Inverse correlation between CD4+ regulatory T-cell population and autoantibody levels in paediatric patients with systemic lupus erythematosus (original) (raw)
Abstract
CD4+ CD25+ regulatory T cells (Tregs) are critical in maintaining self-tolerance and preventing organ-specific autoimmunity. Their role in paediatric systemic lupus erythematosus (SLE), an autoimmune disease characterized by inappropriate regulation of hyperactivated B and T cells, has not been clearly defined. Using flow cytometry to determine cell populations and real-time polymerase chain reaction to assay mRNA expression for FOXP3, CTLA-4, and GITR, we characterized CD4+ CD25+ T cells in paediatric SLE patients and healthy subjects. The frequency of CD4+ CD25+ Tregs was significantly decreased in patients with active SLE compared with patients with inactive SLE and with controls (7·27% ± 2·50%, 9·59% ± 2·80% and 9·78% ± 2·11%, respectively; P = 0·027 and P < 0·001, respectively), and was inversely correlated with disease activity, as assessed with the Systemic Lupus Erythematosus Disease Activity Index 2000 scores (r = −0·59, P = 0·001) and serum anti-double-stranded DNA levels (r = −0·65, P < 0·001). Our preliminary investigations found elevated surface expression of GITR in CD4+ CD25+ T cells, elevated mRNA expression of CTLA-4 in CD4+ T cells and higher amounts of mRNA expression for FOXP3 in CD4+ cells in patients with active SLE compared with patients with inactive disease and controls. We demonstrated reduced CD4+ CD25+ Treg levels were inversely correlated with disease activity, indicating a defective Treg population in paediatric SLE patients. The differences in the expression of FOXP3, CTLA-4 and GITR imply the possible role of CD4+ Tregs in the pathogenesis of SLE.
Keywords: CD4+ CD25+ regulatory T cells, FOXP3, real-time PCR, systemic lupus erythematosus
Introduction
The CD4+ CD25+ regulatory T cells (Tregs), which constitute 5–10% of peripheral CD4+ T cells in normal naive mice and humans, were first described by Sakaguchi et al.1 The direct removal of CD4+ Tregs from healthy animals can break self-tolerance, leading to the development of autoimmune disease, whereas repopulation of these cells can re-establish self-tolerance and prevent autoimmune disease. This observation indicates that Tregs play a critical role in the maintenance of self-tolerance and the prevention of organ-specific autoimmunity.1 These cells constitutively express the α-chain of the interleukin-2 receptor (IL-2R), and the thymus produces them as a functionally mature T-cell subpopulation (naturally occurring Tregs). Other regulatory T-cell subsets have also been described. These are largely T-cell populations induced by in vitro or in vivo manipulation with antigenic stimulation, and they have been termed adaptive or induced Tregs.2
The precise mechanisms of immunological regulation by which Tregs mediate their effect on other activated T cells are controversial and under active investigation. Essential roles for cytokines (interleukin-10 and transforming growth factor-β), cell-to-cell contact interaction, modification of antigen-presenting cells by Tregs, and competition with naive T cells of the same antigen specificity in adhesion to antigen-presenting cells have all been suggested.3 Candidate molecules associated with the activation and immunosuppressive function of Tregs include the negative costimulator cytotoxic T lymphocyte-associated antigen 4 (CTLA-4), which interacts with the B7 family, and the glucocorticoid-induced tumour-necrosis-factor-receptor-family-related receptor (GITR), which is involved in signal transduction.3
Moreover, the suppressive phenotype and the development of regulatory function depend on the expression of FOXP3, a forkhead-winged helix transcription factor gene exclusively expressed by CD4+ CD25+ Tregs. FOXP3 appears to be a master control gene for the development of CD4+ CD25+ Tregs.4– 6 Immune dysregulation polyendocrinopathy X-linked syndrome is caused by mutations in FOXP3 and by defects in the generation and development of the suppressive function of naturally arising CD4+ CD25+ Tregs. The alterations lead to immune dysregulation, severe eczema, elevated levels of immunoglobulin E, eosinophilia and food allergy.7 So far, this is the clearest example of an abnormality in naturally arising Tregs as the primary cause of a human autoimmune disease.3
Systemic lupus erythematosus (SLE) is a disorder of generalized autoimmunity characterized by pathogenic autoantibodies and immune complexes that are attributed to inappropriate regulation of hyperactivated B and T cells, defective clearance of apoptotic cells and immune complexes, and loss of immune tolerance.8 The role of T cells in the pathogenesis of SLE is revealed by a defect of T-cell-mediated immunological tolerance and by their support of autoantibody production by autoantigen-reactive B cells.9 The aetiology of SLE is complex. Multiple susceptibility genes and environmental factors are implicated in the initiation and perpetuation of the activation of T and B lymphocytes. Because spontaneous remission or improvements in the course of the disease are common in patients with SLE,8 dysfunctional regulatory cells may contribute to the onset and exacerbation of SLE.10 However, the characteristics and roles of Tregs in patients with SLE have not been carefully studied. Only limited data showing reduced numbers of CD4+ CD25+ T cells in adults with SLE have been reported.11,12 The functional implications of Tregs in the pathogenesis of SLE and studies in paediatric patients are lacking.
