A model for a umuDC-dependent prokaryotic DNA damage checkpoint (original) (raw)
Abstract
The products of the Escherichia coli umuDC operon are required for translesion synthesis, the mechanistic basis of most mutagenesis caused by UV radiation and many chemicals. The UmuD protein shares homology with LexA, the repressor of SOS-regulated loci, and similarly undergoes a facilitated autodigestion on interaction with the RecA/single-stranded DNA nucleoprotein filaments formed after a cell experiences DNA damage. This cleavage, in which Ser-60 of UmuD acts as the nucleophile, produces UmuD′, the form active in translesion synthesis. Expression of the noncleavable UmuD(S60A) protein and UmuC was found to increase survival after UV irradiation, despite the inability of the UmuD(S60A) protein to participate in translesion synthesis; this survival increase is uvr+ dependent. Additional observations that expression of the UmuD(S60A) protein and UmuC delayed the resumption of DNA replication and cell growth after UV irradiation lead us to propose that the uncleaved UmuD protein and UmuC delay the resumption of DNA replication, thereby allowing nucleotide excision repair additional time to repair the damage accurately before replication is attempted. After a UV dose of 20 J/m2, uncleaved UmuD is the predominant form for approximately 20 min, after which UmuD′ becomes the predominant form, suggesting that the umuDC gene products play two distinct and temporally separated roles in DNA damage tolerance, the first in cell-cycle control and the second in translesion synthesis over unrepaired or irreparable lesions. The relationship of these observations to the eukaryotic DNA damage checkpoint is discussed.
umuDC mutants of Escherichia coli were originally identified by directly screening for derivatives of E. coli that are nonmutable by treatment with UV and various other chemicals (1, 2). The umuDC gene products have subsequently been shown to contribute to _E. coli_’s ability to tolerate DNA damage by participating in the process of translesion synthesis (3–8). Base substitution mutagenesis by UV and various chemicals occurs when, during the process of translesion synthesis, an incorrect nucleotide is inserted opposite the lesion and the chain is subsequently extended (9–12).
The activities of the umuDC gene products are regulated in a complex way as part of the SOS response of E. coli (3, 5, 6). Cells sense that they have experienced DNA damage when RecA proteins bind to regions of single-stranded DNA, which are produced as a consequence of the damage, and undergo a conformational change that activates the RecA coprotease. The RecA coprotease then transduces the DNA-damage signal that leads to the induction of the SOS response by facilitating the inactivation of LexA, the repressor of DNA damage-inducible genes, including umuD+ and umuC+. The inactivation of LexA occurs by a facilitated autodigestion mechanism in which Ser-119 of LexA serves as the nucleophile that cleaves the LexA Ala-84—Gly-85 bond (3, 13). The carboxyl terminal domain of UmuD is structurally related to the carboxyl terminal domain of LexA and similarly undergoes a RecA-facilitated autodigestion in which Ser-60 of UmuD serves as the nucleophile that cleaves the UmuD Cys-24—Gly-25 bond to yield UmuD′ (14–16). This cleavage activates the umuD gene product for its role in translesion synthesis (15, 17). In vivo the noncleavable UmuD(S60A) protein is not capable of participating in UV and chemical mutagenesis, whereas UmuD′ is capable (15). In vitro the addition of uncleaved UmuD to UmuC, RecA, and DNA polymerase III holoenzyme does not result in translesion synthesis (9, 10), whereas the addition of UmuD′ protein does (9–11) .
In addition to participating in translesion synthesis, UmuD′ and UmuC have been shown to inhibit RecA-mediated homologous recombination when they are present at elevated levels, and this has led to the proposal that this modulation of recombination represents an additional mechanism by which the umuDC gene products help E. coli tolerate DNA damage (18). The possibility that the umuDC gene products might play even more roles was raised by our analysis of the cold sensitivity for growth that is observed with strains that overexpress the umuDC operon (19, 20). Our discovery that cold sensitivity for growth caused by overexpression of the umuDC operon is genetically distinct from SOS mutagenesis and involves uncleaved UmuD rather than UmuD′ suggested that the uncleaved UmuD protein and UmuC might play a role in modulating the E. coli cell cycle after DNA damage (20). Interestingly, the umuD(S60A) mutation causes a dominant-negative effect on SOS mutagenesis (15), but does not affect the activity responsible for UmuDC-mediated cold sensitivity (20). In contrast, the umuC125 (A39V) mutant gene product is active in SOS mutagenesis but is severely impaired in its ability to promote _umuDC_-mediated cold sensitivity (21). In this paper, we describe unanticipated results we have obtained in followup experiments that lead us to suggest that, after E. coli has experienced DNA damage, uncleaved UmuD protein and UmuC play a role in regulating DNA synthesis and cell growth in a manner that is reminiscent of the DNA damage checkpoint that has been recognized in eukaryotes (22–24).
MATERIALS AND METHODS
Strains and Plasmids.
The salient features of the strains and plasmids used in this work are described in legend of each figure. Strain GW8101 was constructed by transducing _uvrA6 malE_∷Tn10 from PF1364 (Patricia L. Foster, Boston University, Boston, MA) into GW8023 (20) by using P1(vir) transduction and selecting for tetracycline resistance and increased UV sensitivity. pTO4 was constructed by replacing the 1-kb _Bgl_II fragment of pSE117 (25) with the corresponding restriction fragment from pGW2112 (15) containing the umuD(S60A) mutation. The presence of the umuD(S60A) mutation in pTO4 was verified by DNA sequence analysis. All plasmids used in these studies are medium copy-number pBR322 derivatives.
UV Survival Curves.
