Conditional ablation of T-cell development by a novel viral ion channel transgene (original) (raw)
Abstract
A novel conditional-lethal transgene system is defined in which a mutated influenza A virus ion-channel protein, which is permeable to monovalent cations, is lethal to cells on heterotypic expression and whose activity can be blocked by an antiviral drug (amantadine), is used to reversibly disrupt T-cell development. In vivo expression of the M2 ion channel, as a transgene under control of the T-cell specific p56Lck proximal promoter, resulted in total ablation of T-cell development with the accumulation of three distinct populations of early progenitor cells (CD44+ CD25−; CD44+ CD25+; CD44+ CD25hi) in the thymic rudiment. In vitro development of transgenic fetal thymic progenitors to single-positive T cells could be rescued by antiviral drug treatment. Moreover, there was a radical reduction in B-cell lymphopoiesis, evident at the pre-B-cell stage, with a twofold increase of lymphoid cells ‘in cycle’ in transgenic bone marrow, indicative of major changes in haematopoietic homeostasis. This system may provide a generic protocol for conditional, lineage-specific cell ablation with available tissue-specific promoters for any eukaryotic developmental system, and provide a window on early T-cell development.
Introduction
A variety of genetic procedures are available to disrupt development in vivo and which provide potent tools for identifying regulatory elements, or transcription factors, that are implicated in a developmental programme. These procedures include: gene disruption by homologous recombination,1,2 inducible gene targeting3 (Cre-LoxP), or cell ablation following transgenic expression of toxins or thymidine kinase under the control of tissue-specific promoters.4–6 However, the efficiency of some cell-ablation procedures may be problematic. Herein, we describe a novel conditional-lethal transgene that can be used with any eukaryotic promoter and which may serve as a generic protocol: transgenic expression of a mutated influenza virus ion channel under control of the T-cell-specific p56Lck proximal promoter, which renders expressing cells permeable to monovalent cations (Na+ or K+), results in total ablation of T-cell development with accumulation of early progenitor cells in the thymic rudiment. Ion-channel activity can be blocked specifically by an antiviral drug, thereby rescuing T-cell development.
The M2 protein of influenza A virus is a small (97 amino acids) type III integral membrane protein that exists as a homotetramer.7,8 It forms a proton-selective channel which functions in two stages of virus replication: first, in the uncoating of infecting virion during endocytosis; and second, in reducing the acidity of the trans Golgi of cells to prevent inactivation of newly synthesized haemagglutinin, during its transport to the plasma membrane.9–11 The channel activity is specifically blocked by the anti-influenza virus drug, amantadine.12 The only charged amino acid residue in the transmembrane domain of M2, His37, is highly conserved between different influenza A virus subtypes, and protonation of His37 has been implicated in ion-channel activity.13 Electrophysiological studies, using either transient expression of M2 in frog oocytes14 or inducible expression in murine erythroleukaemic cells (N. Mulrine & A. J. Hay, unpublished), have shown that a mutant M2 (His37→Ala) confers non-selective, monovalent cation current activity at physiological pH, whereas wild-type M2 is a proton-specific channel and regulated by low pH (< 6).
We demonstrate herein that constitutive expression of M2 (H37A) in mammalian cell transfectants is highly toxic and that ion-channel activity, and toxicity, can be efficiently blocked by amantadine. We have exploited these findings to develop a conditional-lethal transgene, whereby expression of M2 (H37A) under control of the T-cell specific, p56Lck proximal promoter15 results in total ablation of T-cell development with the accumulation of immature progenitor cells in the thymic rudiment. T-cell development in fetal thymic organ culture, to the single-positive (SP) stage, can be rescued by amantadine treatment.
Materials and Methods
Transgenic mice
Animals were bred under specific pathogen-free conditions at this Institute. Weybridge strain avian influenza virus (A/Chicken/Germany/27) M2 variant, M2 (H37A) was cloned into vector p1017 containing the p56Lck proximal promoter.15 The _Not_I-excised fragment of p1017-M2 (H37A) was injected into the pro-nuclei of (CBA/Ca × C57BL/10)F2 zygotes, which were implanted into the oviducts of pseudo-pregnant females. Transgenic founders were identified by Southern blot analysis of tail DNA, using an M2 (H37A)-specific probe. Progeny from founder mice established three homozygous transgenic lines: Tg5, Tg15b and Tg15c.
Cell transfectants
Polymerase chain reaction (PCR)-amplified wild-type (Weybridge strain) M2 or M2 (H37A) were cloned into the pTarget T expression vector (Promega Corporation, San Luis Obispo, CA). The primers used for PCR amplification were: 5′-GAATTCCAAAAGCAGGTACATAT-3′ and 5′-GAAGATCTTTTACTCCAGCTCTAT-3′. T-cell thymoma BW5147 was transfected with the plasmid vector by electroporation, then maintained and cloned in the presence of amantadine (10 µg/ml, Sigma-Aldrich Co. Ltd, Poole, UK).
