The APC/C inhibitor, Emi1, is essential for prevention of rereplication (original) (raw)
Abstract
Emi1 (early mitotic inhibitor) inhibits APC/C (anaphase-promoting complex/cyclosome) activity during S and G2 phases, and is believed to be required for proper mitotic entry. We report that Emi1 plays an essential function in cell proliferation by preventing rereplication. Rereplication seen after Emi1 depletion is due to premature activation of APC/C that results in destabilization of geminin and cyclin A, two proteins shown here to play redundant roles in preventing rereplication in mammalian cells. Geminin is known to inhibit the replication initiation factor Cdt1. The rereplication block by cyclin A is mediated through its association with S and G2/M cyclin-dependent kinases (Cdks), Cdk2 and Cdk1, suggesting that phosphorylation of proteins by cyclin A–Cdk is responsible for the block. Rereplication upon Emi1 depletion activates the DNA damage checkpoint pathways. These data suggest that Emi1 plays a critical role in preserving genome integrity by blocking rereplication, revealing a previously unrecognized function of this inhibitor of APC/C.
Keywords: APC/C, Cdk, cyclin A, Emi1, geminin, rereplication
DNA replication initiates from the specific regions of chromosomal DNA called origins. A critical question in the field of genomic stability is how cells manage to restrict the firing of origins to once and only once per cell cycle. Not only is this regulation achieved despite the fact that thousands of origins fire asynchronously all over the genome, but the regulation is selectively breached during certain stages of development as in endoreduplicating trophoblasts. Most of this regulation appears to be executed at the level of prereplicative complex (pre-RC) formation or origin licensing. The origin recognition complex (ORC) recruits Cdc6 and Cdt1 to origins in G1, which in turn load the putative replicative helicase, Mcm2–7, to form pre-RCs (for review, see Machida et al. 2005a). After replication initiation in the subsequent S phase, Mcm2–7 are depleted from origins, but reformation of pre-RCs is prevented until chromosomes are segregated in M phase. Higher eukaryotes, including human cells, express geminin (McGarry and Kirschner 1998) a protein that binds to Cdt1 to prevent loading of Mcm2–7 on post-initiation origins (Wohlschlegel et al. 2000; Tada et al. 2001). Knockdown of geminin by RNA interference (RNAi) is sufficient to induce rereplication in many human cell lines (Melixetian et al. 2004; Zhu et al. 2004), and geminin-null mice show enhanced endoreduplication in trophoblasts of early embryos (Gonzalez et al. 2006). These results suggest that geminin is a major inhibitor of rereplication in mammalian cells. Yeast, however, do not encode geminin, and rereplication is prevented solely by the high levels cyclin-dependent kinase (Cdk) activity seen during S and G2 phases of the cell cycle (Correa-Bordes and Nurse 1995; Nguyen et al. 2001; Wilmes et al. 2004; for review, see Machida et al. 2005a). A role of high Cdk activity in rereplication block has also been suggested in human cells (Itzhaki et al. 1997), although cancer cells that show rereplication after geminin knockdown do so without any obvious mechanism to concurrently inhibit Cdk2 activity. It is therefore currently unclear whether geminin and Cdk play redundant roles in rereplication block, or whether these mechanisms function in different parts of the cell cycle or in different tissues.
The regulated degradation of proteins by proteasomes through a carefully orchestrated polyubiquitination program is a critical component of cell cycle regulation. For example, Cdks are important for progression through S, G2, and M, but their activity is regulated by the periodic accumulation and destruction of different types of cyclins. Levels of cyclins in the cell cycle are regulated by two ubiquitin ligases, SCF and anaphase-promoting complex/cyclosome (APC/C). SCF complex uses an F-box protein as a substrate recognition subunit. For example, SCFFBXW7 polyubiquitinates the G1 cyclin, cyclin E, for degradation in S phase. There are >60 F-box proteins in the human genome but the cellular functions of most of them are not yet known (Jin et al. 2004). APC/C, on the other hand, uses substrate recognition subunits Cdc20 and Cdh1 to polyubiquitinate substrates like cyclins A and B (and geminin) in mitosis and the subsequent G1. Cdc20 activates APC/C in mitosis while Cdh1 activates APC/C in late M and G1 phases. Both these subunits target substrates by recognizing a destruction motif called D-box (Glotzer et al. 1991; Fang et al. 1998), while Cdh1, in addition, recognizes a KEN-box (Pfleger and Kirschner 2000).
Since cyclin A/Cdk kinase activity is essential for DNA replication, the inactivation of APC/C at the G1/S transition is critical for the accumulation of cyclin A for S-phase progression. Emi1 (early mitotic inhibitor) is a cellular inhibitor of APC/C that is not present in yeasts, and is induced by the E2F transcription factor to inactivate APC/C at the G1/S transition (Reimann et al. 2001; Hsu et al. 2002). Emi1-mediated reduction in APC/C activity allows cells to accumulate cyclin A, and then the accumulated cyclin A–Cdk complex in S phase further suppresses APC/C activity by phosphorylating Cdh1 (Lukas et al. 1999; Sorensen et al. 2001). Because of its role in suppression of APC/C and because the latter is critical for mitosis, the primary role of Emi1 is believed to ensure proper mitotic entry. At the onset of mitosis, Emi1 is degraded following phosphorylation by Plk1 and ubiquitination by SCFβ-TrCP (Guardavaccaro et al. 2003; Margottin-Goguet et al. 2003; Hansen et al. 2004; Moshe et al. 2004), and this allows the activation of APC/C that is critical for progression through M phase and the subsequent G1 phase.
Since SCF is involved in many aspects of cell cycle regulation, we initiated a search for F-box proteins that are required for cell proliferation by RNAi screening. This screen identified Emi1, an F-box protein known as FBXO5, to be essential for cell proliferation. Surprisingly, the block to cell proliferation was accompanied by extensive rereplication after Emi1 depletion. This rereplication is due to premature activation of APC/C in S and G2 cells. Among many APC/C substrates, we show that cyclin A and geminin are critical for prevention of rereplication, and that the two proteins act simultaneously in S and G2 cells as redundant barriers to rereplication. These data suggest that an essential function of Emi1 in S and G2 cells is to prevent rereplication via stabilization of inhibitors of rereplication such as cyclin A and geminin.
Results
RNAi screening for F-box proteins required for cell proliferation
We previously developed a simple method to screen for genes required for cell proliferation by RNAi (Machida et al. 2006). We have initiated a search for F-box proteins involved in cell cycle regulation, by using small interfering RNA (siRNA) duplexes against each of 66 human F-box proteins. Each siRNA duplex was transfected into a breast epithelial cell line, MCF10A, in a 96-well format and effect on cell proliferation assessed by measuring BrdU incorporation after 3 d (Y. Machida and A. Dutta, unpubl.). For genes that were essential for cell proliferation in the primary screen, second siRNAs targeting another part of mRNA were designed and used in a secondary RNAi screen. FBXO5, which is also known as the APC inhibitor, Emi1, was one of the F-box proteins whose knockdown reduced BrdU incorporation in both primary and secondary screens (Supplementary Fig. 1), suggesting that Emi1 plays an essential role in cell proliferation.
Emi1 RNAi causes rereplication in human cells
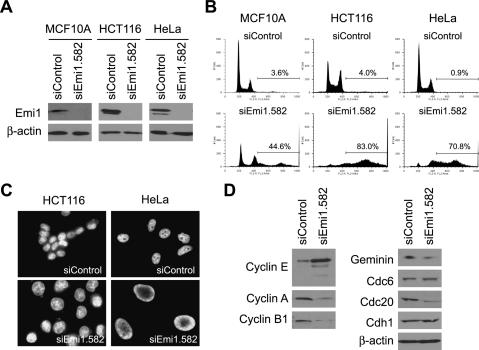
We first confirmed that the Emi1 siRNA oligo (siEmi1. 582), which was used for the siRNA screening, knocked down Emi1 protein levels in MCF10A by Western blot (Fig. 1A). Emi1 depletion is known to cause premature degradation of mitotic cyclins, and result in defects in entry into S or M phases (Reimann et al. 2001; Grosskortenhaus and Sprenger 2002; Hsu et al. 2002; Lee et al. 2006). To test if failure to enter S or M phases is the reason for reduced cell proliferation, we examined the cell cycle status of Emi1-depleted cells by FACS analysis. If the essential role of Emi1 in cell proliferation is in S- or M-phase entry, Emi1-depleted cells are expected to accumulate with 2N or 4N DNA content, respectively. However, RNAi of Emi1 instead caused rereplication in MCF10A as revealed by the accumulation of cells with >4N DNA content (Fig. 1B). Similarly, rereplication was seen after Emi1 depletion in other cell lines such as a colon cancer cell line, HCT116, and a cervical cancer cell line, HeLa (Fig. 1A,B). Since rereplication was observed in the nontransformed cell line, MCF10A, the requirement of Emi1 for rereplication block is not restricted to cancer-derived cell lines. Two additional siRNA duplexes (siEmi1.1298 and siEmi1.1411) targeting different sequences of the Emi1 mRNA also caused rereplication (Supplementary Fig. 2). Previously we have reported that the size of nuclei correlates with the degree of rereplication after geminin depletion (Zhu et al. 2004). Emi1 depletion also resulted in giant nuclei in HCT116 and HeLa (Fig. 1C). In summary, these results suggest that Emi1 is critical to prevent rereplication in human cells.
Figure 1.