UV survival curves were performed essentially as previously described (26). Briefly, cultures growing exponentially in M9 medium (27) supplemented with 0.2% glucose and 0.4% casamino acids were irradiated in 5-ml aliquots in the growth medium in a 100-mm glass Petri dish. Serial dilutions were plated on Luria–Bertani agar as previously described (20, 26) to determine colony-forming units/ml after exposure to a given UV dose. In Figs. 1B and 2B, a representative example of multiple experiments is shown.
Figure 1.
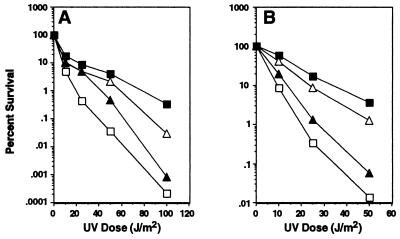
The activities of uncleaved UmuD and UmuD′ in combination with UmuC increase resistance to DNA damage. (A) Survival after UV irradiation of isogenic strains of GW8024 [recA+ Δ(umuDC)595_∷_cat lexA(Def)] (20) that differ in the genotype of umuD and umuC carried on a plasmid: (■) umuD+ umuC+ [pSE117] (25); (▵) umuD′ umuC+ [pGW3751] (56); (▴) umuD(S60A) umuC+ [pTO4] (this work); (□) ΔumuDC [pBR322kan] (20). (B) Same as in A, but strains are GW8023 [recA+ Δ(umuDC)595_∷_cat lexA+] (20).
Figure 2.
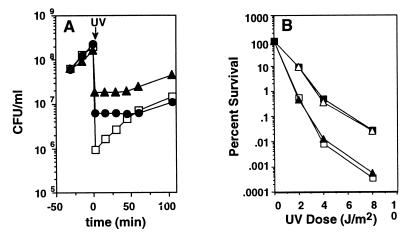
(A) Uncleaved UmuD and UmuC regulate growth in response to DNA damage in exponentially growing E. coli cultures. Effect of various plasmid-encoded umuD+ umuC+ gene products on growth of GW8023 [lexA+, recA+, _Δ(umuDC)595_∷_cat_] after UV irradiation (25 J/m2). (□) ΔumuDC [pBR322kan]; (▴) umuD+ umuC+ [pSE117]; (●) umuD(S60A) umuC+ [pTO4] (this work). (B) The resistance to killing by DNA damage provided by uncleaved UmuD and UmuC depends on nucleotide excision repair. Survival after UV irradiation of isogenic strains of GW8101 [recA+ Δ(umuDC)595_∷_cat lexA+ _uvrA6_] (this work) that differ in the genotype of umuD and umuC carried on a plasmid: (■) umuD+ umuC+ [pSE117] (25); (▵) umuD′ umuC+ [pGW3751] (56); (▴) umuD(S60A) umuC+ [pTO4] (this work); (□) ΔumuDC [pBR322kan] (20).
Measurement of Growth after UV Irradiation.
The effect of UV irradiation on subsequent growth was measured as follows. Growth of bacterial cultures, exposure to UV radiation, and subsequent growth were as described below. Growth of each culture was monitored by plating on Luria–Bertani agar and measuring colony-forming units/ml at various times before and after UV irradiation.
Induced Replisome Reactivation (IRR) Assays.
IRR assays were performed essentially as described by Khidir et al. (28). Briefly, overnight cultures grown in M9 medium (27) supplemented with 0.2% glucose, and 0.4% casamino acids were diluted 1:200 in fresh prewarmed medium and incubated at 37°C until the culture was growing exponentially (ca. 0.1 OD600). The OD600 and rate of DNA synthesis were measured at the indicated times before and after UV irradiation. The rate of DNA synthesis was measured as the amount (cpm) of [3H]thymidine (Amersham Pharmacia) incorporated into trichloroacetic acid insoluble counts during a 2-min pulse. Each exponentially growing culture was irradiated in M9 (0.2% glucose) growth medium with UV (10 J/m2) in a 150-mm Petri dish to minimize the interruption to exponential growth. After UV irradiation, bacterial cultures were grown in the absence of light to prevent reversal of UV-induced DNA damage by photoreactivation.
Measurement of Steady-State Levels of UmuD and UmuD′.
Immunoblot analyses to measure steady-state levels of UmuD and UmuD′ at various times after UV irradiation were performed as previously described (20), except that a 0.10-OD600 unit aliquot of each sample was electrophoresed. Growth of cultures, UV irradiation, and subsequent growth were performed as described above.
RESULTS
Uncleaved UmuD Protein and UmuC Increase Resistance to Killing by UV Radiation.
We had previously found that, when overexpressed from a multicopy plasmid in a lexA(Def) strain, uncleaved UmuD protein and UmuC inhibit aspects of the E. coli cell cycle at 30°C, resulting in a cold sensitivity for growth, whereas UmuD′ and UmuC were far less inhibitory (19, 20). It seemed possible that the effects of the uncleaved UmuD protein and UmuC on the cell cycle we had observed under these overproduction conditions might have resulted from the exaggeration of a physiological role of these proteins that is normally relevant only to cells that have suffered DNA damage.
To test this idea, we used the same lexA(Def) recA+ Δ_umuDC_ cells expressing various plasmid-encoded derivatives of the umuDC operon that we had used in our previous studies but grew them at the permissive temperature of 37°C (20). As shown in Fig. 1A, the derivative expressing the noncleavable UmuD(S60A) protein (15) along with UmuC was more resistant to killing by UV radiation than the isogenic strain that lacked umuDC function. Furthermore, the derivative that directly expressed UmuD′ and UmuC was not as resistant to killing by UV radiation as the one that expressed the wild-type umuDC gene products and thus produces UmuD, UmuD′, and UmuC. Taken together, these observations suggested that, in combination with UmuC, the UmuD and UmuD′ forms of the umuD gene product each confer a separate survival advantage to cells that have suffered DNA damage from UV radiation.
