Effects of secreted oligomers of amyloid β-protein on hippocampal synaptic plasticity: a potent role for trimers (original) (raw)
Abstract
The accumulation of amyloid β-protein (Aβ) in brain regions serving memory and cognition is a central pathogenic feature of Alzheimer's disease (AD). We have shown that small soluble oligomers of human Aβ that are naturally secreted by cultured cells inhibit hippocampal long-term potentiation (LTP) in vitro and in vivo and transiently impair the recall of a complex learned behaviour in rats. These results support the hypothesis that diffusible oligomers of Aβ initiate a synaptic dysfunction that may be an early event in AD. We now report detailed electrophysiological analyses that define conditions under which acute application of soluble Aβ inhibits hippocampal synaptic plasticity in wild-type mice. To ascertain which Aβ assemblies contribute to the impairment of LTP, we fractionated oligomers by size-exclusion chromatography and found that Aβ trimers fully inhibit LTP, whereas dimers and tetramers have an intermediate potency. Natural Aβ oligomers are sensitive to heat denaturation, primarily inhibit the induction phase of LTP, and cause a sustained impairment of LTP even after extensive washout. We observed no effects of Aβ oligomers on presynaptic vesicle release. LTP in juvenile mice is resistant to the effects of Aβ oligomers, as is brain-derived-neurotrophic-factor-induced LTP in adult hippocampus. We conclude that specific assemblies, particularly timers, of naturally secreted Aβ oligomers are potent and selective inhibitors of certain forms of hippocampal LTP.
Alzheimer's disease (AD) is the most common neurodegenerative disease, affecting more than 30 million individuals worldwide. Patients who develop AD initially experience subtle and transient impairments of declarative (particularly episodic) memory and gradually undergo a debilitating erosion of other types of memory and cognitive function. Multiple lines of evidence have converged on the notion that the 42-residue amyloid β-protein (Aβ) plays an essential role in the pathogenesis of AD. Although Aβ is generated throughout life from the normal processing of the β-amyloid precursor protein (APP), heritable forms of AD often alter Aβ production or its propensity to aggregate. In particular, a strong correlation has been established between the levels of soluble Aβ and the severity of dementia in humans (Lue et al. 1999; McLean et al. 1999). The amyloid (or Aβ) cascade hypothesis proposes that the abnormal accumulation of Aβ inhibits synaptic function, gradually induces neuritic and glial changes, and initiates a process of neurodegeneration (Hardy & Selkoe, 2002).
Transgenic mice have provided important insights into the chronology of events leading to the neuritic plaques, gliosis and neurofibrillary tangles that characterize AD. For instance, triple transgenic mice (PS1M146V, APPSWE, tauP301L) develop Aβ pathology prior to tau alterations, despite using the same promoter to drive expression of mutant human APP and tau (Oddo et al. 2003). Those same authors also found that the appearance of Aβ aggregation is correlated with impairments in long-term potentiation (LTP). Moreover, immunizing the triple transgenic mice with anti-Aβ antibodies reduces Aβ accumulation and slows the emergence of tau-containing tangles, suggesting that the build-up of Aβ precedes tau aggregation (Oddo et al. 2004; Billings et al. 2005). It has also been shown that Aβ accelerates tangle-like cytopathology in tau transgenic mice (Gotz et al. 2001; Lewis et al. 2001). In humans, familial forms of AD caused by missense mutations in APP or presenilin (the active site component of γ-secretase (Wolfe et al. 1999) lead to severe tau cytopathology (Sudo et al. 2005). Thus, excessive accumulation of Aβ may be one of the earliest pathogenic events in AD.
We have chosen to study natural oligomers of human Aβ that are secreted by cultured cells expressing APPV717F, a mutant form of APP known to cause an aggressive form of familial AD. The Aβ assemblies secreted by this ‘7PA2’ CHO cell line have been extensively characterized biochemically (Podlisny et al. 1995, 1998; Walsh et al. 2000). Cell-derived Aβ is distinct from the widely used synthetic Aβ preparations in at least four ways that make it attractive for understanding the neurophysiological properties of Aβ. First, the Aβ produced by the 7PA2 cells is naturally generated from human APP and has heterogeneous N- and C-termini similar to those that occur in brain, in contrast to synthetic Aβ peptides of a single defined length. Second, it has biological effects at low nanomolar to high picomolar concentrations, similar to those in human brain and cerebrospinal fluid (Motter et al. 1995; Mehta et al. 2000; Walsh et al. 2002), whereas synthetic Aβ typically needs to be applied to neurons at 2–4 orders of magnitude higher concentrations to achieve similar biological effects. Third, the 7PA2 cells naturally generate stable and soluble oligomers of Aβ, in addition to abundant monomers (Podlisny et al. 1995). We have previously reported that these low-n oligomers inhibit synaptic function, suggesting that cell-derived Aβ oligomers are in a biologically active conformation that may resemble the physical state of some Aβ species in the hippocampus of AD patients (Walsh et al. 2000; Kokubo et al. 2005). Fourth, the cell-derived oligomers interrupt LTP rapidly, robustly and consistently (Walsh et al. 2002, 2005; Klyubin et al. 2005), indicating that the electrophysiological action of Aβ can be readily assayed before significant compensatory effects, inflammatory reaction, neuritic degeneration or apoptosis have occurred. Importantly, the same Aβ oligomers microinjected intraventricularly into healthy behaving rats impair their ability to recall a complex learned behaviour (Cleary et al. 2005).
Here, we report a detailed electrophysiological characterization of the effects of secreted human Aβ on hippocampal synaptic plasticity in wild-type Swiss Webster mice. With improved separation of Aβ oligomers by size-exclusion chromatography (SEC), we show that a trimer species is a particularly potent inhibitor of LTP. Aβ oligomers have the most pronounced effect on induction rather than expression of LTP, yet have little effect on presynaptic release. Moreover, to the best of our knowledge, we show for the first time that not all forms of LTP are affected by Aβ oligomers. Thus, the effects of natural Aβ oligomers are selective for certain forms of synaptic plasticity.
Methods
Aβ preparation
Aβ was collected and prepared from 7PA2-cell conditioned (CM) as previously described (Walsh et al. 2005). 7PA2 cells are a CHO line that stably expresses human APP751 containing the V717F mutation. Cells were grown in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum, penicillin/streptomycin, l-glutamine, and G418 for selection. Once the cells reached approximately 95% confluency, they were washed and cultured overnight (∼15 h) in serum-free medium. CM was collected, spun at 1000 g to remove dead cells and debris, supplemented with a protease inhibitor cocktail (Sigma P1860 at 1:1000) and stored at −80°C. When ∼300 ml of medium had been collected, it was centrifuged (3000 g) at 4°C in YM-3 Centricon tubes to concentrate proteins larger than ∼3 kDa. This procedure concentrated the medium 15-fold, with some loss (∼30–50%) of the ∼4 kDa monomer species through the Centricon filter. The concentrated CM was pooled and aliquoted to produce a large number of identical medium samples for experiments. These aliquots were stored at −80°C until use. For experiments with 2× concentrated CM from 7WD4 CHO cells (stably expressing wild-type human APP751), the medium was spun to 30-fold concentration in YM-3 Centricon tubes, and then diluted 1:15 in artificial cerebrospinal fluid (ACSF).
Immunoprecipitation
Immunoprecipitation of Aβ from 8 ml of CM prepared as above was performed as described (Walsh et al. 2005). A cocktail of protease inhibitors (mg ml−1: leupeptin 1, pepstatin 1, aprotinin 0.1, EDTA 40, and 1,10-phenanthroline 0.4) was added. Samples were precleared with protein A sepharose for 30 min. The CM was then immunoprecipitated overnight with a 1:75 dilution of our polyclonal antibody R1282. The beads were washed with STEN buffers (mm: NaCl 150, Tris 50, EDTA 2, NP-40 0.2%, pH 7.4). Our standard wash protocol is 20 min 0.5 STEN, 20 min STEN + 0.1% SDS, 20 min STEN. The samples were then resuspended in 2× Tricine sample buffer, boiled, and the supernatant was frozen at −80°C or loaded directly onto Tricine SDS-PAGE gels.
Size-exclusion chromatography
To physically separate natural Aβ oligomers, 7PA2 CM was run on two Superdex 75 prep grade 20 × 500 mm columns (∼100 ml volume) arranged in series. Five millilitres of 15× concentrated CM was injected onto the columns and eluted with 50 mm ammonium acetate pH 8.5. An Amersham AKTA fast protein liquid chromatograph (FPLC) (Amersham Biosciences, Piscataway, NJ, USA) was used to collect 1 ml fractions, which were stored at −20°C until lyophilized. Blots of CHO-control CM (not shown) were similar to our previously reported results (Walsh et al. 2005). Individual SEC column fractions were resuspended in 15 ml of ACSF for electrophysiological experiments. Alternatively, the fractions were resuspended in 1× Tricine sample buffer, and half-fractions run on SDS-PAGE. The SEC columns were cleaned with H2O, 44% formic acid, 1 m NaOH and 1 m Tris-base.
Western blots
Samples were electrophoresed on 10–20% Tricine gels (Invitrogen or Bio-Rad), and the proteins transferred to 0.2 μm Optitran nitrocellulose. The membranes were boiled in water or phosphate-buffered saline (to enhance the exposure of Aβ epitopes) and blocked for 1 h in 50% Odyssey blocking buffer diluted in PBS. Blots were probed with the monoclonal antibody 2G3 (Elan), which is specific for Aβ peptides ending at residue 40, or with 6E10 (Signet), which recognizes amino acids 4–8 near the N-terminus of Aβ. Immunoreactive bands were detected and quantified using a Licor Odyssey imaging system.