Emi1 depletion causes rereplication accompanied by a decrease in cyclin A and geminin. (A) Emi1 depletion by Emi1 RNAi. MCF10A, HCT116, and HeLa were transfected with siControl or siEmi1 oligos, and Emi1 protein levels were examined by Western blotting. β-Actin is shown as a loading control. (B) Emi1 depletion causes rereplication. MCF10A, HCT116, and HeLa were transfected with siControl or siEmi1 oligos, and the DNA content of the cells was examined by FACS. The percentages of cells with >4N DNA content are indicated. (C) Emi1 depletion results in giant nuclei. HCT116 and HeLa transfected with siControl or siEmi1 duplexes were examined under a microscope after staining DNA with DAPI. (D) APC/C substrates are decreased after Emi1 RNAi in HeLa. Levels of indicated proteins were assessed by Western blotting.
The known function of Emi1 is inhibition of APC/C in S and G2 phases. APC/C substrates including cyclin A, cyclin B1, geminin, and Cdc20 were decreased after Emi1 RNAi (Fig. 1D), suggesting that APC/C is prematurely activated in these cells. In contrast, the protein level of another APC/C substrate involved in replication initiation, Cdc6, remained unchanged. Cyclin E levels are elevated in Emi1-depleted cells probably due to increased E2F-dependent transcription upon APC/C activation (Sorensen et al. 2000). Since cyclin E–Cdk2 protects Cdc6 from APC/C (Mailand and Diffley 2005), the elevated cyclin E protein level in Emi1-depleted cells could explain the lack of any decrease of Cdc6. The proteasome inhibitor, MG132, blocked decrease of geminin and cyclin A (Supplementary Fig. 3), consistent with the idea that these proteins are targeted for degradation by proteasomes after Emi1 RNAi.
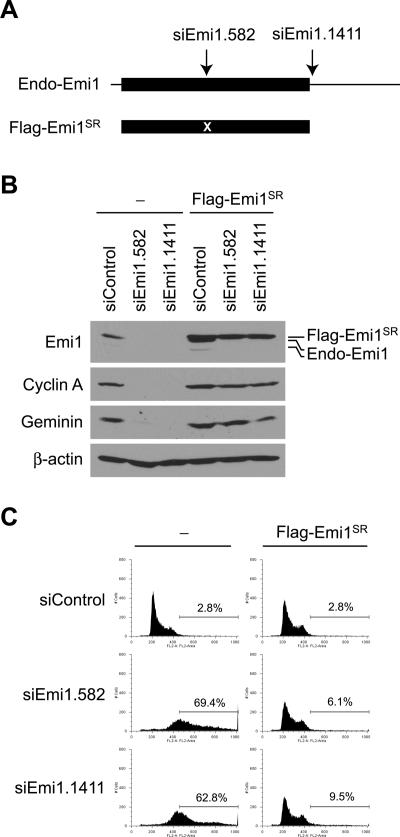
To rule out the possibility that rereplication seen after Emi1 RNAi is due to off-target activity, we tested whether the rereplication phenotype is suppressed by exogenous expression of Flag-tagged Emi1. Seven silent mutations were introduced to the target sequence of siEmi1.582 to make the exogenous Emi1 resistant to siRNA (Flag-Emi1SR; SR for siRNA resistant) (Fig. 2A). In addition, Flag-Emi1SR was expressed from the coding sequence of Emi1 so that Flag-Emi1SR is resistant to siEmi1.1411, whose target site is in the 3′UTR of Emi1 mRNA. Expression of Flag-Emi1SR restored the amount of Emi1 in cells treated with siEmi1.582 and siEmi1.1411 and prevented reduction of APC/C substrates, cyclin A and geminin (Fig. 2B). These cells did not undergo rereplication (Fig. 2C), confirming that rereplication is a specific effect of Emi1 knockdown.
Figure 2.
Rescue of Emi1 RNAi phenotype in cells stably expressing siRNA-resistant Emi1. (A) Schematic diagrams of endogenous Emi1 mRNA (Endo-Emi1) and the region expressed exogenously (Flag-Emi1SR). Flag-Emi1SR is expressed from the coding sequence of Emi1, and contains seven silent mutations in the siEmi1.582 target sequence (marked as “X”). Since the exogenous Emi1 does not possess the 3′UTR sequence, its expression is also not affected by siEmi1.1411, whose target site is in the 3′UTR. Black boxes and lines indicate the Emi1 coding sequence and UTRs, respectively. (B) Restoration of Emi1 levels by exogenous Emi1. HeLa cells were infected with retroviruses expressing Flag-Emi1SR. The cells were transfected with siRNAs (48 h), and the indicated proteins were examined by Western blotting. (Endo-Emi1) Endogenous Emi1. (C) Suppression of rereplication after Emi1 RNAi by Flag-Emi1SR expression. HeLa cells with or without Flag-Emi1SR expression were transfected with the indicated siRNAs and rereplication was analyzed by FACS for DNA content. The percentages of cells with >4N DNA are indicated.
Rereplication in Emi1-depleted cells is due to unscheduled activation of APC/CCdh1
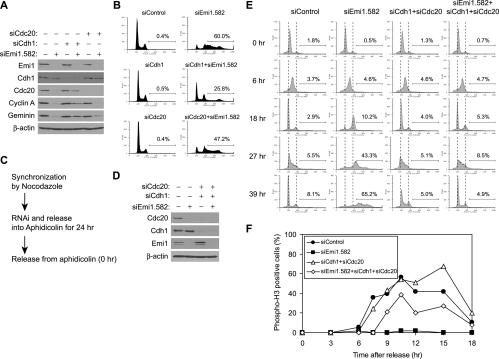
We next tested whether rereplication in Emi1-depleted cells is due to failure to suppress APC/C activity. We codepleted the activator subunits of APC/C, Cdh1, or Cdc20, with Emi1. Cdh1 depletion partially restored the levels of APC/C substrates such as cyclin A and geminin, whereas Cdc20 depletion did not (Fig. 3A), indicating that cyclin A and geminin are degraded via the APC/CCdh1-dependent pathway. The failure of Cdc20 codepletion to restore cyclin A and geminin levels is consistent with the fact that Cdc20 is decreased in Emi1-depleted cells because of degradation by APC/CCdh1 (Figs. 1D, 3A). Cdh1 depletion suppressed rereplication in Emi1-depleted cells, whereas Cdc20 depletion did so minimally (Fig. 3B), indicating that rereplication after Emi1 RNAi is caused via APC/CCdh1 activation. RNAi of Cdh1 or Cdc20 did not affect the cell cycle profile by itself (Fig. 3B), suggesting that suppression of rereplication is not due to failure of mitosis. To confirm this, we codepleted Cdh1 and Cdc20 with Emi1 in cell cycle-synchronized cells by the procedure shown in Figure 3C. Cells released from Nocodazole block were transfected with siRNAs and cultured in the media containing aphidicolin, an inhibitor of DNA polymerase α. Thus, the cells were held at the G1/S transition while the targeted proteins are depleted. Subsequent release of the cells from the aphidicolin block allowed us to follow the cell cycle progression in the absence of the depleted proteins (Fig. 3D,E). Cells treated with control siRNA entered S phase, passed through mitosis, and returned to G1 after 18 h. In contrast, Emi1-depleted cells continued replication and accumulated DNA >4N after 39 h. Cells depleted of both Cdh1 and Cdc20 showed cell cycle progression similar to control cells except for a slight delay in mitotic exit (Fig. 3F). Cdh1 is dispensable for cell proliferation (Sudo et al. 2001), while Cdc20 is believed to be essential for mitosis. The lack of a greater effect of Cdc20 RNAi on mitosis is most likely because RNAi leaves sufficient proteins in the cells to allow the mitotic function of APC/C. Cdh1 and Cdc20 codepletion, however, suppressed rereplication caused by Emi1 RNAi, strengthening our conclusion that Emi1 RNAi induces rereplication through APC/C activation.
Figure 3.
Rereplication in the Emi1-depleted cells is APC/CCdh1 dependent. (A) Codepletion of Cdh1 and/or Cdc20 with Emi1 by RNAi in HeLa. The indicated proteins in RNAi-treated cells were examined by Western blotting. (B) Rereplication after Emi1 depletion requires Cdh1. HeLa cells transfected with the indicated siRNAs were analyzed by FACS for DNA content. The percentages of cells with >4N DNA are indicated. (C) Experimental scheme of RNAi in cells synchronized in mitosis. Cells released from the aphidicolin block were sampled at the times indicated in E and F. (D) Codepletion of Cdh1 and Cdc20 with Emi1 by RNAi using the protocol in C. Cells released from the aphidicolin block for 27 h were examined by Western blotting. (E) Emi1 depletion causes rereplication in an APC/C-dependent manner. HeLa cells treated as in C and released for the indicated times were analyzed by FACS for DNA content. Broken lines indicate the positions of 2N and 4N peaks in asynchronous cells. The percentages of cells with >4N DNA are indicated. (F) Emi1 depletion causes rereplication without cells entering mitosis. HeLa cells treated as in C and released for the indicated times were immunostained with an anti-phospho-histone H3 (mitosis marker) antibody, and the percentages of stained cells are shown.
Emi1-depleted cells undergo rereplication in the absence of mitosis
Rereplication caused by repeated replication initiation in S and G2 phases is clearly different from that produced by cells entering the next cell cycle without chromosome segregation (abortive mitosis). To test whether the Emi1-depleted cells rereplicated in S and G2, we examined whether the cells enter mitosis prior to rereplication. Phosphorylation of histone H3 at Ser10, which is an early mitotic marker, was examined in Emi1-depleted cells released from aphidicolin arrest (Fig. 3F). While control siRNA-treated cells became phospho-histone H3-positive starting at 7.5 h after release, Emi1-depleted cells did not have this mitotic marker during this period. Mitotic entry was partially restored by codepletion of Cdh1 and Cdc20 with Emi1. These results suggest that Emi1 causes rereplication without the cells entering mitosis. Phosphorylation of histone H3 at Ser10 initiates in pericentromeric heterochromatin as foci in late G2 phase (Hendzel et al. 1997). We did not observe such phospho-histone H3 foci either in Emi1-depleted cells.
