Isolation of primitive human hematopoietic progenitors on the basis of aldehyde dehydrogenase activity (original) (raw)
Abstract
Because hematopoietic stem cells are rich in aldehyde dehydrogenase (ALDH) activity, we developed a fluorescent substrate for ALDH, termed BODIPY aminoacetaldehyde (BAAA), and tested its potential for isolating primitive human hematopoietic cells. A population of cells with low orthogonal light scattering and bright fluorescence intensity (SSCloALDHbr cells) could be readily fractionated from human umbilical cord blood cells costained with BAAA and the multidrug-resistance inhibitor verapamil. The SSCloALDHbr population was depleted of lineage-committed cells, 40–90% pure for CD34+CD38lo/− cells, and enriched 50- to 100-fold for primitive hematopoietic progenitors detected in short- and long-term culture analyses. Together, these observations indicate that fractionating human hematopoietic stem cells on the basis of ALDH activity using BAAA is an effective method for isolating primitive human hematopoietic progenitors. This technique may be useful for isolating stem cells from other tissues as well.
The hematopoietic system is continually renewed from hematopoietic stem cells (HSCs) and progenitor cells (1). To isolate HSCs and other progenitor cells, a variety of purification strategies have been developed. Most commonly, cells have been fractionated based on the expression of cell surface markers such as Sca-1, thy-1, CD34, CD38, HLA-DR, and c-kit (2–8). Fractionations based on size and cell density, efflux of metabolic dyes, or resistance to cytotoxic agents also have been used (9–14). Although these strategies have been useful for studying hematopoiesis and for some clinical applications, they suffer from several drawbacks. Many of the HSC characteristics have been found to vary between species, genetic background, or stem cell source (1, 15). Cell populations that have been isolated based on many of these criteria have been heterogeneous, with significant overlap between HSCs and more mature progenitors (16). In addition, the phenotype of cultured or transplanted stem cells may remain stable despite a decline in functional activity (17, 18). Populations with the phenotypic characteristics of HSCs have been isolated that are not able to reconstitute hematopoiesis (19). CD34 and other markers may be found only on some, but not all, HSCs (5, 20–22). Finally, many of the stem cell isolation procedures described to date involve the use of multiple steps, reagents, and costly procedures that do not lend themselves to clinical applications.
Because of these issues, we have been searching for simpler and more reliable methods for isolating primitive human hematopoietic cells. One potential strategy is to develop a fluorescent substrate for an enzyme that is expressed at relatively high levels in stem cells compared with more differentiated cells. Fluorescence-activated cell sorting (FACS) then could be used to isolate the brightest staining cells. One candidate enzyme for such a strategy is aldehyde dehydrogenase (ALDH) (23). ALDH is a cytosolic enzyme that is responsible for oxidizing a variety of aldehydes including vitamin A (24, 25). ALDH is expressed at high levels in hematopoietic progenitors and appears to be responsible for the resistance of HSCs to the alkylating agents cyclophosphamide and 4-hydroxyperoxycyclophosphamide (10, 26–28). Jones et al. (22, 29) demonstrated proof of the principle for this strategy in the murine system. They showed that mouse HSCs could be isolated by staining partially purified bone marrow stem cells with a fluorescent substrate for ALDH, termed dansyl-aminoacetaldehyde (DAAA), and then selecting cells with the highest fluorescence intensity. Although provocative, this approach suffers from several drawbacks. To yield highly purified stem cells, it appeared necessary to combine DAAA staining with several other fractionation steps. In addition, DAAA fluorescence is excited by UV light, which could be mutagenic to cells. The emission spectra of DAAA overlaps with that of other fluorochromes so that DAAA staining cannot be readily combined with other stem cell markers. Last, this method has not been shown to be useful for isolating primitive human HSCs.
Because of these limitations, we designed a fluorescent substrate for ALDH, termed BODIPY aminoacetaldehyde (BAAA), that consists of an aminoacetaldehyde moiety bonded to the BODIPY fluorochrome. We report here that staining with BAAA provides a simple and efficient strategy for isolating primitive human hematopoietic cells.
MATERIALS AND METHODS
Cell Lines.
K562 (American Type Culture Collection), L1210, and L1210/cpa cells (provided by John Hilton, Johns Hopkins Oncology Center, Baltimore) were maintained in suspension in RPMI media 1640 supplemented with 10% FCS and 5 × 10−5 M β-mercaptoethanol (30).
Preparation of Human Umbilical Cord Blood (UCB).
Human UCB was obtained as discarded material from the Department of Obstetrics and Gynecology of the Duke University Medical Center through an Institutional Review Board-approved protocol. Red cells were depleted by diluting UCB with Dulbecco’s PBS/1% Hespan (DuPont) at room temperature. After 1 hr, nonagglutinated cells were harvested, and residual red cells were hemolysed at 37°C in 0.17 M NH4Cl/20 mM Tris⋅HCl/200 μM EDTA, pH 7.2. The recovered cells were washed in Iscove’s modified Dulbecco’s medium/2% FCS. Mononuclear cells then were purified over Ficoll-Hypaque (1.077 g/ml).
