]
- 24.Gipson IK, Spurr-Michaud S, Moccia R, et al. MUC4 and MUC5B transcripts are the prevalent mucin messenger ribonucleic acids of the human endocervix. Biol Reprod 1999;60:58–64. [DOI] [PubMed] [Google Scholar]
- 25.Hollingsworth MA, Strawhecker JM, Caffrey TC, et al. Expression of MUC1, MUC2, MUC3 and MUC4 mucin mRNAs in human pancreatic and intestinal tumor cell lines. Int J Cancer 1994;57:198–203. [DOI] [PubMed] [Google Scholar]
- 26.Balague C, Gambus G, Carrato C, et al. Altered expression of MUC2, MUC4, and MUC5 mucin genes in pancreas tissues and cancer cell lines. Gastroenterology 1994;106:1054–61. [DOI] [PubMed] [Google Scholar]
- 27.Balague C, Audie JP, Porchet N, et al. In situ hybridization shows distinct patterns of mucin gene expression in normal, benign, and malignant pancreas tissues. Gastroenterology 1995;109:953–64. [DOI] [PubMed] [Google Scholar]
- 28.Andrianifahanana M, Moniaux N, Schmied BM, et al. Mucin (MUC) gene expression in human pancreatic adenocarcinoma and chronic pancreatitis: a potential role of MUC4 as a tumor marker of diagnostic significance. Clin Cancer Res 2001;7:4033–40. [PubMed] [Google Scholar]
- 29.Hruban RH, Adsay NV, Albores-Saavedra J, et al. Pancreatic intraepithelial neoplasia: a new nomenclature and classification system for pancreatic duct lesions. Am J Surg Pathol 2001;25:579–86. [DOI] [PubMed] [Google Scholar]
- 30.Swartz MJ, Batra SK, Varshney GC, et al. MUC4 expression increases progressively in pancreatic intraepithelial neoplasia. Am J Clin Pathol 2002;117:791–6. [DOI] [PubMed] [Google Scholar]
- 31.Vandenhaute B, Buisine MP, Debailleul V, et al. Mucin gene expression in biliary epithelial cells. J Hepatol 1997;27:1057–66. [DOI] [PubMed] [Google Scholar]
- 32.Shibahara H, Tamada S, Higashi M, et al. MUC4 is a novel prognostic factor of intrahepatic cholangiocarcinoma-mass forming type. Hepatology 2004;39:220–9. [DOI] [PubMed] [Google Scholar]
- 33.Carraway KL 3rd, Rossi EA, Komatsu M, et al. An intramembrane modulator of the ErbB2 receptor tyrosine kinase that potentiates neuregulin signaling. J Biol Chem 1999;274:5263–6. [DOI] [PubMed] [Google Scholar]
- 34.Ramsauer VP, Carraway CA, Salas PJ, et al. Muc4/sialomucin complex, the intramembrane ErbB2 ligand, translocates ErbB2 to the apical surface in polarized epithelial cells. J Biol Chem 2003;278:30142–7. [DOI] [PubMed] [Google Scholar]
- 35.Carraway KL, Ramsauer VP, Haq B, et al. Cell signaling through membrane mucins. Bioessays 2003;25:66–71. [DOI] [PubMed] [Google Scholar]
- 36.Jepson S, Komatsu M, Haq B, et al. Muc4/sialomucin complex, the intramembrane ErbB2 ligand, induces specific phosphorylation of ErbB2 and enhances expression of p27(kip), but does not activate mitogen-activated kinase or protein kinaseB/Akt pathways. Oncogene 2002;21:7524–32. [DOI] [PubMed] [Google Scholar]
- 37.Polyak K, Kato JY, Solomon MJ, et al. p27Kip1, a cyclin–Cdk inhibitor, links transforming growth factor-beta and contact inhibition to cell cycle arrest. Genes Dev 1994;8:9–22. [DOI] [PubMed] [Google Scholar]
- 38.Ito Y, Takeda T, Sakon M, et al. Expression and clinical significance of the G1–S modulators in carcinoma of the extrahepatic bile duct. Anticancer Res 2000;20:337–44. [PubMed] [Google Scholar]