Because of concern that the protection against killing conferred by uncleaved UmuD protein with UmuC might be an artifact of the high level of expression of these plasmid-encoded proteins in the lexA(Def) background, the same experiments were repeated in a lexA+ background. Many studies of umuDC function have similarly used lexA+ strains in which the gene products were expressed from a multicopy plasmid (15, 18, 29–33) and furthermore, as will become evident, the striking and consistent allele-specific phenotypes we observe for each of the different plasmid-encoded umuDC operons in a variety of experiments argue against their being the result of overproduction-induced artifacts. As shown in Fig. 1B, in the lexA+ background as well, expression of the noncleavable UmuD(S60A) protein with UmuC increased resistance to killing by UV radiation compared with a Δ_umuDC_ strain. Furthermore, direct expression of UmuD′ with UmuC did not result in as high a level of resistance to UV killing as expression of the wild-type umuDC gene products.
Our discovery that uncleaved UmuD together with UmuC could increase resistance to killing by UV radiation was surprising because, in the past, _umuDC_-dependent resistance to killing by UV radiation and other DNA-damaging agents has been attributed entirely to the ability of UmuD′ and UmuC to promote translesion synthesis (3, 34). Cells capable of expressing only uncleaved UmuD protein and UmuC because of a umuD mutation causing a defect in RecA-facilitated UmuD cleavage are nonmutable (15, 17), indicating that they cannot carry out translesion synthesis. Furthermore, uncleaved UmuD cannot substitute for UmuD′ in promoting translesion synthesis in vitro (9, 10). Thus uncleaved UmuD protein and UmuC must increase cellular resistance to killing by UV radiation by a mechanism other than translesion synthesis.
Because of our previous observation that overproduction of uncleaved UmuD protein and UmuC inhibit cell growth at 30°C (19, 20), we investigated whether uncleaved UmuD and UmuC modulate cell growth after UV irradiation. This proved to be the case because, in a lexA+ recA+ Δ_umuDC_ strain, plasmid-encoded expression of either the noncleavable UmuD(S60A) protein (15) together with UmuC, or the similar expression of the umuD+C+ gene products, significantly delayed the recovery of cell growth after UV irradiation compared with the parental strain, which lacks umuDC function (Fig. 2A).
The Resistance to Killing by DNA Damage Provided by Uncleaved UmuD Protein and UmuC Depends on Nucleotide Excision Repair.
If uncleaved UmuD protein and UmuC were to act by delaying progression through the cell cycle after UV-induced DNA damage, this would allow more time for the highly accurate uvr+-dependent nucleotide excision repair system to remove DNA lesions before DNA replication and cell division. A key prediction of this hypothesis is that the increased resistance to UV radiation conferred by uncleaved UmuD protein and UmuC would not be observed in a uvr mutant, which lacks nucleotide excision repair. A second prediction is that, in a uvr background, UmuD′ would be as effective as the wild-type UmuD protein in conferring resistance to killing by UV radiation in combination with UmuC. As shown in Fig. 2B (compare with Fig. 1B), both of these predictions were borne out. In contrast to the corresponding uvrA+ strain (Fig. 1B), the UV resistance of a lexA+ recA+ uvrA6 Δ_umuDC_ strain was unaffected by expression of the noncleavable UmuD(S60A) protein along with UmuC. In addition, the UmuD′ protein and the wild-type UmuD+ protein, when expressed along with UmuC, were equally effective in increasing UV resistance. Thus these data are consistent with the hypothesis that uncleaved UmuD protein and UmuC function to protect the cell from DNA damage by slowing down the E. coli cell cycle, thereby allowing more time for error-free repair mechanisms such as nucleotide excision repair to act.
Uncleaved UmuD Protein and UmuC Regulate DNA Synthesis in Response to DNA Damage.
A plausible mechanism for how the uncleaved UmuD and UmuC could cause a pausing or slowing of the cell cycle after DNA damage so that additional accurate repair could occur would be for them to modulate the rate of DNA synthesis after UV irradiation. This possibility was also suggested by our previous observation that strong overexpression of umuDC at 30°C inhibited DNA synthesis (19). To test this aspect of the model, we examined the influence of uncleaved UmuD protein together with UmuC on the rate of DNA synthesis occurring after DNA damage.
After UV irradiation of E. coli, DNA synthesis is inhibited. Its subsequent recovery is referred to as IRR (28) or “replication restart” (35). The inhibition of DNA synthesis after UV irradiation does not require induction of the SOS response and has been attributed to a direct effect of lesions in the template blocking replisome movement (28). IRR requires the induction of a recA+-regulated protein, but the molecular mechanisms underlying this phenomenon have not been elucidated and, in particular, the relationship of the umuDC gene products to IRR is poorly understood. IRR is still observed in umuC mutants, both in a uvr+ E. coli K-12 strain (28) and in a uvr E. coli B/r strain (33), indicating that there is a umuDC-independent mode of IRR. In contrast, IRR completely depends on umuDC function in E. coli B/r derivatives carrying the recA718 allele (33). This latter observation suggests that there is also a second pathway for IRR that is umuDC dependent (33). However, the existence of the _umuDC_-independent pathway makes it difficult to detect the effect of loss-of-function umuDC mutations on IRR in a wild-type genetic background.