Synthesis and Preparation of BAAA.
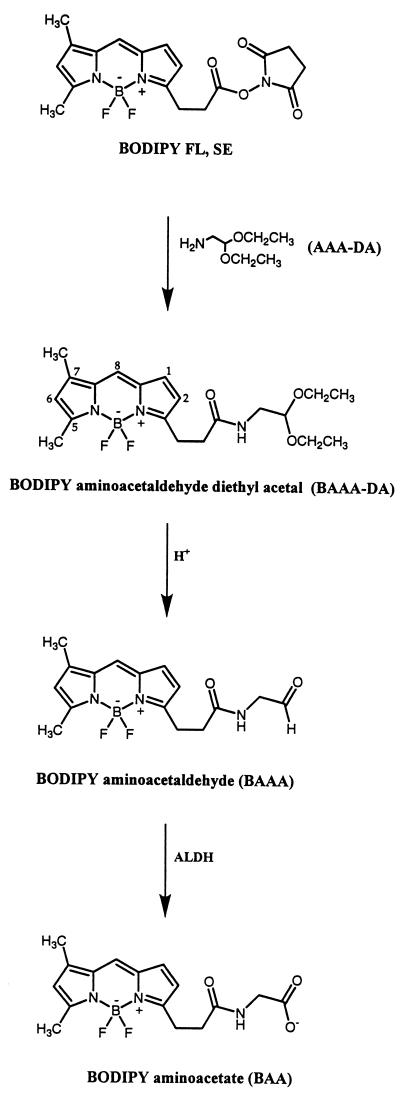
Using an amber vial, a solution of aminoacetaldehyde diethyl acetal (AAA-DA, 19 μmol, 2.8 μl, Aldrich) in dry tetrahydrofuran (THF, 0.1 ml) was added dropwise to a solution of 4,4-difluoro-5,7-dimethyl-4-bora-3a,4a-diaza-s-indacene-3-proprionic acid, succinimidyl ester (BODIPY FL,SE) (13 μmol, 5 mg, Molecular Probes D-2184) in dry THF (0.5 ml). Upon complete addition, the vial was capped and the reaction mixture was stirred (30 min) in the dark. The THF was evaporated, the residue was dissolved in minimal methylene chloride and then filtered through a pipette filled with silica gel (230–400 mesh) by using ethyl acetate/hexane (1:1) as eluent. The product (Rf 0.26), BODIPY-aminoacetaldehyde diethyl acetal (BAAA-DA) (Fig. 1), was recovered in quantitative yield as an orange solid and stored at −20°C. 1H NMR (400 MHz, CDCl3) δ7.08 and 6.12 (two s, 1 H each, C6-H and C8-H), 6.87 and 6.29 (two d, 3_J_ = 4.2 Hz for each, 1 H each, C1-H and C2-H), 5.82 (br t, 1 H, NH), 4.43 [t, 3_J_ = 5.4 Hz, 1 H, CH(OEt)2], 3.68, 3.66, 3.51, and 3.49 (four q, 3_J_ = 7.0 Hz for each, 1 H each, two OCH2), 3.37 (apparent t, J = 5.6 Hz, 2 H, CH2N), 3.28 (t, 3_J_ = 7.5 Hz, 2 H, CH2C=O), 2.64 (t, 3_J_ = 7.6 Hz, 2 H, CH2CH2C=O), 2.56 and 2.26 (two s, 3 H each, C5-CH3 and C7-CH3), and 1.18 (t, 3J = 7.0 Hz, 6 H, two CH2CH3). Proton NMR assignments were based on a 1H-1H two-dimensional homonuclear correlation NMR experiment at 400 MHz. The UV spectrum of BAAA-DA (1.26 × 10−5 M in acetone) gave λmax = 495 nm and an extinction coefficient of 74,595 M−1⋅cm−1.
Figure 1.
Structures of BAAA-DA, BAAA, and BAA. BAAA-DA was synthesized by the bonding of the fluorescent compound BODIPY FL,SE to AAA-DA. BAAA-DA is converted to BAAA in the presence of acid with a _t_1/2 of approximately 15 min under the conditions described in Materials and Methods. When cells are stained with BAAA, ALDH then converts BAAA into BAA, which is retained intracellularly because of its net negative charge. The structures of BAAA-DA and BAAA as well as the kinetics of conversion between these compounds were determined by 1H-NMR spectroscopy.
At least 2 hr before use, 0.5 μmoles of BAAA-DA was solubilized in 100 μl of DMSO then 20 μl of this solution was diluted with an equal volume of 2 N HCl to convert it to BAAA (Fig. 1). After 2 hr, the BAAA was diluted 10-fold with Dulbecco’s PBS. The buffered stock solution of BAAA was added directly to cells to reach a final concentration of 1–20 μM as described in the text.