Thymus organ culture
Fetal thymi (day 16) were separated into their two individual lobes and cultured separately above polycarbonate filters (Nucleopore, Whatman International Ltd, Maidstone, UK), as described previously,16 in RPMI-1640 containing 5% fetal calf serum (FCS), in the presence or absence of amantadine (10 µg/ml). After 9 days, cell suspensions from pooled lobes were surface phenotyped by fluorescence-activated cell sorter (FACS) analysis.
Alternatively, thymic lobes were depleted of haematopoietic precursors by culture with deoxyguanosine (1·35 mm; Sigma) for 6 days. Thymic lobes were washed and reconstituted with adult thymic rudiment cells (5 × 105 cells/lobe) for 24–48 hr in hanging drop cultures. Thereafter, reconstituted thymi were cultured on filters, in the presence or absence of amantadine, for 7–9 days.
Flow cytometry analysis
FACS analyses were carried out using a FACS vantage system (BD Biosciences Immunocytometry Systems, San Jose, CA); all monoclonal antibodies (mAbs) – either fluorescein isothiocyanate (FITC) or phycoerythrin (PE) conjugated – were from BD Biosciences Pharmingen (San Diego, CA).
For cell-surface staining, Fc receptors were blocked by prior incubation with anti-CD16 and anti-CD32 mAbs followed, after washing, by incubation with specific mAb conjugates (anti-CD44, -CD25, -CD4, -CD8, -CD3 or -CD117), at 3°.
For M2 surface expression, cell transfectants were incubated with a polyclonal rabbit antiserum, specific for an N-terminal peptide of M2 (amino acids 1–24) followed, after washing, by incubation with goat anti-rabbit immunoglobulin G (IgG)-FITC.
bcl-2 staining
Intracellular bcl-2 staining was performed as described previously.17 After viable staining with anti-CD25-PE, cells were fixed, for 10 min at room temperature, in phosphate-buffered saline (PBS) containing 4% paraformaldehyde. After washing, cells were resuspended in buffer (PBS + 0·5% saponin + 1% FCS) containing anti-bcl-2 mAb for 30 min, then washed and analysed by FACS.
Bromodeoxyuridine (BrdU) staining
Transgenic mice received intraperitoneal injections of BrdU (1 mg) at 0 hr and 1 hr, were killed 5 hr later and their thymic rudiments were analysed by FACS. Cells were stained for surface CD25 expression followed by intracellular staining for BrdU incorporation, as previously described:18 cells were fixed in 75% ethanol for 30 min and, after overnight incubation in PBS containing 2% paraformaldehyde, were treated with DNAse I (50 U; Sigma) for 10 min at 37°, washed and then incubated for 30 min in PBS containing 10% FCS and 0.5% Tween-20. Finally, fixed cells were incubated with anti-BrdU mAb and analysed by FACS.
DNA analysis
DNA content was determined as described previously19 after staining of fixed cells with 7-aminoactinomycin D.
Results
Conditional-lethal M2 (H37A) cell transfectants
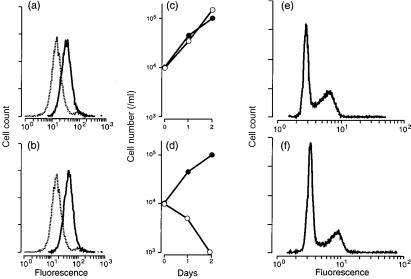
We wished to determine the effects of heterotypic expression of the influenza A virus ion channel, either wild-type M2 or mutant M2 (H37A) protein, on the growth and viability of mammalian cells in vitro. Transfectants of the murine thymoma, BW5147, were established and cloned in the presence of the ion-channel inhibitory drug amantadine, in order to obviate any possible deleterious effects of ion channel expression. We confirmed the equivalent surface expression of M2 and M2 (H37A) proteins in cell transfectants by FACS analysis following indirect immunofluorescent staining with a polyclonal antiserum specific for an M2 N-terminal peptide (Fig. 1a, 1b).
Figure 1.
Expression of wild-type M2 or mutant M2 (H37A) ion channels in transfectants of the murine thymoma BW5147. (a), (b) Fluorescence-activated cell sorter (FACS) analysis of surface expression of non-transfected cells (broken line), or cells transfected (unbroken line) with wild-type M2 (a) or M2 (H37A) (b). (c) (d) Growth of wild-type M2 (c) or M2 (H37A) (d) transfectants in the presence (•) or absence (○) of amantadine. (e), (f) FACS analysis of DNA content for M2 (H37A) transfectants cultured for 6 hr in the presence (e) or absence (f) of amantadine, fixed and stained with 7-aminoactinomycin D.19
Culture of M2 (H37A) transfectants in amantadine-free medium resulted in a loss of cell viability (Fig. 1d) with a decrease in incorporation of [3H]thymidine ([3H]TdR) into acid-precipitable material within 30 min of drug removal (Table 1). In contrast, culture of wild-type M2 transfectants in the presence or absence of amantadine had no effect on cell growth or cell viability (Fig. 1c). FACS analysis showed no difference in the bimodality of DNA content for M2 (H37A)-transfected cells cultured in the presence or absence of the drug for 6 hr (Fig. 1e, 1f). We concluded from these findings that ion channel-mediated toxicity was: rapid; independent of cell cycle status; and not mediated by an apoptotic mechanism, as there was no evidence of cells with < 2c DNA content.