Electrophysiology
Field potential recordings were made from coronal sections of postnatal day 16–28 male and female Swiss Webster mice. Mice were deeply anaesthetized with isoflurane before decapitation, in compliance with Harvard University's Animal Resources and Comparative Medicine policies for use of laboratory animals. The brain was rapidly removed from the skull and submerged in oxygenated (95% O2, 5% CO2) 4°C ACSF (mm: sucrose 206, KCl 2.8, CaCl2 1, MgCl2 1 MgSO4 2, NaH2PO4 1.25, NaHCO3 26, d-glucose 10, sodium ascorbate 0.4, pH 7.4; osmolarity 297) (Moyer & Brown, 1998). After 2 min, the cerebellum was removed and the remaining brain was bisected at the midline. The two hemispheres were glued to the sectioning chamber and re-immersed in 4°C ACSF. Coronal sections (350 μm) were prepared on a Vibratome 1000 Plus using stainless steel razor blades (Electron Microscopy Science). The sections were placed in oxygenated ACSF at 27°C (mm: NaCl 124, KCl 2.8, CaCl2 3.6, MgSO4 2, NaH2PO4 1.25, NaHCO3 26, d-glucose 10, sodium ascorbate 0.4, pH 7.4; osmolarity 306) in a custom slice recovery chamber designed to provide a circulating perfusion of aerated ACSF. The slices were allowed to recover for at least 75 min. The order of treatments was randomized for slices prepared from a given animal.
Glass electrodes (G8510T-3, Warner Instruments) were pulled on a P-97 Sutter Instruments pipette puller to resistances of 3–6 MΩ. Electrodes were filled with ACSF for field recordings. Electrical stimulation to the Schaeffer collaterals of the hippocampus was delivered through a World Precision Instruments bipolar (TM33A05) or unipolar (TM33CCNON) electrode. Field potential recordings were made at room temperature (∼27°C) using an Axon Instruments 200B amplifier and digitized with a Digidata 1322 A. Electrodes were specifically placed just below the surface of the slice to maximize the exposure to circulating Aβ. (It has not yet been determined how quickly or deeply the Aβ can penetrate the slice). Data were stored and analysed on an IBM PC running pClamp 9.1 software. Recordings were sampled at 10 kHz and low-pass filtered at 5 kHz. The slope of the EPSP was estimated from 10 to 60% of the evoked response. The intensity of the stimulus was set to 20–30% of the maximum evoked EPSP or until a population spike was elicited. A typical stimulation intensity was approximately 7.5 μA. Slices were perfused for 20 min in ACSF to establish a steady baseline. During this interval, 1 ml of 15× concentrated CM (with Sigma protease inhibitors) was thawed to 37°C and diluted to 1× in 15 ml ACSF. This 1× CM/ACSF solution was recirculated over the slice using a P720 Instech peristaltic pump at 2.5–3 ml min−1 while being continuously aerated with 95% oxygen. LTP was induced 20 min later by delivering four 100 Hz stimuli every 5 min. The slope of the EPSP was monitored for 1 h after the last high-frequency stimulation.
For chemical-LTP experiments, brain slices were bathed in ACSF containing 0 mm Mg2+, 10 μ m picrotoxin, 200 μ m glycine (Lu et al. 2001). LTP was prevented by 100 μ md,l-2-amino-5-phosphonovaleric acid (AP5).
To demonstrate retention of Aβ on brain slices, three brain sections were continuously perfused for 2 h with recirculating 7PA2 CM or its FPLC fractions. The brain sections were homogenized in 1 ml of Tris-EDTA buffer (50 mm Tris, pH 7.4, 5 mm EDTA and protease inhibitor cocktail as described above) containing 1% Triton X-100. Samples were sonicated for 5 s, and the volume adjusted to 10 ml in Tris-EDTA buffer (without Triton). Aβ was immunoprecipitated overnight with the polyclonal antibody R1282. Immunoprecipitation of the perfusate showed no detectable Aβ remaining in the solution (data not shown), indicating near complete transfer to the tissue and tubing. Recovery of Aβ from slices was considerably less than that found in the starting 7PA2 CM, suggesting some loss to the perfusion tubing or lower efficiency of immunoprecipitation in the presence of brain homogenate.
Chemicals
Reagents for electrophysiological solutions were from Sigma. AP5 was from Tocris, and brain derived neurotrophic factor (BDNF) was acquired from Peprotech and Cell Sciences. BDNF was used on the day of dilution, since we found that the bioactivity of BDNF fell rapidly after solubilizing (Kang & Schuman, 1995).
Statistics
Typical comparisons were done using Student's t test. For comparing the slope of the t values for regression lines, t values were calculated as t = (_C_1−_C_2)/√[(s.d. 1 × s.d. 2/_n_1) + (s.d. 2 × s.d. 2/_n_2)]. P values were calculated from the calculated t value and the degrees of freedom. Error bars indicate the s.e.m.
Results
Naturally secreted Aβ oligomers can be separated with high resolution
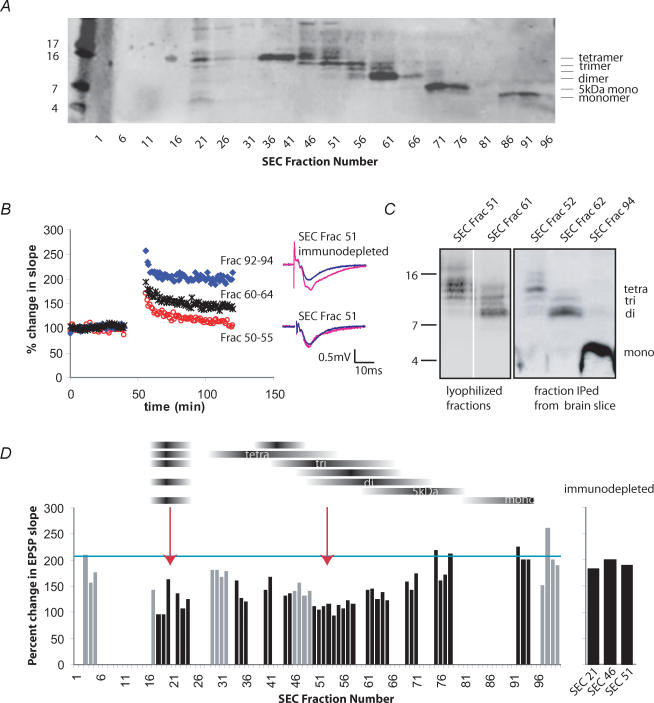
The cell-derived human Aβ used throughout these experiments was obtained from the CM of a CHO cell line that stably expresses human APP751V717F. These cells (7PA2) secrete biochemically well-characterized monomeric and oligomeric Aβ species whose identities have been confirmed by both radiosequencing and selective immunoprecipitation with numerous N- and C-terminal-specific Aβ antibodies (Podlisny et al. 1995; Walsh et al. 2000). We recently reported a method for the fractionation of the 7PA2 CM that employs SEC to separate oligomers from monomers (Walsh et al. 2005). Only fractions containing the oligomers have been shown to impair LTP in vitro and in vivo. To achieve better separation of the oligomeric species, we used two Sephadex 75 SEC columns run in series. The resulting fractions were lyophilized and analysed by Western blotting. The use of the tandem SEC columns allowed for separation of six principal Aβ-immunoreactive species that could be detected by monoclonal antibodies specific for amino acids 4–8 (6E10) and the C-terminus (2G3) (Fig. 1_A_). The fractionation produced a clear laddering by size, such that early eluting fractions contained larger oligomers and later eluting fractions contained mostly monomer, with the exception of a range of Aβ species observed in a very early eluting fraction (no. 21). The appearance of a tetrameric species as well as several low-molecular-weight Aβ bands in this early eluting fraction may indicate a larger but labile multimer that depolymerizes during SDS-PAGE. The improved separation of different-sized Aβ oligomers achieved with this modified SEC method enabled us to assess for the first time the effects of individual oligomeric assemblies on synaptic plasticity.
Figure 1. Amyloid β-protein trimers potently inhibit long-term potentiation in the CA1 region of mouse hippocampal slices.
A, secreted human amyloid β-protein (Aβ) oligomers from 7PA2 (APP751V717F) conditioned medium (CM) were size-separated by fast protein liquid chromatography (FPLC). Column fractions were lyophilized and examined by tricine SDS-PAGE. Blotting with the Aβ40-specific antibody 2G3 revealed the fractionation of a ladder of oligomers from tetramers down to monomers. B, each lyophilized fraction was resuspended in artificial cerebrospinal fluid (ACSF) and perfused over mouse hippocampal slices for 20 min before four high-frequency stimulations (HFS; 100 Hz, 1 s) were given. Field potential recordings were made in the CA1 region. Size-exclusion chromatography (SEC) fractions 50–55, enriched for Aβ trimers, strongly inhibited long-term potentiation (LTP) at 60 min post-HFS (118 ± 8.8% s.e.m., whereas monomer fractions had no effect (202 ± 19.1% Student's t test, P < 0.01, n = 12 and 13, respectively). Fractions 60–64, which were enriched for dimer, showed an intermediate effect that was significantly different from monomer but not trimer (149 ± 6.7%). C, oligomeric assemblies do not change in size after perfusion over hippocampal slices, indicating high stability. Lyophilized SEC fractions were run directly on a Western blot (left panel) or else used for electrophysiology followed by immunoprecipitation (with R1282) of Aβ species from the homogenized slices. Blots were probed with 6E10. Note that 6E10 showed better detection of the Aβ monomer relative to oligomers than did 2G3. D, summary histogram depicting the potentiation of the EPSP slope 60 min post-HFS for many of the SEC fractions (n = 3 independent SEC fractionation runs). The mean potentiation obtained using CHO-control CM (lacking human Aβ) is shown as a blue bar just above 200%. The black horizontal bands above the histogram depict the relative abundance of each oligomeric band across the fractions. Arrows point to the regions of the histogram representing the greatest LTP inhibition (fractions 18–23 and 50–58). Immunodepletion of the inhibitory fractions with Aβ antibody R1282 restored normal LTP (far right bars). Black vertical histogram bars indicate fractions of primary interest in this study, while the grey bars show results from intervening fractions.