Antibody Reagents.
Directly conjugated fluorescent antibodies directed against the following antigens were used: CD14 [Leu-M3; phycoerythrin (PE) and allophycocyanin (APC)], CD34 (HPCA2; PE and APC), CD38 (Leu-17; PE and APC), CD56 (neural cell adhesion molecule 16.2; PE), CD20 (Leu-16; PE), CD3 (Leu-4; PE) all from Becton Dickinson Immunocytometry Systems; CD7 (3A1, Coulter), and CD19 (J4.119, Immunotech, Westbrook, ME).
Cell Staining and FACS.
All cells were stained at densities of 106 cells/ml in Iscove’s modified Dulbecco’s medium with 2% FCS (staining medium). Based on preliminary titration experiments, cell lines were labeled with 20 μM BAAA, and mononuclear UCB cells were labeled with 1 μM BAAA for 30 min. To inhibit multidrug-resistance activity, verapamil (American Regent Laboratories, Shirley, NY) was included at a final concentration of 50 μM. For multiparameter FACS, additional antibody reagents were added directly to cell suspensions at 107 cells/ml in staining medium. To exclude dying cells, 7-aminoactinomycin D (Molecular Probes) was added at a final concentration of 10 μg/ml. Cells were incubated on ice for 20 min and then washed in ice-cold staining media. The cells then were analyzed on a FACSCalibur instrument equipped with 488-nM argon lasers (Becton Dickinson Immunocytometry Systems) or sorted on a FACStarPLUS cell sorter equipped with dual 488-nM Coherent I-90 lasers and an argon-dye laser for APC excitation (Becton Dickinson Immunocytometry Systems). The BODIPY moiety was excited at 488 nm, and its fluorescence was detected by using a standard FITC 530/30 bandpass filter.
Hematopoietic Progenitor Colony Assays (HPCAs) and Long-Term Cultures (LTCs).
UCB cells were stained with BAAA, verapamil, and anti-CD14 as described above, and then SSCloALDHbr CD14− cells were isolated by using the FACStarPLUS cell sorter with the SSClo and ALDHbr gates defined as in Fig. 3. Anti-CD14 was included in these experiments to exclude monocytes that contaminated the SSCloALDHbr cell-sorting gate. HPCAs were performed by plating 100–200 cells in MethoCult GF H4434 containing recombinant human kit ligand, IL-3, granulocyte/macrophage colony-stimulating factor, and erythropoietin (StemCell Technologies, Vancouver). The cells were incubated in a humidified chamber at 37°C with 5% CO2, and then hematopoietic colonies (>100 cells) were scored after 18 days. LTCs were maintained on murine MS-5 stromal cells (graciously provided by Tadashi Sudo, Kirin Pharmaceutical Research Laboratory, Gunma, Japan) (31). MS-5 stromal cells were seeded into 24-well plates (Corning) at 5 × 104 cells/well in MEMα/10% FCS and cultured at 37°C in 5% CO2. When the monolayers approached 80% confluence they were γ-irradiated from a cesium source (40 Gy). For LTCs, the culture media were replaced entirely with MEMα/10% FCS/10% equine serum/5 × 10−5 M β-mercaptoethanol/1 mM pyruvate. LTCs were initiated with 500–2,000 hematopoietic progenitor cells/well and were maintained at 33°C in 5% CO2. At weekly intervals, half the media from each well were removed and replaced with fresh media. Adherent and nonadherent cells were harvested after 5 or 8 weeks and were plated into HPCAs as described above. LTC activity was defined as the number of HPCAs developing after 5–8 weeks in stromal LTCs per 1,000 cells used to establish the cultures.
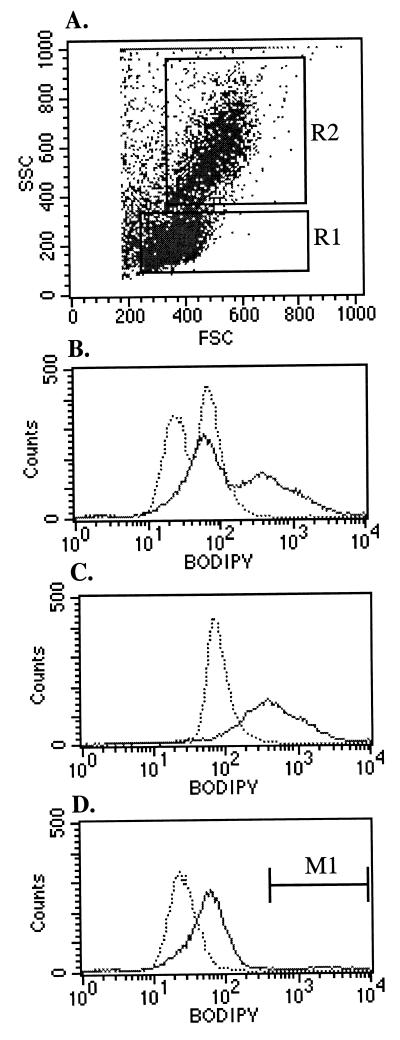
Figure 3.