Table 1. Effects of amantadine (Atd) on [3H]thymidine ([3H]TdR) incorporation by M2 or M2 (H37A) cell transfectants.
| [3H]TdR incorporation* | |||
|---|---|---|---|
| Cells | 0 hr | 3 hr | 6 hr |
| M2 | 2700 | 5000 | 3970 |
| M2+Atd† | 3000 | 5606 | 4700 |
| M2 (H37A) | 1300 | 1073 | 724 |
| M2 (H37A)+Atd† | 2632 | 4634 | 3530 |
Transgenic expression of M2 (H37A)
We wished to extend these studies in vivo, to determine whether M2 (H37A) expression would function as an efficient means of cell ablation, the activity of which could be blocked by drug treatment, thereby providing a conditional-lethal transgene. We selected the p56Lck proximal promoter for tissue-specific expression as its regulation is known to be stringent and activity restricted to defined stages in T-cell differentiation, from early progenitor to SP T cell.15
Three homozygous transgenic lines (differing in copy number) were established: Tg5 (two copies), Tg15b (four copies) and Tg15c (16 copies). Each transgenic line showed a similar phenotype, namely, radical reduction in thymic cellularity as compared to non-transgenic thymi (see Table 2).
Table 2. Cellular profile of thymic tissue in wild type, hemizygous (+/−) or homozygous (+/+) transgenic mice at 2 weeks of age.
| CD4+ (%) | CD8+ (%) | CD4+ CD8+ (%) | CD25+ (%) | CD44+ (%) | CD25+ CD44+ (%) | Cells (× 106) | |
|---|---|---|---|---|---|---|---|
| Wild type | 12 | 6 | 77 | 4 | 7 | 2 | 80–200 |
| Tg5+/− | 10 | 4 | 84 | 9 | 8 | 4 | 1–15 |
| Tg5+/+ | 4 | 4 | 3 | 55 | 44 | 55 | 2–6 |
| Tg15b+/− | 3 | 8 | 80 | 6 | 12 | 6 | 1–12 |
| Tg15b+/+ | 5 | 4 | 6 | 46 | 40 | 46 | 0·3–0·5 |
| Tg15c+/− | 15 | 6 | 75 | 3 | 4 | 3 | 10–30 |
| Tg15c+/+ | 2 | 5 | < 1 | 36 | 44 | 34 | 0·6–1 |
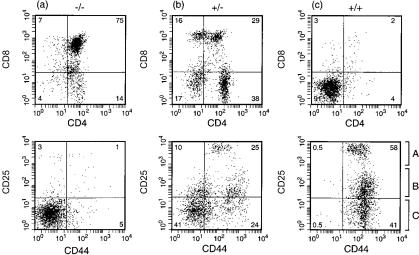
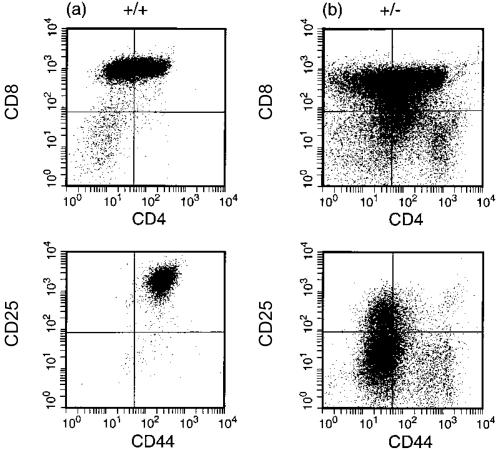
The thymic rudiment of homozygous transgenic mice was devoid of double-positive (DP) (CD4+ CD8+) or SP cells, and contained three distinct populations of double-negative (DN) cells (Fig. 2c): CD44+ CD25hi, CD44+ CD25+ and CD44+ CD25−. For ease of presentation of data, we have designated these three subsets as A, B and C, respectively, and which correspond to DN1 (C) or DN2 (A, B) assignments. (We wish to point out, however, that the distinction between populations A and B is not evident in the DN2 assignment of non-transgenic thymi.)
Figure 2.
Fluorescence-activated cell sorter (FACS) analysis of the surface phenotype of (a) non-transgenic, (b) hemizygous, or (c) homozygous M2 (H37A) transgenic thymi at 2 weeks of age. Equivalent profiles were obtained for the three homozygous transgenic lines.