We aimed to assay the frequency of CD4+ CD25+ T cells in paediatric patients with SLE and to investigate differences in the expression of functionally important molecules of CD4+ Tregs in these patients, in anticipation that the findings could help in understanding the possible role of CD4+ Tregs in the pathogenesis of pediatric SLE.
Materials and methods
Patients and control subjects
From July 2003 to December 2004, 27 paediatric patients (22 girls, five boys) who fulfilled at least four of the 1997 revised criteria for the classification of SLE were enrolled in the study.13 Patients older than 18 years at the time of enrolment were excluded. The disease activity was assessed by measuring serum levels of anti-double-stranded DNA (dsDNA) antibody and by using scores on the Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K).14 Patients with SLEDAI-2K scores of ≥ 4 were defined as having active SLE. Twenty-five healthy controls matched in age and sex were included. The study was approved by the Institutional Review Board at the National Taiwan University Hospital. Informed consent was obtained from all subjects and their parents. The demographic characteristics of all subjects, their disease status and their current medication are shown in Table 1.
Table 1.
Demographic and clinical characteristics of patients and control subjects
| Control | Inactive | Active | |
|---|---|---|---|
| Case no. | 25 | 10 | 17 |
| Sex (Female:Male) | 20:5 | 9:1 | 13:4 |
| Age1 (range) | 12 ± 4 (5–18) | 14 ± 3 (7–18) | 13 ± 4 (5–18) |
| SLEDAI score1 | NA | 2·1 ± 0·3 | 6·1 ± 2·4* |
| Anti-dsDNA1 | NA | 30·9 ± 20·4 | 303·3 ± 241·8* |
| Treg %1 (range) | 9·78 ± 2·11** (6·05–14·43) | 9·59 ± 2·80** (6·25–14·50) | 7·27 ± 2·50 (3·97–13·92) |
| Prednisolone12 (case no., %) | NA | 7·0 ± 2·6 (6, 60·0%) | 13·4 ± 12·8* (15, 88·2%) |
| Hydroxychloroquine12 (case no., %) | NA | 200·0 ± 133·3 (8, 80·0%) | 223·5 ± 114·7 (15, 88·2%) |
| Azathioprine12 (case no., %) | NA | 17·5 ± 26·5 (4, 40·0%) | 19·6 ± 24·5 (7, 41·2%) |
Monoclonal antibodies
Fluorescein isothiocyanate (FITC)-conjugated anti-CD25, phycoerythrin (PE)-conjugated anti-CD69, PE-conjugated anti-CD122 and peridinine chlorophyll protein (PerCP)-conjugated anti-CD4 monoclonal antibodies, and appropriate isotype controls of mouse immunoglobulin G1 (IgG1) were purchased from Becton-Dickinson Immunocytometry System (BD, San Jose, CA), while PE-conjugated anti-GITR antibody was purchased from R & D Systems Inc (Minneapolis, MN).
Flow cytometric analysis
Three-colour fluorochrome-conjugated monoclonal antibody sets were used to stain for T-cell surface markers. All antibodies were used at concentrations titrated for optimal staining. Briefly, a sample of whole blood was incubated in the dark for 30 min, washed with phosphate-buffered saline twice and fixed with 1% paraformaldehyde at room temperature. Fluorocytometry was performed with the FACSCalibur system (BD, San Jose, CA). All fluorocytometric data were subsequently analysed and displayed with cell questpro software (BD, San Jose, CA). Each analysis included measurements from a minimum of 20 000 cells.