Human UCB contains ALDHbr cells. Red cell-depleted UCB mononuclear cells were stained with 1 μM BAAA. (A) The scatter properties of stained UCB. Region R1 defines the SSClo population and R2 defines the monocyte population. (B_–_D) The BAAA staining profile of the total UCB, monocytes, and SSClo cells, respectively. The SSCloALDHbr cells are defined by the M1 cursor in D. In each panel the dotted line is the diethylaminobenzaldehyde-treated negative control and the solid line is the experimental sample. The data are representative of six experiments.
Statistics.
Statistical analysis was performed by using the paired two-tailed Student’s t test.
RESULTS
BAAA Is a Substrate for ALDH.
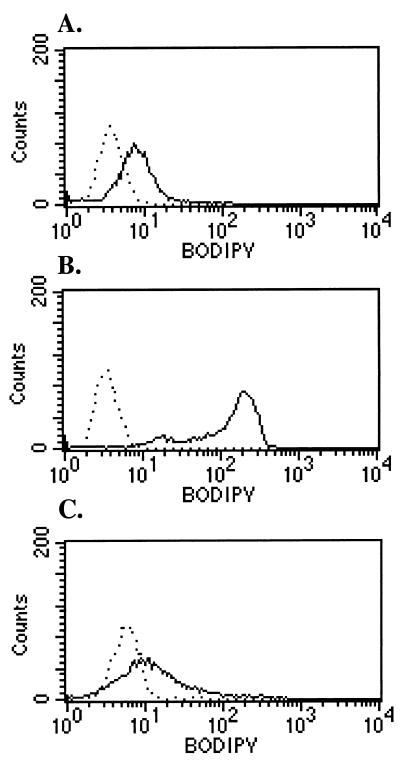
To develop a fluorescent substrate for ALDH, the fluorescent compound BODIPY FL,SE was reacted with AAA-DA to yield the stable compound BAAA-DA (Fig. 1). Immediately before cell staining, this compound was converted to the more labile ALDH substrate BAAA. We hypothesized that BAAA would freely diffuse into cells and then be converted by ALDH into a carboxylate ion (BODIPY amino acetate, BAA), which would be trapped intracellularly (Fig. 1). Consequently, cells expressing high levels of ALDH could be identified based on their bright fluorescence intensity. To test this hypothesis, murine L1210 cells and a cyclophosphamide-resistant derivative termed L1210/cpa were stained with BAAA and examined by flow cytometry (Fig. 2). L1210 cells express low levels of ALDH whereas the L1210/cpa cells express high levels of ALDH (30). Consistent with this, the L1210 cells exhibited dim staining with BAAA (Fig. 2A), whereas the L1210/cpa cells stained brightly with BAAA (Fig. 2B). In both lines, staining with BAAA was significantly inhibited by a 10-fold molar excess of diethylaminobenzaldehyde (DEAB), a specific inhibitor of ALDH (Fig. 2 A and B) (32). To test whether BAAA was a substrate for human ALDH, K562 cells were stained with BAAA (28, 33). K562 cells stained moderately with BAAA (Fig. 2C), and the staining was inhibited by DEAB (Fig. 2C), confirming that BAAA is a substrate for both murine and human ALDH.
Figure 2.
BAAA staining of L1210, L1210/cpa, and K562 cells. L1210 (A) is a murine cell line that expresses little ALDH. L1210/cpa (B) expresses high levels of ALDH, and K562 (C) is a human cell line that expresses ALDH. Cells were stained with 20 μM BAAA and analyzed as described in Materials and Methods. The data are representative of three experiments.
Cells Are Present in Human UCB That Stain Brightly With BAAA.
To determine whether BAAA could stain primary human hematopoietic cells, red cell-depleted UCB mononuclear cells were treated with BAAA and analyzed. UCB was chosen for these studies because of its increasing promise as a source of transplantable HSCs (34). An optimal concentration of 1 μM BAAA was found to best discriminate ALDHbr cells from more dimly staining populations (data not shown and Fig. 3 A and B). Staining was inhibited by DEAB, confirming that the fluorescence emission of the BAAA-stained UCB was the result of ALDH activity (Fig. 3B). The BAAA-stained UCB exhibited two distinct peaks of fluorescence emission (Fig. 3B). The brighter fluorescence emission peak was nearly entirely caused by monocytes (Fig. 3C and data not shown). Because the BODIPY fluorescence from monocytes obscured the signal from hematopoietic progenitor cells, the BAA signal was examined only in cells with low orthogonal light scattering (SSClo cells, defined by gate R1 in Fig. 3A) in all subsequent studies (35). In addition, because in preliminary experiments we found that BAA was a substrate for the high level of multidrug-resistance (MDR) efflux activity in primitive hematopoietic cells (data not shown), MDR was blocked with verapamil during the staining procedure to enhance the discrimination of primitive cells (36). With this approach, a small, but clearly defined, subpopulation of SSClo cells stained brightly with BAAA (SSCloALDHbr cells, defined by region M1 in Fig. 3D).
