Immune responses that adapt the intestinal mucosa to commensal intestinal bacteria (original) (raw)
Abstract
Animals contain an enormous load of non-pathogenic bacteria in the lower intestine, which exploit an environment with a stable temperature and abundant carbon sources. Our load of bacteria outnumbers our own cells. In order to survive with such a high number of organisms in very close proximity to host tissues the intestinal mucosa and its immune system is highly adapted. Mucosal immune responses are induced by small numbers of live commensal organisms penetrating the Peyer's patches and persisting in dendritic cells (DC). These DC can induce immunoglobulin A+ (IgA+) B cells, which recirculate through the lymph and bloodstream to populate the lamina propria and secrete protective IgA. Because DC loaded with commensal bacteria do not penetrate further than the mesenteric lymph nodes, immune induction to commensals is confined to the mucosa, allowing strong mucosal immune responses to be induced whilst the systemic immune system remains relatively ignorant of these organisms.
Keywords: commensal bacteria, IgA, T-lymphocyte, dendritic cell, intestinal mucosa
Adaptation of the intestinal mucosa in the presence of commensal bacteria
The lower intestine of mammals contains an extremely diverse and dense flora of bacteria that are normally non-pathogenic in an immunocompetent host. These commensal intestinal bacteria reach densities of 1012 organisms per ml of luminal contents.1 There are about 1000 species present, mostly anaerobes, but less than half of these species can be successfully cultured ex vivo. This immense load of commensal bacteria means that the number of bacterial cells being carried in the intestine is greater than the number of eukaryotic cells of the host's own body. Furthermore, the overall number of genes in the commensal bacterial flora can be estimated to be at least two orders of magnitude greater than the gene number in the host's own DNA.2 Peaceful coexistence between our bodies and commensal bacteria is a classic example of mutualism, which works despite the abundance of bacterial molecules that can potentially activate Toll-like and other bacterial molecular pattern receptors to trigger damaging innate immune responses and inflammation.3
The bacteria obtain a secure habitat with a stable temperature, rich in carbon and mineral sources, whereas the host benefits through the ability of bacteria to salvage energy from otherwise indigestible dietary constituents (such as cellulose). Bacteria also synthesize short chain fatty acids and vitamin K1, which can be used in host anabolic pathways. Host metabolic pathways are also directly regulated by the influence of commensals on the intestinal epithelium.4 Because the available microbiological niche is full of commensals, it is harder for potentially pathogenic bacteria that can produce exotoxins or are able to adopt a facultative intracellular existence to gain a foothold.5 The existence of this mutualism, established by evolution on both sides, has been long appreciated, but we are only beginning to understand the complex ways in which host and bacteria each adapt to the other's presence.
The adaptation of the intestinal mucosa to the presence of commensal bacteria has been studied by comparing germ-free animals bred and kept in a sterile environment, with the same strain kept using conventional husbandry. Germ-free animals live in flexible-film isolators, which are essentially plastic bubbles inflated with sterile filtered air under positive pressure. All food, water and bedding are autoclaved and introduced to the isolator using aseptic protocols. Founder animals for a germ-free colony must be aseptically delivered by Caesarean section and hand-reared, but thereafter the germ-free animals can be easily interbred within the sterile environment.
Experiments with germ-free colonies of mice and other species have been carried out for over 50 years. There are two important general results obtained from many experiments. First, the germ-free animal is an excellent culture medium: if a single conventionally reared animal is simply placed in the same cage, the germ-free animals will acquire a flora in the intestine and other body surfaces within days.6 This is similar to the way in which a human baby rapidly acquires an intestinal flora after leaving the sterile uterus. Second, sequential histological and molecular biological observations of germ-free animals as they become colonized show that the presence of the bacterial flora causes extensive adaptation of the host.7–12 The composition of the mucosal immune system is changed dramatically (as discussed in more detail below), but there are also important changes in systemic immunity, intestinal epithelial gene expression, intestinal angiogenesis and intestinal motility.2,13,14 Within the setting of these complex adaptive changes, this review will focus on the mechanisms of induction of humoral mucosal immune responses by commensal bacteria, and the function of these responses in containing the commensals within the lumen of the intestine.
Induction of mucosal immune responses
Induction and recirculation of mucosal lymphocytes
Classic papers from Gowans,15–17 Cebra18 and their colleagues showed that mucosal immunoglobulin A (IgA) responses were induced in the Peyer's patches, and that IgA plasmablasts recirculate through the lymph and blood stream to home back to the intestine to mature into IgA-secreting plasma cells in the lamina propria. Similarly, a proportion of intestinal T cells (CD8αβ+ or CD4+ T-cell receptor (TCR)α/β+) are induced in the Peyer's patches and follow a similar route.19–21
These early experiments used adoptive transfer of lymphocytes to distinguish the source of lymphocyte induction.18 Induced lymphocytes were shown to recirculate by two methods: (i) by cannulating the thoracic duct to obtain samples of efferent intestinal lymph and (ii) by surgically modifying the small intestine in rats to create Thiry Vella loops. These segments of small intestine were removed from the main stream with openings onto the skin of the animal, although the vascular and lymphatic connections were not disturbed. Immunization with cholera toxin in one intestinal segment was shown to result in an IgA response in another which had not been exposed to the antigen.