Preparation of peripheral blood mononuclear cells
Heparinized venous whole blood from each donor was layered on a Ficoll-Paque density gradient (Amersham Pharmacia Biotech, Uppsala, Sweden) and centrifuged at room temperature. The peripheral blood mononuclear cell layer was collected, washed and resuspended in Hanks' balanced salt solution (Gibco BRL, Life Technologies, GmbH, Eggenstein, Germany). Cell viability was greater than 95%, as determined by using the trypan blue exclusion assay under the optical microscope.
Isolation of CD4+ Tregs
Human peripheral blood mononuclear cells were centrifuged and resuspended in RPMI-1640 medium (Bio-Whittaker, Boehringer Ingelheim, Belgium) containing 10% heat-inactivated fetal calf serum, 40 μmol/l l-glutamine, 100 U/ml penicillin, 100 U/ml streptomycin and 20 mmol/l HEPES buffer solution (Gibco BRL, Life Technologies) at room temperature. CD4+ T cells were purified by negative selection with immunomagnetic beads (by depleting CD8+, CD11b+, CD16+, CD19+, CD36+ and CD56+ cells), using a human CD4+ T-cell isolation kit according to the manufacturer's instructions. (Miltenyi Biotec, Bergisch Gladbach, Germany). Starting with 1 × 107 to 2 × 107 peripheral blood mononuclear cells, 4·5 × 106 to 5·0 × 106 CD4+ T cells were typically isolated; the purity of CD4+ T cells was always more than 90%.
RNA extraction, first-strand cDNA synthesis, and quantitative real-time polymerase chain reaction
Total mRNA was extracted by using the GeneStrips mRNA isolation kit (RNAture, Irvine, CA) according to the manufacturer's protocol. Then, 50–500 ng of mRNA was reverse transcribed with random primers (Promega, Madison, WI), SuperScript II reverse transcriptase (Invitrogen, Carlsbad, CA) and RNaseOUT ribonuclease inhibitor (Invitrogen) in the same hybridization tube. The primers and probes used in the real-time polymerase chain reaction (PCR) for FOXP3, GITR, and CTLA-4 were selected using Assays-on-Demand Gene Expression Products (Applied Biosystems, Foster City, CA). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Applied Biosystems) was used as an endogenous control.
Real-time PCR reactions of each gene were performed in triplicate, and the results were analysed with an ABI Prism 7700 sequence detector (Applied Biosystems). Levels of mRNA expression for each gene were calculated using the 2−ΔΔCT (comparative CT) method (Applied Biosystems). Efficiencies of target genes (FOXP3, GITR and CTLA-4) and reference (GAPDH) were approximately equal, as proven in individual validation experiments. Quantities of target gene expression in test samples were normalized to the corresponding GAPDH mRNA level of a healthy subject (calibrator).
Statistical analysis
Data were expressed as the mean ± SD unless otherwise specified. Statistical comparisons of flow cytometric data among groups of subjects were performed with the Mann–Whitney _U_-test. Correlation coefficients (r) were generated using the Spearman rank correlation. A _P_-value of less than 0·05 was considered to indicate a statistically significant difference.
Results
Demographic and clinical characteristics of subjects
The control group was matched with the patient group for both age and sex. Within the study group, patients with active SLE disease had significantly higher SLEDAI-2K scores, anti-dsDNA levels and daily prednisolone dose than did patients with inactive SLE disease (Table 1).
Percentages of CD4+ CD25+ Tregs in paediatric SLE
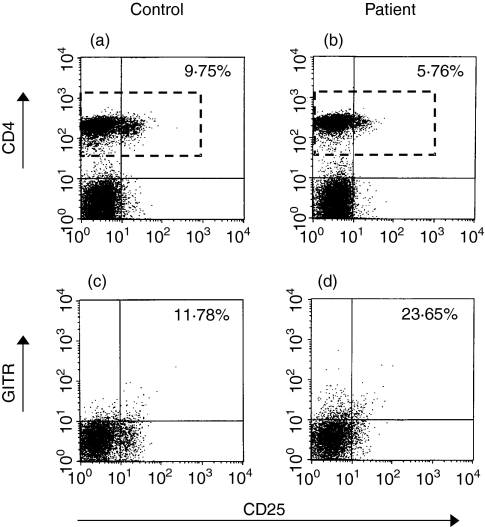
The representative figures for comparison of CD4+ CD25+ T cells between controls and patients are shown in Fig. 1.