SSCloALDHbr UCB Cells Are Highly Enriched for Primitive Hematopoietic Cells.
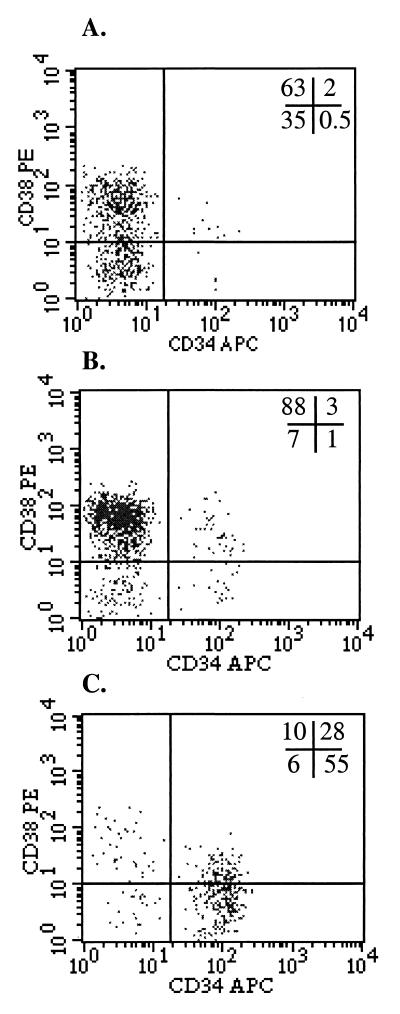
To determine whether the SSCloALDHbr UCB population was enriched for primitive hematopoietic progenitors, several strategies were pursued. First, the SSCloALDHbr population was examined for the presence of cells expressing markers indicative of commitment to a particular hematopoietic lineage. Other than a small number of B cells, the SSCloALDHbr population was largely depleted of cells with mature T cell, natural killer cell, myeloid, erythroid, and platelet lineage markers (Table 1). Next, the SSCloALDHbr population was examined for the presence of CD34+ as well as CD34+CD38lo/− cells, primitive populations of hematopoietic cells that typically comprise approximately 1% and <0.5%, respectively of the UCB (7, 37). The SSCloALDHbr UCB population contained 74 ± 20% (n = 11) CD34+ cells and 46 ± 22% (n = 6) CD34brCD38lo/− cells (Fig. 4C).
Table 1.
Analysis of SCClo ALDHbr UCB for the presence of mature lineage committed cells
| Cell population | Unfractionated UCB, % | SSClo ALDHbrUCB, % |
|---|---|---|
| CD56+ | 6 ± 5 | 0.3 ± 0.5 |
| CD3+ | 59.3 ± 27 | 2.6 ± 2.1 |
| CD19+ | 5.2 ± 4 | 11.8 ± 10 |
| CD20+ | 8.5 ± 4.6 | 5.5 ± 6.7 |
| CD14+ | 13.4 ± 11.1 | 1.8 ± 2 |
| CD33+ | 11.3 ± 8.1 | 2.1 ± 1 |
| E6+ | 10.4 ± 9.5 | 3.1 ± 3.2 |
Figure 4.
SSCloALDHbr UCB is enriched for CD34+CD38lo/− cells. UCB was costained with BAAA, verapamil, and antibodies to CD34 and CD38 and analyzed with FACS. (A) The CD34 and CD38 staining profile of unfractionated UCB. (B) The profile of SSClo cells. (C) The profile of SSCloALDHbr cells. The data are representative of six experiments.