The initial founder and/or early progeny (hemizygous) mice showed the same intermediate phenotype, and thymic cellularity was not as severely reduced. However, the distribution of DP and SP T cells was distinct from age-matched non-transgenic thymi (compare Fig. 2a with Fig. 2b) and a significant number of +/− thymocytes were CD44+ CD25+, expressing both CD4 and CD8. However, when the final homozygous lines were crossed to wild type, this phenotype was not observed (Table 2): the majority of +/− thymocytes were DP, albeit with severely reduced cellularity compared to wild type for all three transgenic lines. Similarly, in spleen there was a reduction in CD3+ cells for +/− mice and a total absence of CD3+ in +/+ mice (Table 3). For Tg5 mice, a reduced cellularity extended to the B-cell compartment with a 10-fold reduction in B220+ cell number, whereas far greater variability in B220+ cells was evident for Tg15b or Tg15c, between individual mice.
Table 3. Cellular profile of splenic tissue in wild type, hemizygous (+/−) or homozygous (+/+) transgenic mice at 2 weeks of age.
| CD3+ (%) | B220+ (%) | Cells (× 106) | |
|---|---|---|---|
| Wild type | 17–35 | 49–53 | 20–50 |
| Tg5+/− | 3–8 | 26–50 | 10–80 |
| Tg5+/+ | < 1* | 28–48 | 2–4 |
| Tg15b+/− | 2–5 | 35–50 | 20–30 |
| Tg15b+/+ | < 1* | 42–52 | 10–30 |
| Tg15c+/− | 4–7 | 72–79 | 20–30 |
| Tg15c+/+ | < 1* | 40–49 | 80–90 |
Phenotype of CD25 CD44 subsets (A, B and C)
The DN1, DN2 and DN3 subsets of wild-type mice are well characterized with respect to precursor-product lineage and cell cycle status.20–22 We wished to confirm that the DN subsets in transgenic thymic rudiments (Fig. 2c; A, B and C) mirrored the normal developmental pathway and did not represent effete ‘end cells’.
Cell cycle status
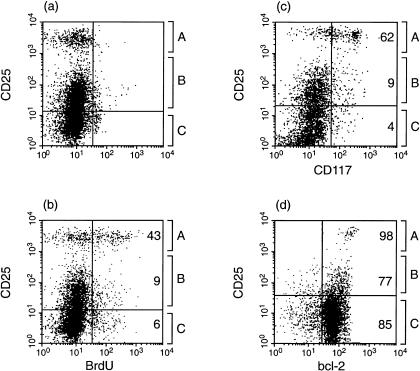
Adult transgenic mice were given BrdU intraperitoneally and their thymi analysed, 5 hr later, by FACS, for CD25 expression and BrdU incorporation. The FACS profiles for experimental and zero-time controls are shown in Fig. 3(a), 3(b) and summarized in Table 4. The highest BrdU labelling index was in the A subset (43%), while there was low-level BrdU incorporation for the B and C populations (6–9%).
Figure 3.
Surface phenotype of the CD25 subsets (CD25−, CD25+, CD25hi) from homozygous Tg5 thymic rudiments. (a) BrdU time-zero control; (b) BrdU-labelled cells 5 hr after intraperitoneal injection; (c) CD117 expression; (d) bcl-2 expression.
Table 4. Summary of the surface phenotype of CD44 CD25 subsets (A,B,C) as defined in Fig. 3.
| CD44 CD25 subsets | |||
|---|---|---|---|
| A | B | C | |
| Total cells (%) | 14 | 44 | 41 |
| BrdU-labelled (%) | 43 | 9 | 6 |
| CD117+ (%) | 62 | 9 | 4 |
| Bcl-2+ (%) | 98 | 77 | 85 |
CD117 expression
CD117 (or c-kit) is expressed at the DN1 and DN2 stages of development in both fetal and adult thymus, and downregulated at the DN3 stage. Figure 3(c) shows that a majority of the A subset are CD117+ in contrast to the low percentage of positive cells in the B and C subsets.
bcl-2 expression
bcl-2 exhibits temporal changes in transcription/translation during early T-cell development:17,23,24 constitutive expression at the DN stage, down-regulation at the DP stage and continued expression from SP to mature, peripheral T cell. Figure 3(d) shows that the majority of cells in A, B and C subsets are bcl-2+.
A summary of the phenotypes of the three A, B and C subsets, present in transgenic thymic rudiments, is shown in Table 4. With the singular exception of CD117 expression, the observed phenotypes mirror what has been reported for DN1 and DN2 cells from non-transgenic thymus.