To determine the biological activity of these fractions, field potential recordings were made in the CA1 region of wild-type mouse hippocampal brain slices. A stable baseline was established for 20 min. Individual SEC fractions were then diluted in 15 ml ACSF and continuously recirculated over the slice for an additional 20 min. Four periods of high-frequency stimulation (HFS), 100 Hz, 1 s spaced 5 min apart, were delivered to the Schaeffer collaterals, and the subsequent potentiation of the evoked postsynaptic potential (EPSP) was followed for 60 min. As expected from our previous work, fractions that contained solely monomers (e.g. 92–94) showed no effect on LTP at 60 min post-HFS, whereas oligomer fractions (50–55, predominantly trimer) and (60–64, predominantly dimer) potently inhibited LTP (Fig. 1_B_).
Our prior studies have shown that cell-derived Aβ oligomers are highly stable and resistant to SDS, sample boiling, urea and formic acid treatment (Walsh et al. 2002). However, it is possible that Aβ oligomers become altered after they have been perfused over brain tissue. To address this, Aβ was immunoprecipitated with polyclonal antibody R1282 from homogenized slices that had been treated with the SEC fractions for 2 h. Compared with adjacent starting fractions that had never been applied to slices, there was no significant change in the size pattern of the oligomeric species after being in contact with the hippocampal slices for 2 h (Fig. 1_C_). These results demonstrate that the cell-secreted Aβ oligomers are highly stable and can be recovered intact after incubation with brain tissue.
Trimers of Aβ inhibit LTP more potently than other low-n oligomers
Based on these results, we tested which oligomeric species were most potent at inhibiting hippocampal LTP. Three independent SEC runs were carried out, and the resulting fractions were all individually tested in LTP experiments. The three replicate results for a given fraction number (always measured at 60 min post-HFS) were averaged and are depicted in the histogram in Fig. 1_D_. The average potentiation achieved in the presence of the parental CHO-control CM (no human APP expression) is shown as a horizontal line just above 200%, while 100% represents no change from the pre-HFS baseline EPSP slope. Gaps in the histogram reflect SEC fractions that were used for other purposes (e.g. Western blotting or immunodepletion (below); small regions of the gel pattern that were of minimal interest or showed overlapping oligomers; or experiments that failed for technical reasons, such as bubbles in the perfusion which perturbed the slice). Although there is some variability that is intrinsic to the technique, the results clearly demonstrate that fractions 50–58 (which correspond principally to a 11–12 kDa trimeric band) are extremely potent inhibitors of LTP (_r_2 = 0.516 anticorrelated with LTP) (Fig. 1_B_ and D), despite being a fainter band by Western blot than are the tetramers (Fig. 1_A_, fractions 36–41) or the dimers (Fig. 1_A_, fractions 61–66). While both the 5 kDa Aβ species (which we have previously identified by mass spectroscopy as an SDS-stable conformer of the 4 kDa monomer; Walsh et al. 2000) and the 4 kDa monomer itself showed little effect (_r_2 = 0.076 and 0.257, respectively), the fractions enriched principally in tetramers or dimers demonstrated an intermediate inhibition of LTP (Fig. 1_B_) (t test compared with monomer, P < 0.05). Fractions immediately surrounding no. 21 that contain several Aβ species (Fig. 1_A_), also caused pronounced inhibition of LTP (Fig. 1_D_). To confirm that the LTP inhibition was specifically due to Aβ, some of the inhibitory SEC fractions were immunodepleted of Aβ with the polyclonal antibody R1282 and then tested for effects on LTP. Immunodepletion of Aβ from these fractions fully restored LTP (Fig. 1_D_, far right panel). Therefore, the inhibition of LTP by these SEC fractions is attributable specifically to Aβ rather than another coeluting protein. Using polydextran standards, we have previously shown that the trimers elute from the column at a molecular weight of ∼12 kDa (Walsh et al. 2005), providing further evidence that it is the trimers per se, not a larger Aβ assembly, that is responsible for the observed inhibition of LTP. We conclude that soluble trimeric assemblies of human Aβ are of particular pathogenic interest because of their potent, complete inhibition of hippocampal LTP. Nevertheless, these data also show that all oligomer-containing fractions tested cause some impairment of LTP.
Cell-derived Aβ oligomers prevent induction but not expression of LTP and are sensitive to heat denaturation
The electrophysiological mechanisms by which soluble Aβ oligomers impair LTP are not well understood. Because multiple Aβ oligomer species inhibited LTP, we proceeded to use whole (unfractionated) CM to characterize the detailed effects of oligomers on synaptic function. We began by asking whether the oligomer-rich 7PA2 CM interferes principally with the induction and/or the expression of LTP. 7PA2 CM was applied to hippocampal slices immediately after the HFS used to induce LTP (Fig. 2_A_). 7PA2 CM was invariably ineffective at inhibiting LTP (measured at 60 min) when applied after the HFS. These data indicate that soluble, low-n Aβ oligomers primarily interfere with the induction of LTP, but not its expression, once the signal transduction cascades which mediate LTP have commenced.
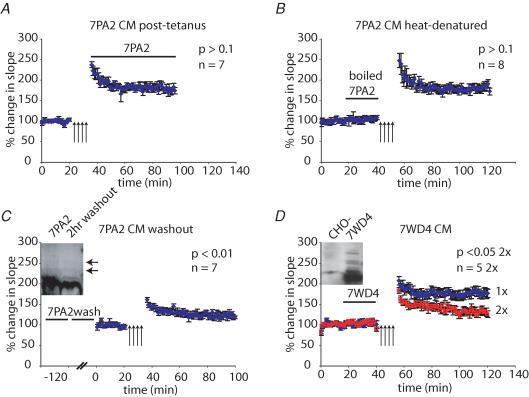
Figure 2. Timing and temperature influence the effect of 7PA2 CM on LTP.
A, 7PA2 CM did not significantly affect LTP (measured 60 min post-HFS) when it was applied immediately after the HFS (180 ± 9.4% Student's t test, P > 0.1, n = 7). B, boiling 7PA2 CM eliminated its inhibitory effect on LTP (182 ± 10.5% P > 0.1, n = 8). C, a 2 h washout of slices pretreated with 7PA2 CM did not prevent the inhibitory effect of the CM on LTP (124 ± 9.4% P < 0.01, n = 7) and it remained similar to the effect of acute application of 7PA2 CM, which we have previously reported as 131 ± 8.8%. The inset shows that a considerable amount of Aβ, including oligomers, was retained in the slice even after a 2 h washout period. D, CM from 7WD4 cells (expressing wild-type human APP751) contained Aβ oligomers (inset, compared to control CHO- CM; probed with 6E10 + 2G3) and inhibited LTP when adjusted to have a similar Aβ concentration as 7PA2 CM (132 ± 14.6% P < 0.05, n = 5 for 2× concentrated).
To assess whether the biological activity of soluble Aβ oligomers is heat sensitive, 7PA2 CM was boiled for 5 min, cooled to room temperature and then applied to hippocampal slices. As shown in Fig. 2_B_, LTP was found to be normal in the presence of the heat-denatured CM. Thus, heating 7PA2 CM prevents its inhibition of LTP, presumably by denaturing the biologically active conformation(s) of Aβ oligomers (Wang et al. 2004). Alternatively, a small-molecule cofactor of Aβ could be released by boiling, rendering the Aβ oligomers inactive.
We next sought to establish whether the effects of 7PA2 CM on LTP could be reversed by extensive washout of the slices. Hippocampal slices were treated with 7PA2 CM for 20 min and then washed by returning the slices to the slice recovery chamber for 2 h. Thereafter, HFS stimulation was performed as previously described. The prolonged washing failed to prevent the inhibition of LTP caused by the 7PA2 CM (Fig. 2_C_). After this LTP assay was completed, the slices were homogenized and subjected to immunoprecipitation/Western blot to determine how much of the Aβ remained in the slice after the washout procedure. As shown in Fig. 2_C_ (inset), some Aβ (oligomers and monomer) was recovered from the slice even after a 2 h washout period, although the amount retained appeared to be less than in a slice that was homogenized immediately after perfusion with 7PA2 CM. Thus, a 2 h washout period did not reverse the inhibition of LTP, nor did it efficiently clear the tissue of Aβ species.
Although the 7PA2 cells have been a consistent and reliable source of bioactive Aβ oligomers, we wished to perform LTP experiments with CM from a distinct cell line to rule out the unlikely possibility that the LTP inhibition is cell-line specific. 7WD4 cells stably overexpress wild-type human APP751 (Xia et al. 1997) and produce soluble Aβ oligomers similar to those of the 7PA2 line (Fig. 2_D_, inset). When prepared identically to 7PA2 CM (i.e. at 1× concentration), the 7WD4 CM did not significantly inhibit LTP, whereas at a 2× concentration, it did cause a significant reduction in hippocampal LTP at 60 min post-HFS (Fig. 2_D_). Therefore, soluble low-n oligomers of human Aβ produced by both 7PA2 and 7WD4 cell lines are capable of inhibiting hippocampal LTP.