Cyclin A and geminin are relevant APC/C substrates involved in prevention of rereplication in some cells
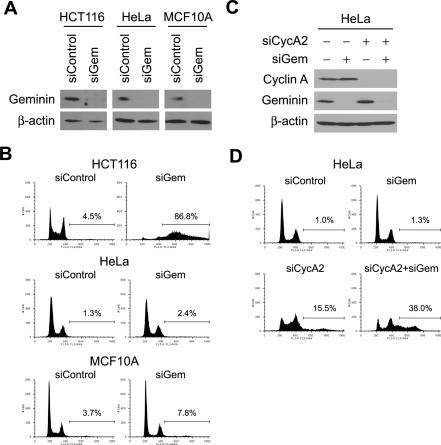
The results described above suggest that Emi1 suppresses rereplication through stabilization of rereplication inhibitors that are APC/C substrates. Geminin is a good candidate for such a protein since it was shown previously that geminin RNAi in HCT116 causes accumulation of cells with DNA >4N (Fig. 4A,B; Melixetian et al. 2004; Zhu et al. 2004). However, in HeLa and MCF10A, depletion of geminin did not induce rereplication even though the knockdown of geminin was as efficient as in HCT116 (Fig. 4A,B), suggesting that, in addition to geminin, there must be other APC/C substrates that block rereplication in these two cell lines. We therefore systematically codepleted APC/C substrates and geminin from HeLa cells looking for induction of rereplication. Among cyclins A1, A2, B1, and B2, depletion of cyclin A2 with geminin induced rereplication in HeLa (Fig. 4C,D; data not shown). Cyclin A2 depletion by itself did not cause as much rereplication as cyclin A2/geminin codepletion, although the cells accumulated in S phase, suggesting that geminin and cyclin A2 are redundant to each other for preventing rereplication in HeLa cells. In summary, depletion of geminin alone is sufficient for rereplication in HCT116 cells but codepletion of cyclin A and geminin is needed to cause rereplication in HeLa cells. Emi1 inactivation (and APC/C activation) simultaneously inactivates both of these redundant barriers to rereplication.
Figure 4.
Cyclin A and geminin prevent rereplication in HeLa. (A) Geminin depletion by geminin RNAi. HCT116, HeLa, and MCF10A were transfected with siControl or siGem oligos and geminin protein levels were examined by Western blotting. β-Actin is shown as a loading control. (B) Geminin depletion is sufficient to induce rereplication in HCT116 but not in HeLa and MCF10A. Cells transfected with siControl or siGem oligos were examined by FACS for DNA content. The percentages of cells with >4N DNA are indicated. (C) Codepletion of cyclin A2 and geminin by RNAi in HeLa. The indicated proteins in RNAi-treated cells were examined by Western blotting. (D) Cyclin A2, in addition to geminin, needs to be depleted to induce rereplication in HeLa. Cells transfected with the indicated siRNAs were examined by FACS for DNA content. The percentages of cells with >4N DNA are indicated.
Destruction of Cyclin A is required for induction of rereplication by Emi1 RNAi
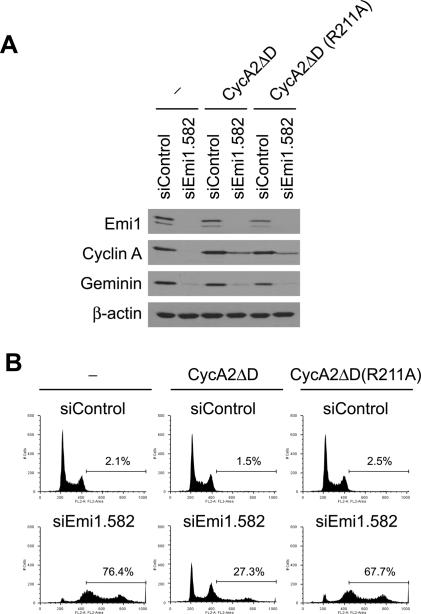
To directly test that cyclin A destruction is critical for rereplication after Emi1 depletion, cyclin A carrying mutations (R47A and L50V) in the destruction-box motif (cyclin A2ΔD) was expressed by a retrovirus vector. While most cyclin A proteins are degraded after Emi1 RNAi in cells infected with control viruses, a significant amount of cyclin A protein remained in cyclin A2ΔD-expressing cells (Fig. 5A). Rereplication after Emi1 RNAi was reduced in the cyclin A2ΔD-expressing cells (Fig. 5B), although Emi1 knockdown and decrease of geminin is similar to control cells. Therefore, cyclin A destabilization is important for the rereplication seen after Emi1 depletion. Geminin was still decreased, again emphasizing that geminin and cyclin A are redundant to each other in blocking rereplication in HeLa cells. Both of them have to be decreased before rereplication is seen.
Figure 5.
Nondegradable cyclin A2 can suppress rereplication induced by Emi1 RNAi in a Cdk-binding-dependent manner. (A) Emi1 RNAi in HeLa cells infected with empty retroviruses (− lanes), cyclin A2ΔD, or cyclin A2ΔD(R211A) viruses. The indicated proteins were examined by Western blotting. (B) Expression of cyclin A2ΔD is sufficient to block rereplication caused by Emi1 depletion. HeLa cells infected with empty retroviruses (− panels), cyclin A2ΔD, or cyclin A2ΔD(R211A) retroviruses. Cells treated with the indicated RNAi were examined by FACS for DNA content. The percentages of cells with >4N DNA are indicated.
To rule out a Cdk-independent function of cyclin A, we next tested whether the ability of cyclin A to suppress rereplication requires association with Cdk. Previously, it was demonstrated that a mutation in R211 disrupts interaction between cyclin A and Cdks (Kobayashi et al. 1992). We generated the cyclin AΔD(R211A) mutant, in which R211 of cyclin AΔD is mutated to alanine. Cyclin AΔD and cyclin AΔD(R211A) were expressed at similar levels (Fig. 5A), yet the R211A mutation impaired the ability of cyclin AΔD to suppress rereplication (Fig. 5B). Therefore, suppression of rereplication by cyclin A requires Cdk association.
Cdk2 and Cdk1 are involved in prevention of rereplication
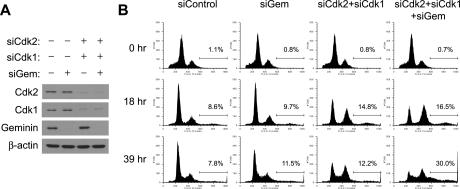
The requirement of Cdk interaction implies that cyclin A–Cdk together are involved in prevention of rereplication. To directly test this we examined whether codepletion of Cdks with geminin causes rereplication. Cyclin A is known to interact with the S-phase Cdk, Cdk2, and the M-phase Cdk, Cdk1. We thus cotransfected siRNAs targeting Cdk2, Cdk1, or geminin into synchronized cells using the procedure summarized in Figure 3C. Cells were released from G1/S block by aphidicolin and harvested after 18 or 39 h. Codepletion of geminin, Cdk2, and Cdk1 proteins in HeLa cells resulted in rereplication after 39 h (Fig. 6A,B). Codepletion of geminin with either Cdk2 or Cdk1 alone showed only marginal rereplication (data not shown), indicating Cdk1 and Cdk2 plays redundant roles in prevention of rereplication. Collectively, these data suggest that cyclin A–Cdk2 and cyclin A–Cdk1 complexes redundantly prevent rereplication in HeLa cells. It is intriguing, in this context, that the high levels of cyclin E in Emi1-depleted cells cannot prevent rereplication, because it suggests that cyclin A–Cdk2 and cyclin E–Cdk2 must have different substrate specificities.
Figure 6.
Cdk2 and Cdk1 are involved in rereplication block in HeLa. (A) Codepletion of Cdk2, Cdk1, and geminin by RNAi using the protocol in Figure 2C. Cells released from the aphidicolin block for 39 h were examined by Western blotting. (B) Codepletion of Cdk2, Cdk1, and geminin induces rereplication in HeLa. Cells treated as in Figure 2C and released for the indicated times were examined by FACS for DNA content. The percentages of cells with >4N DNA are indicated.
Checkpoint pathways are activated in rereplicating cells following Emi1 depletion
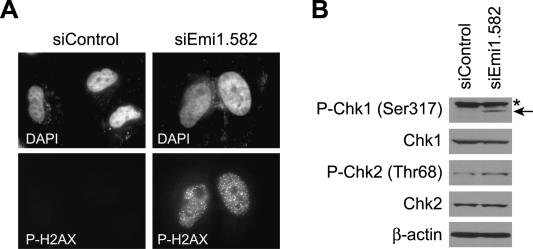
Previously we and others have shown that the DNA damage checkpoint is activated in cells undergoing rereplication after geminin RNAi (Melixetian et al. 2004; Zhu et al. 2004). We thus tested if a similar checkpoint pathway is activated during Emi1 RNAi-induced rereplication. The DNA damage checkpoint is activated through kinases, ATM or ATR, which in turn activate downstream checkpoint kinases, Chk1 and Chk2, by phosphorylation. Phosphorylation of a histone variant, H2AX, by ATM or ATR is known to be an early event during checkpoint activation. Figure 7A shows that, in Emi1-depleted cells, H2AX is phosphorylated at Ser139 and phospho-H2AX forms foci. In addition, phosphorylation of Chk1 at Ser317 was greatly increased and that of Chk2 at Thr68 was also slightly increased (Fig. 7B). These data suggest that rereplication induced by Emi1 depletion activates the DNA damage checkpoint.