Figure 1.
Comparison of flow cytometric analysis of CD4+ CD25+ T-cell populations and of surface expression of GITR in CD4+ CD25+ T cells between control and patient. The peripheral blood was stained with FITC-conjugated anti-CD25, PE-conjugated anti-GITR and PerCP-conjugated anti-CD4. Cells were gated on lymphocytes via their forward- and side-scatter properties. The gating strategy is shown for the analysis of surface CD25 expression in CD4+ T cells (a, b) and surface GITR expression in CD4+CD25+ T cells (c, d). The rectangle boxes in (a) and (b) indicate the gating area for the analysis of surface GITR expression in CD4+ CD25+ T cells. The percentages shown in (a) and (b) were the percentages of CD25+ in CD4+ T cells; the percentages shown in (c) and (d) were the percentages of GITR+ in CD4+ CD25+ T cells. Isotype controls for each sample were run in conjunction with CD25 staining (a, b) and with GITR staining (c, d), respectively.
Patients with active SLE had a significantly decreased percentage of CD25+ lymphocytes in CD4+ T cells compared with patients with inactive SLE and healthy controls [7·27% ± 2·50% (range: 3·97–13·92%) versus 9·59% ± 2·80% (range, 6·25–14·50%) and 9·78% ± 2·11% (range, 6·05–14·43%), respectively; _P_ = 0·027 and _P_ < 0·001, respectively) (Fig. 2). The percentage of CD25+ lymphocytes in CD4+ T cells was not significantly different between patients with inactive SLE and control subjects.
Figure 2.
Comparison of the frequency of CD4+ CD25+ T cells between paediatric patients with SLE and control subjects. The percentage of CD25+ lymphocytes in CD4+ T cells was significantly lower in patients with active SLE than in patients with inactive SLE and control subjects, respectively. Line within the vertical points marks the median (7·00% for patients with active SLE versus 9·32% for patients with active SLE and 9·43% for control subjects, respectively).
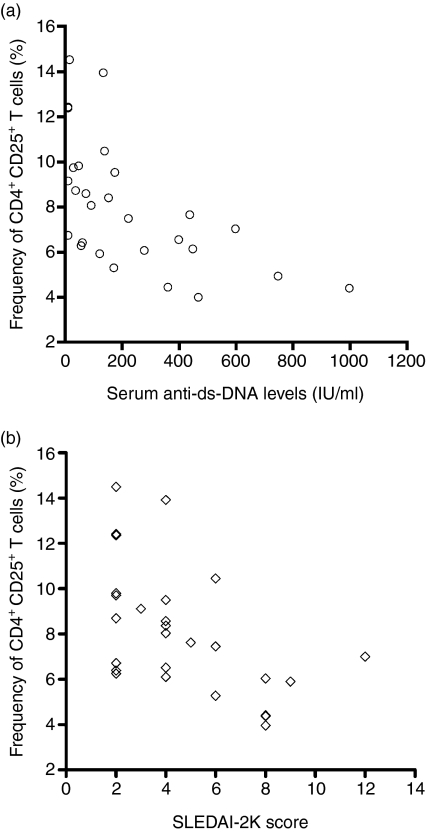
Correlation of disease activity with frequency of CD4+ CD25+ Tregs
The mean anti-dsDNA antibody serum levels and SLEDAI-2K scores of patients with SLE are shown in Table 1.
The frequency of CD4+ CD25+ Tregs was inversely correlated with serum anti-dsDNA levels (r = − 0·65, 95% confidence interval: − 0·37 to − 0·83, P < 0·001) (Fig. 3a), and with SLEDAI-2K score (r = − 0·59, 95% confidence interval: − 0·27 to − 0·79, P = 0·001) (Fig. 3b). Disease activity in patients with SLE was significantly correlated with serum anti-dsDNA levels (r = 0·84, 95% confidence interval: − 0·67 to − 0·92, P < 0·001) (data not shown).
Figure 3.
The relationships between disease activity and the frequency of CD4+ CCD25+ Tregs in pediatric patients with SLE.