The cytokine-mediated mechanisms of IgA induction were initially studied in cell culture during non-specific B-cell stimulation, in which the media was supplemented by purified cytokines, and their spontaneous effect on class switch recombination to IgA was measured.22–26 In the 1980s and 1990s the cytokine and cellular interactions required for an IgA class switch were demonstrated in vivo using murine strain combinations with spontaneous and targeted immunodeficiencies. In some cases the readout was spontaneous production of IgA, which is defective in mice deficient in transforming growth factor-β (TGF-β) signalling (TGFβRII–/–) and the tumour necrosis factor (TNF) family member A proliferation-inducing ligand (APRIL).27 In other studies a specific stimulus has been used to induce IgA: this is usually cholera toxin,28,29 which is a powerful mucosal adjuvant, and the functional outcome of mucosal immune induction can be tested by neutralization of fluid accumulation within hours of injecting a test dose of cholera toxin into a ligated intestinal segment.30–32 The cholera toxin response requires T-cell help, as it is defective in CD4–/– mice33 and animals that are major histocompatibility complex (MHC) class II deficient. Cholera toxin responses are also reduced in interleukin (IL)-4–/– mice34 as well as cytotoxic T-lymphocyte antigen (CTLA)-4-Hγ1 transgenic mice that express a CTLA-4 protein construct under the control of the immunoglobulin heavy chain promoter, which blocks CD28⊼CD80/86 costimulation signals between T cells and antigen-presenting cells.35 This led to the conclusion that the process of IgA induction was substantially T-cell dependent in vivo. However, it remained paradoxical that many of the models (CD4–/–, IL-4–/–, and CTLA-4-Hγ1 transgenics) in which cholera toxin induction of IgA was defective, nonetheless had relatively normal numbers of IgA-secreting plasma cells in the intestinal mucosa.33–35
The importance of dendritic cells for the IgA class switch in addition to interactions between B and T lymphocytes was also initially examined in ex vivo cell culture.36–39 Antigen-presenting cells have been shown to stimulate the class switch (to IgG and IgA) probably through interactions between the TNF family members B cell activating factor (BAFF) and APRIL on the antigen-presenting cells and the BAFF receptor on B cells.40,41In vivo APRIL-deficient mice have decreased spontaneous levels of IgA and reduced specific switching to T-dependent and T-independent immunization protocols.27
Induction of IgA against commensal bacteria
In contrast to toxin induction of IgA, the same process triggered by commensal bacteria is not exclusively CD4-dependent. Measurement of total IgA in mice that are deficient in T cells as a result of targeted deletions of the β and δ chains of the T-cell receptor, showed that the amount of IgA secreted was reduced to about a quarter of that in wild-type animals but there remained a T-cell independent component.42 The binding specificities to Enterobacter cloacae (a dominant aerobe of the commensal intestinal flora in the Zurich colony of specific pathogen-free mice) were identical whether studied in wild-type or T-cell deficient animals.42 In animals deficient for MHC-class II, IgA content has also been shown experimentally to be normal despite disruption of cognate interactions between antigen-presenting cells and T cells.43 T-cell independent mucosal IgA responses have also been found to confer protective immunity when C57BL/6 × 129 mice are challenged with rotavirus.44,45 Humans with defective CD40-mediated signalling have also been described with normal or high levels of serum IgA.46,47
Studies of IgA sequences also suggest indirectly that the response to commensal bacteria does not depend on conventional germinal centre reactions in which the affinity of the antibodies is improved by sequential accumulation of somatic hypermutations.48 This is unlikely to merely reflect excess antigen binding to B-cell receptors, since germinal centres form selectively in Peyer's patches and mesenteric lymph nodes in mice in which the B-cell receptor (BCR) has been deleted, but a low level antigen-independent constitutive signal is delivered by B-cell expression of the Epstein–Barr virus protein LMP2A containing an immunoreceptor tyrosine-based activation motif.49 Experiments with antibiotics in BCR-deficient LMP2A mice suggest that BCR-independent signals from the intestinal flora are sufficient to drive germinal centre formation in the mucosal lymphoid system, although the details are unknown.49 In fact, even germinal centre formation is not obligatory for IgA induction, which occurs efficiently in the TNF receptor I-deficient strain.42 Sequence analysis of the alpha heavy chain and spectratyping of the CDR3 region length also shows that the repertoire of the (VHα) variable region in Peyer's patch or lamina propria tissues of mouse and man is surprisingly restricted given the diversity of the commensal flora.48,50 Somatic mutation of intestinal VH genes increases with age in humans51 although we do not know whether this has occurred by classical affinity maturation of the BCR or alternative signals from intestinal bacteria. Overall, the observations suggest that induction of IgA by commensal bacteria is rather a primitive system in which the production of large amounts of antibody against bacterial surface molecules with relatively low affinity, yet broad specificity, is useful to limit their local colonization or penetration through the epithelial layer.
In adult mice there are two sources of B-cell precursors.52 The bone marrow has stem cells that give rise to the conventional lineage of (B2) B cells. There are also precursors in the pleuroperitoneal cavities for a different (B1) lineage which are distinguished from B2 cells by higher levels of B1 staining for surface IgM, Mac-1 and CD5, and weaker staining with antibodies against B220 and IgD. Actually, the independence of these lineages is controversial, because experiments in mice with B cell receptor signalling abnormalities or a fixed antigen-binding specificity show that whether B cells exhibit the B1 or B2 phenotype is dependent on the specficity and strength of signalling from the B cell receptor.49,53 IgM antibodies derived from B1 cells are reactive with polysaccharide microbial antigens (induced in a T-independent fashion), and are encoded by unmutated VH genes.52 The contribution of B1 cells to intestinal IgA has been estimated by reconstitution of lethally irradiated animals with sources of B1 and B2 cells where there are distinctive allotypic differences in secreted immunoglobulins. These experiments give estimates of about half the intestinal IgA and most of the T-independent IgA being B1 derived.42,54,55 In a different experimental system, germ-free allotype chimeric mice were generated by repetitive antibody depletion of endogenous neonatal B cells followed by transfer of peritoneal cells.56 The final chimeras still had considerable numbers of recipient B1 cells in the peritoneal compartment (15–39%), so the system would underestimate the B1 contribution to secreted antibodies on the basis of their allotypic differences. However, 56–70 days after recolonization with bacteria the donor allotype contribution to intestinal IgA was less than 15%. In a third experimental system the intestinal IgA in MHC class II-deficient animals was studied. Here, the levels of intestinal IgA were relatively normal, despite the T-cell deficiency and disruption of cognate B–T interactions, but intestinal IgA became very reduced when the animals also carried the xid mutation resulting in deficient B1 cells.43 These inconsistent results leave open the exact contribution of B1 cells to intestinal IgA in mice. In man, CD5+ B cells producing polyspecific antibodies form 15–20% of the adult B-cell repertoire and constitute most neonatal B cells, although there is no significant pleuroperitoneal B-cell precursor population as in mice.57–59 It is not possible to assess the contribution of these B1-like cells to intestinal IgA directly in man.