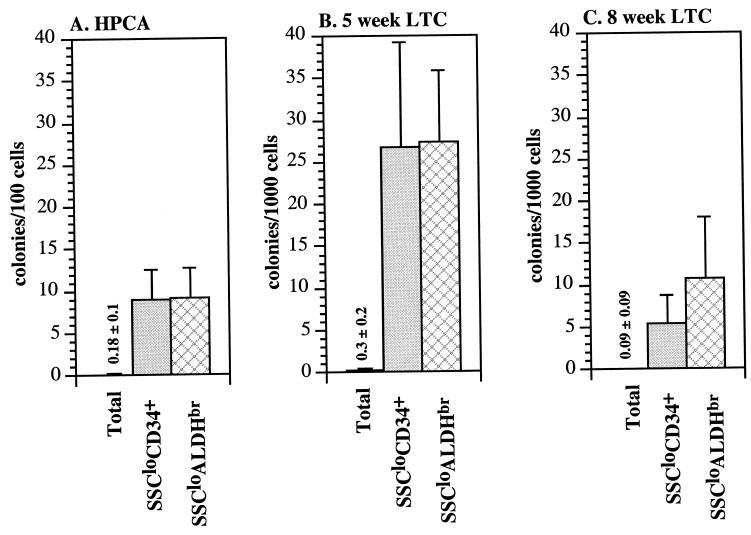
SSCloALDHbr cells then were isolated and placed into both short-term culture and LTC assays that detect primitive myeloerythroid progenitors. The short-term HPCA enumerates relatively mature myeloerythroid progenitors whereas the 5-week and 8-week LTC assays enumerate progressively more primitive progenitors (37). As controls, the SSCloALDHbr cells were compared with unfractionated UCB and to sorted CD34+ UCB cells. The CD34+ and SSCloALDHbr fractions both were enriched approximately 65-fold for HPCAs and nearly 100-fold for 5-week LTC cells compared with the unfractionated UCB (Fig. 5 A and B). In contrast, the SSCloALDHbr population was significantly more enriched in 8-week LTC activity (140-fold) than the CD34+ population (85-fold) (P < 0.05, n = 6, Fig. 5C) relative to unfractionated UCB. In summary, the phenotypic and functional assays demonstrate that the SSCloALDHbr population is highly enriched for primitive hematopoietic cells.
Figure 5.
SSCloALDHbr UCB is enriched in hematopoietic progenitors. Unfractionated, CD34+, and SSCloALDHbr UCB cells were isolated and placed into HPCA (A), 5-week LTC (B), and 8-week LTC (C) assays. As described in Materials and Methods, CD14+ cells also were removed during the isolation of the SSCloALDHbr population because these cells were the major contaminant in preliminary sorting experiments. The bar graphs demonstrate the mean (±SD) of six experiments. The difference in HPCA, 5-week LTC, and 8-week LTC was significantly different (P < 0.05) between unfractionated UCB and both the CD34+ and SSCloALDHbr populations. In addition, the proportion of 8-week LTCs was significantly higher (P < 0.05) in the SSCloALDHbr population compared with the CD34+ population whereas no differences were noted between the HPCA and 5-week LTC proportions.
DISCUSSION
In this report we describe the development of a fluorescent reagent, BAAA, which is a substrate for ALDH in both murine and human cell lines. When combined with inhibition of multidrug resistance, a population termed the SSCloALDHbr population could be readily fractionated from UCB mononuclear cells on the basis of high BAAA staining intensity and low orthogonal light scattering. This population was highly enriched for Lin− cells, CD34+CD38lo/- cells, and a variety of hematopoietic progenitors measured in short-term culture and LTC assays. In aggregate, these observations indicate that the SSCloALDHbr population contains primitive hematopoietic cells and may contain HSCs. This suggestion is supported by the previous findings that murine cells isolated on the basis of ALDH activity durably reconstituted hematopoiesis in mice (22). In addition, human hematopoiesis has been reconstituted by bone marrow cells treated with 4-hydroxyperoxycyclophosphamide (23, 33). Formal proof that SSCloALDHbr cells contain HSCs, however, will depend on demonstrating that either allogeneic or genetically marked SSCloALDHbr cells can durably reconstitute all hematopoietic lineages in human recipients.
There are several potential advantages to using BAAA staining for isolating primitive human hematopoietic cells compared with currently available methods. The BAAA staining method is simple and inexpensive and does not require large amounts of mAbs. In addition, BAA should not enter the nucleus or bind DNA, the fluorchrome is excited by visible light, and BAA appears to be rapidly effluxed once verapamil is removed (data not shown). Consequently, isolating primitive cells with BAAA staining may be safer and less toxic than methods based on staining with dyes that bind to nucleic acids or require UV excitation (20). Another advantage of BAAA staining is that the emission spectrum of BAA does not overlap significantly with other fluorochromes so that BAAA staining can be readily combined with additional markers. Last, because high-level expression of ALDH appears to be an intrinsic property of a variety of stem cells, isolating primitive hematopoietic cells on the basis of ALDH activity may not be affected as significantly by the stem cell source, genetic background, or stem cell manipulation as other stem cell isolation methods (23). Future preclinical studies, including transplantation into immunodeficient mice, as well as clinical trials will help determine whether staining for ALDH activity is superior to other methods for isolating primitive hematopoietic cells (38).