The V717F APP mutation expressed in the 7PA2 cells has been shown to increase the Aβ42/Aβ40 ratio in the CM and enhance oligomerization (Xia et al. 1997). The observed difference in potency of 7PA2 versus 7WD4 CM (Fig. 2_D_) could be due to the type of oligomer species and/or to the levels of APP expression in the two cell lines. To address this issue, immunoprecipitation /Western blots were performed on CM and cell lysates of the two lines. Both 7PA2 CM and 7WD4 CM contained a similar dimer doublet and a trimer band that migrated distinctly from the dimers of synthetic Aβ on Tricine SDS-PAGE gels (Fig. 3_A_). However, the 7WD4 cells consistently showed a lower abundance of total Aβ and altered ratios of the Aβ species. A Licor Odyssey gel analysis system was used to quantify the optical density of each Aβ species from the two cell lines. Importantly, the oligomer ratios were found to be different between the two cell lines, with 7PA2 cells showing a significantly higher trimer/monomer ratio than the 7WD4 cells (Fig. 3_B_). The lower relative abundance Aβ trimers in the 7WD4 CM, which has a reduced potency to inhibit LTP, is consistent with our finding above that trimers are particularly potent at inhibiting LTP.
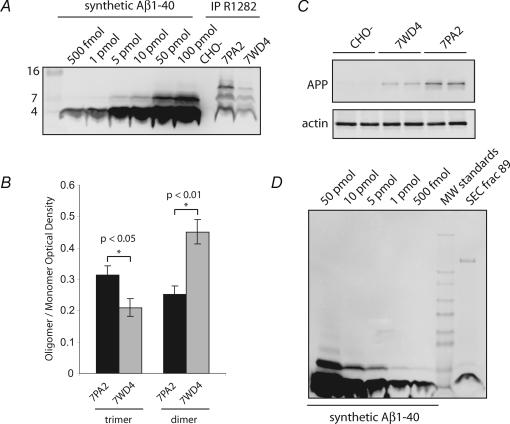
Figure 3. Comparison of 7PA2 CM, 7WD4 CM and synthetic Aβ shows differences in quality and quantity.
A, a titration curve of synthetic Aβ demonstrated the high sensitivity of the immunoprecipitation/Western blot assay. Apparent dimers of synthetic Aβ1–40 were detected at higher concentrations, although they migrated distinctively from the cell-derived Aβ oligomers on Tricine gels. Similar types of Aβ species (monomers, dimers and trimers) were found in 7PA2 and 7WD4 CM, but in different ratios. B, quantifying these ratios demonstrated that compared to 7WD4 CM, 7PA2 cells expressed a higher ratio of trimers relative to monomers (Student's t test, P < 0.05, n = 16 for 7PA2, n = 7 for 7WD4). C, 7PA2 cells also stably expressed β-amyloid precursor protein (APP) at higher levels relative to 7WD4 cells. D, a titration standard of synthetic Aβ was used to estimate the amount of Aβ momomer in SEC fractions (as in Fig. 1). Fractions 89 and 92 (not shown) contained approximately 750 fmol (∼3 ng) of Aβ monomer.
We also evaluated the relative levels of APP expression in the two cell lines by direct Western blotting. When cell lysates were probed with the anti-APP antibody 22C11, we observed that 7PA2 cells had a somewhat higher expression of APP (Fig. 3_C_) (Xia et al. 1997). Therefore, two factors (the better expression of APP in 7PA2 cells and the higher abundance of Aβ trimers due to the V717F mutation) are likely to contribute to the consistently greater inhibition of LTP by 7PA2 CM.
Minute quantities of natural Aβ oligomers are sufficient to impede synaptic function
Next, we wished to establish as accurately as possible the concentration of natural Aβ to which the hippocampal neurons were being subjected during a typical LTP experiment. To this end, a titration standard of synthetic human Aβ was compared to the SEC fractions containing natural human Aβ monomer, using quantification on a Licor Odyssey gel analysis system (Fig. 3_D_). Although there are limitations to this approach (see Discussion), we estimate that the total amount of secreted Aβ monomer in our SEC fractions is ∼750 fmol (∼3 ng/15 ml or ∼50 pm). Based on this estimate of monomer, we calculated the approximate concentration of tetramer, trimer and dimer species based on their relative optical density measurements. By this accounting, tetramer is predicted to be ∼150 pm, trimer ∼100 pm and dimer ∼300 pm, levels that approximate our previous estimates for total oligomer content (Podlisny et al. 1995). Thus, very low concentrations of naturally secreted soluble Aβ oligomers are sufficient to robustly and consistently impair hippocampal LTP.
Several aspects of synaptic function are unaffected by the oligomer-rich CM
Our previous work has shown that 7PA2 CM has no effect on baseline field potential recordings in the absence of a HFS (Fig. 2) (Walsh et al. 2005), demonstrating that basal synaptic transmission is unaffected by Aβ. We tested the effects of 7PA2 CM on short-term plasticity to determine if, like LTP, it was similarly inhibited by Aβ oligomers. Post-tetanic potentiation (PTP) is a short-term enhancement of presynaptic release thought to be caused by residual Ca2+ in the presynaptic terminals following a HFS (Zucker & Regehr, 2002). Slices were treated with AP5 to block NMDA receptors, thereby eliminating postsynaptic potentiation. Slices treated with either control CHO- CM or 7PA2 CM showed an indistinguishable PTP (Fig. 4_A_), suggesting that this aspect of presynaptic function is not affected by soluble Aβ oligomers.
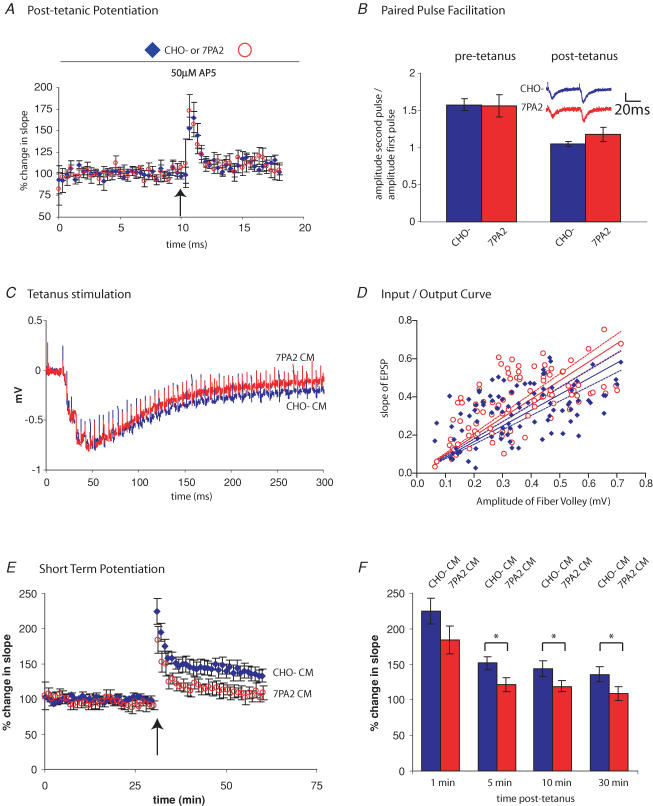
Figure 4. Effects of 7PA2 CM on LTP are not attributable to alterations in post-tetanic potentiation, paired-pulse facilitation or HFS.
A, slices were pretreated with CHO- CM or 7PA2 CM for 20 min, and the NMDA receptor antagonist d,l-2-amino-5-phosphonovaleric acid (AP5) for 10 min. A single HFS (100 Hz, 1 s) was delivered (arrow) to facilitate presynaptic release. The post-tetanic potentiation was indistinguishable for slices treated with either CHO- CM or 7PA2 CM (n = 7 CHO- CM; n = 6 7PA2). B, paired-pulse ratios were measured in slices treated with CHO- CM or 7PA2 CM by delivering two pulses, 50 ms apart. A ratio of the amplitude of second pulse to first pulse was determined (left bars). There was no significant difference between the two treatments (1.57 ± 0.08 for CHO- CM and 1.56 ± 0.15 for 7PA2 CM; Student's t test, P > 0.1, n = 4). A paired-pulse ratio was also measured 1 min after a single HFS (right bars), and no significant difference was observed (1.04 ± 0.03 for CHO- CM and 1.18 ± 0.10 for 7PA2 CM; P > 0.1, n = 4). C, EPSP responses to the first of four HFS (100 Hz, 1 s) were aligned from slices treated with CHO- CM or 7PA2 CM and overlayed. There was no difference in the average peak amplitudes, number of pulses to peak amplitude, or decay kinetics between the two treatments (n = 23 for CHO- CM and for 7PA2 CM). D, an input/output ratio between the amplitude of the fibre volley (presynaptic) and the slope of the EPSP (postsynaptic) over a range of stimulus intensities was performed. A small but significant shift in the slope of the regression curve was detected (P < 0.05). Dotted lines indicate the respective 95% confidence intervals. E, average traces from short-term potentiation (STP) experiments demonstrate a divergence in EPSP slope between slices treated with CHO- CM control and 7PA2 CM. Slices were perfused with CM for 20 min prior to delivering one HFS (100 Hz, 1 s) (arrow). F, histogram depicting the effect of CHO (diamonds) and 7PA2 (circles) CM on STP. Measured as the percentage change in EPSP slope for the given time intervals, we observed that by 5 min, there was a significant difference between CHO- CM- and 7PA2-CM-treated slices (151 ± 8.5% CHO- CM and 121 ± 10.4% 7PA2 CM; t test, P < 0.05, n = 9 and 7, respectively). The difference between CHO- CM and 7PA2 CM persisted for 10 min (144 ± 6.6% CHO- CM and 122 ± 4.9% 7PA2 CM) and 30 min (136 ± 6.1% CHO- CM and 108 ± 5.6% 7PA2 CM).