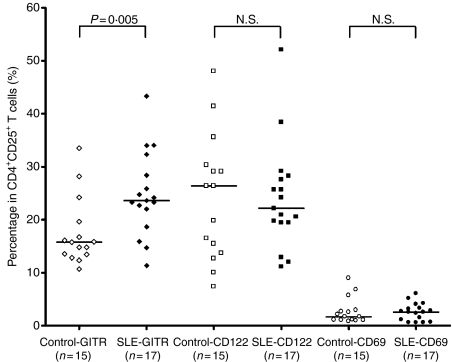
Phenotypic characteristics of CD4+ CD25+ Tregs
Figure 4 shows the results of the comparison of the expression of markers CD69, CD122 and GITR on CD4+ CD25+ T cells of patients (n = 17) and control subjects (n = 15). GITR expression in CD4+ CD25+ T cells was significantly higher in patients than in control subjects, with values of 26·65% ± 6·97% versus 17·49% ± 6·38% (P = 0·005). CD122 and CD69 expression in CD4+ CD25+ T cells did not differ in patients and controls (23·63% ± 10·47% versus 24·11% ± 11·85% for CD122 and 2·96% ± 1·58% versus 2·74 ± 2·46% for CD69, both P > 0·05). The representative figures for comparative surface expression of GITR in CD4+ CD25+ T cells of controls and patients are shown in Fig. 1.
Figure 4.
Surface expression of GITR, CD122, and CD69 in CD4+ CD25+ Tregs. GITR expression was significantly higher in patients with SLE than in control subjects. The expression of CD122 and CD69 did not significantly differ between groups.
In contrast, the CD69 expression in CD4+ T cells was significantly higher in patients compared with controls (2·40% ± 0·95% versus 1·46% ± 0·83%, P = 0·004).
Preferential messenger RNA expression of FOXP3 and CTLA-4 on CD4+ T cells
An illustrative comparison of mRNA expression of FOXP3, CTLA-4 and GITR between CD4+ and CD4– T cells in three patients with active SLE and in three control subjects was performed. In the SLE group, expression of FOXP3 [(2·35 ± 1·15 versus 3·87 ± 2·68 (× 10−2)], CTLA-4 (1·43 ± 0·24 versus 0·31 ± 0·16), and GITR (5·85 ± 2·62 versus 1·91 ± 1·83) was higher in CD4+ T cells than in CD4– T cells. In the control group, levels for FOXP3 [0·86 ± 0·45 versus 1·82 ± 3·54 (× 10−3)] and CTLA-4 [0·73 ± 0·36 versus 9·01 ± 5·48 (× 10−2) were higher for CD4+ T cells than CD4– T cells. In control subjects, GITR expression did not differ in CD4+ T cells and CD4– T cells (2·39 ± 1·99 versus 1·10 ± 0·87). Levels of mRNA expression for CTLA-4 of CD4+ T cells were greater in patients than in controls (1·43 ± 0·24 versus 0·73 ± 0·36). For GITR, levels of mRNA expression did not differ in patients and controls (5·85 ± 2·62 versus 2·39 ± 1·99). Given the small number of subjects, these findings must be regarded as preliminary.
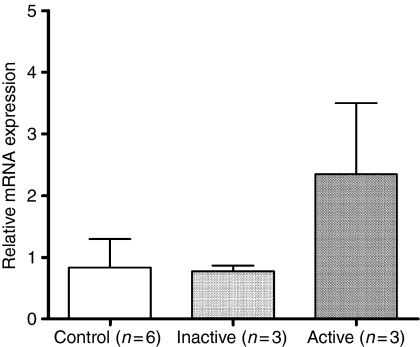
Comparison of mRNA expression of FOXP3 in different disease activity
FOXP3 was expressed in higher amounts in patients with active SLE (2·35 ± 1·15; n = 3) than in those with inactive SLE (0·76 ± 0·09; n = 3) and in controls (0·83 ± 0·46; n = 6). The mRNA expression for FOXP3 was not different between patients with inactive SLE and control subjects (Fig. 5). Again, subject numbers are too small for these findings to be regarded as other than preliminary.
Figure 5.
Comparison of mRNA expression of FOXP3 among diseases with different activities. Patients with active SLE had higher mRNA expression of FOXP3 than that of patients with inactive SLE and control subjects. The mRNA expression of FOXP3 was not different between patients with inactive SLE and control subjects.