Another unresolved issue is where class switch recombination might occur for B1 cells.60 Flow cytometry of the characteristic B2/B1 markers shows that effectively all Peyer's patch B cells have the B2 phenotype and B1 cells in the peritoneum are IgM+. In animals that are deficient for the TGF-β receptor (TGFβRII–/–), B1 lymphocytes do appear in the Peyer's patches, although whether this is prolonging transitory presence in normal circumstances is unknown.61 Class switch recombination has been described in the intestine outside the Peyer's patches62 although isolated lymphoid follicles may be contributing to this process.63 It is has also been suggested that B1 class switch recombination may take place in the mesenteric lymph nodes.48,64
We found that significant intestinal IgA only occurred in strains with some B-cell structures in the intestine, although these could be very disorganized, for example without follicular dendritic cells and germinal centres in mice deficient for the TNF receptor I.42,65 Regardless of relative B1/B2 contributions, the IgA response has a restricted VH repertoire of intestinal immunoglobulin α heavy chains in mouse48 and man.50 This supports the primitive nature of the overall IgA response, mostly induced in the presence of commensal bacteria, as a high capacity, broad specificity, low affinity system, not dependent on conventional B–T interactions for affinity maturation. The system is eminently suitable for producing antibodies that will bind to a very diverse bacterial flora with multiple redundant surface epitopes.42,66
Intestinal IgA can be experimentally induced in C57BL/6 wild-type mice by repeated administration of live bacteria into the intestine.6 Unlike the T-dependent cholera toxin response, which induces serum IgG and IgE in addition to IgA67 the commensal conditioning method of mucosal stimulation is entirely specific for IgA. Conditioning doses of bacteria result in loading of live bacteria in Peyer's patch dendritic cells (DC) – when these DC are isolated and cultured with naive mesenteric B ± T cells, both surface expression and secretion of IgA by the B cells is induced. Neither the in vivo conditioning response nor the ex vivo IgA induction will work when the animals are treated with heat-killed bacteria.6
The mechanisms for the specificity of IgA induction, as opposed to class switch recombination to other isotypes are not clearly understood. TGF-β signalling from diverse mucosal cell types is clearly important from models both in vitro22 and in vivo61 and there may also be direct interactions between antigen-presenting cells and B cells enhanced by the TNF family members BAFF and APRIL.27,40 These unconventional mechanisms of class switch recombination may also be able to occur without prior B-cell receptor engagement.65,68
Despite the enormous amount of IgA that is secreted daily across the intestinal epithelium, there are very few studies that address its function in relationship to commensal intestinal bacteria. Mice that are genetically deficient in the polymeric immunoglobulin receptor (pIgR) that transports IgA and IgM across the epithelial cell layer69–71 have a protein-losing enteropathy in which serum proteins are lost into the intestinal lumen as a result of damage to the paracellular permeability barrier.72 Two functional mechanisms of mucosal IgA secretion have been described. First, IgA antibody-coated commensal bacteria are excluded from penetrating the intestinal epithelium. This observation came from experiments in which the intact mucosa was challenged either by recolonizing germ-free animals or by delivering an experimental dose of intestinal bacteria to animals that already had an established commensal flora.6,9 Overall therefore IgA is protective against penetration of luminal bacteria, presumably by limiting their motility or access to the epithelial surface, but it is possible that IgA receptors of M cells facilitate sampling of live bacteria in the Peyer's patches and isolated lymphoid follicles.73–75 Second, in the absence of IgA, luminal densities of the commensal organisms are not properly controlled.63 The evidence for this comes from activation-induced cytidine deaminase deficient (AID–/–) mice, which have an anaerobic overgrowth in the lower intestine. AID–/– mice are deficient in IgA (and other class-switched isotypes) and affinity matured IgM as a result of defective class switch recombination and somatic hypermutation.
These mechanisms of IgA induction against commensals are only a component of the overall way in which the immune system adapts to the presence of such a large load of intestinal bacteria. In the following section we will discuss host–commensal bacterial mutualism in its wider immune context.
Special features of immune adaptation against commensals
The nature of mucosal immune adaptation
Adaptive immune responses against pathogens must be able to assist the innate immune system in one of the following ways: prevent a virus from entering host cells to establish a productive infection; kill infected host cells; neutralize the effects of an exotoxin; or opsonize pathogens with antibody to enhance their elimination through complement fixation and phagocytosis. Adaptation to commensal bacteria is fundamentally different. Here the important barrier is the epithelial surface with its mucus coat. Commensals live almost entirely within the intestinal lumen or within the mucus coat barrier, whereas pathogens are found within the body, having attached to the epithelial surface or penetrated it. Mutualism dictates that innocent bacteria above the epithelial surface or within the mucus should be tolerated, but bacteria penetrating the epithelial barrier need to be rapidly eliminated.
Mucosal immune adaptation to commensals is not purely an adaptive immune response. Animals without B or T cells (scid or RAG–/–) are able to survive with an intestinal bacterial flora without problems in pathogen-free facilities.76 However, when (innate) biocidal mechanisms of phagocytes are seriously deficient, such as in a strain lacking both inducible nitric oxide and superoxide generation, animals will succumb to systemic sepsis from penetrating commensals.77 In general, intestinal bacterial pathogens have mechanisms of avoiding or subverting phagocyte biocidal activity.78 Commensals that do penetrate allow themselves to be phagocytosed and eliminated;6 otherwise they would trigger inflammation that would destroy their luminal habitat. Retaining susceptibility to host macrophage biocidal mechanisms are probably an evolutionary adaptation of bacteria to ensure commensal status.
Since the essential mechanisms for mutualism with commensals are the physical epithelial/mucus barrier and phagocyte biocidal activity, what part do secreted antibodies or mucosal T cells play? Deficiency of either leads to increased levels of commensals within the mucosa or the draining mesenteric lymph nodes.6,79–81 Luminal commensal bacteria that have been coated by secretory antibody are probably restricted from accessing the mucus layer to reach the epithelial surface or penetrate it.6,79,81,82 T cells probably work both by activating macrophages and amplifying the class switch to IgA.5 Unlike the adaptive responses against pathogens which must be of high affinity and specificity83 the antibody responses against the commensal flora are of broad specificity42 and probably relatively low affinity, although this remains to be measured.
The object of mucosal adaptation to commensals is to accomplish the transition from 1012 bacterial c.f.u./ml to approaching sterility within the 20 µm distance between the luminal mucus surface and the basolateral surface of the enterocytes. In this context, expenditure of energy to increase secretory antibody affinity is probably not justified, as the commensal flora is so diverse, and low numbers of bacteria that do penetrate can easily be eliminated through innate immune mechanisms.
Systemic ignorance of intestinal commensal bacteria
A second issue regarding immune responses to commensal bacteria is whether there is a need to induce tolerance of the systemic immune system. Studying serum IgG responses against commensals, we found that pathogen-free mice had no specific IgG against Enterobacter cloacae, a dominant aerobe in the commensal flora of the colony, whereas this was easily induced 14 days after injecting 104−106 live organisms intravenously.42 In other words, the reason for non-responsiveness in the unmanipulated mice was ignorance rather than tolerance of these organisms. This experiment also shows that if the same commensal bacteria reach the systemic immune system, priming is very efficient: unlike clean mice, Western blots of commensals using human IgG show that humans are mostly primed to commensal bacteria84 presumably because an infection has resulted in a sufficient systemic bacteraemia for priming to occur. Again, this argues against significant systemic tolerance from intestinal commensals – compartmentalizing the system and preserving the ability to mount good systemic responses against commensal epitopes is probably important for good antibacterial immunity.