We next examined the paired-pulse ratio (PPR), which is commonly used as a measure of the probability of synaptic vesicle release. Two stimuli were delivered 50 ms apart, and the EPSP amplitude ratio of the second to the first pulse was calculated. In PPR experiments, it is thought that the second evoked EPSP is typically larger than the first because of residual Ca2+ in the presynaptic terminal that increases the probability of neurotransmitter release. Again, we observed no difference in the PPR between slices treated with control CHO- CM and oligomer-rich 7PA2 CM (Fig. 4_B_). We also looked at the PPR 1 min after a HFS; PPR is typically decreased post-HFS due to an increased probability of transmitter release. No significant difference was found between slices treated with CHO- CM or 7PA2 CM (Fig. 4_B_), suggesting that the probability of neurotransmitter release is not altered by Aβ oligomers.
During the HFS used to elicit LTP, the EPSPs show a characteristic bimodal response, initially facilitating and then depressing. We asked whether Aβ oligomers can reduce the response to the HFS, thereby causing suboptimal stimulation. This scenario seemed unlikely, because 4 HFS is considered to be a saturating LTP stimulus. Indeed, when the HFS profiles were compared, there was no detectable difference between slices treated with CHO- CM or 7PA2 CM (number of stimuli to peak amplitude, peak amplitude, decay). Therefore, although 7PA2 CM inhibits LTP during its induction phase, we found no evidence that Aβ oligomers alter the pattern of EPSPs during the HFS stimulation.
The input/output ratio compares the size of the fibre volley (representing the evoked action potentials in the Schaeffer collaterals) with the size of the evoked EPSP in the CA1 pyramidal neurons. This measure provides an estimate of the amount of presynaptic stimulation required to elicit a given postsynaptic response and imparts information regarding the effectiveness of the synaptic connections. For these experiments, slices were treated with CHO- CM or 7PA2 CM, and the stimulation intensity adjusted between a minimal and maximal evoked EPSP. As shown in Fig. 4_D_, 7PA2-CM-treated slices showed a slight but significant increase in slope in the regression curve, compared to those in CHO- CM (P < 0.05). This result indicates that slices treated with 7PA2 CM show a larger EPSP for a given input stimulation. Preliminary follow-up studies suggest that this is probably due to the secreted APP ectodomain (APPs) that is also found in the 7PA2 CM. However, this small shift is not likely to account for the effects of the oligomer-rich 7PA2 CM on LTP.
Finally, we compared the short-term potentiation after stimulation of hippocampal slices with a single HFS (100 Hz, 1 s). At 5, 10 and 30 min post-HFS, there was a significant difference between slices treated with control CHO- CM and 7PA2 CM (Fig. 4_E_ and F). These data demonstrate that Aβ oligomers inhibit even the earliest stages of synaptic plasticity following a HFS.
Not all forms of LTP are vulnerable to Aβ oligomers
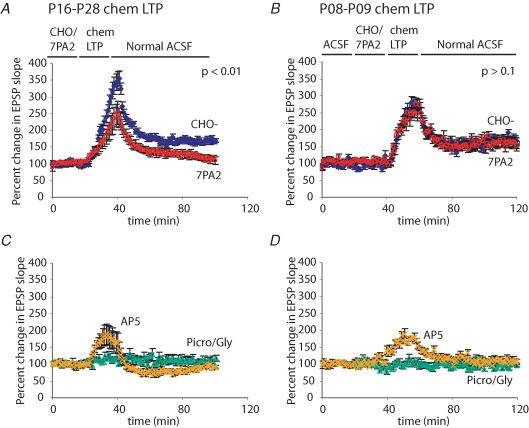
APP transgenic mice typically do not show memory deficits for several months after birth, presumably because sufficient levels of bioactive Aβ species must accumulate to induce neuronal dysfunction. However, an additional explanation could be that very young mice differ in their susceptibility to Aβ, particularly because the mechanisms involved in LTP differ from those in older animals (Yasuda et al. 2003). Because postnatal day (P)8–9 mice have immature synapses that are not capable of following HFS, we modified our LTP protocol to induce a chemical LTP by using picrotoxin, glycine and low Mg2+ to elevate spontaneous synaptic activity in brain slices (Neuhoff et al. 1999; Lu et al. 2001; Ehlers, 2003). In the presence of CHO- CM, this protocol induced a long-lasting potentiation of the EPSP in both P8–9 and P16–28 mice 60 min after induction (Fig. 5_A_ and B). While 7PA2 CM inhibited this chemical LTP in older animals (Fig. 5_A_), there was no significant inhibition in the P8–9 mice (Fig. 5_B_). The chemical LTP was blocked by AP5, demonstrating its dependence on NMDA receptors (Fig. 5_C_ and D), and it was not due to residual picrotoxin or glycine in the slices, as there was no effect on the EPSP slope unless the Mg2+ concentration was lowered during the induction protocol. We conclude that soluble Aβ oligomers do not exert a significant effect on hippocampal LTP in P8–9 mice.
Figure 5. 7PA2 CM inhibits chemical LTP in older but not younger mice.
A, hippocampal slices from postnatal day (P)16–28 mice were treated with CHO- CM or 7PA2 CM for 20 min. The usual ACSF in the perfusate was replaced with ACSF containing 0 mm Mg2+. After 5 min, picrotoxin and glycine were added to the perfusion for an additional 15 min to increase spontaneous synaptic activity. This yielded a large increase in the EPSP, which subsided upon restoration with normal ACSF. Slices treated with CHO- CM showed a persistent enhancement of the EPSP at 60 min after wash-in of normal ACSF, whereas 7PA2 CM caused a significant inhibition of this effect (166 ± 8.2% CHO- CM and 112 ± 8.3% 7PA2 CM; Student's t test, P < 0.01, _n_ = 5 for CHO- CM and _n_ = 8 for 7PA2 CM). _B_, mice of ages P8–9 also responded to the chem-LTP protocol with a persistent enhancement of the EPSP. However, there was no significant difference between CHO- CM- and 7PA2-CM-treated slices at 60 min after restoring normal ACSF (175 ± 10.9% CHO- CM and 155 ± 22.8% 7PA2 CM; _P_ > 0.1, n = 7 for CHO- CM and n = 7 for 7PA2 CM). C, the enhancement of synaptic function was a form of LTP, as it was blocked by the NMDA receptor antagonist AP5 (P < 0.01, n = 5). Also, the effect could not be attributed to residual picrotoxin or glycine in the slice, since chem-LTP could not be induced without perfusing the slice with 0 mm Mg2+ (P < 0.01, n = 4). D, as with the slices from older mice, the LTP was blocked by AP5 (P < 0.05, n = 5), and picrotoxin/glycine treatment alone did not augment EPSPs (P < 0.01, n = 5).
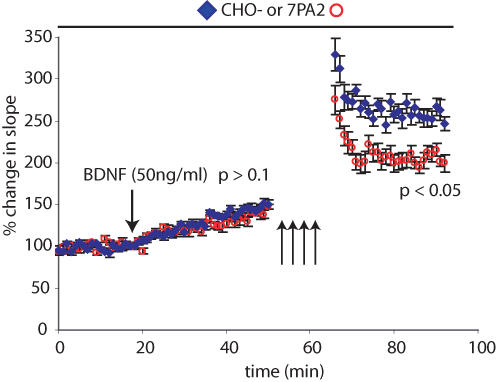
It has been shown previously that BDNF can induce a stable form of LTP that is mechanistically distinct from stimulus-induced LTP (Kang & Schuman, 1995). To test whether this form of LTP was similarly susceptible to Aβ oligomers, brain slices from P16–28 mice were pretreated with CHO- CM or 7PA2 CM for a 20 min baseline period. BDNF was then added to the perfusion medium, resulting in a modest potentiation over 30 min. There was no significant difference between CHO- CM- or 7PA2 CM-treated slices at this juncture (Fig. 6). The slices were then stimulated with 4 HFS. As expected, at 30 min post-HFS, there was already a significant difference between the two treatments (Fig. 6). These results suggest that BDNF-induced LTP is not abrogated by Aβ oligomers, in striking contrast to electrical LTP.
Figure 6. BDNF-induced LTP is resistant to the effects of 7PA2 CM.
Slices were pretreated with CHO- CM or 7PA2 CM for 20 min. Brain-derived neurotrophic factor (BDNF; 50 ng ml−1) (see Methods) was added to the perfusate, and EPSPs were followed for 30 min. Four HFS (100 Hz, 1 s) were then delivered, and the potentiation followed for an additional 30 min. The average slopes of the EPSPs at 30 min post-BDNF were not significantly different between CHO- CM- and 7PA2-CM-treated samples (147 ± 8.1% CHO- CM and 138 ± 3.4% 7PA2 CM; Student's t test, P > 0.1, n = 9 for CHO- CM and n = 8 for 7PA2 CM). However, 7PA2 CM caused significant inhibition after HFS (compared to CHO- CM) at 30 min post-HFS (P < 0.05).
Discussion
It is of great interest to identify very early events in the mechanism of Alzheimer's disease that may be amenable to pharmacological intervention before significant neuronal degeneration has occurred. Soluble Aβ accumulation, altered phosphorylation of tau, and decreases in synaptic density appear to occur early in the course of AD-like cytopathology in mouse models and perhaps also in patients with mild cognitive impairment, a frequent harbinger of AD (Davies et al. 1987; Terry et al. 1991; Hsia et al. 1999; Lue et al. 1999; McLean et al. 1999; Mucke & Masliah, 2000; Koistinaho et al. 2001; Masliah et al. 2001; Hardy & Selkoe, 2002; Lavados et al. 2005). Emerging evidence suggests that a failure of normal synaptic function may be one of the earliest measurable deficits in AD (reviewed in Selkoe, 2002). Acute application of soluble forms of human Aβ can rapidly (within minutes) and, in some cases, reversibly alter synaptic transmission (Chen et al. 2002; Walsh et al. 2002, 2005; Wang et al. 2002; Wang et al. 2004). Injection of Aβ antibodies into adult APP transgenic mice can rapidly restore synaptic function without overt changes in histopathology (DeMattos et al. 2001; Dodart et al. 2002). Moreover, endogenous or exogenous Aβ antibodies can reverse the acute effects of soluble Aβ oligomers on LTP in rats (Klyubin et al. 2005). Such results suggest that certain forms of diffusible Aβ may rapidly and continuously inhibit synaptic function, presumably leading gradually to long-term alterations in synapse structure (Neuhoff et al. 1999). It was recently reported that intrahippocampal application of an Aβ antibody in young APP transgenic mice prior to plaque formation rapidly reduced intracellular Aβ accumulation and subsequently hyperphosphorylated tau aggregation, consistent with the hypothesis that excessive Aβ can induce local neuronal abnormalities (Oddo et al. 2004). Therefore, understanding the acute effects of soluble Aβ on synaptic function may provide important clues to the genesis of the earliest stages of the disease and offer new possibilities for intervention.