Altered B-cell lymphopoiesis
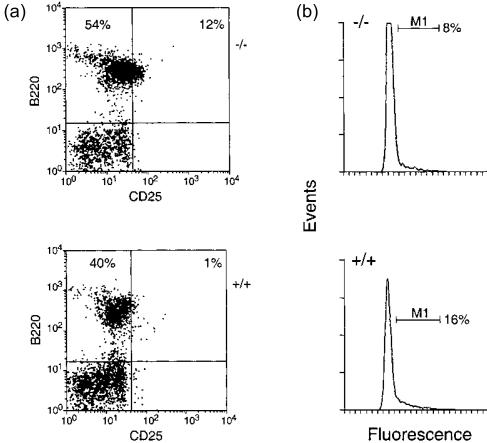
The radical reduction in lymphoid cellularity of Tg5 spleen (Table 3) indicated that M2 (H37A) had a global impact on lymphopoiesis, both for B- and T-cell development. This was further illustrated by FACS analyses of 3-week-old non-transgenic or Tg5 bone marrow (Fig. 4a), indicating a severe reduction in B220+ and CD25+ cells or B220+ cells co-expressing immunoglobulin M (IgM), with a corresponding increase in B220+ CD43+ BP-1+ cells (Table 5). As B220 and CD25 are co-expressed at the pre-B-cell stage, following productive VDJH rearrangement,21,23 Transgene expression had affected lymphopoiesis throughout development from pre- or pro-B cell to mature, peripheral B cell. Moreover, major differences were found in the proportion of ‘cycling’ cells (> 2c DNA) in the lymphoid-gated population (by forward/side scatter) with a significant increase in ‘cycling’ cells with > 2c DNA content in transgenic (13–16%) as compared to wild-type bone marrow (8–9%) (Fig. 4b).
Figure 4.
Altered B-cell lymphopoiesis in Tg5 bone marrow. Three-week-old non-transgenic (−/−) or Tg5 (+/+) bone marrow samples were analysed by fluorescence-activated cell sorter (FACS) for surface expression of CD25 and B220 (a), or DNA content (b).
Table 5. Fluorescence-activated cell sorter (FACS) data from B220+ cells expressing CD43, BP-1 and sIgM surface markers.
| % (B220+) | |||
|---|---|---|---|
| CD43 | BP-1 | sIgM | |
| Non-Tg | 5 | 11 | 14 |
| Tg5 | 22 | 26 | 2 |
Thymomas with a novel surface phenotype
A common feature of the three transgenic lines was the occurrence of ‘aggressive’ thymomas resulting in a high mortality rate in older mice, particularly in female breeding pairs after their second pregnancy, which severely hindered our programme to establish homozygous transgenic lines.
The surface phenotype of a typical thymoma (obtained from either transgenic line 5 or 15b) at homozygosity is shown in (Fig. 5a). A striking feature of such tumours is the uniformly high level of CD25 expression, together with co-expression of CD44, CD4 and CD8 and lack of CD3 co-receptor expression. In contrast, thymomas from initial founders had far greater variability in surface marker expression, both for individual tumours (Fig. 5b) and tumours from different animals.
Figure 5.
Surface Phenotype of thymoma from homozygous (a) or homozygous (b) M2 (H37A) transgenic mice. A similar phenotype was observed for thymomas from homozygous Tg5 or Tg15b mice.
Drug rescue of fetal T-cell development
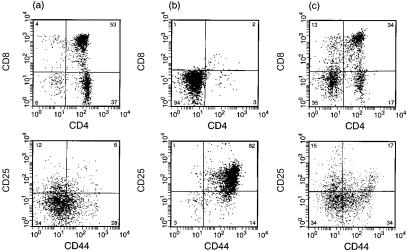
As the toxicity of M2 (H37A) expression in cell transfectants was effectively blocked by amantadine (Fig. 1d), we wished to attempt rescue of transgenic T-cell development by drug treatment of thymic progenitor cells. The entire developmental programme, from progenitor to mature SP T-cell, can be recapitulated under defined in vitro conditions by culturing day-16 fetal thymus lobes. We cultured non-transgenic or homozygous (M2-H37A) transgenic thymic lobes in the presence or absence of amantadine, and examined the surface phenotype of viable cells at day 9. In the absence of drug, the majority of cells were CD44+ CD25+ CD4− CD8− (Fig. 6b), and this was in striking contrast to that of drug-treated lobes (Fig. 6c). Here, development to SP T cells had been rescued by amantadine. By comparison to non-transgenic lobes (Fig. 6a) there were some differences in the proportion of DP to SP T cells, which might be attributed to progenitor frequency at the initiation of culture. As the Lck proximal promoter is active at this time in embryo development, M2 (H37A) would have eliminated a substantial fraction of transgenic progenitor cells prior to in vitro culture.
Figure 6.
Rescue of fetal T-cell development by drug treatment. Thymic lobes from day-16 embryos, either non-transgenic or Tg5, were cultured for 9 days in the presence or absence of amantadine (10 µg/ml) and analysed by fluorescence-activated cell sorter (FACS) for all surface expression. (a) non-transgenic; (b) Tg5 in the absence of amantadine; (c) Tg5 in the presence of amantadine.