- 39.Matsumura N, Yamamoto M, Aruga A, et al. Correlation between expression of MUC1 core protein and outcome after surgery in mass-forming intrahepatic cholangiocarcinoma. Cancer 2002;94:1770–6. [DOI] [PubMed] [Google Scholar]
- 40.Takao S, Uchikura K, Yonezawa S, et al. Mucin core protein expression in extrahepatic bile duct carcinoma is associated with metastases to the liver and poor prognosis. Cancer 1999;86:1966–75. [DOI] [PubMed] [Google Scholar]
- 41.Wittekind C, Compton CC, Greene FL, et al. TNM residual tumor classification revisited. Cancer 2002;94:2511–16. [DOI] [PubMed] [Google Scholar]
- 42.DiGiovanna MP, Stern DF. Activation state-specific monoclonal antibody detects tyrosine phosphorylated p185neu/erbB-2 in a subset of human breast tumors overexpressing this receptor. Cancer Res 1995;55:1946–55. [PubMed] [Google Scholar]
- 43.Singh AP, Moniaux N, Chauhan SC, et al. Inhibition of MUC4 expression suppresses pancreatic tumor cell growth and metastasis. Cancer Res 2004;64:622–30. [DOI] [PubMed] [Google Scholar]
- 44.Half E, Broaddus R, Danenberg KD, et al. HER-2 receptor expression, localization, and activation in colorectal cancer cell lines and human tumors. Int J Cancer 2004;108:540–8. [DOI] [PubMed] [Google Scholar]
- 45.Sgambato A, Migaldi M, Leocata P, et al. Loss of p27Kip1 expression is a strong independent prognostic factor of reduced survival in N0 gastric carcinomas. Cancer 2000;89:2247–57. [DOI] [PubMed] [Google Scholar]
- 46.Chen AJ, Meng QH, Long B, et al. (Relationship between p27 expression and prognosis of colorectal carcinoma.) Ai Zheng 2002;21:1075–7. [PubMed] [Google Scholar]
- 47.Taguchi K, Aishima S, Asayama Y, et al. The role of p27kip1 protein expression on the biological behavior of intrahepatic cholangiocarcinoma. Hepatology 2001;33:1118–23. [DOI] [PubMed] [Google Scholar]
- 48.Moniaux N, Nollet S, Porchet N, et al. Complete sequence of the human mucin MUC4: a putative cell membrane-associated mucin. Biochem J 1999;338:325–33. [PMC free article] [PubMed] [Google Scholar]
- 49.Komatsu M, Yee L, Carraway KL. Overexpression of sialomucin complex, a rat homologue of MUC4, inhibits tumor killing by lymphokine-activated killer cells. Cancer Res 1999;59:2229–36. [PubMed] [Google Scholar]
- 50.Komatsu M, Carraway CA, Fregien NL, et al. Reversible disruption of cell–matrix and cell–cell interactions by overexpression of sialomucin complex. J Biol Chem 1997;272:33245–54. [DOI] [PubMed] [Google Scholar]
- 51.Brayman M, Thathiah A, Carson DD. MUC1: a multifunctional cell surface component of reproductive tissue epithelia. Reprod Biol Endocrinol 2004;2:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Levi E, Klimstra DS, Adsay NV, et al. MUC1 and MUC2 in pancreatic neoplasia. J Clin Pathol 2004;57:456–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McDermott KM, Crocker PR, Harris A, et al. Overexpression of MUC1 reconfigures the binding properties of tumor cells. Int J Cancer 2001;94:783–91. [DOI] [PubMed] [Google Scholar]
- 54.Kam JL, Regimbald LH, Hilgers JH, et al. MUC1 synthetic peptide inhibition of intercellular adhesion molecule-1 and MUC1 binding requires six tandem repeats. Cancer Res 1998;58:5577–81. [PubMed] [Google Scholar]
- 55.Hinoda Y, Ikematsu Y, Horinochi M, et al. Increased expression of MUC1 in advanced pancreatic cancer. J Gastroenterol 2003;38:1162–6. [DOI] [PubMed] [Google Scholar]