Discussion
The decreased frequency of CD4+ CD25+ T cells in adult patients with SLE implies that a deficiency of Tregs is associated with SLE pathogenesis.11,12 Our data demonstrate that the number of CD4+ CD25+ T cells in the peripheral blood of paediatric patients with active SLE is also significantly decreased. When the age-related frequency of CD4+ CD25+ T cells in Taiwanese children (13·% in children aged 2–5 years; 15.7% in those aged 6–15 years)15 is taken into consideration, the difference becomes even more significant. We also demonstrated an inverse correlation between the number of CD4+ CD25+ T cells and disease activity. We therefore suggest that the decreased number of CD4+ CD25+ T cells in the peripheral blood of paediatric patients with SLE constitutes a defective Treg population and contributes to the pathogenesis of SLE.
Activation of anti-dsDNA-producing autoreactive B cells in autoimmune lpr/lpr mice requires help from CD4+ helper T cells and the overcoming of the suppression of CD4+ CD25+ Tregs.16 The activated CD4+ helper T cells presumably rescue self-reactive B cells from apoptosis and stimulate them to form autoantibodies.17 We demonstrated that the frequency of CD4+ CD25+ T cells in our patients with SLE was inversely related to both the serum level of anti-dsDNA and the disease activity. These results imply that a breakdown of tolerance as a result of impaired Treg function contributes to the excessive autoantibody formation seen in active SLE.
FOXP3/FOXP3 programmes the development of the suppressive function of CD4+ CD25+ Tregs.4 – 6 We have shown that a decreased CD4+ CD25+ T-cell population is associated with the pathogenesis of paediatric SLE. However, our preliminary results showed a higher mRNA expression of FOXP3 in active disease. If this latter result is verified in further studies, the inconsistency between the decreased number of Tregs and high expression of FOXP3 would require explanation. There are two possibilities for this situation. Firstly, it had been reported that both normal and mutated FOXP3 alleles are equally expressed in the T cells of healthy carriers of immune dysregulation polyendocrinopathy X-linked syndrome and that reduced numbers of FOXP3-positive Tregs are responsible for the control of pathogenic T cells.18,19 It is therefore possible that some kind of functional compensation (FOXP3 overexpression) might be present in the reduced numbers of Tregs to regain immune control in patients with active SLE. The second possibility is that increased mRNA expression of FOXP3 is associated with steroid usage. According to Christian Karagiannidis et al. FOXP3 mRNA expression is significantly increased in asthmatic patients receiving glucocorticoid.20 Our SLE patients with active disease received more prednisolone than the inactive SLE patients, implying that such treatment might have some impact upon the mRNA expression of FOXP3 (Table 1). However, both explanations leave unanswered the question of why there is active disease when there is ‘intact’ mRNA expression of FOXP3 in Tregs. Further studies are necessary to establish the exact role and functional capacity of Tregs in paediatric patients with active SLE.
GITR is expressed preferentially at high levels on CD4+ CD25+ T cells and plays a key role in the peripheral tolerance mediated by Tregs.21 – 23 GITR is involved in regulating the functions of the CD4+ CD25+ T cell.24 GITR expression on activated T cells and Tregs confers resistance to suppression of Tregs and is required for the most effective immune response.25 The role of GITR in the pathogenesis of SLE is not defined and, to our knowledge, has not been reported. Our preliminary data revealed increased surface GITR expression in paediatric patients with SLE (Fig. 4). However, this result should be interpreted with great caution, not only because of the limited number of subjects involved, but also because the expression of GITR was also increased after T-cell activation. Since GITR is expressed preferentially at high levels on CD4+ CD25+ Tregs, it is possible that GITR expression might reflect the regulation of the suppressive function of CD4+ CD25+ Tregs in active paediatric SLE.
CTLA-4 (CD152) plays two roles in immune regulation: the transduction of negative control to activated T cells, and the activation of the suppressive function of Tregs.3,26 Increased expression of CTLA-4 in patients with SLE has been reported, 27 – 29 which might play a role in the pathogenesis of SLE. Since the preferential expression of CTLA-4 among CD4+ T cells is restricted primarily to Tregs30 it is possible that expression of CTLA-4 is associated with the activation of a Treg-mediated suppressive function required for immune control of active SLE. Our preliminary data are in line with previous reports, but involve very few subjects. Interpretation of these results may also be complicated by the finding that the expression of CTLA-4 was up-regulated after T-cell activation.