Figure 7.
DNA damage checkpoint is activated in Emi1-depleted cells. (A) H2AX phosphorylation and foci formation in Emi1-depleted HeLa cells. Cells were transfected with the indicated siRNAs for 48 h and stained with DAPI and anti-phospho-H2AX (Ser139). (P-H2AX) Phospho-H2AX. (B) Phosphorylation of Chk1 and Chk2 after Emi1 depletion. HeLa cells were transfected with the indicated siRNAs for 48 h and cell lysates were examined by Western blotting for the indicated proteins. (P-Chk1) Phospho-Chk1; (P-Chk2) phospho-Chk2; (*) a cross-reactive band.
Discussion
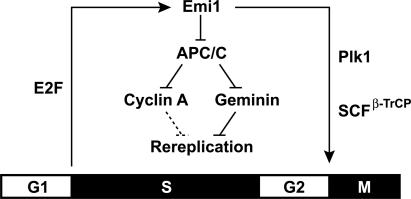
In this paper, we showed that Emi1 is essential for cell proliferation. Although Emi1 has been recognized as a factor important for entry into S and M phases in humans, mice, Drosophila, and Xenopus extracts (Reimann et al. 2001; Grosskortenhaus and Sprenger 2002; Hsu et al. 2002; Lee et al. 2006), our data suggest that an essential role of Emi1 in the cell cycle is prevention of rereplication. At the G1/S transition, Emi1 is induced by E2F and allows accumulation of cyclin A and geminin, which are two redundant mechanisms for rereplication block (Fig. 8). Until it is degraded via ubiquitination by SCFβ-TrCP in early mitosis, Emi1 prevents rereplication by maintaining high levels of geminin and cyclin A. A similar role of Emi1 in preventing rereplication has been independently discovered by Dr. Jonathon Pines (pers. comm.).
Figure 8.
Suppression of rereplication by Emi1 during S and G2 phases. Rereplication block pathways triggered by Emi1. Emi1 is induced at G1/S transition by E2F and inhibits APC/C to stabilize rereplication inhibitors, cyclin A and geminin. Emi1 is degraded upon mitotic entry after phosphorylation by Plk and ubiquitination by SCFβ-TrCP. In some cancer cells—e.g., HCT116—cyclin A-dependent suppression of rereplication is not functional (shown by dashed lines).
Although the effect of Emi1 depletion was examined in many experimental systems (Grosskortenhaus and Sprenger 2002; Hsu et al. 2002; Lee et al. 2006), the rereplication phenotype has not been reported. A part of the reason is that it is difficult to detect rereplication without analysis of DNA content by FACS, which is hard to perform in flies or mice, especially because Emi1-null organisms are embryonic lethal. However, it is important to note that in the previous studies some of the Emi1-deficient cells in flies and in mouse embryos seem to have giant nuclei, a hallmark of rereplication (Grosskortenhaus and Sprenger 2002; Lee et al. 2006). Contrary to our study, Emi1 RNAi in HeLa cells was reported to cause a failure of cells to enter S phase (Hsu et al. 2002). This conclusion was drawn from the decrease in the number of cells incorporating BrdU after Emi1 RNAi. However, reduction in BrdU-positive cells could be due to a decrease in DNA synthesis secondary to rereplication-induced fork stalling or to a cell cycle arrest due to checkpoint activation. Rereplication may have been missed due to the lack of cell cycle analysis by FACS. Alternatively, differences in RNAi efficiency between the two studies may cause differences in the levels of residual Emi1, leading to differences in the terminal phenotype.
APC/C activity and pre-RC formation
Consistent with its role as an inhibitor of APC/C, rereplication after Emi1 depletion requires APC/C. Since Cdc20 itself is an APC/C substrate (Pfleger and Kirschner 2000), under Emi1-depleted conditions, the active APC/C mainly contains Cdh1. APC/CCdh1 is normally active in late M and G1 phases, and prevents accumulation of mitotic cyclins and geminin (Irniger and Nasmyth 1997; McGarry and Kirschner 1998). APC/C activity is important for allowing pre-RC formation in G1. Overexpression of geminin lacking a destruction box inhibited pre-RC formation and impaired DNA replication (Shreeram et al. 2002; Wohlschlegel et al. 2002). Similarly, premature appearance of cyclins in G1 is deleterious to pre-RC formation (Coverley et al. 2002; Ekholm-Reed et al. 2004). Thus, high APC/C activity and the attendant decrease in geminin and cyclin A are necessary to allow pre-RC formation in late M and G1 phases. Our results suggest that the converse is also true: Low APC/C activity prevents pre-RC formation in S and G2 and prevents rereplication. Overexpression of Cdh1 has been reported to cause overreplication (Sorensen et al. 2000). Taken together, our results suggest that Emi1 plays a critical role in prevention of rereplication as a regulator of APC/C activity in S and G2 phases.
Geminin and Cdk as redundant mechanisms for rereplication block
While a role of geminin in preventing rereplication in mammalian cells is well accepted, we show that in some cell lines, cyclin A is equally important. The role of cyclin A in prevention of rereplication is dependent on Cdks. The importance of high Cdk activity in prevention of rereplication is well established in yeasts (cited in the introduction). However, it has been unclear whether Cdk prevents rereplication in mammalian cells or Xenopus egg extracts because alteration of the geminin–Cdt1 balance alone was sufficient to induce rereplication, without any manipulation of the Cdk activity seen in S-phase cells or extracts (Melixetian et al. 2004; Zhu et al. 2004; Arias and Walter 2005). On the other hand, a negative effect of Cdk activity on mammalian pre-RC components has also been reported. For example, Cdk-dependent phosphorylation causes nuclear export of excess Cdc6 protein in human cells (Saha et al. 1998; Fujita et al. 1999; Jiang et al. 1999; Petersen et al. 1999). Phosphorylation of Cdt1 by Cdk targets Cdt1 for polyubiquitination by SCFSkp2 and degradation (Liu et al. 2004; Sugimoto et al. 2004). Overexpression of Cdt1 mutated in a Cy motif that is required for phosphorylation by Cdk2 increases rereplication, supporting the negative effect of Cdks on pre-RC formation (Takeda et al. 2005; Nishitani et al. 2006). In addition, Cdk1 inactivation in a mammalian fibrosarcoma cell line causes rereplication (Itzhaki et al. 1997). In the face of this conflicting evidence of the critical role of geminin or Cdk in rereplication block in vertebrates, it was unclear whether geminin and Cdk are redundant in rereplication block or nonredundant, and function in different phases of the cell cycle. Our study suggests that geminin and Cdk provide redundant mechanisms for preventing rereplication in S and G2 phases, although there is a difference between cell lines (see below). Collectively, we propose a model that the cyclin A–Cdk complex and geminin independently prevent rereplication by inhibiting formation of pre-RCs, and both pathways are coregulated by Emi1 during S and G2 phases.
Difference in rereplication block pathways between cell lines
A redundant role of cyclin A and geminin in preventing rereplication in some cell lines explains the puzzling failure to see rereplication after depletion of geminin in commonly studied cells like HeLa. The presence of two redundant pathways to prevent rereplication is also consistent with the results of H. Hochegger and S. Takeda (pers. comm.). They showed that Cdk inhibition in the chicken DT40 cell line causes rereplication in mitosis but not in G2 phase, indicating the presence of at least one other pathway to prevent rereplication in G2 cells that is neutralized by the activation of APC/C in mitosis. On the other hand, in a Drosophila cell line, geminin and cyclin A are nonredundant in this aspect, so that depletion of either protein leads to rereplication (Mihaylov et al. 2002). Similarly, we and others have shown that depletion of geminin alone leads to rereplication in HCT116 colon cancer cell lines while overexpression of Cdt1 promotes rereplication in lung cancer cells (Vaziri et al. 2003; Takeda et al. 2005). Thus, rereplication-blocking pathways are different between tissues, developmental stages, or types of cancer cells with geminin being the sole inhibitor in some but not all cells. Further study will be required to determine why cyclin A is not able to suppress rereplication in the absence of geminin in HCT116 cells or upon overexpression of Cdt1 in lung cancer cells.
Activation of DNA damage checkpoint after rereplication suggests that rereplication leads to DNA damage or unusual DNA structures. Indeed, a recent study suggested that uncontrolled rereplication causes fork collisions and results in DNA fragmentation in Xenopus egg extracts (Davidson et al. 2006). Therefore, it will be important to examine the effect of rereplication on genome integrity and its potential as a cause of tumorigenesis. In this sense, it is worth noting that overexpression of Cdt1 or Cdc6, which tilts the balance of origin licensing toward rereplication (Vaziri et al. 2003), is associated with genomic instability and poor prognosis in lung cancers and mantle cell lymphoma (Karakaidos et al. 2004; Magda Pinyol et al. 2006). In addition, the lack of the Cdk-dependent rereplication block pathway in HCT116 reported in this study suggests that loss of some but not all rereplication-blocking pathways may be seen in cancer cells, predisposing them to genomic instability. Emi1 overexpression has been reported to accompany genomic instability, and Emi1 is overexpressed in tumors (Hsu et al. 2002; Lehman et al. 2006). Our results suggest, however, that deregulation of APC activity through functional inactivation of Emi1 might also contribute to genome instability in cancer cells. Finally, given that Emi1 regulates two redundant mechanisms for preventing rereplication, we speculate that inactivation of Emi1 may be important in physiological situations where vertebrate cells undergo endoreduplication as part of normal development.