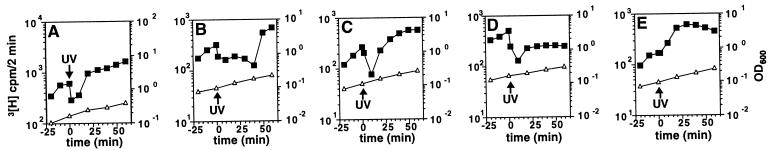
In an effort to detect the _umuDC_-dependent pathway in the presence of the _umuDC_-independent pathway, we increased the dosages of the various umuDC derivatives by expressing them from multicopy plasmids in a lexA(Def) background. In this genetic background, as in other genetic backgrounds (28, 33), inhibition and recovery of DNA synthesis after UV irradiation occurred in the absence of umuDC function (Fig. 3A). However, expression of elevated levels of the umuD+C+ gene products resulted in a readily detectable delay in the resumption of DNA synthesis after UV irradiation compared with the strain lacking umuDC (Fig. 3B). This delay is apparently because of the presence of uncleaved UmuD together with UmuC because direct expression of the UmuD′ protein together with UmuC did not cause such a delay (Fig. 3C), whereas expression of the noncleavable UmuD(S60A) protein together with UmuC prevented the resumption of DNA synthesis after UV within the time frame of the experiment (Fig. 3D). These results indicate that, although the initial inhibition of DNA synthesis after UV irradiation can occur in the absence of uncleaved UmuD and UmuC, these proteins have the potential ability to maintain the inhibition of DNA synthesis until it is alleviated by a key regulatory step, namely the conversion of uncleaved UmuD to UmuD′ by RecA-mediated cleavage. Such a delay in the resumption of DNA synthesis after DNA damage could provide a survival advantage after UV irradiation by allowing additional time for nucleotide excision repair to remove DNA lesions before the DNA was replicated.
Figure 3.
Uncleaved UmuD and UmuC regulate DNA synthesis in response to DNA damage. The effect of various umuD umuC gene products on inhibition and recovery (IRR) of DNA synthesis caused by UV irradiation in isogenic strains of GW8024 [lexA(Def) recA+ _Δ(umuDC)595_∷_cat_] that differ in the genotype of umuD umuC carried on a plasmid. The rate of DNA synthesis (amount of [3H]thymidine incorporated into TCA insoluble counts during a 2-min pulse; ■) and OD600 (▵) were measured at various times before and after UV irradiation (indicated by arrow) in isogenic strains that differ only in the genotype of umuD umuC carried on a plasmid: (A) ΔumuDC [pBR322kan] (20); (B) umuD+ umuC+ [pSE117] (25); (C) umuD′ umuC+ [pGW3751] (56); (D) umuD(S60A) umuC+ [pTO4] (this work); (E) umuD+ umuC125 [pLM109] (21).
Remarkably, although inhibition of DNA synthesis occurs in the absence of umuD and umuC (Fig. 3A), expression of the UmuC125 protein along with the UmuD protein prevented inhibition of DNA synthesis after UV irradiation (Fig. 3E). This finding suggests that the change in UmuC function caused by the umuC125 allele, a single missense mutation that changes Ala-39 to Val (21), enables it to modulate DNA polymerase III in such a way that DNA replication is not inhibited when the cell suffers DNA damage. The umuC125 allele has the unusual property of not causing cold sensitivity for growth when overexpressed with umuD+, while still being proficient in SOS mutagenesis (21), a property indicating that it can still participate in translesion synthesis. Furthermore the presence of the umuC125 mutation increases the sensitivity of E. coli to killing by UV radiation (21). Because the umuC125 mutant is apparently proficient in SOS mutagenesis, our observations suggest that the hitherto unexplained UV sensitivity of the umuC125 mutant is caused, at least in part, by the insensitivity of its DNA replication system to inhibition after DNA damage.
Temporal Regulation of the Activities of the umuDC Gene Products.
Although the umuDC operon is induced by DNA damage, like other SOS-controlled operons (3) it is expressed constitutively at a basal level. E. coli that have not suffered DNA damage contain approximately 200 UmuD molecules/cell (36). Although the levels of UmuC in an uninduced cell were reported to be below the level of detection (36), a reasonable estimate based on the UmuD induction ratio would be approximately 20 UmuC molecules/cell. Thus, even before the SOS system has been induced, there would appear to be sufficient numbers of these proteins available to begin to interact with the replication machinery and influence its behavior after DNA damage has occurred.
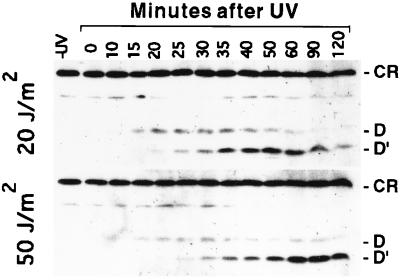
Taken together with previous observations (14–16), the results presented above indicate that RecA-mediated cleavage of uncleaved UmuD to yield UmuD′ must be a key regulatory event that controls the various activities of the umuDC gene products in a wild-type cell. To test whether the kinetics of UmuD cleavage in vivo after a DNA-damaging treatment are compatible with the hypothesis that there are different physiological roles for uncleaved UmuD and UmuD′, we measured the steady-state levels of UmuD and UmuD′ at various times after a UV dose of 20 J/m2 (Fig. 4). After UV irradiation, uncleaved UmuD accumulates and predominates over UmuD′ for 20 min. This is followed by a period in which UmuD′ accumulates. The higher level of UmuD′ that accumulates during these later times relative to UmuD during the earlier time points may reflect the relative resistance of UmuD′ to proteolytic degradation compared with UmuD (30, 37). We observed that irradiation of cells with a modestly higher dose of UV (50 J/m2) resulted in a striking delay in the peak of UmuD′ production of about 40 min compared with that observed with 20 J/m2 (Fig. 4). Such a delay could allow time for more error-free repair to occur at the higher level of DNA damage. Thus our measurements indicate that cells do indeed experience two distinguishable phases after a DNA-damaging treatment, an earlier one in which UmuD predominates and a later one in which UmuD′ predominates.