There are several potential applications to this strategy for identifying and isolating HSCs. Enumerating SSCloALDHbr cells may be a more reliable means for quantitating the transplantable stem cells in bone marrow, peripheral blood, and UCB (39). Isolating SSCloALDHbr cells also may be an effective method for purging autologous bone marrow or peripheral blood stem cell collections of tumor cells (23, 40). This method could replace the use of 4-hydroxyperoxycyclophosphamide for tumor purging because it results in less nonspecific cell toxicity and can be adjusted during the stem cell isolation procedure. Because T cells appear to be depleted during the isolation of SSCloALDHbr cells, the BAAA staining method may effectively remove the T cells capable of causing graft versus host disease in allogeneic transplant applications. This method also may be useful for evaluating tumor samples to determine whether they are resistant to cyclophosphamide and other oxazaphosphorines. Last, because ALDH is expressed in a wide variety of stem cells, the BAAA staining method may have broad applicability to isolating stem cells such as embryonic stem cells or gut stem cells for analytical and clinical purposes (23, 41).
Acknowledgments
We gratefully acknowledge Dr. Mike Cook of the Duke Comprehensive Cancer Center Flow Cytometry Core for his expert assistance in cell sorting. We also thank the staff of the Duke University Medical Center Labor and Delivery Suite as well as the laboratory of Dr. Joanne Kurtzberg for collection of UCB samples. The NMR work was conducted at the Shared Instrumentation Facility of the Duke University Medical Center. This work was supported by National Institutes of Health Grant 1 PO1 AI 40981-01 (C.S.) and Public Health Service Grant R01-CA16783 (O.M.C.) from the National Cancer Institute.
ABBREVIATIONS
ALDH
aldehyde dehydrogenase
BAAA
BODIPY aminoacetaldehyde
HSC
hematopoietic stem cell
FACS
fluorescence-activated cell sorting
DAAA
dansyl-aminoacetaldehyde
UCB
umbilical cord blood
BAAA-DA
BODIPY-aminoacetaldehyde diethyl acetal
PE
phycoerythrin
APC
allophycocyanin
HPCA
hematopoietic progenitor colony assay
LTC
long-term culture
AAA-DA
aminoacetaldehyde diethyl acetal
BAA
BODIPY amino acetate
References
- 1.Morrison S J, Wright D E, Cheshier S H, Weissman I L. Curr Opin Immunol. 1997;9:216–221. doi: 10.1016/s0952-7915(97)80138-0. [DOI] [PubMed] [Google Scholar]
- 2.Spangrude G, Weissman I. Science. 1988;241:58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- 3.Civin C I, Strauss L, Brovall C, Fackler M, Schwartz J. J Immunol. 1984;133:157–165. [PubMed] [Google Scholar]
- 4.Krause D, Ito T, Fackler M, Smith O, Collector M, Sharkis S, Mary S. Blood. 1994;84:691–701. [PubMed] [Google Scholar]
- 5.Osawa M, Hanada K, Hamada H, Nakauchi H. Science. 1996;273:242–245. doi: 10.1126/science.273.5272.242. [DOI] [PubMed] [Google Scholar]
- 6.Okada S, Nakauchi H, Nagayoshi K, Nishikawa S, Nishikawa S, Miura Y, Suda T. Blood. 1991;78:1706–1712. [PubMed] [Google Scholar]
- 7.Lansdorp P M, Schmitt C, Sutherland H J, Craig W H, Dragowska W, Thomas T E, Eaves C J. Prog Clin Biol Res. 1992;377:475–486. [PubMed] [Google Scholar]
- 8.Sutherland H J, Eaves C J, Eaves A C, Dragowska W, Lansdorp P M. Blood. 1989;74:1563–1570. [PubMed] [Google Scholar]
- 9.Hodgson G, Bradley T. Nature (London) 1979;281:381–382. doi: 10.1038/281381a0. [DOI] [PubMed] [Google Scholar]
- 10.Smith C, Gasparetto C, Collins N, Gillio A, Muench M, O’Reilly R, Moore M. Blood. 1991;77:2122–2128. [PubMed] [Google Scholar]
- 11.Berardi A C, Wang A, Levine J D, Lopez P, Scadden D T. Science. 1995;267:104–108. doi: 10.1126/science.7528940. [DOI] [PubMed] [Google Scholar]
- 12.Jones R, Wagner J, Celano P, Zicha M, Sharkis S. Nature (London) 1990;347:188–190. doi: 10.1038/347188a0. [DOI] [PubMed] [Google Scholar]