Immune geography of responses to commensal bacteria
The way in which the mucosal immune system accomplishes the trick of inducing a local immune response to commensals without needing to suppress a systemic immune response lies in the distinct immune geography of the intestines compared with the rest of the body. Here the critical barrier is formed by the mesenteric lymph nodes. The evidence for this is that when mice are challenged with high doses of commensal bacteria into the intestines, small numbers of live bacteria can be detected in dendritic cells of the Peyer's patches and (later) in the mesenteric lymph nodes.6 As long as the mesenteric lymph nodes are intact, dendritic cells loaded with commensal bacteria do not penetrate any further and cannot reach systemic secondary lymphoid structures. If the mesenteric lymph nodes are absent a single intestinal challenge with commensals gives culturable organisms in the spleen, and repeated challenge causes dramatic splenomegaly and enlargement of the splenic marginal zones.6
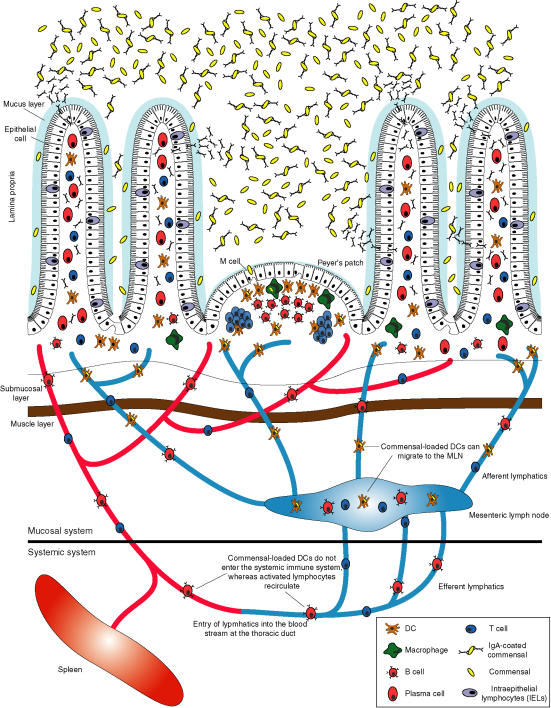
The immune geography of the intestinal immune system therefore depends on restricting mucosal induction by particulate bacteria to the mucosal immune system itself. This is a supreme example of compartmentalizing an immune response, as the mucosa contains about three-quarters of the total immune system in animals colonized by intestinal bacteria. This compartmentalization, shown in Fig. 1, is accomplished through the following features:
Figure 1.
Intestinal immune geography of responses to commensal bacteria. Commensal bacteria are found in high densities within the lumen of the lower intestine but are largely restricted from gaining access due to the physical epithelial and mucus barriers. However, small numbers of commensals are allowed to penetrate through the epithelial barrier into specialized inductive sites known as Peyer's patches or isolated lymphoid follicles, where they are picked up by dendritic cells or phagocytosed and destroyed by macrophages. DC presenting commensal bacterial antigens can traffic only as far as the mesenteric lymph nodes, which form the barrier between the mucosal and the systemic immune system. In contrast, activated lymphocytes (T and B cells) can circulate through the lymph and bloodstream and traffic back to the mucosa to populate sites remote from the inductive sites. IgA-secreting plasma cells are found in the lamina propria, where the secreted dimeric IgA can pass through the epithelial cells by binding to the poly immunoglobulin receptor (pIgR) on the basolateral membrane, leading to its internalization, transport to the luminal surface, enzymatic cleavage of the pIgR and thus release of secretory IgA into the intestinal lumen. Commensal bacteria coated with IgA are then restricted from passing through the intestinal mucosa and their luminal densities are modulated.
- By having specialized induction sites in which the appropriate signals are available for correct class switch recombination (IgA) and imprinting for lymphocyte homing signals. These are necessary to allow breaks in the tight mucosal barrier in which commensals and other luminal antigens can be sampled because the mucus layer and epithelial glycocalyx is reduced. IgA coating of commensals may actually facilitate uptake of bacteria through M cells expressing IgA receptors.73–75
- By induction upstream of the mesenteric lymph nodes by DC that can travel from the Peyer's patches to the mesenteric lymph nodes, but do not re-circulate within the body. This avoids unnecessary systemic priming by powerful and abundant particulate antigens that contain endogenous adjuvant because of their content of Toll-like receptor ligands. Because the innate immune system can deal with these bacteria through phagocytic microbiocidal activity, excessive induction of adaptive immunity with accompanying polyclonal responses might break tolerance to host antigens.
- By the effector cells being able to re-circulate to populate sites remote from the inductive sites. This allows the remainder of the mucosa to be populated by cells that contribute to limiting the penetration of commensals, yet large numbers of live commensals do not need to penetrate to induce immune responses and most of the mucosa underneath the epithelium can remain almost sterile.
Dendritic cells (DC) have been shown to protrude processes between epithelial cells of the intestinal (ileal) villus, particularly the DC subset that express the chemokine receptor CX3CR1.85,86 These DC are able to sample commensals or pathogens at the epithelial surface and also transport them to the mesenteric lymph nodes. This provides a mechanism of monitoring organisms that penetrate the surface mucus layer, or bacterial colonies that proliferate within the mucus as an organized biofilm consortium. Only tiny proportions (approximately 0·0001%) of a challenge dose of intestinal bacteria actually reach DC either in the villi or the Peyer's patches. In our experiments, penetration into the Peyer's patches was by far the most efficient pathway6 although this may to some extent reflect the experimental setup, because a challenge dose of bacteria is prevented from reaching the villous epithelial surface by the combination of the mucus and the epithelial glyocalyx. Nevertheless, we propose that the DC in the Peyer's patches and isolated lymphoid follicles promiscuously sample intestinal luminal commensals, whereas lamina propria DC sample those bacteria within the surface mucus layer.
These features allow the commensals to be sensed in small numbers, causing induction of an IgA response. Repeated challenge of experimental animals with live intestinal commensals selectively increases the levels of mucosal secretory and serum IgA.6 These increased secretory IgA levels in turn limit the penetration of intestinal bacteria from an experimental challenge, so the system works through a negative feedback mechanism.6 Such immune exclusion may also be the way in which IgA secreted through the milk during lactation can delay the induction of IgA in the neonatal mucosal immune system in experiments where scid/+ neonates are suckled either by scid/scid or wild-type dams.87
As discussed earlier adaptive immunity is not essential to tolerate commensal intestinal bacteria. Experiments in which germ-free animals are re-colonized by an intestinal flora show that antibody-deficient mice have a longer-lasting leak of commensals through the intestinal mucosa compared with wild-type controls, but even the antibody-deficient strain can adapt to stop bacterial penetration 35 days after recolonization.6 Thus, the negative-feedback mechanism of sensing commensal bacteria and inducing IgA to limit their translocation from the intestinal lumen into mucosal and systemic tissues is a layer of the protection mechanism, but is not essential. Antibody-dependent mechanisms of adaptation are therefore only a part of overall adaptation. This also includes T-cell driven macrophage activation5 reprogramming of epithelial gene expression2,14,88 and compensatory defence from intestinal epithelial lymphocytes.89 These all supplement the permeability barrier formed by epithelial cells interconnected by tight junctions with a surface covering of mucus and antibacterial peptides, including defensins and cathelicidins.90,91 Multilayered protection is probably an essential evolutionary failsafe mechanism in the face of such an abundant antigen challenge.