Figure 4.
The appearance of UmuD and UmuD′ in response to DNA damage are temporally regulated by RecA, and the timing of the peak production of UmuD′ depends on the amount of DNA damage the cells have suffered. Immunoblot analyses were used to measure steady-state levels of UmuD and UmuD′ at various times after exponentially growing cultures of GW2771 [lexA+, recA+, umuD+ umuC+] were irradiated with UV(20 J/m2 or 50 J/m2). The positions of UmuD (D), UmuD′ (D′) and a crossreacting species (CR), which was used as a loading control, are indicated.
The delay in the appearance of UmuD′ can be explained, at least in part, by the observation that RecA-mediated cleavage of UmuD, both in vivo (16) and in vitro (14), is slow compared with LexA (38). Furthermore, there are additional factors that regulate UmuD cleavage in vivo. This is illustrated by the fact that kinetics of RecA-mediated cleavage of UmuD that we observed using a lexA+ strain were significantly faster than those reported by Bailone et al. (39), who used a lexA(Def) strain in their studies. The difference in the kinetics of UmuD cleavage between these strains is likely to be caused by one or more SOS-regulated factors, which could be expressed at higher levels in a lexA(Def) strain. At least one of these is the SOS-inducible dinI gene product, which has been shown to slow the rate of UmuD cleavage in vivo (40). The complexity of the regulatory circuitry is undoubtedly further complicated by the differential susceptibility to proteolytic degradation of the various forms of the umuD gene product; Frank et al. (30) have shown that UmuD2 is degraded by Lon and UmuD′ is degraded by ClpXP when it is in the UmuD′⋅UmuD heterodimer. The conversion of UmuD to UmuD′ by RecA-mediated cleavage may have evolved to act as a molecular timing mechanism that enables the cell to switch from one mode of dealing with DNA damage to another.
DISCUSSION
The unexpected results reported in this paper lead us to propose a model in which, after E. coli suffers damage to its DNA and its replication forks become blocked by lesions in the DNA (28), the umuDC gene products play two distinct and temporally separated roles in DNA damage tolerance. The first role, which requires the uncleaved UmuD protein and UmuC, delays the recovery of DNA replication and cell growth after DNA damage. Such a delay in the resumption of DNA synthesis and cell growth increases the ability of cells to survive the DNA damage by allowing additional time for accurate repair systems to remove or process the damage before replication is attempted. In support of this aspect of the model, we have shown that the protection from UV-induced DNA damage provided by uncleaved UmuD in combination with UmuC depends on a functional uvrA gene product, an essential component of the error-free uvr+-dependent nucleotide excision repair system in E. coli. Moreover, by preventing cells from attempting to replicate damaged DNA templates, uncleaved UmuD and UmuC might prevent secondary consequences that could be even more difficult to deal with than the original lesions in the DNA.
The RecA-mediated cleavage of UmuD to UmuD′ then acts as a molecular switch that permits the umuDC gene products to carry out their second role, in which UmuD′ and UmuC aid in the resumption of DNA replication by participating in translesion synthesis. This damage-tolerance mechanism permits cells to survive by replicating over unrepaired or irreparable lesions that would otherwise block DNA synthesis. Our analyses of the kinetics of the conversion of UmuD to UmuD′ in wild-type cells after DNA damage suggest that the role of the umuD gene product in controlling DNA replication and cell growth would be carried out during the first few minutes after DNA damage, during which UmuD predominates over UmuD′, and that the translesion synthesis role would take place subsequently when UmuD′ predominates over UmuD. We have also found that higher levels of DNA damage result in a shift of the peak of UmuD′ production to a significantly later time after the DNA-damaging treatment relative to what is seen at a lower level of damage. This observation suggests that there is a regulatory mechanism that responds to increased levels of DNA damage by modulating the RecA-mediated molecular switch, thereby prolonging the period in which the levels of UmuD are high relative to those of UmuD′ and hence the period during which the resumption of DNA replication is delayed.
Interactions of the umuDC gene products with components of the cell’s replicative machine, DNA polymerase III holoenzyme, seem likely to be involved in both of these roles. Effects of UmuD′ and UmuC in permitting translesion synthesis have been observed in a purified system containing only DNA polymerase III holoenzyme and RecA in addition to UmuD′ and UmuC (9–11). An interaction of the uncleaved UmuD protein and UmuC with components of DNA polymerase III holoenzyme would be an economical way of accounting for the inhibition of DNA synthesis caused by the uncleaved UmuD protein and UmuC that we have described in this paper. However, it is clear that under normal physiological conditions, the mere presence of uncleaved UmuD and UmuC in an undamaged cell is not enough to result in the inhibition of DNA replication. There must be some additional event that occurs as a result of DNA damage that permits the umuDC gene products to interact productively with DNA polymerase III.