Discussion
Novel features of the mutant influenza virus ion channel protein, M2 (H37A), as a conditional-lethal transgene, are threefold:
- Its expression confers permeability to Na+ or K+ ions at physiological pH, which is lethal to eukaryotic cells.
- The ease of genetic manipulation of such a small protein (97 amino acids) with available promoters and its efficient targeting to the surface membrane.
- The ability to specifically inhibit ion channel activity with the antiviral drug amantadine and thereby maintain cell viability.
We should emphasize herein, that this is the first report of the toxicity of a viral ion-channel protein on constitutive expression in mammalian cells. Wild-type M2 is a low pH-regulated, proton-specific channel and its expression in mammalian cells is not deleterious to cell growth or viability (Fig. 1c). This is consistent with known electrophysiological data that M2 is functionally ‘closed’ at physiological pH. In contrast, the mutant channel M2 (H37A) is ‘open’ to monovalent cations (Na+ or K+) at physiological pH and is therefore toxic on heterotypic expression in mammalian cells.
M2 (H37A) has a signal sequence for targeting to the eukaryotic- cell surface and ion-channel expression results in efficient cell ablation, irrespective of transgene copy number. This contrasts with many available toxic transgenic candidates in which the effect(s) of the transgene are secondary to host cellular metabolism and/or with variable efficiency in cell ablation. But more significantly, the ability to block ion-channel activity with antiviral drugs12 confers a conditional feature to M2 (H37A) transgene expression. We failed to detect DP or SP T cells in the periphery, indicating efficient toxicity of the transgene.
Our work with M2 (H37A) transfectants indicates that the ‘off-rate’ for amantadine is rapid, as shown by the brisk decline in biosynthetic labelling and cell viability in its absence (Fig. 1). Conversely, the toxic action of the ion channel is efficiently blocked by drug treatment of cell transfectants or fetal thymic progenitor cells. This provides some considerable scope for the future development of M2 conditional-lethal transgenes, using either M2 (H37A) or other variant ion channels.
The accumulation of three distinct populations of DN progenitor cells in the transgenic thymic rudiment, which we designated A (CD44+ CD25hi), B (CD44+ CD25+) and C (CD44+ CD25−), is (we assume) coincident with activation of the p56Lck proximal promoter at the transition point from the CD44+ CD25hi to the CD44− CD25+ (or DN3) stage of development. The phenotype of the ABC populations (Fig. 3; Table 4) is generally consistent with published data for DN1 and DN2 cells from adult non-transgenic mice. The highest BrdU labelling index was in the A subset (43%), indicative of a rapidly cycling population of progenitor cells. Similar findings have been reported for CD44+ CD25+ and CD44− CD25+ cells in non-transgenic thymi.22 Similarly, each of these three subsets exhibited a high index for bcl-2 expression (Table 4). However, we found major differences in CD117 expression between the ABC subsets. In non-transgenic thymus, it has been reported that both DN1 (CD44+ CD25−) and DN2 (CD44+ CD25+) cells express CD117, while in M2 (H37A) transgenic mice we found low-level expression in CD44+ CD25− (C; 4%) and CD44+ CD25+ (B; 9%) subsets. Such differences in phenotypic expression may well be the result of altered homeostasis (to wild type) in the face of efficient cell ablation in transgenic mice at the DN2 to DN3 transition point. The intermediate hemizygous phenotype of founder mice (Fig. 2b) was common to all three transgenic lines despite differences in trangene copy number (two, four, or 16), suggesting the absence of position-effect variegation. Finally, we consider that the distinction between subsets A and B is novel and not evident for non-transgenic (DN2) cells.
The occurrence of thymomas provides a novel aspect to this system viz the contrasting phenotype of thymomas from homozygous (+/+) and ‘early founder’ (+/−) mice (Fig. 4a, 4b). The uniform surface phenotype of +/+ thymomas (CD44+ CD25hi) corresponds to the A subset present in transgenic thymic rudiments (Fig. 2c) – a cycling population of cells with a high index for BrdU labelling, which we assume are at the penultimate stage of development, prior to downregulation of CD44 and toxic expression of M2 (H37A). It is of interest that these thymomas express CD4 and CD8 in the absence of T-cell receptor-β (TCR-β) rearrangement or CD3 expression. In contrast, thymomas from +/− mice have a less defined phenotype, both for CD4 and CD8 and with variable expression of CD25. This may reflect either differences in clonality of the two tumour types, or alternatively that cell ablation is less stringent in +/− mice.
The homeostatic changes evident in transgenic bone marrow, with a significant increase in number of cells ‘in cycle’ (Fig. 5), may provide some basis both for the observed incidence of thymomas and radical reduction in number of peripheral B220+ B cells (Table 3). There is no published evidence to suggest that the Lck proximal promoter is expressed during B-cell development. However, the significant reduction in B220+ CD25+ pre-B cells in transgenic bone marrow (from 12 to 1%) has to be accounted for. In view of the toxic efficiency of M2 (H37A), we consider it unlikely that, if the transgene was expressed during early B-cell development, it would not ablate splenic B220+ cells. It is possible that the Lck proximal promoter is expressed on a subset of pro- or pre-B cells, or that such a subset is critically dependent on early T-cell development.