Conclusions
In this article we have reviewed the evidence that the mucosal immune system contributes to protection against penetration by commensal intestinal bacteria. The contrast between germ-free animals and those with commensal bacteria in the intestine is stark: whereas the mucosal immune system of germ-free animals is hypoplastic, after the introduction of commensal bacteria the majority of the all the body's leucocytes are in the intestine. To maintain constant mutualism with commensal bacteria is probably the greatest challenge facing the immune system. This challenge is constant, and to meet it vigorous immune responses are induced.
Because DCs loaded with commensal bacteria that induce responses to commensals do not penetrate further than the draining mesenteric lymph nodes, protective mucosal immunity to commensals is separated from systemic immunity. In this way the systemic immune system can be kept completely ignorant of commensals in pathogen-free animals, and probably relatively so in most circumstances, avoiding unnecessary and potentially dangerous responses potentially capable of triggering autoimmunity.
The geography of the mucosal immune response therefore depends on induction mainly in intestinal follicles or mesenteric lymph nodes and recirculation of the induced lymphocytes via the lymph and blood stream to populate other parts of the intestinal lamina propria. It has been considered that this recirculation integrates mucosal immunity at different sites. However, the most important advantage may be that the compromise of allowing immune induction to commensals primarily in the leaky follicles permits the lamina propria to be kept almost sterile by the combination of a physical permeability barrier formed by the epithelium and its overlying mucus and antibacterial defences, including secretory antibodies and secreted peptides. Within this system secretory IgA is the commensal–bacterial responsive negative feedback mechanism, able to respond with specific immune exclusion to different densities and compositions of the intestinal flora.
Abbreviations
APRIL
A proliferation-inducing ligand
BAFF
B cell activating factor
References
- 1.Mackie R, Sghir A, Gaskins HR. Developmental microbial ecology of the neonatal gastrointestinal tract. Am J Clin Nutr. 1999;69:1035S–1045S. doi: 10.1093/ajcn/69.5.1035s. [DOI] [PubMed] [Google Scholar]
- 2.Hooper LV, Wong MH, Thelin A, Hansson L, Falk PG, Gordon JI. Molecular analysis of commensal host-microbial relationships in the intestine (cites personal communication from Joshua Lederberg) Science. 2001;291:881–4. doi: 10.1126/science.291.5505.881. [DOI] [PubMed] [Google Scholar]
- 3.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by Toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–41. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Backhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA. 2004;101:15718–23. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Macpherson AJ, Martinic MM, Harris N. The functions of mucosal T cells in containing the indigenous flora of the intestine. Cell Mol Life Sci. 2003;59:2088–96. doi: 10.1007/s000180200009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Macpherson AJ, Uhr T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science. 2004;303:1662–5. doi: 10.1126/science.1091334. [DOI] [PubMed] [Google Scholar]
- 7.Cebra JJ. Influences of microbiota on intestinal immune system development. Am J Clin Nutr. 1999;69:1046S–1051S. doi: 10.1093/ajcn/69.5.1046s. [DOI] [PubMed] [Google Scholar]
- 8.Crabbe PA, Nash DR, Bazin H, Eyssen H, Heremans JF. Immunohistochemical observations on lymphoid tissues from conventional and germ-free mice. Laboratory Invest. 1970;22:448–57. [PubMed] [Google Scholar]
- 9.Shroff KE, Meslin K, Cebra JJ. Commensal enteric bacteria engender a self-limiting humoral mucosal immune response while permanently colonizing the gut. Infection Immunity. 1995;63:3904–13. doi: 10.1128/iai.63.10.3904-3913.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Helgeland L, Vaage JT, Rolstad B, Halstensen TS, Midtvedt T, Brandtzaeg P. Regional phenotypic specialization of intraepithelial lymphocytes in the rat intestine does not depend on microbial colonization. Scand J Immunol. 1997;46:349–57. doi: 10.1046/j.1365-3083.1997.d01-133.x. [DOI] [PubMed] [Google Scholar]
- 11.Helgeland L, Vaage JT, Rolstad B, Midtvedt T, Brandtzaeg P. Microbial colonization influences composition and T-cell receptor V beta repertoire of intraepithelial lymphocytes in rat intestine. Immunology. 1996;89:494–501. doi: 10.1046/j.1365-2567.1996.d01-783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamanaka T, Helgeland L, Farstad IN, Fukushima H, Midtvedt T, Brandtzaeg P. Microbial colonization drives lymphocyte accumulation and differentiation in the follicle-associated epithelium of Peyer's patches. J Immunol. 2003;170:816–22. doi: 10.4049/jimmunol.170.2.816. [DOI] [PubMed] [Google Scholar]
- 13.Stappenbeck TS, Hooper LV, Gordon JI. Developmental regulation of intestinal angiogenesis by indigenous microbes via Paneth cells. Proc Natl Acad Sci U S A. 2002;99:15451–5. doi: 10.1073/pnas.202604299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hooper LV, Gordon JI. Commensal host-bacterial relationships in the gut. Science. 2001;292:1115–8. doi: 10.1126/science.1058709. [DOI] [PubMed] [Google Scholar]
- 15.Gowans JL, Knight EJ. The route of recirculation of lymphocytes in the rat. Proc Roy Soc B. 1964;159:257–82. doi: 10.1098/rspb.1964.0001. [DOI] [PubMed] [Google Scholar]