Differences between the structures of the UmuD and UmuD′ proteins could account for the very different biochemical and physiological roles of these two proteins. Analysis of the crystal structure of UmuD′ has revealed that the UmuD′ monomer has a globular carboxyl terminus but an unstructured N terminus (41). NMR analysis has shown that the UmuD′2 dimer found in solution is the one in which the extreme carboxyl termini participate in the interface, and that the N termini (residues 25–39 using UmuD numbering) are flexible in solution (42). Comparative analyses of the behavior of monocysteine derivatives of UmuD2 and UmuD′2 (refs. 43–46; A. Guzzo and G.C.W., unpublished results) indicate that, although the two dimers share certain similarities, there must be substantial differences between their conformations These differences between the UmuD and UmuD′ dimers could result in different types of interactions with the DNA replication machinery that could then result in the differential physiological effects that we have observed.
From studies of mutant strains with reduced rates of excision repair both Witkin (47, 48) and Bridges (49, 50) have previously suggested that E. coli might delay its cell cycle to enable a certain amount of repair to occur. Bridges (50) has hypothesized that the replication rate after DNA damage might be controlled by the rate of RecA-coated to -uncoated single-stranded regions of DNA in the replication fork, whereas we are suggesting that uncleaved UmuD and UmuC may serve as ultimate effectors that control the rate of DNA replication by interacting with the components of the replicative DNA polymerase. Bridges has also discussed the hypothesis that a bacterium is able to actively monitor the level of DNA damage it has suffered and delay the progression of its cell cycle until the damage has been completely repaired (50). Our observations raise the interesting possibility that the “delay” in resumption of DNA replication is actually a timed pause rather than a pause whose lifting depends on some condition being satisfied. The length of the timed pause would be determined by the amount of DNA damage and implemented by the SOS circuitry. We presume that the length of the timed pause for a given amount of damage would have been set through evolutionary selection.
If the UmuD and UmuC proteins play such a previously unrecognized role in increasing DNA damage tolerance in E. coli by regulating DNA synthesis and growth in response to DNA damage, this may offer a possible explanation for the finding that several bacterial species have umuD umuC homologs but are poorly mutable by UV radiation (51). Perhaps it is a _umuDC_-dependent role in regulating the cell cycle after damage that has been evolutionarily important for these bacteria, rather than a role in the potentially mutagenic process of translesion synthesis.
The model we have proposed for the uncleaved UmuD protein and UmuC has strong parallels to the DNA-damage checkpoint system that has been recognized as operating in eukaryotic cells (22–24). Checkpoint surveillance mechanisms “are usually not required for cell cycle events but enforce their proper order, especially critical after acute damage or error” (24). In our model, the primary signal that triggers the operation of the checkpoint is single-stranded DNA generated by the cell’s initial attempt to replicate its damaged DNA (38). The sensing of the signal is accomplished by RecA polymerizing along the single-stranded DNA. The signal is then transduced by the resulting RecA/ssDNA nucleoprotein filament facilitating the autodigestion of LexA, which in turn results in the induction of SOS-regulated genes including the umuDC operon (3, 6). As discussed above, it seems plausible that uncleaved UmuD protein and UmuC act as the ultimate effectors that execute the checkpoint by directly interacting with components of the DNA polymerase III holoenzyme and delaying the resumption of DNA replication.
An interesting aspect of the model we are proposing is that the event that relieves the checkpoint, namely the RecA-mediated cleavage of UmuD, simultaneously produces a protein, UmuD′, that allows cells to tolerate any remaining DNA damage by carrying out translesion synthesis and thus aids in the resumption of DNA replication. Like other signal transduction systems, eukaryotic DNA damage checkpoints adapt so that, even though damage remains unrepaired, after an interval of arrest the cell may resume progress through the cell cycle (23, 52). Viewed in this light, the cleavage of UmuD can be seen as a form of checkpoint adaptation that generates a capacity to tolerate unrepaired or irreparable DNA damage.
The possibility that cells might generate mutations by inserting noncognate nucleotides in the nascent strand during translesion synthesis has been cited as one of the reasons for their having a DNA-damage checkpoint system (23). However, if the _umuDC-_dependent checkpoint system indeed orders the sequence of events so that accurate nucleotide excision repair occurs before translesion synthesis, then translesion synthesis can be viewed as a desirable process that, for the price of an increase in mutation frequency, enables the cell to cope with irreparable damage that would otherwise prove lethal. The associated increase in mutation frequency might even prove beneficial to an organism in times of stress (53, 54). The observation that in a rad1 strain the Saccharomyces cerevisiae genes RAD9, RAD17, RAD24, and MEC3 are not only required for a checkpoint that slows the progression of an ongoing S phase in response to DNA damage, but are also required for UV-induced mutagenesis (55), raises the possibility that eukaryotic DNA-damage checkpoint systems might use a similar principle.
The eukaryotic DNA damage checkpoint can act at three stages in the cell cycle: at the G1/S transition, during progression through S, and at the G2/M boundary (23). Because E. coli grown under the conditions used in these experiments initiate a new round of DNA replication before finishing the previous one, they synthesize DNA continuously throughout the cell cycle. Thus the _umuDC_-dependent checkpoint we have proposed most closely resembles a eukaryotic S phase checkpoint. However, in eukaryotes, genes necessary for arrest at one stage of the cell cycle are also necessary for arrest at other stages (23). In nature, many prokaryotes spend a great deal of time in stationary phase and must accumulate DNA damage from exogenous and endogenous sources that could potentially pose a problem when they finally experience a nutritional upshift and are able to resume an active cell cycle. It will be interesting to test whether the _umuDC_-dependent DNA damage checkpoint we have proposed also acts as cells make the transition from stationary phase to active growth. In eukaryotes, this would most closely correspond to a checkpoint operating as cells make the transition from G0 back into an active cell cycle.