One obstacle to understanding how Aβ inhibits LTP is the ambiguity over which species are responsible. We previously reported that soluble low-n Aβ oligomers (but not monomers) can inhibit LTP (Walsh et al. 2002, 2005). Using an improved SEC method to separate naturally secreted Aβ oligomers by size, we now demonstrate that a ∼12 kDa trimeric species is particularly potent at inhibiting LTP. While it is unknown whether a similar Aβ trimer is found in the AD brain, we have previously reported that low-n oligomers are detectable in human cerebral spinal fluid (Walsh et al. 2000). Other low-n oligomers, including dimers and tetramers, produced a partial inhibition, but consistently less than did the trimer. Both the 4 kDa classical monomeric band and the band migrating just above it at 5 kDa in 7PA2 medium have been confirmed by mass spectrometry to be conformers of the Aβ monomer (Walsh et al. 2000), and intensive efforts are underway to obtain mass spectrometry data on the other oligomer species. We also noted that a few early eluting fractions (21) contained several low-n Aβ species. It is unknown at this time whether this represents a larger oligomer that breaks down during SDS PAGE, or whether this fraction may contain an unknown Aβ binding protein. Intriguingly, these early fractions did show a fairly strong inhibition of LTP. Additional studies will be needed to characterize these fractions.
Several groups have made important advances in the preparation of synthetic Aβ to generate low-n oligomers (Lambert et al. 1998; Barghorn et al. 2005). It has now been shown that nanomolar concentrations of these synthetic Aβ42 assemblies can inhibit LTP. Moreover, Ashe and colleagues have proposed that the appearance of a 12-mer Aβ species in APP transgenic mice correlates with disease progression (K. Ashe, personal communication). It will therefore, be important to identify commonalities among these different sources, different concentrations, and different sizes of Aβ assemblies, to improve our understanding of Aβ pathology. Nevertheless, it also important to pursue the study of Aβ with various distinct preparations, and to resist the temptation to identify a single amyloidgenic Aβ species, as it is currently unclear how any of the Aβ multimers mediates the inhibition of LTP.
We show here that very low concentrations of cell-derived human Aβ are sufficient to inhibit hippocampal LTP. Using a standard curve of synthetic Aβ, we estimate that the concentration of Aβ oligomers in the SEC fractions that were applied to the hippocampus is likely to be only ∼100–300 pm in the 15 ml of circulating medium. Although there is some error inherent in this method, for example, the efficiency of resuspending a lyophilized sample in a small volume for electrophoresis and the potentially different affinities of the antibody for synthetic and cell-derived Aβ, other methods of measuring secreted Aβ such as ELISA have similar limitations. The ∼3 ng of Aβ monomer in any one SEC fraction will underestimate the amount of monomer in the whole CM, because (a) the monomer is distributed over several SEC fractions (b) some monomer is lost during the Centricon concentration and SEC steps, and (c) inhibitors of the Aβ-degrading enzymes could not be included in the CM used for electrophysiology. However, Aβ oligomers are resistant to degradation by Insuline Degrading Enzyme (IDE) (Wang et al. 2002) and certain other proteases, and thus we can approximate the oligomer concentrations in whole medium. Our previous estimate of total oligomer concentration of <1 nm is consistent with the findings shown here. Moreover, we can now approximate the relative abundance of each oligomer and demonstrate their relative effects on LTP. Interestingly, Aβ trimers showed the strongest inhibition of LTP and yet were the weakest band on the gel, as detected by both a C-terminal (2G3) and amino acids 4–8 directed antibody (6E10) (data not shown). In summary, hippocampal synapses are exquisitely sensitive to very small quantities of naturally secreted, diffusible oligomers of human Aβ.
Because LTP requires the activation of multiple, sequential signal transduction cascades (reviewed in Lynch, 2004), we tested whether the oligomer-rich 7PA2 CM disrupts early and/or late events. Our results indicate that the Aβ oligomers inhibit the induction but not the expression of LTP. Application of 7PA2 CM shortly after the HFS had no effect on early LTP (1 h post-HFS). This result suggests that Aβ oligomers disrupt the primary steps in the signal transduction cascades that initiate LTP. Two studies have reported that application of micromolar quantities of synthetic Aβ to hippocampal slices immediately after a HFS can interfere with late LTP, although just as we report, early LTP was not substantially affected (Kim et al. 2001; Chen et al. 2002). Our use of cell-derived Aβ at far lower concentrations may account for this difference. Nevertheless, all three studies suggest that the most pronounced effect of Aβ is on the initial events during LTP induction.
The inhibitory effect of 7PA2 CM was also found to be sensitive to heat denaturation. It has been hypothesized that the β-sheet conformation of Aβ may contribute to its cytotoxicity (Glenner & Wong, 1984; Kirschner et al. 1986), and high temperatures may destroy this conformation. By SDS-PAGE, we have observed no difference in the migration of trimer and dimer bands in samples that have been boiled or allowed to equilibrate to room temperature before loading onto a gel (data not shown). Therefore, it may not be possible to detect changes in the conformation of these oligomers as a shift in size on an SDS-PAGE gel. The continued development of conformation-specific antibodies will be an important tool (Chang et al. 2003; Kayed et al. 2003), since the relative abundance of oligomers alone may not be sufficient to conclude whether the Aβ species are in an active conformation. In this regard, we have tested the conformation-specific antibody A11, reported by Kayed et al. (2003), but it does not recognize our naturally secreted low-n oligomers, consistent with the authors' report that it recognizes intermediate assemblies of >40 kDa.
We also demonstrate here that despite a 2 h washout period, 7PA2 CM continues to inhibit LTP in brain slices. By assaying the retention of Aβ on those slices, we show that a substantial amount of Aβ remains in the tissue even after it was washed extensively. With longer wash periods, additional Aβ clearance may be possible. Moreover, the slice preparations may not clear Aβ as effectively as the intact brain, where both microglial uptake and Aβ transport across the blood–brain barrier may actively decrease Aβ levels (Deane et al. 2003; Streit, 2004). Nevertheless, our data demonstrate that Aβ oligomers strongly adhere to brain tissue and continue to impair synaptic function.
Like the 7PA2 cell line, 7WD4 cells, which stably express wild-type APP751, secrete an array of soluble Aβ oligomers. We found that the 7WD4 CM also inhibited LTP, although higher concentrations were required to obtain similar quantities of Aβ oligomers and thus similar effects on LTP. The reduced potency of 7WD4 CM may be explained by two factors: the lower expression of APP (and lower total Aβ levels) in the 7WD4 cells, and their decreased trimer/monomer ratio versus the 7PA2 cells. The difference in oligomer ratios is presumably caused by the reported Aβ42-elevating effect of the V717F mutation in the 7PA2 cells (Xia et al. 1997). This mutation is known to cause an aggressive form of AD in humans, and it may similarly result in more potent Aβ species in the AD family carrying it. While additional studies will be necessary to answer these question, our results make the important observation that Aβ oligomers from two different cell lines cause an inhibition of LTP in a dose-dependent fashion.
Because LTP is so profoundly affected by soluble Aβ oligomers, we examined other electrophysiological features of synaptic function and plasticity. Importantly, post-tetanic potentiation, paired-pulse ratios and the profile of the synaptic potential evoked during HFS were all found to be normal in the presence of the oligomer-rich 7PA2 medium. Therefore, there was no indication that presynaptic vesicle release was affected, nor the ability of the pyramidal cells to follow the presynaptic stimulation. Based on the tests that we performed, our data is agreement with previous work using Aβ-derived diffusible ligands (ADDLs) or APP transgenic mice (Wang et al. 2002; Zhang et al. 2005). Nevertheless, we cannot rule out that a more extensive study of presynaptic function might detect an effect of Aβ on presynaptic release. We did observe a small but significant difference between the CHO- CM (control) and 7PA2 CM-treated slices in the slope of the regression line of the input/output curve. However, the shift was in the opposite direction than that expected if 7PA2 CM were inhibiting synaptic transmission. Preliminary studies with a cell line that only expresses the soluble APP ectodomain APPs-α indicate that this product of APP processing in the 7PA2 CM may account for this finding. Our experiments did not detect any significant effect of 7PA2 CM on presynaptic vesicle release. However, short-term potentiation measured 5 min after HFS was significantly reduced in the presence of 7PA2 CM. The latter results are consistent with other work in hippocampal slices (Wang et al. 2004) but differ somewhat from the effects of the same CM in vivo (Klyubin et al. 2005), where short-term potentiation (STP) was not significantly affected. There are many potential explanations for this difference including the exact quantity of Aβ oligomers applied in vivo and in vitro. Nevertheless, our results suggest that as with LTP, Aβ oligomers interfere with the early stages of synaptic plasticity following a HFS.