Materials and methods
Plasmids
A coding sequence of human cyclin A2 and Emi1 was cloned into a retrovirus vector pMMP-puro (Pulsipher et al. 1998) and pMSCVpuro (Clontech), respectively, and mutagenesis was performed using QuickChange kit (Stratagene) according to the manufacturer’s instructions. Mutations that confer resistance to siEmi1.582 were introduced to the Emi1 coding sequence using primers 5′-GAAGAAGGTAGCCTCCTCGAGGAAAA CTTTGGAGATAGCCTCCAATCCTGCCTGCTACAAATAC-3′ and 5′-GTATTTGTAGCAGGCAGGATTGGAGGCTATCTCC AAAGTTTTCCTCGAGGAGGCTACCTTCTTC-3′.
Cell culture, retrovirus infection, and FACS
HeLa cells were cultured in DMEM containing 10% donor calf serum. HCT116 cells were cultured in McCoy’s 5A containing 10% fetal calf serum. MCF10A is an immortalized breast epithelial cell line derived from fibrocystic disease that was cultured in DMEM/F12 containing 5% donor calf serum, 0.02 μg/mL EGF (Sigma), 1 μg/mL Insulin (Sigma), 1.4 μM hydrocortisone (Sigma), and 0.1 μg/mL Cholera toxin. For synchronization of cells in mitosis, cells were treated with 40 ng/mL of Nocodazole for 8 h and rounded cells were collected. For synchronization of cells at G1/S, aphidicolin (1 μg/mL) was added to the medium. Cells were released from the block by extensive washing with PBS. Retroviruses were produced in the 293GPG cell line (Ory et al. 1996) or the Phoenix cell line (Pear et al. 1993) by transient transfection of retrovirus plasmids. Cells were infected with retroviral supernatants and selected in puromycin (1 μg/mL). FACS analysis was performed as described previously (Machida et al. 2005b).
RNAi screening
RNAi screening for genes involved in cell proliferation was performed as described previously (Machida et al. 2006). Single siRNA oligos were designed against human F-box proteins based on the criteria reported previously (Reynolds et al. 2004) and purchased from Samchuly. Five-thousand MCF10A cells were transfected with 4 pmol of siRNA duplex using Lipofectamine 2000 (Invitrogen) in a 96-well plate. After a 3-d culture, cells were incubated with 10 μM BrdU for 15 min and incorporation of BrdU per well was measured by ELISA using HRP-coupled anti-BrdU antibody (Roche). The screen was performed with three technical replicates and repeated twice. Genes whose knockdown reduced BrdU incorporation as much as a positive control siRNA (targeting a replication factor, ORC2) were considered as hits. siControl, which targets a luciferase gene, was used as a negative control. For FBXO5/Emi1, a hit gene in the primary siRNA screen, additional siRNA duplexes were designed against different sequences of the mRNA and used for the secondary siRNA screen.
RNAi
siRNA transfection was performed using Oligofectamine (Invitrogen) as described previously (Machida et al. 2005b). Lipofectamine RNAiMAX (Invitrogen) was used for siRNA transfection into Nocodazole-arrested cells. The target sequences of siRNAs were as follows: siControl (GL2), 5′-AACGUACGCGGA AUACUUCGA-3′; siEmi1.582, 5′-GAGAAUUUCGGUGACA GUCUA-3′; siEmi1.1298, 5′-UACGAAGUGUCUCUGUAA UUA-3′; siEmi1.1411, 5′-UACGAAGAUUGUGAUCUCUUA -3′; siCyclin A2, 5′-AAAGCUGGCCUGAAUCAUUAA-3′; and siCdk1, 5′-AAAGGAACTTCGTCATCCAAA-3′. The sequences of Geminin, Cdk2, Cdh1, Cdc20, and ORC2 siRNAs were reported previously (Brummelkamp et al. 2002; Bashir et al. 2004; Zhang et al. 2004; Zhu et al. 2004; Machida et al. 2005b).
Experimental scheme of RNAi in cells synchronized in mitosis (Fig. 3): HeLa cells were synchronized in metaphase by Nocodazole treatment (40 ng/mL) for 8 h. Rounded cells were collected, transfected with siRNAs, and released into aphidicolin for 24 h. Cells were released from the aphidicolin block, and samples were taken after indicated times in Figure 3, E and F.
Western blotting
For Western blotting, cells were lysed in lysis buffer (50 mM Tris-HCl, 150 mM NaCl, 0.1% NP-40, 5 mM EDTA) supplemented with protease inhibitor mix (Sigma). The antibodies used were anti-Emi1 (Invitrogen); anti-cyclin E, anti-cyclin A, anti-cyclin B1, anti-Cdk2, anti-Cdk1, anti-Cdc20, and anti-Cdc6 (Santa Cruz Biotechnology); anti-Cdh1 (BD Biosciences); anti-β-actin and anti-Chk2 (Sigma); anti-Chk1, anti-phospho-Chk1 (Ser317), anti-phospho-Chk2 (Thr68) (Cell Signaling Technology). Rabbit anti-geminin antiserum was reported previously (Wohlschlegel et al. 2000).
Immunostaining
Cells cultured on slides were fixed with 4% paraformaldehyde for 10 min and permeabilized with 0.2% TX-100 for 10 min. Following blocking with 3% BSA in PBS for 1 h, cells were incubated with antibodies diluted in PBS containing 3% BSA for 1 h. After three washes with PBS, cells were incubated with FITC-labeled anti-rabbit IgG antibody (DAKO) diluted in PBS containing 3% BSA for 30 min and washed three times with PBS. Cells were mounted with Vectashield containing DAPI (Vector Laboratory). The antibodies used were anti-phospho-histone H3 (Ser10) antibody (Millipore) and anti-phospho-H2AX (Ser139) (Cell Signaling Technology).
Acknowledgments
We thank members of the Dutta laboratory for helpful discussions. This work was supported by RO1 CA60499.
Footnotes
References
- Arias E.E., Walter J.C., Walter J.C. Replication-dependent destruction of Cdt1 limits DNA replication to a single round per cell cycle in Xenopusegg extracts. Genes & Dev. 2005;19:114–126. doi: 10.1101/gad.1255805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashir T., Dorrello N.V., Amador V., Guardavaccaro D., Pagano M., Dorrello N.V., Amador V., Guardavaccaro D., Pagano M., Amador V., Guardavaccaro D., Pagano M., Guardavaccaro D., Pagano M., Pagano M. Control of the SCF(Skp2–Cks1) ubiquitin ligase by the APC/C(Cdh1) ubiquitin ligase. Nature. 2004;428:190–193. doi: 10.1038/nature02330. [DOI] [PubMed] [Google Scholar]
- Brummelkamp T.R., Bernards R., Agami R., Bernards R., Agami R., Agami R. A system for stable expression of short interfering RNAs in mammalian cells. Science. 2002;296:550–553. doi: 10.1126/science.1068999. [DOI] [PubMed] [Google Scholar]
- Correa-Bordes J., Nurse P., Nurse P. p25rum1 orders S phase and mitosis by acting as an inhibitor of the p34cdc2 mitotic kinase. Cell. 1995;83:1001–1009. doi: 10.1016/0092-8674(95)90215-5. [DOI] [PubMed] [Google Scholar]
- Coverley D., Laman H., Laskey R.A., Laman H., Laskey R.A., Laskey R.A. Distinct roles for cyclins E and A during DNA replication complex assembly and activation. Nat. Cell Biol. 2002;4:523–528. doi: 10.1038/ncb813. [DOI] [PubMed] [Google Scholar]
- Davidson I.F., Li A., Blow J.J., Li A., Blow J.J., Blow J.J. Deregulated replication licensing causes DNA fragmentation consistent with head-to-tail fork collision. Mol. Cell. 2006;24:433–443. doi: 10.1016/j.molcel.2006.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekholm-Reed S., Mendez J., Tedesco D., Zetterberg A., Stillman B., Reed S.I., Mendez J., Tedesco D., Zetterberg A., Stillman B., Reed S.I., Tedesco D., Zetterberg A., Stillman B., Reed S.I., Zetterberg A., Stillman B., Reed S.I., Stillman B., Reed S.I., Reed S.I. Deregulation of cyclin E in human cells interferes with prereplication complex assembly. J. Cell Biol. 2004;165:789–800. doi: 10.1083/jcb.200404092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang G., Yu H., Kirschner M.W., Yu H., Kirschner M.W., Kirschner M.W. Direct binding of CDC20 protein family members activates the anaphase-promoting complex in mitosis and G1. Mol. Cell. 1998;2:163–171. doi: 10.1016/s1097-2765(00)80126-4. [DOI] [PubMed] [Google Scholar]
- Fujita M., Yamada C., Goto H., Yokoyama N., Kuzushima K., Inagaki M., Tsurumi T., Yamada C., Goto H., Yokoyama N., Kuzushima K., Inagaki M., Tsurumi T., Goto H., Yokoyama N., Kuzushima K., Inagaki M., Tsurumi T., Yokoyama N., Kuzushima K., Inagaki M., Tsurumi T., Kuzushima K., Inagaki M., Tsurumi T., Inagaki M., Tsurumi T., Tsurumi T. Cell cycle regulation of human CDC6 protein. Intracellular localization, interaction with the human mcm complex, and CDC2 kinase-mediated hyperphosphorylation. J. Biol. Chem. 1999;274:25927–25932. doi: 10.1074/jbc.274.36.25927. [DOI] [PubMed] [Google Scholar]