In the model we are proposing for a DNA-damage checkpoint in E. coli, the proteins and regulatory systems involved in sensing the damage, transducing the signal, and implementing and relieving the checkpoint are intertwined to an extraordinary extent. For example, the molecular details of the cleavage event that governs the conversion of the umuD gene product from its checkpoint form, UmuD, to its translesion synthesis form, UmuD′ are virtually identical to the molecular details of the cleavage of LexA, a key event in the signal transduction system responsible for SOS induction and checkpoint implementation (3). In another example, the RecA protein not only plays two distinct regulatory roles by facilitating the cleavage of LexA and UmuD but is also required, apparently in a mechanistic way, for the process of translesion synthesis (3). Finally, although not yet proven, it seems plausible that the various forms of the umuDC gene products exert both their checkpoint and translesion synthesis functions through interactions with components of DNA polymerase III.
Acknowledgments
The authors thank Mark Sutton, Chon Martinez, Roberto Kolter, and Phil Hanawalt for their comments and suggestions on the manuscript. In addition, the authors thank Patricia L. Foster for providing strain PF1364. This work was supported by Public Health Service Grants CA21615 from the National Cancer Institute to G.C.W. and 5-P01-ES03926 from the National Institutes of Environmental Health Sciences. B.T.S. was supported in part by a predoctoral training grant from the National Institutes of Health.
ABBREVIATION
IRR
induced replisome reactivation
References
- 1.Kato T, Shinoura Y. Mol Gen Genet. 1977;156:121–131. doi: 10.1007/BF00283484. [DOI] [PubMed] [Google Scholar]
- 2.Steinborn G. Mol Gen Genet. 1978;165:87–93. doi: 10.1007/BF00270380. [DOI] [PubMed] [Google Scholar]
- 3.Friedberg E C, Walker G C, Siede W. DNA Repair and Mutagenesis. Washington, DC: Am. Soc. Microbiol.; 1995. [Google Scholar]
- 4.Koch W H, Woodgate R. In: DNA Damage and Repair, Vol. 1: DNA Repair in Prokaryotes and Lower Eukaryotes. Nickoloff J A, Hoekstra M F, editors. Totowa, NJ: Humana; 1997. pp. 107–134. [Google Scholar]
- 5.Smith B T, Walker G C. Genetics. 1998;148:1599–1610. doi: 10.1093/genetics/148.4.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walker G C. Microbiol Rev. 1984;48:60–93. doi: 10.1128/mr.48.1.60-93.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walker G C. Trends Biochem Sci. 1995;20:416–420. doi: 10.1016/s0968-0004(00)89091-x. [DOI] [PubMed] [Google Scholar]
- 8.Woodgate R, Levine A S. Cancer Surv. 1996;28:117–140. [PubMed] [Google Scholar]
- 9.Rajagopalan M, Lu C, Woodgate R, O’Donnell M, Goodman M F, Echols H. Proc Natl Acad Sci USA. 1992;89:10777–10781. doi: 10.1073/pnas.89.22.10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reuven N B, Tomer G, Livneh Z. Mol Cell. 1998;2:191–199. doi: 10.1016/s1097-2765(00)80129-x. [DOI] [PubMed] [Google Scholar]
- 11.Tang M, Bruck I, Eritja R, Turner J, Frank E G, Woodgate R, O’Donnell M, Goodman M F. Proc Natl Acad Sci USA. 1998;95:9755–9760. doi: 10.1073/pnas.95.17.9755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walker G C. Proc Natl Acad Sci USA. 1998;95:10248–10350. [Google Scholar]
- 13.Slilaty S N, Little J W. Proc Natl Acad Sci USA. 1987;84:3987–3991. doi: 10.1073/pnas.84.12.3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burckhardt S E, Woodgate R, Scheuermann R H, Echols H. Proc Natl Acad Sci USA. 1988;85:1811–1815. doi: 10.1073/pnas.85.6.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nohmi T, Battista J R, Dodson L A, Walker G C. Proc Natl Acad Sci USA. 1988;85:1816–1820. doi: 10.1073/pnas.85.6.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shinagawa H, Iwasaki H, Kato T, Nakata A. Proc Natl Acad Sci USA. 1988;85:1806–1810. doi: 10.1073/pnas.85.6.1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Battista J R, Ohta T, Nohmi T, Sun W, Walker G C. Proc Natl Acad Sci USA. 1990;87:7190–7194. doi: 10.1073/pnas.87.18.7190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sommer S, Bailone A, Devoret R. Mol Microbiol. 1993;10:963–971. doi: 10.1111/j.1365-2958.1993.tb00968.x. [DOI] [PubMed] [Google Scholar]
- 19.Marsh L, Walker G C. J Bacteriol. 1985;162:155–161. doi: 10.1128/jb.162.1.155-161.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Opperman T, Murli S, Walker G C. J Bacteriol. 1996;178:4400–4411. doi: 10.1128/jb.178.15.4400-4411.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marsh L, Nohmi T, Hinton S, Walker G C. Mutat Res. 1991;250:183–197. doi: 10.1016/0027-5107(91)90175-n. [DOI] [PubMed] [Google Scholar]