The detection and isolation of CD25+ Tregs in humans is complicated by the fact that CD25 is a marker of recent T-cell activation.31 Most CD4+ CD25+ Tregs from peripheral blood do not express CD69.32 Our finding of a low expression of CD69 in CD4+ CD25+ T cells and the same CD69 expression in CD4+ CD25+ T cells from patients with SLE and control subjects is consistent with previous findings. CD122 is expressed by only 6% of CD4+ CD25– T cells, by 28% of CD4+ CD25low T cells, and by more than 85% of CD4+ CD25hi T cells.33 In our study, CD122 expression was 23·63% ± 10·47% for patients with SLE and 24·11% ± 11·85% for control subjects, respectively, indicating that the CD4+ CD25+ T cells we studied comprised both CD25low and CD25hi T cells.
In summary, we found decreased CD4+ CD25+ T cells in paediatric patients with active SLE and an inverse correlation with increased disease activity and anti-dsDNA level, suggesting a role for a decreased Treg population in paediatric patients with SLE. The higher mRNA expression for FOXP3 found in active SLE could be the result of either functional compensation in CD4+ CD25+ Tregs or up-regulation of FOXP3 because of increased usage of corticosteroids. Our preliminary results also imply a fundamental role for GITR and CTLA-4 – possibly associated with regulation and activation, respectively, of CD4+ Treg suppressive functions – in the pathogenesis of paediatric SLE. This again requires confirmation in future studies.
References
- 1.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–64. [PubMed] [Google Scholar]
- 2.Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat Rev Immunol. 2003;3:253–7. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- 3.Sakaguchi S. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–62. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 4.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–6. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 5.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4:337–42. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 6.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–61. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 7.Gambineri E, Torgerson TR, Ochs HD. Immune dysregulation, polyendocrinopathy, enteropathy, and X-linked inheritance (IPEX), a syndrome of systemic autoimmunity caused by mutations of FOXP3, a critical regulator of T-cell homeostasis. Curr Opin Rheumatol. 2003;15:430–5. doi: 10.1097/00002281-200307000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Mok CC, Lau CS. Pathogenesis of systemic lupus erythematosus. J Clin Pathol. 2003;56:481–90. doi: 10.1136/jcp.56.7.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoffman RW. T cells in the pathogenesis of systemic lupus erythematosus. Clin Immunol. 2004;113:4–13. doi: 10.1016/j.clim.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Ohtsuka K, Gray JD, Stimmler MM, Toro B, Horwitz DA. Decreased production of TGF-beta by lymphocytes from patients with systemic lupus erythematosus. J Immunol. 1998;160:2539–45. [PubMed] [Google Scholar]
- 11.Crispin JC, Martinez A, Alcocer-Varela J. Quantification of regulatory T cells in patients with systemic lupus erythematosus. J Autoimmun. 2003;21:273–6. doi: 10.1016/s0896-8411(03)00121-5. [DOI] [PubMed] [Google Scholar]
- 12.Liu MF, Wang CR, Fung LL, Wu CR. Decreased CD4+CD25+ T cells in peripheral blood of patients with systemic lupus erythematosus. Scand J Immunol. 2004;59:198–202. doi: 10.1111/j.0300-9475.2004.01370.x. [DOI] [PubMed] [Google Scholar]
- 13.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 14.Gladman DD, Ibañez D, Urowitz MB. Systemic lupus erythematosus disease activity index 2000. J Rheumatol. 2002;29:288–91. [PubMed] [Google Scholar]
- 15.Lin SC, Chou CC, Tsai MJ, et al. Age-related changes in blood lymphocyte subsets of Chinese children. Pediatr Allergy Immunol. 1998;9:215–20. doi: 10.1111/j.1399-3038.1998.tb00376.x. [DOI] [PubMed] [Google Scholar]