- Glotzer M., Murray A.W., Kirschner M.W., Murray A.W., Kirschner M.W., Kirschner M.W. Cyclin is degraded by the ubiquitin pathway. Nature. 1991;349:132–138. doi: 10.1038/349132a0. [DOI] [PubMed] [Google Scholar]
- Gonzalez M.A., Tachibana K.E., Adams D.J., van der Weyden L., Hemberger M., Coleman N., Bradley A., Laskey R.A., Tachibana K.E., Adams D.J., van der Weyden L., Hemberger M., Coleman N., Bradley A., Laskey R.A., Adams D.J., van der Weyden L., Hemberger M., Coleman N., Bradley A., Laskey R.A., van der Weyden L., Hemberger M., Coleman N., Bradley A., Laskey R.A., Hemberger M., Coleman N., Bradley A., Laskey R.A., Coleman N., Bradley A., Laskey R.A., Bradley A., Laskey R.A., Laskey R.A. Geminin is essential to prevent endoreduplication and to form pluripotent cells during mammalian development. Genes & Dev. 2006;20:1880–1884. doi: 10.1101/gad.379706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosskortenhaus R., Sprenger F., Sprenger F. Rca1 inhibits APC–Cdh1(Fzr) and is required to prevent cyclin degradation in G2. Dev. Cell. 2002;2:29–40. doi: 10.1016/s1534-5807(01)00104-6. [DOI] [PubMed] [Google Scholar]
- Guardavaccaro D., Kudo Y., Boulaire J., Barchi M., Busino L., Donzelli M., Margottin-Goguet F., Jackson P.K., Yamasaki L., Pagano M., Kudo Y., Boulaire J., Barchi M., Busino L., Donzelli M., Margottin-Goguet F., Jackson P.K., Yamasaki L., Pagano M., Boulaire J., Barchi M., Busino L., Donzelli M., Margottin-Goguet F., Jackson P.K., Yamasaki L., Pagano M., Barchi M., Busino L., Donzelli M., Margottin-Goguet F., Jackson P.K., Yamasaki L., Pagano M., Busino L., Donzelli M., Margottin-Goguet F., Jackson P.K., Yamasaki L., Pagano M., Donzelli M., Margottin-Goguet F., Jackson P.K., Yamasaki L., Pagano M., Margottin-Goguet F., Jackson P.K., Yamasaki L., Pagano M., Jackson P.K., Yamasaki L., Pagano M., Yamasaki L., Pagano M., Pagano M. Control of meiotic and mitotic progression by the F box protein β-Trcp1 in vivo. Dev. Cell. 2003;4:799–812. doi: 10.1016/s1534-5807(03)00154-0. [DOI] [PubMed] [Google Scholar]
- Hansen D.V., Loktev A.V., Ban K.H., Jackson P.K., Loktev A.V., Ban K.H., Jackson P.K., Ban K.H., Jackson P.K., Jackson P.K. Plk1 regulates activation of the anaphase promoting complex by phosphorylating and triggering SCFβTrCP-dependent destruction of the APC Inhibitor Emi1. Mol. Biol. Cell. 2004;15:5623–5634. doi: 10.1091/mbc.E04-07-0598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendzel M.J., Wei Y., Mancini M.A., Van Hooser A., Ranalli T., Brinkley B.R., Bazett-Jones D.P., Allis C.D., Wei Y., Mancini M.A., Van Hooser A., Ranalli T., Brinkley B.R., Bazett-Jones D.P., Allis C.D., Mancini M.A., Van Hooser A., Ranalli T., Brinkley B.R., Bazett-Jones D.P., Allis C.D., Van Hooser A., Ranalli T., Brinkley B.R., Bazett-Jones D.P., Allis C.D., Ranalli T., Brinkley B.R., Bazett-Jones D.P., Allis C.D., Brinkley B.R., Bazett-Jones D.P., Allis C.D., Bazett-Jones D.P., Allis C.D., Allis C.D. Mitosis-specific phosphorylation of histone H3 initiates primarily within pericentromeric heterochromatin during G2 and spreads in an ordered fashion coincident with mitotic chromosome condensation. Chromosoma. 1997;106:348–360. doi: 10.1007/s004120050256. [DOI] [PubMed] [Google Scholar]
- Hsu J.Y., Reimann J.D., Sorensen C.S., Lukas J., Jackson P.K., Reimann J.D., Sorensen C.S., Lukas J., Jackson P.K., Sorensen C.S., Lukas J., Jackson P.K., Lukas J., Jackson P.K., Jackson P.K. E2F-dependent accumulation of hEmi1 regulates S phase entry by inhibiting APC(Cdh1) Nat. Cell Biol. 2002;4:358–366. doi: 10.1038/ncb785. [DOI] [PubMed] [Google Scholar]
- Irniger S., Nasmyth K., Nasmyth K. The anaphase-promoting complex is required in G1 arrested yeast cells to inhibit B-type cyclin accumulation and to prevent uncontrolled entry into S-phase. J. Cell Sci. 1997;110:1523–1531. doi: 10.1242/jcs.110.13.1523. [DOI] [PubMed] [Google Scholar]
- Itzhaki J.E., Gilbert C.S., Porter A.C., Gilbert C.S., Porter A.C., Porter A.C. Construction by gene targeting in human cells of a ‘conditional’ CDC2 mutant that rereplicates its DNA. Nat. Genet. 1997;15:258–265. doi: 10.1038/ng0397-258. [DOI] [PubMed] [Google Scholar]
- Jiang W., Wells N.J., Hunter T., Wells N.J., Hunter T., Hunter T. Multistep regulation of DNA replication by Cdk phosphorylation of HsCdc6. Proc. Natl. Acad. Sci. 1999;96:6193–6198. doi: 10.1073/pnas.96.11.6193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J., Cardozo T., Lovering R.C., Elledge S.J., Pagano M., Harper J.W., Cardozo T., Lovering R.C., Elledge S.J., Pagano M., Harper J.W., Lovering R.C., Elledge S.J., Pagano M., Harper J.W., Elledge S.J., Pagano M., Harper J.W., Pagano M., Harper J.W., Harper J.W. Systematic analysis and nomenclature of mammalian F-box proteins. Genes & Dev. 2004;18:2573–2580. doi: 10.1101/gad.1255304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakaidos P., Taraviras S., Vassiliou L.V., Zacharatos P., Kastrinakis N.G., Kougiou D., Kouloukoussa M., Nishitani H., Papavassiliou A.G., Lygerou Z., Taraviras S., Vassiliou L.V., Zacharatos P., Kastrinakis N.G., Kougiou D., Kouloukoussa M., Nishitani H., Papavassiliou A.G., Lygerou Z., Vassiliou L.V., Zacharatos P., Kastrinakis N.G., Kougiou D., Kouloukoussa M., Nishitani H., Papavassiliou A.G., Lygerou Z., Zacharatos P., Kastrinakis N.G., Kougiou D., Kouloukoussa M., Nishitani H., Papavassiliou A.G., Lygerou Z., Kastrinakis N.G., Kougiou D., Kouloukoussa M., Nishitani H., Papavassiliou A.G., Lygerou Z., Kougiou D., Kouloukoussa M., Nishitani H., Papavassiliou A.G., Lygerou Z., Kouloukoussa M., Nishitani H., Papavassiliou A.G., Lygerou Z., Nishitani H., Papavassiliou A.G., Lygerou Z., Papavassiliou A.G., Lygerou Z., Lygerou Z., et al. Overexpression of the replication licensing regulators hCdt1 and hCdc6 characterizes a subset of non-small-cell lung carcinomas: Synergistic effect with mutant p53 on tumor growth and chromosomal instability—Evidence of E2F-1 transcriptional control over hCdt1. Am. J. Pathol. 2004;165:1351–1365. doi: 10.1016/S0002-9440(10)63393-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H., Stewart E., Poon R., Adamczewski J.P., Gannon J., Hunt T., Stewart E., Poon R., Adamczewski J.P., Gannon J., Hunt T., Poon R., Adamczewski J.P., Gannon J., Hunt T., Adamczewski J.P., Gannon J., Hunt T., Gannon J., Hunt T., Hunt T. Identification of the domains in cyclin A required for binding to, and activation of, p34cdc2 and p32cdk2 protein kinase subunits. Mol. Biol. Cell. 1992;3:1279–1294. doi: 10.1091/mbc.3.11.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H., Lee D.J., Oh S.P., Park H.D., Nam H.H., Kim J.M., Lim D.S., Lee D.J., Oh S.P., Park H.D., Nam H.H., Kim J.M., Lim D.S., Oh S.P., Park H.D., Nam H.H., Kim J.M., Lim D.S., Park H.D., Nam H.H., Kim J.M., Lim D.S., Nam H.H., Kim J.M., Lim D.S., Kim J.M., Lim D.S., Lim D.S. Mouse emi1 has an essential function in mitotic progression during early embryogenesis. Mol. Cell. Biol. 2006;26:5373–5381. doi: 10.1128/MCB.00043-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman N.L., Verschuren E.W., Hsu J.Y., Cherry A.M., Jackson P.K., Verschuren E.W., Hsu J.Y., Cherry A.M., Jackson P.K., Hsu J.Y., Cherry A.M., Jackson P.K., Cherry A.M., Jackson P.K., Jackson P.K. Overexpression of the anaphase promoting complex/cyclosome inhibitor Emi1 leads to tetraploidy and genomic instability of p53-deficient cells. Cell Cycle. 2006;5:1569–1573. doi: 10.4161/cc.5.14.2925. [DOI] [PubMed] [Google Scholar]