- 22.Elledge S J. Science. 1996;274:1664–1672. doi: 10.1126/science.274.5293.1664. [DOI] [PubMed] [Google Scholar]
- 23.Paulovich A G, Toczyski D P, Hartwell L H. Cell. 1997;88:315–321. doi: 10.1016/s0092-8674(00)81870-x. [DOI] [PubMed] [Google Scholar]
- 24.Weinert T. Cell. 1998;94:555–558. doi: 10.1016/s0092-8674(00)81597-4. [DOI] [PubMed] [Google Scholar]
- 25.Elledge S J, Walker G C. J Mol Biol. 1983;164:175–192. doi: 10.1016/0022-2836(83)90074-8. [DOI] [PubMed] [Google Scholar]
- 26.Donnelly C E, Murli S, Walker G C. Mutat Res. 1994;309:225–233. doi: 10.1016/0027-5107(94)90096-5. [DOI] [PubMed] [Google Scholar]
- 27.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 28.Khidhir M A, Casaregola S, Holland I B. Mol Gen Genet. 1985;199:133–140. doi: 10.1007/BF00327522. [DOI] [PubMed] [Google Scholar]
- 29.Woodgate R, Singh M, Kulaeva O I, Frank E G, Levine A S, Koch W H. J Bacteriol. 1994;176:5011–5021. doi: 10.1128/jb.176.16.5011-5021.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frank E G, Ennis D G, Gonzalez M, Levine A S, Woodgate R. Proc Natl Acad Sci USA. 1996;93:10291–10296. doi: 10.1073/pnas.93.19.10291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohta T, Sutton M D, Guzzo A, Cole S, Ferentz A E, Walker G C. J Bacteriol. 1999;181:177–185. doi: 10.1128/jb.181.1.177-185.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hiom K J, Sedgwick S G. Mol Gen Genet. 1992;231:265–275. doi: 10.1007/BF00279800. [DOI] [PubMed] [Google Scholar]
- 33.Witkin E M, Roegner-Maniscalco V, Sweasy J B, McCall J O. Proc Natl Acad Sci USA. 1987;84:6805–6809. doi: 10.1073/pnas.84.19.6805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Woodgate R, Sedgwick S. Mol Microbiol. 1992;6:2213–2218. doi: 10.1111/j.1365-2958.1992.tb01397.x. [DOI] [PubMed] [Google Scholar]
- 35.Echols H, Goodman M F. Annu Rev Biochem. 1991;60:477–511. doi: 10.1146/annurev.bi.60.070191.002401. [DOI] [PubMed] [Google Scholar]
- 36.Woodgate R, Ennis D G. Mol Gen Genet. 1991;229:10–16. doi: 10.1007/BF00264207. [DOI] [PubMed] [Google Scholar]
- 37.Gonzalez M, Frank E G, Levine A S, Woodgate R. Genes Dev. 1998;12:3889–3899. doi: 10.1101/gad.12.24.3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sassanfar M, Roberts J W. J Mol Biol. 1990;212:79–96. doi: 10.1016/0022-2836(90)90306-7. [DOI] [PubMed] [Google Scholar]
- 39.Bailone A, Sommer S, Knezevic J, Devoret R. Biochimie. 1991;73:471–478. doi: 10.1016/0300-9084(91)90114-g. [DOI] [PubMed] [Google Scholar]
- 40.Yasuda T, Morimatsu K, Horii T, Nagata T, Ohmori H. EMBO J. 1998;17:3207–3216. doi: 10.1093/emboj/17.11.3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peat T S, Frank E G, McDonald J P, Levine A S, Woodgate R, Hendrickson W A. Structure (London) 1996;4:1401–1412. doi: 10.1016/s0969-2126(96)00148-7. [DOI] [PubMed] [Google Scholar]
- 42.Ferentz A E, Opperman T, Walker G C, Wagner G. Nat Struct Biol. 1997;4:979–983. doi: 10.1038/nsb1297-979. [DOI] [PubMed] [Google Scholar]
- 43.Lee M H, Ohta T, Walker G C. J Bacteriol. 1994;176:4825–4837. doi: 10.1128/jb.176.16.4825-4837.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee M H, Guzzo A, Walker G C. J Bacteriol. 1996;178:7304–7307. doi: 10.1128/jb.178.24.7304-7307.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee M H, Walker G C. J Bacteriol. 1996;178:7285–7294. doi: 10.1128/jb.178.24.7285-7294.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guzzo A, Lee M H, Oda K, Walker G C. J Bacteriol. 1996;178:7295–7303. doi: 10.1128/jb.178.24.7295-7303.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.George D L, Witkin E M. Mol Gen Genet. 1974;133:283–291. doi: 10.1007/BF00332704. [DOI] [PubMed] [Google Scholar]
- 48.George D L, Witkin E M. Mutat Res. 1975;28:347–354. doi: 10.1016/0027-5107(75)90229-8. [DOI] [PubMed] [Google Scholar]
- 49.Bridges B A. Mutat Res. 1975;29:489–492. [Google Scholar]
- 50.Bridges B A. BioEssays. 1995;17:63–70. doi: 10.1002/bies.950170112. [DOI] [PubMed] [Google Scholar]
- 51.Sedgwick S G, Ho C, Woodgate R. J Bacteriol. 1991;173:5604–5611. doi: 10.1128/jb.173.18.5604-5611.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sandell L L, Zakian V A. Cell. 1993;75:729–739. doi: 10.1016/0092-8674(93)90493-a. [DOI] [PubMed] [Google Scholar]
- 53.Echols H. Cell. 1981;25:1–2. doi: 10.1016/0092-8674(81)90223-3. [DOI] [PubMed] [Google Scholar]
- 54.Taddei F, Matic I, Godelle B, Radman M. Trends Microbiol. 1997;5:427–429. doi: 10.1016/S0966-842X(97)01157-8. [DOI] [PubMed] [Google Scholar]
- 55.Paulovich A G, Armour C D, Hartwell L H. Genetics. 1998;150:75–93. doi: 10.1093/genetics/150.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Donnelly C E, Walker G C. J Bacteriol. 1992;174:3133–3139. doi: 10.1128/jb.174.10.3133-3139.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]