The occurrence, in older mice, of thymomas with the surface phenotype CD25+ CD44+ is also consistent with altered homeostasis and oncogenic transformation prior to Lck promoter activation. We consider that expression of CD4 and CD8 on such tumours may not necessarily correlate with a particular developmental stage. M2 (H37A) expression was not detected in these thymomas by either Western blotting or reverse transcription–polymerase chain reaction (RT–PCR) (C. A. Smith et al., unpublished).
We propose that M2 (H37A) may provide a generic protocol as a conditional-lethal transgene (particularly as the antiviral drug amantadine can cross the blood–brain barrier) and therefore be of some relevance in the broader context of vertebrate development. Moreover, the rapid ‘off rate’ for interaction of the drug with the ion channel may provide a model system useful for identification of novel gene products that are expressed during early T-cell development, by subtractive analysis of transgenic progenitor cells cultured in the presence or absence of the drug.
In conclusion, we wish to emphasize the efficiency of M2 (H37A) as a conditional-lethal transgene. It is estimated that 2 × 106 mature, single-positive T cells exit the adult thymus daily with a further 10-fold progenitor cells subject to positive-negative selection in the thymus.25 In M2 (H37A) transgenic mice we failed to detect DP or SP T cells in either the transgenic thymic rudiment or spleen. It is not surprising therefore that such mice are severely immunocomprised and subject to opportunistic infections with, for example, Pneumocystis carinii (C. A. Smith et al., unpublished). However, T-cell development can be rescued by treatment of progenitor cells with amantadine and may therefore provide a useful resource for identifying novel regulatory elements in early lymphoid development. We conclude, however, with a caveat: administration of amantadine to mice after birth (2–3 weeks old) did not rescue T-cell development (DP or SP T cells) in either the thymic rudiment or the spleen (C. A. Smith et al., unpublished). However, it is known that establishment of the thymic epithelial architecture and T-cell development are interdependent events26 and this may account for our findings of fetal, but not adult, T-cell rescue.
References
- 1.Frohman MA, Martin GR. Cut, paste and save: new approaches to altering specific genes in mice. Cell. 1989;56:145–7. doi: 10.1016/0092-8674(89)90887-8. [DOI] [PubMed] [Google Scholar]
- 2.Capecchi MR. Altering the genome by homologous recombination. Science. 1989;244:1288–92. doi: 10.1126/science.2660260. [DOI] [PubMed] [Google Scholar]
- 3.Kuhn R, Schwenk F, Aguet M, Rajewsky K. Inducible gene targeting in mice. Science. 1995;269:1427–9. doi: 10.1126/science.7660125. [DOI] [PubMed] [Google Scholar]
- 4.Palmiter RD, Behringer RR, Quaife CJ, Maxwell F, Maxwell IH, Brinster RL. Cell lineage ablation in transgenic mice by cell-specific expression of a toxin gene. Cell. 1987;50:435–43. doi: 10.1016/0092-8674(87)90497-1. [DOI] [PubMed] [Google Scholar]
- 5.Breitman ML, Clapoff S, Rossant J, Tsui L-C, Glode LM, Maxwell IH, Bernstein A. Genetic ablation: targeted expression of a toxin gene causes microphthalmia in transgenic mice. Science. 1987;238:1563–5. doi: 10.1126/science.3685993. [DOI] [PubMed] [Google Scholar]
- 6.Borelli E, Heyman R, Hsi M, Evans RM. Targeting of an inducible toxic phenotype in animal cells. Proc Natl Acad Sci USA. 1988;85:7572–6. doi: 10.1073/pnas.85.20.7572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lamb RA, Zebedee SL, Richardson CD. Influenza virus M2 protein is an integral membrane protein expressed on the infected-cell surface. Cell. 1985;40:627–33. doi: 10.1016/0092-8674(85)90211-9. [DOI] [PubMed] [Google Scholar]
- 8.Sugrue RJ, Hay AJ. Structural characteristics of the M2 protein of influenza A viruses: evidence that it forms a tetrameric channel. Virology. 1991;180:617–24. doi: 10.1016/0042-6822(91)90075-M. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pinto LH, Holsinger LJ, Lamb RA. Influenza virus M2 protein has ion channel activity. Cell. 1992;69:517–28. doi: 10.1016/0092-8674(92)90452-i. [DOI] [PubMed] [Google Scholar]