- Liu E., Li X., Yan F., Zhao Q., Wu X., Li X., Yan F., Zhao Q., Wu X., Yan F., Zhao Q., Wu X., Zhao Q., Wu X., Wu X. Cyclin-dependent kinases phosphorylate human Cdt1 and induce its degradation. J. Biol. Chem. 2004;279:17283–17288. doi: 10.1074/jbc.C300549200. [DOI] [PubMed] [Google Scholar]
- Lukas C., Sorensen C.S., Kramer E., Santoni-Rugiu E., Lindeneg C., Peters J.M., Bartek J., Lukas J., Sorensen C.S., Kramer E., Santoni-Rugiu E., Lindeneg C., Peters J.M., Bartek J., Lukas J., Kramer E., Santoni-Rugiu E., Lindeneg C., Peters J.M., Bartek J., Lukas J., Santoni-Rugiu E., Lindeneg C., Peters J.M., Bartek J., Lukas J., Lindeneg C., Peters J.M., Bartek J., Lukas J., Peters J.M., Bartek J., Lukas J., Bartek J., Lukas J., Lukas J. Accumulation of cyclin B1 requires E2F and cyclin-A-dependent rearrangement of the anaphase-promoting complex. Nature. 1999;401:815–818. doi: 10.1038/44611. [DOI] [PubMed] [Google Scholar]
- Machida Y.J., Hamlin J.L., Dutta A., Hamlin J.L., Dutta A., Dutta A. Right place, right time, and only once: Replication initiation in metazoans. Cell. 2005a;123:13–24. doi: 10.1016/j.cell.2005.09.019. [DOI] [PubMed] [Google Scholar]
- Machida Y.J., Teer J.K., Dutta A., Teer J.K., Dutta A., Dutta A. Acute reduction of an origin recognition complex (ORC) subunit in human cells reveals a requirement of ORC for Cdk2 activation. J. Biol. Chem. 2005b;280:27624–27630. doi: 10.1074/jbc.M502615200. [DOI] [PubMed] [Google Scholar]
- Machida Y.J., Chen Y., Machida Y., Malhotra A., Sarkar S., Dutta A., Chen Y., Machida Y., Malhotra A., Sarkar S., Dutta A., Machida Y., Malhotra A., Sarkar S., Dutta A., Malhotra A., Sarkar S., Dutta A., Sarkar S., Dutta A., Dutta A. Targeted comparative RNA interference analysis reveals differential requirement of genes essential for cell proliferation. Mol. Biol. Cell. 2006;17:4837–4845. doi: 10.1091/mbc.E06-04-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magda Pinyol I.S., Silvia B., Fernandez V., Colomo L., Campo E., Jares E., Silvia B., Fernandez V., Colomo L., Campo E., Jares E., Fernandez V., Colomo L., Campo E., Jares E., Colomo L., Campo E., Jares E., Campo E., Jares E., Jares E. Unbalanced expression of licensing DNA replication factors occurs in a subset of mantle cell lymphomas with genomic instability. Int. J. Cancer. 2006;119:2768–2774. doi: 10.1002/ijc.22146. [DOI] [PubMed] [Google Scholar]
- Mailand N., Diffley J.F., Diffley J.F. CDKs promote DNA replication origin licensing in human cells by protecting Cdc6 from APC/C-dependent proteolysis. Cell. 2005;122:915–926. doi: 10.1016/j.cell.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Margottin-Goguet F., Hsu J.Y., Loktev A., Hsieh H.M., Reimann J.D., Jackson P.K., Hsu J.Y., Loktev A., Hsieh H.M., Reimann J.D., Jackson P.K., Loktev A., Hsieh H.M., Reimann J.D., Jackson P.K., Hsieh H.M., Reimann J.D., Jackson P.K., Reimann J.D., Jackson P.K., Jackson P.K. Prophase destruction of Emi1 by the SCF(βTrCP/Slimb) ubiquitin ligase activates the anaphase promoting complex to allow progression beyond prometaphase. Dev. Cell. 2003;4:813–826. doi: 10.1016/s1534-5807(03)00153-9. [DOI] [PubMed] [Google Scholar]
- McGarry T.J., Kirschner M.W., Kirschner M.W. Geminin, an inhibitor of DNA replication, is degraded during mitosis. Cell. 1998;93:1043–1053. doi: 10.1016/s0092-8674(00)81209-x. [DOI] [PubMed] [Google Scholar]
- Melixetian M., Ballabeni A., Masiero L., Gasparini P., Zamponi R., Bartek J., Lukas J., Helin K., Ballabeni A., Masiero L., Gasparini P., Zamponi R., Bartek J., Lukas J., Helin K., Masiero L., Gasparini P., Zamponi R., Bartek J., Lukas J., Helin K., Gasparini P., Zamponi R., Bartek J., Lukas J., Helin K., Zamponi R., Bartek J., Lukas J., Helin K., Bartek J., Lukas J., Helin K., Lukas J., Helin K., Helin K. Loss of Geminin induces rereplication in the presence of functional p53. J. Cell Biol. 2004;165:473–482. doi: 10.1083/jcb.200403106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihaylov I.S., Kondo T., Jones L., Ryzhikov S., Tanaka J., Zheng J., Higa L.A., Minamino N., Cooley L., Zhang H., Kondo T., Jones L., Ryzhikov S., Tanaka J., Zheng J., Higa L.A., Minamino N., Cooley L., Zhang H., Jones L., Ryzhikov S., Tanaka J., Zheng J., Higa L.A., Minamino N., Cooley L., Zhang H., Ryzhikov S., Tanaka J., Zheng J., Higa L.A., Minamino N., Cooley L., Zhang H., Tanaka J., Zheng J., Higa L.A., Minamino N., Cooley L., Zhang H., Zheng J., Higa L.A., Minamino N., Cooley L., Zhang H., Higa L.A., Minamino N., Cooley L., Zhang H., Minamino N., Cooley L., Zhang H., Cooley L., Zhang H., Zhang H. Control of DNA replication and chromosome ploidy by geminin and cyclin A. Mol. Cell. Biol. 2002;22:1868–1880. doi: 10.1128/MCB.22.6.1868-1880.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moshe Y., Boulaire J., Pagano M., Hershko A., Boulaire J., Pagano M., Hershko A., Pagano M., Hershko A., Hershko A. Role of Polo-like kinase in the degradation of early mitotic inhibitor 1, a regulator of the anaphase promoting complex/cyclosome. Proc. Natl. Acad. Sci. 2004;101:7937–7942. doi: 10.1073/pnas.0402442101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen V.Q., Co C., Li J.J., Co C., Li J.J., Li J.J. Cyclin-dependent kinases prevent DNA re-replication through multiple mechanisms. Nature. 2001;411:1068–1073. doi: 10.1038/35082600. [DOI] [PubMed] [Google Scholar]
- Nishitani H., Sugimoto N., Roukos V., Nakanishi Y., Saijo M., Obuse C., Tsurimoto T., Nakayama K.I., Nakayama K., Fujita M., Sugimoto N., Roukos V., Nakanishi Y., Saijo M., Obuse C., Tsurimoto T., Nakayama K.I., Nakayama K., Fujita M., Roukos V., Nakanishi Y., Saijo M., Obuse C., Tsurimoto T., Nakayama K.I., Nakayama K., Fujita M., Nakanishi Y., Saijo M., Obuse C., Tsurimoto T., Nakayama K.I., Nakayama K., Fujita M., Saijo M., Obuse C., Tsurimoto T., Nakayama K.I., Nakayama K., Fujita M., Obuse C., Tsurimoto T., Nakayama K.I., Nakayama K., Fujita M., Tsurimoto T., Nakayama K.I., Nakayama K., Fujita M., Nakayama K.I., Nakayama K., Fujita M., Nakayama K., Fujita M., Fujita M., et al. Two E3 ubiquitin ligases, SCF–Skp2 and DDB1–Cul4, target human Cdt1 for proteolysis. EMBO J. 2006;25:1126–1136. doi: 10.1038/sj.emboj.7601002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ory D.S., Neugeboren B.A., Mulligan R.C., Neugeboren B.A., Mulligan R.C., Mulligan R.C. A stable human-derived packaging cell line for production of high titer retrovirus/vesicular stomatitis virus G pseudotypes. Proc. Natl. Acad. Sci. 1996;93:11400–11406. doi: 10.1073/pnas.93.21.11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pear W.S., Nolan G.P., Scott M.L., Baltimore D., Nolan G.P., Scott M.L., Baltimore D., Scott M.L., Baltimore D., Baltimore D. Production of high-titer helper-free retroviruses by transient transfection. Proc. Natl. Acad. Sci. 1993;90:8392–8396. doi: 10.1073/pnas.90.18.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen B.O., Lukas J., Sorensen C.S., Bartek J., Helin K., Lukas J., Sorensen C.S., Bartek J., Helin K., Sorensen C.S., Bartek J., Helin K., Bartek J., Helin K., Helin K. Phosphorylation of mammalian CDC6 by cyclin A/CDK2 regulates its subcellular localization. EMBO J. 1999;18:396–410. doi: 10.1093/emboj/18.2.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfleger C.M., Kirschner M.W., Kirschner M.W. The KEN box: An APC recognition signal distinct from the D box targeted by Cdh1. Genes & Dev. 2000;14:655–665. [PMC free article] [PubMed] [Google Scholar]
- Pulsipher M., Kupfer G.M., Naf D., Suliman A., Lee J.S., Jakobs P., Grompe M., Joenje H., Sieff C., Guinan E., Kupfer G.M., Naf D., Suliman A., Lee J.S., Jakobs P., Grompe M., Joenje H., Sieff C., Guinan E., Naf D., Suliman A., Lee J.S., Jakobs P., Grompe M., Joenje H., Sieff C., Guinan E., Suliman A., Lee J.S., Jakobs P., Grompe M., Joenje H., Sieff C., Guinan E., Lee J.S., Jakobs P., Grompe M., Joenje H., Sieff C., Guinan E., Jakobs P., Grompe M., Joenje H., Sieff C., Guinan E., Grompe M., Joenje H., Sieff C., Guinan E., Joenje H., Sieff C., Guinan E., Sieff C., Guinan E., Guinan E., et al. Subtyping analysis of Fanconi anemia by immunoblotting and retroviral gene transfer. Mol. Med. 1998;4:468–479. [PMC free article] [PubMed] [Google Scholar]