To further address the mechanisms by which Aβ inhibits LTP, we examined whether early postnatal mice were similarly susceptible to 7PA2 CM. Juvenile mice generally express a different complement of cell-surface receptors than older mice and employ different signal transduction cascades for LTP (Yasuda et al. 2003), both factors that could affect Aβ-mediated synaptic inhibition. Unlike the P16–28 mice used throughout most of this study, P8–9 mice showed no significant difference in synaptic potentiation between CHO- CM and 7PA2 CM. While the reason for this difference remains unknown, these results do reveal that 7PA2 CM does not invariably inhibit LTP, suggesting that there is molecular specificity to the inhibition. It cannot be concluded that Aβ has no effect on young neurons, but rather that LTP is unaffected by short applications of Aβ in young hippocampal neurons. These results may have important implications for dissociated neuronal culture studies, in which it is common to use immature neurons.
BDNF can induce a stable potentiation of synapses that is independent of stimulus-induced LTP (Kang & Schuman, 1995). We therefore examined whether this form of LTP is similarly affected by 7PA2 CM. Intriguingly, this pathway was resistant to inhibition by Aβ oligomers in the same slices that then showed impaired HFS-induced LTP. Although the BDNF signal transduction pathway has not been fully elucidated, it is known that the autophosphorylation of TrkB activates the Erk/MAPK and PI3K pathways, ultimately leading to CREB phosphorylation (Lu & Chow, 1999). It remains to be determined whether BDNF activates the LTP downstream of an Aβ block or whether BDNF activates a parallel pathway.
In conclusion, we report that the acute application of cell-derived soluble oligomers of human Aβ can rapidly inhibit LTP in normal mouse hippocampus. Fractionation of the medium suggests that the Aβ trimer is particularly potent, even at very low concentrations (∼100 pm). We have also determined that several measures of synaptic function remain normal in the presence of 7PA2 CM, despite the highly reproducible inhibition of LTP. Finally, young mice appear to be resilient to the immediate effects of Aβ on LTP, and BDNF-induced LTP is also spared. These results suggest that small diffusible Aβ oligomers specifically target certain molecular components that mediate synaptic plasticity. The chronic failure of certain synapses to function normally in the ongoing presence of natural Aβ oligomers might be expected to lead to a loss of synaptic spines (Papa & Segal, 1996; Collin et al. 1997; Kossel et al. 1997; Neuhoff et al. 1999), and thus is likely to contribute to the downstream neuropathology of AD.
Acknowledgments
This work was supported by National Institutes of Health grants AG05134 (D.J.S.) and T32 NS07484-04 (M.T.). We thank Marcia Podlisny for experimental assistance and critical review of the manuscript.
References
- Barghorn S, Nimmrich V, Striebinger A, Krantz C, Keller P, Janson B, Bahr M, Schmidt M, Bitner RS, Harlan J, Barlow E, Ebert U, Hillen H. Globular amyloid beta-peptide oligomer – a homogenous and stable neuropathological protein in Alzheimer's disease. J Neurochem. 2005;95:834–847. doi: 10.1111/j.1471-4159.2005.03407.x. [DOI] [PubMed] [Google Scholar]
- Billings LM, Oddo S, Green KN, McGaugh JL, LaFerla FM. Intraneuronal Abeta causes the onset of early Alzheimer's disease-related cognitive deficits in transgenic mice. Neuron. 2005;45:675–688. doi: 10.1016/j.neuron.2005.01.040. [DOI] [PubMed] [Google Scholar]
- Chang L, Bakhos L, Wang Z, Venton DL, Klein WL. Femtomole immunodetection of synthetic and endogenous amyloid-beta oligomers and its application to Alzheimer's disease drug candidate screening. J Mol Neurosci. 2003;20:305–313. doi: 10.1385/JMN:20:3:305. [DOI] [PubMed] [Google Scholar]
- Chen QS, Wei WZ, Shimahara T, Xie CW. Alzheimer amyloid beta-peptide inhibits the late phase of long-term potentiation through calcineurin-dependent mechanisms in the hippocampal dentate gyrus. Neurobiol Learn Mem. 2002;77:354–371. doi: 10.1006/nlme.2001.4034. [DOI] [PubMed] [Google Scholar]
- Cleary JP, Walsh DM, Hofmeister JJ, Shankar GM, Kuskowski MA, Selkoe DJ, Ashe KH. Natural oligomers of the amyloid-beta protein specifically disrupt cognitive function. Nat Neurosci. 2005;8:79–84. doi: 10.1038/nn1372. [DOI] [PubMed] [Google Scholar]
- Collin C, Miyaguchi K, Segal M. Dendritic spine density and LTP induction in cultured hippocampal slices. J Neurophysiol. 1997;77:1614–1623. doi: 10.1152/jn.1997.77.3.1614. [DOI] [PubMed] [Google Scholar]
- Davies CA, Mann DM, Sumpter PQ, Yates PO. A quantitative morphometric analysis of the neuronal and synaptic content of the frontal and temporal cortex in patients with Alzheimer's disease. J Neurol Sci. 1987;78:151–164. doi: 10.1016/0022-510x(87)90057-8. [DOI] [PubMed] [Google Scholar]
- Deane R, Du Yan S, Submamaryan RK, LaRue B, Jovanovic S, Hogg E, Welch D, Manness L, Lin C, Yu J, Zhu H, Ghiso J, Frangione B, Stern A, Schmidt AM, Armstrong DL, Arnold B, Liliensiek B, Nawroth P, Hofman F, Kindy M, Stern D, Zlokovic B. RAGE mediates amyloid-beta peptide transport across the blood–brain barrier and accumulation in brain. Nat Med. 2003;9:907–913. doi: 10.1038/nm890. [DOI] [PubMed] [Google Scholar]
- DeMattos RB, Bales KR, Cummins DJ, Dodart JC, Paul SM, Holtzman DM. Peripheral anti-A beta antibody alters CNS and plasma A beta clearance and decreases brain A beta burden in a mouse model of Alzheimer's disease. Proc Natl Acad Sci U S A. 2001;98:8850–8855. doi: 10.1073/pnas.151261398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodart JC, Bales KR, Gannon KS, Greene SJ, DeMattos RB, Mathis C, DeLong CA, Wu S, Wu X, Holtzman DM, Paul SM. Immunization reverses memory deficits without reducing brain Abeta burden in Alzheimer's disease model. Nat Neurosci. 2002;5:452–457. doi: 10.1038/nn842. [DOI] [PubMed] [Google Scholar]
- Ehlers MD. Activity level controls postsynaptic composition and signaling via the ubiquitin-proteasome system. Nat Neurosci. 2003;6:231–242. doi: 10.1038/nn1013. [DOI] [PubMed] [Google Scholar]
- Glenner GG, Wong CW. Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984;120:885–890. doi: 10.1016/s0006-291x(84)80190-4. [DOI] [PubMed] [Google Scholar]
- Gotz J, Chen F, Van Dorpe J, Nitsch RM. Formation of neurofibrillary tangles in P301l tau transgenic mice induced by Abeta 42 fibrils. Science. 2001;293:1491–1495. doi: 10.1126/science.1062097. [DOI] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Hsia AY, Masliah E, McConlogue L, Yu GQ, Tatsuno G, Hu K, Kholodenko D, Malenka RC, Nicoll RA, Mucke L. Plaque-independent disruption of neural circuits in Alzheimer's disease mouse models. Proc Natl Acad Sci U S A. 1999;96:3228–3233. doi: 10.1073/pnas.96.6.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HJ, Schuman EM. Neurotrophin-induced modulation of synaptic transmission in the adult hippocampus. J Physiol Paris. 1995;89:11–22. doi: 10.1016/0928-4257(96)80547-x. [DOI] [PubMed] [Google Scholar]
- Kayed R, Head E, Thompson JL, McIntire TM, Milton SC, Cotman CW, Glabe CG. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science. 2003;300:486–489. doi: 10.1126/science.1079469. [DOI] [PubMed] [Google Scholar]
- Kim JH, Anwyl R, Suh YH, Djamgoz MB, Rowan MJ. Use-dependent effects of amyloidogenic fragments of (beta)-amyloid precursor protein on synaptic plasticity in rat hippocampus in vivo. J Neurosci. 2001;21:1327–1333. doi: 10.1523/JNEUROSCI.21-04-01327.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschner DA, Abraham C, Selkoe DJ. X-ray diffraction from intraneuronal paired helical filaments and extraneuronal amyloid fibers in Alzheimer disease indicates cross-beta conformation. Proc Natl Acad Sci U S A. 1986;83:503–507. doi: 10.1073/pnas.83.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klyubin I, Walsh DM, Lemere CA, Cullen WK, Shankar GM, Betts V, Spooner ET, Jiang L, Anwyl R, Selkoe DJ, Rowan MJ. Amyloid beta protein immunotherapy neutralizes Abeta oligomers that disrupt synaptic plasticity in vivo. Nat Med. 2005;11:556–561. doi: 10.1038/nm1234. [DOI] [PubMed] [Google Scholar]
- Koistinaho M, Ort M, Cimadevilla JM, Vondrous R, Cordell B, Koistinaho J, Bures J, Higgins LS. Specific spatial learning deficits become severe with age in beta-amyloid precursor protein transgenic mice that harbor diffuse beta-amyloid deposits but do not form plaques. Proc Natl Acad Sci U S A. 2001;98:14675–14680. doi: 10.1073/pnas.261562998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokubo H, Kayed R, Glabe CG, Yamaguchi H. Soluble Abeta oligomers ultrastructurally localize to cell processes and might be related to synaptic dysfunction in Alzheimer's disease brain. Brain Res. 2005;1031:222–228. doi: 10.1016/j.brainres.2004.10.041. [DOI] [PubMed] [Google Scholar]