- 16.Husband AJ, Gowans JL. The origin and antigen-dependent distribution of IgA-containing cells in the intestine. J Exp Med. 1978;148:1146–60. doi: 10.1084/jem.148.5.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pierce NF, Gowans JL. Cellular kinetics of the intestinal immune response to cholera toxoid in rats. J Exp Med. 1975;142:1550–63. doi: 10.1084/jem.142.6.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Craig SW, Cebra JJ. Peyer's patches: an enriched source of precursors for IgA-producing immunocytes in the rabbit. J Exp Med. 1971;134:188–200. doi: 10.1084/jem.134.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guy-Grand D, Griscelli C, Vassalli P. The mouse gut T lymphocyte, a novel type of T cell. Nature, origin, and traffic in mice in normal and graft-versus-host conditions. J Exp Med. 1978;148:1661–77. doi: 10.1084/jem.148.6.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guy-Grand D, Vassalli P. Gut injury in mouse graft-versus-host reaction. Study of its occurrence and mechanisms. J Clin Invest. 1986;77:1584–95. doi: 10.1172/JCI112474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arstila T, Arstila TP, Calbo S, Selz F, Malassis-Seris M, Vassalli P, Kourilsky P, Guy-Grand D. Identical T cell clones are located within the mouse gut epithelium and lamina propia and circulate in the thoracic duct lymph. J Exp Med. 2000;191:823–34. doi: 10.1084/jem.191.5.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coffman RL, Lebman DA, Shrader B. Transforming growth factor β specifically enhances IgA production by lipopolysaccharide stimulated murine B lymphocytes. J Exp Med. 1989;170:1039–44. doi: 10.1084/jem.170.3.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kunimoto DY, Harriman GR, Strober W. Regulation of IgA differentiation in CH12LX B cells by lymphokines: IL-4 induces membrane IgM-positive CH12LX cells to express membrane IgA and IL-5 induces membrane IgA-positive CH12LX cells to secrete IgA. J Immunol. 1988;141:713–20. [PubMed] [Google Scholar]
- 24.Kunimoto DY, Nordan RP, Strober W. IL-6 is a potent cofactor of IL-1 in IgM synthesis and of IL-5 in IgA synthesis. J Immunol. 1989;143:2230–5. [PubMed] [Google Scholar]
- 25.Beagley KW, Eldridge JH, Lee F, et al. Interleukins and IgA synthesis. Human and murine interleukin 6 induce high rate IgA secretion in IgA-committed B cells. J Exp Med. 1989;169:2133–48. doi: 10.1084/jem.169.6.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beagley KW, Eldridge JH, Kiyono H, Everson MP, Koopman WJ, Honjo T, McGhee JR. Recombinant murine IL-5 induces high rate IgA synthesis in cycling IgA-positive Peyer's patch B cells. J Immunol. 1988;141:2035–42. [PubMed] [Google Scholar]
- 27.Castigli E, Scott S, Dedeoglu F, Bryce P, Jabara H, Bhan AK, Mizoguchi E, Geha RS. Impaired IgA class switching in APRIL-deficient mice. Proc Natl Acad Sci U S A. 2004;101:3903–8. doi: 10.1073/pnas.0307348101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elson CO, Ealding W. Cholera toxin feeding did not induce oral tolerance in mice and abrogated oral tolerance to an unrelated protein antigen. J Immunol. 1984;133:2892–7. [PubMed] [Google Scholar]
- 29.Elson CO, Ealding W. Generalized systemic and mucosal immunity in mice after mucosal stimulation with cholera toxin. J Immunol. 1984;132:2736–41. [PubMed] [Google Scholar]
- 30.Lycke N, Holmgren J. Intestinal mucosal memory and presence of memory cells in lamina propria and Peyer's patches in mice 2 years after oral immunization with cholera toxin. Scand J Immunol. 1986;23:611–6. doi: 10.1111/j.1365-3083.1986.tb01995.x. [DOI] [PubMed] [Google Scholar]
- 31.Lycke N, Eriksen L, Holmgren J. Protection against cholera toxin after oral immunisation is thymus dependent and associated with intestinal production of neutralising IgA antitoxin. Scand J Immunol. 1987;25:413–9. doi: 10.1111/j.1365-3083.1987.tb02208.x. [DOI] [PubMed] [Google Scholar]
- 32.Lycke N, Erlandsson L, Ekman L, Schon K, Leanderson T. Lack of J chain inhibits the transport of gut IgA and abrogates the development of intestinal antitoxic protection. J Immunol. 1999;163:913–9. [PubMed] [Google Scholar]
- 33.Hörnquist CE, Ekman L, Grdic KD, Schön K, Lycke NY. Paradoxical IgA immunity in CD4-deficient mice. J Immunol. 1995;155:2877–87. [PubMed] [Google Scholar]
- 34.Vajdy M, Kosco-Vilbois MH, Kopf M, Kohler G, Lycke N. Impaired mucosal immune responses in interleukin 4-targeted mice. J Exp Med. 1995;181:41–53. doi: 10.1084/jem.181.1.41. [DOI] [PubMed] [Google Scholar]
- 35.Gardby E, Lane P, Lycke NY. Requirements for B7-CD28 costimulation in mucosal IgA responses: paradoxes observed in CTLA4-H gamma 1 transgenic mice. J Immunol. 1998;161:49–59. [PubMed] [Google Scholar]
- 36.Weinstein PD, Cebra JJ. The preference for switching to IgA expression by Peyer's patch germinal centre B cells is likely due to the influence of their microenvironment. J Immunol. 1991;147:4126–35. [PubMed] [Google Scholar]
- 37.Weinstein PD, Schweitzer PA, Cebra-Thomas A, Cebra JJ. Molecular genetic features reflecting the preference for isotype switching to IgA expression by Peyer's patch germinal center B cells. Int Immunol. 1991;3:1253–63. doi: 10.1093/intimm/3.12.1253. [DOI] [PubMed] [Google Scholar]
- 38.Schrader CE, Cebra JJ. Dendritic cell dependent expression of IgA by clones in T/B microcultures. Adv Exp Med Biol. 1993;329:59–64. doi: 10.1007/978-1-4615-2930-9_10. [DOI] [PubMed] [Google Scholar]
- 39.Fayette J, Dubois B, Vandenabeele S, et al. Human dendritic cells skew isotype switching of CD40-activated naive B cells towards IgA1 and IgA2. J Exp Med. 1997;185:1909–18. doi: 10.1084/jem.185.11.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Litinskiy MB, Nardelli B, Hilbert DM, Schaffer A, Casali P, Cerutti A. Antigen presenting cells induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat Immunol. 2002;3:822–9. doi: 10.1038/ni829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Castigli E, Wilson SA, Scott S, et al. TACI and BAFF-R mediate isotype switching in B cells. J Exp Med. 2005;201:35–9. doi: 10.1084/jem.20032000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Macpherson AJ, Gatto D, Sainsbury E, Harriman GR, Hengartner H, Zinkernagel RM. A primitive T cell-independent mechanism of intestinal mucosal IgA responses to commensal bacteria. Science. 2000;288:2222–6. doi: 10.1126/science.288.5474.2222. [DOI] [PubMed] [Google Scholar]