- 10.Schroeder C, Ford CM, Wharton SA, Hay AJ. Functional reconstitution in lipid vesicles of influenza virus M2 protein expressed by baculovirus: evidence for proton transfer activity. J Gen Virol. 1994;75:3477–84. doi: 10.1099/0022-1317-75-12-3477. [DOI] [PubMed] [Google Scholar]
- 11.Chizhmakov IV, Geraghty FM, Ogden DC, Hayhurst A, Antoniou M, Hay AJ. Selective proton permeability and pH regulation of the influenza virus M2 channel expressed in mouse erythroleukaemia cells. J Physiol. 1996;494:329–36. doi: 10.1113/jphysiol.1996.sp021495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hay AJ, Wolstenholme AJ, Skehel JJ, Smith MH. The molecular basis of the specific anti-influenza action of amantadine. EMBO J. 1985;4:3021–4. doi: 10.1002/j.1460-2075.1985.tb04038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang C, Lamb RA, Pinto LH. Activation of the M2 ion channel of influenza virus. a role for the transmembrane domain histidine residue. Biophys J. 1995;69:1363–71. doi: 10.1016/S0006-3495(95)80003-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gandhi CS, Shuck K, Lear JD, Dieckmann GR, DeGrado WF, Lamb RA, Pinto LH. Cu (II) inhibition of the proton translocation machinery of the influenza A virus M2 protein. J Biol Chem. 1999;274:5474–82. doi: 10.1074/jbc.274.9.5474. [DOI] [PubMed] [Google Scholar]
- 15.Chaffin KE, Beals CR, Wilkie TM, Forbush KA, Simon MI, Perlmutter RM. Dissection of thymocyte signaling pathways by in vivo expression of pertussis toxin ADP-ribosyltransferase. EMBO J. 1990;9:3821–9. doi: 10.1002/j.1460-2075.1990.tb07600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jenkinson EJ, Franchi LL, Kingston R, Owen JJ. Effect of deoxyguanosine on lymphopoiesis in the developing thymus rudiment in vitro: application in the production of chimeric thymus rudiments. Eur J Immunol. 1982;12:583–7. doi: 10.1002/eji.1830120710. [DOI] [PubMed] [Google Scholar]
- 17.Von Freeden-Jeffry U, Solvason N, Howard M, Murray R. The earliest T lineage-committed cells depend on IL-7 for bcl-2 expression and normal cell cycle progression. Immunity. 1997;7:147–54. doi: 10.1016/s1074-7613(00)80517-8. [DOI] [PubMed] [Google Scholar]
- 18.Tough DF, Sprent J. Turnover of naive-and memory-phenotype T-cells. J Exp Med. 1994;179:1127–35. doi: 10.1084/jem.179.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rabinovitch PS, Torres RM, Engel D. Simultaneous cell cycle analysis and two-color surface immunofluorescence using 7-amino-actinomycin D and single laser excitation: applications to study of cell activation and the cell cycle analysis of murine Ly-1 B cells. J Immunol. 1986;136:2769–75. [PubMed] [Google Scholar]
- 20.Godfrey DI, Kennedy J, Suda T, Zlotnik A. A developmental pathway involving four phenotypically and functionally distinct subsets of CD3−CD4−CD8− triple-negative adult mouse thymocytes defined by CD44 and CD25 expression. J Immunol. 1993;150:4244–52. [PubMed] [Google Scholar]
- 21.Pénit C, Vasseur F, Papiernik M. In vivo dynamics of CD4− CD8− thymocytes. Proliferation, renewal and differentiation of different cell subsets studied by DNA synthetic labeling and surface antigen detection. Eur J Immunol. 1988;18:1343–50. doi: 10.1002/eji.1830180907. [DOI] [PubMed] [Google Scholar]
- 22.Pénit C, Lucas B, Vasseur F. Cell expansion and growth arrest phases during the transition from precursor (CD4−8−) to immature (CD4+8+) thymocytes in normal and genetically modified mice. J Immunol. 1995;154:5103–13. [PubMed] [Google Scholar]
- 23.Moore NC, Anderson G, Williams GT, Owen JJT, Jenkinson EJ. Developmental regulation of bcl-2 expression in the thymus. Immunology. 1994;81:115–9. [PMC free article] [PubMed] [Google Scholar]
- 24.Linette GP, Grusby MJ, Hedrick SM, Hansen TH, Glimcher LH, Korsmeyer SJ. Bcl-2 is upregulated at the CD4+ CD8+ stage during positive selection and promotes thymocyte differentiation at several control points. Immunity. 1994;1:197–205. doi: 10.1016/1074-7613(94)90098-1. [DOI] [PubMed] [Google Scholar]
- 25.Egerton M, Shortman K, Scollay R. The kinetics of immature thymocyte development in vivo. Int Immunol. 1990;2:501–7. doi: 10.1093/intimm/2.6.501. [DOI] [PubMed] [Google Scholar]
- 26.Van Ewijk W, Holländer G, Terhorst C, Wang B. Stepwise development of thymic microenvironments in vivo is regulated by thymocyte subsets. Development. 2000;127:1583–91. doi: 10.1242/dev.127.8.1583. [DOI] [PubMed] [Google Scholar]