- 13.Visser J, de Vries P. Blood Cells. 1988;14:369–384. [PubMed] [Google Scholar]
- 14.Goodell M A, Brose K, Paradis G, Conner A S, Mulligan R C. J Exp Med. 1996;183:1797–1806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Randall T D, Lund F E, Howard M C, Weissman I L. Blood. 1996;87:4057–4067. [PubMed] [Google Scholar]
- 16.Li C, Johnson G. J Exp Med. 1992;175:1443–1447. doi: 10.1084/jem.175.6.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spangrude G, Brooks D, Tumas D. Blood. 1995;85:1006–1016. [PubMed] [Google Scholar]
- 18.Yonemura Y, Ku H, Hirayama F, Souza L M, Ogawa M. Proc Natl Acad Sci USA. 1996;93:4040–4044. doi: 10.1073/pnas.93.9.4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Randall T D, Weissman I L. Stem Cells. 1998;16:38–48. doi: 10.1002/stem.160038. [DOI] [PubMed] [Google Scholar]
- 20.Goodell M A, Rosenzweig M, Kim H, Marks D F, DeMaria M, Paradis G, Grupp S A, Sieff C A, Mulligan R C, Johnson R P. Nat Med. 1997;3:1337–1345. doi: 10.1038/nm1297-1337. [DOI] [PubMed] [Google Scholar]
- 21.Bhatia M, Bonnet D, Murdoch B, Gan O I, Dick J E. Nat Med. 1998;4:1038–1045. doi: 10.1038/2023. [DOI] [PubMed] [Google Scholar]
- 22.Jones R J, Collector M I, Barber J P, Vala M S, Fackler M J, May W S, Griffin C A, Hawkins A L, Zehnbauer B A, Hilton J, et al. Blood. 1996;88:487–491. [PubMed] [Google Scholar]
- 23.Colvin O. In: Pharmacologic Purging of Bone Marrow. Thomas E, Blume K, Forman S, editors. Malden, MA: Blackwell Science; 1999. pp. 217–224. [Google Scholar]
- 24.Labrecque J, Bhat P, Lacroix A. Biochem Cell Biol. 1993;71:85–89. doi: 10.1139/o93-013. [DOI] [PubMed] [Google Scholar]
- 25.Riveros-Rosas H, Julian-Sanchez A, Pina E. Arch Med Res. 1997;28:453–471. [PubMed] [Google Scholar]
- 26.Gordon M, Goldman J, Gordon-Smith E. Leukemia Res. 1985;9:1017–1021. doi: 10.1016/0145-2126(85)90072-4. [DOI] [PubMed] [Google Scholar]
- 27.Sahovic E, Colvin M, Hilton J, Ogawa M. Cancer Res. 1988;48:1223–1226. [PubMed] [Google Scholar]
- 28.Moreb J S, Turner C, Sreerama L, Zucali J R, Sladek N E, Schweder M. Leukemia Lymphoma. 1995;20:77–84. doi: 10.3109/10428199509054756. [DOI] [PubMed] [Google Scholar]
- 29.Jones R J, Barber J P, Vala M S, Collector M I, Kaufmann S H, Ludeman S M, Colvin O M, Hilton J. Blood. 1995;85:2742–2746. [PubMed] [Google Scholar]
- 30.Hilton J. Cancer Res. 1984;44:5156–5160. [PubMed] [Google Scholar]
- 31.Isaad C, Croisille L, Katz A, Vaimchenker W, Coulombei L. Blood. 1993;81:2916–2924. [PubMed] [Google Scholar]
- 32.Russo J, Hauquitz D, Hilton J. Biochem Pharmacol. 1988;37:1639–1642. doi: 10.1016/0006-2952(88)90030-5. [DOI] [PubMed] [Google Scholar]
- 33.Russo J, Hilton J, Colvin O. In: The Role of Aldehyde Dehydrogenase Isozymes in Cellular Resistance to the Alklating Agent Cyclophosphamide. Weiner H, Flynn T, editors. New York: Liss; 1989. pp. 65–79. [PubMed] [Google Scholar]
- 34.Kurtzberg J. J Hematother. 1996;5:95–96. doi: 10.1089/scd.1.1996.5.95. [DOI] [PubMed] [Google Scholar]
- 35.Ratajczak M Z, Pletcher C H, Marlicz W, Machalinski B, Moore J, Wasik M, Ratajczak J, Gewirtz A M. Leukemia. 1998;12:942–950. doi: 10.1038/sj.leu.2401027. [DOI] [PubMed] [Google Scholar]
- 36.Chaudhary P M, Roninson I B. Cell. 1991;66:85–94. doi: 10.1016/0092-8674(91)90141-k. [DOI] [PubMed] [Google Scholar]
- 37.Hao Q, Thiemann F T, Peterson D, Smorgorzewska E M, Crooks G M. Blood. 1996;87:3306–3313. [PubMed] [Google Scholar]
- 38.Dick J E. Semin Immunol. 1996;8:197–206. doi: 10.1006/smim.1996.0025. [DOI] [PubMed] [Google Scholar]
- 39.Albella B, Segovia J, Bueren J. Blood. 1997;90:464–470. [PubMed] [Google Scholar]
- 40.Michallet M, Philip I, Coiffier B, Salles G, Sebban C, Godinot H, Roubi N, Lopez F, Bessueille L, Mazars P, et al. Blood. 1998;92:728a. (abstr.). [Google Scholar]
- 41.Thomson J, Itskovitz-Eldor J, Shapiro S, Waknitz M, Swiergiel J, Marshall V, Jones J. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]