- 43.Snider DP, Liang H, Switzer I, Underdown BJ. IgA production in MHC class II-deficient mice is primarily a function of B-1a cells. Int Immunol. 1999;11:191–8. doi: 10.1093/intimm/11.2.191. [DOI] [PubMed] [Google Scholar]
- 44.Franco MA, Greenberg HB. Immunity to rotavirus infection in mice. J Infect Dis. 1999;179:S466–9. doi: 10.1086/314805. [DOI] [PubMed] [Google Scholar]
- 45.Franco M, Greenberg HB. Immunity to rotavirus in T cell deficient mice. Virology. 1997;238:169–79. doi: 10.1006/viro.1997.8843. [DOI] [PubMed] [Google Scholar]
- 46.Jain A, Ma CA, Lopez-Granados E, et al. Specific NEMO mutations impair CD40-mediated c-Rel activation and B cell terminal differentiation. J Clin Invest. 2004;114:1593–602. doi: 10.1172/JCI21345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Atkinson TP, Smith CA, Hsu YM, et al. Leukocyte transfusion-associated granulocyte responses in a patient with X-linked hyper-IgM syndrome. J Clin Immunol. 1998;18:430–9. doi: 10.1023/a:1023286807853. [DOI] [PubMed] [Google Scholar]
- 48.Stoel M, Jiang H-Q, van Diemen CC, et al. Restricted IgA repertoire in both B-1 and B-2 cell-derived gut plasmablasts. J Immunol. 2005;174:1046–54. doi: 10.4049/jimmunol.174.2.1046. [DOI] [PubMed] [Google Scholar]
- 49.Casola S, Otipoby KL, Alimzhanov M, Humme S, Uyttersprot N, Kutok JL, Carroll MC, Rajewsky K. B cell receptor signal strength determines B cell fate. Nat Immunol. 2004;5:317–27. doi: 10.1038/ni1036. [DOI] [PubMed] [Google Scholar]
- 50.Holtmeier W, Hennemann A, Caspary WF. IgA and IgM V (H) repertoires in human colon: evidence for clonally expanded B cells that are widely disseminated. Gastroenterology. 2000;119:1253–66. doi: 10.1053/gast.2000.20219. [DOI] [PubMed] [Google Scholar]
- 51.Dunn-Walters DK, Boursier L, Spencer J. Hypermutation, diversity and dissemination of human intestinal lamina propria plasma cells. Eur J Immunol. 1997;27:2959–64. doi: 10.1002/eji.1830271131. [DOI] [PubMed] [Google Scholar]
- 52.Martin F, Kearney JF. B1 cells: similarities and differences with other B cell subsets. Curr Opin Immunol. 2001;13:195–201. doi: 10.1016/s0952-7915(00)00204-1. [DOI] [PubMed] [Google Scholar]
- 53.Lam KP, Rajewsky K. B cell antigen receptor specificity and surface density together determine B-1 versus B-2 cell development. J Exp Med. 1999;190:471–8. doi: 10.1084/jem.190.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kroese FGM, Butcher EC, Stall AM, Lalor PA, Adams S, Herzenberg LA. Many of the IgA producing plasma cells in the murine gut are derived from self-replenishing precursors in the peritoneal cavity. Int Immunol. 1988;1:75–84. doi: 10.1093/intimm/1.1.75. [DOI] [PubMed] [Google Scholar]
- 55.Kroese FG, Ammerlaan WA, Kantor AB. Evidence that intestinal IgA plasma cells in mu, kappa transgenic mice are derived from B-1 (Ly-1 B) cells. Int Immunol. 1993;5:1317–27. doi: 10.1093/intimm/5.10.1317. [DOI] [PubMed] [Google Scholar]
- 56.Thurnheer MC, Zuercher AW, Cebra JJ, Bos NA. B1 cells contribute to serum igM but not to intestinal IgA production in gnotobiotic Ig allotype chimeric mice. J Immunol. 2003;170:4564–71. doi: 10.4049/jimmunol.170.9.4564. [DOI] [PubMed] [Google Scholar]
- 57.Antin JH, Emerson SG, Martin P, Gadol N, Ault KA. Leu-1+ (CD5+) B cells. A major lymphoid subpopulation in human fetal spleen: phenotypic and functional studies. J Immunol. 1986;136:505–10. [PubMed] [Google Scholar]
- 58.Casali P, Notkins AL. CD5+ B lymphocytes, polyreactive antibodies and the human B-cell repertoire. Immunol Today. 1989;10:364–8. doi: 10.1016/0167-5699(89)90268-5. [DOI] [PubMed] [Google Scholar]
- 59.Hardy RR, Hayakawa K, Shimizu M, Yamasaki K, Kishimoto T. Rheumatoid factor secretion from human Leu-1+ B cells. Science. 1987;236:81–3. doi: 10.1126/science.3105057. [DOI] [PubMed] [Google Scholar]
- 60.Brandtzaeg P, Pabst R. Let's go mucosal: communication on slippery ground. Trends Immunol. 2004;25:570–7. doi: 10.1016/j.it.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 61.Cazac BB, Roes J. TGF-beta receptor controls B cell responsiveness and induction of IgA in vivo. Immunity. 2000;13:443–51. doi: 10.1016/s1074-7613(00)00044-3. [DOI] [PubMed] [Google Scholar]
- 62.Fagarasan S, Kinoshita K, Muramatsu M, Ikuta K, Honjo T. In situ class switching and differentiation to IgA-producing cells in the gut lamina propria. Nature. 2001;413:639–43. doi: 10.1038/35098100. [DOI] [PubMed] [Google Scholar]
- 63.Fagarasan S, Muramatsu M, Suzuki K, Nagaoka H, Hiai H, Honjo T. Critical roles of activation-induced cytidine deaminase in the homeostasis of gut flora. Science. 2002;298:1424–7. doi: 10.1126/science.1077336. [DOI] [PubMed] [Google Scholar]
- 64.Bos NA, Bun JC, Popma SH, Cebra ER, Deenen GJ, van der Cammen MJ, Kroese FG, Cebra JJ. Monoclonal immunoglobulin A derived from peritoneal B cells is encoded by both germ line and somatically mutated VH genes and is reactive with commensal bacteria. Infect Immun. 1996;64:616–23. doi: 10.1128/iai.64.2.616-623.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Macpherson AJ, Lamarre A, McCoy K, Dougan G, Harriman G, Hengartner H, Zinkernagel R. IgA B cell and IgA antibody production in the absence of mu and delta heavy chain expression early in B cell ontongeny. Nat Immunol. 2001;2:625–31. doi: 10.1038/89775. [DOI] [PubMed] [Google Scholar]
- 66.Bouvet JP, Fischetti VA. Diversity of antibody-mediated immunity at the mucosal barrier. Infect Immun. 1999;67:2687–91. doi: 10.1128/iai.67.6.2687-2691.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Snider DP, Marshall JS, Perdue MH, Liang H. Production of IgE antibody and allergic sensitization of intestinal and peripheral tissues after oral immunization with protein Ag and cholera toxin. J Immunol. 1994;153:647–57. [PubMed] [Google Scholar]