- Reimann J.D., Freed E., Hsu J.Y., Kramer E.R., Peters J.M., Jackson P.K., Freed E., Hsu J.Y., Kramer E.R., Peters J.M., Jackson P.K., Hsu J.Y., Kramer E.R., Peters J.M., Jackson P.K., Kramer E.R., Peters J.M., Jackson P.K., Peters J.M., Jackson P.K., Jackson P.K. Emi1 is a mitotic regulator that interacts with Cdc20 and inhibits the anaphase promoting complex. Cell. 2001;105:645–655. doi: 10.1016/s0092-8674(01)00361-0. [DOI] [PubMed] [Google Scholar]
- Reynolds A., Leake D., Boese Q., Scaringe S., Marshall W.S., Khvorova A., Leake D., Boese Q., Scaringe S., Marshall W.S., Khvorova A., Boese Q., Scaringe S., Marshall W.S., Khvorova A., Scaringe S., Marshall W.S., Khvorova A., Marshall W.S., Khvorova A., Khvorova A. Rational siRNA design for RNA interference. Nat. Biotechnol. 2004;22:326–330. doi: 10.1038/nbt936. [DOI] [PubMed] [Google Scholar]
- Saha P., Chen J., Thome K.C., Lawlis S.J., Hou Z.H., Hendricks M., Parvin J.D., Dutta A., Chen J., Thome K.C., Lawlis S.J., Hou Z.H., Hendricks M., Parvin J.D., Dutta A., Thome K.C., Lawlis S.J., Hou Z.H., Hendricks M., Parvin J.D., Dutta A., Lawlis S.J., Hou Z.H., Hendricks M., Parvin J.D., Dutta A., Hou Z.H., Hendricks M., Parvin J.D., Dutta A., Hendricks M., Parvin J.D., Dutta A., Parvin J.D., Dutta A., Dutta A. Human CDC6/Cdc18 associates with Orc1 and cyclin–cdk and is selectively eliminated from the nucleus at the onset of S phase. Mol. Cell. Biol. 1998;18:2758–2767. doi: 10.1128/mcb.18.5.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shreeram S., Sparks A., Lane D.P., Blow J.J., Sparks A., Lane D.P., Blow J.J., Lane D.P., Blow J.J., Blow J.J. Cell type-specific responses of human cells to inhibition of replication licensing. Oncogene. 2002;21:6624–6632. doi: 10.1038/sj.onc.1205910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen C.S., Lukas C., Kramer E.R., Peters J.M., Bartek J., Lukas J., Lukas C., Kramer E.R., Peters J.M., Bartek J., Lukas J., Kramer E.R., Peters J.M., Bartek J., Lukas J., Peters J.M., Bartek J., Lukas J., Bartek J., Lukas J., Lukas J. Nonperiodic activity of the human anaphase-promoting complex–Cdh1 ubiquitin ligase results in continuous DNA synthesis uncoupled from mitosis. Mol. Cell. Biol. 2000;20:7613–7623. doi: 10.1128/mcb.20.20.7613-7623.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen C.S., Lukas C., Kramer E.R., Peters J.M., Bartek J., Lukas J., Lukas C., Kramer E.R., Peters J.M., Bartek J., Lukas J., Kramer E.R., Peters J.M., Bartek J., Lukas J., Peters J.M., Bartek J., Lukas J., Bartek J., Lukas J., Lukas J. A conserved cyclin-binding domain determines functional interplay between anaphase-promoting complex–Cdh1 and cyclin A–Cdk2 during cell cycle progression. Mol. Cell. Biol. 2001;21:3692–3703. doi: 10.1128/MCB.21.11.3692-3703.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudo T., Ota Y., Kotani S., Nakao M., Takami Y., Takeda S., Saya H., Ota Y., Kotani S., Nakao M., Takami Y., Takeda S., Saya H., Kotani S., Nakao M., Takami Y., Takeda S., Saya H., Nakao M., Takami Y., Takeda S., Saya H., Takami Y., Takeda S., Saya H., Takeda S., Saya H., Saya H. Activation of Cdh1-dependent APC is required for G1 cell cycle arrest and DNA damage-induced G2 checkpoint in vertebrate cells. EMBO J. 2001;20:6499–6508. doi: 10.1093/emboj/20.22.6499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto N., Tatsumi Y., Tsurumi T., Matsukage A., Kiyono T., Nishitani H., Fujita M., Tatsumi Y., Tsurumi T., Matsukage A., Kiyono T., Nishitani H., Fujita M., Tsurumi T., Matsukage A., Kiyono T., Nishitani H., Fujita M., Matsukage A., Kiyono T., Nishitani H., Fujita M., Kiyono T., Nishitani H., Fujita M., Nishitani H., Fujita M., Fujita M. Cdt1 phosphorylation by cyclin A-dependent kinases negatively regulates its function without affecting geminin binding. J. Biol. Chem. 2004;279:19691–19697. doi: 10.1074/jbc.M313175200. [DOI] [PubMed] [Google Scholar]
- Tada S., Li A., Maiorano D., Mechali M., Blow J.J., Li A., Maiorano D., Mechali M., Blow J.J., Maiorano D., Mechali M., Blow J.J., Mechali M., Blow J.J., Blow J.J. Repression of origin assembly in metaphase depends on inhibition of RLF-B/Cdt1 by geminin. Nat. Cell Biol. 2001;3:107–113. doi: 10.1038/35055000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda D.Y., Parvin J.D., Dutta A., Parvin J.D., Dutta A., Dutta A. Degradation of Cdt1 during S phase is Skp2-independent and is required for efficient progression of mammalian cells through S phase. J. Biol. Chem. 2005;280:23416–23423. doi: 10.1074/jbc.M501208200. [DOI] [PubMed] [Google Scholar]
- Vaziri C., Saxena S., Jeon Y., Lee C., Murata K., Machida Y., Wagle N., Hwang D.S., Dutta A., Saxena S., Jeon Y., Lee C., Murata K., Machida Y., Wagle N., Hwang D.S., Dutta A., Jeon Y., Lee C., Murata K., Machida Y., Wagle N., Hwang D.S., Dutta A., Lee C., Murata K., Machida Y., Wagle N., Hwang D.S., Dutta A., Murata K., Machida Y., Wagle N., Hwang D.S., Dutta A., Machida Y., Wagle N., Hwang D.S., Dutta A., Wagle N., Hwang D.S., Dutta A., Hwang D.S., Dutta A., Dutta A. A p53-dependent checkpoint pathway prevents rereplication. Mol. Cell. 2003;11:997–1008. doi: 10.1016/s1097-2765(03)00099-6. [DOI] [PubMed] [Google Scholar]
- Wilmes G.M., Archambault V., Austin R.J., Jacobson M.D., Bell S.P., Cross F.R., Archambault V., Austin R.J., Jacobson M.D., Bell S.P., Cross F.R., Austin R.J., Jacobson M.D., Bell S.P., Cross F.R., Jacobson M.D., Bell S.P., Cross F.R., Bell S.P., Cross F.R., Cross F.R. Interaction of the S-phase cyclin Clb5 with an ‘RXL’ docking sequence in the initiator protein Orc6 provides an origin-localized replication control switch. Genes & Dev. 2004;18:981–991. doi: 10.1101/gad.1202304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlschlegel J.A., Dwyer B.T., Dhar S.K., Cvetic C., Walter J.C., Dutta A., Dwyer B.T., Dhar S.K., Cvetic C., Walter J.C., Dutta A., Dhar S.K., Cvetic C., Walter J.C., Dutta A., Cvetic C., Walter J.C., Dutta A., Walter J.C., Dutta A., Dutta A. Inhibition of eukaryotic DNA replication by geminin binding to Cdt1. Science. 2000;290:2309–2312. doi: 10.1126/science.290.5500.2309. [DOI] [PubMed] [Google Scholar]
- Wohlschlegel J.A., Kutok J.L., Weng A.P., Dutta A., Kutok J.L., Weng A.P., Dutta A., Weng A.P., Dutta A., Dutta A. Expression of geminin as a marker of cell proliferation in normal tissues and malignancies. Am. J. Pathol. 2002;161:267–273. doi: 10.1016/S0002-9440(10)64178-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G.J., Safran M., Wei W., Sorensen E., Lassota P., Zhelev N., Neuberg D.S., Shapiro G., Kaelin W.G., Jr., Safran M., Wei W., Sorensen E., Lassota P., Zhelev N., Neuberg D.S., Shapiro G., Kaelin W.G., Jr., Wei W., Sorensen E., Lassota P., Zhelev N., Neuberg D.S., Shapiro G., Kaelin W.G., Jr., Sorensen E., Lassota P., Zhelev N., Neuberg D.S., Shapiro G., Kaelin W.G., Jr., Lassota P., Zhelev N., Neuberg D.S., Shapiro G., Kaelin W.G., Jr., Zhelev N., Neuberg D.S., Shapiro G., Kaelin W.G., Jr., Neuberg D.S., Shapiro G., Kaelin W.G., Jr., Shapiro G., Kaelin W.G., Jr., Kaelin W.G., Jr. Bioluminescent imaging of Cdk2 inhibition in vivo. Nat. Med. 2004;10:643–648. doi: 10.1038/nm1047. [DOI] [PubMed] [Google Scholar]
- Zhu W., Chen Y., Dutta A., Chen Y., Dutta A., Dutta A. Rereplication by depletion of geminin is seen regardless of p53 status and activates a G2/M checkpoint. Mol. Cell. Biol. 2004;24:7140–7150. doi: 10.1128/MCB.24.16.7140-7150.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]