- Kossel AH, Williams CV, Schweizer M, Kater SB. Afferent innervation influences the development of dendritic branches and spines via both activity-dependent and non-activity-dependent mechanisms. J Neurosci. 1997;17:6314–6324. doi: 10.1523/JNEUROSCI.17-16-06314.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Liosatos M, Morgan TE, Rozovsky I, Trommer B, Viola KL, Wals P, Zhang C, Finch CE, Krafft GA, Klein WL. Diffusible, nonfibrillar ligands derived from Abeta1–42 are potent central nervous system neurotoxins. Proc Natl Acad Sci U S A. 1998;95:6448–6453. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavados M, Farias G, Rothhammer F, Guillon M, Mujica MC, Maccioni C, Maccioni RB. ApoE alleles and tau markers in patients with different levels of cognitive impairment. Arch Med Res. 2005;36:474–479. doi: 10.1016/j.arcmed.2005.03.036. [DOI] [PubMed] [Google Scholar]
- Lewis J, Dickson DW, Lin WL, Chisholm L, Corral A, Jones G, Yen SH, Sahara N, Skipper L, Yager D, Eckman C, Hardy J, Hutton M, McGowan E. Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. Science. 2001;293:1487–1491. doi: 10.1126/science.1058189. [DOI] [PubMed] [Google Scholar]
- Lu B, Chow A. Neurotrophins and hippocampal synaptic transmission and plasticity. J Neurosci Res. 1999;58:76–87. [PubMed] [Google Scholar]
- Lu W, Man H, Ju W, Trimble WS, MacDonald JF, Wang YT. Activation of synaptic NMDA receptors induces membrane insertion of new AMPA receptors and LTP in cultured hippocampal neurons. Neuron. 2001;29:243–254. doi: 10.1016/s0896-6273(01)00194-5. [DOI] [PubMed] [Google Scholar]
- Lue LF, Kuo YM, Roher AE, Brachova L, Shen Y, Sue L, Beach T, Kurth JH, Rydel RE, Rogers J. Soluble amyloid beta peptide concentration as a predictor of synaptic change in Alzheimer's disease. Am J Pathol. 1999;155:853–862. doi: 10.1016/s0002-9440(10)65184-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch MA. Long-term potentiation and memory. Physiol Rev. 2004;84:87–136. doi: 10.1152/physrev.00014.2003. [DOI] [PubMed] [Google Scholar]
- Masliah E, Mallory M, Alford M, DeTeresa R, Hansen LA, McKeel DW, Jr, Morris JC. Altered expression of synaptic proteins occurs early during progression of Alzheimer's disease. Neurology. 2001;56:127–129. doi: 10.1212/wnl.56.1.127. [DOI] [PubMed] [Google Scholar]
- McLean CA, Cherny RA, Fraser FW, Fuller SJ, Smith MJ, Beyreuther K, Bush AI, Masters CL. Soluble pool of Abeta amyloid as a determinant of severity of neurodegeneration in Alzheimer's disease. Ann Neurol. 1999;46:860–866. doi: 10.1002/1531-8249(199912)46:6<860::aid-ana8>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Mehta PD, Pirttila T, Mehta SP, Sersen EA, Aisen PS, Wisniewski HM. Plasma and cerebrospinal fluid levels of amyloid beta proteins 1–40 and 1–42 in Alzheimer disease. Arch Neurol. 2000;57:100–105. doi: 10.1001/archneur.57.1.100. [DOI] [PubMed] [Google Scholar]
- Motter R, Vigo-Pelfrey C, Kholodenko D, Barbour R, Johnson-Wood K, Galasko D, Chang L, Miller B, Clark C, Green R, et al. Reduction of beta-amyloid peptide42 in the cerebrospinal fluid of patients with Alzheimer's disease. Ann Neurol. 1995;38:643–648. doi: 10.1002/ana.410380413. [DOI] [PubMed] [Google Scholar]
- Moyer JR, Jr, Brown TH. Methods for whole-cell recording from visually preselected neurons of perirhinal cortex in brain slices from young and aging rats. J Neurosci Meth. 1998;86:35–54. doi: 10.1016/s0165-0270(98)00143-5. [DOI] [PubMed] [Google Scholar]
- Mucke L, Masliah E, Yu GQ, Mallory M, Rockenstein EM, Tatsuno G, Hu K, Kholodenko D, Johnson-Wood K, McConlogue L. High-level neuronal expression of abeta 1–42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J Neurosci. 2000;20:4050–4058. doi: 10.1523/JNEUROSCI.20-11-04050.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhoff H, Roeper J, Schweizer M. Activity-dependent formation of perforated synapses in cultured hippocampal neurons. Eur J Neurosci. 1999;11:4241–4250. doi: 10.1046/j.1460-9568.1999.00856.x. [DOI] [PubMed] [Google Scholar]
- Oddo S, Billings L, Kesslak JP, Cribbs DH, LaFerla FM. Abeta immunotherapy leads to clearance of early, but not late, hyperphosphorylated tau aggregates via the proteasome. Neuron. 2004;43:321–332. doi: 10.1016/j.neuron.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM. Triple-transgenic model of Alzheimer's disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- Papa M, Segal M. Morphological plasticity in dendritic spines of cultured hippocampal neurons. Neuroscience. 1996;71:1005–1011. doi: 10.1016/0306-4522(95)00490-4. [DOI] [PubMed] [Google Scholar]
- Podlisny MB, Ostaszewski BL, Squazzo SL, Koo EH, Rydell RE, Teplow DB, Selkoe DJ. Aggregation of secreted amyloid beta-protein into sodium dodecyl sulfate-stable oligomers in cell culture. J Biol Chem. 1995;270:9564–9570. doi: 10.1074/jbc.270.16.9564. [DOI] [PubMed] [Google Scholar]
- Podlisny MB, Walsh DM, Amarante P, Ostaszewski BL, Stimson ER, Maggio JE, Teplow DB, Selkoe DJ. Oligomerization of endogenous and synthetic amyloid beta-protein at nanomolar levels in cell culture and stabilization of monomer by Congo red. Biochemistry. 1998;37:3602–3611. doi: 10.1021/bi972029u. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer's disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- Streit WJ. Microglia and Alzheimer's disease pathogenesis. J Neurosci Res. 2004;77:1–8. doi: 10.1002/jnr.20093. [DOI] [PubMed] [Google Scholar]
- Sudo S, Shiozawa M, Cairns NJ, Wada Y. Aberrant accentuation of neurofibrillary degeneration in the hippocampus of Alzheimer's disease with amyloid precursor protein 717 and presenilin-1 gene mutations. J Neurol Sci. 2005;234:55–65. doi: 10.1016/j.jns.2005.03.043. [DOI] [PubMed] [Google Scholar]
- Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, Hansen LA, Katzman R. Physical basis of cognitive alterations in Alzheimer's disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, Rowan MJ, Selkoe DJ. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Townsend M, Podlisny MB, Shankar GM, Fadeeva JV, Agnaf OE, Hartley DM, Selkoe DJ. Certain inhibitors of synthetic amyloid beta-peptide (Abeta) fibrillogenesis block oligomerization of natural Abeta and thereby rescue long-term potentiation. J Neurosci. 2005;25:2455–2462. doi: 10.1523/JNEUROSCI.4391-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh DM, Tseng BP, Rydel RE, Podlisny MB, Selkoe DJ. The oligomerization of amyloid beta-protein begins intracellularly in cells derived from human brain. Biochemistry. 2000;39:10831–10839. doi: 10.1021/bi001048s. [DOI] [PubMed] [Google Scholar]
- Wang HW, Pasternak JF, Kuo H, Ristic H, Lambert MP, Chromy B, Viola KL, Klein WL, Stine WB, Krafft GA, Trommer BL. Soluble oligomers of beta amyloid (1–42) inhibit long-term potentiation but not long-term depression in rat dentate gyrus. Brain Res. 2002;924:133–140. [Google Scholar]
- Wang Q, Walsh DM, Rowan MJ, Selkoe DJ, Anwyl R. Block of long-term potentiation by naturally secreted and synthetic amyloid beta-peptide in hippocampal slices is mediated via activation of the kinases c-Jun N-terminal kinase, cyclin-dependent kinase 5, and p38 mitogen-activated protein kinase as well as metabotropic glutamate receptor type 5. J Neurosci. 2004;24:3370–3378. doi: 10.1523/JNEUROSCI.1633-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe MS, Xia W, Ostaszewski BL, Diehl TS, Kimberly WT, Selkoe DJ. Two transmembrane aspartates in presenilin-1 required for presenilin endoproteolysis and gamma-secretase activity. Nature. 1999;398:513–517. doi: 10.1038/19077. [DOI] [PubMed] [Google Scholar]
- Xia W, Zhang J, Kholodenko D, Citron M, Podlisny MB, Teplow DB, Haass C, Seubert P, Koo EH, Selkoe DJ. Enhanced production and oligomerization of the 42-residue amyloid beta-protein by Chinese hamster ovary cells stably expressing mutant presenilins. J Biol Chem. 1997;272:7977–7982. doi: 10.1074/jbc.272.12.7977. [DOI] [PubMed] [Google Scholar]
- Yasuda H, Barth AL, Stellwagen D, Malenka RC. A developmental switch in the signaling cascades for LTP induction. Nat Neurosci. 2003;6:15–16. doi: 10.1038/nn985. [DOI] [PubMed] [Google Scholar]
- Zhang H, Gong B, Liu S, Fa M, Ninan I, Staniszewski A, Arancio O. Synaptic fatigue is more pronounced in the APP/PS1 transgenic mouse model of Alzheimer's disease. Curr Alzheimer Res. 2005;2:137–140. doi: 10.2174/1567205053585936. [DOI] [PubMed] [Google Scholar]
- Zucker RS, Regehr WG. Short-term synaptic plasticity. Calcium- and activity-dependent synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]