- 68.Hasan M, Polic B, Bralic M, Jonjic S, Rajewsky K. Incomplete block of B cell development and immunoglobulin production in mice carrying the muMT mutation on the BALB/c background. Eur J Immunol. 2002;32:3463–71. doi: 10.1002/1521-4141(200212)32:12<3463::AID-IMMU3463>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 69.Brandtzaeg P. Mucosal and glandular distribution of immunoglobulin components. differential localization of free and bound SC in secretory epithelial cells. J Immunol. 1974;112:1553–9. [PubMed] [Google Scholar]
- 70.Brandtzaeg P. Polymeric IgA is complexed with secretory component (SC) on the surface of human intestinal epithelial cells. Scand J Immunol. 1978;8:39–52. doi: 10.1111/j.1365-3083.1978.tb00494.x. [DOI] [PubMed] [Google Scholar]
- 71.Johansen FE, Brandtzaeg P. Transcriptional regulation of the mucosal IgA system. Trends Immunol. 2004;25:150–7. doi: 10.1016/j.it.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 72.Johansen FE, Pekna M, Norderhaug IN, Haneberg B, Hietala MA, Krajci P, Betsholtz C, Brandtzaeg P. Absence of epithelial immunoglobulin A transport, with increased mucosal leakiness, in polymeric immunoglobulin receptor/secretory component-deficient mice. J Exp Med. 1999;190:915–22. doi: 10.1084/jem.190.7.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Roy MJ, Varvayanis M. Development of dome epithelium in gut-associated lymphoid tissues. association of IgA with M cells. Cell Tissue Res. 1987;248:645–51. doi: 10.1007/BF00216495. [DOI] [PubMed] [Google Scholar]
- 74.Weltzin R, Lucia-Jandris P, Michetti P, Fields BN, Kraehenbuhl JP, Neutra MR. Binding and transepithelial transport of immunoglobulins by intestinal M cells: demonstration using monoclonal IgA antibodies against enteric viral proteins. J Cell Biol. 1989;108:1673–85. doi: 10.1083/jcb.108.5.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mantis NJ, Cheung MC, Chintalacharuvu KR, Rey J, Corthesy B, Neutra MR. Selective adherence of IgA to murine Peyer's patch M cells: evidence for a novel IgA receptor. J Immunol. 2002;169:1844–51. doi: 10.4049/jimmunol.169.4.1844. [DOI] [PubMed] [Google Scholar]
- 76.Macpherson AJ, Uhr T. Compartmentalisation of mucosal immune responses to commensal intestinal bacteria. In: Weiner HL, Mayer L, Strober W, editors. Oral Tolerance: Mechanisms and Applications. Vol. 1029. New York: Annals New York Academy of Science; 2004. pp. 36–43. [DOI] [PubMed] [Google Scholar]
- 77.Shiloh MU, MacMicking JD, Nicholson S, Brause JE, Potter S, Marino M, Fang F, Dinauer M, Nathan C. Phenotype of mice and macrophages deficient in both phagocyte oxidase and inducible nitric oxide synthase. Immunity. 1999;10:29–38. doi: 10.1016/s1074-7613(00)80004-7. [DOI] [PubMed] [Google Scholar]
- 78.Sansonetti P. Phagocytosis of bacterial pathogens: implications in the host response. Semin Immunol. 2001;13:381–90. doi: 10.1006/smim.2001.0335. [DOI] [PubMed] [Google Scholar]
- 79.Owens WE, Berg RD. Bacterial translocation from the gastrointestinal tract of athymic (nu/nu) mice. Infect Immun. 1980;27:461–7. doi: 10.1128/iai.27.2.461-467.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Berg LJ, Pullen AM, Fazekas de St Groth B, Mathis D, Benoist C, Davis MM. Antigen/MHC-specific T cells are preferentially exported from the thymus in the presence of their MHC ligand. Cell. 1989;58:1035–46. doi: 10.1016/0092-8674(89)90502-3. [DOI] [PubMed] [Google Scholar]
- 81.Gautreaux MD, Gelder FB, Deitch EA, Berg RD. Adoptive transfer of T lymphocytes to T-cell-depleted mice inhibits Escherichia coli translocation from the gastrointestinal tract. Infect Immun. 1995;63:3827–34. doi: 10.1128/iai.63.10.3827-3834.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Berg RD. The indigenous gastrointestinal microflora. Trends Microbiol. 1996;4:430–5. doi: 10.1016/0966-842x(96)10057-3. [DOI] [PubMed] [Google Scholar]
- 83.Bachmann MF, Kalinke U, Althage A, et al. The role of antibody concentration and avidity in antiviral protection. Science. 1997;276:2024–7. doi: 10.1126/science.276.5321.2024. [DOI] [PubMed] [Google Scholar]
- 84.Macpherson A, Khoo UY, Forgacs I, Philpott-Howard J, Bjarnason I. Mucosal antibodies in inflammatory bowel disease are directed against intestinal bacteria. Gut. 1996;38:365–75. doi: 10.1136/gut.38.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rescigno M, Urbano M, Valzasina B, et al. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol. 2001;2:361–7. doi: 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- 86.Niess JH, Brand S, Gu X, et al. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 2005;307:254–8. doi: 10.1126/science.1102901. [DOI] [PubMed] [Google Scholar]
- 87.Kramer DR, Cebra JJ. Early appearance of ‘natural’ mucosal IgA responses and germinal centers in suckling mice developing in the absence of maternal antibodies. J Immunol. 1995;154:2051–62. [PubMed] [Google Scholar]
- 88.Hooper LV, Stappenbeck TS, Hong CV, Gordon JI. Angiogenins: a new class of microbicidal proteins involved in innate immunity. Nat Immunol. 2003;4:269–73. doi: 10.1038/ni888. [DOI] [PubMed] [Google Scholar]
- 89.Brandtzaeg P, Nilssen DE. Mucosal aspects of primary B cell deficiency and gastrointestinal infections. Curr Opin Gastroenterol. 1995;11:532–40. [Google Scholar]
- 90.Lehrer RI, Ganz T. Cathelicidins: a family of endogenous antimicrobial peptides. Curr Opin Hematol. 2002;9:18–22. doi: 10.1097/00062752-200201000-00004. [DOI] [PubMed] [Google Scholar]
- 91.Lehrer RI, Ganz T. Defensins of vertebrate animals. Curr Opin Immunol. 2002;14:96–102. doi: 10.1016/s0952-7915(01)00303-x. [DOI] [PubMed] [Google Scholar]