- 16.Seo SJ, Fields ML, Buckler JL, et al. The impact of T helper and T regulatory cells on the regulation of anti-double-stranded DNA B cells. Immunity. 2002;16:535–46. doi: 10.1016/s1074-7613(02)00298-4. [DOI] [PubMed] [Google Scholar]
- 17.Tsubata T, Wu J, Honjo T. B-cell apoptosis induced by antigen receptor crosslinking is blocked by a T-cell signal through CD40. Nature. 1993;364:645–8. doi: 10.1038/364645a0. [DOI] [PubMed] [Google Scholar]
- 18.Tommasini A, Ferrari S, Moratto D, et al. X-chromosome inactivation analysis in a female carrier of FOXP3 mutation. Clin Exp Immunol. 2002;130:127–30. doi: 10.1046/j.1365-2249.2002.01940.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fehérvari Z, Sakaguchi S. CD4+ Tregs and immune control. J Clin Invest. 2004;114:1209–17. doi: 10.1172/JCI23395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karagiannidis C, Akdis M, Holopainen P, et al. Glucocorticoids upregulate FOXP3 expression and regulatory T cells in asthma. J Allergy Clin Immunol. 2004;114:1425–33. doi: 10.1016/j.jaci.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 21.McHugh RS, Whitters MJ, Piccirillo CA, et al. CD4+CD25+ immunoregulatory T cells: gene expression analysis reveals a functional role for the glucocorticoid-induced TNF receptor. Immunity. 2002;16:311–23. doi: 10.1016/s1074-7613(02)00280-7. [DOI] [PubMed] [Google Scholar]
- 22.Shimizu J, Yamazaki S, Takahashi T, Ishida Y, Sakaguchi S. Stimulation of CD25+CD4+ regulatory T cells through GITR breaks immunological self-tolerance. Nat Immunol. 2002;3:135–42. doi: 10.1038/ni759. [DOI] [PubMed] [Google Scholar]
- 23.Ji HB, Liao GX, Faubion WA, et al. Cutting edge: the natural ligand for glucocorticoid-induced TNF receptor-related protein abrogates regulatory T cell suppression. J Immunol. 2004;172:5823–7. doi: 10.4049/jimmunol.172.10.5823. [DOI] [PubMed] [Google Scholar]
- 24.Chen W, Wahl SM. TGF-beta: the missing link in CD4+CD25+ regulatory T cell-mediated immunosuppression. Cytokine Growth Factor Rev. 2003;14:85–9. doi: 10.1016/s1359-6101(03)00003-0. [DOI] [PubMed] [Google Scholar]
- 25.Harald von Boehmer. Mechanisms of suppression by suppressor T cells. Nat Immunol. 2005;6:338–44. doi: 10.1038/ni1180. [DOI] [PubMed] [Google Scholar]
- 26.Bluestone JA. Commentary: is CTLA-4 a master switch for peripheral T cell tolerance? J Immunol. 1997;158:1989–93. [PubMed] [Google Scholar]
- 27.Liu MF, Liu HS, Wang CR, Lei HY. Expression of CTLA-4 molecule in peripheral blood T lymphocytes from patients with systemic lupus erythematosus. J Clin Immunol. 1998;18:392–8. doi: 10.1023/a:1023226621966. [DOI] [PubMed] [Google Scholar]
- 28.Liu MF, Wang CR, Chen PC, Fung LL. Increased expression of soluble cytotoxic T-lymphocyte-associated antigen-4 molecule in patients with systemic lupus erythematosus. Scand J Immunol. 2003;57:568–72. doi: 10.1046/j.1365-3083.2003.01232.x. [DOI] [PubMed] [Google Scholar]
- 29.Hirashima M, Fukazawa T, Abe K, Morita Y, Kusaoi M, Hashimoto H. Expression and activity analyses of CTLA4 in peripheral blood lymphocytes in systemic lupus erythematosus patients. Lupus. 2004;13:24–31. doi: 10.1191/0961203304lu488oa. [DOI] [PubMed] [Google Scholar]
- 30.Read S, Malmström V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25+CD4+ regulatory cells that control intestinal inflammation. J Exp Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wing K, Ekmark A, Karlsson H, Rudin A, Suri-Payer E. Characterization of human CD25+CD4+ T cells in thymus, cord and adult blood. Immunology. 2002;106:190–9. doi: 10.1046/j.1365-2567.2002.01412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ng WF, Duggan PJ, Ponchel F, et al. Human CD4+CD25+ cells: a naturally occurring population of regulatory T cells. Blood. 2001;98:2736–44. doi: 10.1182/blood.v98.9.2736. [DOI] [PubMed] [Google Scholar]
- 33.Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. CD4+CD25high regulatory cells in human peripheral blood. J Immunol. 2001;167:1245–53. doi: 10.4049/jimmunol.167.3.1245. [DOI] [PubMed] [Google Scholar]